Abstract
The frequent coexistence of depression in epileptic patients raises the issue of simultaneous use of antidepressants along with antiepileptic drugs in the management of such cases. However, it is necessary to evaluate the safety of these antiepileptic/antidepressant drug combinations. The present study investigates the effect of the antidepressant paroxetine (a selective serotonin reuptake inhibitor) administered alone or in combination with the antiepileptic drug sodium valproate on chemoconvulsions induced by picrotoxin (PTX). Seizure score was recorded in vivo, and the levels of thiobarbituric acid-reactive substances and gamma aminobutyric acid (GABA) were measured in the nucleus accumbens of the tested groups of mice. The results show enhancement of seizure severity with significant reduction in GABA levels upon PTX treatment that were reversed by its combination with sodium valproate. On the other hand, paroxetine administered in combination with sodium valproate provided significant protection against PTX-induced convulsions as well as a significant increase in GABA levels in selected brain areas. These results favor their application in management of epilepsy-depression comorbidities.
Introduction
Epilepsy is one of the most common neurological disorders characterized by recurring excessive neuronal discharge, exhibited by transient episodes of motor, sensory, or psychic dysfunction, with or without unconsciousness or convulsive movements. In addition, epilepsy may be associated with neurodegeneration, presumably due to abnormal lipid peroxidation.Citation1,Citation2 On the other hand, comorbid depression is common in patients with epilepsy. A review of available studiesCitation3 suggested a high prevalence of mood (affective) disorders, especially major depression (8%–48%), followed by anxiety (5%–32%), in patients with epilepsy. Although the mechanisms underlying the epilepsy-depression relationship have not been clearly identified, depression in epileptic cases is multifaceted with many interacting neurobiological and psychosocial determinants. These include clinical features of epilepsy (seizure frequency, type, foci, or lateralization of foci) and neurochemical or iatrogenic mechanisms.Citation4,Citation5
Serotonin and norepinephrine are the most studied neurotransmitters in mood disorders. A decrease in the functional activity of central serotonergic and cate-cholaminergic systems is probably associated in major depressive episodes.Citation6 Applying an appropriate strategy with drugs targeting both neurotransmitter systems may improve the efficacy of the antidepressant treatment. Antidepressant drugs are either selective serotonin reuptake inhibitors (SSRIs), eg, paroxetine, or mixed serotonin/noradrenaline reuptake inhibitors (SNRIs), eg, venlafaxine and duloxetine.Citation7,Citation8 SSRIs, such as paroxetine and citalopram, are effective in treating depressed patients.Citation9,Citation10 However, other neurotransmitter systems may also be involved in the pathogenesis of depression as a gamma aminobutyric acid (GABA)ergic system that is considered to be implicated in the pathogenetic mechanisms of mood disorders.Citation11
The high prevalence of depression in epileptic patients may necessitate the addition of an antidepressant agent in therapy. However, the use of antidepressant drugs in epileptics has been a matter of debate for clinicians because of reports that these drugs may have frank convulsant or proconvulsant effects that increase seizure incidence.Citation12 This might happen due to modulation of pre- and/or postsynaptic receptor function and rate of release of neurotransmitters such as +-amino butyric acid, noradrenaline, dopamine, or serotonin,Citation13,Citation14 so it is important to recognize and assess possible implications of antiepileptic/antidepressant drug combinations in the management of epileptic cases complicated by depression.
The rationale of the present study is to (1) evaluate the seizure score of the antiepileptic drug sodium valproate with the antidepressant paroxetine in the management of chemically induced seizures in chronically restrained mice, and (2) study the effect of these drug combinations on thiobarbituric acid-reactive substances (TBARS) as a marker of lipid peroxidation and GABA levels in nucleus accumbens in tested mice.
Materials and methods
Drugs and chemicals
Picrotoxin (PTX; Sigma-Aldrich Co, St Louis, MO), paroxetine HCl (GlaxoSmithKline, Brentford, Middlesex, UK), sodium valproate (2-propyl pentanoic acid-Na salt; Sigma-Aldrich Co), GABA and L-norvaline standards (Sigma-Aldrich Co), ethanol (high-performance liquid chromatography [HPLC] grade; Merck and Co, Inc, Whitehouse Station, NJ), triethylamine (Merck and Co, Inc), phenylisothiocyanate (PITC; Sigma-Aldrich Co), hydrochloric acid (32%, Merck and Co, Inc), acetonitrile (Merck and Co, Inc), glacial acetic acid (Sigma-Aldrich Co), and sodium acetate anhydrous (Merck and Co, Inc).
Animals
Albino mice (20–25 g) were divided into four groups with 12 mice each. They were housed in cages with a natural light-dark cycle and fed on a standard pellet diet and water ad libitum.
Picrotoxin-induced convulsions
All mice were given a single subcutaneous dose (3.5 mg/kg body weight) of PTX either in the absence of any treatment (control group) or following administration of a single dose of the test drug(s) (treated groups). Accordingly, the study comprised the following four groups:
Control group receiving neither antiepileptic nor antidepressant treatment.
Sodium valproate-treated group: received sodium valproate dissolved in water (30 mg/kg body weight intraperitoneal) per Siddiqui, A et al Citation15
Paroxetine-treated group: was administered paroxetine dissolved in saline (1 mg/kg body weight intraperitoneal). This dose was selected according to David, D et al.Citation16
Sodium valproate/paroxetine-treated group: given sodium valproate and paroxetine treatment in doses of 30 mg/kg body weight intraperitoneal and 1 mg/kg body weight intraperitoneal, respectively.
In the treated groups, PTX was injected after a suitable latency corresponding to the time expected to reach a peak effect following administration of the respective test drug(s). The latency of both tested drugs was estimated to be 1 hour after their intraperitoneal administration, as determined by the pilot study.
Immediately after administration of PTX, the animal was observed for 30 minutes. The onset of convulsive behavior as well as the nature and severity of convulsions were carefully recorded using the scoring system 1–7 as follows: hyperlocomotion or piloerection (erection of the skin hair), 1; stunning (immobile) or catatonic posture (assuming a fixed posture and inability to move), 2; clonic body tremors (a series of involuntary muscular contractions due to sudden stretching of the muscle), 3; prolonged clonic tremors, 4; tonic forelimb convulsions followed by clonus, 5; repetitive tonic (prolonged muscular contraction) forelimb convulsions followed by clonus, 6; and tonic extension of both forelimbs and hindlimbs followed by clonus, 7; a mean cumulative score was calculated for each treatment group for comparisons and statistical analysis. At the end of the PTX study for each group, the animals were returned to their cages to continue with the chronic restraint stress study.
Chronic restraint stress procedure
Each mouse of the respective group was placed in a wire mesh restrainer 6 hours daily for 21 days. At the end of the restraint period, the mice were moved to their cages.
Measurement of nucleus accumbens TBARS as a marker of lipid peroxidation
At the end of the 21 days of restraint stress, nucleus accumbens was excised out of the brain and rinsed with cold 0.14 M NaCl, and part of it was homogenized in 25% ice cold 50 mM Tris-HCl buffer, pH 7.4.Citation17 One hundred and fifty microliters of the tissue supernatant of samples was diluted to 500 μL with deionized water. A total of 250 μL of 1.34% thiobarbituric acid was added to all the tubes, followed by the addition of an equal volume of 40% trichloroacetic acid. The mixture was shaken and incubated for 30 minutes in a boiling water bath. Tubes were allowed to cool to room temperature and the absorbance was read at 532 nm using zero concentration as blank.Citation18
Determination of GABA in homogenates of nucleus accumbens isolated from tested mice
The GABA level in the tissue homogenates of the nucleus accumbens was determined.Citation8,Citation19–Citation21 The HPLC method with precolumn PITC derivatization was used for the determination of GABA levels in the homogenate of the nucleus accumbens of the brains of mice of different groups. The measurement scale of the data was in nmol/mg tissue protein.
Parts of the homogenates of nucleus accumbens were centrifuged in a cooling (4°C) centrifuge at 15,000 rpm for 10 minutes. The supernatant was aspirated and transferred to an Eppendorff tube, while the pellet was kept at −70°C until assayed for its total protein content.Citation22 According to Gunawan et al,Citation19 each sample was derivatized via drying 100 μL of the aspirated supernatant in the centrivap, under vacuum. The residue was dissolved in 20 μL of ethanol-water-triethylamine (2:2:1) and evaporated to dryness under vacuum. A 30 μL mixture of ethanol-water-triethylamine-PITC (7:1:1:1) was added to the residue and allowed to react for 20 minutes at room temperature to form the PITC derivatives of the amino acids. Excess reagent was then evaporated under vacuum. The mobile phase of HPLC consisted of solvents A and B: solvent A: 0.1 M sodium acetate buffer (pH = 5.8), solvent B: acetonitrile: water (60:40, v:v). A mixture of 80% solvent A and 20% solvent B was adjusted for the “isocratic” HPLC separations. Flow rate was set at 0.6 mL/minutes. The injected sample was 20 μL. The peaks were detected at 254 nm wave length. Standard curves for GABA and norvaline were plotted using norvaline 2 nmol/20 μL as an internal standard. The ratio of the peak area of each concentration of each standard to the peak area of the internal standard was determined and entered against the concentration of the standard, in a simple regression procedure.
Protein determination
The total protein content of nucleus accumbens homogenate was determined.Citation22
Data analysis
The results were presented as medians with 25 and 75 percentiles for seizure score and mean ± standard deviation for seizure onset. Additionally, the contents of TBARS as a marker of lipid peroxidation and GABA in nucleus accumbens of tested mice of all groups were expressed in nmol/mg tissue protein as mean ± standard deviation. Data were analyzed using one-way analysis of variance with Tukey t-test at a 95% confidence level with significant differences between groups at P < 0.05.
Results
Effect of different drug treatment regimens on PTX-induced convulsions
Single sodium valproate treatment significantly (P < 0.05) delayed the onset and reduced the severity of PTX-induced convulsions compared with the control group. On the other hand, treatment with paroxetine alone significantly (P < 0.05) delayed the onset and reduced the severity of convulsions compared with the control group. However, it was significantly (P < 0.05) less effective than single sodium valproate treatment with respect to prolonging onset latency or reducing severity of convulsions. Treatment with both drugs significantly (P < 0.05) delayed the onset and reduced the severity score of convulsions in comparison with all other groups ().
Table 1 Effect of different drug treatment regimens on picrotoxin (PTX)-induced convulsions
Effect of tested drugs on TBARS in nmol/mg tissue protein in nucleus accumbens of mice exposed to chronic restraint model
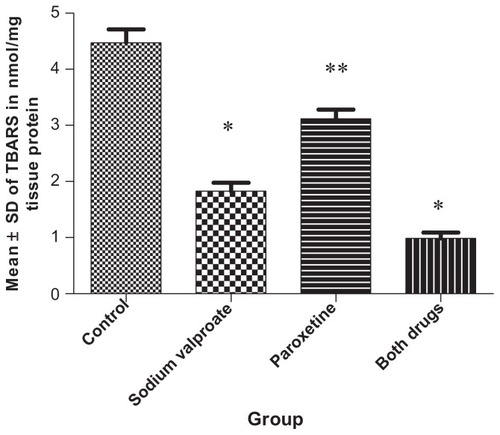
Sodium valproate administration to group 2 significantly (P < 0.05) lowered TBARS level compared with the control group. Coadministration of paroxetine with sodium valproate significantly (P < 0.05) reduced TBARS level compared with the control, sodium valproate, or paroxetine groups ().
Figure 1 Effect of tested drugs on thiobarbituric acid-reactive substances (TBARS) in nmol/mg tissue protein in nucleus accumbens of mice exposed to a chronic restraint model.
Notes: Changes in cortical TBARS levels upon treatment with either sodium valproate or paroxetine given alone or in combination with each other. Administration of sodium valproate resulted in a significant (P < 0.05) reduction in the TBARS content of nucleus accumbens compared with groups 1 and 3, while paroxetine in combination with sodium valproate (group 4) significantly reduced TBARS content when compared with either the valproate-only treated group or the paroxetine-treated group (groups 2 and 3). *P < 0.05 significant decrease compared with groups 1 and 3; **P < 0.05 significant increase compared with groups 2 and 4.
Effect of tested drugs on the GABA level in the nucleus accumbens of tested mice
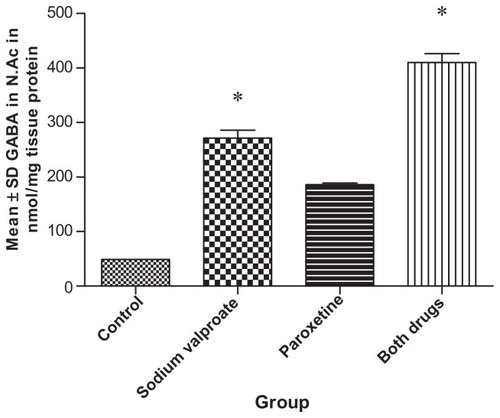
Sodium valproate administration to group 2 significantly (P < 0.05) increased GABA level compared with the control group. Coadministration of paroxetine with sodium valproate significantly (P < 0.05) increased GABA level if compared with the control, sodium valproate, or paroxetine groups ().
Figure 2 Effect of tested drugs on the gamma aminobutyric acid (GABA) level in the nucleus accumbens of tested mice.
Note: Sodium valproate significantly (P < 0.05) increased the GABA concentration in the nucleus accumbens of mice of group 2 compared with group 1. GABA concentration of chronic mild stress mice was significantly increased (P < 0.05) by both sodium valproate and paroxetine compared with all tested groups. *P < 0.05 significant increase compared with groups 1 and 3.
Discussion
The high incidence of psychiatric comorbidities, especially depression and anxiety, seen in epileptic patients may require treatment of both disorders at the same time with a combination of antiepileptic and psychotropic drugs. Therefore, the safety of these drug combinations should be evaluated in order to optimize the treatment of epilepsy. Interestingly, some antiepileptic drugs have a complex of proconvulsant and anticonvulsant activities.Citation23 On the other hand, although the risk of antidepressant-induced seizures, in general, is very low, most, if not all, antidepressant agents have a propensity to lower the seizure threshold, and most are associated with a clinical risk of seizures. The mechanism by which antidepressants cause seizures, however, is still not well established. Recently, it has been suggested that the proconvulsant effects of antidepressants may be attributed to their local anesthetic, antimuscarinic, or anti-histaminic properties.Citation20
Although many studies report that use of older antidepressants, eg, monoamine oxidase inhibitors and tricyclic antidepressants, is frequently associated with the risk of seizures,Citation24,Citation25 the newer antidepressants, eg, SSRIs and SNRIs, are claimed to exhibit a better tolerability profile for most patients. The safety of such agents should still be thoroughly assessed for clinical application in epileptic cases.
There is great debate about the use of SSRIs in the management of depression complicating epileptic cases. There are old reports about the increase in seizure frequency and intensity by SSRIs.Citation26,Citation27 Surprisingly, though the reports about proconvulsive actions of SSRIs are ever increasing, these agents are still widely used in epileptics to treat accompanying depression.Citation28
Meanwhile, there is rising interest to study the possible mechanism of action of many antidepressants on GABA. This amino acid is the major inhibitory neurotransmitter in the brain that diminishes the activity of its target neurons. It also modulates the activity of several neurotransmitters, including dopamine, serotonin, and norepinephrine. It is synthesized in a single step from its precursor glutamate by glutamic acid decarboxylase and metabolized by successive transamination and oxidation to yield succinic semialdehyde and succinic acid, respectively. As part of the transamination reaction, a recycling system is formed in which α-ketoglutaric acid is converted to the GABA precursor glutamate by GABA-glutamic acid transaminase.Citation29
The cornerstone of the GABA hypothesis of bipolar disorder is that GABA provides an inhibitory action to both norepinephrine and dopamine systems.Citation30 Although this widely expressed neurotransmitter has been thought to exert a tonic inhibitory effect on norepinephrine systems, an earlier studyCitation31 suggests that GABA may in fact facilitate norepinephrine activity. It also reported that plasma GABA levels are relatively reduced in depressed patients. The current theory of GABA and depression is that low plasma levels of GABA may identify an inheritable tendency for mood disorders such as depression or bipolar disease.Citation32 Hence, the role of GABA in mood disorders and its interactions with serotonin and norepinephrine systems is worthy of further study. The present study investigated the alterations of GABA content in the nucleus accumbens of chronic restrained mice exposed to a chemoconvulsive substance (PTX) and treated by sodium valproate and/or paroxetine.
Additionally, the models of chemoconvulsion and chronic restraint used in this study evaluate the effect of paroxetine used alone or in combination with a widely used antiepileptic drug sodium valproate on seizure threshold and also on an oxidative stress marker, namely TBARS. The results indicate that sodium valproate alone significantly attenuated PTX-induced convulsions, decreased TBARS levels, and increased GABA contents in nucleus accumbens of tested mice. This may be attributed to an inhibitory effect of sodium valproate on dopamine-induced hyperactivity in nucleus accumbens. This inhibitory effect is similar to that by GABA to control dopaminergic function in this area of the brain. Additionally, this antiepileptic drug has been shown to inhibit the activity of succinic semialdehyde dehydrogenase and GABA transaminase enzymes involved in GABA metabolism. This action will result in an elevation of GABA content. PTX, the GABA antagonist, when injected prior to valproate blocked the reduction in locomotor activity caused by valproate. This further supports the hypothesis that the effects of valproate are mediated by the stimulation of GABA receptors.Citation33
The oxidative stress and modulation of antioxidant enzyme activity may contribute to the central deleterious consequences of chronic stress.Citation34,Citation35 One of the neurochemical complications associated with epilepsy is increased lipid peroxide levels, especially TBARS, in the brain.Citation36 Enhanced lipid peroxidation can induce seizure activity by direct activation of glutamine synthase, thereby permitting an abnormal buildup of the excitatory neurotransmitter glutamic acid.Citation37
A single-blind, placebo-controlled, crossover trialCitation38 demonstrated a beneficial combination of paroxetine and other antiepileptic drugs. The authors investigated possible interactions between paroxetine, a serotonin reuptake inhibitor, and carbamazepine, valproate, and phenytoin. It was carried out in 20 outpatients with epilepsy. Patients on long-term treatment with carbamazepine, valproate, or phenytoin were given a 7-day placebo treatment, followed by paroxetine cotreatment for 16 days. Side effects were infrequent and mild. None of the patients experienced epileptic seizures during this clinical study. These results support findings of paroxetine in this experimental research. Its beneficial effect, when combined with valproate, could be related to a significant reduction in TBARS and a significant increase in GABA contents in crucial brain areas such as nucleus accumbens.
Preliminary findings of clinical studies, demonstrated in a report of the American Neuropsychiatric Association Committee on Research, denoted some neuroprotective effects of drugs, eg, paroxetine and valproic acid, in neurodegenerative diseases. A recommendation was reported by these studies to perform further clinical drug trials on the use of these well-known medications, not only for their psychotropic effects but also for neuroprotection in neurodegenerative diseases.Citation39
Conclusion
In conclusion, the management of epilepsy is a difficult task when associated with other neuropsychiatric disorders. Therefore, extreme caution should be exercised with respect to selection of the proper antidepressants to treat epilepsy-depression comorbidities. The SSRI paroxetine could be recommended in the management of such cases, in combination with sodium valproate, as it may reduce seizure frequency and intensity most likely as a result of decreased brain levels of the oxidative stress marker TBARS with an increase in GABA content of nucleus accumbens of mice exposed to a chemoconvulsive model.
Acknowledgment
This research was supported by the Medical Research Service of the Ain Shams University, Cairo, Egypt. It was supported by the HPLC Laboratory of the Pharmacology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
Disclosure
The author reports no conflicts of interest in this work.
References
- TurkdoganDToplanSKarakocYLipid peroxidation and antioxidative enzyme activities in childhood epilepsyJ Child Neurol200217967367612503643
- HamedSAAbdellahMMTrace elements and electrolytes homeostasis and their relation to antioxidant enzyme activity in brain hyperexcitability of epileptic patientsJ Pharmacol Sci200496434935915599105
- HermannBPSeidenbergMBellBWoodardARuteckiPShethRComorbid psychiatric symptoms in temporal lobe epilepsy: association with chronicity of epilepsy and impact on quality of lifeEpilepsy Behav20001318419012609152
- HardenCLThe co-morbidity of depression and epilepsy. Epidemiology, etiology, and treatmentNeurology200259S485512270969
- AhernTHJavorsMAEaglesDAThe effect of chronic norepinephrine transporter inactivation on seizure susceptibility in miceNeuropsychopharmacol20052721319
- BlierPDe MontignyCCurrent advances and trends in the treatment of depressionTrends Pharmacol Sci1994152202267940983
- ArtigasFSelective serotonin/noradrenaline reuptake inhibitors (SNRIs)CNS Drugs19947989
- VetulaniJNalepaIAntidepressants: past, present and futureEur J Pharmacol200040535136311033340
- DelgadoPLPriceLHHeningerGRCharneyDSNeurochemistry of Affective DisordersHandbook of Affective DisordersPaytkelESLivingston, NYChurchill1992219253
- DeakinBDursunSOptimizing antidepressant treatment: efficacy and tolerabilityInt Clin Psychopharmacol200217Suppl 1S132412369607
- DikeosDGPapadimitriouGNGenetic investigation of dopamine and GABA in mood disordersAnn Gen Hosp Psychiatry20032S83
- PrueterCNorraCMood disorders and their treatment in patients with epilepsyJ Neuropsychiatry Clin Neurosci2005171202815746479
- BuckleyNAMcManusPRFatal toxicity of serotoninergic and other antidepressant drugs: analysis of United Kingdom mortality dataBr Med J20023251332133312468481
- MontgomerySALoftHSanchezCEscitalopram (S-enantiomer of citalopram): clinical efficacy and onset of action predicted from a rat modelPharmacol Toxicol200188528228611393591
- SiddiquiANazmiAKarimSKhanRPillaiKPalKEffect of Melatonin and Valproate In Epilepsy and DepressionInd J Pharmacol200133378381
- DavidDBourinMJegoGPrzybylskiCJollietPGardierAEffects of acute treatment with paroxetine, citalopram and venlafaxine in vivo on noradrenaline and serotonin outflow: a microdialysis study in Swiss miceBr J Pharmacol200314061128113614530210
- BenjaminJIwataRHazalettJKinetics of entry of proteins into the myelin membraneJ Neurochem19783110771085702136
- GutteridgeJMQuinlanGJMalondialdhyde formation from lipid peroxides in the thiobarbituric acid test: the role of lipid radicals, iron salt and metal chelatorsJ Appl Biochem198354–52932996679543
- GunawanSWaltonNTreimanDHigh performance liquid chromatography determination of selected amino acids in rat brain by precolumn derivatization with phenylisothiocyanateJ Chroma19905031177187
- MontgomerySAAntidepressants and seizures: emphasis on newer agents and clinical implicationsInt J Clin Pract200559121435144016351676
- RossettiVLombardADetermination of glutamate decarboxylase by high-performance liquid chromatographyJ Chromatogr B19966816367
- BradfordMMA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem197672248254942051
- DaileyJWNaritokuDKAntidepressants and seizure: clinical anecdotes overshadow neuroscienceBiochem Pharmacol199652132313298937441
- TrimbleMNon-monoamine oxidase inhibitor antidepressants and epilepsy: a reviewEpilepsia197819241250354920
- EdwardsJGAntidepressants and convulsionsLancet197921368136992708
- SkowronDMStimelGLAntidepressants and the risk of seizuresPharmacotherapy19921218221549533
- RosensteinDLNelsonJCJacobsSCSeizures associated with antidepressants: a reviewJ Clin Psychiatry1993542892998253696
- KannerAMThe behavioral aspects of epilepsy: an overview of controversial issuesEpilepsy Behav20011101105
- BrambillaPPerezJBaraleFSchettiniGSoaresJCGABAergic dysfunction in mood disordersMol Psychiatry20038872173712888801
- ShinsukeWAkifumiINobumasaKTadafumiKPossible relationship between mitochondrial DNA polymorphisms and lithium response in bipolar disorderThe International Journal of Neuropsychopharmacology2003642142410.1017/S146114570300377814604458
- PettyFKramerGLFultonMMoellerFGRushAJLow plasma GABA is a trait-like marker for bipolar illnessNeuropsychopharmacology1993921251328216695
- SanacoraGMasonGFRothmanDLKrystalJHIncreased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitorsAm J Psychiatry2002159466366511925309
- KuruvillaAUretskyNEffect of sodium valproate on motor function regulated by the activation of GABA receptorsPsychopharmacol198172167172
- SunandaBSShankaranarayanaRRajuTRChronic restraint stress impairs acquisition and retention of spatial memory taskCurrent Science2000791115811584
- GrilloCAPiroliGGRosellDRHoskinEKMcewenBSReaganLPRegion specific increases in oxidative stress and superoxide dismutase in the hippocampus of diabetic rats subjected to stressNeuroscience2003121113314012946706
- SudhaKRaoAVRaoAOxidative stress and antioxidants in epilepsyClinica Chimiva Acta20013031924
- ScharfmanHESollasALBergerREGoodmanJHElectrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sproutingJ Neurophysiol2003902536254714534276
- AndersenBBMikkelsenMVesteragerADamMKristensenHBPedersenBNo influence of the antidepressant paroxetine on carbamazepine, valproate and phenytoinEpilepsy Res1991102–32012041840138
- LauterbachEMendezMPsychopharmacological neuroprotection in neurodegenerative diseases, part III: criteria-based assessment: A report of the ANPA Committee on ResearchJ Neuropsychiatry Clin Neurosci200123324226021948886