 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
It was recently reported that iron oxide nanoparticles attenuated antigen-specific humoral responses and T cell cytokine expression in ovalbumin-sensitized mice. It is presently unclear whether iron oxide nanoparticles influence T helper 1 cell-mediated immunity. The present study aimed to investigate the effect of iron oxide nanoparticles on delayed-type hypersensitivity (DTH), whose pathophysiology requires the participation of T helper 1 cells and macrophages.
Methods
DTH was elicited by a subcutaneous challenge with ovalbumin to the footpads of mice sensitized with ovalbumin. Iron oxide nanoparticles (0.2–10 mg iron/kg) were administered intravenously 1 hour prior to ovalbumin sensitization. Local inflammatory responses were examined by footpad swelling and histological analysis. The expression of cytokines by splenocytes was measured by enzyme-linked immunosorbent assay.
Results
Administration of iron oxide nanoparticles, in a dose-dependent fashion, significantly attenuated inflammatory reactions associated with DTH, including the footpad swelling, the infiltration of T cells and macrophages, and the expression of interferon-γ, interleukin-6, and tumor necrosis factor-α in the inflammatory site. Iron oxide nanoparticles also demonstrated a suppressive effect on ovalbumin-stimulated production of interferon-γ by splenocytes and the phagocytic activity of splenic CD11b+ cells.
Conclusion
These results demonstrated that a single dose of iron oxide nanoparticles attenuated DTH reactions by suppressing the infiltration and functional activity of T helper 1 cells and macrophages in response to antigen stimulation.
Introduction
Engineered nanoparticles have been increasingly applied in many fields, including photonics, catalysis, magnetics, and biomedical applications. Among various nanomaterials, iron oxide nanoparticles possess a unique property of superparamagnetism that confers advantages for biomedical applications, including the generation of heat in alternating magnetic fields, and an ability to guide specific targeting by an external magnetic field. This property plays a central role in the development of iron oxide nanoparticles for contrast enhancement, targeted delivery of drugs or genes, tissue engineering, cancer thermal therapy, magnetic transfection, chelation therapy, and tissue engineering.Citation1–Citation7 Given the widespread potential applications of iron oxide nanoparticles and their impending commercialization, exposure of humans and animals to iron oxide nanoparticles is likely to increase significantly in the near future. Therefore, evaluation of the health impact of iron oxide nanoparticles is important.
Medicinal preparations of superparamagnetic iron oxide nanoparticles have been used as diagnostic contrasting agents for magnetic resonance imaging of focal liver lesions.Citation7–Citation10 It has been well documented that systemically administered iron oxide nanoparticles are rapidly engulfed by the reticuloendothelial system, with the spleen being one of the major organs for particle distribution.Citation7,Citation11,Citation12 Accordingly, the impact of iron oxide nanoparticles on the functionality of immune cells has become a focus of research. Exposure of primary macrophages to iron oxide nanoparticles results in a marked induction of oxidative stress and apoptosis.Citation13 In addition, iron oxide nanoparticles suppress the phagocytic activity of RAW 264.7 cells – a murine macrophage line – and increase the production of tumor necrosis factor-α (TNF-α) and nitric oxide.Citation14 Murine studies have also demonstrated that intratracheal instillation of iron oxide nanoparticles elicits proinflammatory and prooxidative effects, as evidenced by a marked infiltration of inflammatory cells in lung tissues and a diminished level of intracellular glutathione in bronchoalveolar lavage cells.Citation15 In addition to macrophages, T cells are also a sensitive target in the immune system to iron oxide nanoparticles. Oral and intravenous administrations of iron oxide nanoparticles alter T cell cellularity in normal nonsensitized mice.Citation16,Citation17 It has been reported that serum levels of interleukin-2 (IL-2), IL-10, and interferon-γ (IFN-γ) are elevated in mice intravenously administered with iron oxide nanoparticles.Citation16 Moreover, it was recently reported that exposure to iron oxide nano particles suppresses humoral immunity and influences antigen-specific T cell reactivity.Citation18,Citation19 Collectively, these results clearly demonstrate that the functionality of macrophages and T cells is modulated by iron oxide nanoparticles upon in vitro and in vivo exposure.
A previous study showed that both T helper 1 (Th1)- and Th2-type immune responses are suppressed by iron oxide nanoparticle administration, in which Th2 cells appear less sensitive.Citation19 Moreover, direct exposure of ovalbumin-primed splenocytes to iron oxide nanoparticles primarily inhibits antigen-specific Th1 cytokine expression.Citation18 On the basis of these results, it was hypothesized that iron oxide nanoparticles may modulate Th1 cell-mediated immune responses. A murine model of delayed-type hypersensitivity (DTH), whose pathophysiology requires the functions of antigen-activated Th1 cells and macrophages,Citation20 was employed to investigate the effect of iron oxide nanoparticles on Th1 cell-mediated immunity.
Materials and methods
Reagents and chemicals
All reagents were purchased from Sigma-Aldrich Corporation (St Louis, MO) unless otherwise stated. Enzyme-linked immunosorbent assay sets for cytokine measurement were purchased from BD Biosciences (San Jose, CA). Cell culture supplies were obtained from Thermo Scientific HyClone (Logan, UT). Resovist® (Bayer Schering Pharma AG, Berlin, Germany) – a commercial preparation of carboxydextran-coated iron oxide nanoparticles containing 28 mg iron/mL – was used in the present study. The iron oxide nanoparticles of Resovist exhibited a monodisperse population of particles with an average diameter of 58.7 nm, as measured using a particle size analyzer (Zetasizer Nano-S, Malvern Instruments Ltd, Malvern, Worcestershire, UK).Citation18 The crystalline core of ferucarbotran (Resovist) is composed of magnetite (Fe3O4) and maghemite (γ-Fe2O3).Citation7
Protocol of animal experiments
Male BALB/c mice (5–6 weeks old) were purchased from the Animal Breeding Center of the National Taiwan University Hospital (Taipei, Taiwan). On arrival, the mice were randomized, transferred to plastic cages containing a sawdust bedding (five mice per cage), and quarantined for at least 1 week. The mice were given standard laboratory food and water ad libitum. The mice were randomly divided into the following groups (five mice per group; ): (1) nonsensitized (but ovalbumin-challenged) group; (2) untreated ovalbumin-sensitized and challenged group; (3) vehicle-treated plus ovalbumin-sensitized and challenged group; and (4) iron oxide nanoparticle-treated plus ovalbumin-sensitized and challenged group. A single dose of iron oxide nanoparticles (0.2–10 mg iron/kg; 0.2 mL/mouse) and/or vehicle (saline) were administered to mice via the tail vein 1 hour prior to ovalbumin sensitization on day zero. Except for the nonsensitized group, each mouse was sensitized with ovalbumin by a subcutaneous injection using 0.1 mL sensitization solution (100 μg ovalbumin and 1 mg alum in saline). The hind footpads of all mice were challenged subcutaneously with 20 μL of 10 μg ovalbumin in saline on day seven. DTH reactions represented by the degree of footpad swelling were measured using an electronic caliper, Model No. 4120 (King Life Technology Company Ltd, Taipei, Taiwan) before and 24 hours after the ovalbumin challenge. The paw swelling was determined as the following:
Figure 1 Protocol of iron oxide nanoparticle administration and ovalbumin sensitization and challenge. BALB/c mice were randomly divided into the following groups (five mice per group): (1) nonsensitized (but ovalbumin-challenged) group; (2) untreated ovalbumin-sensitized and challenged group; (3) vehicle-treated (saline 0.2 mL/mouse) plus ovalbumin-sensitized and challenged group; and (4) iron oxide nanoparticle-treated (0.2–10 mg iron/kg, 0.2 mL/mouse) plus ovalbumin-sensitized and challenged group. The dosing regimen for iron oxide nanoparticle administration and antigen sensitization and challenge are described in the materials and methods.
Abbreviation: OVA, ovalbumin.

The mice were then sacrificed, and their serum samples, spleens, and footpads were harvested for further measurements. All animal experiments were approved by the Institutional Animal Care and Use Committee of the National Taiwan University.
Histological analysis of footpads
The footpads of mice were fixed with 10% neutral buffered formalin for 1 day followed by decalcification for 2 days. The tissue specimens were embedded in paraffin, cut into 3–4 μm sections, and stained with hematoxylin and eosin for routine histopathology.
Immunohistochemical staining
The tissue sections were deparaffinized and then rehydrated following a standard procedure. The rehydrated slides were immersed in Trilogy™ (Cell Marque, Hot Springs, AR) at 121°C for 15 minutes for antigen retrieval. The endogenous peroxidase activity was then quenched with 3% hydrogen peroxide in methanol and blocked with normal horse serum. Antimouse CD3, F4/80, Foxp3, IFN-γ, IL-6, or TNF-α monoclonal antibodies were applied onto each section overnight. The slides were treated with super enhancer and then incubated with polyhorseradish peroxidase reagent. For visualization, the slides were treated with the peroxidase substrate 3-amino-9-ethylcarbazole followed by hematoxylin counter staining (blue color). The number of positive cells showing dark-red color was counted manually.
Splenocyte cultures and measurement of splenocyte viability
Spleens from the mice of the same group were aseptically isolated, pooled, and made into single cell suspensions as described previously.Citation19 The viability of splenocytes was determined by the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (methylthiazol tetrazolium) assay described previously.Citation21 Splenocytes (5 × 106 cells/mL) were seeded into 96-well plates. The cells were either left unstimulated or stimulated with ovalbumin for 68 hours. A methylthiazol tetrazolium stock solution (5 mg/mL in phosphate buffered saline) was then added to each well (10 μL/well) and incubated for 4 hours. The formed formazan was dissolved with 0.1 N acid-isopropanol (100 μL/well); the optical density was measured at 570 nm (and at 630 nm as a background reference) using a microplate reader (Dynatech Laboratories Inc, Chantilly, VA).
Measurement of cytokines by enzymelinked immunosorbent assay
Splenocytes (5 × 106 cells/mL) were cultured in 48-well plates (250 μL/well) followed by stimulation with ovalbumin (50 μg/mL) for 72 hours. The supernatants were harvested and quantified for IL-4 and IFN-γ by enzyme-linked immunosorbent assay.Citation22
Measurement of the phagocytic activity of splenic CD11b+ cells
Splenic CD11b+ cells were isolated using a commercial kit (BD IMag™ Cell Separation System; BD Biosciences) following the supplier’s protocol. The purity of isolated cells was confirmed to be ≥90% by flow cytometry (FACSCalibur™; BD Biosciences) using a fluorescein isothiocyanate-conjugated antimouse CD11b monoclonal antibody (clone M1/70; BD Biosciences). The phagocytic activity of the splenic CD11b+ cells was measured using pH-sensitive pHrodo® Escherichia coli BioParticles™ conjugate (Invitrogen Life Technologies, Carlsbad, CA) following the manufacturer’s instructions. In brief, the CD11b+ cells (5 × 105 cells/mL) were seeded into 96-well plates (0.1 mL/well) and cocultured with pHrodo E. coli BioParticles conjugate for 3 hours. After washing, cells were collected, and the single cell fluorescence of 5000 cells for each sample was measured at emission of 575 nm using a FACSCalibur flow cytometer.
Statistical analysis
The mean ± standard error was determined for each treatment group in the individual experiments. Homogeneous data were then evaluated by a parametric analysis of variance, and Dunnett’s two-tailed t-test was used to compare the results for the treatment groups with those of the control group. P < 0.05 was defined as statistical significance.
Results
Administration of iron oxide nanoparticles suppressed DTH reactions
The effect of iron oxide nanoparticles on DTH reactions was firstly investigated by measuring the swelling of footpads in mice sensitized and challenged with ovalbumin. Ovalbumin challenge markedly increased the thickness of footpads in ovalbumin-sensitized mice compared to the nonsensitized group (), signifying a successful induction of DTH. Iron oxide nanoparticles (0.6 and 10 mg iron/kg) showed a significant suppressive effect on footpad swelling, in which the inhibitory magnitude of the two doses was comparable, whereas the low dose (0.2 mg iron/kg) was ineffective (). Histological examination using hematoxylin and eosin staining showed a marked infiltration of mononuclear cells in subcutaneous tissues of the footpads in the untreated ovalbumin-sensitized and vehicle-treated groups, which appeared to be diminished by iron oxide nanoparticle administration (0.6 and 10 mg iron/kg; ). To more specifically characterize the types of inflammatory cells influenced by iron oxide nanoparticles, the number of total T cells (CD3+), regulatory T cells (Foxp3+), and macrophages (F4/80+) and the expression of IFN-γ, IL-6, and TNF-α in the footpads was measured by immunohistochemical staining. The signal of IFN-γ, IL-6, and TNF-α was primarily detected perivascularly in the dermis layer, which was markedly suppressed by iron oxide nanoparticle treatment (0.6 and 10 mg iron/kg; and ). Furthermore, the number of CD3+ and F4/80+ cells was significantly suppressed in mice administered with iron oxide nanoparticles (0.6 and 10 mg iron/kg) compared to the vehicle-treated control (). In contrast, the infiltration of Foxp3+ cells was not altered by iron oxide nanoparticles ().
Table 1 Immunohistochemical staining of CD3+, F4/80+, Foxp3, interferon-γ, interleukin-6, and tumor necrosis factor-α+ cells in the footpads of ovalbumin-sensitized and challenged mice
Figure 2 Inhibition by iron oxide nanoparticle administration of the footpad swelling and inflammatory cell infiltration associated with delayed-type hypersensitivity. BALB/c mice were treated as the protocol depicted in . Delayed-type hypersensitivity reactions in the footpad were induced by a subcutaneous ovalbumin challenge. (A) The thickness of footpads was measured before and 24 hours after the ovalbumin challenge. (B) The tissue sections were stained with hematoxylin and eosin (original magnification, ×200). Boxes show subcutaneous regions with heavy infiltration of mononuclear cells in the untreated ovalbumin-sensitized group and vehicle-treated group, which appears to be diminished in the iron oxide nanoparticle-treated (0.6 and 10 mg/kg) groups.
Notes: The data are expressed as the mean ± standard error of six to eight samples per group. *P < 0.05 compared to the vehicle-treated group. Results are representative of three independent experiments.
Abbreviations: Fe, iron; NS, nonsensitized group; OVA, untreated ovalbumin-sensitized and challenged group; VH, vehicle-treated plus ovalbumin-sensitized and challenged group.
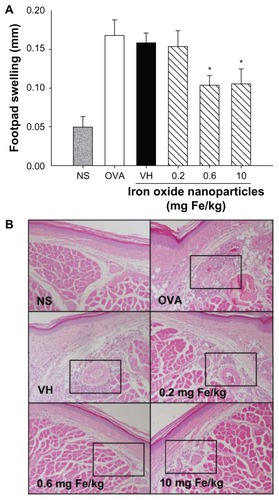
Figure 3 Iron oxide nanoparticles inhibited the expression of interferon-γ and tumor necrosis factor-α in footpads. (A) Representative light micrograph of interferon-γ-stained footpad sections is shown (original magnification, ×200). The box shows subcutaneous regions with interferon-γ signals in the untreated ovalbumin-sensitized group. Representative light micrographs of (B) interferon-γ-stained and (C) tumor necrosis factor-α-stained footpad sections are shown (original magnification, ×400).
Notes: Arrows indicate positive cells. Quantified data are presented in .
Abbreviations: Fe, iron; NS, nonsensitized group; OVA, untreated ovalbumin-sensitized and challenged group; VH, vehicle-treated plus ovalbumin-sensitized and challenged group.
Differential effects of iron oxide nanoparticles on T cell functionality
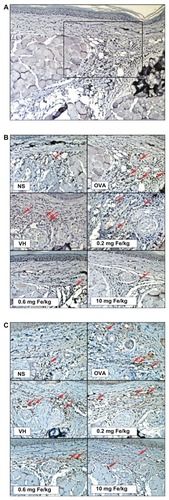
Cytokines expressed by T cells play an essential role dictating antigen-specific immune reactions, including humoral and cell-mediated immunity. To investigate the influence of iron oxide nanoparticles on the functionality of T cells in DTH reactions, splenocytes harvested from nonsensitized and ovalbumin-sensitized mice were restimulated with ovalbumin (50 μg/mL) in culture for 72 hours to induce the production of antigen-specific cytokines. As shown in , both IFN-γ and IL-4 were markedly induced by ovalbumin stimulation in the ovalbumin-sensitized groups compared to the nonsensitized group, demonstrating a successful induction of antigen-specific T cell reactivity. Consistent with the reduced footpad swelling, iron oxide nanoparticles (0.6 and 10 mg iron/kg) demonstrated a suppressive effect on the production of IFN-γ (). By contrast, administration of iron oxide nanoparticles (0.6 and 10 mg iron/kg) enhanced the production of IL-4 (). As the expression of cytokines was influenced by iron oxide nanoparticle treatment, the viability of ovalbumin-stimulated splenocytes was further examined. The results from the methylthiazol tetrazolium assays showed that the viability of splenocytes was unaltered in mice administered with iron oxide nanoparticles ().
Figure 4 Differential effects of iron oxide nanoparticles on the production of antigen-specific interferon-γ and interleukin-4 by splenocytes. Splenocytes isolated from each group of mice were cultured in the presence of ovalbumin (50 μg/mL) for 72 hours, and the supernatants were collected for measurement of (A) interferon-γ and (B) interleukin-4 by enzyme-linked immunosorbent assay.
Note: Data are expressed as the mean ± standard error of six samples pooled from two experiments; *P < 0.05 compared to the vehicle-treated group.
Abbreviations: Fe, iron; NS, nonsensitized group; OVA, untreated ovalbumin-sensitized and challenged group; VH, vehicle-treated plus ovalbumin-sensitized and challenged group.
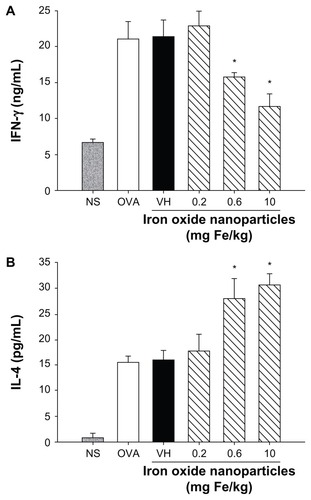
Figure 5 Effect of iron oxide nanoparticles on the viability of splenocytes. Splenocytes isolated from each group of mice were cultured in the presence of ovalbumin (50 μg/mL) for 72 hours. The viability of splenocytes was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay.
Notes: Data are expressed as the mean ± standard error of quadruplicate cultures. Results are representative of three independent experiments.
Abbreviations: Fe, iron; NS, nonsensitized group; OD, optical density; OVA (key), ovalbumin; OVA (X-axis), untreated ovalbumin-sensitized and challenged group; VH, vehicle-treated plus ovalbumin-sensitized and challenged group.
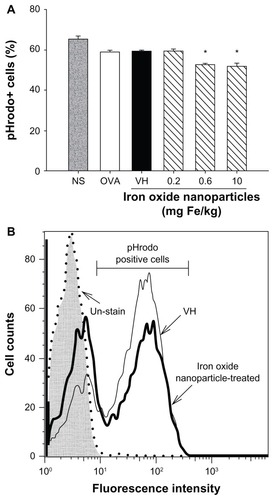
Effects of iron oxide nanoparticles on phagocytic activity of CD11b+ splenocytes
To further examine the influence of iron oxide nanoparticles on the functionality of macrophages, the phagocytic activity of splenic CD11b+ cells was examined. The percentage of splenic CD11b+ cells engulfing the fluorescent probe pHrodo was significantly attenuated in mice administered with iron oxide nanoparticles (0.6 and 10 mg iron/kg; ). Representative histograms of the pHrodo uptake by splenic CD11b+ cells in the vehicle-treated and iron oxide nanoparticle-treated (10 mg iron/kg) groups are illustrated in .
Figure 6 Iron oxide nanoparticles suppressed the phagocytic activity of splenic CD11b+ cells. (A) CD11b+ cells from the spleen were isolated using anti-CD11b magnetic beads. The phagocytic activity of CD11b+ cells was measured by flow cytometry using pH-sensitive pHrodo® Escherichia coli BioParticles™ conjugate (Invitrogen Life Technologies, Carlsbad, CA) as described in the materials and methods. (B) Representative histograms of the uptake of pHrodo by splenic CD11b+ cells in vehicle-treated and iron oxide nanoparticle-treated (10 mg/kg) groups are illustrated.
Notes: Data are expressed as the mean ± standard error of triplicate samples per group; *P < 0.05 compared to the vehicle-treated group. Results are representative of three independent experiments.
Abbreviations: Fe, iron; NS, nonsensitized group; OVA, untreated ovalbumin-sensitized and challenged group; VH, vehicle-treated plus ovalbumin-sensitized and challenged group.
Discussion
Although iron oxide nanoparticles have been reported to affect the functionality of T cells and antigen-presenting cells,Citation12–Citation16,Citation18,Citation19,Citation23,Citation24 evidence pertaining to their effects on Th1 cell-mediated immunity in vivo remains mostly unknown. It was previously reported that systemic administration of ovalbumin-sensitized mice with iron oxide nanoparticles suppresses, the serum production of ovalbumin-specific immunoglobulin G1 and immunoglobulin G2a, and the expression of both IFN-γ and IL-4 by splenocytes.Citation19 Moreover, a direct exposure of ovalbumin-primed splenocytes to iron oxide nanoparticles results in a reduced production of IFN-γ, but not IL-4.Citation18 Because IFN-γ is a key cytokine expressed by Th1 cells, these results suggest that Th1 cells are potentially a more sensitive target to iron oxide nanoparticles compared to Th2 cells. It was therefore hypothesized that iron oxide nanoparticles may inhibit the DTH reactions in which Th1 cells play a central role in the elicitation of antigen-specific immune responses. This hypothesis was substantiated by the results in the present study, which show that the footpad swelling was suppressed by a single intravenous administration of iron oxide nanoparticles. Concordantly, the infiltration of T cells and the expression of IFN-γ in footpads were attenuated by iron oxide nanoparticle treatment.
It was previously reported that high doses (30 and 60 mg iron/kg) of iron oxide nanoparticles cause an overall suppressive effect on the expression of both Th1 and Th2 cytokines by splenocytes of ovalbumin-sensitized mice, whereas a low dose (10 mg iron/kg) affects the production of IFN-γ, but not IL-4.Citation19 Hence, 10 mg of iron/kg was employed in the present study, and the results confirmed the sensitivity of IFN-γ to the dose of iron oxide nanoparticles. The study further extended to a lower dose (0.6 mg iron/kg) in mice with DTH, which enhanced the production of IL-4 (). These results suggest that the dose of iron oxide nanoparticles plays a critical role dictating the effects of iron oxide nanoparticles on T cells, in which doses ≤10 mg iron/kg may shift the Th1/Th2 immunobalance toward the Th2-dominant direction in ovalbumin-sensitized mice. The potential impact of iron oxide nanoparticles on Th2 cell-mediated immunity, such as allergy, warrants further investigations using appropriate models.
The recommended doses of iron oxide nanoparticles as contrast agents to enhance the signal of magnetic resonance imaging are 25.2 mg iron (0.9 mL of Resovist) and 39.2 mg iron (1.4 mL) for adults weighing <60 kg and ≥60 kg, respectively.Citation25,Citation26 Hence, the clinical dose range of Resovist is greater than 0.42 mg iron/kg for adults weighing <60 kg and less than 0.65 mg iron/kg for adults weighing ≥60 kg. In the present study, the dose of 0.6 mg iron/kg was effective to curb DTH reactions, demonstrating the impact of iron oxide nanoparticles at a clinically relevant dose on Th1 cell-mediated immunity.
Previous studies reported that iron oxide nanoparticles activated macrophages and induced a proinflammatory effect when administered locally to the airways.Citation15,Citation27 In contrast, the present study showed a suppressive effect on the functionality of both macrophages and Th1 cells by systemically administered iron oxide nanoparticles. These results suggest that the way of administration may be a critical factor influencing the immunomodulatory effects of iron oxide nanoparticles. In addition, the nature of immune responses could be another key factor in that no antigen sensitization was applied in the airway studies, whereas the DTH reactions were antigen-specific. It is well established that both T cells and macrophages are involved in the pathophysiology of DTH, in which IFN-γ expressed by antigen-activated Th1 cells stimulates macrophages that subsequently trigger inflammatory responses.Citation20,Citation28 In light of this premise, it was speculated that the downregulated activities of macrophages in iron oxide nanoparticle-treated mice could be due to the suppressive effect of the nanoparticles on T cells. Obviously, the potential impact of iron oxide nanoparticles on the functionality of macrophages may be dictated by different routes of exposure. As demonstrated in the present study for the scenario of systemic exposure, iron oxide nanoparticles suppress the function of both macrophages and T cells in response to antigen stimulation. As Th cells play an essential role in the regulation of different arms of antigen-specific immunity, addressing the impact on the functionality of Th cells would be crucial for assessing the nanotoxicology of iron oxide nanoparticles when systemic exposure is concerned.
In addition to the route of exposure, antigen sensitization and challenge is another potential factor influencing the different effects induced by systemic and intratracheal administrations of iron oxide nanoparticles.
The observed inhibition of DTH responses by iron oxide nanoparticles is in line with a recent report showing a similar suppressive effect by fullerene nanoparticles.Citation29 It is noticed that both studies employed intravenous injection for nanoparticle administration. However, the underlying mechanisms are quite different. The study of fullerene nanoparticles showed an enhanced number of regulatory T cells and an attenuated expression of the proinflammatory cyto kines IL-6 and IL-17, whereas the expression of TNF-α was paradoxically enhanced.Citation29 Hence, the mechanism of action for fullerene nanoparticles was attributed to an upregulation of the regulatory immunity. By contrast, the present data showed a concordant attenuation of the functionality of both Th1 cells and their downstream effector cells, namely macrophages, whereas the number of Foxp3+ cells in footpads was unaltered. The suppression of macrophage functions was further substantiated by the present data showing a significant attenuation of the expression of IL-6 and TNF-α in footpads and the phagocytic activity in splenic CD11b+ cells of iron oxide nanoparticle-treated mice. Collectively, despite different mechanisms of action, both fullerene and iron oxide nanoparticles elicit a similar suppressive effect on DTH, suggesting the potential health impact on Th1 immunity by systemic exposure of nanoparticles.
Conclusion
The present study demonstrated that a single administration of iron oxide nanoparticles at a clinically relevant dose attenuated DTH reactions. Iron oxide nanoparticles suppressed the systemic production of IFN-γ – a Th1 signature cytokine – by splenocytes, as well as locally in the inflamed footpads. In addition to T cells, the functionality of macrophages associated with DTH was attenuated, including the infiltration and expression of the proinflammatory cytokines IL-6 and TNF-α in the inflammatory site. These results provide critical insights into the health impact of iron oxide nanoparticles on cell-mediated immunity. The potential immunotoxicity of iron oxide nanoparticles warrants further investigation.
Acknowledgments
This work was supported by grants NSC98-2320-B-002-036-MY3 and NSC99-2313-B264-001-MY3 from the National Science Council, Executive Yuan (Taipei, Taiwan) and KMU-ER013 from Kaohsiung Medical University Research Foundation (Kaohsiung City, Taiwan).
Disclosure
The authors report no conflicts of interest in this work.
References
- ItoAShinkaiMHondaHKobayashiTMedical application of functionalized magnetic nanoparticlesJ Biosci Bioeng2005100111116233845
- HuberDLSynthesis, properties, and applications of iron nanoparticlesSmall20051548250117193474
- GuptaAKGuptaMSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials200526183995402115626447
- HautotDPankhurstQAMorrisCMCurtisABurnJDobsonJPreliminary observation of elevated levels of nanocrystalline iron oxide in the basal ganglia of neuroferritinopathy patientsBiochim Biophys Acta200717721212517097860
- LiuGMenPHarrisPLRolstonRKPerryGSmithMANanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalanceNeurosci Lett2006406318919316919875
- BulteJWDouglasTWitwerBMagnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cellsNat Biotechnol200119121141114711731783
- HammBStaksTTaupitzMContrast-enhanced MR imaging of liver and spleen: first experience in humans with a new superpara-magnetic iron oxideJ Magn Reson Imaging1994456596687981510
- VoglTJHammerstinglRPegiosWThe value of the livers-pecific superparamagnetic contrast medium AMI-25 for the detection and differential diagnosis of primary liver tumors versus metastasesRofo19941604319328 German8161744
- JosephsonLLewisJJacobsPHahnPFStarkDDThe effects of iron oxides on proton relaxivityMagn Reson Imaging1988666476532850434
- McLachlanSJMorrisMRLucasMAPhase I clinical evaluation of a new iron oxide MR contrast agentJ Magn Reson Imaging1994433013078061425
- ReimerPMullerMMarxCT1 effects of a bolus-injectable super-paramagnetic iron oxide, SH U 555 A: dependence on field strength and plasma concentration – preliminary clinical experience with dynamic T1-weighted MR imagingRadiology199820938318369844683
- WangJChenYChenBPharmacokinetic parameters and tissue distribution of magnetic Fe(3)O(4) nanoparticles in miceInt J Nanomedicine2010586186621042548
- LunovOSyrovetsTBucheleBThe effect of carboxydextran-coated superparamagnetic iron oxide nanoparticles on c-Jun N-terminal kinase-mediated apoptosis in human macrophagesBiomaterials201031195063507120381862
- HsiaoJKChuHHWangYHMacrophage physiological function after superparamagnetic iron oxide labelingNMR Biomed200821882082918470957
- ParkEJKimHKimYYiJChoiKParkKInflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in miceToxicology20102751–3657120540983
- ChenBAJinNWangJThe effect of magnetic nanoparticles of Fe(3)O(4) on immune function in normal ICR miceInt J Nanomedicine2010559359920856834
- WangJChenBJinNThe changes of T lymphocytes and cytokines in ICR mice fed with Fe3O4 magnetic nanoparticlesInt J Nanomedicine2011660561021674017
- ShenCCLiangHJWangCCLiaoMHJanTRA role of cellular glutathione in the differential effects of iron oxide nanoparticles on antigen-specific T cell cytokine expressionInt J Nanomedicine201162791279822114506
- ShenCCWangCCLiaoMHJanTRA single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c miceInt J Nanomedicine201161229123521753874
- CherDJMosmannTRTwo types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clonesJ Immunol198713811368836942953788
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- JanTRSuSTWuHYLiaoMHSuppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c miceInt Immunopharmacol20077677378017466911
- BlankFGerberPRothen-RutishauserBBiomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cellsNanotoxicology20115460662121231795
- NaqviSSamimMAbdinMConcentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stressInt J Nanomedicine2010598398921187917
- ReimerPBalzerTFerucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applicationsEur Radiol20031361266127612764641
- Resovist® [package insert]BerlinBayer Schering Pharma AG2002
- ZhuMTFengWYWangBComparative study of pulmonary responses to nano- and submicron-sized ferric oxide in ratsToxicology20082472–310211118394769
- KobayashiKKanedaKKasamaTImmunopathogenesis of delayed-type hypersensitivityMicrosc Res Tech200153424124511340669
- YamashitaKSakaiMTakemotoNAttenuation of delayed-type hypersensitivity by fullerene treatmentToxicology20092611–2192419376187