Abstract
Aim
To compare the effects of dietary fibers on hepatic cellular signaling in mice.
Methods
Mice were randomly divided into four groups (n = 9/group): high-fat diet (HFD) control, cellulose, psyllium, and sugarcane fiber (SCF) groups. All mice were fed a HFD with or without 10% dietary fiber (w/w) for 12 weeks. Body weight, food intake, fasting glucose, and fasting insulin levels were measured. At the end of the study, hepatic fibroblast growth factor (FGF) 21, AMP-activated protein kinase (AMPK) and insulin signaling protein content were determined.
Results
Hepatic FGF21 content was significantly lowered, but βKlotho, fibroblast growth factor receptor 1, fibroblast growth factor receptor 3, and peroxisome proliferator-activated receptor alpha proteins were significantly increased in the SCF group compared with those in the HFD group (P < 0.01). SCF supplementation also significantly enhanced insulin and AMPK signaling, as well as decreased hepatic triglyceride and cholesterol in comparison with the HFD mice. The study has shown that dietary fiber, especially SCF, significantly attenuates lipid accumulation in the liver by enhancing hepatic FGF21, insulin, and AMPK signaling in mice fed a HFD.
Conclusion
This study suggests that the modulation of gastrointestinal factors by dietary fibers may play a key role in both enhancing hepatic multiple cellular signaling and reducing lipid accumulation.
Introduction
Nonalcoholic fatty liver disease (NAFLD), characterized by excessive lipid accumulation in the liver, is a significant health problem affecting 70 million adults in the USA (30% of the adult population). An estimated 20% of these individuals have the most severe form of NAFLD – nonalcoholic steatohepatitis. Although the mechanisms underlying the disease development and progression are awaiting clarification,Citation1 insulin resistance and obesity-related inflammation, among other possible genetic, dietary, and lifestyle factors, are thought to play a key role.Citation2,Citation3
Hormones such as insulin and glucagon have well-established roles in controlling substrate utilization and energy balance in response to nutritional status. Briefly, insulin activates the insulin receptor tyrosine kinase (IR), which phosphorylates and recruits different substrate adaptors such as the insulin receptor substrate (IRS) family of proteins. Tyrosine-phosphorylated IRS then displays binding sites for numerous signaling partners. A key action of insulin is to stimulate glucose uptake into cells by inducing translocation of the glucose transporter 4 from intracellular storage to the plasma membrane.Citation4 Phosphatidylinositol 3 kinase (PI 3K) and protein kinase B (Akt) are known to play a role in glucose transporter 4 translocation.Citation5
Fibroblast growth factors (FGFs) are a large family of polypeptide growth factors that are found in organisms ranging from nematodes to humans. In particular, hormone-like FGF19, FGF21, and FGF23 have been shown to be involved in glucose, lipid, bile acid, phosphate, and vitamin D metabolism via βKlotho, a protein that binds FGF21 and facilitates FGF21 activation of FGF receptors (FGFRs – such as FGFR1 and FGFR3). However, the cellular mechanisms underlying their functions as metabolic regulators are still being defined.Citation6 FGF21 has recently been identified as an important regulator of glucose and lipid metabolism in animal models.Citation7,Citation8 FGF21, induced in the liver in response to fasting, has been shown to increase hepatic lipid oxidation via increased gene expression of peroxisome proliferator-activated receptor γ coactivator protein 1 alpha (PGC-1α), a key transcriptional regulator of energy homeostasis, and causes corresponding increases in fatty acid oxidation, tricarboxylic acid cycle flux, and gluconeogenesis without increasing glycogenolysis.Citation9 FGF21 is also regulated by peroxisome proliferator-activated receptor alpha (PPARα) activation, which plays a critical role in regulating metabolism during ketosis.Citation9,Citation10 It has been observed that transgenic mice with overexpression of FGF21 are resistant to diet-induced obesity and metabolic disturbance.Citation11 Administration of FGF21 also enhances insulin sensitivity in insulin-resistant animals. FGF21’s acute glucose-lowering and insulin sensitizing effects are potentially associated with its metabolic actions in the liver and adipose tissues.Citation12 In diabetic animals, FGF21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways.Citation13 A study has shown that FGF21 regulates energy homeostasis in adipocytes through activation of AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1), resulting in enhanced mitochondrial oxidative function.Citation14 Paradoxically, elevated FGF21 has also been identified as an independent risk factor related to metabolic syndrome.Citation15,Citation16
Dietary fiber consumption may facilitate weight loss and improve lipid profiles and glucose metabolism.Citation17–Citation19 However, the cellular signaling mechanisms of dietary fiber-mediated improvements in glucose and lipid metabolism remain largely unknown. Our previous study showed the various effects of dietary fibers on metabolism in a diet-induced obesity (DIO) animal. Dietary fibers incorporated into high-fat diets (HFDs) such as sugarcane fiber (SCF) and psyllium (PSY) appeared to attenuate weight gain, enhance insulin sensitivity, modulate leptin and glucagon-like peptide 1 (GLP-1) secretion, and down-regulate gastric ghrelin gene expression.Citation20 Given the importance of insulin, FGF21, and AMPK signaling in both glucose and fatty acid metabolism, we hypothesized that dietary fiber-mediated changes in insulin sensitivity are involved in these cellular signaling pathways. Thus, using the tissues from a previously reported cohort of mice fed an HFD,Citation20 we compared the effects of three dietary fibers on hepatic FGF21, insulin, and AMPK signaling.
Materials and methods
Animals
The design of the animal study and the results for carbohydrate metabolism and body weight were previously reported.Citation20 As described, thirty-six male 4-week-old C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). After arrival, the animals were housed one per cage with ad libitum access to rodent chow and water for a 2-week acclimation period under specific pathogen-free conditions with a 12-hour light–dark cycle. Animals were then randomly divided into four treatment groups: HFD alone, HFD containing 10% (wt/wt) cellulose (CEL), HFD containing 10% (w/w) PSY, and HFD containing 10% (w/w) SCF. At the end of the 12-week study, the mice were fasted overnight and then euthanized via carbon dioxide inhalation followed by decapitation at the basal condition (n = 5) or insulin-stimulated condition (n = 4, at 10 minutes, insulin injection at a dose of 2 U/kg body weight) for each group. Liver and other tissues were collected, quickly placed in a liquid nitrogen container, and then stored at −80°C until analysis. This animal protocol was approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center.
Sources of dietary fiber
PSY husk powder (.75% soluble fiber, average particle size >100 μm) was obtained from Source Naturals (Scotts Valley, CA). CEL powder (100% insoluble fiber, particle size ≤100 μm) was obtained from NutriCology (Hayward, CA). Nano-sized SCF (85% insoluble, 70% of particles <1 μm) was prepared by Dr Kun Lian at the Center for Advanced Microstructures and Devices at Louisiana State University. Briefly, after the sugarcane was crushed to extract the juice, a proprietary method was used to reduce the remaining components (bagasse) to nanometer-sized particles (Figure S1). This processing technology maintains the integrity of the constituents of the original bagasse as described previously.Citation20
Blood chemistry and hormone analysis
After 4 hours of fasting, blood samples were collected from the orbital sinus of anesthetized mice at week 11 of the study. Plasma insulin, glucose levels, plasma triglyceride, and cholesterol concentrations were determined as previously described.Citation20,Citation21 Homeostasis model assessment – insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = [I0 (μU/mL) × G0 (mmol/L)]/22.5.Citation22
Lipid extract from liver for triglyceride and cholesterol measurements
Liver lipid content was determined by the Folch procedure.Citation21 Briefly, liver tissues (about 25 mg) were added to five volumes of phosphate-buffered saline (PBS) (w/v), minced with scissors in an Eppendorf (Hamburg, Germany) microcentrifuge tube, and homogenized. Liver lysates were added to ten volumes of an extract solvent containing 2:1 chloroform:methanol (v/v). After vortexing, the tubes were centrifuged at 5000 g for 10 minutes. Aliquots of 100 μL were removed from the bottom phase, transferred to a new tube, and dried under nitrogen gas. After drying, 100 μL of PBS was added to the tube along with 5 μL of the mixture used to measure triglyceride or cholesterol content using a tryglyceride reagent kit (Sidma-Aldrich, St Louis, MO) or a cholesterol quantitation kit (BioVision, Milpitas, CA) as per manufacturers’ instructions. The results were normalized by protein concentration.
Immunoblotting analysis
Liver tissue lysates were prepared by homogenization in buffer A (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4; 1% NP-40 (Calbiochem, Darmstadt, Germany); 137 mM NaCl; 1 mM henylmethanesulfonylfluoride; 10 μg/mL aprotinin; 1 μg/mL pepstatin; 5 μg/mL leupeptin) using a PRO200 homogenizer (PRO Scientific Inc, Oxford, CT). The samples were centrifuged at 14 000 g for 20 minutes at 4°C and protein concentrations of the supernatants were determined using a protein assay kit (Bio-Rad Laboratories, Inc, Hercules, CA). Supernatants (50 μg) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and subjected to immunoblotting. Protein abundances were detected with antibodies against insulin receptor substrate 1 (IRS-1), insulin receptor (IR)-p(Tyr1150–1151), IRS-1 p(Tyr612), Akt1, Akt1 p(ser473), AMPKα p(Thr172), AMPKα1, AMPKα2, FGFR1, PGC-1α, SIRT1, ACC p(cer79), ACC, and PPARα (Upstate, Lake Placid, NY), FGFR3 (Bioworld Inc, Visalia, CA), antiphosphotyrosine 20 (PY 20), insulin receptor beta (IR β), sterol regulatory element-binding protein (SREBP) 1c, and βKlotho antibodies (Santa Cruz Biotech Inc, Santa Cruz, CA), and β-actin (Affinity BioReagents Inc, Golden, CO) using Chemiluminescence Reagent Plus from PerkinElmer Life Science (Boston, MA), and quantified via a Bio-Rad universal hood II densitometer with Quantity One software (v 4.5; Bio-Rad, Hercules, CA). The highly conserved structural protein β-actin was used to normalize protein data and specific protein phosphorylation was normalized by its corresponding protein, as stated in the figure captions.
Liver PI 3K activity assay
Hepatic IRS-1-associated PI 3K activities at baseline (0 minutes) and 10-minutes post-insulin stimulation (2 U/kg body weight via intraperitoneal injection) were assessed as previously described.Citation23 Briefly, 500 μg of liver lysate protein was immunoprecipitated with 3 μg of IRS-1 antibody and protein A agarose. IRS-1 immune complexes were incubated (10 minutes, 22°C) in 50 μL of 20 mM Tris/HCl (pH 7.0) buffer containing 50 μM [γ-32P]adenosine 5′-triphosphate (5 μCi; PerkinElmer), 10 mM MgCl2, 2 mM MnCl2, 100 mM NaCl, 2 mM ethylenediaminetetraacetic acid, and 10 μg of phosphatidylinositol (PI). After thin-layer chromatography, isotope-labeled phosphatidylinositol 3-phosphate (PI-3P) was visualized by autoradiography and quantitated by densitometer.
Liver FGF21 content assessment
Liver tissues (~25 mg) were minced with scissors in ten volumes of homogenization buffer (w/v) in a microcentrifuge tube and homogenized using a Bio-Gen Pro 200 micro-homogenizer (PRO Scientific, Oxford, CT). Samples were centrifuged at 15 000 g for 15 minutes. For FGF21 measurement, 50 μL of supernatant was used with a Mouse FGF-21 ELISA Kit (R&D Systems, Minneapolis, MN), according to instructions of the manufacturer. The FGF21 standard ranges were from 82 to 6667 pg/mL. Intra- and interassay coefficients of variation (CVs) of FGF21 were 4.5% and 6.1%, respectively. The result of FGF21 quality control was 278 pg/mL (range 191–319 pg/mL).
RNA isolation, reverse transcription, and quantitative polymerase chain reaction (PCR)
RNA was isolated from liver tissues using TRIzol® reagent (Invitrogen, Carlsbad, CA) and purified using an RNeasy Mini Kit (Qiagen, Valencia, CA). Isolated RNA was reverse transcribed into cDNA by following the protocol of the SuperScript III First-Strand Synthesis System (Invitrogen). The primer sequences of candidate genes were designed using Primer Express® Software (v 3.0; Applied Biosystems, Foster, CA) and are shown in . All primers were ordered from Integrated DNA Technologies, Inc (Coralville, IA). The quantitative PCR reaction was conducted in 384-well PCR reaction plates on an ABI Prism Sequence Detector 7900 (Applied Biosystems) with Bio-Rad iTaqTM SYBR Green Supermix ROX Kits (Hercules, CA). PCR amplification was performed in duplicate and was normalized to β-actin.
Table 1 Sequence of listing primers
Statistical analysis
Results were expressed as mean ± standard error of the mean. Differences between two groups were tested with Student’s unpaired t test. Differences among the four groups were tested using one-way analysis of variance and least-significant difference or Tamhane’s T2 tests.Citation24
A P value ≤0.05 was considered statistically significant.
Results
Effects of dietary fibers on glucose and lipid metabolism, hormones, and body weight in mice fed a HFD
Data on body weight, blood glucose, insulin, GLP-1 and leptin from this animal cohort have been reported previously, and are outlined in for demonstration purposes.Citation20 In brief, after the 12-week intervention, it was observed that SCF- and PSY-supplemented mice had significantly reduced fasting plasma glucose and insulin and significantly enhanced insulin sensitivity, as measured by HOMA-IR, in comparison with mice fed only a HFD (P < 0.05 and P < 0.01, respectively; ). Moreover, SCF significantly reduced fasting plasma triglyceride levels and body weight but only modestly decreased plasma cholesterol. In contrast, CEL-supplemented mice had significantly reduced insulin sensitivity in comparison with mice fed only a HFD (P < 0.05). Mice in PSY and SCF supplement groups had reduced plasma leptin and increased GLP-1 levels compared with those in the HFD group, but there was no difference between the CEL and HFD groups. Interestingly, intervention with all of the dietary fibers did not appear to significantly alter caloric intake ().
Table 2 Clinical parameters in mice with dietary fiber supplementation
Effects of dietary fibers on hepatic lipid content, FGF21 signaling and gene expression of FGF21 signaling in mice
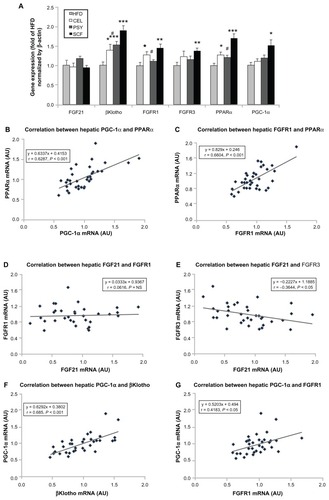
Mice supplemented with PSY had moderately decreased liver triglyceride content and mice supplemented with SCF had significantly reduced liver triglyceride content in comparison with mice in the HFD and CEL groups and there was no significant difference between the HFD and CEL groups. SCF also significantly reduced liver cholesterol content in comparison with CEL (P < 0.05, ). However, the lipid content in the liver did not significantly differ between PSY and SCF animals when compared with control. It was also observed that all fibers significantly increased cholesterol content in feces. PSY and SCF greatly increased glycerol content in feces, but CEL did not alter feces glycerol levels when compared with the HFD group (Figure S2B and C). FGF21 levels in the hepatic lysates were significantly lower in the SCF mice than in the HFD and CEL mice (). To evaluate the effects of dietary fibers on FGF21 signaling, hepatic FGF21 signaling proteins were also measured by western blotting. The data show that SCF significantly increased βKlotho, FGFR1, FGFR3, and PPARα protein abundance (first three proteins P < 0.01; PPARα P < 0.001). CEL and PSY significantly increased PPARα content when compared with the HFD alone (P < 0.05, ). All the dietary fibers modestly increased hepatic PGC-1α content in comparison with the HFD alone, but there were no significant differences between these groups (P = NS). Except for unaltered FGF21 mRNA, gene expression analysis shows a similar pattern to FGF21 signaling protein content measured by western blotting in the dietary supplemented mice: SCF significantly increased gene expression of βKlotho, FGFR1, FGFR3, PPARα, and PGC-1α in the livers of mice supplemented with this, compared with mice in the HFD group (); PSY only significantly increased βKlotho, while CEL significantly increased βKlotho, FGFR1, and PPARα mRNA content. None of the dietary fibers significantly altered hepatic FGF21 gene expression (). In addition, the gene expression of βKlotho, FGFR1, and PPARα in the PSY group was significantly lower than in the SCF group. There were no significant correlations between FGF21 protein and FGF21 mRNA, between FGF21 mRNA and PGC-1α mRNA, or between FGF21 mRNA and PPARα mRNA (r = 0.06, 0.269, and 0.07, respectively, P = NS). However, there were significant correlations in the gene expression between hepatic PGC-1α and PPARα () and between PPARα and FGFR1 (). There was no relationship between hepatic FGF21 and FGFR1 () and significant negative correlation between FGF21 and FGFR3 in all groups of mice (). There were significant relationships in the gene expression between PGC-1α and βKlotho () and between PGC-1α and FGFR1 ().
Figure 1 Effects of dietary fiber on hepatic lipid content in mice fed a HFD. Triglyceride and cholesterol levels in liver were measured after lipid extraction as described in the methods. (A) shows liver triglyceride concentration and (B) shows liver cholesterol levels. The results were normalized by protein concentrations.
Notes: Mean ± standard error of the mean (n = 9/group). *P < 0.05, SCF-supplemented group vs HFD-only group; #P < 0.05, SCF vs CEL.
Abbreviations: CEL, cellulose; HFD, high-fat diet; PSY, psyllium; SCF, sugar cane fiber.
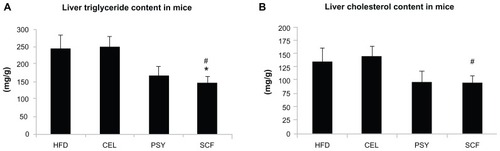
Figure 2 Hepatic fibroblast growth factor (FGF) 21 signaling in mice treated with dietary fiber. (A) FGF21 content of liver lysates, as measured by Rat/Mouse Fibroblast Growth Factor 21 (FGF21) ELISA Kit from Millipore (Billerica, MA). Hepatic FGF21 proteins were measured by western blotting. Liver lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, then transferred into nitrocellulose membranes. βKlotho, fibroblast growth factor receptor (FGFR) 1, FGFR3, peroxisome proliferator-activated receptor alpha (PPARα), and peroxisome proliferator-activated receptor γ coactivator protein 1 alpha (PGC-1α) were determined using their corresponding specific antibodies, shown in (B). Results were normalized with β-actin.
Notes: Mean ± standard error of the mean (n = 9/group). *P < 0.05; **P < 0.01; ***P < 0.001, dietary fiber group vs HFD group; #P < 0.05; ##P < 0.01, CEL or PSY vs SCF group.
Abbreviations: CEL, cellulose; HFD, high-fat diet; PSY, psyllium; SCF, sugar cane fiber.
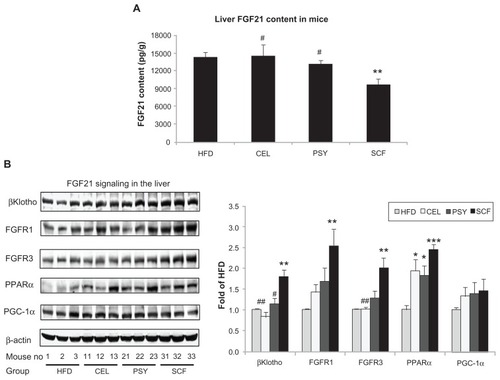
Figure 3 Gene expression of fibroblast growth factor (FGF) 21 signaling pathway in the liver and correlation between the gene levels. Hepatic FGF21 signaling-related gene expression was analyzed by real-time polymerase chain reaction assay. (A) FGF21 and its related gene expression levels; (B–G) show the correlations among genes.
Notes: Mean ± standard error of the mean (n = 9/group). *P < 0.05; **P < 0.01; ***P < 0.001, dietary fiber-supplemented group vs HFD-only group; #P < 0.05, CEL or PSY vs SCF group.
Abbreviations: AU, arbitrary unit; CEL, cellulose; HFD, high-fat diet; PPARα, peroxisome proliferator-activated receptor alpha; PGC-1α, peroxisome proliferator-activated receptor γ coactivator protein 1 alpha; PSY, psyllium; SCF, sugar cane fiber.
Effects of dietary fibers on hepatic insulin signaling in mice
Since SCF and PSY significantly reduced glucose and insulin concentrations and increased insulin sensitivity, we further evaluated the effects of these dietary fibers on hepatic insulin signaling in all groups of animals. It was shown that SCF supplementation significantly increased tyrosine phosphorylation of IRS-1, IR, and Akt1 in comparison with an unsupplemented HFD (P < 0.01 and P < 0.001, ), while PSY significantly increased basal and insulin-stimulated PY 20 content (). CEL greatly increased basal and insulin-stimulated phosphorylation of Akt1 content. CEL and PSY did not alter phosphorylation of PY 20, IRS-1, or IR. Thus, SCF supplementation significantly increased hepatic phosphorylation of PY 20, IR, and Akt1 and significantly increased insulin-stimulated phosphorylation of IRS-1, but not basal. SCF also greatly increased PI 3K activity at basal and insulin-stimulated conditions in mice (P < 0.001 and P < 0.01, ). CEL modestly increased PI 3K activity relative to the HFD group. PSY had significantly reduced insulin-stimulated PI 3K activity when compared with the SCF group (P < 0.0001, ).
Figure 4 Effects of dietary fibers on insulin signaling protein abundance and phosphatidylinositol 3 kinase (PI 3K) activity in mice livers. Liver lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and insulin signaling pathway proteins were detected with corresponding specific antibodies as shown in the figures. (A) shows that phosphorylation of antiphosphotyrosine 20 (PY 20), insulin receptor substrate (IRS) 1, insulin receptor beta (IR β), and Akt1 were normalized by their corresponding protein contents and PY 20 was normalized by β-actin. (B) Hepatic IRS-1-associated PI 3K activity in mice. Liver lysates from basal and insulin-stimulated mice were immunoprecipitated with IRS-1 antibody and protein A agarose. The immune complexes were incubated with reaction buffer containing [γ-32P]adenosine 5′-triphosphate, MgCl2, MnCl2, and phosphatidylinositol for 20 minutes. Autoradiograph was performed after thin-layer chromatography.
Notes: Data presented as mean ± standard error of the mean (n = 9/group). *P < 0.05; **P < 0.01; ***P < 0.001, dietary fiber-supplemented group vs HFD-only group; #P < 0.05; ##P < 0.01; ###P < 0.001; CEL or PSY vs SCF group.
Abbreviations: CEL, cellulose; HFD, high-fat diet; PSY, psyllium; SCF, sugar cane fiber.
![Figure 4 Effects of dietary fibers on insulin signaling protein abundance and phosphatidylinositol 3 kinase (PI 3K) activity in mice livers. Liver lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and insulin signaling pathway proteins were detected with corresponding specific antibodies as shown in the figures. (A) shows that phosphorylation of antiphosphotyrosine 20 (PY 20), insulin receptor substrate (IRS) 1, insulin receptor beta (IR β), and Akt1 were normalized by their corresponding protein contents and PY 20 was normalized by β-actin. (B) Hepatic IRS-1-associated PI 3K activity in mice. Liver lysates from basal and insulin-stimulated mice were immunoprecipitated with IRS-1 antibody and protein A agarose. The immune complexes were incubated with reaction buffer containing [γ-32P]adenosine 5′-triphosphate, MgCl2, MnCl2, and phosphatidylinositol for 20 minutes. Autoradiograph was performed after thin-layer chromatography.Notes: Data presented as mean ± standard error of the mean (n = 9/group). *P < 0.05; **P < 0.01; ***P < 0.001, dietary fiber-supplemented group vs HFD-only group; #P < 0.05; ##P < 0.01; ###P < 0.001; CEL or PSY vs SCF group.Abbreviations: CEL, cellulose; HFD, high-fat diet; PSY, psyllium; SCF, sugar cane fiber.](/cms/asset/74db24e1-6278-44f0-bfd4-efdc5843401a/dijn_a_30887_f0004_b.jpg)
Effects of dietary fiber supplementation on hepatic AMPK signaling in mice
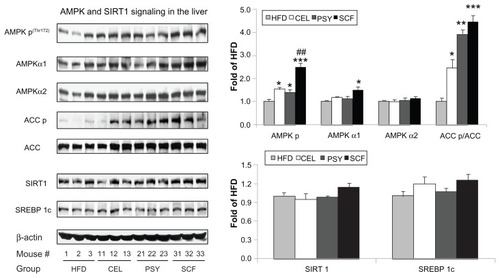
FGF21 regulates energy homeostasis in adipocytes through the activation of AMPK and SIRT1, resulting in enhanced mitochondrial oxidative function.Citation11 AMPK signaling pathway proteins in the liver were measured and data show that supplementation with all three dietary fibers significantly increased phosphorylation of AMPK [AMPK p(Thr172)]. Phosphorylated AMPK abundance was increased about 2.5-fold in the SCF group and 1.5~1.4 fold in the CEL and PSY groups compared with the HFD group (P < 0.001 and P < 0.05, respectively). Only SCF significantly increased AMPKα1 protein abundance (P < 0.05), but none of the three fibers were found to have altered AMPKα2 protein content when the supplemented animals were compared with those in the HFD group. Mice fed dietary fibers had significantly higher levels of ACC phosphorylation than the HFD animals. The ratio of ACC p to ACC increased 2.45-fold (P < 0.05) in the CEL, 3.91-fold (P < 0.01) in the PSY, and 4.45-fold (P < 0.001) in the SCF supplemented groups in comparison with the HFD group. Although hepatic SIRT1 and SREBP 1c were modestly increased in the SCF mice, there were no significant differences among all the groups ().
Figure 5 Effects of dietary fibers on liver AMP-activated protein kinase (AMPK) signaling in mice. The protein abundances of AMPKα1, AMPKα2, ACC, SIRT1, sterol regulatory element-binding protein (SREBP) 1c and phosphorylated AMPKα, and ACC were measured by immunoblotting.
Notes: Data are shown as mean ± standard error of the mean (n = 9/group). *P < 0.05; **P < 0.01; ***P < 0.001, dietary fiber-supplemented group vs HFD-only group; ##P < 0.01, CEL or PSY vs SCF group.
Abbreviations: CEL, cellulose; HFD, high-fat diet; PSY, psyllium; SCF, sugar cane fiber.
Discussion
Dietary fiber has an important role in contributing to metabolic health in humans. Proposed possible mechanisms for metabolic improvements with dietary fiber include reduced glucose and lipid absorption, increased hepatic extraction of insulin, improved insulin sensitivity at the cellular level, increased binding of bile acids, and promotion of satiety.Citation17,Citation25,Citation26 These effects may be related to its physical properties, structure, and the fiber compositions, particle sizes, amount and type of fiber, viscosity, and soluble and insoluble content.Citation17,Citation20,Citation25 Colonic fermentation of naturally available high-fiber foods can also be mainly attributed to soluble fiber, whereas no difference between soluble and insoluble dietary fiber consumption on the regulation of body weight has been observed.Citation27 It was also noticed that the consumption of not only soluble dietary fiber but also insoluble cereal dietary fiber and whole grains is consistently associated with a reduced risk of type 2 diabetes in a large prospective cohort study.Citation28 Indeed, our prior study revealed that the effects of SCF (85% insoluble fiber) on reducing glucose and lipid levels was greater than PSY (75% soluble fiber).Citation20 These effects were felt to be mediated in part by SCF’s nano-sized particles and by its bioactive components. Dietary fiber consumption was demonstrated to contribute to a number of metabolic effects in addition to changes in body weight. These favorable effects included improved insulin sensitivity, modulation of the secretion of certain gut hormones, and alteration of various metabolic and inflammatory markers associated with metabolic syndrome.Citation18,Citation29
Comparison of the effects of dietary fibers on hepatic insulin signaling
Obesity is a condition that impairs multiple cellular signaling pathways, resulting in resistance to FGF21, insulin, and leptin.Citation15,Citation16,Citation20 In this study, we observed that SCF and PSY supplementation significantly enhanced hepatic insulin signaling () in comparison with a HFD alone. SCF, which significantly increased both basal and insulin-stimulated tyrosine phosphorylation of IRS-1, IR β, and Akt1, appears to be the most effective of the three dietary fibers studied. In contrast, CEL supplementation in this study affected neither plasma glucose nor hepatic insulin signaling. In insulin-resistant states, elevated free fatty acid flux into the intestine, downregulation of intestinal insulin signaling, and upregulation of microsomal triglyceride transfer protein all appear to stimulate intestinal lipoprotein production. Gut peptides – that is, GLP-1 and GLP-2 – may be important regulators of intestinal lipid absorption and lipoprotein production.Citation30 Therefore, SCF and PSY, by significantly elevating plasma GLP-1 levels, could be said to mediate the favorable lipid effects via modulation of these incretins.Citation20 GLP-1 also mediates its effect on gastrointestinal motility, appetite, food intake, and glucagon secretion directly in an amylin-independent fashion.Citation31 Moreover, long-term treatment with GLP-1 or its analog can improve insulin sensitivity, as it was observed that GLP-1 and exendin-4 (a peptide analog of GLP-1) increased insulin-mediated glucose uptake in intact and tumor necrosis factor-alpha-treated 3T3-L1 adipocytes by increasing phosphorylated IR β, IRS-1, Akt, and glycogen synthase kinase 3.Citation32 A single administration of a recombinant adenovirus expressing GLP-1 was shown to result in the long-term remission of diabetes in ob/ob mice by improving insulin sensitivity via restoration of insulin signaling and reduction of hepatic gluconeogenesis.Citation33
SCF significantly enhanced hepatic FGF21 signaling
Other studies have shown that transgenic mice with over expression of FGF21 were resistant to DIO and metabolic disturbances, while FGF21 administration enhanced insulin sensitivity in insulin resistant animals.Citation11,Citation12 However, it was recently reported that increased levels of FGF21 can be an independent risk factor related to metabolic syndrome and can also represent an independent predictor of liver steatosis.Citation34,Citation35 The paradoxical increase of serum FGF21 levels in obese individuals may be explained by a compensatory response or resistance to FGF21.Citation36 In the present study, SCF supplementation significantly reduced hepatic FGF21 abundance and increased βKlotho, FGFR1, FGFR3, and PPARα contents in comparison with an unsupplemented HFD, indicating FGF21 negative feedback regulates its receptors, while PSY and CEL modestly altered liver FGF21 signaling. Although plasma FGF21 concentrations were not available in this study, liver FGF21 content measured using enzyme-linked immunosorbent assay may mirror plasma FGF21 levels, since the liver is the major source of circulating FGF21. Conversely, no significant relationship between hepatic FGF21 protein abundance and FGF21 mRNA levels was observed. The effects of dietary fiber on post-translational modification, such as alteration in the rates of protein synthesis and degradation, are not precisely known.
Another possible mechanism is that SCF may have significantly reduced hepatic FGF21 content by enhancing FGF21 signaling. Consistent with findings that FGF21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in DIO mice,Citation37 our data reveal that SCF supplementation significantly enhanced both liver FGF21 and insulin signaling in mice in comparison with a HFD alone. Based on the evidence that hyperinsulinemia induces skeletal muscle production of FGF21Citation16 and a PI 3K/Akt1 signaling pathway-dependent mechanism also regulates FGF21,Citation38 the effects of SCF on enhancing insulin signaling and attenuating insulin resistance may greatly help to reduce liver FGF21 content. Alternatively, FGF21 treatment has been found to induce mRNA and protein expression of PGC-1α, suggesting that PGC-1α may play a role in regulating FGF21 action.Citation39 However, our finding that all dietary fibers did not significantly alter PGC-1α indicates that the effect of SCF on FGF21 is not dependent on PGC-1α because SCF significantly reduced rather than increased hepatic FGF21 content.
All animals supplemented with dietary fiber demonstrated significantly increased hepatic phosphorylation of AMPK independent of SIRT1 and SREBP 1c. It is well documented that activation of AMPK can greatly increase fatty acid oxidation and inhibit lipid synthesis and glucose production by modulating the transcription of genes involved in lipogenesis and mitochondrial biogenesis.Citation40,Citation41 HFD supplementation with dietary fiber, especially SCF, dramatically increased hepatic phosphorylation of AMPK and ACC in comparison with a HFD alone (P < 0.05 in CEL and PSY and P < 0.001 in SCF, respectively). However, none of these fibers were shown to affect SIRT1 and SREBP 1c content.
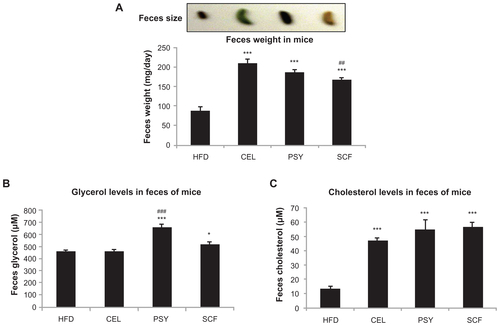
Our data suggest that the effects of dietary fiber on activation of hepatic AMPK signaling may not be associated with the SIRT1 and SREBP 1c pathways. Enhanced AMPK signaling in the SCF-treated mice could also account for the reduction of plasma glucose and lipid levels and the attenuation of hepatic lipid accumulation. Short-chain fatty acids produced during the colonic fermentation of fiber beneficially influence circulating concentrations of free fatty acids and gut hormones involved in the regulation of blood glucose and body mass.Citation42 Butyrate – a major end product of dietary starch and fiber produced naturally during digestion by anaerobic bacteria in the cecum and colon – may be a contributing mechanism for increased endogenous secretions of total GLP-1 and peptide YY (PYY) in rodents.Citation43 A more recent study revealed that different fiber sources can induce different physiological responses in overweight cats, including the reduction of energy digestibility, greater inclination to favor glucose metabolism (with SCF), or gut health improvement (with beet pulp), according to their different solubilities and fermentation rates.Citation44 On the other hand, to serve as a sorbent material in the gut, smaller particles of dietary fiber may be more active than the coarse, because more small-sized particles than large-sized particles greatly increases the surface area in the same weight of fiber. Therefore, both fiber source and particle size may play an important role in fermentability and energy intake. Indeed, in this study, dietary fiber not only significantly increased the amount of feces through its bulk effect (Figure S2A) but also reduced lipid absorption. We observed that the glycerol content of feces was significantly higher in the PSY and SCF groups and the cholesterol content of feces was significantly higher in all three fiber groups than in the HFD group (Figure S2A–C).
Unlike CEL and PSY, the effects of SCF on enhanced cellular signaling may also be attributed to its bioactive components such as policosanol, a natural compound derived from sugar cane wax, and other unidentified molecules. Policosanol has been demonstrated to activate AMPK and 3-hydroxy-3-methyl-glutaryl coenzyme A reductase in hepatoma cells and in mouse liver after intragastric administration.Citation45 Moreover, nano-sized SCF was more effective than non-nano-sized CEL and PSY because the smaller particles are more activated than the coarser particles. Although we did not measure butyrate levels, our data show that SCF significantly increased hepatic AMPK phosphorylation and plasma GLP-1 concentrations, supporting the notion that butyrate may induce AMPK activation through expression of GLP−1.Citation46
It has been found that the ability of GLP-1 to reduce food intake has a central mechanism, as GLP-1 centrally administered in the lateral ventricles of rats significantly reduced food intake, strongly suggesting that GLP-1 acts through a direct central nervous system-mediated mechanism to control food intake.Citation47 Therefore, the reason why SCF did not affect food intake in our study may be that the endogenously elevated GLP-1 from SCF ingestion was insufficient to affect satiety when compared with central injection of exogenous GLP-1. Taken together, SCF enhanced multiple cellular signaling and promoted cross-talking in signaling pathways that may be mediated by gastrointestinal factors such as GLP-1, ghrelin, and butyrate.
Conclusion
SCF appears to have enhanced metabolic effects over CEL and PSY in improving glucose and lipid metabolism in mice fed a HFD. SCF significantly enhanced hepatic FGF21, insulin and AMPK signaling in this study. This has extended our insight into the possible mechanisms that may underlie the beneficial effects of dietary fiber, suggesting that gastrointestinal factors such as ghrelin, GLP-1, and butyrate may play a crucial role in enhancing FGF21, insulin, and AMPK signaling, consequently attenuating NAFLD and insulin resistance.
Disclosure
Other than the funding outlined in the Acknowledgments, the authors declare no conflicts of interest in this work.
Authors’ contributions
ZQW designed the study, wrote, and edited the manuscript. XHZ and YMY researched data. KL invented the technology of SCF. ZEF, AB, and WTC reviewed and edited the manuscript. All authors read and approved the final version of the manuscript. ZQW had primary responsibility for the final content.
Acknowledgments
This project was supported in part by P50AT002776-01 (WTC) from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), which funds the Botanical Research Center of Pennington Biomedical Research Center and The Biotech Center of Rutgers University. In addition, support was obtained from American Sugarcane League as an award to ZQW. The authors would like to thank Susan Newman for her assistance with the real-time PCR assay at Genomics Core Facility, which is supported in part by COBRE (NIH P20-RR021945) and NORC (NIH 1P30-DK072476) center grants from the National Institutes of Health. We acknowledge the assistance of Russell Tipton (Pennington Biomedical Research Center) in preparing the manuscript.
Supplementary figures
Figure S1 Sugar cane fiber processing conditions.
Notes: After blending, a proprietary method was used to reduce the remaining components (bagasse) to nanometer-sized particles. The particle sizes of sugarcane fiber were: blended > condition 15 > condition 30 > condition 45 > condition 60, in which 70% of particles were less than 1 μm.
Figure S2 Feces weight and lipid contents in mice fed various dietary fibers. (A) Feces size and weight. The different colors are due to food dye. Feces were collected over a 24-hour period and weighed. (B) and (C) show the glycerol and cholesterol content of mouse feces. Lipids were extracted from feces using chloroform:methanol (1:2, v/v). Glycerol and cholesterol levels in the feces were determined using using a free glycerol determination kit, cat # FG0100 (Sidma-Aldrich, St Louis, MO) or a cholesterol quantitation kit, cat # K603-100 (BioVision, Milpitas, CA), respectively.
Notes: Data are shown as mean ± standard error of the mean (n = 9/group). *P < 0.05; ***P < 0.001, dietary fiber groups vs unsupplemented high-fat diet group. ##P < 0.05; ###P < 0.001, comparison between dietary groups.
Abbreviations: CEL, cellulose; HFD, high-fat diet; PSY, psyllium; SCF, sugar cane fiber.
References
- ZivkovicAMGermanJBSanyalAJComparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver diseaseAm J Clin Nutr200786228530017684197
- ChanJMRimmEBColditzGAStampferMJWillettWCObesity, fat distribution, and weight gain as risk factors for clinical diabetes in menDiabetes Care19941799619697988316
- SilvermanJFO’BrienKFLongSLiver pathology in morbidly obese patients with and without diabetesAm J Gastroenterol19908510134913552220728
- FritscheLWeigertCHäringHULehmannRHow insulin receptor substrate proteins regulate the metabolic capacity of the liver – implications for health and diseaseCurr Med Chem200815131316132918537611
- LizcanoJMAlessiDRThe insulin signalling pathwayCurr Biol2002127R23623811937037
- KharitonenkovAFGFs and metabolismCurr Opin Pharmacol20099680581019683963
- NishimuraTNakatakeYKonishiMItohNIdentification of a novel FGF, FGF-21, preferentially expressed in the liverBiochim Biophys Acta20001492120320610858549
- MaiKBobbertTGrothCPhysiological modulation of circulating FGF21: relevance of free fatty acids and insulinAm J Physiol Endocrinol Metab20102991E12613020424140
- PotthoffMJInagakiTSatapatiSFGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation responseProc Natl Acad Sci U S A200910626108531085819541642
- InagakiTDutchakPZhaoGEndocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21Cell Metab20075641542517550777
- KliewerSAMangelsdorfDJFibroblast growth factor 21: from pharmacology to physiologyAm J Clin Nutr2010911254S257S19906798
- XuJStanislausSChinookoswongNAcute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models – Association with liver and adipose tissue effectsAm J Physiol Endocrinol Metab2009297E1105111419706786
- WenteWEfanovAMBrennerMFibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathwaysDiabetes20065592470247816936195
- ChauMDGaoJYangQWuZGromadaJFibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathwayProc Natl Acad Sci U S A201010728125531255820616029
- Cuevas-RamosDAlmeda-ValdesPAguilar-SalinasCACuevas-RamosGCuevas-SosaAAGomez-PerezFJThe role of fibroblast growth factor 21 (FGF21) on energy balance, glucose and lipid metabolismCurr Diabetes Rev20095421622019531026
- HojmanPPedersenMNielsenARFibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemiaDiabetes200958122797280119720803
- LyonMRReichertRGThe effect of a novel viscous polysaccharide along with lifestyle changes on short-term weight loss and associated risk factors in overweight and obese adults: an observational retrospective clinical program analysisAltern Med Rev2010151687520359270
- WolframTIsmail-BeigiFEfficacy of high-fiber diets in the management of type 2 diabetes mellitusEndocr Pract201117113214220713332
- ZhangZLanzaEKris-EthertonPMA high legume low glycemic index diet improves serum lipid profiles in menLipids201045976577520734238
- WangZQZuberiARZhangXHEffects of dietary fibers on weight gain, carbohydrate metabolism, and gastric ghrelin gene expression in mice fed a high-fat dietMetabolism200756121635164217998014
- FolchJLeesMSloane StanleyGHA simple method for the isolation and purification of total lipides from animal tissuesJ Biol Chem1957226149750913428781
- MatthewsDRHoskerJPRudenskiASNaylorBATreacherDFTurnerRCHomeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in manDiabetologia19852874124193899825
- WangZQZhangXHRussellJCHulverMCefaluWTChromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp ratsJ Nutr2006136241542016424121
- UmpierrezGEJonesSSmileyDInsulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: a randomized controlled trialDiabetes Care20093271164116919366972
- SchulzeMBSchulzMHeidemannCSchienkiewitzAHoffmannKBoeingHFiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysisArch Intern Med2007167995696517502538
- TuomilehtoJLindströmJErikssonJGPrevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose toleranceN Engl J Med2001344181343135011333990
- SchroederNGallaherDDArndtEAMarquartLInfluence of whole grain barley, whole grain wheat, and refined rice-based foods on short-term satiety and energy intakeAppetite200953336336919643157
- de MunterJSHuFBSpiegelmanDFranzMvan DamRMWhole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic reviewPLoS Med200748e26117760498
- WeickertMOPfeifferAFMetabolic effects of dietary fiber consumption and prevention of diabetesJ Nutr2008138343944218287346
- AdeliKLewisGFIntestinal lipoprotein overproduction in insulin-resistant statesCurr Opin Lipidol200819322122818460911
- AsmarMNew physiological effects of the incretin hormones GLP-1 and GIPDan Med Bull2011582B424821299928
- GaoHWangXZhangZGLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 in 3T3-L1 adipocytesEndocrine2007321909517992607
- LeeYSShinSShigiharaTGlucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesisDiabetes20075661671167917369525
- FisherFMChuiPCAntonellisPJObesity is a fibroblast growth factor 21 (FGF21)-resistant stateDiabetes201059112781278920682689
- YilmazYErenFYonalOIncreased serum FGF21 levels in patients with nonalcoholic fatty liver diseaseEur J Clin Invest2010401088789220624171
- ZhangXYeungDCKarpisekMSerum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humansDiabetes20085751246125318252893
- XuJLloydDJHaleCFibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese miceDiabetes200958125025918840786
- IzumiyaYBinaHAOuchiNAkasakiYKharitonenkovAWalshKFGF21 is an Akt-regulated myokineFEBS Lett2008582273805381018948104
- FisherFMEstallJLAdamsACIntegrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivoEndocrinology201115282996300421712364
- ViolletBGuigasBLeclercJAMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectivesActa Physiol (Oxf)20091961819819245656
- WinderWWHardieDGAMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetesAm J Physiol19992771 Pt 1E11010409121
- TariniJWoleverTMThe fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjectsAppl Physiol Nutr Metab201035191620130660
- ZhouJMartinRJTulleyRTDietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodentsAm J Physiol Endocrinol Metab20082955E1160116618796545
- FischerMMKesslerAMde SáLRFiber fermentability effects on energy and macronutrient digestibility, fecal parameters, postprandial metabolite responses, and colon histology of overweight catsJ Anim Sci2012Epub Jan13
- BanerjeeSGhoshalSPorterTDActivation of AMP-kinase by policosanol requires peroxisomal metabolismLipids201146431132121359855
- PengLLiZRGreenRSHolzmanIRLinJButyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayersJ Nutr200913991619162519625695
- AsarianLCorpESHrupkaBGearyNIntracerebroventricular glucagon-like peptide-1 7–36 amide inhibits sham feeding in rats without eliciting satietyPhysiol Behav19986433673729748106