Abstract
RNA interference (RNAi) is a promising strategy to suppress the expression of disease-relevant genes and induce post-transcriptional gene silencing. Their simplicity and stability endow RNAi with great advantages in molecular medicine. Several RNAi-based drugs are in various stages of clinical investigation. This review summarizes the ongoing research endeavors on RNAi in molecular medicine, delivery systems for RNAi-based drugs, and a compendium of RNAi drugs in different stages of clinical development. Of special interest are RNAi-based drug target discovery and validation, delivery systems for RNAi-based drugs, such as nanoparticles, rabies virus protein-based vehicles, and bacteriophages for RNA packaging.
Overview of RNA interference
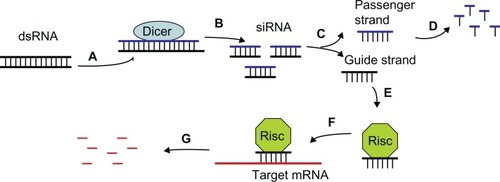
RNA plays key roles in organisms beyond the traditional role of being a messenger bridging genetic information and biosynthesis of protein. Endogenous or exogenous double-stranded RNA can be cleaved into 21–23-nucleotide small interfering RNA (siRNA) by the endonuclease, Dicer. siRNA can unzip into a guide strand and a passenger strand. The latter will be subsequently degraded. The guide strand is then incorporated into the RNA-induced silencing complex and binds to the target mRNA, inducing mRNA degradation by Argonaute, a component of the RNA-induced silencing complex which affects protein synthesis (). This phenomenon is known as RNA interference (RNAi)Citation1–Citation4 and is well established in diverse organisms.
Figure 1 Schematic representation of RNAi. (A and B) Double-stranded RNA is cleaved into siRNA by Dicer. (C and D). siRNA is unzipped into a guide strand and passenger strand. The latter is subsequently degraded. (E–G). The guide strand is incorporated into a RISC and binds to target mRNA, inducing degradation of mRNA.
Abbreviation: RISC, RNA-induced silencing complex.
There are three major types of small RNA used to silence gene function by RNAi technology, ie, microRNA (miRNA), siRNA, and short hairpin RNAs (shRNA).Citation5,Citation6 Although all small RNAs are 22–24 nucleotides in length,Citation7–Citation9 there are some important differences between them. First, siRNA, processed from exogenously introduced strictly base-paired siRNA duplexes, are completely complementary to their target mRNA and facilitate cleavage of bases 10–12 at the 5′ end of the guide strand,Citation10,Citation11 resulting in transient silence of the target gene. Second, shRNA, inserting in a Pol III expression cassette, can be transfected or packaged into a recombinant virus and introduced into target cells.Citation5 The advantage of shRNA is prolonged expression of the RNAi effect. Third, miRNA or chimeric miRNA/shRNA, derived from endogenously encoded shRNAs,Citation5 can mediate gene silencing post-transcriptionally, and bind partially to complementary miRNA target sequences located in the 3′ untranslated regions of target mRNAs,Citation9 but the “seed” region located in bases 2–8 is highly complementary.Citation12 The seed region can result in deadenylation, translational repression, and decay of mRNAs.Citation9,Citation13,Citation14 Interestingly, either strand of miRNA can silence gene expression post-transcriptionallyCitation6 and some translational repression mediated by miRNA is reversible.Citation15 miRNA are the latest major breakthrough in the field of RNAi and represents an important tool for RNAi to overcome some of the limitations of shRNA.Citation5
RNAi as a tool for discovery of drug targets
Most drugs function via blocking of their targets. The simplicity and stability of RNAi in gene silencing make it a powerful tool in drug research and development.Citation16 RNAi-based drug target discovery includes the following steps.
Identification of target genes
The selection of target gene is crucial and, to great extent, determines whether subsequent RNAi silencing can be effective or not. The abundance of the target gene, the regulation of its expression, and the half-life of its products are several important parameters that should be factored into.Citation17 For example, mutation of somatic cells and their unchecked proliferation are the main factors underlying tumors. Based on this phenomenon, the RNAi library and high throughput screening can be harnessed to find the genes or proteins involved in tumor development to pinpoint drug target candidates.Citation18
Designing siRNA
Once the target is defined, siRNA can be used to evaluate potential candidates. siRNA-mediated target RNA cleavage is highly sequence-specific. Any mismatches in the siRNA duplex can abolish potential target RNA cleavage.Citation17 Therefore, the success of RNAi-based therapeutics is strictly dependent on siRNA which can silence target genes specifically and effectively. There are various online algorithms available for appropriate design of siRNAs.Citation5 Some empirical characteristics of efficacious siRNA are summarized as follows:
G/C content should be relatively low,Citation17,Citation19 ranging between 30% and 52%,Citation19 because a high G content tends to form G quartet structuresCitation17
Low internal stability of siRNA at the 5′ antisense end is a prerequisite for effective silencing;Citation19 internal repeats or palindromes in siRNA sequences may form internal fold structures;Citation19 the silencing potential and effective concentration of siRNA can be reduced by these hairpin-like structuresCitation19
Silencing efficiency is inversely proportional to high internal repeat stabilityCitation19
The sense strand has an A at positions 3 and 19Citation5,Citation19,Citation20
The sense strand has a U at position 10Citation5,Citation19,Citation20
The sense strand lacks a G or C at position 19Citation19
The sense strand lacks a G at position 13.Citation5,Citation19
The efficiency of siRNA can be improved if the above-mentioned criteria are met.
Constructing an RNAi library
A library of artificial RNAi can induce RNAi to suppress expression of diverse genes. An RNAi library can be specific or random. A specific RNAi library is designed according to known genes, whereas a random RNAi library consists of random DNA fragments obtained by cleaving genomic DNA in specific reaction conditions.Citation21 RNAi libraries have very much facilitated RNAi drug research.
SiRNA-transfected target cells
RNAi-based screening has singled out numerous candidate cancer targets, among which many have been or will be validated by disease-relevant in vitro phenotypic assays. However, few have been validated in vivo, largely due to the lack of effective vehicles and high cost.Citation16 siRNA has been shown to be effective in several localized disease models. However, weak stability and low transmission efficiency during in vivo delivery have severely limited its application.Citation16
The “off-target” effect of RNAi is a severe drawback. To realize the benefit of RNAi fully, this effect must be eliminated or minimized.Citation16 An ideal drug target would have high on-target efficacy and low toxicity.Citation5 Generally, toxicity is caused by saturation of the endogenous RNAi machinery following transfection of siRNA or expression of shRNA. Reducing the intracellular concentration and/or cell-specific expression of shRNA can minimize side effects.Citation5,Citation22 This can be achieved by using a regulated RNAi expression cassette delivered either by a nonviral or a viral vector.Citation5 The delivery vehicle is crucial to achieve the above goal and should be capable of circumventing the reticuloendothelial system, be effectively taken up by the target tissues, and be able to escape from the endosome after endocytosis.Citation23
Expression cassette for RNAi
Under the control of RNA polymerase III promoters, the expression of shRNA can introduce siRNA into cells.Citation22,Citation24,Citation25 Heritable gene knockdown can be achieved by incorporating the shRNA cassette into viral vectors in cells and even in whole animals.Citation22,Citation26–Citation28 Inducible systems which silence RNA expression by RNA polymerase III promoters containing operator sequences (tetO) of the Escherichia coli tetracycline resistance (tet) operonCitation22 have been developed.Citation29 This tetracycline-inducible gene regulation system has become a powerful device for transient and dose-dependent regulation of target gene expression in vitro and in vivo.Citation30 A lentiviral based-system in which a tetO sequence is located between the H1 promoter and shRNA was developed. This system can cooperate with another cassette consisting of the codon-optimized tetRCitation31,Citation32 fused with enhanced green fluorescent protein by the T2A peptide under the control of the ubiquitin promoter,Citation33 which can be used to treat type 2 diabetes mellitus.Citation22 In the absence of the doxorubicin inducer, tetR binding to tetO can block shRNA transcription. Addition of doxorubicin or tetracycline can release tetR, thereby facilitating initiation of shRNA expression.Citation22
Delivery systems
Nanoparticles have recently been recognized as a principal delivery vehicle for gene therapy.Citation34 Nanotechnology holds great promise for medicines for a number of reasons, ie, multiple selectivity, desirable solubility, and permeability, a favorable pharmacokinetic profile, tunable tissue specificity, higher stability under physiological conditions, and ready scale-up during manufacture.Citation35,Citation36 Polymers are the basis of nanomedicine and their characteristics are constantly being improved to achieve better efficacy. Polymers can protect siRNA from serum nuclease degradation and escort the siRNA to the desired cells.Citation37 One drawback of synthetic nanoparticles is their relatively lower transfection efficiency.Citation38 This can be improved by modification. The primary, secondary, and tertiary amines within the cationic polymer polyethylenimine (PEI) enable it to complex with DNA or siRNA, creating a “proton sponge effect”,Citation37,Citation39 which can result in accumulation of PEI-siRNA complexes in the liver, lung, spleen, and kidney, and also in severe cytotoxicity, which limits its wider application.Citation37 Poly(ethylene glycol) (PEG)-modified PEI polymers can reduce this adverse effect, and achieve higher transfection efficiency, more solubility and stability, and a longer circulation time in vivo, as well as fewer non-specific interactions with serum protein.Citation40 An example of this is a PEG-PEI/siRNA nanoparticle targeting CD44v6, a risk factor for lymph node metastasis and highly expressed in patients with gastric cancer and liver metastasis.Citation41,Citation42 The morphology of the PEG-PEI/siRNA nanoparticle has been monitored by scanning electron microscopy, and the size and surface charge of the nanoparticle assessed by zeta potential measurement. The transfection efficiency, cytotoxicity, and interactions with the target were measured using SGC7901 human gastric carcinoma cells as a model. The results showed that the N/P charge ratio between the PEG-PEI amino groups and the siRNA phosphate groups largely determines the transfection efficiency. The peak transfection efficiency of the PEG-PEI/siRNA nanoparticle was 72.53% ± 2.38% at an N/P of 15, and the cytotoxicity of PEG-PEI was lower than that of PEI using the MTT assay. Optimal silencing of CD44v6 expression was achieved at a PEG-PEI/siRNA N/P ratio of 15.Citation40 More recently, nanoparticle-based delivery systems have largely been viral or nonviral.Citation5
Viral delivery systems
Viruses can integrate shRNA expression cassettes into the genome, thereby inducing prolonged gene silencing. Viruses can be customized to specific tissue tropism.Citation37 Delivery systems based on retrovirus, lentivirus, adenovirus, and adenovirus-related vectors have been validated in transgenic systems.Citation38 They have the advantages of delivering siRNAs to nondividing cells and of improving biosafety by diminishing the risk of producing replication-competent viruses and evading dysregulation of endogenous genes by promoters and viral enhancers.Citation43–Citation45
Lentiviral vectors, in contrast with adenovirus, can introduce short RNA into bone marrow cells and blood,Citation46 and are widely used in the biomedical field. Amyotrophic lateral sclerosis is a fatal and incurable degenerative disorder of motor neurons in the brainstem, spinal cord, and motor cortex, leading to generalized weakness and muscle atrophy.Citation47 Amyotrophic lateral sclerosis has a high incidence of approximately 6/100,000, and about 90% of cases are sporadic, with the remaining 10% being familial.Citation47 About 20% of familial cases arise from mutations in the Cu/Zn superoxide dismutase (SDS1) gene. SOD1G93A and SOD1mis mutants were designed as a control for SOD1 silencing, and a specific shRNA (shSOD1) was applicable to most mutant SOD1 proteins. In transgenic SOD1G93A mice, intraspinal injection of a lentiviral vector (LV-shSOD1) led to a long-lasting and substantial decrease in mutant SOD1 protein, thereby delaying the onset and progression of amyotrophic lateral sclerosis.Citation47
The transmissible spongiform encephalopathies or prion diseases are fatal neurodegenerative disorders prevalent among sheep, cattle, cervids, and humans. Transmissible spongiform encephalopathies arise from a cellular prion protein which misfolds and forms a protease-resistant isoform. The cellular prion protein is widespread among mammalian cells, and is highly expressed on glial and neuronal cells in the central nervous system.Citation48,Citation49 Prions migrate to the central nervous system, where a conformational change in the cellular prion protein causes a protease-resistant isoform.Citation50 Decreasing the cellular prion protein in mice infected with transmissible spongiform encephalopathy can reduce the incidence and severity of the disease, delay its development,Citation51,Citation52 and even reverse its pathological progression.Citation53 RNAi can effectively knock down the cellular prion protein in mice, but the effect on the outcome of prion disease remains to be determined.Citation54 Lentiviral vectors encoding prion protein-specific shRNAs can be injected intracranially to knock down cellular prion protein expression,Citation52 but the clinical application of this delivery method is limited due to irreversibility of cellular prion protein suppression.Citation55
The chemokine receptor CCR5, a human immunodeficiency virus-type 1 (HIV-1) coreceptor, is essential for CCR5 tropic HIV-1 infection and serves as a desirable therapeutic target for inhibiting HIV-1.Citation56–Citation59 Hindering CCR5 expression protects against HIV-1 infection at the primary stage of the life cycle of the virus. Δ32CCR5, mutants with a 32-bp deletion in the CCR5 gene, do not express CCR5 and are highly effective in preventing HIV-1 infection.Citation60,Citation61 Therefore, it is a promising strategy for reducing CCR5 expression in a stable manner when treating HIV-infected patients.Citation62 A hu-BLT (bone marrow/liver/thymus humanized) mouse model showed that engraftment of lentiviral vector-mediated CCR5 shRNA led to stable and efficient CCR5 knockdown in multiple lymphoid organs, and CCR5 expression was downregulated in systemic lymphoid organs without causing obvious adverse effects.Citation62 In addition, the anti-HIV drug, BLT-HIV (rHIV7-shl-TAR-CCR5RZ) produced by Benitec Ltd, using lentivirus as a delivery tool has now entered into Phase Ib investigation.
The major drawbacks of viruses are their ready elimination by preexisting bloodstream antibodies, and their role in raising cytoxicity. Furthermore, viruses can activate coagulation or complement factors, and can induce neutralizing antibody responses that prevent repeated administration.
Nonviral delivery systems
The advantages of nonviral delivery systems, compared with viral vectors, are their ease of synthesis, low toxicity, and limited immune response.Citation63 Nonviral vectors mainly contain liposomes and bacteriophages.Citation37
Liposome delivery systems
The delivery systems based on liposomes can protect the nuclease, penetrate the cell membrane, and deliver RNA to target cells.Citation38 This method can decrease immunogenicity and is much safer.Citation64 Well designed lipid delivery systems can bypass the endosome and release siRNA. The endosomal pathway is the main obstacle to drug delivery into the cytoplasm. siRNA can be released by neutralization.Citation38
More recently, a transvascular method which delivered siRNA across the blood-brain barrier by intracranial injection was reported.Citation65 The siRNA was fused to a short peptide of the rabies virus glycoprotein which can bind to acetylcholine receptors on neuronal cells,Citation66 and nine d-arginines were added to the C-terminal of the short peptide (RVG-9r), enabling it to interact electrostatically with siRNA. In this way, siRNA has been successfully delivered to neurons within the mouse brain and been shown to inhibit protein expression and protect against viral encephalitis. RVG-9r peptide within cationic liposomes can knock down cellular prion protein expression and dramatically decrease expression of the protease-resistant isoform in neurons infected with transmissible spongiform encephalopathy in vitro.Citation55 This combination integrates the advantages of resistance of cationic liposomes to serum degradation and the target specificity of the RVG-9r peptide.
Stable nucleic acid-lipid particles (SNALPs) developed by Tekmira Pharmaceuticals, represent an efficacious siRNA delivery system. SNALPs are composed of a lipid bilayer containing a mixture of fusogenic and cationic lipids that enable cellular uptake and endosomal release of a nucleic acid payload. SNALPs can also be coated with a diffusible PEG-lipid conjugate providing a neutral or hydrophilic surface, and stabilizes the particle during formulation. The exterior coating also shields the cationic bilayer in vivo, blocking rapid systemic clearance.Citation67
In the study of hepatitis B virus (HBV), HBV263 is a siRNA molecule of HBV. HBV263M that placed one ribonuleotide on the 5′ end of the antisense strand of HBV263 was incorporated into lipid nanoparticles to form SNALPs.Citation67 HBV263M-SNALP was intravenously injected into mice carrying replicating HBV to evaluate its biodistribution, half-life, immunostimulatory properties, and efficacy. The results showed that HBV263M-SNALP had improved efficacy and a longer half-life, and reduced serum HBV DNA to >1.0 log10 after three days of intravenous injections at a dose of 3 mg/kg/day. Furthermore, HBV263M-SNALP reduced toxicity, dosing frequency, and immunostimulatory side effects, and had more robust and persistent biological activity.Citation67 SNALP delivery systems for SNALP-apolipoprotein B and ALN-TTR01 have been used in several clinical trials due to their advantages.
Gold nanoparticle delivery systems
Excellent biocompatibility and easy surface chemistry make gold nanoparticles one of the most widely investigated nanomaterials, and they have received increasing attention in a wide range of biomedical fields,Citation68 including radiotherapy, photothermal cancer therapy, biomolecular sensing, drug delivery, imaging, and regulation of DNA.Citation69–Citation71 Gold nanoparticles have emerged, especially in drug delivery, as promising candidates because of their excellent biocompatibility, high surface-to-volume ratio, versatility in synthesis, optical properties, and easy surface functionalization.Citation70,Citation71
Until recently, diverse types of gold nanoparticles, including antisense oligonucleotide, plasmid DNA, and siRNA, have been explored for their ability to deliver nucleic acid drugs into cells.Citation71–Citation78 Cationic lipid-coated gold nanoparticles have been developed for efficient intracellular delivery of therapeutic siRNA. In this system, gold nanoparticles served as a scaffolding material, inducing self-assembly of lipid elements, with an exterior cationic lipid shell surrounding a core of clustered gold nanoparticles. Lipid-coated gold nanoparticles possessing a positively charged shell layer are believed to condense siRNA molecules effectively into stable nanosized polyelectrolyte complexes, enabling a gene silencing effect and efficient cellular internalization.Citation79
To validate further the efficacy of lipid-coated gold nanoparticles in the treatment of hepatitis B, siHBV targeting viral open reading frames encoding an X protein was complexed with the lipid-coated gold nanoparticles to induce an antiviral response. The gene silencing effect of lipid-coated gold nanoparticles-siHBV polyelectrolyte complexes was evaluated in HBV-expressing HepG2.2.15 cells.Citation79 After treatment with 200 nM of lipid-coated gold nanoparticles-siHBV complexes, release of HBV surface antigen decreased markedly to 37.0% ± 6.6%, showing that these complexes were able to inhibit HBV replication efficiently.Citation79 Furthermore, gold nanoparticles have been used in clinical diagnostics and therapeutics, such as cancer, tuberculosis, Alzheimer’s disease, HIV, and sciatic nerve repair.Citation80–Citation84
Bacteriophage delivery systems
Bacteriophages are promising delivery systems, as exemplified by the Bacillus subtilis phage phi29 encoding a 117-nucleotide packaging RNA molecule.Citation85 Packaging RNA can constitute a nanoparticle approximately 11 nm in size by folding into a unique and stable secondary or tertiary structure.Citation86 Further, it can encapsulate DNA into procapsids, thereby forming 10–30 nm polymers by base-pairing between two interlocking right-hand and left-hand loops.Citation87 A packaging RNA monomer has two functional domains, ie, a double-stranded helical DNA packaging domain and an intermolecular interacting domain.Citation87–Citation91 Although the intermolecular regions can hardly tolerate sequence variation, the double helix allows changes in lengths and sequences without compromising the structure of packaging RNA, as long as the double helix is maintained.Citation90,Citation92,Citation93 SiRNA is a double-stranded RNA helix which can replace the 3′/5′ helical region of the packaging RNA without affecting folding of siRNA and packaging RNA, or the functions of the inserted moiety.Citation87 This method can deliver siRNA to cancer cells without degradation, with a longer half-life and significant efficacy.Citation87
Several featuresCitation87,Citation94,Citation95 of RNA nanoparticles make nanodelivery platforms attractive:
Two-dimensional, three-dimensional, and four-dimensional structures, which are diverse and stable
Various biochemical/biological functions
Metabolic stability accomplished by modification
Ideal biodegradability, biocompatibility, and noninduction of antibodies, modularized design, and ready scale-up.Citation34,Citation96
These delivery systems can be tailored to the characteristics of focal tissues, making artificial polymer-based and virus-based vehicles dispensable. For example, based on the fact that transferrin protein receptor expression was significantly upregulated in human tumors, a 70 nm nanoparticle containing transferrin protein was generated and could deliver siRNA to tumor cells by systemic injection. Target gene expression can be specifically silenced by RNAi.Citation97 Other novel immunoliposome methods can integrate nanoparticles and lymphocyte function-associated antigen-1 integrin, a molecule which is highly expressed on all leukocytes. A lymphocyte function-associated antigen-1 integrin-targeted and stabilized nanoparticle could lead to selective uptake of siRNA by T cells and macrophages, which are the principal target cells of HIV. Anti-CCR5 siRNA/lymphocyte function-associated antigen-1 integrin-targeted and stabilized nanoparticles could silence leucocyte-specific genes, thereby preventing HIV infection in BLT mice.Citation98
RNAi drugs and future opportunities
RNAi have been tested in various fields of medicine because of their specificity, simplicity, and stability in gene silencing.Citation99 RNAi drugs in different stages of clinical investigation are summarized in . Many obstacles remain to be overcome to bring RNAi-based therapeutics successfully to the bedside, eg, precise targeting to avoid potentially fatal off-target effectsCitation38 and overcoming the potential cytotoxicity and immunoreactivity of delivery systems. Hopefully, more indepth exploration of the above questions will expedite realization of the full potential of RNAi-based therapeutics.
Table 1 RNA interference drugs in clinical trials
Acknowledgements
This work was funded by the National Megaprojects for Key Infectious Diseases (2008ZX10003-006, 2012ZX10003-003), National Natural Science Foundation (81071316), New Century Excellent Talents in Universities (NCET-11), Fellowship of Southwest University (kb2009010 and ky2011003), Fundamental Research Funds for the Central Universities (XDJK2009A003), and the Natural Science Foundation Project of CQ CSTC (2010BB5002).
Disclosure
The authors report no conflicts of interest in this work.
References
- FireAXuSMontgomeryMKKostasSADriverSEMelloCCPotent and specific genetic interference by double-stranded RNA in Caenorhabditis elegansNature199839166698068119486653
- HannonGJRNA interferenceNature2002418689424425112110901
- MeisterGTuschlTMechanisms of gene silencing by double-stranded RNANature2004431700634334915372041
- NovinaCDSharpPAThe RNAi revolutionNature2004430699616116415241403
- PushparajPNAarthiJJManikandanJKumarSDsiRNA, miRNA, and shRNA: in vivo applicationsJ Dent Res20088711992100318946005
- DavidsonBLMcCrayPBJrCurrent prospects for RNA interference-based therapiesNat Rev Genet201112532934021499294
- Valencia-SanchezMALiuJHannonGJParkerRControl of translation and mRNA degradation by miRNAs and siRNAsGenes Dev200620551552416510870
- BartelDPMicroRNAs: genomics, biogenesis, mechanism, and functionCell2004116228129714744438
- LundESheetsMDImbodenSBDahlbergJELimiting Ago protein restricts RNAi and microRNA biogenesis during early development in Xenopus laevisGenes Dev201125111121113121576259
- ElbashirSMLendeckelWTuschlTRNA interference is mediated by 21- and 22-nucleotide RNAsGenes Dev200115218820011157775
- ZamorePDTuschlTSharpPABartelDPRNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervalsCell20001011253310778853
- LewisBPShihIHJones-RhoadesMWBartelDPBurgeCBPrediction of mammalian microRNA targetsCell2003115778779814697198
- EulalioAHuntzingerEIzaurraldeEGetting to the root of miRNA-mediated gene silencingCell2008132191418191211
- FabianMRSonenbergNFilipowiczWRegulation of mRNA translation and stability by microRNAsAnnu Rev Biochem20107935137920533884
- BhattacharyyaSNHabermacherRMartineUClossEIFilipowiczWRelief of microRNA-mediated translational repression in human cells subjected to stressCell200612561111112416777601
- LiuGWong-StaalFLiQXRecent development of RNAi in drug target discovery and validationDrug Discov Today Technol200633293300
- ElbashirSMHarborthJWeberKTuschlTAnalysis of gene function in somatic mammalian cells using small interfering RNAsMethods200226219921312054897
- LinaWChonggangYDevelopment and applications of RNAi technology in drug researchChin Bull Life Sci200719199213
- ReynoldsALeakeDBoeseQScaringeSMarshallWSKhvorovaARational siRNA design for RNA interferenceNat Biotechnol200422332633014758366
- HarborthJElbashirSMVandenburghKSequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencingAntisense Nucleic Acid Drug Dev20031328310512804036
- ShiraneDSugaoKNamikiSTanabeMIinoMHiroseKEnzymatic production of RNAi libraries from cDNAsNat Genet200436219019614704669
- HeroldMJvan den BrandtJSeiblerJReichardtHMInducible and reversible gene silencing by stable integration of an shRNA-encoding lentivirus in transgenic ratsProc Natl Acad Sci U S A200810547185071851219017805
- LiJHuangLTargeted delivery of RNAi therapeutics for cancer therapyNanomedicine (Lond)20105101483148621143026
- TuschlTExpanding small RNA interferenceNat Biotechnol200220544644811981553
- BrummelkampTRBernardsRAgamiRA system for stable expression of short interfering RNAs in mammalian cellsScience2002296556755055311910072
- TiscorniaGSingerOIkawaMVermaIMA general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNAProc Natl Acad Sci U S A200310041844184812552109
- RubinsonDADillonCPKwiatkowskiAVA lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interferenceNat Genet200333340140612590264
- ChenZStocktonJMathisDBenoistCModeling CTLA4-linked autoimmunity with RNA interference in miceProc Natl Acad Sci U S A200610344164001640517060611
- MatsukuraSJonesPATakaiDEstablishment of conditional vectors for hairpin siRNA knockdownsNucleic Acids Res20033115e7712888529
- ShengYLinCCYueJGeneration and characterization of a Tet-On (rtTA-M2) transgenic ratBMC Dev Biol2010101720158911
- SeiblerJKleinriddersAKüter-LuksBNiehavesSBrüningJCSchwenkFReversible gene knockdown in mice using a tight, inducible shRNA expression systemNucleic Acids Res2007357e5417376804
- AnastassiadisKKimJDaigleNSprengelRSchölerHRStewartAFA predictable ligand regulated expression strategy for stably integrated transgenes in mammalian cells in cultureGene2002298215917212426104
- SzymczakALVignaliDADevelopment of 2A peptide-based strategies in the design of multicistronic vectorsExpert Opin Biol Ther20055562763815934839
- AbdelmawlaSGuoSZhangLPharmacological characterization of chemically synthesized monomeric phi29 pRNA nanoparticles for systemic deliveryMol Ther20111971312132221468004
- WoodleMCLuPYNanoparticles deliver RNAi therapyMater Today200588 Suppl 13441
- AllenTMCullisPRDrug delivery systems: entering the mainstreamScience200430356651818182215031496
- van den BoornJGSchleeMCochCHartmannGSiRNA delivery with exosome nanoparticlesNat Biotechnol201129432532621478846
- LaresMRRossiJJOuelletDLRNAi and small interfering RNAs in human disease therapeutic applicationsTrends Biotechnol2010281157057920833440
- SuttonDKimSShuaiXEfficient suppression of secretory clusterin levels by polymer-siRNA nanocomplexes enhances ionizing radiation lethality in human MCF-7 breast cancer cells in vitroInt J Nanomedicine20061215516217722531
- WuYWangWChenYThe investigation of polymer-siRNA nanoparticle for gene therapy of gastric cancer in vitroInt J Nanomedicine2010512913620309399
- YamamichiKUeharaYKitamuraNNakaneYHiokiKIncreased expression of CD44v6 mRNA significantly correlates with distant metastasis and poor prognosis in gastric cancerInt J Cancer19987932562629645347
- KurozumiKNishidaTNakaoKNakaharaMTsujimotoMExpression of CD44 variant 6 and lymphatic invasion: importance to lymph node metastasis in gastric cancerWorld J Surg19982288538579673558
- MiyoshiHBlomerUTakahashiMGageFHVermaIMDevelopment of a self-inactivating lentivirus vectorJ Virol19987210815081579733856
- VandenDriesscheTThorrezLNaldiniLLentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivoBlood2002100381382212130491
- Hacein-Bey-AbinaSVon KalleCSchmidtMLMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1Science2003302564441541914564000
- HannonGJRossiJJUnlocking the potential of the human genome with RNA interferenceNature2004431700637137815372045
- RaoulCAbbas-TerkiTBensadounJCLentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALSNat Med2005114342342815768028
- McKinleyMPBoltonDCPrusinerSBA protease-resistant protein is a structural component of the Scrapie prionCell198335157626414721
- BoltonDMcKinleyMPrusinerSIdentification of a protein that purifies with the scrapie prionScience19822184579130913116815801
- GieseABrownDRGroschupMHFeldmannCHaistIKretzschmarHARole of microglia in neuronal cell death in prion diseaseBrain Pathol1998834494579669696
- BuelerHRaeberASailerAFischerMAguzziAWeissmannCHigh prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP geneMol Med19941119308790598
- PfeiferAEigenbrodSAl-KhadraSLentivector-mediated RNAi efficiently suppresses prion protein and prolongs survival of scrapie-infected miceJ Clin Invest2006116123204321017143329
- MallucciGDickinsonALinehanJKlöhnPCBrandnerSCollingeJDepleting neuronal PrP in prion infection prevents disease and reverses spongiosisScience2003302564687187414593181
- GallozziMChapuisJLe ProvostFPrnp knockdown in transgenic mice using RNA interferenceTransgenic Res200817578379118350371
- PulfordBReimNBellALiposome-siRNA-peptide complexes cross the blood-brain barrier and significantly decrease PrP on neuronal cells and PrP in infected cell culturesPLoS One201056e1108520559428
- BergerEAMurphyPMFarberJMChemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and diseaseAnn Rev Immunol199917165770010358771
- MooreJPTrkolaADragicTCo-receptors for HIV-1 entryCurr Opin Immunol1997945515629287172
- MosierDVirus and target cell evolution in human immunodeficiency virus type 1 infectionImmunol Res200021225325810852125
- SimmonsGReevesJDHibbittsSCo-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligandsImmunol Rev2000177111212611138769
- O’BrienSJNelsonGWHuman genes that limit AIDSNat Genet200436656557415167933
- SmithMWDeanMCarringtonMContrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE StudyScience199727753289599659252328
- ShimizuSHongPArumugamBA highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse modelBlood201011581534154420018916
- ZhangSZhaoBJiangHWangBMaBCationic lipids and polymers mediated vectors for delivery of siRNAJ Control Release2007123111017716771
- AndersenMOHowardKAPaludanSRBesenbacherFKjemsJDelivery of siRNA from lyophilized polymeric surfacesBiomaterials200829450651217950838
- KumarPWuHMcBrideJLTransvascular delivery of small interfering RNA to the central nervous systemNature20074487149394317572664
- LafonMRabies virus receptorsJ Neurovirol2005111828715804965
- MorrisseyDVLockridgeJAShawLPotent and persistent in vivo anti-HBV activity of chemically modified siRNAsNat Biotechnol20052381002100716041363
- RichardsDIvanisevicAInorganic material coatings and their effect on cytotoxicityChem Soc Rev20124162052206022116515
- RippelRASeifalianAMGold revolution – gold nanoparticles for modern medicine and surgeryJ Nanosci Nanotechnol20111153740374821780364
- GhoshPHanGDeMKimCKRotelloVMGold nanoparticles in delivery applicationsAdv Drug Deliv Rev200860111307131518555555
- GuoSHuangYJiangQEnhanced gene delivery and siRNA silencing by gold nanoparticles coated with charge-reversal polyelectrolyteACS Nano2010495505551120707386
- ChenAMTaratulaOWeiDLabile catalytic packaging of DNA/siRNA: control of gold nanoparticles “out” of DNA/siRNA complexesACS Nano2010473679368820521827
- ThomasMKlibanovAMConjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cellsProc Natl Acad Sci U S A2003100169138914312886020
- RosiNLGiljohannDAThaxtonCSLytton-JeanAKRHanMSMirkinCAOligonucleotide-modified gold nanoparticles for intracellular gene regulationScience200631257761027103016709779
- ElbakryAZakyALieblRRachelRGoepferichABreunigMLayer-by-layer assembled gold nanoparticles for siRNA deliveryNano Lett2009952059206419331425
- LeeJSGreenJJLoveKTSunshineJLangerRAndersonDGGold, poly(beta-amino ester) nanoparticles for small interfering RNA deliveryNano Lett2009962402240619422265
- SongWJDuJZSunTMZhangPZWangJGold nanoparticles capped with polyethyleneimine for enhanced siRNA deliverySmall20106223924619924738
- ZuckermanJEChoiCHJHanHDavisMEPolycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membraneProc Natl Acad Sci U S A201210983137314222315430
- KongWBaeKJoSKimJParkTCationic lipid-coated gold nanoparticles as efficient and non-cytotoxic intracellular siRNA delivery vehiclesPharm Res201229236237421842305
- SokolovKFollenMAaronJReal-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticlesCancer Res20036391999200412727808
- XiDLuoXNingQLuQYaoKLiuZThe detection of HBV DNA with gold nanoparticle gene probesJournal of Nanjing Medical University2007214207212
- BaptistaPVKoziol-MontewkaMPaluch-OlesJDoriaGFrancoRGold nanoparticle probe-based assay for rapid and direct detection of Mycobacterium tuberculosis DNA in clinical samplesClin Chem20065271433143416798971
- GeorganopoulouDGChangLNamJMNanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s diseaseProc Natl Acad Sci U S A200510272273227615695586
- MahmoudKALuongJHTImpedance method for detecting HIV-1 protease and screening for its inhibitors using ferrocene-peptide conjugate/Au nanoparticle/single-walled carbon nanotube modified electrodeAnal Chem200880187056706218707132
- GuoPEricksonSAndersonDA small viral RNA is required for in vitro packaging of bacteriophage phi 29 DNAScience198723648026906943107124
- HoeprichSGuoPComputer modeling of three-dimensional structure of DNA-packaging RNA (pRNA) monomer, dimer, and hexamer of Phi29 DNA packaging motorJ Biol Chem200227723207942080311886855
- KhaledAGuoSLiFGuoPControllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnologyNano Lett2005591797180816159227
- ChenCShengSShaoZGuoPA dimer as a building block in assembling RNAJ Biol Chem200027523175101751610748150
- ZhangCLeeCSGuoPThe proximate 5′ and 3′ ends of the 120-base viral RNA (pRNA) are crucial for the packaging of bacteriophage Ø29 DNAVirology1994201177858178491
- GarverKGuoPBoundary of pRNA functional domains and minimum pRNA sequence requirement for specific connector binding and DNA packaging of phage phi29RNA199739106810799292504
- ReidRJBodleyJWAndersonDCharacterization of the prohead-pRNA interaction of bacteriophage phi 29J Biol Chem19942697515751628106496
- ChenCZhangCGuoPSequence requirement for hand-in-hand interaction in formation of RNA dimers and hexamers to gear phi29 DNA translocation motorRNA19995680581810376879
- ZhangCTrottierMGuoPCircularly permuted viral pRNA active and specific in the packaging of bacteriophage φ 29 DNAVirology199520724424517533964
- GuoSTschammerNMohammedSGuoPSpecific delivery of therapeutic RNAs to cancer cells via the dimerization mechanism of phi29 motor pRNAHum Gene Ther20051691097110916149908
- GuoSHuangFGuoPConstruction of folate-conjugated pRNA of bacteriophage phi29 DNA packaging motor for delivery of chimeric siRNA to nasopharyngeal carcinoma cellsGene Ther2006131081482016482206
- LiuJGuoSCinierMFabrication of stable and RNase-resistant RNA nanoparticles active in gearing the nanomotors for viral DNA packagingACS Nano20105123724621155596
- DavisMEZuckermanJEChoiCHJEvidence of RNAi in humans from systemically administered siRNA via targeted nanoparticlesNature201046472911067107020305636
- KimSSPeerDKumarPRNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT miceMol Ther201018237037619997090
- XinpingLApplications of RNA interference technology to drug studyChinese Pharmacological Bulletin2005214400403
- TheFreeLibrary.com [homepage on the Internet]OPKO Health Announces Update on Phase III Clinical Trial of BevasiranibPennsylvaniaFarlex, Inc.2012 [updated 2009 May 6; cited 2012 Apr 10]. Available from: http://www.thefreelibrary.com/OPKO+Health+Announces+Update+on+Phase+III+Clinical+Trial+of...-a0195014938. Accessed Jun 25, 2012.
- Quarkpharma.com [homepage on the Internet]Quark Pharmaceuticals Presented At ARVO Data Showing That PF-04523655 Enters Retinal Cells And Elicits Its Pharmacologic Effect Via Target Gene Knock-Down Without Activating TLR3CaliforniaQuark Pharmaceuticals, Inc.2007 [updated 2009 May 11; cited 2012 Apr 10]. Available from: http://www.quarkpharma.com/qbi-en/newslist/arvo/. Accessed Jun 25, 2012.
- Tekmirapharm.com [homepage on the Internet]Tekmira Provides Corporate Update and Announces First Quarter 2012 ResultsBritish ColumbiaTekmira Pharmaceuticals Corp.2012 [updated 2012 May 15; cited 2012 Apr 10]. Available from: http://investor.tekmirapharm.com/releasedetail.cfm?releaseid=673804. Accessed Jun 22, 2012
- Tekmirapharm.com [homepage on the Internet]Tekmira Announces Initiation of TKM-Ebola Phase 1 Clinical TrialBritish ColumbiaTekmira Pharmaceuticals Corp.2012 [updated 2012 Feb 8; cited 2012 Apr 10]. Available from: http://files.shareholder.com/downloads/ABEA-50QJTB/1754071349x0x541056/d731bed8-39f3-4fe2-a962-c86e2f72e681/TKMR_News_2012_2_8_General_Releases.pdf.Accessed Jun 24, 2012
- Pharmaceuticals A Phase 2b Study of ALN-RSV01 in Lung Transplant Patients Infected With Respiratory Syncytial Virus (RSV) In: Clinical-Trialsgov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2012 [updated 2012 May 30]. Available from: http://clinicaltrials.gov/ct2/show/NCT01065935?term=Alnylam&rank=5. ClinicalTrials.gov Identifier: NCT01065935. Accessed Jul 11, 2012
- Quarkpharma.com [homepage on the Internet]. Quark Pharmaceuticals Reports Favorable Interim Results from Phase I Clinical Study of QPI-1007 California: Quark Pharmaceuticals, Inc.; c 2007 [updated 2012 Jan 4; cited 2012 Apr 10]. Available from: http://www.quarkpharma.com/qbi-en/newslist/clinicalstudyqpi-1007/. Accessed Jun 21, 2012.
- Pharmaceuticals A. Dose Escalation Trial to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Intravenous ALN-VSP02 In Patients With Advanced Solid Tumors With Liver Involvement In: ClinicalTrialsgov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2011 [updated 2011 Aug 23]. Available from: http://clinicaltrials.gov/ct2/show/NCT00882180?term=Alnylam&rank=3. ClinicalTrials.gov Identifier: NCT00882180. Accessed Jul 11, 2012
- Calando.com [homepage on the Internet]Arrowhead Research Subsidiary, Calando Pharmaceuticals, Provides First Proof of RNA Interference in Humans with Systemically Administered siRNA Therapeutic Clinical Trial Results Published inNature CaliforniaCalando Pharmaceuticals2012 [updated 2010 Mar 22; cited 2012 Apr 11]. Available from: http://www.calandopharma.com/newspdfs/NR--Calando_data--3-22-10_final.pdf. Accessed Jun 19, 2012
- Project PC. Study of TD101, a Small Interfering RNA (siRNA) Designed for Treatment of Pachyonychia Congenita In: ClinicalTrials gov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2008 [updated 2008 Nov 18]. Available from: http://clinicaltrials.gov/ct2/show/NCT00716014. ClinicalTrials.gov Identifier: NCT00716014. Accessed Jul 11, 2012.
- Genomeweb.com [homepage on the Internet]Allergan Drops Development of siRNA Rx for AMD on Poor Phase II DataNew YorkGenomeWeb LLC.2012 [updated 2009 May 28; cited 2012 Apr 11]. Available from: http://www.genomeweb.com/rnai/allergan-drops-development-sirna-rx-amd-poor-phase-ii-data. Accessed June 21, 2012
- Quarkpharma.com [homepage on the Internet]. QPI-1002. California: Quark Pharmaceuticals, Inc.; c 2007 [updated 2010 Aug 18; cited 2012 Apr 10]. Available from: http://www.quarkpharma.com/qbi-en/products/QPI-1002da/. Accessed Jun 15, 2012
- TheFreeLibrary.com [homepage on the Internet]. Santaris Pharma A/S Advances a Second Drug From Its Cardiometabolic Program, SPC4955, Inhibiting apoB, into Phase 1 Clinical Trials for the Treatment of High Cholesterol. Pennsylvania: Farlex, Inc.; c 2012 [updated 2011 May 11; cited 2012 Apr 10]. Available from: http://www.thefreeli-brary.com/Santaris+Pharma+A%2fS+Advances+a+Second+Drug+From+Its+Cardiometabolic...-a0256048995. Accessed May 11, 2012
- Pharmaceuticals A. Trial to Evaluate Safety and Tolerability of ALN-PCS02 in Subjects With Elevated LDL-Cholesterol (LDL-C) In: ClinicalTrialsgov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2012 [updated 2012 Apr 20]. Available from: http://clinicaltrials.gov/ct2/show/NCT01437059?term=PCS02&rank=1. ClinicalTrials.gov Identifier: NCT01437059. Accessed Jul 11, 2012
- Santaris.com [homepage on the Internet]. Santaris Pharma A/S advances new cholesterol-lowering drug, SPC5001 inhibiting exciting new target PCSK9, into Phase 1 clinical trials for the treatment of high cholesterol. San Diego: Santaris Pharma A/S; c 2011 [updated 2011 May 4; cited 2012 Apr 9]. Available from: http://www.santaris.com/news/2011/05/04/santaris-pharma-advances-new-cholesterol-lowering-drug-spc5001-inhibiting-exciting-n. Accessed Jun 25, 2012
- Tekmirapharm.com [homepage on the Internet]. Tekmira Pharmaceuticals Completes ApoB SNALP Phase 1 Clinical Trial. British Columbia: Tekmira Pharmaceuticals Corp.; c 2012 [updated 2010 Jan 7; cited 2012 Apr 10]. Available from: http://files.shareholder.com/downloads/ABEA-50QJTB/1754071349x0x425312/9b404e8f-82e2-48a8-ba61-7132-8539afd9/TKM_News_2010_1_7_General_Releases.pdf. Accessed Jun 29, 2012
- Giiresearch.com [homepage on the Internet]. Top Companies and Products in the RNA/DNA Therapy Products Market Forecasts: The challenge of turning technology into therapy. Kawasaki: Global Information, Inc.; c 1997–2007 [updated 2011 Aug 12; cited 2012 Apr 11]. Available from: http://www.giiresearch.com/report/kt55538-therapy-prod.html. Accessed Jun 26, 2012
- Abnnewswire.net [homepage on the Internet]. rHIV7-shI-TAR-CCR5RZ. New York: ABN Newswire; c 2012 [updated 2010 Nov 1; cited 2012 Apr 11]. Available from: http://www.abnnewswire.net/search.asp?cp=utf-8&lang=_c&site=press_c&client=press-search-cs&output=xml_no_dtd&proxystylesheet=press-search-cs&proxyreload=1&filter=p&getfields=*&way=abn&way=web&qu=rHIV7-shI-TAR-CCR5RZ&stype=press_c&image2.x=33&image2.y=10. Accessed Jun 18, 2012.
- Pharmaceuticals A. Trial to Evaluate Safety and Tolerability of ALN-TTR01 in Transthyretin (TTR) Amyloidosis In: ClinicalTrials gov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2012 [updated 2012 May 23]. Available from: http://clinicaltrials.gov/ct2/show/NCT01148953?term=Alnylam&rank=4. ClinicalTrials.gov Identifier: NCT01148953. Accessed Jul 11, 2012.