?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Biodegradable polymers can be applied to a variety of implants for controlled and local drug delivery. The aim of this study is to develop a biodegradable and nanoporous polymeric platform for a wide spectrum of drug-eluting implants with special focus on stent-coating applications. It was synthesized by poly(DL-lactide-co-glycolide) (PLGA 65:35, PLGA 75:25) and polycaprolactone (PCL) in a multilayer configuration by means of a spin-coating technique. The antiplatelet drug dipyridamole was loaded into the surface nanopores of the platform. Surface characterization was made by atomic force microscopy (AFM) and spectroscopic ellipsometry (SE). Platelet adhesion and drug-release kinetic studies were then carried out. The study revealed that the multilayer films are highly nanoporous, whereas the single layers of PLGA are atomically smooth and spherulites are formed in PCL. Their nanoporosity (pore diameter, depth, density, surface roughness) can be tailored by tuning the growth parameters (eg, spinning speed, polymer concentration), essential for drug-delivery performance. The origin of pore formation may be attributed to the phase separation of polymer blends via the spinodal decomposition mechanism. SE studies revealed the structural characteristics, film thickness, and optical properties even of the single layers in the triple-layer construct, providing substantial information for drug loading and complement AFM findings. Platelet adhesion studies showed that the dipyridamole-loaded coatings inhibit platelet aggregation that is a prerequisite for clotting. Finally, the films exhibited sustained release profiles of dipyridamole over 70 days. These results indicate that the current multilayer phase therapeutic approach constitutes an effective drug-delivery platform for drug-eluting implants and especially for cardiovascular stent applications.
One of the fields that has benefited from nanomedicine is the design of new drug- delivery and -release systems. These novel systems can be combined with state-of-the-art implant technology and, in turn, give birth to drug-eluting implants that release therapeutic agents at the site of implantation. One of the most promising categories of drug-eluting coatings is considered to be nanoporous platforms with pore sizes less than 0.1 μm. Porosity of such low dimensions contributes to the material’s high active surface and drug loading, leading to desirable drug release profiles for each medical application.Citation1,Citation2
A wide range of different materials and fabrication methods have been used to manufacture nanoporous coatings.Citation3 Biocompatible metals and their alloys, such as titanium and aluminum, are subjected to a self-ordering porous formation that is based on electrochemical processes.Citation4 Polymers and especially block copolymers have been widely used to compose nanoporous drug-delivery platforms aimed at clinical applications.Citation5,Citation6 However, their use has been hindered because of limited biodegradability and slow degradation rates.Citation7 These properties were found to cause undesirable side effects, such as hypersensitivity reactions at the site of implantation, inflammation, and thrombus formation, which can lead to tissue damage and even implant failure.Citation8
In this study, we designed and developed biodegradable polymeric matrices in a multilayer configuration characterized by a diversity of nanopores for controlled drug loading and release. Two classes of poly(DL-lactide-co-glycolide) (PLGA 65:35, PLGA 75:25) and polycaprolactone (PCL) with different degradation rates constitute the nanolayers of the platform, and they were deposited by spin coating. Although this technique has been widely used for the deposition of thin films for various applications,Citation9,Citation10 to the best of our knowledge this is the first time that it has been used for the synthesis of drug-eluting applications. In particular, novel nanoporous materials with a variety of nanopore characteristics (depth, density, and diameter) were manufactured to serve as drug reservoirs with multiplex loading capacities. The major goals in designing drug-delivery systems are to control nanopore size, surface properties, and release of pharmacologically active agents in order to achieve the site-specific action of the drug at the nanoporosity therapeutically optimal rate and dose regimen. The control of the nanoporosity of the engineered nanomaterials was achieved by the implementation of highly sensitive techniques, such as atomic force microscopy (AFM) and spectroscopic ellipsometry (SE) in correlation with variations in deposition parameters.
These multilayer polymeric nanocoatings may serve as a drug-eluting platform for a wide spectrum of implants (eg, orthopedic, cardiovascular, retinal etc). The design of the platform and the selection of polymers and drugs should be made in line with the specific medical application. In this study, the platform was designed for stent-coating needs.
Several drug candidates, such as immune-suppressive agents, anti-inflammatory, and cellular proliferation inhibitors, have been employed for stent coating and evaluated in clinical trials. Sirolimus-eluting (Cypher®; Cordis, Miami Lakes, FL) and paclitaxel-eluting (Taxus®; Boston Scientific, Natick, MA) stents have been extensively used for coronary angioplasty.Citation11,Citation12
In our study, dipyridamole (DPM), an antiplatelet drug known to inhibit clotting,Citation13 was encapsulated into the external layer of the polymeric matrix and the release of the drug was monitored over time. We used platelet adhesion studies to assess their antithrombogenic effect.
Methods
Materials
Dipyridamole was obtained from Sigma-Aldrich (Athens, Greece). NaCl, KCl, KH2PO4, and Na2HPO4 were obtained from Merck (Darmstadt, Germany). PLGA with different lactide:glycolide contents (65:35 with average molecular weight [Mw] = 40,000–75,000 and 75:25 with average Mw = 66,000–107,000) and PCL (with average Mw = 48,000– 90,000) were purchased from Sigma-Aldrich. All solutions were prepared with deionized water.
Fabrication of multilayer films
The samples were fabricated by spin coating onto silicon (Si) and stainless steel substrates inside a nitrogen-filled glovebox. For the fabrication of polymeric single layers, a solution of the corresponding polymer was prepared with a total concentration of 10 mg mL−1 in chloroform. The solution was spin coated under various experimental conditions (rotation speed = 3–95 × g and spinning time = 18–30 seconds). The substrates were cleaned prior to spin coating with isopropanol and methanol and blow dried using N2 flow. Each layer was left overnight to slow dry any residual solvent left before the deposition of the next polymeric layer.
Drug-loading studies
A solution of drug (PLGA [65:35] 1:3, weight [w]/w) was prepared with a total concentration of 13.3 mg mL−1 in chloroform. The initially added amount of the drug was 1.0 mg. The amount of the drug remaining in the film after the spin-coating method was determined by washing the substrate with 3 mL of CHCl3 and measuring the free drug with a UV-1700 ultraviolet (UV)-visible spectrophotometer (Shimadzu, Kyoto, Japan) at 292 nm.
Atomic force microscopy for platelet adhesion studies
The polymeric layers were imaged by an AFM Solver P-47H (NT-MDT, Moscow, Russia) scanning probe microscope at ambient environmental conditions. For platelet adhesion studies, human platelet-rich plasma (PRP) was prepared after the centrifugation of whole blood drawn by venopuncture (kept in tubes with 3.8% citrate acid) from healthy donors at 4 × g for 7–10 minutes at room temperature. The polymeric films were cleaned by N2 flow and incubated in PRP at room temperature. AFM was then applied for platelet visualization onto the drug-free triple layers (PLGA [65:35]–PLGA [75:25]–PCL) and DPM-loaded ones (PLGA [65:35] + DPM–PLGA [75:25]–PCL) at 1- and 2-hour intervals in the tapping mode for better image acquisition and avoidance of platelet damage.Citation14 The quantities that were used for the evaluation of surface roughness of the multilayer films before and during platelet adhesion were peak-to-valley (Ry) distance and root-mean-square roughness (Rq). Ten areas of the samples were chosen at random to obtain statistical averages of the Rq and Ry parameters by Student’s t-test.
Spectroscopic ellipsometry studies
The optical properties of the PLGA and PCL films were studied with the SE technique using a phase-modulated spectroscopic ellipsometer (by Horiba/Jobin-Yvon), covering the extended spectral range from near-infrared to far UV (1.5–6.5 eV). It has already been established that SE is a suitable technique for measuring the optical constants of the materials.Citation15 However, few data have been reported on the optical characterization of polymeric thin films using SE. Ellipsometry measures changes in the reflectance and phase difference between the parallel (Rp) and perpendicular (Rs) components of a polarized light beam upon reflection from a material surface. Using the following equation:
The intensity ratio of Rp and Rs can be related to the amplitude ratio (Ψ) and the phase difference (Δ) between the two components of polarized light.Citation15 Because ellipsometry measures the ratio of two values originating from the same signal, the data collected are accurate and reproducible. Moreover, the changes in polarization measured by ellipsometry are extremely sensitive to thickness (down to monolayer level), microstructure, and optical properties of the film under study.
In vitro drug release studies
The multilayer polymeric films (n = 7 indicates the number of substrates coated with the polymeric films) loaded with dipyridamole were immersed in phosphate buffered saline (PBS) at 37°C for 70 days in order to determine the drug-release kinetics. The release studies were conducted in 24-well cell culture clusters (Costar; Corning, Manassas, VA) containing 1.0 mL of PBS (pH 7.4). Sodium azide (0.05% w/volume) was added to the medium in order to prevent bacterial growth. The medium was removed (completely) at each sampling time (2, 6, and 12 hours and 1, 2, 3, 5, 7, 14, 21, 28, 35, 49, 56, and 70 days) assayed with UV at 292 nm and fresh medium introduced.
Results
Surface nanotopography and structural properties of single- and multilayerpolymeric films
The surface morphology of the polymeric films was investigated by AFM. A series of single-layer polymeric films was first fabricated. illustrates the topography image of a single-layer film made of PLGA (75:25), while the topography image of a single-layer film made of PCL is depicted in . The AFM images revealed that the PLGA films were atomically smooth with a surface roughness of 0.2 nm under variable experimental conditions. In contrast to the PLGA films, the PCL films () were less smooth with a surface roughness of ca. 5.5 nm. The surface topography demonstrated the formation of spherulites with various sizes (5–40 nm) as an outcome of the crystallization process that takes places after thin-film deposition. In , the corresponding X cross sections of indicate that the holes between the spherulites of the PCL single layers reaches ca. 8 nm and the pore depth in the dual layers is ca. 10 nm.
Figure 1 AFM topography images of: (A) PLGA single layer that was spin coated at 53 × g for 30 seconds. (B) PCL single layer that was spin coated at 53 × g for 30 seconds. (C) Dual layer (PLGA [75:25]–PCL) that was spin coated at 53 × g for 30 seconds. (D) Triple layer (PLGA [65:35]–PLGA [75:25]–PCL) that was spin coated at 53 × g for 30 seconds. The scan size is 2.5 μm × 2.5 μm. (E) Corresponding X cross sections of Figure 1B and C that reveal the dimensions of the holes between the spherulites of PCL single layers and the pore sizes of PLGA–PCL dual layers, respectively.
Abbreviations: PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone.
![Figure 1 AFM topography images of: (A) PLGA single layer that was spin coated at 53 × g for 30 seconds. (B) PCL single layer that was spin coated at 53 × g for 30 seconds. (C) Dual layer (PLGA [75:25]–PCL) that was spin coated at 53 × g for 30 seconds. (D) Triple layer (PLGA [65:35]–PLGA [75:25]–PCL) that was spin coated at 53 × g for 30 seconds. The scan size is 2.5 μm × 2.5 μm. (E) Corresponding X cross sections of Figure 1B and C that reveal the dimensions of the holes between the spherulites of PCL single layers and the pore sizes of PLGA–PCL dual layers, respectively.Abbreviations: PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone.](/cms/asset/ab4183f6-19f6-445a-ae25-f42ca490d201/dijn_a_31185_f0001_c.jpg)
After fabricating single-layer samples, dual-polymeric layers made of PLGA (as the outer layer) and PCL (as the inner layer) were constructed. presents the topography image of a dual-layer polymeric film. In the dual-layer polymeric films, the formation of nanopores was evident. Characterization of the pores revealed variation in diameter (20–170 nm) and in depth (2–17 nm). The pore density was estimated between 40 and 70 pores/μm2 under different experimental conditions. Triple-layer polymeric films composed of PLGA (65:35; outer layer), PLGA (75:25; intermediate layer), and PCL (inner layer) were then manufactured. presents the topography image of a triple-layer film. Nanopores were observed in the triple layers as well. Hence, the AFM data demonstrated the formation of nanopores with smaller diameter (20–150 nm) and depth compared to the pores formed onto the surface of dual-layer polymeric matrices. The pore density of these triple layers was estimated between 20 and 80 pores/μm2. The AFM data involving the surface roughness parameters and pore characteristics of all layers are presented at .
Table 1 AFM data of the surface characteristics of the drug-free polymeric layers
In an attempt to investigate to what extent the process of pore formation can be controlled, the triple-layer films were fabricated under variable experimental conditions: (a) By altering the spinning speed at 24 × g and 53 × g, two series of triple-layer films were developed. depicts the AFM topography images of triple-layer films, spin coated at 24 × g for 30 seconds and at 53 × g for 30 seconds. Both samples had a polymer concentration of 10 mg mL−1; (b) by variations in polymer concentration of the outer layer (5 mg mL−1 and 10 mg mL−1). shows the topography images of triple-layer films, one with a polymer concentration of 10 mg mL−1 and the other with a concentration of 5 mg mL−1. Both samples were spin coated at 24 × g for 30 seconds. These two parameters were correlated with the surface roughness, pore depth, diameter, and pore density of the engineered triple layers ().
Table 2 AFM data of triple layers (PLGA [65:35]–PLGA [75:25]–PCL) fabricated under variable experimental conditions
Figure 2 AFM topography images of the triple layer (PLGA [65:35]–PLGA [75:25]–PCL) fabricated under variable experimental conditions. (A) Polymer concentration of 10 mg mL−1, spin coated at 24 × g for 30 seconds; (B) polymer concentration of 10 mg mL−1, spin coated at 53 × g for 30 seconds; and (C) polymer concentration of 5 mg mL−1, spin coated at 24 × g for 30 seconds.
Note: The scan size is 10 μm × 10 μm.
Abbreviations: PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone.
![Figure 2 AFM topography images of the triple layer (PLGA [65:35]–PLGA [75:25]–PCL) fabricated under variable experimental conditions. (A) Polymer concentration of 10 mg mL−1, spin coated at 24 × g for 30 seconds; (B) polymer concentration of 10 mg mL−1, spin coated at 53 × g for 30 seconds; and (C) polymer concentration of 5 mg mL−1, spin coated at 24 × g for 30 seconds.Note: The scan size is 10 μm × 10 μm.Abbreviations: PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone.](/cms/asset/165536cf-c919-4e2e-ba53-ef747ade15db/dijn_a_31185_f0002_c.jpg)
Spectroscopic ellipsometry studies for optical characterization of polymeric layers
SE was successfully employed for determining the optical properties, thickness, and structural characteristics of the spin-coated polymeric films, either in the form of single-, dual-, or triple-layer structure. The optical model that was applied to analyze the measured data consisted of the appropriate number of layers for each case. For determining the optical properties and the thickness of the films, a standard fitting procedure was applied in which the dielectric function ɛ(ω) of the polymeric films was described using the Tauc–Lorentz (TL) oscillator dispersion equation, where the imaginary part of the dielectric function is given by
where ω is the photon energy, ωg the fundamental band gap energy, A the amplitude of the oscillator, ω0 the Lorentz resonant energy, C its broadening term, and the real part ɛ1(ω) is obtained by the Kramer–Kronig integration.Citation16
The real part of the refractive index (n) and the extinction coefficient (k) of the complex refractive index are related to the dielectric function by the following equations:Citation16
and
shows the experimentally measured pseudodielectric function <ɛ(ω)> (curves with symbols) of single PLGA 75:25, PCL, dual-(PLGA [75:25]–PCL) 75:25–PCL films, and triple-(PLGA [65:35]–PLGA [75:25]–PCL) films that were spin coated at 53 × g. The <ɛ(ω)> accounts for the effect of film thickness and the substrate in addition to the dielectric response of the bulk film, and this is evident by the multiple reflections that appear in the spectra. The multiplex reflections are expected to be eliminated when the penetration depth of light becomes smaller than the film thickness. However, in our cases the whole spectra are dominated by multiple reflections since the PLGA and PCL start to absorb at energies above the upper experimental energy limit. In , solid lines at the respective plots correspond to the simulated spectra derived by the applied fitting procedure. By this procedure, the films’ thicknesses, the bulk dielectric functions, and the refractive indices of the produced PLGA and PCL films were calculated. In the corresponding real part of the complex refractive index n, referred to the bulk materials, has been plotted; we omitted the contribution of the thickness and the substrate, and the best-fit parameters of the TL oscillator model were used to reproduce the dielectric function or refractive index of the PLGA and PCL materials.
Figure 3 The experimental pseudodielectric functions (symbols) and the corresponding simulated ones (solid lines) determined by the use of best-fit results or the single-layer polymeric films: (A) PLGA 75:25–c-Si, (B) PCL–c-Si, (C) the dual-layer PLGA (PLGA [75:25]–PCL-c-Si), and (D) the triple-layer (PLGA [65:35]–PLGA [75:25]–PCL-c-Si).
Note: The insets in the figures show the geometrical model, which was applied in each case, and the respective thicknesses of the polymeric films derived by the fitting analysis.
Abbreviations: PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone; Si, silicon.
![Figure 3 The experimental pseudodielectric functions (symbols) and the corresponding simulated ones (solid lines) determined by the use of best-fit results or the single-layer polymeric films: (A) PLGA 75:25–c-Si, (B) PCL–c-Si, (C) the dual-layer PLGA (PLGA [75:25]–PCL-c-Si), and (D) the triple-layer (PLGA [65:35]–PLGA [75:25]–PCL-c-Si).Note: The insets in the figures show the geometrical model, which was applied in each case, and the respective thicknesses of the polymeric films derived by the fitting analysis.Abbreviations: PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone; Si, silicon.](/cms/asset/69d7ad56-dd2e-4c5c-8e4e-93d70aa7d1d3/dijn_a_31185_f0003_c.jpg)
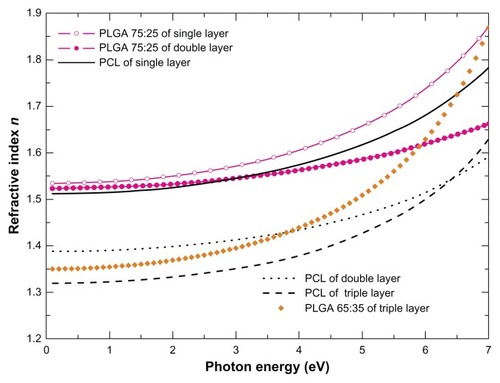
Figure 4 The real part of the bulk complex refractive index n calculated using best-fit parameters of the SE data analysis for the single-layer polymeric films PLGA 75:25 and PCL, the PLGA 75:25 and PCL from the dual-layer film, and the PLGA 65:35 and PCL from the triple-layer film.
Abbreviations: SE, spectroscopic ellipsometry; PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone.
The AFM data obtained for the single-layer films were in agreement with the SE data analysis since for the case of PLGA films there was no need to introduce an additional layer in the applied geometrical model (air/film/substrate) for the analysis in order to take into account the surface roughness. Indeed, the PLGA films were found to be atomically smooth.
In contrast, better fitting analysis was established by the introduction of a four-phase model (air/surface layer/film/substrate) for the case of PCL films (see also inset in ). With this model analysis, the surface roughness was represented by a top layer that is composed of polymeric material (PCL) and air (voids) with an equal percentage of 50%. The calculated effective thicknesses of these rough layers for the variable PCL films varied from 2.5 to 8.6 nm.
The formation of nanopores or voids in the dual- (PLGA 75:25–PCL) and triple-layer films (PLGA 65:25–PLGA 75:25–PCL) was also verified by the SE studies. More specifically, from where the bulk refractive index n has been plotted versus photon energy, nanopores formation is evident from the reduction of the n values in both PLGA and PCL films that consist of dual- and triple-layer polymeric film compared to those derived for the single-layer films. The decrease in n values may be due to the reduction in the density of the material and partially due to the surface and interface roughness in PCL and PCL–PLGA layers. Moreover, it is noticeable that a more drastic reduction in the n values was deduced for the PCL films compared to the PLGA. Therefore, we can interpret the SE results with nanopore formation in the dual- and triple-layer films.
Platelet adhesion studies onto drug-free and drug-loaded triple-polymeric layers
In order to perform platelet adhesion studies, drug-free and dipyridamole-loaded triple-layer films (PLGA 65:35–PLGA 75:25–PCL) were synthesized ( and , respectively). The DPM-loaded triple layer was characterized by a slight increase of surface roughness (Ry 12 nm and Rq 1.4 nm) compared to the drug-free one (Ry 9.5 nm and Rq 0.3 nm) (). As it is essential to monitor the polymer degradation of this triple layer when it comes in contact with plasma, the DPM-loaded triple layer was placed in PBS solution for 15 minutes (). Interestingly, the PLGA polymer starts to degrade even at this initial stage causing the initiation of drug release and an increase of pores diameter and surface roughness of the material (Ry: 310nm and Rq: 64nm of the sample in plasma versus Ry: 12nm and Rq: 1.4nm in ambient). The topography images of both drug-free and drug-loaded films after 1 hour and 2 hours of platelet adhesion are shown in .
Table 3 AFM data of platelet adhesion onto drug-free and drug-loaded triple layer (PLGA [65:35]–PLGA [75:25]–PCL)
Figure 5 AFM topography images of the triple layer (PLGA [65:35]–PLGA [75:25]–PCL) loaded with dipyridamole: (A) as fabricated by spin coating at 53 × g for 30 seconds and (B) after its degradation in PBS solution for 15 minutes.
Note: The scan size is 2.5 μm × 2.5 μm.
Abbreviations: AFM, atomic force microscopy; PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone; PBS, phosphate buffered saline
![Figure 5 AFM topography images of the triple layer (PLGA [65:35]–PLGA [75:25]–PCL) loaded with dipyridamole: (A) as fabricated by spin coating at 53 × g for 30 seconds and (B) after its degradation in PBS solution for 15 minutes.Note: The scan size is 2.5 μm × 2.5 μm.Abbreviations: AFM, atomic force microscopy; PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone; PBS, phosphate buffered saline](/cms/asset/05a48cb7-a55e-4bc9-9c6c-30c092a6be48/dijn_a_31185_f0005_c.jpg)
Figure 6 AFM topography images of platelets onto (A and B) drug-free and (C and D) dipyridamole-loaded triple layers (PLGA [65:35]–PLGA [75:25]–PCL). Both samples were spin coated at 53 × g for 30 seconds. (A) drug-free sample after 1 hour of platelet adhesion; (B) drug-free sample after 2 hours of platelet adhesion; (C) drug-loaded sample after 1 hour of platelet adhesion; and (D) drug-loaded sample after 2 hours of platelet adhesion with a typical cross section (inset).
Note: The scan size is 20 μm × 20 μm.
Abbreviations: AFM, atomic force microscopy; PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone.
![Figure 6 AFM topography images of platelets onto (A and B) drug-free and (C and D) dipyridamole-loaded triple layers (PLGA [65:35]–PLGA [75:25]–PCL). Both samples were spin coated at 53 × g for 30 seconds. (A) drug-free sample after 1 hour of platelet adhesion; (B) drug-free sample after 2 hours of platelet adhesion; (C) drug-loaded sample after 1 hour of platelet adhesion; and (D) drug-loaded sample after 2 hours of platelet adhesion with a typical cross section (inset).Note: The scan size is 20 μm × 20 μm.Abbreviations: AFM, atomic force microscopy; PLGA, poly (DL-lactide-co-glycolide); PCL, polycaprolactone.](/cms/asset/1d6b66ae-4fca-4c34-a5df-ffe9812c278c/dijn_a_31185_f0006_c.jpg)
Platelets after their contact with biomaterials are activated and undergo the following morphological changes: (i) their shape gets flatter, (ii) the granules are gathered into the center of the cell, forming the pseudo nucleus (egg-like type), and (iii) broad pseudopodia (filopods) extend, which are essential for platelets spreading onto surfaces and for aggregation.Citation17 These structural alterations of platelets during activation can be imaged by AFM in detail, as described in previous studies.Citation18 The cells then release biochemical compounds (eg, serotonin, thromboglobulin, platelet factor IV, thromboxane) for further activation and aggregation.Citation17
In , the platelets after 1 hour of adhesion onto the drug-free sample were found at different stages of activation: (a) egg-like type platelets, less activated, denoted by circles; (b) more activated platelets with pseudonucleus and pseudopodia, as presented by the blue squares; and (c) platelet aggregation (height ca. 411 nm) within the red squares, a stage that precedes clot formation. In , the AFM topography image of platelets after 2 hours of adhesion onto the drug-free sample is depicted. It can be easily noticed that the encircled platelets form aggregations reaching the height of ca. 565 nm. In , the platelets onto the drug-loaded samples after 1 hour of adhesion develop pseudopodia and form clusters with a height of ca. 245 nm, and after 2 hours the height of platelet clusters reaches approximately 245 nm (as measured by the corresponding cross section, inset of ).
The surface roughness (Rq and Ry) parameters after 1 hour and 2 hours of platelet adhesion on the drug-free sample and DPM-loaded triple-layer films are listed in .
Drug release kinetics
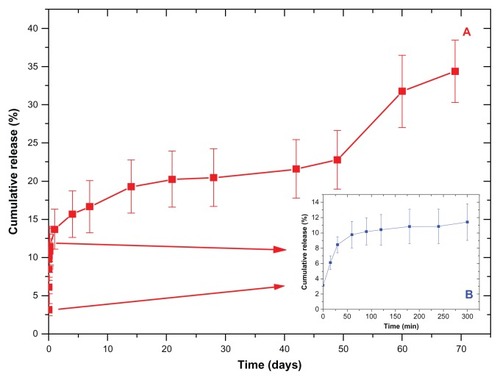
The amount of drug remaining in the substrate after the spinning process was estimated to be 2.82% ± 0.40% of the initial amount. The cumulative release profiles from the triple-layer sample are depicted in . The release profiles can be divided into four main stages: burst release during the first 5 hours (inset to ); an exponential release rate during the first week; approximately constant release rate during weeks 2–7; and finally an increased release after week 7 to week 10. This indicates that the assembled therapeutic multilayer was slowly disassembled with a release of DPM in a controlled manner, possibly along with a change in the multilayer thickness. It is likely that the degradation of the hydrophobic core (PLGA) induces the formation of particles, and subsequent partitioning of DPM from the hydrophobic core to an aqueous medium could be mainly responsible for the resulting DPM release patterns.
Discussion
There are clinical needs for the development of biomaterials for implants with diverse functionalities, such as controllable and multiplex drug release from their surface, in order to serve different goals in the fight against diseases. For example, in cardiovascular stents the antiplatelet drugs for avoidance of thrombosis and the antiproliferative drugs for inhibition of smooth muscle cells proliferation and restenosis should be eluted at different time intervals and in a controllable manner. Furthermore, few studies have shown that the nonbiodegradable polymeric drug reservoirs that comprise the drug-eluting stents (DES) may cause a chronic inflammation and hypersensitivity of the vessel wall.Citation20,Citation21
For that reason, in our study biodegradable polymers were selected for manufacturing the drug-delivery platform. Both PLGA and PCL, which are fully biodegradable due to hydrolysis of their ester bonds during their degradation, were used to form the layers of the platform. Targeting their potential application as coatings for DES, the polymers were deposited with a specific order that is in accordance with their degradation rates. In order to achieve a sustained drug elution profile, we designed the construct in a way that the outer polymeric layer will have the fastest degradation rate, while the inner polymeric layer will need much more time to degrade. The PCL was selected to serve the inner layer since its degradation rate is much slower (greater than 24 months) compared to that of PLGA (around 3–6 months).Citation22
As regards the two PLGAs with lactide:glycolide ratios of 65:35 and 75:25, it has been shown that the time required for degradation of PLGA is related to the monomers’ ratio used in production – the higher the content of glycolide units, the lower the time required for degradation.Citation23 As a result, the intermediate layer made of PLGA 75:25 requires more time to degrade compared to the outer layer made of PLGA 65:35.
In a fundamental drug-release study of biomaterials, an uncontrolled drug release occurred when drug adhered to the outer surface rather than being deposited within the pores.Citation24 There are difficulties with control of loading and release of drug from nanometer-sized pores, which is a significant challenge due to their small size. Thus, in this study we describe an experimental procedure for manufacturing multilayer polymeric thin films with tailored nanoporosity for drug loading by the implementation of spin coating. It was noticeable by the data provided by the surface characterization techniques AFM and SE that the PLGA–PCL multilayers were highly nanoporous in contrast to the smooth PLGA and spherulite-characterized PCL single layers.
The origin of pore formation may be attributed to a demixing process, which generally involves the spontaneous phase separation of polymer blend occurring under the specific conditions of spin coating. Similar holes or pores have been reported with a polymer blend phase separating via a spinodal decomposition mechanism.Citation25 At the initial stages of spin coating, the upper part of the substrate film was dissolved, forming a blend solution since chloroform is a common solvent for the selected polymers. This hypothesis was verified by the SE data showing that the thickness of the substrate film was reduced after the deposition of the subsequent film (as depicted in ).
In addition, Affrossman et alCitation26 reported that morphology evolution is strongly depended on the polymer blend ratio and the film thickness. If the weight content of one component in a polymer blend is much higher (about 90%) than the other component, then small holes are observed. When this fraction decreases to about 70%, the diameter of the holes increases and the holes start to coalesce. An analogous behavior is observed in our case comparing ; in the concentration of the added PLGA (65:35) solution is 10 mg mL−1, while in the concentration is 5 mg mL−1. Since the amount of the added solution is the same in both cases, the reduction in the film thickness and the percentage analogy of the two types of PLGA in the latter case explains its characteristic morphology ().
The pore distribution in the nanoporous biomaterials is a key factor for determination of drug release kinetics and directional control. By varying the size (ie, diameter, volume) and number of the porous reservoirs, a range of therapeutic agent loading levels can be achieved.Citation25 In the case of DES where the drug targets different sites of the vessel, it is necessary to control the porosity of the stent nanocoatings for directional and tailored drug loading and resultant release. In this study we found that by tuning the spin-coating parameters, the pore distribution and dimensions, interconnectivity, and density of the materials can be controlled. The AFM images demonstrated that by increasing the spinning speed, the pore diameter and surface roughness were increased, whereas the pore density was decreased. The pore depth was not found to be affected by this parameter, as shown in . Moreover, the decrease in the polymer concentration of the outer layer of PLGA (65:35), keeping the rotation speed and spinning time constant, resulted in an increase in pore diameter, pore density, and surface roughness of the engineered biomaterials (as presented at ).
In controlled polymeric drug-delivery systems, the drug-delivery rates are mainly determined by the dynamics of polymer degradation, which is strongly related to polymer structure, morphology, and properties. All these can be correlated to the optical properties of the polymeric films. The study of the optical properties and the consequent creation of a database containing specific optical constants of the candidate polymeric films for such systems can provide a powerful tool for a nondestructive and creditable methodology to evaluate their performance. Hence, one can consider that the complete elucidation of the optical response of the engineered systems is essential for the achievement of their functionality as well as for the prediction of their effectiveness.
In this work we show successful implementation of SE for determining the thicknesses, nanoporosity and the optical constants of the spin-coated polymeric films. In regards to thickness measurements, the SE data revealed () that the thickness of the substrate film was reduced after the deposition of the subsequent film due to a spinodal decomposition mechanism.
Dipyridamole was selected for loading within the nanopores of the polymeric multilayers to examine their antiplatelet effect in order to be used as potential coatings for cardiovascular implants. The ultimate goal was to inhibit thrombus formation, which may lead to implant failure and patient complications. The DPM burst effect and release profile during the first week was typical of diffusion-controlled systems. The third and fourth phase of the constant release rate presumably involved degradation of PLGA combined with diffusion of the remaining drug that was more firmly attached to polymer.
As polymer degradation is an important factor that determines drug elution, the AFM enables us to image the degraded DPM-loaded triple-polymeric layer after placing it in PBS solution for 15 minutes. At this initial stage, an increase in surface roughness of the drug-loaded layer was observed due to the degradation of PLGA, the formation of particles occurred, and there was resultant partitioning of the drug from PLGA, causing drug release.
Our previous studies have shown that AFM is a useful tool for imaging platelets in real time, with high precision and without destroying sensitive cells.Citation27,Citation28 The AFM images of platelets onto drug-free, triple-layer, polymeric films showed that the platelets are highly activated, forming pseudopodia and aggregates. From the AFM data presented in , it can be deduced that there was a time-dependent increase of Rq and Ry parameters, indicative of platelet aggregation. Our previous studies showed that the process of platelet adhesion onto nanomaterials that are atomically smooth or have low nanoroughness is time-dependent, resulting in an increase in Ry due to platelet aggregation.Citation28,Citation29
In contrast, in the case of the DPM-loaded triple-layer configuration of the biodegradable polymers, after 1 hour of platelet adhesion, although the cells develop pseudopodia and aggregations, the height is lower and remains at the same level after 2 hours. These findings provide evidence that dipyridamole loaded into the outer layer inhibits the platelet tendency to form high clusters, which are a prestage of thrombus. Moreover, although the triple-layer drug-loaded films are rougher compared to the drug-free films (Ry 12.5 nm versus 9.5 nm and Rq 1.4 nm versus 0.3 nm, respectively) after 1 hour of platelet adhesion there is a decrease in surface roughness (as depicted by the decrease in Ry and Rq values). This flattening of the surface biomaterials due to platelet adhesion is a phenomenon that shows how platelets behave toward nanomaterials with grooves, pores, and other morphologic irregularities.
Conclusion
In this study, a multilayer and biodegradable polymeric platform with diverse nanopores for drug-eluting implants was successfully developed. The design of the multilayer drug-eluting coatings was based on polymer degradation rates and properties in order to achieve a long-term and controlled drug elution profile; the spin-coating technique was implemented for this purpose. The spontaneous phase separation of polymer blends during spin-coating conditions leads to the creation of nanopores via the spinodal decomposition mechanism. The formation of nanopores onto the biomaterial surface with tailored characteristics (pore diameter, depth, density, surface roughness) was achieved by tuning the growth parameters.
The complementary AFM and SE studies for determination of structural characteristics, film thickness, and optical properties provide essential information for drug-loading capacities. A case study of dipyridamole loading within the nanopores and platelet studies for evaluation of their antiplatelet effect was carried out. It was found that the dipyridamole-loaded coatings inhibit the platelets tendency to form high aggregations.
In-parallel, drug release kinetics studies shown the release profiles of DPM that indicate that the biodegradable multilayer was slowly degraded with releasing DPM in a controlled manner. By fine tuning the dipyridamole release amount in a desired period, it would be possible to prevent thrombosis and recreate physiologically normal arterial conditions after DPM release.
Acknowledgments
This work was partially supported by NanoArthroxondros project 09SYN-41-1150.
Disclosure
The authors disclose no conflicts of interest.
References
- KangHKimDJParkSYooJRyuYSControlled drug release using nanoporous anodic aluminum oxide on stentThin Solid Films200751551845187
- OroszKEGuptaSHassinkMDelivery of antiangiogenic and antioxidant drugs of ophthalmic interest through a nanoporous inorganic filterMol Vis20041055556515332016
- LosicDVellemanLKantKSelf-ordering electrochemistry: a simple approach for engineering nanopore and nanotube arrays for emerging applicationsAust J Chem201164294301
- AninweneGEIIYaoCWebsterTJEnhanced osteoblast adhesion to drug-coated anodized nanotubular titanium surfacesInt J Nanomedicine2008325726418686785
- NuxollEEHillmyerMAWangRLeightonCSiegelRAComposite block polymer-microfabricated silicon nanoporous membraneACS Appl Mater Interfaces2009188889320160882
- BieleckaULutsykPJanusKSworakowskiJBartkowiakWEffect of solution aging on morphology and electrical characteristics of regioregular P3HT FETs fabricated by spin-coating and spray coatingOrg Electr20111217681776
- MansourHSohnMGhananeemADeLucaPMaterials for pharmaceutical dosage forms: molecular pharmaceutics and controlled release drug delivery aspectsInt J Mol Sci2010113298332220957095
- HaraHNakamuraMPalmazJCSchwartzRSRole of stent design and coatings on restenosis and thrombosisAdv Drug Deliv Rev20065837738616650911
- KaragiannidisPKassavetisSPitsalidisCLogothetidisSThermal annealing effect on the nanomechanical properties and structure of P3HT:PCBM thin filmsThin Solid Films201151941054109
- SouzaFLopesKNascentePLeiteENanostructured hematite thin films produced by spin-coating deposition solution: Application in water splittingSolar Energy Mater and Solar Cells200993362368
- AcharyaGParkKMechanisms of controlled drug release from drug-eluting stentsAdv Drug Deliv Rev20065838734016546289
- GargSSerruysPCoronary stents: current statusJ Am Coll Cardiol20105614220620709
- MammenEFAn overview of dipyridamoleThrombosis Res19905713
- MüllerDAndersonKBiomolecular imaging using atomic force microscopyTrends in Biotech200220545549
- AzzamRMABosharaNEllipsometry and polarized lightAzzamRMAEllipsometry and Polarized LightNorth HollandAmsterdam1977San Diego
- JellisonGEModineFAParameterization of the optical functions of amorphous materials in the interband regionAppl Phys Lett1996693371373
- HartwigJHPlatelet structureMichelsonADPlateletsAcademic PressSan Diego20023745
- KaragkiozakiVLogothetidisSKalfagiannisNAFM probing platelets activation behavior on titanium nitride nanocoatings for biomedical applicationsJ Nanomedicine200956472
- BavryAKumbhaniDHeltonTBorekPMoodGBhattDLate thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trialsAm J Med20061191056106117145250
- LiistroFColomboALate acute thrombosis after paclitaxel eluting stent implantationHeart20018626226411514475
- ArmentanoIDottoriMFortunatiEMattioliSKennyJMBiodegradable polymer matrix nanocomposites for tissue engineering: a reviewPolym Degrad Stab20109521262146
- LuLGarciaCAMikosAGIn vitro degradation of thin poly(DL-lactic-co-glycolic acid) filmsJ Biomed Mater Res19994623624410380002
- GultepeENageshaDSridharSAmijiMNanoporous inorganic membranes or coatings for sustained drug delivery in implantable devicesAdv Drug Deliv Rev20106230531519922749
- NormanJDesalTMethods for fabrication of nanoscale topography for tissue engineering scaffoldsAnn Biomed Eng20063418910116525765
- AffrossmanSHennGO’NeillSPethrickPStammMSurface topography and composition of deuterated polystyrene-poly (bromostyrene) blendsMacromolecules19962950105016
- HughesGNanostructure-mediated drug deliveryJ Nanomedicine200512230
- KaragkiozakiVLogothetidisSLousinianSGiannoglouGImpact of surface electric properties of carbon-based thin films on platelets activation for nano-medical and nano-sensing applicationsInt J Nanomedicine2008346146919337414
- KaragkiozakiVLogothetidisSLaskarakisAGiannoglouGLousinianSAFM Study of the thrombogenicity of carbon-based coatings for cardiovascular applicationsMater Sci Eng B20081521621
- KaragkiozakiVLogothetidisSKassavetisSGiannoglouGNanomedicine for the reduction of the thrombogenicity of stent coatingsInt J Nanomedicine2010523924820463940