Abstract
In the rapid development of nanoscience and nanotechnology, many researchers have discovered that metal oxide nanoparticles have very useful pharmacological effects. Cuprous oxide nanoparticles (CONPs) can selectively induce apoptosis and suppress the proliferation of tumor cells, showing great potential as a clinical cancer therapy. Treatment with CONPs caused a G1/G0 cell cycle arrest in tumor cells. Furthermore, CONPs enclosed in vesicles entered, or were taken up by mitochondria, which damaged their membranes, thereby inducing apoptosis. CONPs can also produce reactive oxygen species (ROS) and initiate lipid peroxidation of the liposomal membrane, thereby regulating many signaling pathways and influencing the vital movements of cells. Our results demonstrate that CONPs have selective cytotoxicity towards tumor cells, and indicate that CONPs might be a potential nanomedicine for cancer therapy.
Introduction
Nanoscience and nanotechnology have advanced greatly in recent years, and a large amount of research has focused on determining the properties and potential functions of nanomaterials.Citation1–Citation3 Nanomaterials show many unique and useful properties and can be applied in the areas of medicine, biology, and life science research.Citation4–Citation8 Nanomaterials have distinct magnetic properties, a large surface-to-volume ratio, and fluorescence, among others. They play important roles, such as developers, drug carriers, and tracers.Citation9–Citation14 Research has also shown that nanoparticles can affect the functions of cells and biomolecules. Nanomaterials can be toxic to cells and harmful to humans and other organisms. Many papers have reported that ZnO, TiO2, Ag, iron core-gold shell nanoparticles, and Au nanorodsCitation15–Citation18 can specifically induce the apoptosis of tumor cells. These results agree well with the idea in traditional Chinese medicine (TCM) that “poisonous drugs sometimes can be used to treat serious and stubborn diseases.” The TCM text, Shen Nong’s Herbal, describes a medicine containing azurite ore, a copper mineral that has potential antitumor properties. Arsenic trioxide, another traditional Chinese medicine, has the same antitumor property, and it has been used to treat acute promyelocytic leukemia successfully.Citation19–Citation21 Although Shen Nong’s Herbal describes both as cancer agents, azurite ore, in particular, shows poor solubility in water, making it difficult to investigate its antitumor property directly. However, nanoscience and nanotechnology methods offer a chance to solve this challenging issue.
CONPs are a new type of p-type semiconductor, have active electron-cavity pairs and good catalytic activity, and have been extensively applied in various fields, such as electrochemistry and physics.Citation22–Citation26 However, only a few studies have focused on the biological effects of CONPs, which are important in understanding the biological activity of nanomaterials. CONPs containing copper might also have pharmacological functions similar to those of azurite ore. Furthermore, cuprous oxide can bind to the hydrosulfide group, as arsenic trioxide does. We predict that CONPs may show therapeutic efficacy against cancer. However, the potential anticancer properties of CONPs remain an undeveloped area that is of medical interest.
In this study, we prepared CONPs to explore their pharmacological effects on cancerous HeLa cells, melanoma cells YUMAC, normal human 293T cells, and mouse embryonic fibroblast (MEF) cells. We discover that CONPs could selectively induce apoptosis and suppress the proliferation of tumor cells. From the transmission electron microscopy images, we found that CONPs could target the mitochondria and cause damage to their membranes. Furthermore, we carried out lipid peroxidation measurements and a dithiothreitol (DTT) protection experiment to test the effects of ROS on apoptosis. Our data show that CONPs have the potential for use in anticancer therapy.
Material and methods
Preparation of CONPs
All of the chemical reagents used in this experiment were of analytical grade. CONPs were synthesized as follows: First, 0.35 mL of 0.1 M CuSO4 (aq) was added to 3 mL of 0.1 M cetyltrimethylammonium bromide (CTAB) (aq) in a test tube, and the solutions were allowed to mix for about 5 minutes. Then, 10 mL of fresh, chilled 0.04 M NaBH4 (aq) was added to the mixture. The mixture was stirred at 26°C–28°C. After approximately 18 hours, the mixture turned yellow. Finally, the product was harvested from the solution by centrifugation at 12,000 rpm for 15–20 minutes and washed several times with ethanol and deionized water. After removing the supernatant, the product was dried in a vacuum dryer for 12–18 hours at 50°C. The dried product was stored in a hermetic container at 4°C.Citation22,Citation27
Characterization of CONPs
The prepared CONPs were characterized by powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). The XRD pattern was obtained using a D/MAX-rB X-ray powder diffractometer (Rigaku, Japan) using Cu Ka radiation (average wavelength is 0.15418 nm), and the operation voltage and current were maintained at 30 kV and 30 mA, respectively. The morphologies of the CONPs were examined by TEM at an acceleration voltage of 100 kV. Dynamic light scattering size analyses of CONPs in suspensions was carried out using a Nano HT Zetasizer (Malvern Instruments, Malvern, UK). The UV-visible spectra of the dispersions were obtained with a visible/UV-spectrophotometer (UV2450, Shimadu, Japan). Fourier transform infrared (FTIR) spectra of the samples were recorded using an Alpha-T spectrometer (Nicolet Nexus470, Nicolet, USA).
Cytotoxicity assay
The HeLa cells, 293T cells, and MEF cells used in this research were grown in Dulbecco’s modified Eagle’s medium (DMEM), and the melanoma cell line YUMAC were grown in Opti-MEM (Gibco™, Invitrogen Corp, Calsbad, CA). Both mediums were supplemented with 2 mg/mL sodium bicarbonate, 4.5 mg/mL glucose, 100 μg/mL streptomycin sulfate, 40 μg/mL gentamicin, 100 U/mL penicillin. DMEM were supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, and the Opti-MEM were 5%. An environment of humidified air containing 5% CO2 was maintained at 37°C. HeLa cells, 293T cells, and melanoma cell line YUMAC were stored by our laboratory. The original source of the HeLa cells and 293T cells was the Cell Bank of Typical Culture Collection (Chinese Academy of Sciences, Shanghai, China). The YUMAC cells were provided generously by Yale University School of Medicine (New Haven, CT). The MEF cells were obtained from day 13 C57BL/6 mouse embryos. To evaluate the cytotoxicity of the CONPs, cells in the log phase were seeded onto a 96-well culture plate with 2000 cells per well and incubated at 37°C in a CO2 incubator for 24 hours until the cells adhered to the plate. Serial dilutions of CONPs were added, and after 48 hours, cell viability was measured using the 3-(4,5-di-methylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. For this assay, 200 μL of 5 mg/mL MTT solution (prepared in medium without blood serum) was added to the cells in the culture and incubated at 37°C in a CO2 incubator for 4 hours. Dimethyl sulfoxide was then used to dissolve the formazan crystals. Finally, the absorbance was measured at 570 nm using an ELISA plate reader.Citation28
Apoptosis detection by Annexin V staining
Cells in the log phase were seeded onto a 6-well culture plate at a density of 1 × 105 cells per well and incubated at 37°C in a CO2 incubator in the presence of CONPs. The cells were treated with 0, 10, or 20 μg/mL CONPs. After 48 hours, apoptosis and necrosis were analyzed with the Annexin V-fluorescein isothiocyanate apoptosis detection kit 1 following the manufacturer’s instructions. The samples were analyzed using a BD FACSCanto instrument (BD Biosciences, Franklin Lakes, NJ).
Flow cytometry of cell cycle progression
The cells were harvested using 0.25% trypsin with 1 mM of EDTA solution and fixed for 12 hours in 70% ethanol at 4°C. The fixed cells were then centrifuged at 3000 rpm for 15 minutes to remove the ethanol thoroughly. The cells were then washed twice with 3 mL of phosphate-buffered saline (PBS), resuspended in 1 mL of propidium iodide (PI) staining solution, and incubated for 15 minutes at room temperature. The staining solution consisted of 20 μg/mL PI and 0.2 mg/mL RNase A in PBS. The samples were subsequently analyzed using a BD FACSCanto instrument (BD Biosciences, Franklin Lakes, NJ).
Transmission electron microscopy (TEM) assay
The HeLa cells were grown in a large petri dish. When cells had adhered to the dish, a solution of CONPs was added to a final concentration of 30 μg/mL. The cells were incubated at 37°C in a CO2 incubator. After approximately 4 hours, the cells were harvested, washed twice with PBS, and incubated with 4°C formaldehyde overnight to fix the cells. The cells were then dehydrated with increasing concentrations of ethanol (50%, 60%, 70%, 80%, 90%, and 100%) for 15 minutes each, stained with 2% uranyl acetate in 70% ethanol overnight at room temperature, and then embedded in Epon. The embedded samples were sectioned into 60-nm-thick slices using a sliding ultramicrotome. Thin sections supported by copper grids were examined using a Hitachi TEM system operated at 100 kV (Hitachi, Tokyo, Japan).
Dithiothreitol protection experiment
ROS may play an important role in lipid peroxidation and the suppression of cell growth. Dithiothreitol (DTT) is a hypotoxic reducing agent that can inactivate ROS and protect the hydrosulfide groups of protein. The cells were cultured as described above in the cytotoxicity assay section, except 20 μg/mL of fresh DTT was added to the cell medium or lipid peroxidation reaction mixture. After 48 hours, an MTT assay was used to determine the extent of the protective effects of DTT.
Lipid peroxidation measurement
The free-radical modulation activity of CONPs was determined using a lipid peroxidation assay. Briefly, lipid peroxidation was induced in liposomes prepared from soybean lecithin and cholesterol by ultrasonic irradiation. The CONPs were added to the prepared liposomes and the control samples were supplemented with PBS. The samples were incubated at 37°C for 1 hour in a dark incubator. The reaction was inhibited by adding an equal volume of stop solution (0.25 M HCl, 1.5% (wt/vol) trichloroacetic acid, and 0.375% (wt/vol) thiobarbituric acid). These reaction mixtures were boiled for 15 minutes, cooled, and then centrifuged at 12,000 rpm for 15 minutes. The absorbance of the resulting solution was measured at 532 nm.Citation15,Citation28
Statistical analysis
Data were analyzed using SigmaPlot 10.0 software (Systat Software Inc, San Jose, CA). Statistical differences between the control and treatment groups for the in vitro experiments comparing the toxicity of the CONPs were determined using an analysis of paired t-test with significance set at P < 0.05.
Results and discussion
Characterization of CONPs
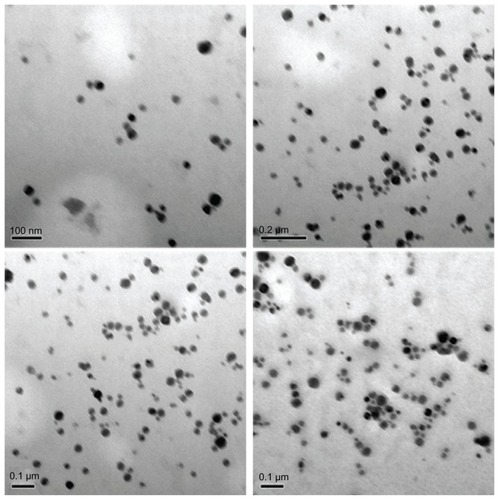
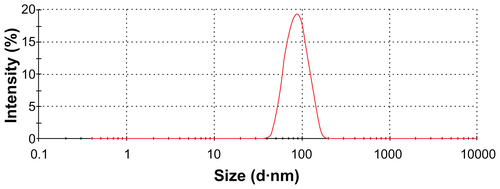
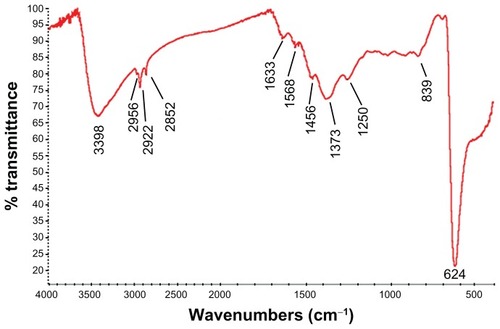
The XRD patterns () showed that the CONPs were crystalline, and the diffraction pattern and interplanar spacing of the CONPs were similar to those in the standard diffraction pattern of cuprous oxide.Citation22 Furthermore, no characteristic peaks of impurities were detected, suggesting that high-quality CONPs were obtained. The UV-vis spectrum of the CONPs showed a wide absorption band between 200 nm and 500 nm (). presents the FTIR spectra of the CONPs, showing their composition and quality. The band at 624 cm−1 correlates with the stretching vibrations of cuprous oxide. The bands at approximately 2922 cm−1 and 1373 cm−1 are probably due to the carbonate moieties, water, or other organic compounds in the air that were adsorbed by the CONPs, as these bands are generally observed when FTIR samples are measured in air.Citation15 The TEM images showed that the CONPs were all spherical and homogeneous (). The diameter of the CONPs in the suspensions ranged from approximately 40 nm to 110 nm (Figure S1). From our characterization of the CONPs, we can see that the CONPs prepared in our experiment were of good quality.
Figure 1 X-ray patterns of the CONPs.
Note: Results revealed the crystal structure of the CONPs, and no characteristic peaks of impurities were detected.
Abbreviation: CONPs, cuprous oxide nanoparticles.
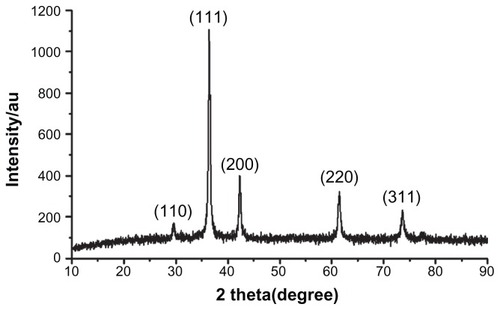
Figure 2 UV-vis spectra of the CONPs.
Note: CONPs were dissolved in deionized water and deionized water was used as a reference.
Abbreviation: CONPs, cuprous oxide nanoparticles.
Figure 3 FTIR transmission spectra of the CONPs. The band at 624 cm−1 correlates to the stretching vibration of the CONPs.
Notes: Moreover, there were also some stretching bands. The other bands at 3398 cm−1, 2992 cm−1, 1373 cm−1, and 839 cm−1 are probably due to the carbonate moieties and water that are generally observed, when FTIR samples are measured in air.
Abbreviations: CONPs, cuprous oxide nanoparticles; FTIR, Fourier transform-infrared.
Cytotoxicity assay
In TCM, copper or cuprous compounds have been used to treat cancer and other diseases for thousands of years.Citation29 These cuprous compounds may have properties similar to those of another effective antitumor drug, arsenic trioxide.Citation19–Citation21 Copper is a known heavy metal that is toxic to human cells and has been shown to have significant side effects on the human body. However, with the development of nanoscience and nanotechnology, research has shown that nanoparticles can target specific organs, have a lower toxicity against the whole organism, and have good dissolubility in water. Therefore, CONPs may be an ideal combination of TCM and nanoscience. Despite the strong potential of CONPs to be a novel therapy tool, no study has reported on the interactions of CONPs in the relevant in vitro or in vivo biological settings.
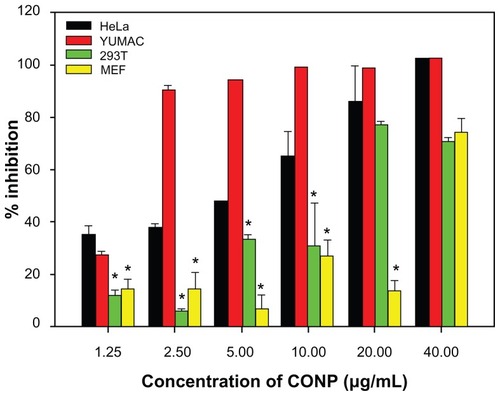
To solve this fundamental issue and to investigate the potential uses of CONPs as cancer therapeutics, an MTT assay was used to evaluate how CONPs affect the viability of HeLa cells and melanoma cells with normal human 293T cells and MEF cells as control. CONPs exhibited dose-dependent cytotoxicity in HeLa cells with a half-maximal inhibitory concentration (IC50) of 8.28 μg/mL, and in melanoma cells with IC50 of 1.533 μg/mL, whereas the IC50 values in MEF cells and 293T cells were 27.00 μg/mL and 14.22 μg/mL, respectively. The cancerous HeLa cells and melanoma cells displayed a strikingly greater sensitivity to the CONPs than the normal human (293T) or mouse (MEF) cell lines did (P < 0.005) (). At low concentrations, lower than 5 μg/mL, there was a discriminative difference in the level of cytotoxicity between tumor cells and normal cells. Differential cytotoxicity is important because one of the greatest challenges facing chemotherapy is the inability of anticancer drugs to distinguish effectively between tumor cells and normal cells. In our study, low concentrations of CONPs selectively killed tumor cells, suggesting that CONPs may be useful as a potential anticancer drug. In addition to CONPs, ZnO nanoparticles also have selective toxicity against human myeloblastic leukemia cells (HL60) and show less toxicity against normal peripheral blood mononuclear cells.Citation15 Therefore, CONPs may have a potential utility in the treatment of cancer, as do ZnO nanoparticles. Research has also shown that iron core-gold shell NPs have cancer cell-specific cytotoxicity.Citation16 Referring to the periodic table of elements, it is not difficult to understand these results. Zn(II) and Cu(I) have a similar extranuclear electron assignment, which may contribute to their similar properties. Therefore, we can conjecture that the chemical and physical properties of these nanomedicines provide the basis of their biological action. Furthermore, some research has reported that the tumor cells were unable to clear the nanoparticles, but this result was different in the normal cells.Citation30 In our experiment, the inability to clear CONPs might be one of the mechanisms that caused CONPs to show selective cytotoxicity against tumor cells.
Figure 5 Differential cytotoxicity of CONPs on tumor cell (melanoma cells YUMAC), paired control tumor cell (HeLa), and normal cells (293T and MEF) detected by MTT assay.
Notes: HeLa cells and melanoma cells were more sensitive, and the two normal cell lines (293T and MEF cells) were tolerant, especially at low concentrations. Cells were treated with the CONPs for 48 hours (*P < 0.005, n = 3).
Abbreviations: CONPs, cuprous oxide nanoparticles; MEF, mouse embryonic fibroblast.
Apoptosis and cell cycle progression
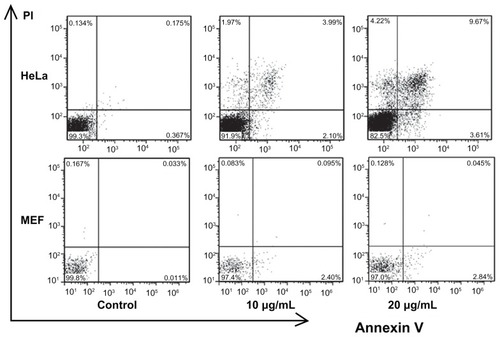
Several studies have indicated that different nanoparticles can suppress cell viability by different mechanisms. Specific kinds of nanoparticles can induce apoptosis in cells, but other kinds of nanoparticles can make cells undergo necrosis.Citation15,Citation16,Citation31 To investigate the mechanism of CONPs cytotoxicity, we performed flow cytometry to analyze the effects of CONPs on cell death and cell cycle. First, Annexin V- and PI-based apoptosis and necrosis discrimination assays were performed on HeLa and MEF cells exposed to 10 μg/mL and 20 μg/mL of CONPs for 48 hours (). The results showed an increase in apoptosis in HeLa cells, which was dependent on the dose of CONPs, and a similar increase in necrosis was observed. However, decreased apoptosis and necrosis were observed in the MEF cells in accordance with the cytotoxicity assays. These results showed that CONPs have selective antitumor properties.
Figure 6 Flow cytometry analysis of HeLa and MEF cells treated with different concentrations of CONPs for 48 hours and then stained with Annexin V and PI.
Notes: Significant increases in apoptosis were observed in HeLa cells exposed to the 10 μg/mL and 20 μg/mL CONPs for 48 hours. However, decreased apoptosis was observed in the MEF cells. The upper-left quadrant in each panel shows the necrotic population, the upper-right quadrant shows the late apoptotic population, and the lower-right quadrant shows the early apoptotic population.
Abbreviations: CONPs, cuprous oxide nanoparticles; MEF, mouse embryonic fibroblast; PI, propidium iodine.
Apoptosis is a cell suicide mechanism that enables organisms to control cell numbers and to eliminate cells that threaten survival. The apoptotic cascade can be triggered through two major pathways. Extracellular signals, such as members of the tumor necrosis factor (TNF) family or the Fas/FasL pathway, can activate the receptor-mediated extrinsic pathway. Alternatively, stress signals, such as DNA damage, hypoxia, and loss-of-survival signals, may trigger the mitochondrial intrinsic pathway.Citation32,Citation33 CONPs are p-type semiconductors and have active electron-cavity pairs. CONPs can produce ROS, break disulfide bonds, and bind to the hydrosulfide group in proteins. Because of their fundamental chemical and physical properties, CONPs could exert oxidative stress on cells and cause membrane damage and DNA breaks. Because many proteins in the cell contain disulfide bonds or hydrosulfide groups, CONPs may target these proteins and influence their activity, thus regulating tumor-specific signal pathways, exhibiting specific pharmacological activity, or inducing the apoptosis of tumor cells, which were similar to the effects of arsenic trioxide. Moreover, cellular respiration is the basis of every vital movement in the cell. The electron transport chain performs an important role in cellular respiration and contains several protein complexes that have cuprous active centers. CONPs may also release cuprous ions and influence the electron transfer through the respiratory chain, causing tumor cells to lack energy and start the apoptotic pathway.
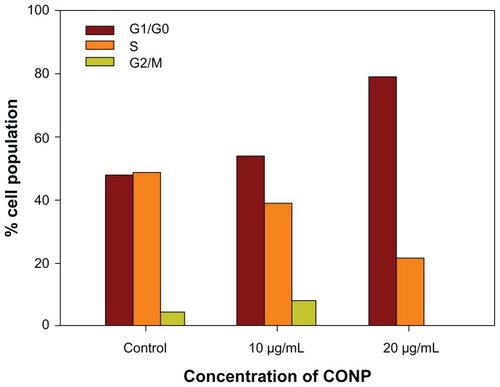
Cell cycle progression analysis was performed and showed that HeLa cells were arrested strongly by CONPs in a dose-dependent manner. Approximately 58% of cells exposed to 10 μg/mL of CONPs and 80% of cells exposed to 20 μg/mL of CONPs were arrested in G1/G0 48 hours after CONPs exposure (). Furthermore, the number of cells in S phase decreased as the concentration of CONPs increased. However, the iron-core gold-shell nanoparticles also suppressed cell proliferation and cells were delayed in S phase,Citation16 whereas the cells exposed to CONPs were delayed in G1/G0. Furthermore, treatment with Ag nanoparticles caused a cell cycle arrest in G2/M phase, possibly due to the repair of damaged DNA.Citation34 These results show that different nanoparticles have different effects on the cell cycle, which may be important in exploring the mechanism of their biological actions.
Figure 7 Cell cycle progression assay of HeLa cells treated with different concentrations of CONPs for 48 hours.
Notes: Proliferation of HeLa cells was strongly suppressed by CONPs in a dose-dependent manner. Cells were arrested in the G1/G0 phase after 48 hours of treatment and were less able to proliferate.
Abbreviation: CONPs, cuprous oxide nanoparticles.
Our experimental data show that CONPs also suppressed the proliferation of tumor cells and caused a cell cycle arrest in G1/G0. These effects showed that CONPs might inhibit DNA replication. DNA replication relies on many factors, including the DNA replication machinery, cyclin/ cyclin-dependent kinase (CDK) complexes, the production of anti-oncogenes (such as p53), growth factor-mediated cellular signaling, and others. CONPs may influence one or more of these factors to suppress DNA replication. CONPs are a novel type of p-type semiconductor that have active electron-cavity pairs and could produce ROS that upregulate p53-mediated cellular signaling, which may be a mechanism by which the cells are arrested in the G1/G0 phase.Citation35 From the similar properties of cuprousion, we could infer that CONPs may also break disulfide bonds and bind to hydrosulfide groups. Because numerous proteins contain disulfide bonds and hydrosulfide groups, these proteins may be targets of the CONPs, which could explain how the CONPs influenced cell signaling. Moreover, disulfide bonds and hydrosulfide groups are also located on the cell membrane; therefore, CONPs might bind to the cell membrane and imitate contact inhibition to suppress cell proliferation.
Transmission electron microscopy assay
To determine the mechanism of apoptosis, it is essential to know whether the CONPs crossed the cell membrane. We therefore used TEM to analyze HeLa cells that had been treated with 30 μg/mL CONPs for 3–4 hours. As shown in , we observed that several CONPs were enclosed in small vesicles within the cells. In addition, numerous CONPs were found in the cytoplasm. The CONPs in the cytoplasm had two possible sources. First, the CONPs may have penetrated the cell membrane without breaking it. Alternatively, the CONPs were taken up by cells in small vesicles, but then they broke the membrane of the vesicles and were released into the cytoplasm. Furthermore, all the images showed that CONPs could not efficiently enter the nucleus, lysosomes, or endoplasmic reticulum. However, the CONPs could cause endoplasmic reticulum tumescence ().
Figure 8 Transmission electron images of HeLa cells treated with a solution of CONPs (30 μg/mL) for 4 hours. The arrows indicate the accumulation of the CONPs. (A) The CONPs entered a mitochondrion and broke its outer membrane. CONPs existed in vesicles and in the cytoplasm. (B) The endoplasmic reticulum swelled visibly. A structure similar to an autophagy body phagocytized a group of CONPs. (C) The vesicles containing the CONPs appeared to target the outer membrane of the mitochondrion, and the shape of the mitochondrion had changed. (D) CONPs localized in small vesicles and entered or were entering the mitochondrion. (E and F) CONPs broke the membrane of the mitochondrion and caused structural changes.
Abbreviations: CONPs, cuprous oxide nanoparticles; M, mitochondrion; ER, endoplasmic reticulum.
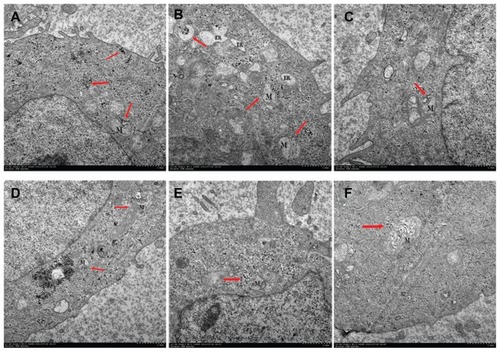
It is important to note that the CONPs within the vesicles could target the mitochondria, enter the mitochondria, and cause their rupture (). How could the CONPs within the vesicles target the mitochondria? Referring to the TEM images, the whole process might be described as follows: The CONPs, which may adsorb the serum proteins in the medium, were taken up by the cells, which was mediated by special receptors and resulted in CONPs entering the cell in small vesicles. The vesicles containing the CONPs may express special proteins, which could bind to the mitochondrial outer membrane, that were transported in the cytoplasm and targeted the mitochondria. The membranes of the vesicles and the mitochondria would then fuse, and the CONPs would be released into the mitochondria. As we discussed previously, CONPs can produce ROS and may bind to proteins with hydrosulfide groups. The membrane of the mitochondria was broken by the CONPs immediately afterwards. Tumor cells may lose the ability to clear the nanoparticles that had been taken up by these cells. However, normal cells can clear the nanoparticles efficiently,Citation30 and were resistant to the CONPs. The CONPs were therefore selectively toxic to the tumor cells and targeted the mitochondria. Alternatively, show that the outer membrane of the mitochondria invaginated at the surface touching the vesicles containing CONPs and pseudopodium-like structures, which might take up the vesicles and is similar to cell phagocytosis. Thereby, the phagocytosis of mitochondria might be observed in our TEM images, and the vesicles were actively taken up by the mitochondria. Further studies should be performed to determine the mechanism by which the CONPs entered the mitochondria.
Similarly, gold nanorods bound to CTAB could also target the mitochondria of tumor cells. However, gold nanorods bound to CTAB targeted the mitochondria without vesicles.Citation30 Mitochondrial damage resulted in cytochrome C release and the formation of the apoptosome, a multimeric protein complex containing Apaf-1, cytochrome C, and caspase-9, which initiates the apoptotic pathway. Furthermore, damaging the mitochondria would result in a lack of energy and could lead to cell death.
Our research shows that CONPs could target the mitochondria and have selective toxicity against tumor cells. The inability to clear the CONPs might be one of the mechanisms to induce apoptosis of tumor cells selectively.
Dithiothreitol protection experiment and lipid peroxidation measurement
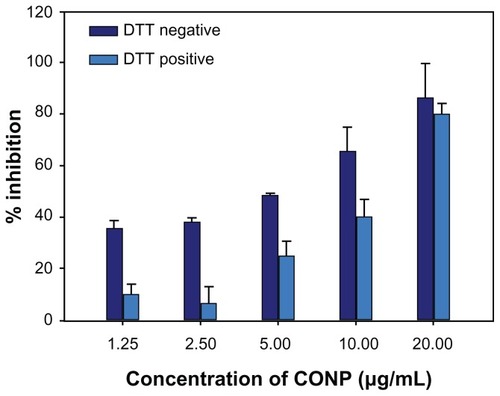
ROS have a very close relationship with various vital movements and participate in immunological and pathological reactions, cell injury and death, and tumor generation. Recent researches have shown that metal oxide nanoparticles can exhibit spontaneous or light-activated ROS production, depending on the material composition and surface characteristics.Citation15,Citation36 To determine the role of ROS in cell death, DTT protection experiments and lipid peroxidation measurements were performed. DTT is commonly used in biochemical studies to prevent the oxidation of thiol groups and to reduce disulfides to dithiols, which can inactivate ROS.Citation37–Citation39 Our study results show that DTT could significantly prevent the cytotoxicity observed in cells treated with low concentrations of CONPs. However, DTT was unable to prevent significantly the cytotoxicity observed at higher concentrations (P < 0.01) (). These studies indicate that the pharmacological actions of CONPs might result from the generation of ROS, which in turn are responsible for inducing apoptosis.
Figure 9 DTT protection experiment on HeLa cells for 48 hours. The MTT assay was then performed to evaluate protective effects of DTT.
Notes: The results show that DTT could increase cell viability at low concentrations but reduced viability at higher concentrations (P < 0.01, n = 3).
Abbreviations: CONPs, cuprous oxide nanoparticles; DTT, dithiothreitol; MTT, 3-(4,5-di-methylthiazol-2yl)-2,5-diphenyl tetrazolium bromide.
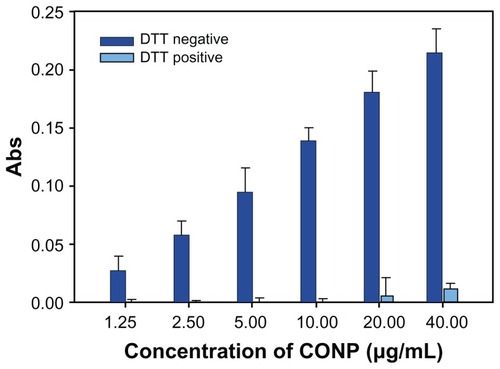
To highlight ROS production, we performed lipid peroxidation experiments. The oxidation of fatty acids leads to the generation of lipid peroxides, thereby initiating a chain reaction that results in the disruption of the plasma and organelle membranes and subsequent cell death. Lipid peroxidation experiments show that DTT effectively inhibited the generation of the lipid peroxidation product malondialdehyde (MDA). MDA could be observed at low levels only when a concentration of greater than 20 μg/mL CONPs was used (). However, higher levels of MDA were observed when DTT was not in the reaction mixture.
Figure 10 Lipid peroxidation measurement of CONPs in the liposomes prepared from soybean lecithin and cholesterol.
Notes: CONPs increased the lipid peroxidation activity and produced ROS, but DTT could significantly block the generation of ROS (P < 0.01, n = 3).
Abbreviations: CONPs, cuprous oxide nanoparticles; DTT, dithiothreitol; ROS, reactive oxygen species.
ROS can result in oxidative stress to cells, modulate the ability of thioredoxin (TRX) to interact and inhibit ASK1, and upregulate the JNK-, p38-, and p53-mediated signaling pathways, resulting in damaged lipids, proteins, DNA and other cellular components.Citation6 Oxidative modifications of redox-sensitive transcription factors, including NF-κB and HIF-1α, and intermediate signaling molecules, such as PKC, ERK, JNK, and MAPKs, have also been shown to be involved in the ROS-mediated modulation of cell growth and survival.Citation38,Citation40 CONPs could induce the production of ROS and influence the vital movements of the cell. ROS were most likely involved in the apoptosis of tumor cells.
Conclusion
We investigated the selective cytotoxicity of CONPs on cancerous cells. CONPs can effectively induce apoptosis and suppress the cell cycle progression of tumor cells. By using TEM, we found that the CONPs could enter cells and specifically target the mitochondria, which may be one of the mechanisms by which CONPs induced apoptosis. We also examined the generation of ROS after treatment with CONPs and the effects of ROS on apoptosis using DTT protection and lipid peroxidation assays. The results indicate that CONPs could induce ROS production and increase lipid peroxidation. These changes might influence many cell signaling pathways and initiate apoptotic pathways.
Our work showed the potential utility of CONPs for the treatment of cancer owing to the selective toxicity of CONPs against tumor cells. However, further studies should be performed to learn more about the properties of these nanoparticles.
Acknowledgments
The authors would like to thank Xing-Hua Pan (Department of Genetics, Yale University, New Haven, CT), Yan Zhang, Guo-Ting Feng, and Fu Gao for their useful discussion and technical assistance with the sample analysis. The present study was financed by the National Basic Research Program of China (Grant Number: 2010CB945600, 2011CB966200, 2009CB941100), the National Natural Science Foundation of China (Grant Number: 30971462, 31171309, 30600545), the National New Drug Development of Major Science and Technology Projects (Grant Number: 2011ZXJ09104-10C, 2009ZXJ09003-028), and the Creativity and Innovation Training Program of Second Military Medical University (Grant Number: MS2011039, MS2010075).
Disclosure
The authors report no conflicts of interest.
References
- ServiceRFNanotechnology grows upScience2004181732173415205504
- SahooSKParveenSPandaJJThe present and future of nanotechnology in human health careNanomedicine20073203117379166
- TwanLVladimirSKarelUPolymeric nanomedicines for image-guided drug delivery and tumor-targeted combination therapyNano Today20105197212
- YangFTangQZhongXSurface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticlesInt J Nanomedicine2012783584422359460
- LeePZhangRLiVEnhancement of anticancer efficacy using modified lipophilic nanoparticle drug encapsulationInt J Nanomedicine2012773173722359452
- MurphyEAMajetiBKBarnesLANanoparticle-mediated drug delivery to tumor vasculature suppresses metastasisProc Natl Acad Sci U S A20081059343934818607000
- SiddiquiIAAdhamiVMChristopherJChamcheu MukhtarHImpact of nanotechnology in cancer: emphasis on nanochemopreventionInt J Nanomedicine2012759160522346353
- StevensMMGhadialiJECohenBEProtein kinase-actuated resonance energy transfer in quantum dot-peptide conjugatesACS Nano201044915491920731464
- DurmusNGTaylorENInciFFructose-enhanced reduction of bacterial growth on nanorough surfacesInt J Nanomedicine2012753754522334783
- ShenSChowPSChenFTanRBSubmicron particles of SBA-15 modified with MgO as carriers for controlled drug deliveryChem Pharm Bull20075598599117603186
- SeoWSLeeJHSunXFeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agentsNat Mater2006597197617115025
- VisariaRKGriffinRJWilliamsBWEnhancement of tumor thermal therapy using gold nanoparticle–assisted tumor necrosis factor-A deliveryMol Cancer Ther200651014102016648573
- CartwrightLPoulsenMSNielsenHMIn vitro placental model optimization for nanoparticle transport studiesInt J Nanomedicine2012749751022334780
- NeeshmaDJuewenLProgrammable assembly of DNA-functionalized liposomes by DNAACS Nano201151304131221230009
- MariappanPKrishnamoorthyKKadarkaraithangamJGovindasamyMSelective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidationNanomedicine2011718419221034861
- WuYNChenDHShiXYCancer-cell-specific cytotoxicity of non-oxidized iron elements in iron core-gold shell NPsNanomedicine2011742042721272668
- BeckerALOrlottiNIFoliniMRedox-active polymer microcapsules for the delivery of a survivin-specific siRNA in prostate cancer cellsACS Nano201151335134421226510
- XiaTZhaoYSagerTDecreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryosACS Nano201151223123521250651
- ShenZXShiZZFangJAll-trans retinoic acid_As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemiaProc Natl Acad Sci U S A20041015328533515044693
- ChenZWangZYChenSJAcute promyelocytic leukemia: cellular and molecular basis of differentiation and apoptosisPharmacol Ther1997761411499535176
- WangZYChenZDifferentiation and apoptosis induction therapy in acute promyelocytic leukemiaLancet Oncol2000110110611905660
- QiWJHuangCZChenLQCuprous oxide nanospheres as probes for light scattering imaging analysis of live cells and for conformation identification of proteinsTalanta2010801400140520006105
- LiuPYangSFangMLuoXCaiWComplex nanostructures synthesized from nanoparticle colloids under an external electric fieldNanoscale201113933394021850336
- YuGHuXLiuDElectrodeposition of submicron/nanoscale Cu2O/Cu junctions in an ultrathin CuSO4 solution layerJ Electroanal Chem2010638225230
- HassanienRAl-SaidSASillerLSmooth and conductive DNA-templated Cu2O nanowires: growth morphology, spectroscopic and electrical characterizationNanotechnology20122307560122261265
- YanXYTongXLZhangYFCuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reactionChem Commun Camb2012481892189422227804
- ChiYZhouSWanFFlow cytometric analysis of the anticancer effects of copper acetateJournal of China-Japan Friendship Hospital199486366
- DanielCJCaseyCRMilesFBDavidNMRodneyAIn vitro proliferating cell models to study cytotoxicity of silica nanowiresNanomedicine20106849219447209
- HajraKMLiuJRApoptosome dysfunction in human cancerApoptosis2004669170415505412
- SzetoHHCell-permeable, mitochondrial-targeted, peptide antioxidantsAAPS J20062E27728316796378
- AshaRaniPVLowKMGHandeMPValiyaveettilSCytotoxicity and genotoxicity of silver nanoparticles in human cellsACS Nano2009327929019236062
- KumarRDwivediPDDhawanADasMAnsariKMCitrinin-generated reactive oxygen species cause cell cycle arrest leading to apoptosis via the intrinsic mitochondrial pathway in mouse skinToxicol Sci2011255756621622943
- WangLMLiuYLiWJiangXMChenCYSelective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapyNano Lett2011277278021186824
- MisawaMTakahashiJGeneration of reactive oxygen species induced by gold nanoparticles under x-ray and UV irradiationsNanomedicine2011760461421333754
- SauvainJJRiedikerMDeslarzesSNanoparticle reactivity toward dithiothreitolNanotoxicology20082121129
- XiaTKovochichMBrantJComparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigmNano Lett200611794180716895376
- MehmetZSaimÖTetsuyaKToruSA novel, simple, organic free preparation and characterization of water dispersible photoluminescent Cu2O nanocubesMater Lett200963400402
- YongHHSungZKSuhnHKWooHPApoptosis in pyrogallol-treated Calu-6 cells is correlated with the changes of intracellular GSH levels rather than ROS levelsLung Cancer20085930131417920721
- WuMHuangJXuSLingTXieYShenWReactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healingExp Eye Res20098987688619635476
- KuoCHMichaelHHMorphologically controlled synthesis of Cu2O nanocrystals and their propertiesNano Today20105106116