?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
In this study, a pH and temperature dual-sensitive liposome gel based on a novel cleavable hydrazone-based pH-sensitive methoxy polyethylene glycol 2000-hydrazone-cholesteryl hemisuccinate (mPEG-Hz-CHEMS) polymer was used for vaginal administration.
Methods
The pH-sensitive, cleavable mPEG-Hz-CHEMS was designed as a modified pH-sensitive liposome that would selectively degrade under locally acidic vaginal conditions. The novel pH-sensitive liposome was engineered to form a thermogel at body temperature and to degrade in an acidic environment.
Results
A dual-sensitive liposome gel with a high encapsulation efficiency of arctigenin was formed and improved the solubility of arctigenin characterized by Fourier transform infrared spectroscopy and differential scanning calorimetry. The dual-sensitive liposome gel with a sol-gel transition at body temperature was degraded in a pH-dependent manner, and was stable for a long period of time at neutral and basic pH, but cleavable under acidic conditions (pH 5.0). Arctigenin encapsulated in a dual-sensitive liposome gel was more stable and less toxic than arctigenin loaded into pH-sensitive liposomes. In vitro drug release results indicated that dual-sensitive liposome gels showed constant release of arctigenin over 3 days, but showed sustained release of arctigenin in buffers at pH 7.4 and pH 9.0.
Conclusion
This research has shed some light on a pH and temperature dual-sensitive liposome gel using a cleavable mPEG-Hz-CHEMS polymer for vaginal delivery.
Introduction
Arctigenin, a major bioactive component of Fructus arctii, occurs naturally in Bardanae fructus, Arctium lappa L., Saussurea medusa, Torreya nucifera, and Ipomea cairica. As a phenylpropanoid dibenzyl butyrolactone lignan, it has been reported to exhibit antioxidant, antihuman immunodeficiency virus, antitumor, and anti-inflammatory activity.Citation1–Citation5
Conventional vaginal delivery systems, such as creams, gels, tablets, and foams, are easily removed by the self-cleansing action of the vaginal tract. The efficacy of all of these treatments is limited by their residence time in the genitourinary tract.Citation6 Moreover, the physiology arising from the protective mechanism of the genitourinary tract limiting residence time and impairing therapeutic drug efficacy makes multiple and frequent administrations necessary for effective treatment.Citation7 Uncomfortable vaginal delivery and the need for repeated dosing are challenges in terms of patient compliance. To overcome these limitations, novel vaginal delivery systems (microspheres, bioadhesive gels, and tablets) have attracted considerable attention for prolonging drug contact with a mucosal surface without inducing local adverse effects on the epithelium.
Thermosensitive gel systems composed of poloxamers are easily administered to the vagina without difficulty or leakage, and are retained in the vagina for a long time. Furthermore, poloxamer gel formulations have been reported to produce significantly higher initial plasma concentrations and faster time to peak concentration values than a solid suppository, indicating that a drug in poloxamer gel form could be absorbed more rapidly than the solid form. Poloxamer gel systems are more physically stable, convenient, and effective vaginal dosage formulations. Over the past few years, gels containing liposomes have been advocated in the literature as vaginal drug delivery systems.Citation8,Citation9 The purpose of these formulations is to maintain liposomes at the delivery site and to avoid their rapid clearance from the system in order to achieve sustained drug release.
Hydrazone-based pH-sensitive methoxy polyethylene glycol 2000-hydrazone-cholesteryl hemisuccinate (mPEG2000-Hz-CHEMS) was synthesized and used to manufacture liposomes in our previous research. This material was stable at pH 7.4 but sensitive to mild acidic conditions (pH 5.5). mPEG2000-Hz-CHEMS-modified paclitaxel-containing pH-sensitive liposomes and the accelerated blood clearance phenomenon have also been investigated. However, further research on the use of mPEG2000-Hz-CHEMS in liposome gels for vaginal delivery has not been reported. This is interesting and novel for the use of mPEG2000-Hz-CHEMS in the pH and temperature dual-sensitive vaginal liposome gel delivery system.Citation10–Citation12
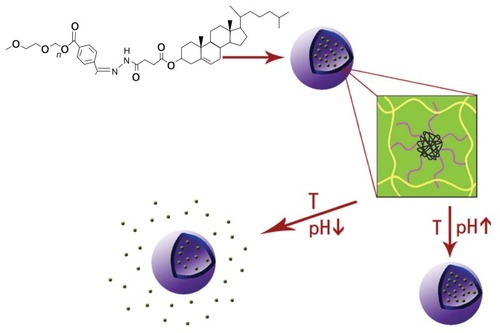
In this study, a pH and temperature dual-sensitive mucoadhesive vaginal gel formulation was developed for gynecological purposes. A schematic diagram of the mPEGHz-CHEMS cleavable dual-sensitive liposome gel strategy is shown in . The pH-sensitive cleavable mPEG-Hz-CHEMS was designed as a modified pH-sensitive liposome that would selectively degrade under locally acidic vaginal conditions. The novel pH-sensitive liposome was engineered to form a thermogel at body temperature and to degrade in an acidic environment. To this end, we prepared pH and temperature dual-sensitive cleavable arctigenin-encapsulating liposome gels, composed of phosphatidylcholine, mPEG-Hz-CHEMS, and cholesterol. The physicochemical properties of the pH-sensitive liposomes and the dual-sensitive liposome gel were characterized, and the in vitro release and cytotoxicity of these arctigenin-encapsulated dual-sensitive liposome gels was investigated.
Materials and methods
Materials
Soybean phosphatidylcholine (S100PC) was purchased from Dongshang Co, Ltd (Shanghai, China). Cholesterol was obtained from China Medicine Shanghai Chemical Reagent Corporation (Shanghai, China). Arctigenin was supplied by Yantai Science and Biotechnology Co, Ltd (Yantai, China). mPEG2000-Hz-CHEMS were synthesized in our laboratory. All other chemicals were of analytical grade.
Preparation of pH-sensitive liposomes
pH-sensitive liposomes were prepared according to the thin-film hydration method. Briefly, a pH-sensitive lipid mixture of S100PC, DOPE, cholesterol, and mPEG2000-Hz-CHEMS (molar ratio 5:3:1:1) was dissolved in chloroform. Arctigenin-loaded pH-sensitive liposomes were prepared by dissolving arctigenin in the organic phase of chloroform and methanol (1:3, v/v) and mixing with S100PC, DOPE, cholesterol, and mPEG2000-Hz-CHEMS. The organic phase was removed at 40°C in a rotary flask. The flask was evaporated under reduced pressure. The dry lipid formed was hydrated with phosphate-buffered saline (pH 7.4). The liposomal suspension was filtered through 0.2 mm polycarbonate filters and stored at 4°C until use. Nonsensitive arctigenin-loaded liposomes without mPEG2000-Hz-CHEMS were also prepared as the control.
Determination of entrapment efficiency
The particle size and zeta potential of the liposomes were measured and analyzed using a 3000H Zetasizer (Malvern Instruments, Worcestershire, UK). The entrapment efficiency was defined as the ratio of the amount of arctigenin encapsulated in the liposome to that of the total arctigenin in the liposomal dispersion. The drug-loading rate is defined as the ratio of the amount of arctigenin encapsulated in the liposome to that of the total number of freeze-dried liposomes. An aliquot of liposome was treated with a 50-fold volume of methanol to disrupt the structure of the liposome. The amount of encapsulated arctigenin was measured using reverse-phase high-pressure liquid chromatography. The concentration of arctigenin in the liposomes was also determined by high-pressure liquid chromatography (HP1100, Agilent, Santa Clara, CA). The mobile phase was a mixture of methanol and water (55:45 v/v). The column was a Kromasil C18 (4.6 mm × 250 mm). The flow rate was 1.0 mL/minute, the detection wavelength was 280 nm (ultraviolet detector, HP1100, Agilent, USA), the column temperature was 30°C, and the volume of the sample injected was 20 μL.
The entrapment efficiency was determined from three separately prepared liposome suspensions as the mean ± standard deviation. The entrapment efficiency was calculated from the following equation:
Preparation of pH and temperature dual-sensitive gel
Thermosensitive gels were prepared by addition of P188 and P407 at various ratios, as described previously.Citation13 Briefly, various ratios of poloxamers were dissolved in distilled water and kept at 4°C until a clear solution was obtained. For dual-sensitive liposome gels, poloxamers were dissolved in pH-sensitive liposome dispersions instead of distilled water. The gelation temperature was defined as the temperature at which the liquid phase makes a transition to gel.Citation13 In brief, a 10 mL transparent vial containing a magnetic bar and each formulation was placed in a water bath. The vial was heated at a constant rate while stirring. The gelation temperature was measured when the magnetic bar stopped moving due to gelation.
Characterization of pH and temperature dual-sensitive gels
Fourier transform infrared spectrophotometry
Functional group characterization was carried out by Fourier transform infrared analysis using a Fourier transform infrared spectrometer (Travel IR, Danbury, CT, US SensIR Technologies, Danbury, CT). The samples were prepared by compressing the free drug, lyophilized arctigenin, arctigenin-loaded pH-sensitive liposomes, and arctigenin-loaded dual-sensitive liposome gel into pellets with potassium bromide.
Differential scanning calorimetry
Formation of the blank gel, arctigenin-loaded pH-sensitive liposomes, and arctigenin-loaded dual-sensitive liposome gel was confirmed by differential scanning calorimetry. Each sample, weighing 3 mg, was heated in hermetically sealed aluminum pans at a rate of 10°C per minute up to 250°C in a dynamic nitrogen atmosphere. All samples were analyzed in duplicate.
Measurement of gelation temperature
Gelation temperature was defined as the temperature at which the liquid phase makes a transition to gel, as described previously. Citation13 In brief, one milliliter of each polymer solution was transferred to each 4 mL vial, and the vials were kept at 4°C overnight. The vials were then placed in a water bath and equilibrated at 15°C for 15 minutes. The sol-gel and gel-sol transitions of the polymer solutions were tested using the tube inversion method in the temperature range of 15°C–80°C, while the polymer solutions were heated at a rate of 2°C every 5 minutes.Citation14 The transition temperature at which a polymer solution stopped flowing after tube inversion was recorded as the gelation temperature.
pH-sensitive gel test
pH-sensitive liposomes were prepared at a concentration of 20% in phosphate-buffered saline (pH 7.4). A 1 mL aliquot of polymer solution was placed in a series of 5 mL vials, and the vials were kept at 4°C overnight. Later, the polymer solutions were incubated at 37°C for 10 minutes for gelation. After gelation, 3 mL of phosphate buffer (pH 5.0, 7.4, and 9.0) was added to the vials. After gel erosion for a predetermined time, the weights of the gels were measured.
In vitro drug release
The in vitro release of arctigenin from different vaginal gels was determined using a dialysis bag placed in a sealed glass vial under constant shaking. One gram of each gel formulation (500 μg of arctigenin) was packed into the dialysis bags sealed with closures of 50 mm. The dialysis bags containing arctigenin-loaded dual-sensitive liposome gel were placed in a water bath at 37°C until the gel formed. The release medium was 45 mL of 0.1 M phosphate buffer (pH 5, 7.4, and 9), providing sink conditions for arctigenin. The medium was maintained at 37°C and shaken at 100 rpm. At various time intervals, 1 mL of dissolution fluid was collected. The sample filtrate was analyzed by reverse-phase high-pressure liquid chromatography. The mobile phase was a mixture of methanol and water (55:45 v/v). The column was a Kromasil C18 (4.6 mm × 250 mm). The flow rate was 1.0 mL/minute, the detection wavelength was 280 nm (ultraviolet detector, HP1100, Agilent), the column temperature was 30°C, and the injected volume of the sample was 20 μL.
Cytotoxicity assay
To evaluate the cytotoxicity of the arctigenin gel by MTT assay, HEK293 cells were seeded at a density of 1 × 104 cells/well in a 96-well plate overnight. A 100 μL aliquot of arctigenin solution (0.8 mg/mL), arctigenin-loaded pH-sensitive liposomes, arctigenin-loaded dual-sensitive liposome gel, diluted arctigenin-loaded dual-sensitive liposome gel (1:2, 1:4 in phosphate-buffered saline), and free arctigenin in diluted gel (1:4 in phosphate-buffered saline) were added to each well and incubated overnight; positive controls (80 μg as arctigenin) and negative controls (phosphate-buffered saline alone) were placed in additional wells and incubated overnight. After incubation, 100 μL of 5 mg/mL MTT in Dulbecco’s Modified Eagle’s Medium was added to each well and incubated for 4 hours at 37°C. To dissolve the formazan crystal, 100 μL of MTT solubilization solution was added. After shaking the 96-well plate for 30 minutes, absorbance was measured at 570 nm using a microplate reader.
Statistical analysis
Statistical comparisons were performed using the Student’s t-test for two groups, and one-way analysis of variance for multiple groups. P < 0.05 was considered to be indicative of statistical significance.
Results and discussion
Characteristics of mPEG-Hz-CHEMS-modified pH-sensitive liposomes
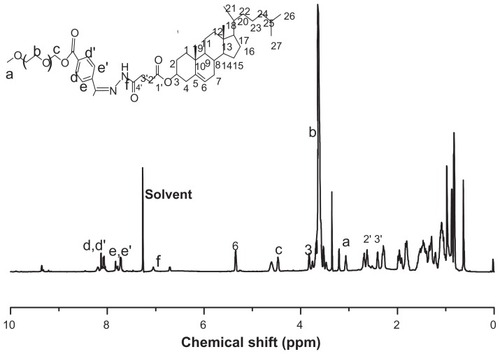
If the arctigenin-loaded dual-sensitive liposome gel was applied to the vaginal membrane, the liposomes or drug released would be taken up into the cells and tissues of the vaginal membrane. Thus, the particle size, zeta potential, and entrapment efficiency of liposomes would be important factors for vaginal delivery of the drug. The structure and 1H-NMR spectrum of the mPEG-Hz-CHEMS is shown in . As shown in , the mean particle size of arctigenin-loaded pH-sensitive liposomes was 126.6 ± 3.8 nm. The mean zeta potential was about −18.1 ± 2.3 mV. The entrapment efficiency was determined to be above 90%.
Table 1 The physicochemical parameters of AC-loading pH-sensitive liposomes and plain AC liposomes
Characterization of dual-sensitive liposome gel
Formation of dual-sensitive liposome gel was confirmed using different techniques, including Fourier transform infrared spectrophotometry and differential scanning calorimetry. shows the Fourier transform infrared spectra for blank gel (A), arctigenin-loaded pH-sensitive liposomes (B), and arctigenin- loaded pH-sensitive liposome gel (C). It can be clearly observed that the arctigenin-loaded pH-sensitive liposome showed a sharp C=O stretch band at 1740 cm−1. In the case of the arctigenin-loaded pH-sensitive liposome gel, the carbonyl-stretching band of the drug disappeared, which suggested that the arctigenin-loaded pH-sensitive liposome was involved in the gel. The differential scanning calorimetry thermograms shown in indicate the thermal behavior of free arctigenin (A), arctigenin-loaded pH-sensitive liposomes (B), and arctigenin-loaded pH-sensitive liposome gel (C) in a range of 30°C–280°C. The arctigenin-loaded pH-sensitive liposomes and arctigenin-loaded pH-sensitive liposome gel show peaks at about 48°C in the differential scanning calorimetry curve. However, complete disappearance of the endothermic peak corresponding to arctigenin was seen for arctigenin-loaded pH-sensitive liposomes and arctigenin-loaded pH-sensitive liposome gel, indicating molecular encapsulation of the arctigenin inside the liposomes and gel.
Figure 3 FT-IR spectra of blank gels (A), AC liposomes (B), AC dual-sensitive gels (C).
Abbreviation: AC, arctigenin.
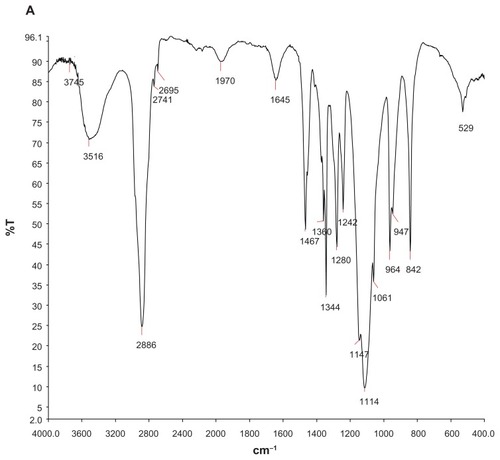
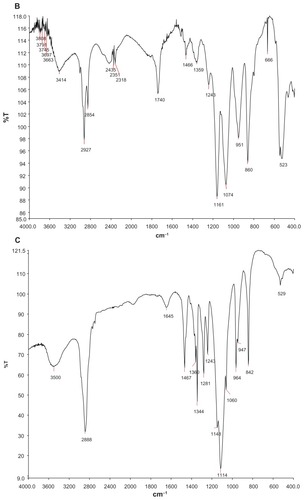
Figure 4 DSC curves of AC (A), AC liposomes (B), AC dual-sensitive gels (C).
Abbreviation: AC, arctigenin.
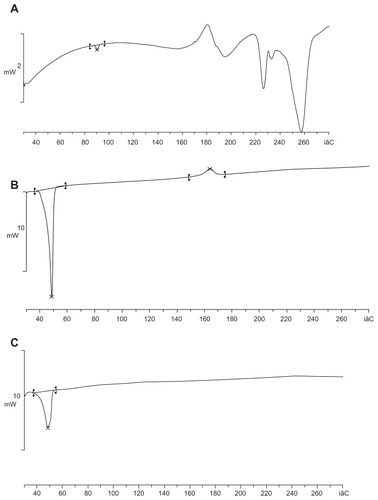
Arctigenin-loaded dual-sensitive liposome gel was prepared using P407:P188 at various ratios. With the temperature-sensitive P407 and P188 gelling polymers, the concentration of poloxamers (P407:P188, 12:20, or 15:15 w/w%) was previously demonstrated to form gels,Citation13,Citation15 and gelling temperatures were lowered by increasing the amount of P188 at an equal content of P407. shows the solgel transition of arctigenin dual-sensitive gels over gelation temperature (A), at gelation temperature (B), and under gelation temperature (C). In the absence of pH-sensitive liposomes, the mixtures of P407:P188 at weight ratios of 1:2 and 1:1 gelled at 39.6°C ± 3.02°C and 37.6°C ± 1.78°C, respectively. In our study, the pH-sensitive liposomes did not significantly affect the gelation temperatures because when pH-sensitive liposomes were added to the thermosensitive gels, the gelation temperatures decreased slightly.
Figure 5 The sol-gel transition observation of AC dual-sensitive gels over the gelation temperature (A), gelation (B), and under the gelation temperature (C).
Abbreviation: AC, arctigenin.
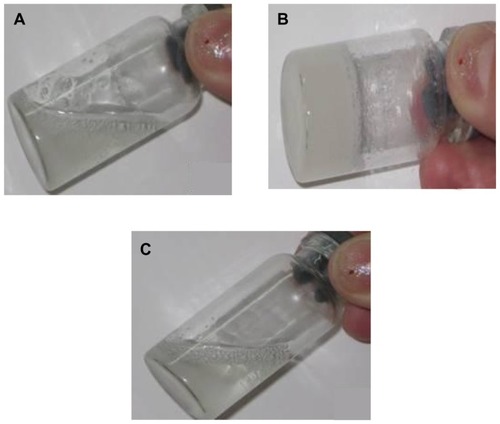
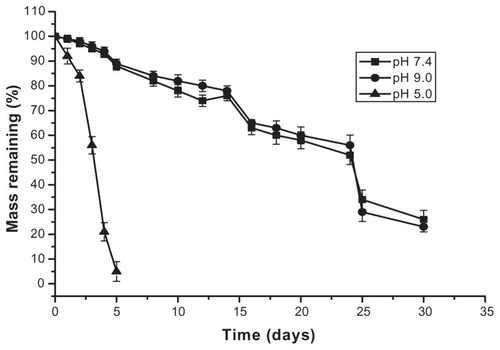
The pH-sensitive test for gel is clearly observed in . Arctigenin-loaded dual-sensitive liposome gels in the pH 5.0 study were completely eroded in 4 days, whereas the gels in the pH 7.4 and 9.0 studies eroded in around 5 weeks. The presence of the gel phase and pH around body temperature (37°C) indicates that this material could be a promising candidate for a vaginal drug delivery system that can be formulated at room temperature and would form a gel in situ upon vaginal administration.
In vitro drug release
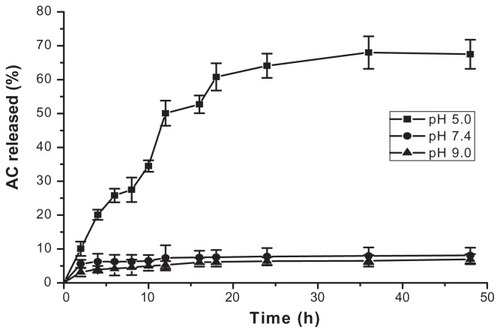
In vitro release experiments were performed at 37°C for 2 days in different pH buffers to study pH-dependent drug release behavior. The cumulative release of arctigenin, as shown in , indicates that the arctigenin-loaded dual-sensitive liposome gel had prolonged release in pH 7.4 and 9.0 buffers. At pH 5.0, the gels showed constant release, and drug release was completed within 3 days. It has been reported previously that the rate of dissolution of gel is actually the controlling factor in drug release.Citation16 In this respect, the gel pH-sensitive pattern would explain the constant release at pH 5.0.Citation14 The arctigenin-loaded dual-sensitive liposome gel looks interesting for vaginal drug delivery in view of vaginal pH.
Cytotoxicity assay
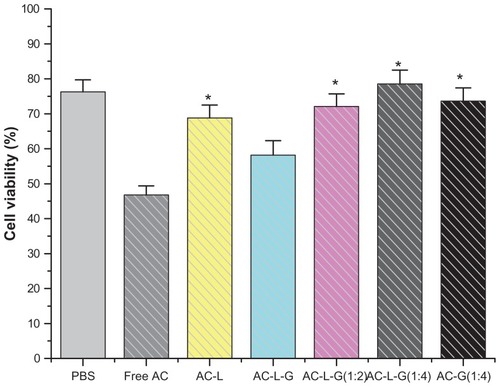
The cytotoxicity of free arctigenin and the formulated arctigenin was investigated in HEK293 cells using MTT assays (). Liposome gel was prepared by mixing P407 and P188 as described earlier in this paper, and was diluted with phosphate-buffered saline (1:2 and 1:4, v/v), because polymer gels should be diluted with a proper dilution ratio or extracted in medium in the cytotoxicity tests of polymer gels.Citation17 Cell viability (%) in the presence of free arctigenin, arctigenin-loaded pH-sensitive liposomes, arctigenin-loaded dual-sensitive liposome gel, diluted samples of arctigenin-loaded dual-sensitive liposome gel with phosphate-buffered saline (1:2 and 1:4, v/v), and phosphate-buffered saline alone were 46.8 ± 2.6, 68.8 ± 3.7, 58.2 ± 4.1, 72.1 ± 3.6, 78.5 ± 4.0, 73.6 ± 3.8, and 76.3 ± 3.4, respectively. Arctigenin encapsulated in pH-sensitive liposomes or arctigenin-loaded dual-sensitive liposome gel was less toxic than free arctigenin. Furthermore, the cell viability of diluted arctigenin-loaded dual-sensitive liposome gel (1:2 and 1:4, v/v) was higher than for the intact dual-sensitive liposome gel. The cytotoxicity of free arctigenin in diluted gels (1:4 in phosphate-buffered saline) was also lower than for free arctigenin. Intact liposome gel without dilution appears to be somewhat toxic compared with arctigenin loaded into liposomes due to oxygen deficiency produced by the thickness of the gels.
Conclusion
In this work, we have prepared a thermosensitive and pH-sensitive gel formulation for vaginal administration using novel cleavable mPEG-Hz-CHEMS. A dual-sensitive liposome gel with high encapsulation efficiency of arctigenin was formed and improved the solubility of arctigenin. The dual-sensitive liposome gel with a sol-gel transition at body temperature was degraded in a pH-dependent manner which was stable for a long period of time at neutral and basic pH, but cleavable under acidic conditions (pH 5.0). The arctigenin encapsulated in the dual-sensitive liposome gels was stable and less toxic than arctigenin loaded into pH-sensitive liposomes. These results suggest that a vaginal gel containing arctigenin would be useful for effective and convenient treatment of vaginal candidiasis with reduced toxicity, and may be helpful in developing effective pharmaceutical formulations for vaginal delivery of hydrophobic drugs.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Plan, 2010CB735600 and 2012CB724003), Taishan Scholar Project, and Yantai University Research Program (YX10B29). The authors also thank Qineng Ping, Qiang Zhang, and Can Zhang for their efforts on behalf of the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- AwaleSLuJKalauniSKIdentification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvationCancer Res2006661751175716452235
- ChoMKJangYPKimYCKimSGArctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: the role in TNF-α inhibitionInt Immunopharmacol200441419142915313439
- MatsumotoTHosono-NishiyamaKYamadaHAnti-proliferative and apoptotic effects of butyrolactone lignans from Arctium lappa on leukemic cellsPlanta Med20067227627816534737
- YangL-MLinS-JYangT-HLeeKHSynthesis and anti-HIV activity of dibenzyl butyrolactone lignansBioorg Med Chem Lett19968941944
- ZhaoFWangLLiuKIn vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L, through inhibition on iNOS pathwayJ Ethnopharmacol200912245746219429312
- HardyEJiménezALde PáduaKSZaneveldLJWomen’s preferences for vaginal antimicrobial contraceptives. III. Choice of a formulation, applicator, and packagingContraception1998582452499866007
- KimYTShinBKGarripelliVKA thermosensitive vaginal gel formulation with HPγCD for the pH-dependent release and solubilization of amphotericin BEur J Pharm Sci20104139940620654712
- PavelicZSkalko-BasnetNJalsenjakICharacterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitisInt J Pharm200530114014816024188
- PavelicZSkalko-BasnetNSchubertRLiposomal gels for vaginal drug deliveryInt J Pharm200121913914911337174
- ChenDLiuWShenYEffects of novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-response double smart mPEG-Hz-CHEMS on ABC phenomenonInt J Nanomedicine201162053206121976980
- ChenDJiangXHuangYZhangCPingQpH-Sensitive mPEG-Hz-cholesterol conjugates as a liposome delivery systemJ Bioact Compat Polym201025527542
- ChenDJiangXLiuJJinXZhangCPingQIn vivo evaluation of novel pH-sensitive mPEG-Hz-Chol conjugate in liposomes: pharmacokinetics, tissue distribution, efficacy assessmentArtif Cells Blood Substit Immobil Biotechnol20103813614220337549
- ParkJSOhYKYoonHKimJMKimCKIn situ gelling and mucoadhesive polymer vehicles for controlled intranasal delivery of plasmid DNAJ Biomed Mater Res20025914415111745547
- GarripelliVKKimJKNamgungRKimWJRepkaMAJoSA novel thermosensitive polymer with pH-dependent degradation for drug deliveryActa Biomater2010647748519596093
- ChangJYOhYKKongHSProlonged antifungal effects of clotrimazole-containing mucoadhesive thermosensitive gels on vaginitisJ Control Release200282395012106975
- BilensoyERoufMAVuralISenMHincalAAMucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole: beta-cyclodextrin complexAAPS Pharm Sci Tech20067E38
- KimHKKimYTChoYHCytotoxicity evaluation on hydrogels for medical devices based on the International Organization for StandardizationJournal of Korean Pharmaceutical Sciences200939127131