Abstract
Background
The purpose of this study was to demonstrate the potential of magnetic poly(methyl methacrylate) (PMMA) core/polyethyleneimine (PEI) shell (mag-PEI) nanoparticles, which possess high saturation magnetization for gene delivery. By using mag-PEI nanoparticles as a gene carrier, this study focused on evaluation of transfection efficiency under magnetic induction. The potential role of this newly synthesized nanosphere for therapeutic delivery of the tryptophan hydroxylase-2 (TPH-2) gene was also investigated in cultured neuronal LAN-5 cells.
Methods
The mag-PEI nanoparticles were prepared by one-step emulsifier-free emulsion polymerization, generating highly loaded and monodispersed magnetic polymeric nanoparticles bearing an amine group. The physicochemical properties of the mag-PEI nanoparticles and DNA-bound mag-PEI nanoparticles were investigated using the gel retardation assay, atomic force microscopy, and zeta size measurements. The gene transfection efficiencies of mag-PEI nanoparticles were evaluated at different transfection times. Confocal laser scanning microscopy confirmed intracellular uptake of the magnetoplex. The optimal conditions for transfection of TPH-2 were selected for therapeutic gene transfection. We isolated the TPH-2 gene from the total RNA of the human medulla oblongata and cloned it into an expression vector. The plasmid containing TPH-2 was subsequently bound onto the surfaces of the mag-PEI nanoparticles via electrostatic interaction. Finally, the mag-PEI nanoparticle magnetoplex was delivered into LAN-5 cells. Reverse-transcriptase polymerase chain reaction was performed to evaluate TPH-2 expression in a quantitative manner.
Results
The study demonstrated the role of newly synthesized high-magnetization mag-PEI nanoparticles for gene transfection in vitro. The expression signals of a model gene, luciferase, and a therapeutic gene, TPH-2, were enhanced under magnetic-assisted transfection. An in vitro study in neuronal cells confirmed that using mag-PEI nanoparticles as a DNA carrier for gene delivery provided high transfection efficiency with low cytotoxicity.
Conclusion
The mag-PEI nanoparticle is a promising alternative gene transfection reagent due to its ease of use, effectiveness, and low cellular toxicity. The mag-PEI nanoparticle is not only practical for gene transfection in cultured neuronal cells but may also be suitable for transfection in other cells as well.
Introduction
Magnetic nanoparticles have previously been used in biomedical applications, especially in the area of medical imaging,Citation1 and drug and gene delivery.Citation2 Magnetic-assisted gene transfection could improve transfection efficiency by using magnetic force induction to introduce a therapeutic gene into a target cell. Application of an external magnetic field for gene delivery was first reported by Mah et al.Citation3 Magnetic microparticles were coated with adenoassociated virus encoding green fluorescent protein. It was demonstrated that adenoassociated virus conjugated with magnetic microparticles enhanced transduction efficiency both in vitro and in vivo. Since then, several intensive studies of magnetic-based gene delivery have been performed.Citation4,Citation5 Magnetic-assisted gene delivery can be applied to transfection reagents and gene therapeutic vehicles, and the first report focusing on the use of magnetic-assisted targeted gene delivery used polyethyleneimine (PEI)-coated nanoparticles for in vitro gene transfection.Citation6 The study demonstrated the advantages of magnetic-assisted transfection in terms of reducing incubation time and DNA dose. To date, magnetic-assisted transfection has been demonstrated as one of the approaches for nucleic acid transfer, including DNA and RNA interference, in various cell lines. For example, the combination of cationic lipid-coated magnetic nanoparticles with transferrin and PEI was developed for transfection in a human cervical cancer cell line. This system enhanced the transfection efficiency by approximately 300-fold compared with control transfection reagents in the presence of an external magnetic field.Citation5 A hybrid nanoparticle system consisting of superparamagnetic nanoparticles and PEI was used as a vehicle to transfer the interleukin-10 gene into vascular endothelial cells.Citation4 This particle showed high transgene expression using a very low vector concentration and in a very short incubation time. This system is promising for treatment of patients with vascular disorders who require fast and target-specific delivery of the genes concerned. Apart from being an effective transfection reagent, incorporation of magnetic nanoparticles into lipid-based or polymeric-based carriers has also been considered as an alternative approach for improvement of non-viral vector-based gene therapy.Citation7,Citation8 At present, many research groups are aiming to develop a vehicle which could facilitate gene therapy in several genetic disorders, including the hematological,Citation9 cardiovascular,Citation10 and immunogenic systems.Citation11
Non-viral approaches for nucleic acid delivery have also become a novel strategy for treating neurological disease.Citation12 Neuron-targeted nucleic acid therapy remains one of the few options available for the treatment of neurodegenerative disease. In previous studies, viral vectors were used as the gene carrier for transfer of nucleic acid into target neuron cells, and adenoassociated virus was the most common viral vector for gene transfection.Citation13–Citation15 However, there has been a recent focus on non-viral vector-based gene vectors for neuron systems, with some reported examples, including lipid-based and polymeric-based carriers. PEGylated immunoliposome-mediated brain-specific delivery of a gene encoding tyrosine hydroxylase for the treatment of patients with Parkinson’s disease has been studied successfully in an animal model.Citation16 Modified transfection reagents, ie, PEI-PEG and Tet1 complexes, demonstrated increased luciferase expression levels in neural progenitor cells compared with unmodified PEI-PEG complexes.Citation17
In this study, we investigated the use of novel synthesized magnetic nanoparticles for gene delivery in neuronal cells. Magnetic PEI/poly(methyl methacrylate) (PMMA) core-shell (mag-PEI) nanoparticles were prepared using ultrasonication-assisted emulsifier-free emulsion polymerization. Loading of magnetic nanoparticles enhanced gene transfection efficiency by accelerating the cellular uptake of nanoparticles. The physicochemical properties and morphology of the mag-PEI nanoparticles were characterized, and a feasibility study was performed to evaluate the gene transfection efficiency of the mag-PEI nanoparticles using plasmid pGL3-basic containing cytomegalovirus (CMV) promoter/enhancer encoding the luciferase reporter gene (pGL3-CMV). In vitro transfection of pGL3-CMV could be measured quantitatively using the luciferase assay system. Different N/P ratios of magnetoplex were prepared to investigate the transfection efficiency at different transfection times with and without magnetic induction. The cytotoxicity of the mag-PEI nanoparticles was examined using the MTT assay. Transfection under magnetic induction strongly promotes cell internalization, as shown by confocal laser scanning microscopy. Optimal conditions were selected for transfection of pGL3-CMV, a plasmid containing tryptophan hydroxylase-2 (TPH-2), a rate-limiting enzyme for production of the serotonin neurotransmitter.Citation18 This study proposes an alternative nanocarrier, which is applicable for neuronal gene therapy.
Materials and methods
Materials
Ferrous chloride tetrahydrate (FeCl2·4H2O), ferric chloride hexahydrate (FeCl3·6H2O), methyl methacrylate (MMA), and t-butyl hydroperoxide were purchased from Fluka (St Louis, MO). PEI (molecular weight of 25 kDa) was purchased from Sigma-Aldrich (St Louis, MO). All chemicals were of analytical grade and used for synthesis of magnetic core/shell nanoparticles. Lipofectamine 2000™ was purchased from Invitrogen (Carlsbad, CA). A PolyMAG and magnetoFACTOR-96 plate was purchased from Chemicell GmbH (Berlin, Germany). Plasmid pGL3-basic containing CMV promoter/enhancer, which is an expression vector for human cell lines, was used to monitor transfection efficiency.Citation19 Plasmid DNA was propagated in Escherichia coli, which were grown in Lysogeny broth (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl), and supplemented with ampicillin under shaking conditions of 250 rounds per minute at 37°C. The plasmid was extracted using the PureLink™ Hipure Plasmid DNA purification kit (Invitrogen). The extracted plasmid was observed by electrophoresis on 1.0% agarose gel. Plasmid purity and concentration were determined by measuring light absorbance at 260 nm and 280 nm using a SpectraMax M2 microplate reader (MDS Inc, Sunnyvale, CA). Primers for reverse-transcriptase polymerase chain reaction (RT-PCR) of GAPDH and TPH-2 genes are listed in .
Table 1 Polymerase chain reaction primers used for tryptophan hydroxylase-2 cloning and semiquantitative assay of GAPDH and tryptophan hydroxylase-2 gene expressions
Preparation of magnetic core/shell nanoparticles
Mag-PEI nanoparticles with a PMMA core and PEI shell were prepared by emulsion polymerization.Citation20 In brief, iron oxide at a concentration of 25 mg was dispersed thoroughly with 2 g of MMA using an ultrasonicator for 5 minutes. For a total of 50 g of solution, the iron oxide-MMA dispersion was mixed with 47 g of PEI solution containing 0.5 g of PEI using a homogenizer (Sonics Vibra cell, amplitude 40%). The dispersion was homogenized for 15 minutes and then transferred into a water-jacketed flask equipped with a condenser, a magnetic stirrer, and a nitrogen inlet. The dispersion was purged with nitrogen for 30 minutes, followed by addition of t-butyl hydroperoxide aqueous solution (1 g, 0.5 mM) to initiate polymerization. The mixture was then continuously stirred at 80°C for 2 hours in a nitrogen environment. After the reaction, the particle dispersion was purified by repeated centrifugation (13,000 rpm), decantation, and redispersion until the conductivity of the supernatant was close to that of the distilled water used. The amine density on the surface of the nanoparticles was evaluated using a typical acid-base titration method.Citation21 The titration was carried out with an Autotitrator (Mettler Toledo, T50, Columbus, OH) and a pH glass sensor (Mettler Toledo, DGi115-SC) using 0.01 M NaOH standardized by potassium hydrogen phthalate as a titrant. The sample preparation was performed using an aqueous solution composed of 0.5 mL of the sample suspension (30–40 mg/mL), 50 mL of deionized water, and 0.40 mL of 0.1 M HCl. Each value reported was an average of at least three measurements. The characteristics of mag-PEI nanoparticles were then observed through a transmission electron microscope at an accelerating voltage of 80 kV.
Preparation of magnetoplex
For the feasibility study of mag-PEI nanoparticles in gene delivery, plasmid DN and pGL3-CMV encoding the luciferase reporter gene at a concentration of 1 mg/mL was mixed with mag-PEI nanoparticles at the same concentration to form the mag-PEI nanoparticle/DNA magnetoplex. The magnetoplex was prepared at various N/P ratios, ie, 0.4/1, 0.8/1, 1.6/1, 4.3/1, 8.7/1, and 17.5/1. The solutions of magnetoplex were subsequently incubated at room temperature for 30 minutes before use. The optimal N/P ratio from pGL3-CMV transfection was used for pGL3-CMV-TPH-2 transfection, in which the magnetoplex was prepared in the same manner.
Gel retardation assay
After forming the magnetoplex, loading dye was added and mixed before loading into 1.0% agarose gel. Electrophoresis was carried out at 100 V for 60 minutes. Agarose gel was stained in 1 μg/mL ethidium bromide. The presence of plasmid DNA was visible under an ultraviolet transilluminator (Syngene, Cambridge, UK). The shifted bands, corresponding to free plasmid, were determined.
Atomic force microscopy analysis
Atomic force microscopic images of magnetoplex were obtained using a dynamic force microscope (Seiko SPA4000, Tokyo, Japan). All samples were prepared by dropping the magnetoplex solution onto a mica surface for air-drying. All images were obtained with a scanning speed of 1.0 Hz over a 2 μm × 2 μm area.
Size and zeta potential analysis
The mean zeta hydrodynamic diameter, polydispersity index, and surface charge of the magnetoplex were determined by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, Worcestershire, UK) at room temperature. The magnetoplex was prepared and combined to achieve 1 mL in deionized water. All samples were measured in triplicate.
Cell culture
In this study, human neuroblastoma (LAN-5) cells were used as the neuronal cell culture model. The cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (HyClone, South Logan, UT) and incubated for 24 hours at 37°C with 5% CO2 before use.
Transfection and cytotoxicity
To evaluate the transfection efficiency of the mag-PEI nanoparticles, LAN-5 cells were seeded into a 96-well plate at a density of 5 × 104 cells per well. Before transfection, the medium was removed, the cells were rinsed with phosphate-buffered saline twice, and then plated and incubated with serum-free Dulbecco’s modified Eagle’s medium. Cells were incubated with the magnetoplex at 37°C for 15, 30, 60, 120, and 180 minutes with or without magnetoFACTOR-96, in serum-free medium which was then replaced with growth medium. Twenty-four hours after transfection,Citation19 luciferase activity was determined in accordance with the manufacturer’s recommendations (Promega, Madison, WI). Luciferase activity was quantified as relative light units using a luciferase assay system (Promega). Luciferase activity was normalized for protein concentration using the Bradford assay. The commercial transfection reagents, Lipofectamine 2000 and PolyMAG, were used as positive controls for comparison of their transfection efficiency with our synthesized mag-PEI nanoparticles. Naked DNA (DNA transfected without a gene carrier) was used as the negative control for transfections. The Lipofectamine/DNA complex and PolyMAG/DNA magnetoplex were prepared according to the manufacturer’s directions.
MTT assays were performed to evaluate cell viability after treatment with magnetoplex. LAN-5 cells were seeded at the same density used for transfection. The cells were cultured at 37°C under 5% CO2 overnight. The assay was performed 24 hours after transfection according to the manufacturer’s recommendation. Percentage viability was calculated for cells transfected with naked DNA.
Magnetoplex internalization into cells
LAN-5 cells were seeded onto glass coverslips in 6-well plates at densities of 7.5 × 105 cells per well. Before transfection, the medium was removed, the cells were rinsed with phosphate-buffered saline twice, and then plated and incubated with serum-free Dulbecco’s modified Eagle’s medium. Cells were incubated with rhodamine-B-isothiocyanate (RITC)-labeled mag-PEI nanoparticle/DNA magnetoplexes at 37°C for 60 and 180 minutes with and without a magnetoFACTOR-96 plate in serum-free medium which was then replaced with growth medium. Twenty-four hours after transfection, the transfected cells were stained with acridine orange then washed with phosphate-buffered saline twice and visualized under a confocal laser scanning microscope (LSM 700, Carl Zeiss Inc, Oberkochen, Germany) with a 100× objective lens under 405 nm excitation for acridine orange and 561 nm excitation for RITC. The results were analyzed using LSM 700 ZEN software.
Isolation of TPH-2, cloning, and construction of expression vector
cDNA for the TPH-2 gene was synthesized by RT-PCR using human brain medulla oblongata total RNA (Clontech cDNA panels, BD Biosciences, Franklin Lakes, NJ) as a template. The RT-PCR reaction was performed using ImPromt-II™ reverse transcriptase in accordance with the manufacturer’s recommendations (Promega). The resulting cDNAs were used as a template for PCR using TPH-2-NheI_pGL-CMV and TPH-2-XbaI_pGL-CMV as forward and reverse primers, respectively (). The specific PCR products were then cloned into the pGEM-T™ easy vector (Promega) to verify TPH-2 sequences by restriction enzyme digestion using HindIII (Fermentas, Glen Burnie, MD) and XbaI (NEB, Hitchin, UK) as the restriction enzyme and confirmed this result by DNA sequencing. The TPH-2 gene was finally cloned into pGL3-basic containing CMV promoter/enhancer, generating pGL3-CMV-TPH-2.
Monitoring of TPH-2 expression by RT-PCR
To determine TPH-2 expression, LAN-5 cells were seeded into 6-well plates at a density of 7.5 × 105 cells per well. pGL3-CMV-TPH-2 was mixed with mag-PEI nanoparticles to prepare magnetoplex at an N/P ratio of 0.8, which was previously optimized. PolyMAG and Lipofectamine 2000 were used as positive controls and naked DNA was used as a negative control for transfection. Cells were incubated with the magnetoplex at 37°C for 60 minutes with and without external magnetic induction in serum-free medium which was then replaced with growth medium. Twenty-four hours after transfection, RNA extraction with TRIzol (Invitrogen) was performed according to the manufacturer’s recommendations. The quantity and integrity of the RNA obtained were evaluated by spectrophotometry and gel electrophoresis stained with ethidium bromide. The RNA samples obtained were then treated with a deoxyribonuclease I amplification grade kit (P romega) at 37°C for 30 minutes to eliminate any contaminated DNA. Two steps of RT-PCR were carried out using Impromt II reverse transcription to synthesize first-strand cDNA. Taq polymerase (NEB) was then used for PCR under the following conditions: 95°C over 2 minutes for the TPH-2 gene and 94°C over 5 minutes for the GAPDH gene, followed by 35 cycles of denaturation (95°C over 30 seconds for the TPH-2 gene and 94°C over 15 seconds for the GAPDH gene), annealing (60°C over 30 seconds for the TPH-2 gene and 55°C over 15 seconds for the GAPDH gene), extension (68°C over 30 seconds for the TPH-2 gene and 72°C over 15 seconds for the GAPDH gene), and finally a single extension (68°C over 10 minutes for the TPH-2 gene and 72°C for 15 minutes for the GAPDH gene). A control negative RT-PCR was performed in the absence of reverse transcriptase to check for DNA contamination in the RNA preparation. Each TPH-2 expression was normalized against expression of the GADPH gene to eliminate the effect of the cell population. Each relative TPH-2 expression was then compared with naked DNA transfected cells.
Statistical analysis
Experiments were carried out in triplicate. The independent Student’s t-test was used for the statistical analysis, with P < 0.05 considered to be statistically significant.
Results and discussion
Fabrication of core shell nanoparticles
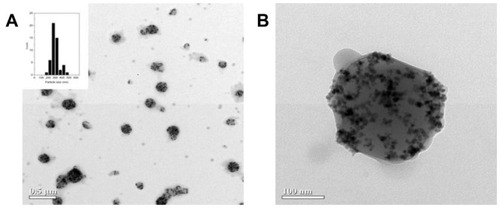
Transmission electron microscopy revealed that we could obtain magnetic polymeric core/shell nanospheres, ie, mag-PEI nanoparticles, with high magnetic nanoparticle loading (). The size distribution was found to be narrow, as indicated in the histogram. The zeta potential determined by dynamic light scattering indicated that the mag-PEI nanoparticles had positive surface charges around 39.3 ± 1.9 mV.
Gel retardation assay
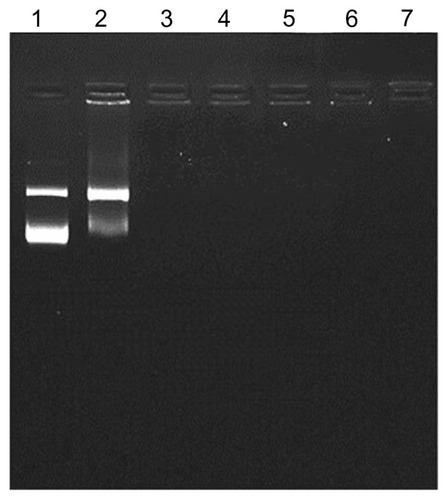
DNA binding affinity and magnetoplex formation were confirmed using the gel retardation assay. One microgram of plasmid pGL3-basic containing the CMV promoter/enhancer was applied to a prepared magnetoplex with mag-PEI nanoparticles at different N/P ratios. Trailing of DNA disappeared in the gel at an N/P ratio of 0.8/1 (). The results showed that plasmid DNA was adsorbed onto the mag-PEI nanoparticle surface by electrostatic interaction, resulting in the magnetoplex. Our cationic mag-PEI nanoparticles could neutralize the negative charge of plasmid DNA and increase the mag-PEI nanoparticle-induced cationic properties of the magnetoplex, corresponding to the results of the dynamic light scattering analysis ().
Table 2 Size and zeta potential of mag-PEI nanoparticle/DNA magnetoplex at N/P ratios of 0.4/1, 0.8/1, 1.6/1, 4.3/1, 8.7/1, and 17.5/1
Figure 2 Gel retardation assay.
Notes: One microgram of plasmid DNA was applied to the magnetoplex with mag- PEI nanoparticles at different N/P ratios. Lane 1 is the control DNA without mag-PEI nanoparticles. Lanes 2–7 represent mag-PEI NP/DNA magnetoplexes with N/P ratios of 0.4/1, 0.8/1, 1.6/1, 4.3/1, 8.7/1, and 17.5/1.
Abbreviation: Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell.
Magnetoplex formation
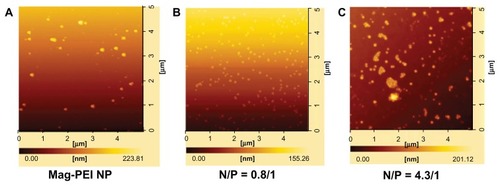
The morphology and size of the magnetoplex were analyzed under atomic force microscopy at two different N/P ratios, ie, 0.8 and 4.3. Atomic force microscopy detected that the magnetoplex appearance was spherical, corresponding to the core structures, ie, mag-PEI nanoparticles (). It is likely that addition of more mag-PEI nanoparticles with N/P ratios in the range of 0.8/1–4.3/1 could improve the magnetoplex condensation. This result correlated well with size analyzed by dynamic light scattering (). However, magnetoplex distribution changed in response to changes in the N/P ratio, as shown at ratios of 0.8/1 and 4.3/1 (). As a result, use of excess mag-PEI nanoparticles caused aggregation of the magnetoplex (), which may have interrupted cell transfection. Therefore, the magnetoplex formed at an N/P ratio of 0.8/1 was selected for cell transfection in further studies.
Size and zeta potential analysis
The size and zeta potential of the magnetic nanoparticles were determined at pH 7.4. During magnetoplex formation, a dynamic change in size and charge occurred at N/P ratios in the range of 0.4–17.5 (). The size of the mag-PEI/ DNA was larger than that of mag-PEI, indicating that adsorption of DNA had occurred on the particle surface. With a constant amount of DNA, the total charges at each N/P ratio were dependent on the amount of mag-PEI nanoparticles added to the DNA solution. At N/P ratios in the 0.4–1.6 range, the charges increased according to the amount of mag-PEI nanoparticles added. However, at N/P ratios in the range of 4.3–17.5, the excess amount of mag-PEI nanoparticles destabilized the complex, as indicated by a decrease in zeta potential.
Optimal transfection conditions and transfection efficiency
Gene transfection was investigated in the human LAN-5 neuroblastoma cell line. Cells were transfected with the magnetoplex at an optimal N/P ratio of 0.8. Gene transfection was performed by incubation of the magnetoplex with cells for 15, 30, 60, 120, and 180 minutes in the presence and absence of an external magnetic plate. Transfection via Lipofectamine 2000 and PolyMAG, two commercial transfection reagents, was carried out in the positive controls. Luciferase signals expressed in transfected cells were determined quantitatively. At all tested N/P ratios, the results confirm that magnetic-induced transfection was a very effective system for gene transfection (). Luciferase expression levels were enhanced when DNA transfections were stimulated under magnetic force using the magnetoFACTOR-96 plate. Our results show that incorporation of magnetic nanoparticles in polymeric-based vectors is an effective strategy to elevate the transfection signal and shorten the transfection time. The efficiency of gene transfection was increased through physical stimulation by an external magnetic field. Among the N/P ratios in the range of 0.4–17.5, the highest transfection efficiency was obtained at an N/P ratio of 0.8. This result indicates that transfection efficiency was affected by several physicochemical properties of the magnetoplex. With a low amount of mag-PEI nanoparticles (N/P ratio < 0.8), the DNA strands are not completely adsorbed onto the nanoparticles. Therefore, the DNA delivered into the cells is not properly protected and easily digested by intracellular enzymes. The N/P ratio of 0.8 is probably the optimal condition, including for size, zeta potential, and complex stability. Although at an N/P ratio of 1.6–4.3 the magnetoplex also has an appropriate size and zeta potential, it can also cause cell membrane damage due to the greater number of nanoparticles with a positive surface charge added to the system. Furthermore, the atomic force microscopy results indicated that the magnetoplex at an N/P ratio of 4.3 was agglomerated, which was an unsuitable condition for transfection. Therefore, to obtain high transfection efficiency, several factors needed to be compromised.
Figure 4 Transfection efficiency (A) and cytotoxicity (B) of mag-PEI nanoparticles at 15, 30, 60, 120, and 180 minutes in LAN-5 cells.
Notes: The transfection efficiency and cytotoxicity was compared with positive control Lipofectamine 2000™, PolyMAG, and negative controls (naked DNA, plasmid pGL-3-basic containing CMV promoter/enhancer). *Significant differences between cells transfected with and without a magnetic plate in each transfection reagent (P < 0.05). The gray and white bars show the results of cells incubated with or without magnetic induction, respectively.
Abbreviation: Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell.
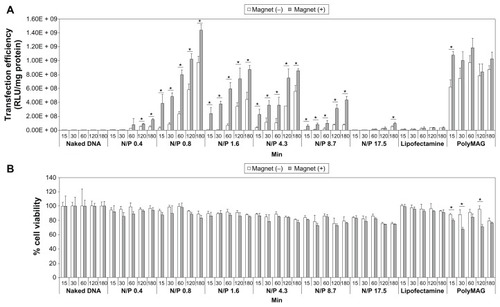
Unlike for PolyMAG, the results indicate that the increased transfection efficiency for mag-PEI nanoparticles is time-dependent. PolyMAG is a commercially available carrier enhancing the transfection signal within a short induction time, and expression levels are fairly constant at different incubation times. The difference in improvement of transfection over time is probably due to the difference in magnetic properties between PolyMAG and mag-PEI nanoparticles. PolyMAG has very strong magnetic properties, which strongly enforces cell internalization of particles into the cell within a short time. However, after 120 minutes of induction, the transfection efficiency obtained from mag-PEI nanoparticles was about the same level as that obtained from PolyMAG, and was increased after 180 minutes of induction time. Apparently, for LAN-5 cells, a magnetic-assisted transfection system is more effective than a liposome-based system like Lipofectamine 2000, and there was no statistically significant difference between cells transfected with and without a magnetic plate.
Evaluation of cytotoxicity
In this study, the toxicity of mag-PEI nanoparticles towards LAN-5 cells was investigated using the MTT assay. Cells were treated with the magnetoplex under the same conditions as the transfection procedures. The viability of LAN-5 cells after transfection was in the range of 80%–100% when incubated with magnetoplex at N/P ratios of 0.4/1, 0.8/1, 1.6/1, 4.3/1, 8.7/1, and 17.5/1 for 15, 30, 60, 120, and 180 minutes (). Viability of cells exposed to magnetic induction was lower than that of unexposed cells. However, the differences were not statistically significant. Therefore, this result verifies that the cytotoxicity of mag-PEI nanoparticles is very low, making these particles suitable for use in gene therapy.
Cellular internalization
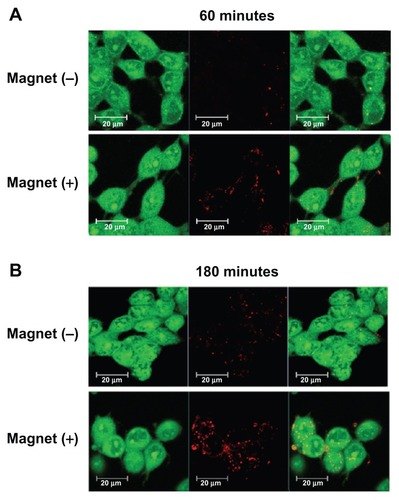
Visualization of uptake of mag-PEI nanoparticles into LAN-5 cells was observed by confocal laser scanning microscopy. The RITC-labeled mag-PEI nanoparticle/DNA magnetoplex at an N/P ratio of 0.8/1 was incubated with the cells for 60 and 180 minutes. The incubations were done separately with and without external magnetic induction. At 24 hours after transfection, confocal laser scanning microscopy images revealed the degree of intensity of the magnetoplex entering into LAN-5 cells (). At both 60 and 180 minutes of incubation, the intensities were significantly increased when transfection was performed under magnetic induction. The results indicate that the magnetoplex distributed into the intracellular compartment, the cytoplasm, and the region of the nucleus. Internalization was confirmed by confocal Z-stack image scanning (data not shown). The result corresponded well with the luciferase activity in . This provides more evidence of acceleration of the transfection period through magnetoplex transfection in neuronal cells.
Figure 5 Confocal image of LAN-5 cells 24 hours after transfection. Cells incubated with or without a magnetic plate for (A) 60 minutes and (B) 180 minutes were used for investigation of the cellular uptake of mag-PEI nanoparticles.
Note: Green, acridine orange-stained live cells; red, RITC-stained mag-PEI nanoparticles.
Abbreviations: RITC, rhodamine-B-isothiocyanate; Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell.
TPH-2 cloning
cDNA synthesized from human brain medulla oblongata total RNA was used as a template for synthesizing the TPH-2 gene fragment. PCR was performed using the specific primers described in . The PCR product showed a specific band at 1.5 kilobases under an ultraviolet transilluminator (Syngene, Cambridge, UK). The band was cut and ligated into a pGEM®-T vector (Promega). DNA sequencing verified that the isolated PCR product had 99.5% similarity to Homo sapiens TPH-2 mRNA. The TPH-2 gene was then finally transferred into pGL3-CMV basic containing the CMV promoter/enhancer.Citation19 The resulting plasmid was used for gene transfection into LAN-5 cells.
Role of mag-PEI nanoparticles as a carrier for TPH-2 expression
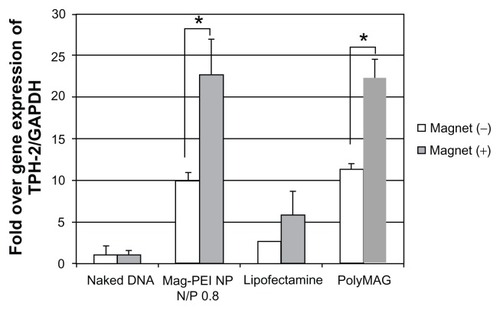
The aforementioned data indicate that mag-PEI nanoparticles are a promising carrier for magnetic-assisted transfection due to their effectiveness, with low cytotoxicity and a short transfection time. We are continuing to test mag-PEI nanoparticles at an N/P ratio of 0.8/1 as a carrier for delivery of the neuronal TPH-2 therapeutic gene into LAN-5 cells. The magnetic induction time was fixed at 60 minutes. After transfection, the cells were incubated for 24 hours and total RNA was isolated by the TRIzol reagent, as described earlier. Expression of the TPH-2 gene was measured by RT-PCR using isolated total RNA as a template. PCR products from the housekeeping gene, GAPDH, were used to normalize the gene expression values. As a result, mag-PEI nanoparticles showed efficiency in induction of TPH-2 expression comparable with that of PolyMAG (). Cells transfected with the mag-PEI nanoparticle/pGL3-CMV-TPH-2 magnetoplex under magnetic induction showed a signal that was 13 times stronger than that obtained without induction. We compared the effectiveness of mag-PEI nanoparticles for therapeutic gene delivery with that of a liposome-based system, ie, Lipofectamine 2000. The results show that the difference between TPH-2 expression in cells transfected with and without magnetic induction was not significantly different. Therefore, this study demonstrates the potential of our synthesized nanoparticle for magnet-assisted gene transfection. Mag-PEI nanoparticles successfully enhanced the transfection efficiency of TPH-2 gene delivery.
Figure 6 Semiquantitative reverse-transcriptase polymerase chain reaction result shows expression of the TPH-2 gene in LAN-5 24 hours after transfection by mag-PEI nanoparticles compared with positive control Lipofectamine 2000™ and PolyMAG, and negative control (naked DNA).
Notes: *Significant differences between cells transfected with and without magnetic plate in each transfection reagent (P < 0.05). The gray bars and white bars show the results of cells incubated with and without magnetic induction, respectively.
Abbreviations: Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell; TPH-2, tryptophan hydroxylase-2.
Conclusion
In this study, we demonstrated the potential of mag-PEI nanoparticles, possessing high saturation magnetization, for gene transfection in vitro. The mag-PEI nanoparticles at an N/P ratio of 0.8/1 showed the highest transfection efficiency and low cytotoxicity in neuronal LAN-5 cells. The results obtained from the luciferase assay were consistent with those of the cell internalization investigation by confocal laser scanning microscopy. Significant acceleration of transfection efficiency within a short induction time revealed that mag-PEI nanoparticles are a promising alternative carrier for gene delivery. This newly improved magnetic nanoparticle is suitable for magnetic-assisted transfection, which may be further applied in gene therapy for neuropsychiatric and other diseases.
Acknowledgments
This work was supported by research grants from the Thailand Research Fund to NS (TRG5480020), National Nanotechnology Center of National Science and Technology Development Agency, Korea Foundation for Advanced Studies at Chulalongkorn University, and the Chulalongkorn University Centenary Academic Development Project. This study was also supported in part by a scholarship from the Thailand Graduate Institute of Science and Technology to KK (TGIST 01-53-055). We acknowledge James M Brimson (Department of Clinical Chemistry, Faculty of Allied Health Sciences, Chulalongkorn University) for critical reading of the manuscript. We thank the Innovation Center for Research and Development of Medical Diagnostic Technology Project, Faculty of Allied Health Sciences, Chulalongkorn University for allowing us to use the confocal microscope for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- SunCLeeJSZhangMMagnetic nanoparticles in MR imaging and drug deliveryAdv Drug Deliv Rev2008601252126518558452
- McBainSCYiuHHDobsonJMagnetic nanoparticles for gene and drug deliveryInt J Nanomedicine2008316918018686777
- MahCFraitesTJZolotukhinIImproved method of recombinant AAV2 delivery for systemic targeted gene therapyMol Ther2002610611212095310
- NamgungRSinghaKHybrid superparamagnetic iron oxide nanoparticle-branched polyethylenimine magnetoplexes for gene transfection of vascular endothelial cellsBiomaterials2010314204421320170956
- PanXGuanbJYoodJWEpsteinAJLeeLJLeeRJCationic lipid-coated magnetic nanoparticles associated with transferrin for gene deliveryInt J Pharm200835826327018384982
- SchererFAntonMSchillingerUMagnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivoGene Ther2002910210911857068
- BoyerCWhittakerMRBulmusVLiuJDavisTPThe design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applicationsNPG Asia Materials201022330
- SamadikhahHRMajidiANikkhahMHosseinkhaniSPreparation, characterization, and efficient transfection of cationic liposomes and nanomagnetic cationic liposomesInt J Nanomedicine201162275228322072865
- RajDDavidoffAMNathwaniACSelf-complementary adeno-associated viral vectors for gene therapy of hemophilia B: progress and challengesExpert Rev Hematol2011453954921939421
- WeiQHuangXLLinJYFeiYJLiuZXZhangXAAdeno associated viral vector-delivered and hypoxia response element-regulated CD151 expression in ischemic rat heartActa Pharmacol Sin20113220120821240296
- WangCRShiauALChenSYIntra-articular lentivirus-mediated delivery of galectin-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritisGene Ther2010171225123320520649
- BergenJMParkIKHornerPJPunSHNonviral approaches for neuronal delivery of nucleic acidsPharm Res20072598399817932730
- HudryEDamDVKulikWAdeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of alzheimer’s diseaseMol Ther201018445319654569
- JansonCMcPheeSHaselgroveJClinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brainHum Gene Ther2002131391141212162821
- KunzeMHuberAKrajewskiAEfficient gene transfer to periodontal ligament cells and human gingival fibroblasts by adeno-associated virus vectorsJ Dent20093750250819362764
- ZhangYSchlachetzkiFZhangYFBoadoRJPardridgeWMNormalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoterHum Gene Ther20041533935015053859
- KwonEJLasieneJJacobsonBEParkIKHornerPJPunSHTargeted nonviral delivery vehicles to neural progenitor cells in the mouse subventricular zoneBiomaterials2010312417242420004466
- ZhangXBeaulieuJMSotnikovaTDGainetdinovRRCaronMGTryptophan hydroxylase-2 controls brain serotonin synthesisScience200430521715247473
- TencomnaoTRakkhitawatthanaVSukhontasingKEvaluation of a novel luciferase reporter construct: a positive control plasmid for reporter gene assayAficanr Journal of Biotechnology200871321242127
- PimphaNChaleawlert-UmponSSunintaboonPCore/shell polymethyl methacrylate/polyethyleneimine particles incorporating large amounts of iron oxide nanoparticles prepared by emulsifier-free emulsion polymerizationPolymer20125320152022
- BalázsNSiposPLimitations of pH-potentiometric titration for the determination of the degree of deacetylation of chitosanCarbohydr Res200734212413017145045
- DivyaCSPillaiMRAntitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosisMol Carcinog20064532033216526022