Abstract
Nanotherapy is a part of nanomedicine that involves nanoparticles as carriers to deliver drugs to target locations. This novel targeting approach has been found to resolve various problems, especially those associated with cancer treatment. In nanotherapy, the carrier plays a crucial role in handling many of the existing challenges, including drug protection before early-stage degradations of active substances, allowing them to reach targeted cells and overcome cell resistance mechanisms. The present review comprises the following sections: the first part presents the introduction of pharmacoeconomics as a branch of healthcare economics, the second part covers various beneficial aspects of the use of nanocarriers for in vitro, in vivo, and pre- and clinical studies, as well as discussion on drug resistance problem and present solutions to overcome it. In the third part, progress in drug manufacturing and optimization of the process of nanoparticle synthesis were discussed. Finally, pharmacokinetic and toxicological properties of nanoformulations due to up-to-date studies were summarized. In this review, the most recent developments in the field of nanotechnology’s economic impact, particularly beneficial applications in medicine were presented. Primarily focus on cancer treatment, but also discussion on other fields of application, which are strongly associated with cancer epidemiology and treatment, was made. In addition, the current limitations of nanomedicine and its huge potential to improve and develop the health care system were presented.
Introduction
Nowadays, increasing evidence indicates that nanomedicine might have revolutionized therapeutic and diagnostic procedures, especially cancer treatment. This new technology provides a new toolset impacting the prevention of diseases by applying novel molecular diagnostic disease markers, early diagnosis of the neoplastic lesions in molecular imaging, and the treatment by enabling precise and effective therapies based on a personalized medication regimen.Citation1,Citation2 Furthermore, there is evidence suggesting that combining nanomedicine with pharmacoeconomic evaluations could help reduce costs in managing cancer patients, for instance, by shortening the time of hospitalization or bringing down the number of necessary tests to be carried out. Another important fact worth mentioning is that the efficacy of drugs used with nanocarriers may substantially reduce cytotoxicity, preventing the occurrence of side effects by dose reduction and lower accumulation of therapeutic compounds in healthy body sites.Citation3,Citation4 The considerations above provide a sound basis for holding nanotechnology in future medical developments capable of delivering highly efficacious and safe products. These new approaches should be available at reasonable costs and help restrict healthcare costs while maintaining clinical efficacy.Citation3,Citation5
From a pharmacoeconomic point of view, the development of new drug substances and products such as nanosystems and their introduction into the pharmaceutical market could contribute to more affordable care. Specifically, the potential for reducing adverse events plays a significant role in new encapsulated therapeutics, which results in fewer medical procedures and leads to the reduction in personnel costs. It also gives greater chances of remission and allows patients to return to professional life.Citation5,Citation6
Moreover, it should be emphasized that the application of nanotechnology in the medical field has many advantages since nanoparticles make a significant contribution as drug delivery systems due to their unique properties like the small size and large surface area.Citation7–Citation9 The nanoformulation of drugs increases efficacy by enhancing the drug’s cellular uptake in the cellular targets; hence, it achieves better biodistribution. Nanosized formulations, in comparison with conventional forms of drugs, exhibit better control of drug release kinetics, which lead to an increased active concentration and bioavailability. Another important factor is that the nanodrugs could induce a marked suppression of tumor growth, prolongation of total survival time in cancer patients, and targeted delivery, which might enhance cytotoxic effects on neoplastic cells and restrict adverse effects in the whole body.Citation10,Citation11 All the above advantages make nanotechnology much cheaper than conventional therapies, which can also be reflected in the pharmacoeconomic aspect as the reduction or total avoidance of costs associated with medical (hospitalization, medical devices, monitoring therapy), and non-medical procedures (accommodation, transportation or the informal care).
It is worth noting that a broad literature review was undertaken. This paper presents existing evidence available regarding the effectiveness and expected pharmacoeconomic benefits of the alternative options of commonly used chemotherapeutic drugs to treat different types of cancers. Some factors may influence the results of the treatment regimen applied, such as patients’ age, stage of the disease, therapy onset, benefit duration, and also time to recurrence. Pharmacoeconomic analyses of alternative therapy options will improve decision-making and will help to optimize the use of already limited health care resources allocated to the care of cancer patients.Citation12 This paper aims to identify potential benefits from applying pharmacoeconomic to the rapidly evolving area of nanotechnology, especially in the domain of drug development for cancer treatment, which is presented in .
Pharmacoeconomics – a Use Case of Nanocarriers Evaluation
Pharmacoeconomics is considered as a branch of health economics, which identifies, measures, and compares the costs and consequences of drug therapy for healthcare systems and society.Citation13–Citation15 Moreover, it provides essential guidance on the management of limited healthcare resources and medical practice. Given the limited financial resources, health economics, particularly pharmacoeconomic analysis, is becoming a frequently used criterion for decision-making in modern healthcare policy.Citation16,Citation17 Therefore, searching for novel therapeutic options characterized by high efficacy with restricted side effects remains a highly desired goal.Citation18
Pharmacoeconomics applies the principles of health economics to the field of pharmaceutical policy. Also, it uses a broad range of techniques for health economic evaluation in the specific context of drug management.Citation19,Citation20 In effect, the introduction of novel forms of drugs, such as those encapsulated in carriers, lies in pharmacoeconomic purposes.
If think about conducting a pharmacoeconomic analysis, we should follow a clearly defined stepwise approach: a) Define the pharmacoeconomic problem – we should state the problem and select the objectives; b) Identify the perspective of the study – the most popular are: patients, provider, payer, and society; c) Identify the relevant interventions – we need to answer a significant question: “Have all relevant interventions been identified (including non-drug interventions)?”; Use decision trees or treatment models; d) Select the appropriate pharmacoeconomic method – CEA, CMA, CUA, CBA; e) Select the primary data source and study design – retrospective/prospective clinical trial data, economic (naturalistic) trial data; f) Select the secondary data sources – such as databases, literature, clinical expertise; g) Select appropriate analysis technique – modeling, meta-analysis; h) Identify the measures and the outcomes of alternative interventions – health outcomes and resource outcomes for beneficial as well as adverse effects; i) Use analytical methods – to establish the probability of outcome events and to answer the research question – such as efficacy rates, the incidence of adverse drug reactions, and decision trees; j) Estimate costs and effectiveness – reduce costs and outcomes; perform incremental cost analysis; k) Perform sensitivity analysis – determine the effect of varying uncertain variables over a range of results/assumptions; l) Interpret and present results – describe assumptions, methods, data sources; study limitations including significant omissions stated; interpret results.Citation21
There are four most popular analyses to estimate the outcomes, and each of the methods is associated with a different type of pharmacoeconomic analysis, see .
Table 1 Types of Pharmacoeconomic Studies.Citation21–Citation24
In pharmacoeconomic analysis, costs are crucial elements that should be taken into consideration. They can be classified as direct (medical and non-medical), indirect and intangible costs. Financial costs relate to monetary payments associated with the price of a good or service traded on the market. Economic costs match the broader concept of resource consumption, irrespective of whether such resources are traded in the market.Citation13,Citation24 In , we summarize and specify the types of costs that are considered in pharmacoeconomics. These costs together with the expected pharmacoeconomic efficacy measure when applying nanocarriers in cancer treatment are shown in .
Table 2 Type of Costs in Pharmacoeconomic Analysis.Citation13,Citation22,Citation24
Table 3 Efficacy of Selected Drugs and Expected Pharmacoeconomic Benefits Due to Their Nanoformulations.Citation115,Citation140–Citation167
For any pharmacoeconomic analysis, the perspective is critical since it determines what costs and benefits will be measured: 1. Societal – all costs and consequences that occurred during the treatment, 2. Third-party payer–payers are represented by insurance companies, employers, or the government; the direct costs are included, but also indirect costs can also be included, 3. Hospital/physician (healthcare providers) – providers include hospitals, private-practice physicians, or managed-care organizations; from this perspective, direct medical costs are included, 4. Patient – all costs borne by the patient for any product or services and are not covered by any insurance; there are direct, indirect, and intangible costs (out of pocket). According to the aforementioned, those costs/analysis should be taken into consideration if we are thinking about the safe application of nanocarriers in modern therapy.Citation13,Citation25
The Cancer Burden in the World
The National Cancer Institute defines cancer as a set of diseases in which abnormal cells divide without control and can spread to various tissues. Cancers can manifest in different parts of the body – leading to a range of different cancer types.Citation26 Based on the available data, it is assessed that cancer is one of the leading causes of death. In 2018, 9.6 million people were estimated to have died of various forms of cancer. Globally, WHO roughly estimates that 1 in 6 deaths is due to cancer. Considering the income – approximately 70% of deaths from cancer occur in low- and middle-income countries. The most common cancers, in terms of frequency and number of deaths, are lung, breast, and colorectal.Citation27
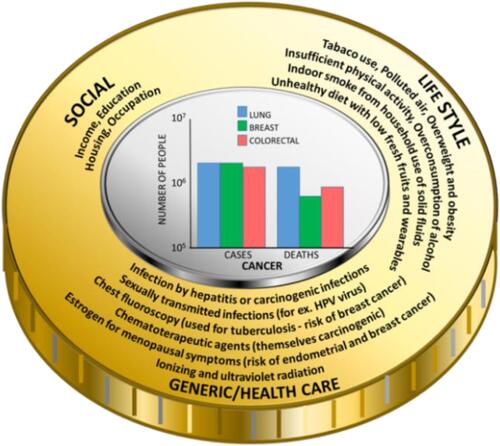
Cancer burden is associated with risk factors belonging to three main groups, which are: socio-economical, life-style, and genetic/health predisposition comprising prolonging and chronic inflammation caused by the existence of microbial infections. Besides the fact that microbes might induce chronic inflammation, it was evidenced that they are able to produce carcinogenic bacterial metabolites, which caused mutation of genetic materials.Citation28 It means that disturbance in one of these groups triggers a cascade of processes leading to the development of cancer. Researchers have found several risk factors that may increase the chance of getting lung, breast, and colorectal cancer ().
In the case of lung cancer, the number one risk factor is smoking. People who smoke cigarettes are about 15, even up to 30 times more likely to get or die of lung cancer than people who do not smoke. Smoking only a few cigarettes a day or occasionally increases the risk of developing lung cancer. The longer a person smokes, and the more cigarettes are smoked each day, the more risk becomes apparent. It is a misleading belief that smoking can only cause lung cancer. Smoking also causes several other neoplasms, such as cancer of the mouth and throat, esophagus, stomach, colon and rectum, liver, pancreas, kidney, urinary bladder, and even acute myeloid leukemia.Citation29 Moreover, it should be emphasized that tuberculosis, pneumonia, and chronic bronchitis are examples of pathology, which have a profound role in the emergence of cancer. In effect, in the case of lung cancer, prolonging microbial infections are major inflammation-inducing factors, which is known to be the cause of cancer development.Citation30
Risk factors for breast cancer can be divided into modifiable and non-modifiable.Citation31 To have a lower risk of getting breast cancer, every woman should be physically active and keep the body weight normal, if possible – avoid taking contraceptives and hormone replacement therapy, have the first pregnancy before age 30, breastfeed, and have a full-term pregnancy. Smoking, being exposed to chemicals, drinking alcohol, and having changes in other hormones due to night shift working may also increase breast cancer risk.Citation31 Non-modifiable risk factors include age, genetic mutations, reproductive history, dense breasts, personal and family history of breast cancer, and previous treatment using radiotherapy. Important is the fact that there is evidence linking chronic inflammation, which might be caused by microbial infection, to breast cancer risk, development and progression.Citation32 For instance, it is established that breast cancer was one example of among other 15 incident cancer, in which the risk of developing one year after Staphylococcus aureus bacteremia (SAB) was significantly increased compared to the general population.Citation33 Screening for this aspect in cancers in populations with developed SAB infection might allow for earlier disease detection. Additionally, the presence of chronic infection also affects the human microbiota. Recent studies have found that people who have a good response to immunotherapy to treat their cancer appear to have a different microbiome composition than those who do not respond that well.Citation34
Figure 2 The burden of cancer: risk factors and the frequency of diagnosed cases and deaths (in the centerCitation35).
The risk of getting colorectal cancer increases as the patient gets older.Citation36 About 90% of cases occur in people in their 50s or older. Other risk factors include inflammatory bowel disease (Crohn’s disease, ulcerative colitis), a personal or family history of colorectal cancer or colorectal polyps, and a genetic syndrome, such as hereditary non-polyposis colorectal cancer (Lynch syndrome).Citation36 Moreover, in recent decades, there has been accumulating information in the published literature about the link between CRC and microbial infection. It has been announced that both viruses and bacteria can cause CRC via prolonged infection and accompanying inflammation, as well as induction of mutagenesis that leads to uncontrolled epithelial cell proliferation. Based on data from clinical and laboratory trials, among the aforementioned microbial agents, a crucial role was noted for Streptococcus bovis, Helicobacter pylori, Escherichia coli, Klebsiella pneumoniae, and Fusobacterium.Citation37
It should be kept in mind that lifestyle factors may also contribute to an increased risk of colorectal cancer, such as lack of regular physical activity, low amount of fruit and vegetables in the diet, a low-fiber and high-fat diet or a diet high in processed meat, overweight and obesity, alcohol consumption and tobacco use.Citation36
Noteworthy is the fact that between 30% and 50% of cancers can be prevented by avoiding risk factors and implementing existing evidence-based prevention strategies. Cancer burden can also be reduced by early detection of cancer and the management of patients who develop cancer. Many cancers are curable if they are diagnosed early and treated properly.Citation38 Additionally, it should be emphasized that inflammation is often associated with cancer development and progression.Citation39 The triggering of chronic inflammation that increases cancer risk includes bacterial infections. In effect, the application of nanotechnology products that possess proved antimicrobial properties might have important implications for cancer preventions ().
Table 4 The Examples of Nanotechnology-Based Applications with Proved Antimicrobial Properties
Adequate prevention measures and early detection and treatment might substantially reduce cancer mortality rate. There are two components for efficient detection: 1. Early diagnosis – cancer that is diagnosed at an early stage, when it is not too large and has not spread, is more likely to better respond to effective treatment and can result in a greater improvement in survival rates, decrease in mortality, and less expensive treatment; 2. Screening – aims to detect cancer before the symptoms appear. The definition says that it is the presumptive identification of unrecognized disease or defects through tests, examinations, or other procedures that can be applied rapidly.Citation60
However, implementation of the above preventive measures mentioned above in most cases cannot be accomplished due to the failure of systemic approaches.
Different Aspects of the Use of Nanocarriers – Prevention, Diagnostic and Therapeutic Application
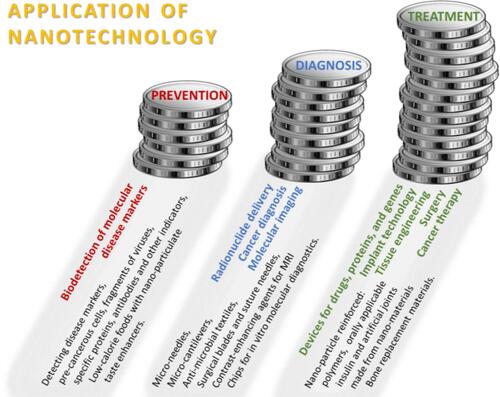
Recently, increasing evidence demonstrates that nanoparticle-based targeting strategy is effective and promising at a diagnostic and therapeutic level and might include many kinds of cancers, such as colorectal cancer, breast cancer, ovarian cancer, or lung cancer.Citation61,Citation62 Nanotechnology can be used in the prevention of disease, diagnosis, and treatment, especially by enabling early disease detection and diagnosis, as well as a precise and effective therapy, which is vital for developing personalized treatment strategy. In effect, implementing the aforementioned new concept of personalized medicine potentially offers an efficient cure for virtually any type of malignancy. Various applications of nanotechnology concerning prevention, diagnosis and treatment fields of use are shown in .
Figure 3 Application of nanotechnology.Citation1
Many types of nanodevices could be clinically applicable, in different kinds of detection, such as imaging contrast agents, immunoassays, or targeted drug delivery systems. In , commonly used nanodevices and their primary areas of application are presented.
Table 5 Types of Nanodevices Used in Clinical Application
Treatments Using Drug Delivery Systems
An accurate cancer diagnosis is essential for adequate and effective treatment because each type of cancer requires a specific treatment regimen that encompasses one or more actions, such as surgery, radiotherapy, and chemotherapy. Determining treatment goals and palliative care is an essential first step, and health services should be integrated and patient-oriented. The fundamental aim is to cure cancer or to prolong life. Improving the patient’s quality of life is not insignificant, and it can be achieved by supportive or palliative care with minimization of side effects of drugs as well as via psychosocial help.Citation73
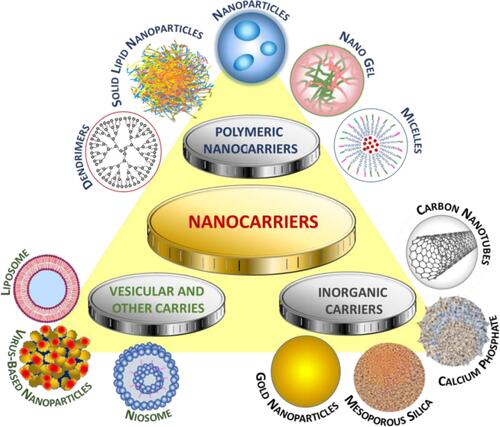
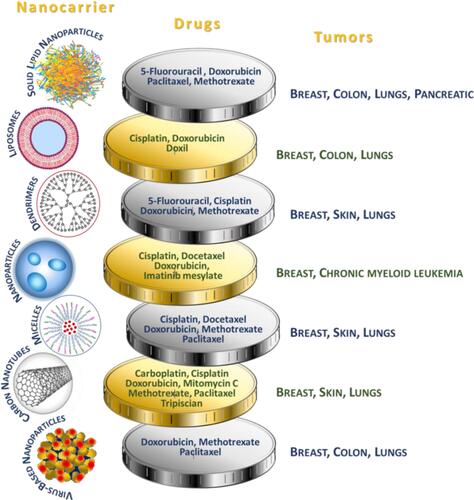
Nanocarriers used in drug delivery systems are typically about a size below 500 nm. They are made of organic (lipid, liposome, dendrimer, polymeric) or inorganic (carbon nanotubes, iron oxides, metallic) materials, as well as their hybrids of varying sizes, shape, and surface characteristics.Citation74 Examples of the most widespread anticancer drugs as part of drug delivery systems, specifying the nanocarriers and type of cancer, are presented in .
Figure 4 Use of nanocarriers.Citation73
To achieve targeted drug delivery with maximum pharmacokinetic activity at pathology sites, constant progress in drug delivery systems using nanotechnology strategies has been noted. The use of drug carriers offers several benefits in terms of the chemical and biological properties of the drug. From a chemical point of view, the application of nanocarriers exerts an impact on drug solubility and penetration ability. Moreover, surface characteristics, immobilization of homing molecules, as well as the sensitivity of carriers to different stimuli determine specific-site delivery, modulate drug release, exert the impact on biodistribution and retention process, as well as influence the immunomodulatory properties of carriers. The above-mentioned features show that a strong association between physicochemical and biological properties exists.
Improving Drug Solubility
The most crucial goal of nanoencapsulation is to solve the problem of poor drug solubility.Citation74 The majority of the currently used drugs are poorly water-soluble molecules, which is why various methods of immobilization and encapsulation of drugs in nanoparticles are used to increase their bioavailability. Drug solubility can be increased by the supplementation of additives (DMSO), which, however, can be toxic even at low doses.Citation75 From the pharmacoeconomic point of view, insoluble drug delivery technologies have many benefits, including reduction of dose and related toxicity, improved formulation, cost reduction, patent protection, or patient compliance.Citation76
Nanocarriers for hydrophobic drugs delivery are most often built of biodegradable monomers or preformed polymers (polymeric nanoparticles), amphiphilic building blocks that due to their organization create structures having the drug located in the hydrophobic interior of a hydrophilic vehicle (polymeric micelles, liposomes), or are structures exhibiting the guest-host properties (dendrimers, carbon nanotubes).
Polymer nanoparticles can be divided due to their organization into (1) nanospheres in which the drug is trapped or dispersed in a polymer matrix, and (2) nanocapsules that consist of a drug dissolved or dispersed in an oily or aqueous core that is surrounded by a solid polymer membrane. A plethora of drugs, including anticancer (Paclitaxel, 5-Fluorouracil), anti-inflammatory (Ibuprofen, Diclofenac), and antibiotics (Rifabutin, Benzathine penicillin G) are described as formulations based on polymeric nanoparticles. A number of PNPs bearing hydrophobic anticancer drugs are at various stages of clinical trials.Citation73,Citation74
In another of the strategies involving the use of polymeric micelles, water-insoluble drugs exhibit affinity for the hydrophobic region of micelles formed from diblock hydrophobic-hydrophilic polymers. As a result of drug encapsulation, a hydrophilic nanocarrier is created, which due to the typically low critical micelle concentration, remains stable even after dilution by body fluids. Drug-containing polymeric micelles, such as Genexol PM® containing Paclitaxel, already exist on the market.Citation76
Another example of delivery of poorly soluble drugs is the liposomal formulation, where lipophilic drugs can be dissolved in the lipid segment of the phospholipid bilayer membrane. Liposomal carriers are very flexible when it comes to their structure and functionality. Lipid formulations of anticancer drugs have been successfully marketed, such as Endo®-TAG-1 which is a product containing Paclitaxel that uses positively charged phospholipid vesicles for pancreatic cancer treatment.Citation77,Citation78
Finally, hydrophilic dendrimeric polymers are recognized as suitable carriers because drugs can be encapsulated in their interior. The presence of empty cavities can be controlled by affecting the polymer conformation by changing the pH, the type of solvent, as well as the design of the polymer structure itself. At the same time, the encapsulation mechanisms can utilize electrostatic, hydrophobic, acid-base interactions, or hydrogen bonds between the drug and the polymer. Although there is no dendrimer-based product on the cancer drug market, research shows that some known dendrimeric vehicles are good candidates. For example, it was reported that polyamidoamine branched polymers with hydrophobic Paclitaxel, in addition to better drug solubility, showed 10-fold higher anticancer activity compared to free drug, which is attributed to better uptake by tumor cells.Citation79
Interestingly, in the latest literature, there are such bioinspired solutions for drug delivery as the use of amphiphilic proteins to stabilize the hydrophobic drug and induce biosilicification on its surface, which leads to the formation of drug-core silica-shell nanoparticles.Citation80
Other interesting examples are hydrogels, biocompatible crosslinked hydrophilic polymer networks already well known for being a good hydrophilic drug delivery system, which can be modified to encapsulate hydrophobic drugs, for example, by having hydrophilic moieties or molecules having empty cavities in their structure, or even containing polymeric micelles or nanoparticles with the encapsulated drug.Citation81
Targeted Drugs Delivery – the Passive and Active Crossing of Biological Barriers
A key element for the effectiveness of the drug is to successfully access diseased sites. This can be improved or enabled by the use of nanosized drug delivery systems, which themselves are capable of crossing biological barriers or allow the encapsulated drug to traverse them to achieve maximum effect at the target. Depending on the method of administration (intravenous, oral, or inhalation), the nanocarrier must cross various barriers on the way to the tissues or organs and subsequently to the cells or organelles, which takes place via two modes of transport, “passive” and “active”. Targeted drug delivery systems (TDDSs) have many advantages, including (1) reducing the exposure of healthy cells to cytotoxic compounds, (2) overcoming the increasingly common drug resistance of tumors, and (3) reduction of side effects of therapy, which directly translates into profits from a pharmacoeconomic point of view.Citation82
“Passive”, non-specific targeting is associated with reduced nanoparticle sizes and surface properties, such as hydrophobicity, surface charge, or non-specific adhesion, which may result in reaching organs having porous endothelial capillaries (liver, spleen), helping to cross specialized epithelial, and penetrating the cell cytoplasm.Citation83 For example, in the case of cancer, the phenomenon of increased permeability and retention (EPR effect) can be observed, which is based on selective penetration into cancer cells compared to normal tissues due to the size of nanoparticles. This is caused by the leaky nature of the tumor-bearing blood vessels that have endothelial cell linings of 100 to 700 nm, which is 10- to 70-fold more than the normal endothelium. This, combined with the weak drainage system typical of solid tumors, leads to the accumulation of drug-loaded nanoparticles in the neoplasm.
Furthermore, due to the increased metabolism of tumor cells, their surroundings are characterized by acidic pH and slightly increased temperature, which can be used in the design of stimuli-responsive nanocarriers. Finally, tumors will release specific enzymes, such as metalloproteases, into their adjacent environment, which in addition to function as tumor markers, can also be recognized by functionalized drug delivery systems.Citation73
Unfortunately, for some organs, the delivery of drugs passively using nanosystems is significantly impeded due to the poor permeability of biological barriers, such as the blood–brain barrier (BBB). In these cases, “active” transport methods can improve traversing through membranes.Citation84 “Active targeting” relies on the increased selectivity of the drug-loaded nanocarrier through its surface functionalization with a ligand showing an affinity for the pathological site. Such ligands, including antibodies, peptides, proteins, glycoproteins, growth factors, nutrient compounds, vitamins, or nucleic acids, are bound by receptors that are overexpressed on cancer cells. Then, receptor-mediated endocytosis ensures cellular uptake of nanocarriers providing higher drug concentration in the cytoplasm.Citation73 An interesting example of a ligand is folic acid, whose receptor (FR) is overexpressed in many types of cancer, such as breast, lung, ovarian, and colorectal tumors.Citation85 Among classical targets, there are transferrin receptors (TfR) or nicotinic acetylcholine receptors typical for the vasculature of brain tumors.Citation86
Furthermore, targeting tumor endothelium on which there are numerous moieties, such as vascular endothelial growth factors (VEGFs) or vascular cell adhesion molecules (VCAMs) can be a complementary strategy to drug delivery, as it involves the destruction of endothelial walls, and thus cutting off oxygen and nutrient access leading to cell death.Citation73
Another advantage of nanocarrier functionalization is the conjugation of the carrier with a fluorescent marker that allows tracking of both the carrier and the drug in vitro and in vivo studies, which can be used in theranostics.Citation87
Nowadays, most of the clinical trials using nanocarriers apply “passive” transport,Citation85 and the use of the EPR effect in the design of drug delivery systems has become standard. Some of these products are commercially available, such as Doxil®, a liposomal formulation of the cytotoxic Doxorubicin, or Caelyx®, a PEGylated liposomal formulation of this drug. Besides, many studies are documenting the in vivo antitumor activity of nanosystems using an “active” mechanism of cell penetration, and some of them are at the clinical trials, including a liposomal nanoplatform containing Doxorubicin with scFv antibody as a ligand targeting the human epidermal growth factor (HER2) receptor in advanced breast cancer, and a polymeric nanoplatform having Docetaxel with nucleic acid-based protein-ligand (ACUPA) targeting prostate-specific membrane antigen (PSMA) in solid tumors.Citation86
Increasing Drug Stability and Controlled Release
Drug delivery systems (DDSs) have many advantages over using free medicines. Often, one type of carrier significantly improves a given therapy by improving several chemical properties of the formulation, thereby increasing the stability of the formulation and drug during storage, the stability of the formulation in vivo, and also allowing for prolonged release of the drug.
Maintaining the unchanged properties of the drug during storage and extending its suitability for use in the drug delivery systems can be very helpful. For example, it was reported that a carrier made of cyclodextrin could result in increased thermal stability and reduced drug volatility.Citation88 Another case was described by Hsiao and coworkers, who showed that chlorophyll, a valuable bioactive compound known for its sensitivity to oxygen, high temperature, and light, has been encapsulated in polycaprolactone, gaining greater stability and therefore being more convenient for storage.Citation86
The drug delivery system can lead to increased drug stability in vivo and protect it from degradation before and after it gets into systemic circulation by decreasing metabolic clearance in blood and gastrointestinal tract (GIT) or renal reticuloendothelial system (RES) clearance. However, it is very important to maintain constant nanoparticle parameters, such as size, morphology, size distribution, porosity, or crystallinity, because their disturbance can lead to altered pharmacological properties of the drug-loaded nanosystem. Some active moieties, such as DNA or siRNA, possess disadvantaged physicochemical properties (molecular weight, charge, susceptibility to degradation by enzymes) and have to be applied clinically together with appropriate nanocarriers.Citation88,Citation89 Specifically, in the case of immobilization of enzymes on nanocarriers, in addition to increased stability, they are attributed to such benefits as reduced protein degradation, resistance to mass transfer, high mechanical strength, and minimum diffusional problems.Citation90 One should also mention the “stealth” technology used for liposomes, which consists of attaching a synthetic polymer poly(ethylene glycol) (PEG) to the liposome structure. This modification extends the presence of intact pegylated nanocarriers in the blood through reduced uptake by the mononuclear phagocyte system (MPS).Citation91
The formulation must be stable to external factors mimicking conditions in the body, and therefore without the evaluation nanomaterials cannot be used clinically.Citation83 For instance, Villamizar-Sarmiento et al carried out a comprehensive study and confirmed that the prepared nanomedicines based on poly(styrene sulfonate) polymer had unchanged hydrodynamic sizes and zeta potential for over a dozen days at a varying salt concentration (NaCl), pH, and temperature, and was durable despite freeze-drying and redissolving in water.Citation92 Similarly, Kanwar et al studied structural changes of nanostructured lipid carriers (NLCs) under stress conditions, such as changing electrolyte concentration, pH, and stabilizing polymer addition. Interestingly, NLCs are resistant to changes in the environment, which is important for their pharmaceutical applications.Citation93
The immobilization of a drug, both hydrophobic and hydrophilic, helps to ensure its controlled slow-release and avoid burst effect, which would not have been possible without the carrier.Citation94 As a result of slow controlled drug release, the active substance has a prolonged circulation in the body and is released at pathological target sites. In one of the strategies, due to the specific chemical properties of the designed nanocarrier, its durability can be controlled in vivo by local stimuli, such as abnormal pH,Citation95 temperature,Citation96 or ionic strengthCitation97 (so-called stimuli-responsive materials). For instance, Guo et al reported the synthesis of carriers consisting of cationic liposomes coated with carboxymethyl chitosan, stable under physiological conditions, but in an acidic environment specific to the tumor (pH=6.5) quickly transformed into a cationic form, which aided tumor-specific cellular uptake. Moreover, in the presented studies synergistic use of two active molecules, the anti-cancer drug (doxorubicin) and oncogenic protein inhibitor (MDM2), was possible using the dual-drug delivery system.Citation98 Recently, Razavi et al described multi-stimuli-responsive block copolymers based on poly(N-(2-(dimethylamino)ethyl)-methacrylate) (PDMAEMA) and poly(methyl methacrylate) (PMMA) chains terminated with spiropyran, wherein the size of the nanoparticles, as well as the release of doxorubicin, was controlled through pH, light, and temperature.Citation99
The Efficiency of Encapsulation/Immobilization of Drugs in Carriers
From a pharmacological point of view, it is important to ensure efficient drug encapsulation to avoid such in vivo side effects of the use of nanocarriers in excess such as agglomeration resulting in excretion from the body by the immune system, high blood pressure, renal failure or systemic toxicity.Citation100 Unfortunately, the majority of currently known drug delivery systems are characterized by a low loading efficiency (less than 10%), which is associated with the use of a large amount of carrier.Citation101 To achieve good loading efficiency, the kind of materials used (characterized mainly by a large surface area) and their surface modification and the method of drug encapsulation/immobilization are important. In general, the mechanism of drug loading through non-covalent interactions most often results in low loaded drug carriers, and covalent or coordination bonds result in high drug loading efficiency. Such non-covalent bonds are electrostatic interactions, π-π stacking, hydrogen bonding, or hydrophobic/hydrophilic interactions of the drug with the surface of the carrier. For example, the most popular carrier, liposomal, depending on its morphology, is characterized by hydrophobic or hydrophilic drug–carrier interactions. In the case of polymer nanoparticles or dendrimers, they may form structures that allow the drug to become entrapped in a micellar or hollow structure, respectively, or to bind the drug via a chemical linker. Typically, enzymatically or chemically cleavable linkers are used, such as amide, ester, disulfide bonds, or phosphate esters. There are also examples of specific linkers sensitive to the stimulus or enzyme typical of the tumor environment. For example, disulfide bonds can be broken by glutathione, an enzyme that is overexpressed on cancer cells.Citation102,Citation103
Due to the type of nanocarriers’ structure, the following types of high drug loading nanomedicines can be distinguished: 1. Inert porous material as a carrier (silica, carbon, or protein nanoparticles); 2. Polymer-drug conjugates (PDCs); 3. Coordination polymer nanoparticles (metal-organic frameworks); 4. Carrier-free nanomedicines (drug nanocrystals, amphiphilic drug–drug conjugates).Citation103 The PDCs systems used are solid dispersion of the drug in a hydrophilic polymer, and nanoconjugates of an amphiphilic or hydrophilic polymer with the drug. Recently, various PDCs carrier improvement strategies have been introduced to enhance loading efficiencies, such as the use of: 1. Multi-arm polymer conjugated with drug;Citation104 2. The hydrophobicCitation105 as well as the hydrophilicCitation106 drugs as part of the core-shell carrier structure; 3. Two drugs with opposite hydrophilicity linked via a hydrophilic carrier (spacer);Citation107 4. Encapsulation in core-crosslinked polymer.Citation108,Citation109
Another class of nanomaterials that overcomes the problem of low drug loading is nanocages (protein, gold, carbon, silica, or DNA NCs), which have a hollow structure and can contain up to thousands of drug particles inside.Citation97 A different way to increase the effectiveness of drug loading is surface modification. For example, porous iron oxide nanoparticles (IONPs) coated with materials, such as silica, surfactants, carbon, and polymers are used as drug carriers. Moreover, the introduction of functional groups on the surface allows its further modification, for example, with proteins, which further increases the affinity for drugs.Citation103 Another example describes calcium phosphosilicate nanoparticles (CPSNPs) as phospho-drug nanocarriers (5-Fluorouracil) where due to metal-ligand complexes between the phosphate group and calcium, efficient drug encapsulation is possible.Citation99
It turns out that the effectiveness of the encapsulation procedure depends on many factors, and in the literature, comprehensive analyses can be found regarding specific carriers in combination with various medicines and encapsulation methods. For example, the fact that the route of immobilization should be selected depending on the type of medicine was described by Krukiewicz et al where two different loading methods have been tested with two various active substances. For quercetin, the highest loading was achieved by immobilization on a polypyrrole matrix during the electropolymerization process, while in the case of a second drug tested, ciprofloxacin, incorporation during post-modification (polymer oxidation) was more efficient.Citation110 Furthermore, Perotto et al reported that in addition to such medicine characteristics as hydrophilicity and molecular weight, the charge of the drug might have the most significant impact on its encapsulation, as in the case of positively charged methylene blue-achieving up to 88% encapsulation efficiency in keratin nanoparticles.Citation111 Besides, the study of curcumin encapsulation into poly ε-caprolactone NPs was carried out by Nagy and coworkers using Box–Behnken experimental design, where the variables in the encapsulation procedure were the initial amount of the drug, the volume ratio of the organic and aqueous phases, as well as the composition of the organic phase. It was found that the volume of the organic phase containing a drug used for nanoprecipitation of the polymer was crucial for efficient drug loading.Citation112
In the latest literature, one can also find reports about drugs encapsulated in high loading carriers by environmentally friendly methods. That is, due to aromatic–aromatic interactions and the formation of ionic pairs, hydrophilic and aromatic low molecular weight drugs (HALMD) were encapsulated in a poly(styrene sulfonate) (PSS) with the yield of about 50%.Citation113
Application, Mechanism of Action, and Drug-Resistance of Selected Chemotherapeutics
Doxorubicin (DOX) is commonly used in various types of malignancies, such as sarcoma, leukemia, lymphomas, breast, lung, and ovarian cancer. There are two different mechanisms of action: intercalation of doxorubicin into DNA and inhibition of topoisomerase II leading to changes in chromatin structure; generation of free radicals and oxidative damage to biomolecules. Repeated doxorubicin administration leads to drug-resistant cancer cells; it also increases drug cytotoxicity. The interaction between signaling pathways can promote drug resistance through the induction of proliferation, cell cycle progression, and prevention of apoptosis. Doxorubicin-induced drug resistance and tumor growth can occur through the adaptive role of the MAPK/ERK pathway in the effort to protect tumor cells. The mechanism of drug resistance of the Anatomical Therapeutic Chemical Classification System (ATC) is related to the expression of multidrug-resistant 1 (MDR1) transporters. MDR1 transporters pump Dox molecules out of cells, reducing intracellular concentration of drug and inhibiting chemotherapeutic efficacy.Citation114,Citation115
5-Fluorouracil (5-FU) could be applied to treat solid tumors of the gastrointestinal tract, breast, head, and neck, as well as the pancreas. Mechanism of action involves blocking DNA synthesis and replication through inhibition of thymidylate synthase and incorporation of 5-FU metabolites into RNA and DNA. 5‑FU resistance abrogated the anticancer effect amplified by the Chk1 inhibition, even in p53-deficient cancer cells. Chk1 inhibition might be effective in sensitizing 5‑FU resistant cancer cells to 5‑FU because Chk1 activation is reported to be related to the resistance to chemotherapy. It has also been observed that the synergistic cytotoxic potential for Chk1 inhibition during 5‑FU treatment in p53‑deficient colon cancer cells with or without 5‑FU resistance.Citation116,Citation117
Paclitaxel (PTX) is used against many forms of cancer, for example, ovarian, breast, lung, Kaposi sarcoma, cervical, and pancreatic cancer. Mechanism of action relates to targeting microtubules – it disrupts the major function of microtubules, which is the production of the mitotic spindle during cell division, as well as maintenance of the cell structure, motility, and cytoplasmic movement within the cell. A weakened mitotic checkpoint confers only short-term resistance to mitotic arrest but also the activation of a mitotic checkpoint followed by mitotic slippage resulting in optimal cell killing. There are some identified markers of resistance or sensitivity to paclitaxel, such as proteasome subunits, cyclin-G1 (CCNG1), and solute carrier genes. The cytotoxicity of nanoparticles using tamarind seed polysaccharide and paclitaxel by epichlorohydrin crosslinking (PST-PTX) in cancer cell lines and resistant cancer cell lines were determined by MTT assay. Quantitative analysis of cell death was determined by Annexin V dead cell assay, Caspase 3/7 assay, and expression of pro-apoptotic protein Bax. Overexpression of the ABCB1 gene confers resistance to nab-paclitaxel in urothelial cancer cells.Citation118,Citation119
Each of these drugs has a different field of application, mechanism of action, and also various explanations of drug-resistance. Cells become resistant to different drugs through various mechanisms of modification of drug targets, alteration in drug metabolism, and genetic changes of cells to target pathways.Citation120 However, it is worth noting that despite these differences, resistance to drugs continues to be a principal problem in oncology, affecting most cancer patients.
Improving Activity and Help to Overcome the Drug-Resistance
Currently, major treatments for cancer management include cytotoxic chemotherapy, surgery, targeted therapy, radiation therapy, endocrine therapy, and also immunotherapy. Despite the efforts and achievements made in treating cancers during the last few decades, resistance to classical chemotherapeutic agents and novel targeted drugs remains a major problem in cancer therapies.Citation121 Drug resistance, also the one existing before treatment (intrinsic) or generated after therapy (acquired), is responsible for most relapses of cancer, which are the major causes of death of the disease. Heterogeneity among patients and tumors and the comprehensiveness of cancer to circumvent therapies make drug resistance even more difficult to deal with. A better understanding of the mechanisms of drug resistance is required to provide guidance to future cancer treatment and achieve better results.Citation121 The complexity of drug resistance development suggests that combinational and personalized treatment might provide better approaches and improved efficacy for fighting drug resistance in cancer.Citation122
Cancer presents difficult challenges that would benefit from uniting experts from a broad cross-section of related and unrelated fields. Combining extant approaches with novel ones could help in raising this challenging health problem, enabling the development of therapeutics to stop disease progression and prolong patient lives.Citation122 Regardless of the research approaches, based on the results from clinical trials and research publications on the application of nanoparticles as drug delivery systems in the treatment of cancer, the main benefits are the enhancement of vascular and gastrointestinal permeability and selectivity of drugs/compounds to tumor cells. Abdifetah et al,Citation123 in their summary of the review, note the fact that due to the application of nanoparticles, the improved permeability and selectivity resulted in the overall improvement of cellular drug uptake, the inhibition of drug hepatic first-pass metabolism and P-gp efflux, the increase in drug solubility and stability, and the decrease in the rate of the drug excretion. As a consequence, a reduced dosage can be achieved without compromising the efficacy, which minimizes potential drug toxicity. Still, regardless of the therapeutic and research progress made, some of the challenges in cancer therapy, such as multidrug resistance (MDR), are being further investigated to better understand the molecular mechanisms and optimize the therapies concerning efficacy and safety. According to El-Readi et al,Citation124 due to the tumorous tissue specifics such as their abnormal blood vessels and pathologic processes that hinder effective cancer chemotherapy, the design and application of new methodologies for drug delivery like NPs are vital. MDR is known to be a result of synergistic processes taking place directly in cancer tissues and tumorous cells. In , different mechanisms synergistically, causing multidrug resistance (MDR) are summarized.
Figure 5 Multidrug resistance in cancer mechanism overview.Citation125
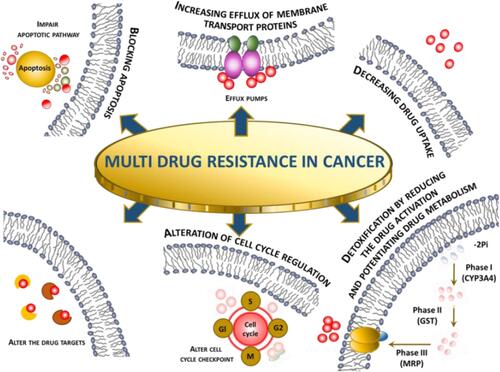
The influence on membrane transport is one of the most important mechanisms in the development of resistance against anticancer drugs. The reduction of drug concentration can be achieved by reduced drug uptake or increased extrusion of the molecules. The overexpression of P-glycoprotein is responsible for efflux. The use of nanoparticles loaded with docetaxel (PLGA-PEG) has proven to be effective in overcoming the MDR as referenced in the article.Citation126 The authors also listed other advantages of the application of NPs in the therapy over the standard dosage forms; for example, nanosized drug carriers minimize the elimination of the molecules substantially through the liver or kidney. Other properties like improved permeability and accumulation of nanoparticles loaded with drugs are passively targeting tumor tissues resulting in lower systemic toxicity.
Another successful application of targeted anticancer nanocarriers using biocarriers is presented in the article by Radu et al.Citation127 Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) carriers were obtained via the emulsification-diffusion method, loaded with 5-fluorouracil and therapeutic potential on human adenocarcinoma cells was investigated. As a result, it was observed that the drug-loaded carrier could significantly decrease cell viability, showing the high potential of destroying human adenocarcinoma cells. Overall, significant progress has been made in the field of nanocarriers in cancer treatment resulting in improved pharmacokinetic properties, better antitumor efficacy, and lower risk associated with the development of undesirable drug effects. Physicochemical properties of the therapeutic nanocarriers and pathophysiological tumor characteristics still need to be investigated to get deeper insights into the mechanisms allowing effective and safe cancer treatments. Arranja et al reported a list of clinically used nanomedicines containing mainly liposomes, polymer-drug conjugates, and polymeric micelles.Citation128 In contrast to traditional chemotherapy, nanomedicines are characterized by prolonged circulation half-lives, increased bioavailability, and better tumor disposition; however, they rely mainly on the EPR effect. To increase our understanding of actively targeted nanodrugs, the authors suggest and discuss the application of strategies from theranostics. The main aim of this approach is to integrate molecular imaging properties into therapeutic agent formulations to monitor tumor accumulation and therapeutic efficacy of nanomedicines at the same application time. More controlled targeted drug delivery should further optimize therapeutic effects minimizing unwanted cytotoxicity in the off-target tissues.
Cancer multidrug resistance (MDR) to chemotherapy is a crucial barrier in the effective treatment of malignancies, which may lead to therapeutic failure of the treatment regimen. Nanotechnology ensures a novel and unconventional approach to circumvent MDR. In , recent literature examples of application nanocarriers to overcome MDR are presented. Mechanisms and advantages of various types of nanocarriers were discussed below as well as potential approaches to overcome these limitations.
Table 6 Mechanism of Overcoming Drug Resistance and Benefits of Nanocarrier Use
Establishing a practical nanotechnology-based drug delivery systems may help in the future to improve the bioavailability and therapeutic efficacy of antitumor drugs while providing better accumulation at the target site compared with conventional antitumor drug delivery systems.
Pharmacoeconomic Aspect of Drug Carriers
The efficacy of selected drugs due to their equivalents in nanocarriers could have an impact on reducing or minimizing costs in pharmacoeconomic analysis, especially in shortening the time of hospitalization or a smaller number of tests carried out. We could also avoid some intangible costs, such as pain, suffering, or anxiety – if the patient stays shorter in the ward and could be faster at home. What is more, we can reduce the number of inpatient days, resulting in decreased risk of infections and medication side effects, improve quality of treatment, and increase hospital profit through more efficient bed management.Citation133
As a result of the use of drug carriers we can observe the following benefits: 1. The economic benefits result from the savings associated with a more cost-effective medical procedure; 2. Clinical benefits are defined as the direct positive effects of the applied therapy, measured by primary or secondary endpoints. The size of clinical benefits is a measure of the clinical effectiveness of the examined medical procedures; 3. Unmeasurable benefits concern the reduction of pain, anxiety, and improvement of life comfort and its duration.
Comparing the use of traditional therapy with alternative therapy, such as nanocarrier-based-therapy, we can evaluate examples of systemic treatment parameters in oncology such as Evaluation of response to treatment (%); Percentage of corresponding patients (%); Percentage of total remissions (%); Time to relapse (months, years); Percentage of reduction in risk of recurrence (%); Percentage of 5-year survival rate (%); Percentage of responses to treatment (%); Percentage of total pathological remissions (%); Total survival time (months); Median survival (months); Indicators of quality of life and reduction of symptoms, such as VAS procedure.Citation134
Clinical studies have demonstrated the effects of using PEGylated-liposomal doxorubicin in adjuvant chemotherapy for advanced and metastatic breast cancer (). Reflected in , results review the clinical application of PLD in the adjuvant chemotherapy of breast cancer and illustrate the therapeutic effects of pegylated liposomal doxorubicin in various treatment regimens. These clinical studies, which presented therapeutic strategies for applying listed drugs to such adjuvant chemotherapy, show a significant improvement in the treatment results in terms of increased survival time as well as progression-free survival time. Both of these indicators are crucial in the effective treatment of oncological patients.
Table 7 The Effects of Using Pegylated Liposomal Doxorubicin (PLD) for Adjuvant Chemotherapy of Advanced and Metastatic Breast Cancer
Over the past decade, the application of nanomaterials for the treatment of cancer features high sensitivity, specificity, and efficacy. Nanomaterials could be applied to employ specific ligands to target cancer cells predictably and deliver encapsulated load capacity effectively. Besides, nanomaterials can also be created for enhanced drug loading, greater half-life in the body, sustained release, and selective distribution by transforming their size, composition, morphology, and surface area. For instance, carbon-based materials, polymeric nanomaterials, metallic nanoparticles, dendrimers, and liposomes have been developed as smart drug delivery systems for cancer treatment, showing improved pharmacokinetic and pharmacodynamic parameters over standard formulations because of their nanosize and individual physicochemical properties.
The data presented in , suggest that nanotechnology will provide new opportunities for cancer management. Moreover, a range of nanoparticles demonstrate significant efficacy for anticancer therapies, and their application can also be discussed in the pharmacoeconomic context. Considering that all the presented benefits from the use of nanomaterials make nanotechnology much cheaper than conventional treatment, it can also be reflected in the expected pharmacoeconomic efficacy. This could result in the reduction or total avoidance of costs in the management of cancer patients, particularly by reducing costs of interventions, shortening the time of hospitalization or avoided expenditure on illness which results in fewer medical procedures carried out, leads to the reduction of personnel costs and allows patients to return to professional life.
Clinical Application of Drug Carriers
The website clinicaltrials.gov was searched on 09.12.2020. The search was conducted using the keywords: cancer and nanoparticle. The start and end dates of the study were determined from 01.01.2015 to 09.12.2020. The status of the study was also defined – only studies with “completed” status were taken into consideration. As a result of this search, 13 studies meeting the above criteria were found. The search strategy is presented in .
Table 8 Terms and Synonyms Searched in Clinical Trials Database
To summarize, in , all studies are interventional (clinical trials), which are presented on the clinicaltrials.gov website. Each study involves a different number of patients, ranging from 2 to 146 participants. Different types of cancer were investigated, and the degree of severity is also taken into account, whether or not it is metastatic cancer. Each study describes arms – experimental or placebo, as well as treatment/other intervention. The selected endpoints – primary, secondary, or other – are included in the studies as per the protocols.
Table 9 Characteristics of Searched Clinical Studies
Unfortunately, so far, no results have been published for any of the thirteen studies, so we cannot draw any conclusions, but we can state that the use of nanoparticles in medicine, in the treatment of cancer, is becoming increasingly popular.
Business Criteria for the Development of Drug Carriers
During the manufacturing of drug forms, different methods should be considered. The selection of manufacturing methods often depends on the final product’s requirements in terms of clinical efficacy, including size distribution, chemical composition, and drug release characteristics together, which dictates the pharmacokinetic demonstration of adsorption, distribution, metabolism, and elimination (ADME).Citation181
Reducing Cost/Reagents/Green Synthesis
It is estimated that the development of a new nanodrugs takes only about 3–4 years and $20- $50 million. In comparison, discovering new active molecules takes more than 10 years, costing an average of about $500 millions.Citation182
In order to perform the procedure for obtaining a drug delivery system designed in a laboratory on an industrial scale, careful optimization of the synthesis must be carried out in order to reduce the production costs. For example, Ding et al carried out a tedious optimization of polymer synthesis for protein therapy by changing time, solvent, and equivalents of reagents. As a result, the cost of a polymer prepared on a few hundred grams scale, following the principles of green chemistry, was reduced by almost 90%.Citation183
Furthermore, in industrial-scale production, the time of synthesis directly translates into cost; thus, it is important to choose the most time-efficientCitation184 and inexpensive production method.Citation185 Finally, affordable, non-toxic, and common solvents, such as water, are most desirable.Citation111 Interestingly, to reduce the time and cost of formulation development, computational methods are used to predict in vitro/in vivo properties of carriers, such as stability, solubility, and potential toxicity.Citation186
It is also worth noting that multifunctional carriers with targeting and imaging properties as well as multistep synthesis and greater regulatory hurdles thereof are worth the cost due to their numerous advantages, such as reducing side effects, dosing frequency, use in theranostics, and even reducing the toxicity of the drug, as proved by Cheng et al.Citation187 Despite higher production costs, recent analyses show that the use of targeted drug delivery systems for cancer patients leads to long-term reduced healthcare utilization and expense.Citation188
Transfer of Drug Carriers Synthesis Methods from Lab to Industry - Challenges
Despite increased interest in nanodrugs in recent years, the transfer of methods to the market is still a challenge due to the difficult industrial transfer.Citation189,Citation190 In general, procedures of nanocarriers synthesis are sensitive to reaction conditions and the characteristics of nanomaterials (size, charge, shape, morphology, and dispersity) can be easily disturbed due to scaling-up and thus formulation and effectiveness of nanodrugs may change.Citation191 Furthermore, these parameters are very important for the in vivo stability and toxicity of nanocarriers.Citation192
In an industrial plant, the particle size can be affected by the available chemical reactor volume, stirring velocity, and time, as well as the energy used during the synthesis. These fluctuations in features may further lead to decreased efficiency of drug loading.Citation193,Citation194 One of the examples of difficulties associated with large-scale production can be Doxil, the first nanodrug authorized in 1995, whose sales were suspended in 2011–2014 due to production and sterility problems.Citation195 Furthermore, it was described in the literature how scaling-up generated new minor impurity, which was found to be cytotoxic and changed the colloidal and structural properties of nanoparticles.Citation196
Another challenge regarding the industrial transfer of nanodrugs is an insufficient number of guidelines for the characterization of nanoparticles concerning their safety and non-toxicity and lack of strict legal regulations.Citation188,Citation197 Given the listed challenges, to obtain the desired features during the synthesis of drug formulations, the Food and Drug Administration (FDA) introduced in the 2000s a method of quality by design, which provides product quality controls at every stage of the process (by using pH or ionic strength sensors). In this way, the key parameters of the drug carrier synthesis must be obtained via standardized procedures and scalable chemical equipment. Since the synthesis conditions in the industrial plant are different from in the laboratory, each stage of the synthesis must be transferred according to Chemistry, Manufacturing and Controls (CMCs) and follow good manufacturing practice (GMP) requirements.Citation191,Citation198 However, as it is not easy to control the process taking into account so many parameters that nanoparticles desire, a reproducibility problem arises.Citation195 As a consequence, each batch of produced material must be thoroughly tested to ensure its characteristics, safety, and non-toxicity.Citation190 Some researchers suggest that routine testing of large-scale formulations in animal models would be desirable.Citation191
One may notice a deficit of simple industrial procedures for the synthesis of nanomaterials regarding the limited possibilities of the industrial plant.Citation199 Methods for the synthesis of nanomaterials described in the literature have many limitations, such as the difficult removal of a toxic organic solvent (in solvent emulsification-diffusion technique applied to lipids) or challenging maintaining sterility of the product.Citation185,Citation191,Citation200 Furthermore, some methods to produce nanoformulations, such as freeze-drying and spray-drying used to fabricate nanoencapsulates in powder form, are expensive and may affect particle size.Citation201 Large-scale preparation of nanocarriers that will be biodegradable in vivo is another challenge.Citation201,Citation202 Therefore, top-down processes (consisting of mechanical fragmentation of the product) are still more common than the bottom-up approach (generating nanoparticles starting from molecules or atoms).Citation203 However, some production methods seem to be more useful than others for large-scale applications, such as supercritical reverse-phase evaporation or microfluidic mixers.Citation191,Citation192
Furthermore, as usually creating a new drug delivery system is a reformulation of a previously known drug, pharmaceutical companies often do not consider this process worth the time and costs compared to profits and prefer investing in the search for new drugs by simply screening libraries of small compounds.Citation188,Citation199
Green Synthesis in Drug Carriers Manufacturing
“Green nanomedicine” is a new field of drug delivery systems based on nanomaterials, which provides tools for more economical nanocarriers synthesis. However, currently, only a few literature examples of research can be found in which at least a few of the dozen “green chemistry” postulates have been met. Among syntheses of such drug carriers as nanometallic compounds, polymer nanocomposites, and quantum dots one can find examples of the use of safer reagents, solvents or auxiliaries, the design of safer, atom economical syntheses, application of renewable energy sources, or the synthesis of biodegradable carriers. Among the described nanosystems, protein and lipid compounds are the safest of known drug carriers.Citation204,Citation205
A very important aspect is the choice of the synthesis method among those available.Citation206 A separate group of non-toxic reactions in nanomedicine are methods that use plant extracts as reagents. For example, Palai et al described the synthesis of a decorated graphene nanocomposite, where the aqueous neem leaf extract was used to reduce graphene oxide, while the synthetic procedure was modified to reduce the number of toxic gases and impurities generated.Citation207
One of the latest examples of the use of eco-friendly reagents was delivered by Uthappa et al, who described the green synthesis of natural diatoms modified with polydopamine as a drug delivery system, in which additionally the synthesis time was reduced and no toxic reagents and solvents were used.Citation87 Furthermore, Hasan et al described the eco-friendly synthesis of silver nanoparticles in which the reduction process by chemical compounds has been replaced by a reduction by a biopolymer (dextrin).Citation208 An alternative to green solvents may be the use of ionic liquids.Citation209
Despite the existence of more adaptive techniques, such as reverse-phase evaporation or thin-film hydration, a green technique, energy-saving probe sonification method using only water as a solvent, was chosen for the production of niosomes by Khan et al.Citation113 Next, Ca2+ cross-linked Fe-guanosine monophosphate (Fe-GMP) hydrogel for doxorubicin delivery was prepared by facile mixing of appropriate components at ambient conditions.Citation210 Finally, it is important to select those biocompatible from among the available polymers (poly(sodium 4-styrene sulfonate), PSS), and assure that the encapsulation of the drug takes place using a simple green method, for example, by mixing of two aqueous phases containing the polymer and the drug, respectively.Citation92 From the producer’s point of view, the more “green” the process, the cheaper and safer for the final product due to the lack of toxic impurities.
Pharmacokinetic and Toxicological Studies of Nanoparticles as a Delivery System
Pharmacokinetics, often described as what an organism does to a drug, is a branch of pharmacology dealing with the study of the activity of compounds in the body over a period of time with a primary focus on processes by which medicinal products and drugs are absorbed, distributed, metabolized, and finally excreted (ADME). Pharmacokinetics depends on many factors that are related to the physicochemical properties of the complex substance as well as to patient-related conditions like gender, age, individual physiology, or genetics. Knowledge of pharmacokinetics is crucial for targeted and safe application of drugs to achieve the maximum therapeutic effect and the minimum risk associated with the occurrence of adverse effects.
An ideal drug should be highly specific concerning the pathologic processes and changes without any toxicity to healthy organs, tissues, or cells. The most desired properties of an active compound should directly lead to proper absorption and drug distribution, low metabolism, decent elimination, and low toxicity.
Pharmacokinetic key parameters used for defining and describing the ADME processes include bioavailability (by determining the area under the plasma concentration–time curve), elimination half-life (t1/2), the volume of distribution (Vd), and clearance (CL).Citation123 These factors play a crucial role in the determination of the concentration of the drug in the body at a specific therapeutic target. Pharmacokinetics is applied to estimate the exposure and the most important parameters used to define the optimal dosage form and the dosing regimen in clinical practice to achieve maximum efficacy and lowest toxicity.Citation211
Pharmacokinetic Aspect of the Application of Nanoparticles as Delivery Systems
Drugs encounter many barriers in living organisms from the time of administration in a specific dosage form until the therapeutic molecules reach the target. Advances in technology allow us to make structural changes that make significant improvements in drug properties and help overcome the limitations of reduced drug efficacy and potential safety issues. Advances in nanotechnology over the past decades did revolutionize drug delivery systems by improving their pharmacokinetic and pharmacodynamic properties, such as higher solubility, duration of exposure, and targeted delivery to the site of action.Citation212
The tabulation below briefly summarizes the main differences in pharmacokinetic properties of small drug molecules and the desirable drug-loaded nanoparticles ().
Table 10 Pharmacokinetic Properties Comparison Between Small Molecule Drugs and Drug-Loaded Nanoparticles
There are many different types of nanoparticles used as carriers for therapeutic compounds, as shown in ., each of them having different properties.
As mentioned in previous sections, nanoparticles differ in their surface charge, particle size and shape, efficiency, loading capacity, and stability, leading to substantial variability in pharmacologic effects and the safety of different nanocarriers. Petschauer et al summarize in their review the main factors affecting the pharmacokinetic (PK) and pharmacodynamic (PD) properties of anticancer carrier-mediated agents in patients.Citation213 The discussion includes the following elements: Uptake by the mononuclear phagocyte system; Delivery of the compounds in tumors: nanoparticles (NPs) can get into tumors’ tissue due to the leaky vasculature, which results in enhanced permeability and retention effect.; Particle size and shape: NPs between 100 and 200 nm have been observed to be most efficient in uptake by tumors; in turn, particles smaller than 50 nm showed short circulation time, and NPs greater than 300 nm prevented particles from taking advantage of the EPR effect, leading to lower tumor accumulation; Surface modification and charge (Conjugation of PEG to the surface of NPs increases circulation time and bioavailability – measured by Area Under The Curve – AUC; Uncharged particles have less mononuclear phagocyte system uptake, which results in longer circulation time); The concentration of NPs administered: a higher concentration level of particles per dose given increases the drug exposure in both plasma and tumor.
Besides, the authors stress the fact of the existence of a relationship between NP clearance and patient age, gender, disease conditions like liver or renal impairments, or concomitant medications. Another point to consider is the possibility to predict pharmacokinetic properties of PEGylated liposomal NPs based on the monocyte and dendritic cells function.
Advances in computational sciences over the past decade allow researchers to focus on mathematical and statistical approaches. Dogra et al describe a novel modeling approach aiming to predict whole-body nanoparticle pharmacokinetics and their tumor delivery.Citation214 The identified main factors governing NP kinetics in the tumor interstitium were nanoparticle size, tumor vascular fraction, tumor vascular porosity, nanoparticle degradation rate, and tumor blood viscosity. Since the number of potential factors having an influence on the ADME processes in the living organism is huge by nature, mathematical modeling in this parameter space is proposed as an efficient alternative to traditional experiments.
The authors discuss the impact and particular values of parameters to optimize the delivery of NPs into tumor tissue. Garofalo et al present another methodology combining computer-aided drug design from the domain of computational chemistry and drug delivery techniques.Citation215 The multidisciplinary approach gives promising results in overcoming some of the main challenges, such as poor selectivity for the target or poor ADME properties. The authors discuss selected applications of the new approach, aiming to provide insights into a novel rational design of anticancer therapies. According to the authors, the computer-aided drug delivery system design should be combined with “wet” laboratory techniques that allow better prediction of drug delivery systems in vivo and helps in designing drug molecules that increase therapeutic targeting and reduce the optimal dosage.
Despite the fact that nanoparticles demonstrate excellent potential as drug delivery agents, the nano-protein interaction and the formation of a protein corona have been found to interfere with the nanoparticle delivery. In recently published studies, Zhang et al provided a brief summary of the latest developments on the nano-protein interactions between NPs and enzymes of the digestion and initiated an engaging discussion on the possibility of the use of the digestive enzyme corona for the targeted delivery in the colon.Citation216 The authors described physicochemical properties that are closely linked to the oral absorption of NPs, which include: size, zeta potential and surface molecules, which are greatly affected by the interaction of nano-enzymes and the formation of the enzyme corona. Moreover, it has been shown that the uptake of NPs by epithelial cells is significantly increased after the formation of the enzyme corona. The interaction of nano-enzymes is thus a major challenge for oral delivery of NPs and might exert an impact on pharmacological properties. On the other hand, a nano-enzyme interaction could also be applied to advanced oral delivery. As epithelial absorption of NPs is inhibited by the enzyme corona, a great number of NPs have a high chance of passing into the colon in the form of the NP-corona complex. After that, inside the colon, the enzyme corona and indeed NPs could be degraded and metabolized throughout the greatest microbiota in the organism, resulting in the release of loaded drugs straight into the colon area. The same problem has been previously discussed by Peng et al.Citation217 They synthesized the cationic NPs (CNPs) based on poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and examined the interaction of CNPs with digestive enzyme and its impact on cellular uptake. Author’s results show for the first time the formation of the enzyme corona and its inhibitory effect on CNP uptake by epithelial cells. In another paper, Peng et al assessed the interaction between proteins and nanomaterials, which results that, in the in vivo performance of nanomaterials, are significantly different from these in vitro. It has been shown that the protein–nanomaterial interaction may induce remarkable changes in the properties of nanomaterials as well as their associated proteins.Citation218 These changes in properties will eventually lead to undesirable outcomes, which include: 1. Fast clearance of the bloodstream owing to opsonin adsorption; 2. Capillary blockage risk from the increased size after adsorption of serum proteins; 3. The loss of ability to target due to the original surface ligand being covered by the protein corona; 4. Possible toxicity due to the change in conformation of bound proteins. On the one hand, the above interactions are a major challenge for the safe and effective use of nanomaterials in clinical way, but, on the other hand, these interactions could be the possibility of decorating nanomaterial-based drug delivery systems. Consequently, in vivo transport and subsequent behavior of the protein-nanomaterial complex is much more controlled and indeed such a complex holds greater promise for being transferred to the practical products. In effect, it could be supposed that in the near future, these new smart products will be on the market for clinical use.
Toxicity of Drug Delivery Systems
Toxicity remains a challenge even when applying nanoparticles as drug carriers. Highly complex interactions between the molecules, cells, and the host environment are influenced by nanoparticles with many questions arising concerning their long-term safety.
Khan et al describe some of the potential NPs toxicities, which depend on various factors and types of particles used.Citation219 One of them, as pointed out by the authors, is the ability to organize around the protein concentration. This particular feature depends on particle size, curvature, shape and surface charge, functional groups, and free energy. Based on these properties, there is at least a theoretical possibility for NPs to generate adverse and unexpected outcomes through protein unfolding, crosslinking, or causing loss of enzymatic activity.
It becomes evident that despite the promising results and improvement of pharmacokinetic properties of anticancer drug-loaded NPs, long-term research and further studies must be rolled out to better understand complex interactions at the molecular level in vivo.
The Problems of Nanotechnology in Practical Use. The Limitations and Concerns of Different Types of Nanoparticles for Drug Delivery Applications
In view of this paper, the use of nanotechnology in practice may face some challenges. The biggest concern is that the health and safety implications of the specific properties of nanoparticles have not yet been addressed by the regulatory authorities. The new European chemicals policy REACH does not consider side effects. Nanoparticles raise a number of safety and regulatory issues that governments are now beginning to address. A review of recent regulations and ongoing monitoring by authorities is necessary.Citation220 Moreover, some problems such as toxicity demonstrated by some nanoparticles cannot be overlooked when considering the application of nanomedicine in routine clinical practice. Recently, nanoparticles are mostly used together with natural products to reduce toxicity problems. The green chemistry pathway in the design of drug-containing nanoparticles is being extensively promoted due to the fact that it minimizes harmful components in the process of biosynthesis. Therefore, the use of “green” nanoparticles for delivering drugs can potentially reduce the side effects of drugs.Citation189
The use of an optimal nanoparticle drug delivery system is mainly determined by the biophysical and biochemical properties of the targeted drugs that are selected for treatment and could help to improve the successful delivery of nanosystems and optimize the pharmacoeconomic impacts.Citation189
Recently, various nanotechnology-based solutions for drug delivery in the field of medicine have attracted great interest. Despite the above, unfortunately there are still many concerns about the safety application of nanoparticles as drug delivery systems.Citation221
Studies carried out on nanotechnology have proven that every type of nanoparticles has some limitations in practical use. The NPs’ toxic effects are in general associated with the poor biocompatibility of the nanomaterials that were used to develop them. Carbon nanotubes (CNTs) are the type of NPs with more toxic potential observed. They have been found to be lung carcinogenic, but they are also toxic to CNS, blood and GIT. Heavy metals may accumulate in the liver and kidneys and can be toxic to the CNS and GIT. Silicates also have a significant potential to accumulate in the liver and lungs, leading to fibrosis. Direct toxicity of liposomes may be caused primarily by their size, charge or composition. For instance, cationic liposomes may interact with lipoproteins, serum proteins or even with the extracellular matrix, resulting in aggregation or release of agents that are loaded before they reach target cells, causing the systemic toxicity. At doses much higher than those administered (multiple injections of ≥100 mg/kg lipid), liposomes have been demonstrated to cause RES impairment, granulomas, hepatomegaly or even splenomegaly. Furthermore, the increase in lipid dose has been demonstrated to deplete plasma of different proteins. While the identification and importance of all deleted proteins remain unclear, it is possible that their loss will cause impairment in normal homeostasis. Metallic NPs could lead to peribronchitis, granulomas, interstitial fibrosis, collagen deposition, adenocarcinoma and pleural lesions. Nanoemulsions could be responsible for interference with the close linkage in GIT and direct cytotoxicity. Carbon NPs exhibit the oxidative stress, depletion of glutathione, an increase in the number of dermal cells, and also thickening of the skin and rash. Dendrimers and gold nanoshells demonstrate toxicity induced by macrophages, plasma protein depletion, aggregation of platelets and also their pathway of synthesis is complicated.Citation222,Citation223
In view of the above, the awareness of particle levels that may cause health effects is imperative for both workers and exposed patients.Citation222
Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems
Nanomedicine adopts the use of nanotechnology for highly specific medical interventions for the prevention, diagnosis and treatment of diseases, all of which are presented in this paper. The development of nanomedicines tends to improve the therapeutic efficacy, reduce the dose that is therapeutically effective, and decrease the risk of developing side effects.Citation224 Nanocarriers as DDS are designed to reduce the cost of administering the drug, improve the compliance and help patients to recover as soon as possible. All of these aspects are reflected in pharmacoeconomics, a discipline that aims to provide reliable information on the cost of therapies and to choose the best one, considering its effectiveness at the lowest possible costs. In the above paper, nanotechnology solutions and standard therapies, their costs and effectiveness were discussed.Citation224
The clinical development of nanomedicine encompass many aspects, there are some key issues to look out for: biological development (appropriate in vivo structural stability of the nanomedicine after application); process of manufacturing (production on a large scale according to GMP standards, which includes: reproducibility, techniques, infrastructure, experience, and costs of the whole process; tests used to control the quality for characterization which includes: charge, size, morphology, dispersion, encapsulation, modification of the surface, stability and purity); biocompatibility and safety concerns (development of much more targeted toxicoassays for nanomedicines; appropriate understanding of nanocarriers interactions with cells and tissues; reduced level of nanoparticles accumulation in targeted cells, tissues or organs); intellectual property (understand of the nanomedicine patent complexity); government regulations (development of clear nanomedicine regulatory guidelines); and total cost-effectiveness compared to standard treatment regimens (restricted understanding of the nanomedicine’s biological interactions with the patient’s biological environment, leading to an impossibility to apply a pharmacoeconomic approach).Citation225–Citation229
Such determinants could be substantial obstacles that limit the market emergence of nanomedicines, despite their therapeutic efficacy.
Conclusion
The availability of evidence resulting from the application of pharmacoeconomics can be useful in health policy decision-making. It can be applied by healthcare professionals such as policymakers, primary healthcare providers, health-care administrators, and health managers.
Pharmacoeconomics can certainly help in decision-making when evaluating the affordability and access to the right medication for the right patient at the right time, comparing alternative drugs from the same therapeutic class or drugs with a similar mechanism of action, and establishing accountability that the claims by a manufacturer regarding a drug are justified. Proper application of pharmacoeconomics will allow the pharmacy practitioners and administrators to make better and more informed decisions regarding the products and services.
Based on the published literature, the engagement of nanoforms at different stages, including prevention, diagnosis, and treatment, might provide significant benefits from an economic as well as treatment perspective. Those include but are not limited to the factors like faster diagnosis, increase in the viability of patients during antitumor therapy, overcoming the mechanisms of resistance in neoplastic cells, or enhancing therapeutic efficacy via synergistic or additive interactions.
The use of drug nanocarriers is a unique opportunity for an economically attractive improvement of known drugs because the development of novel nanoformulations is much cheaper and faster than the discovery of new drugs. Despite higher production costs, greater regulatory hurdles and difficult industrial transfer, they are worth the cost due to their numerous advantages.
Nanoencapsulation can increase the bioavailability of poorly soluble drugs, facilitate access to pathologically altered sites by improving the crossing of biological barriers and increased selectivity of drug-loaded nanocarriers, provide better storage and in vivo stability, and enable slow, controlled release of the drug in the human body. From a pharmaceutical and economic point of view, all of these benefits can reduce dose and associated toxicity, dosing frequency, side effects and costs, improve formulation, protect patents, and enhance patient compliance. In addition, the use of drug nanocarriers has found wide application in theranostics.
However, there are doubts about the use of drug carriers regarding the risks associated with the excess of nanocarriers used, such as high blood pressure or systemic toxicity. These side effects can be countered by selecting an appropriate carrier material as well as proper drug-carrier binding to ensure a low drug-to-carrier ratio in the formulation. Furthermore, changes in the stability and toxicity of carriers associated with industrial production can be avoided due to carefully optimized synthesis, including product control at every stage of its production, as well as the preparing guidelines for the synthesis of nanomaterials.
It is impossible to say which is better: discovering new drug nanocarriers or searching for new, more effective active substances. But surely to improve the well-known drugs with serious side effects through the use of their nanoformulations is very desirable and cost-effective from the point of view of pharmacoeconomics.
Nowadays, nanotechnology has many advantages, which includes: great bioadhesive properties, high biocompatibility, low toxicity, high encapsulation efficiency and also great drug-loading capacity. Analyzing the above features of nanoformulations it can be concluded that nanoparticles hold a huge potential as drug delivery systems, imaging agents and also in phototherapy. Despite these advantages, there still remain many issues that need to be resolved before nanoparticles can be used in a safe and comprehensive clinical way. Some aspects need further studies, such as: to the generation of nanoparticles with desired sizes; control of the thickness of each layer of the nanoparticles and the impact of it on the therapeutic efficacy; development of more stable nanoparticle; optimization of the drug release profile from nanomaterials; presently, the release rates differ significantly and depend on how drugs are integrated into nanomaterials (mostly by: surface adsorption, conjugation or encapsulation); safety and clinical applications; their biodistribution and long-term toxicity profile. Finally, to understand the mechanisms for metabolism, accumulation and biodegradation of nanoparticles in in vivo studies; discover interactions of nanoparticles with other materials, substances, drugs, and living organisms.
Furthermore, current studies have been limited to the in vitro stage and do not show in-depth toxicology and pharmacokinetic parameters. However, as time passes, the science and publication aspects are broadening, and we are getting much more data that allow us to evaluate and predict the effects of the nanocarriers formulations.
Moreover, from a manufacturing point of view, optimization of the synthesis parameter, encapsulation efficacy, and improved stabilization of nanoproducts will also provide a better understanding of their mode of action and potentially predict the risks of eventual use. In effect, it might be a substantial achievement in reducing the direct and indirect costs of therapy.
Undoubtedly, implementation of new therapeutic options, such as nanotherapy, will be associated with to date still unknown risks; however, expansion and development of the currently performed studies will consequently eliminate the existing gap in our knowledge and understanding of relevant mechanisms when applying nanotechnology to drug development and related costs.
Given the above, more attention should be paid to the pharmacoeconomic aspects of the nanocarriers, to properly assess the risk and benefit balance of the very promising technology presented in this review.
Abbreviations
5-FU, 5-Fluorouracil; p53, cellular tumor antigen p53; ABCB1, ATP-binding cassette subfamily B member 1; ACUPA, Acid-based protein-ligand; ADME, Absorption, distribution, metabolism, and excretion; AEs, adverse events; AgNPs, silver nanoparticles; AGuIX NPs, Polysiloxane gadolinium-chelates based nanoparticles; ALK, Anaplastic lymphoma kinase; ALT, Alanine aminotransferase; ANG 1005, Angiopep-2 paclitaxel conjugate; AST, Aspartate transaminase; ATC, Anatomical Therapeutic Chemical Classification System; ATC, Anaplastic thyroid cancer; AUC, Area under the curve; BBB, blood–brain barrier; BCL2L12, BCL-2-related proline-rich protein; BCRP, Breast cancer resistance protein; BiOBr NPs, Polyethylenimine grafted bismuth oxybromide nanoplates with Fe3+; BNPs, bioadhesive nanoparticles; BSA, Bovine serum albumin; C1-PNPs, pyridinium amphophile-loaded PLGA (poly (lactic-co-glycolic acid) nanoparticles; CA, Carcinoma antigen; CBA, Cost-benefit analysis; CCNG1, Cyclin-G1; CD NPs, Cyclodextrin nanoparticles; CEA, Cost-effectiveness analysis; Chk1, checkpoint kinase 1; CL, Clearance; CMA, Cost-minimization analysis; Cmax, the maximum concentration; CMCs, chemistry, manufacturing and controls; CNFs, carbon nanofibers; CNS, Central nervous system; CNTs, carbon nanotubes; CPSNPs, calcium phosphosilicate nanoparticles; CR, complete clinical response; CRC, colorectal cancer; CS@MoS2, Chitosan-assisted MoS2; CSC, cancer stem cells; CTC, circulating tumour cell; CTCAE, Common Terminology Criteria for Adverse Events; CUA, cost-utility analysis; CuS NPs, copper sulfide nanoparticles; DDSs, drug delivery systems; DKK4, Dickkopf WNT signaling pathway inhibitor 4; DMSO, Dimethyl sulfoxide; DNA, Deoxyribonucleic acid; DOX, Doxorubicin; DPYD, Dihydropyrimidine dehydrogenase; DLT, Dose-limiting toxicity; dvPtNPs, dualvalent platinum nanoparticles; ECG, Electrocardiogram; EGF, Epidermal growth factor; EORTC QLQ-C30, The European Organisation for Research and Treatment of Cancer Quality of Life 30-item questionnaire; EPR effect, enhanced permeability and retention effect; Eudragit S100, anionic copolymers based on methacrylic acid and methyl methacrylate; FACT-G, functional Assessment of Cancer Therapy – General questionnaire; FDA, Food and Drug Administration; FFPE, Formalin-fixed paraffin embedded; FKBP5, FKBP Prolyl Isomerase 5; FR, Folate receptor; GBM, Glioblastoma multiforme; GC, Gastric cancer; GIT, Gastrointestinal tract; GMP, Good manufacturing practice; GNR@LDH-PEG NPs, Poly(ethylene glycol) (PEG) modified core-shell GNR@ (gold nanorod) layered double hydroxide nanoparticles; GNSs, Gold nanospheres; GO, Graphene oxide; GR, Glucocorticoid receptor; GS, Glioblastoma stem-like cell cultures; GSH, Glutathione; GSH-AgNPs, Glutathione-stabilized silver nanoparticles; Gy, Gray (SI unit); HA, Hyaluronic acid; HAAH, Human aspartyl (asparaginyl) β-hydroxylase; (HA)-PBCA-NPs, hyaluronic acid coated poly(butyl cyanoacrylate) nanoparticles; HALMD, hydrophilic and aromatic low molecular weight drugs; HER2, human epidermal growth factor 2; HIF‐1α, Hypoxia-inducible factor 1-alpha; HO-GC, hydrotropic oligomer-conjugated glycol chitosan; HPG, Hyperbranched polyglycerol; HPG-C10-PEG, hyperbranched polyglycerol-C10- poly(ethylene glycol); ICER, Incremental cost-effectiveness ratio; ICPMS, inductively coupled plasma mass spectrometry; IONPs, Iron oxide nanoparticles; i.v., intravenosa; MDCK-MDR1, Madin darby canine kidney (MDCK) cells with the MDR1 gene; MDM2, mouse double minute 2 homolog; MDR, Multidrug resistance; MDR1, Multidrug-resistant 1 transporters; Mg(OH)2 NPs, magnesium hydroxide nanoparticles; MIC, Minimum inhibitory concentration; MnO2 NPs, Manganese oxide nanoparticles; MNPs, magnetic nanoparticles; MoS2/PDA-RGD, molybdenum disulfide/polydopamine-arginine-glycine-aspartic acid; mPEG-CHO-chitosan NPs, Methoxypoly(ethylene glycol) conjugated chitosan nanoparticles; MPS, Mononuclear phagocyte system; MRI, Magnetic resonance imaging; MRSA, Methicillin-resistant Staphylococcus aureus; MTD, The maximum tolerated dose; MTT, The (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay; mTOR, mechanistic target of rapamycin; NaCl, sodium chloride; NCI’s CTCAE, The National Cancer Institute Common Terminology Criteria for Adverse Events; NCs, Neocarzinostatin; NCQDs, Nitrogen-doped carbon quantum dots; NIH, National Institutes of Health; NIR, Near-infrared irradiation; NK 105, Paclitaxel-incorporating micellar nanoparticle formulation; NLCs, nanostructured lipid carriers; NPs, Nanoparticles; NRS-11, The Numeric Rating Scale; ORR, Overall response rate; OSA, Octyl-modified bovine serum albumin; OS, Overall survival; PBCA-NPs, Poly(butyl cyanoacrylate) nanoparticles; PCE, Tetrachloroethene; PCL, Poly(ε-caprolactone); PCMB-Dox NPs, PEGylated carborane-conjugated amphiphilic copolymer doxorubicin nanoparticles; PD, Pharmacodynamics; PD, Progressive disease; PDA-PEG-Van NPs, Polydopamine-based nanoparticles modified with PEG and vancomycin; PDCs, Polymer-drug conjugates; PDL1, programmed death-ligand 1; PDMAEMA, Poly(N-(2-(dimethylamino)ethyl)-methacrylate); PEG, Poly(ethylene glycol); PEG, Polyaspartate micellar nanoparticles; PEG-PCL, Poly(ethylene glycol)-poly(ε-caprolactone); PEG-PE, Poly(ethylene glycol)phosphatidyl ethanolamine; PEI-C18-HPG, polyethyleneimine (PEI)-C18-HPG; PET, Positron emission tomography; PFS, Progression free survival; PG, Pharmacogenomic; P-gp, permeability glycoprotein; pH, potential of hydrogen; PIPAC, Pressurized intraperitoneal aerosol chemotherapy; PK, Pharmacokinetics; PLA, Polylactide; PLD, PEGylated liposomal doxorubicin; PLGA, Poly(lactic-co-glycolic acid); PLGA-PEG, Poly(lactic acid-co-glycolic acid)-poly(ethylene glycol); PLMB-Dox NPs, Doxorubicin-loaded carborane-conjugated polymeric nanoparticles; PMMA, Poly(methyl methacrylate); p.o., Per os; PR, Partial response; PSMA, Prostate-specific membrane antigen; PSS, Poly(sodium 4-styrene sulfonate); PST-PTX, Poly(styrene)-paclitaxel; PTX, Paclitaxel; RECIST, Response Evaluation Criteria in Solid Tumours; RES, Renal reticuloendothelial system; rGO-Au NPs, Reduced-graphene-oxide functionalized with gold nanoparticles; RNA, Ribonucleic acid; ROS, Reactive oxygen species; RP2D, The recommended Phase II dose (in clinical trials); SAB, Staphylococcus aureus bacteremia; SAE, Serious adverse event; s.c., Subcutaneously; scFv, Single-chain fragment variable; SD, Stable disease; siRNA, Small interfering RNA; SLNs, solid lipid nanoparticles; SNA, Spherical nucleic acid; SNPs/SiNPs, silica nanoparticles; SPF, Sun protection factor; t1/2, Half-life; TDDSs, targeted drug delivery systems; TFAP2E, transcription factor AP-2 epsilon; TfR 1, transferrin receptor 1; Tmax, The time it takes to reach Cmax; Tri-Ag NPs, Citrate-coated triangular nanoparticles; Tv-Ag NPs, Toxicodendron vernicifluum silver nanoparticles; UPLC-MS/MS, Ultra-performance liquid chromatography–tandem mass spectrometry; UV, Ultraviolet; VAS, Vasectomy; VCAMs, vascular cell adhesion molecules; VEGFs, vascular endothelial growth factors; Vd, volume of distribution; VOI, volumes of interest; QALYs, quality-adjusted life-years.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Alharbi KK, Al-Sheikh YA. Role and implications of nanodiagnostics in the changing trends of clinical diagnosis. Saudi J Biol Sci. 2014;21(2):109–117. doi:10.1016/j.sjbs.2013.11.001
- Fornaguera C, García-Celma MJ. Personalized nanomedicine: a revolution at the nanoscale. J Pers Med. 2017;7(4):12. doi:10.3390/jpm7040012
- Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine (Lond). 2019;14(1):93–126. doi:10.2217/nnm-2018-0120
- Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015;21(4):223–232. doi:10.1016/j.molmed.2015.01.001
- Bosetti R. Cost-effectiveness of nanomedicine: the path to a future successful and dominant market? Nanomedicine (Lond). 2015;10(12):1851–1853. doi:10.2217/nnm.15.74
- Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6:360. doi:10.3389/fchem.2018.00360
- Acebes-Fernández V, Landeria-Viñuela A, Juanes-Velasco P, et al. Nanomedicine and onco-immunotherapy: from the bench to bedside to biomarkers. Nanomaterials (Basel). 2020;10(7):1274. doi:10.3390/nano10071274
- Sun Q, Ojha T, Kiessling F, Lammers T, Shi Y. Enhancing tumor penetration of nanomedicines. Biomacromolecules. 2017;18(5):1449–1459. doi:10.1021/acs.biomac.7b00068
- Su H, Wang Y, Gu Y, Bowman L, Zhao J, Ding M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J Appl Toxicol. 2018;38(1):3–24. doi:10.1002/jat.3476
- Salvioni L, Rizzuto MA, Bertolini JA, Pandolfi L, Colombo M, Prosperi D. Thirty years of cancer nanomedicine: success, frustration, and hope. Cancers (Basel). 2019;11(12):1855. doi:10.3390/cancers11121855
- Alshehri S, Imam SS, Rizwanullah M, et al. Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: preclinical promise and translational challenges. Pharmaceutics. 2020;13(1):24. doi:10.3390/pharmaceutics13010024
- Pallis A, Tsiantou V, Simou E, Maniadakis N. Pharmacoeconomic considerations in the treatment of breast cancer. Clinicoecon Outcomes Res. 2010;2:47–61. doi:10.2147/CEOR.S4220
- Rai M, Goyal R, Vohora D, Singh G. Chapter 33 - Pharmacoeconomics in healthcare. In: Vohora D, Singh G, editors. Pharmaceutical Medicine and Translational Clinical Research. 1st ed. Academic Press; 2018:465–472.
- Haddad FS, McLawhorn AS. Guidelines for reporting health economic research. Bone Joint J. 2016;98-B(2):147–151. doi:10.1302/0301-620X.98B2.37643
- Ho MY, Chan KK, Peacock S, Cheung WY. Improving the quality of abstract reporting for economic analyses in oncology. Curr Oncol. 2012;19(6):e428–35. doi:10.3747/co.19.1152
- Kumar S, Baldi A. Pharmacoeconomics: principles, methods and economic evaluation of drug therapies. Pharm Tech Med. 2013;2(5):362–369.
- Bracco A, Krol M. Economic evaluations in European reimbursement submission guidelines: current status and comparisons. Expert Rev Pharmacoecon Outcomes Res. 2013;13(5):579–595. doi:10.1586/14737167.2013.837766
- Zafari Z, Bryan S, Sin DD, Conte T, Khakban R, Sadatsafavi M. A systematic review of health economics simulation models of chronic obstructive pulmonary disease. Value Health. 2017;20(1):152–162. doi:10.1016/j.jval.2016.08.003
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi:10.1001/jama.2016.12195
- Ghadge O, Khale A. Pharmacoeconomics – a brief review. Indian Pharmacist. 2012:29–36.
- Zaidi S, Babar Z. Chapter 9 - Applying pharmacoeconomics in community and hospital pharmacy research. In: Adis, Cham, editor. Pharmacy Practice Research Methods. Springer International Publishing AG; 2015.doi:10.1007/978-3-319-14672-0_9
- Tömöri G, Bács Z. Application of cost analysis methods in pharmacoeconomic decisions. Proc Econ Financ. 2015;32:416–422. doi:10.1016/S2212-5671(15)01412-4
- Catić T. Pharmacoeconomics – short overview and perspectives in Bosnia and Herzegovina. Pharmacia. 2015;18(1):15–21.
- Kumar R. Health economics and cost-effectiveness research with special reference to hemato-oncology. Med J Armed Forces India. 2013;69(3):273–277. doi:10.1016/j.mjafi.2013.06.003
- Gangone S, Godala D, Kashetti SV. A review of pharmacoeconomics and a discussion on branded, branded generics and generics. Pharmacoeconomics. 2017;2(2):1–5. doi:10.4172/2472-1042.1000112
- National Cancer Institute. Available from: https://www.cancer.gov/about-cancer/understanding/what-is-cancer. Accessed January 29, 2020.
- Available from: https://ourworldindata.org/cancer#note-1. Accessed January 29, 2020.
- Parsonnet J. Bacterial infection as a cause of cancer. Environ Health Perspect. 1995;103 Suppl 8(Suppl8):263–268. doi:10.1289/ehp.95103s8263
- Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm. Accessed April 22, 2020.
- Keikha M, Esfahani BN. The relationship between tuberculosis and lung cancer. Adv Biomed Res. 2018;7:58. doi:10.4103/abr.abr_182_17
- Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/cancer/breast/basic_info/risk_factors.htm. Accessed April 22, 2020.
- Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;3418-9:21. doi:10.1200/JCO.2009.21.9782
- Gotland N, Hansen M-LU, Mejer N, et al. Increased risk of incident cancer after Staphylococcus aureus Bacteremia: a Matched Cohort Study. Open Forum Infect Dis. 2016;3(suppl_1). doi:10.1097/MD.0000000000019984
- Shui L, Yang X, Li J, Yi C, Sun Q, Zhu H. Gut microbiome as a potential factor for modulating resistance to cancer immunotherapy. Front Immunol. 2019;10:2989. doi:10.3389/fimmu.2019.02989
- World Health Organization. Cancer Mortality Database. Available from: https://www-dep.iarc.fr/WHOdb/WHOdb.htm. Accessed December 16, 2020.
- Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/cancer/colorectal/basic_info/risk_factors.htm. Accessed April 22, 2020.
- Antonic V, Stojadinovic A, Kester KE, et al. Significance of infectious agents in colorectal cancer development. Review. J Cancer. 2013;4(3):227–240. doi:10.7150/jca.5835
- World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed January 29, 2020.
- Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi:10.4103/aam.aam_56_18
- Gao Y, Chen Y, Cao Y, Mo A, Peng Q. Potentials of nanotechnology in treatment of methicillin-resistant Staphylococcus aureus. Eur J Med Chem. 2021;213:113056. doi:10.1016/j.ejmech.2020.113056
- Xia MY, Xie Y, Yu CH, et al. Graphene-based nanomaterials: the promising active agents for antibiotics-independent antibacterial applications. J Control Release. 2019;307:16–31. doi:10.1016/j.jconrel.2019.06.011
- Galar A, Weil AA, Dudzinski DM, Muñoz P, Siedner MJ. Methicillin-resistant Staphylococcus aureus prosthetic valve endocarditis: pathophysiology, epidemiology, clinical presentation, diagnosis, and management. Clin Microbiol Rev. 2019;32(2):e00041–18. doi:10.1128/CMR.00041-18
- Gerlach D, Guo Y, De Castro C, et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature. 2018;563(7733):705–709. doi:10.1038/s41586-018-0730-x
- Liu J, Dong J, Zhang T, Peng Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J Control Release. 2018;286:64–73. doi:10.1016/j.jconrel.2018.07.034
- Lu B-Y, Zhu G-Y, Yu C-H, et al. Functionalized graphene oxide nanosheets with unique three-in one properties for efficient and tunable antibacterial applications. Nano Res. 2021;14(1):185–190. doi:10.1007/s12274-020-3064-6
- Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42(2):199–209. doi:10.1093/jac/42.2.199
- Katayama Y, Sekine M, Hishinuma T, Aiba Y, Hiramatsu K. Complete reconstitution of the vancomycin-intermediate Staphylococcus aureus phenotype of strain Mu50 in vancomycin-susceptible S. aureus. Antimicrob Agents Chemother. 2016;60(6):3730–3742. doi:10.1128/AAC.00420-16
- Howden BP, Stinear TP, Allen DL, Johnson PD, Ward PB, Davies JK. Genomic analysis reveals a point mutation in the two-component sensor gene graS that leads to intermediate vancomycin resistance in clinical Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(10):3755–3762. doi:10.1128/AAC.01613-07
- Assis LM, Nedeljković M, Dessen A. New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist Update. 2017;31:1–14. doi:10.1016/j.drup.2017.03.001
- Zhou K, Li C, Chen DM, et al. A review on nanosystems as an effective approach against infections of Staphylococcus aureus. Int J Nanomedicine. 2018;13:7333–7347. doi:10.2147/IJN.S169935
- Wang L, Chen YP, Miller KP, et al. Functionalised nanoparticles complexed with antibiotic efficiently kill MRSA and other bacteria. Chem Commun (Camb). 2014;50(81):12030–12033. doi:10.1039/c4cc04936e
- Li H, Yin D, Li W, Tang Q, Zou L, Peng Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf B Biointerfaces. 2020;199:111502. doi:10.1016/j.colsurfb.2020.111502
- Al Attas SG, Al-Hazmi F, Alwafi R. Bactericidal efficacy of new types of magnesium hydroxide and calcium carbonate nanoparticles. Mol Genet Microbiol Virol. 2019;34:252–262. doi:10.3103/S0891416819040086
- Safarov T, Kiran B, Bagirova M, Allahverdiyev AM, Abamor ES. An overview of nanotechnology-based treatment approaches against Helicobacter Pylori. Expert Rev Anti Infect Ther. 2019;17(10):829–840. doi:10.1080/14787210.2019.1677464
- Zhang Q, Wu W, Zhang J, Xia X. Eradication of Helicobacter pylori: the power of nanosized formulations. Nanomedicine (Lond). 2020;15(5):527–542. doi:10.2217/nnm-2019-0329
- Chen Y, Gao Y, Liu L, Mo A, Peng Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Control Release. 2020;328:251–262. doi:10.1016/j.jconrel.2020.08.055
- Guo P, Xue HY, Buttaro BA, Tran NT, Wong HL. Enhanced eradication of intracellular and biofilm-residing methicillin-resistant Staphylococcus aureus (MRSA) reservoirs with hybrid nanoparticles delivering rifampicin. Int J Pharm. 2020;589:119784. doi:10.1016/j.ijpharm.2020.119784
- Zorraquín-Peña I, Cueva C, González de Llano D, Bartolomé B, Moreno-Arribas MV. Glutathione-stabilized silver nanoparticles: antibacterial activity against periodontal bacteria, and cytotoxicity and inflammatory response in oral cells. Biomedicines. 2020;8(10):375. doi:10.3390/biomedicines8100375
- Nam G, Rangasamy S, Purushothaman B, Song JM. The application of bactericidal silver nanoparticles in wound treatment. Nanomater Naotechnol. 2015;5(23):1–14. doi:10.5772/60918
- World Health Organization. Available from: https://www.who.int/cancer/detection/variouscancer/en/. Accessed January 29, 2020.
- Zhang Q, Zhang F, Li S, et al. A multifunctional nanotherapy for targeted treatment of colon cancer by simultaneously regulating tumor microenvironment. Theranostics. 2019;9(13):3732–3753. doi:10.7150/thno.34377
- Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi:10.1038/nrc.2016.108
- Jain KK. Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert Rev Mol Diagn. 2003;3(2):153–161. doi:10.1586/14737159.3.2.153
- Fortina P, Kricka LJ, Surrey S, Grodzinski P. Nanobiotechnology: the promise and reality of new approaches to molecular recognition. Trends Biotechnol. 2005;23(4):168–173. doi:10.1016/j.tibtech.2005.02.007
- Wu G, Datar RH, Hansen KM, Thundat T, Cote RJ, Majumdar A. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nat Biotechnol. 2001;19(9):856–860. doi:10.1038/nbt0901-856
- West JL, Halas NJ. Engineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeutics. Annu Rev Biomed Eng. 2003;5:285–292. doi:10.1146/annurev.bioeng.5.011303.120723
- Jain KK. Nanotechnology in clinical laboratory diagnostics. Clin Chim Acta. 2005;358(1–2):37–54. doi:10.1016/j.cccn.2005.03.014
- Storhoff JJ, Lucas AD, Garimella V, Bao YP, Müller UR. Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat Biotechnol. 2004;22(7):883–887. doi:10.1038/nbt977
- Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):1–6. doi:10.1186/1477-3155-2-3
- Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005;23(10):1294–1301. doi:10.1038/nbt1138
- Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol. 2001;19(7):631–635. doi:10.1038/90228
- Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21(1):41–46. doi:10.1038/nbt764
- Din FU, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine. 2017;12:7291–7309. doi:10.2147/IJN.S146315
- Ahmed N, Mora-Huertas CE, Jaafar-Maalej C, Fessi H, Elaissari A. Polymeric drug delivery systems for encapsulating hydrophobic drugs. In: Drug Delivery Strategies for Poorly Water-Soluble Drugs (Advances in Pharmaceutical Technology). 1st ed. Willey; 2013: chap 5.
- Onetto N, Canetta R, Winograd B, et al. Overview of Taxol safety. J Natl Cancer Inst Monogr. 1993;15:131–139.
- Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5(5):442–453. doi:10.1016/j.apsb.2015.07.003
- Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4(4):403–416. doi:10.1517/17425247.4.4.403
- van Hoogevest P, Liu X, Fahr A. Drug delivery strategies for poorly water-soluble drugs: the industrial perspective. Expert Opin Drug Deliv. 2011;8(11):1481–1500. doi:10.1517/17425247.2011.614228
- Markowicz-Piasecka M, Mikiciuk-Olasik E. Chapter 2 - Dendrimers in drug delivery. In: Grumezescu AM, editor. Nanobiomaterials in Drug Delivery Applications of Nanobiomaterials. Elsevier Ltd.; 2016.
- Yang G, Liu Y, Wang H, et al. Bioinspired core-shell nanoparticles for hydrophobic drug delivery. Angew Chem Int Ed Engl. 2019;58(40):14357–14364. doi:10.1002/anie.201908357
- Larrañeta E, Stewart S, Ervine M, Al-Kasasbeh R, Donnelly RF. Hydrogels for hydrophobic drug delivery. Classification, synthesis and applications. J Funct Biomater. 2018;9(1):13. doi:10.3390/jfb9010013
- Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–1198. doi:10.1111/jphp.13098
- Rabanel JM, Aoun V, Elkin I, Mokhtar M, Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr Med Chem. 2012;19(19):3070–3102. doi:10.2174/092986712800784702
- Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood-brain barrier. Int J Nanomedicine. 2014;9:795–811. doi:10.2147/IJN.S52236
- Narmani A, Rezvani M, Farhood B, et al. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res. 2019;80(4):404–424. doi:10.1002/ddr.21545
- Hsiao C-J, Lin J-F, Wen H-Y, et al. Enhancement of the stability of chlorophyll using chlorophyll-encapsulated polycaprolactone microparticles based on droplet microfluidics. Food Chem. 2020;306:1–6. doi:10.1016/j.foodchem.2019.125300
- Uthappaa UT, Kigga M, Sriram G, et al. Facile green synthetic approach of bio inspired polydopamine coated diatoms as a drug vehicle for controlled drug release and active catalyst for dye degradation. Microporous Mesoporous Mater. 2019;288:1–10. doi:10.1016/j.micromeso.2019.109572
- Bruschi ML. Chapter 6 - Drug delivery systems: principles, local of administration, materials, characterization, applications, advances and the use of natural products. In: Bruschi ML, editor. Strategies to Modify the Drug Release from Pharmaceutical Systems. 1st ed. Elsevier; 2015.
- Midha K, Diwan P, Marwah S, Sood A, Arora G. Nanocarrier: a boom or a bane in the medical industry. Acta Sci Pharm Sci. 2019;3(5):8–23.
- Hassan ME, Yang Q, Xiao Z, et al. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech. 2019;9(12):1–16. doi:10.1007/s13205-019-1969-0
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315.
- Villamizar-Sarmiento MG, Molina-Soto EF, Guerrero J, et al. A new methodology to create polymeric nanocarriers containing hydrophilic low molecular-weight drugs: a green strategy providing a very high drug loading. Mol Pharm. 2019;16(7):2892–2901. doi:10.1021/acs.molpharmaceut.9b00097
- Kanwar R, Gradzielski M, Prevost S, Kaur G, Appavou MS, Mehta SK. Physicochemical stimuli as tuning parameters to modulate the structure and stability of nanostructured lipid carriers and release kinetics of encapsulated antileprosy drugs. J Mater Chem B. 2019;7(42):6539–6555. doi:10.1039/c9tb01330j
- Moustafa A, Moustafa MMAR, Zilinskas GJ, Gillies ER. Covalent drug immobilization in poly(ester amide) nanoparticles for controlled release. Can J Chem Eng. 2015;93(12):2098–2106. doi:10.1002/cjce.22323
- Goyal AK, Rath G, Faujdar C, Malik B. Chapter 2 - Application and perspective of pH-responsive nano drug delivery systems. In: Mohapatra S, Ranjan S, Dasgupta N, Mishra R, Thomas S, editors. Applications of Targeted Nano Drugs and Delivery System Nanoscience and Nanotechnology in Drug Delivery. 1st ed. Elsevier; 2019.
- Bikram M, West JL. Thermo-responsive systems for controlled drug delivery. Expert Opin Drug Deliv. 2008;5(10):1077–1091. doi:10.1517/17425247.5.10.1077
- Garcia M. 14 - Ionic-strength-responsive polymers for drug delivery applications. In: Makhlouf ASH, Abu-Thabit NY, editors. Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications Volume 2: Advanced Nanocarriers for Therapeutics. 1st ed. Elsevier; 2019.
- Guo L, Xu Y, Zhou A, et al. A stimuli-responsive combination therapy for recovering p53-inactivation associated drug resistance. Mater Sci Eng C. 2020;108:1–11. doi:10.1016/j.msec.2019.110403
- Razavi B, Abdollahi A, Roghani-Mamaqani H, Salami-Kalajahi M. Light-, temperature-, and pH-responsive micellar assemblies of spiropyran-initiated amphiphilic block copolymers: kinetics of photochromism, responsiveness, and smart drug delivery. Mater Sci Eng C Mater Biol Appl. 2020;109:110524. doi:10.1016/j.msec.2019.110524
- Karimi M, Zangabad PS, Mehdizadeh F, et al. Nanocaged platforms: modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale. 2017;9(4):1356–1392. doi:10.1039/c6nr07315h
- Shen S, Wu Y, Liu Y, Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine. 2017;12:4085–4109. doi:10.2147/IJN.S132780
- Mitra A, Lee CH, Cheng K. Advanced Drug Delivery. Wiley; 2013:550.
- Loc WS, Linton SS, Wilczynski ZR, et al. Effective encapsulation and biological activity of phosphorylated chemotherapeutics in calcium phosphosilicate nanoparticles for the treatment of pancreatic cancer. Nanomedicine. 2017;13(7):2313–2324. doi:10.1016/j.nano.2017.06.017
- Zhao H, Rubio B, Sapra P, et al. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem. 2008;19(4):849–859. doi:10.1021/bc700333s
- Tian R, Wang H, Niu R, Ding D. Drug delivery with nanospherical supramolecular cell penetrating peptide-taxol conjugates containing a high drug loading. J Colloid Interface Sci. 2015;453:15–20. doi:10.1016/j.jcis.2015.04.028
- Phan UT, Nguyen KT, Vo TV, Duan W, Tran PH, Tran TD. Investigation of Fucoidan-Oleic acid conjugate for delivery of Curcumin and Paclitaxel. Anticancer Agents Med Chem. 2016;16(10):1281–1287. doi:10.2174/1567201810666131124140259
- Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine. 2018;14(5):1629–1641. doi:10.1016/j.nano.2018.04.009
- Han HS, Choi KY, Ko H, et al. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J Control Release. 2015;200:158–166. doi:10.1016/j.jconrel.2014.12.032
- Tran TTD, Tran PHL. Nanoconjugation and encapsulation strategies for improving drug delivery and therapeutic efficacy of poorly water-soluble drugs. Pharmaceutics. 2019;11(7):1–41. doi:10.3390/pharmaceutics11070325
- Krukiewicz K, Stokfisz A, Zak JK. Two approaches to the model drug immobilization into conjugated polymer matrix. Mater Sci Eng C Mater Biol Appl. 2015;54:176–181. doi:10.1016/j.msec.2015.05.017
- Perotto G, Sandri G, Pignatelli C, Milanesiband G, Athanassiou A. Water-based synthesis of keratin micro- and nanoparticles with tunable mucoadhesive properties for drug delivery. J Mater Chem B. 2019;7(28):4385–4392. doi:10.1039/C9TB00443B
- Nagy NZ, Varga Z, Mihály J, Kasza G, Iván B, Kiss É. Highly efficient encapsulation of curcumin into and pH-controlled drug release from poly(ε-caprolactone) nanoparticles stabilized with a novel amphiphilic hyperbranched polyglycerol. Express Polym Lett. 2020;14(1):90–101. doi:10.3144/expresspolymlett.2020.8
- Khan DH, Bashir S, Correia A, et al. Utilization of green formulation technique and efficacy estimation on cell line studies for dual anticancer drug therapy with niosomes. Int J Pharm. 2019;572:1–41. doi:10.1016/j.ijpharm.2019.118764
- Taymaz-Nikerel H, Karabekmez ME, Eraslan S, Kırdar B. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci Rep. 2018;8(1):13672. doi:10.1038/s41598-018-31939-9
- Lelle M, Freidel C, Kaloyanova S, et al. Overcoming drug resistance by cell-penetrating peptide-mediated delivery of a doxorubicin dimer with high DNA-binding affinity. Eur J Med Chem. 2017;130:336–345. doi:10.1016/j.ejmech.2017.02.056
- Chen Y, Sun L, Guo D, Wu Z, Chen W. Co-delivery of hypoxia inducible factor-1α small interfering RNA and 5-fluorouracil to overcome drug resistance in gastric cancer SGC-7901 cells. J Gene Med. 2017;19(12):e2998. doi:10.1002/jgm.2998
- Chen L, She X, Wang T, et al. Overcoming acquired drug resistance in colorectal cancer cells by targeted delivery of 5-FU with EGF grafted hollow mesoporous silica nanoparticles. Nanoscale. 2015;7(33):14080–14092. doi:10.1039/c5nr03527a
- Kampan NC, Madondo MT, McNally OM, Quinn M, Plebanski M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed Res Int. 2015;2015:413076. doi:10.1155/2015/413076
- Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi:10.1091/mbc.E14-04-0916
- Arora HC, Jensen MP, Yuan Y, et al. Nanocarriers enhance Doxorubicin uptake in drug-resistant ovarian cancer cells. Cancer Res. 2012;72(3):769–778. doi:10.1158/0008-5472.CAN-11-2890
- Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–160. doi:10.20517/cdr.2019.10
- Cagan R, Meyer P. Rethinking cancer: current challenges and opportunities in cancer research. Dis Model Mech. 2017;10(4):349–352. doi:10.1242/dmm.030007
- Abdifetah O, Na-Bangchang K. Pharmacokinetic studies of nanoparticles as a delivery system for conventional drugs and herb-derived compounds for cancer therapy: a systematic review. Int J Nanomedicine. 2019;14:5659–5677. doi:10.2147/IJN.S213229
- El-Readi MZ, Althubiti AM. Cancer nanomedicine: a new era of successful targeted therapy. J Nanomater. 2019;2019:13. doi:10.1155/2019/4927312
- Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7(3):339–348. doi:10.15171/apb.2017.041
- Wang Y, Li J, Chen JJ, Gao X, Huang Z, Shen Q. Multifunctional nanoparticles loading with docetaxel and GDC0941 for reversing multidrug resistance mediated by PI3K/Akt signal pathway. Mol Pharm. 2017;14(4):1120–1132. doi:10.1021/acs.molpharmaceut.6b01045
- Radu IC, Hudita A, Zaharia C, et al. Poly(3-hydroxybutyrate-CO-3-hydroxyvalerate) PHBHV biocompatible nanocarriers for 5-FU delivery targeting colorectal cancer. Drug Deliv. 2019;26(1):318–327. doi:10.1080/10717544.2019.1582729
- Arranja AG, Pathak V, Lammers T, Shi Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol Res. 2017;115:87–95. doi:10.1016/j.phrs.2016.11.014
- Wang K, Wang S, Chen K, Zhao Y, Ma X, Wang L. Doxorubicin-loaded melanin particles for enhanced chemotherapy in drug-resistant anaplastic thyroid cancer cells. J Nanomater. 2018. doi:10.1155/2018/2603712
- Hong YD, Zhang J, Zhuang M, et al. Efficacy of decitabine-loaded gelatinases-stimuli nanoparticles in overcoming cancer drug resistance is mediated via its enhanced demethylating activity to transcription factor AP-2 epsilon. Oncotarget. 2017;8(70):114495–114505. doi:10.18632/oncotarget.21274
- Reshma PL, Unnikrishnan BS, Preethi GU, et al. Overcoming drug-resistance in lung cancer cells by paclitaxel loaded galactoxyloglucan nanoparticles. Int J Biol Macromol. 2019;136:266–274. doi:10.1016/j.ijbiomac.2019.06.075
- Jackson J, Leung D, Burt H. The use of ultrasound to increase the uptake and cytotoxicity of dual taxane and P-glycoprotein inhibitor loaded, solid core nanoparticles in drug resistant cells. Ultrasonics. 2020;101:106033. doi:10.1016/j.ultras.2019.106033
- Baek H, Cho M, Kim S, Hwang H, Song M, Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS One. 2018;13(4):e0195901. doi:10.1371/journal.pone.0195901
- Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol. 2019;54(2):407–419. doi:10.3892/ijo.2018.4661
- Rau KM, Lin YC, Chen YY, et al. Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study. BMC Cancer. 2015;15:423. doi:10.1186/s12885-015-1433-4
- Pircher M, Mlineritsch B, Fridrik MA, et al. Lapatinib-plus-pegylated liposomal doxorubicin in advanced HER2-positive breast cancer following trastuzumab: a phase II trial. Anticancer Res. 2015;35(1):517–521.
- Sparano JA, Makhson AN, Semiglazov VF, et al. Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: results from a randomized Phase III study. J Clin Oncol. 2009;27(27):4522–4529. doi:10.1200/JCO.2008.20.5013
- Basso U, Roma A, Brunello A, et al. Bi-weekly liposomal doxorubicin for advanced breast cancer in elderly women (≥ 70 years). J Geriatr Oncol. 2013;4(4):340–345. doi:10.1016/j.jgo.2013.07.004
- Addeo R, Faiola V, Guarrasi R, et al. Liposomal pegylated doxorubicin plus vinorelbine combination as first-line chemotherapy for metastatic breast cancer in elderly women > or = 65 years of age. Cancer Chemother Pharmacol. 2008;62(2):285–292. doi:10.1007/s00280-007-0605-6
- Zhao N, Woodle MC, Mixson AJ. Advances in delivery systems for doxorubicin. J Nanomed Nanotechnol. 2018;9(5):1–9. doi:10.4172/2157-7439.1000519
- Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp Hematol Oncol. 2012;1(1):10. doi:10.1186/2162-3619-1-10
- Jeong YI, Jin SG, Kim IY, et al. Doxorubicin-incorporated nanoparticles composed of poly(ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloids Surf B Biointerfaces. 2010;79(1):149–155. doi:10.1016/j.colsurfb.2010.03.037
- Xiong H, Zhou D, Qi Y, et al. Doxorubicin-loaded carborane-conjugated polymeric nanoparticles as delivery system for combination cancer therapy. Biomacromolecules. 2015;16(12):3980–3988. doi:10.1021/acs.biomac.5b01311
- Zhang Y, Yang C, Wang W, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep. 2016;6:21225. doi:10.1038/srep21225
- Liu C, Liu F, Feng L, Li M, Zhang J, Zhang N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials. 2013;34(10):2547–2564. doi:10.1016/j.biomaterials.2012.12.038
- Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi:10.1016/j.biomaterials.2013.11.083
- Hadla M, Palazzolo S, Corona G, et al. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine (Lond). 2016;11(18):2431–2441. doi:10.2217/nnm-2016-0154
- Wuang SC, Neoh KG, Kang ET, Leckband DE, Pack DW. Acid-sensitive magnetic nanoparticles as potential drug depots. AiChE J. 2011;57(6):1638–1645. doi:10.1002/aic.12373
- Gautier J, Munnier E, Paillard A, et al. A pharmaceutical study of doxorubicin-loaded PEGylated nanoparticles for magnetic drug targeting. Int J Pharm. 2012;423(1):16–25. doi:10.1016/j.ijpharm.2011.06.010
- Chandran SP, Natarajan SB, Chandraseharan S, Shahimi MSBM. Nano drug delivery strategy of 5-fluorouracil for the treatment of colorectal cancer. J Cancer Res Pract. 2017;4:45–48. doi:10.1016/j.jcrpr.2017.02.002
- Subudhi MB, Jain A, Hurkat P, Shilpi S, Gulbake A, Jain SK. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-Fluorouracil. Materials (Basel). 2015;8(3):832–849. doi:10.3390/ma8030832
- Patel MN, Lakkadwala S, Majrad MS, et al. Characterization and evaluation of 5-fluorouracil-loaded solid lipid nanoparticles prepared via a temperature-modulated solidification technique. AAPS PharmSciTech. 2014;15(6):1498–1508. doi:10.1208/s12249-014-0168-x
- Yassin AE, Anwer MK, Mowafy HA, El-Bagory IM, Bayomi MA, Alsarra IA. Optimization of 5-fluorouracil solid-lipid nanoparticles: a preliminary study to treat colon cancer. Int J Med Sci. 2010;7(6):398–408. doi:10.7150/ijms.7.398
- Wang JJ, Zeng ZW, Xiao RZ, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765–774. doi:10.2147/IJN.S17296
- Tummala S, Satish Kumar MN, Prakash A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm J. 2015;23(3):308–314. doi:10.1016/j.jsps.2014.11.010
- Wang Y, Li P, Chen L, Gao W, Zeng F, Kong LX. Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles. Drug Deliv. 2015;22(2):191–198. doi:10.3109/10717544.2013.875603
- Ma P, Mumper RJ. Paclitaxel nano-delivery systems: a Comprehensive Review. J Nanomed Nanotechnol. 2013;4(2):1–35. doi:10.4172/2157-7439.1000164
- Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS PharmSciTech. 2008;9(2):486–493. doi:10.1208/s12249-008-9063-7
- Yoshizawa Y, Kono Y, Ogawara K, Kimura T, Higaki K. PEG liposomalization of paclitaxel improved its in vivo disposition and anti-tumor efficacy. Int J Pharm. 2011;412(1–2):132–141. doi:10.1016/j.ijpharm.2011.04.008
- Jin C, Wu H, Liu J, Bai L, Guo G. The effect of paclitaxel-loaded nanoparticles with radiation on hypoxic MCF-7 cells. J Clin Pharm Ther. 2007;32(1):41–47. doi:10.1111/j.1365-2710.2007.00796.x
- Jin C, Bai L, Wu H, Liu J, Guo G, Chen J. Paclitaxel-loaded poly(D,L-lactide-co-glycolide) nanoparticles for radiotherapy in hypoxic human tumor cells in vitro. Cancer Biol Ther. 2008;7(6):911–916. doi:10.4161/cbt.7.6.5912
- Danhier F, Lecouturier N, Vroman B, et al. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Control Release. 2009;133(1):11–17. doi:10.1016/j.jconrel.2008.09.086
- Dong Y, Feng SS. Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int J Pharm. 2007;342(1–2):208–214. doi:10.1016/j.ijpharm.2007.04.031
- Chakravarthi SS, Robinson DH. Enhanced cellular association of paclitaxel delivered in chitosan-PLGA particles. Int J Pharm. 2011;409(1–2):111–120. doi:10.1016/j.ijpharm.2011.02.034
- Mei L, Sun H, Jin X, et al. Modified paclitaxel-loaded nanoparticles for inhibition of hyperplasia in a rabbit arterial balloon injury model. Pharm Res. 2007;24(5):955–962. doi:10.1007/s11095-006-9214-z
- Zhao L, Feng SS. Enhanced oral bioavailability of paclitaxel formulated in vitamin E-TPGS emulsified nanoparticles of biodegradable polymers: in vitro and in vivo studies. J Pharm Sci. 2010;99(8):3552–3560. doi:10.1002/jps.22113
- Liu Y, Pan J, Feng SS. Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: effects of surfactants on particles size, characteristics and in vitro performance. Int J Pharm. 2010;395(1–2):243–250. doi:10.1016/j.ijpharm.2010.05.008
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03505528?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=1. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02646319?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=2. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02442531?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=3. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03304210?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=4. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02227940?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=5. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03120832?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=7. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02392637?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=6. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02820454?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=8. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02762981?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=9. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03101358?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=10. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03712423?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=11. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02668536?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=12. Accessed December 09, 2020.
- National Institutes of Health (NIH) U.S. National Library of Medicine ClinicalTrialsgov. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03020017?term=nanoparticle&recrs=e&cond=Cancer&strd_s=01%2F01%2F2015&strd_e=09%2F12%2F2020&draw=2&rank=13. Accessed December 09, 2020.
- Worsham RD, Thomas V, Farid SS. Potential of continuous manufacturing for liposomal drug products. Biotechnol J. 2019;14(2):e1700740. doi:10.1002/biot.201700740
- Verma RK, Garg S. Current status of drug delivery technologies and future directions. Pharm Technol On-Line. 2001;25(2):1–14.
- Ding X, Miller PG, Hwang MP, Fu J, Wang Y. Scale-up synthesis of a polymer designed for protein therapy. Eur Polym J. 2019;117:353–362. doi:10.1016/j.eurpolymj.2019.05.032
- Galindo-Rodríguez SA, Puel F, Briançon S, Allémann E, Doelker E, Fessi H. Comparative scale-up of three methods for producing ibuprofen-loaded nanoparticles. Eur J Pharm Sci. 2005;25(4–5):357–367. doi:10.1016/j.ejps.2005.03.013
- Rafiee Z, Jafari SM. Application of Lipid Nanocarriers for the Food Industry. Cham: Springer; 2019:623–665.
- Bajracharya R, Song JG, Back SY, Han HK. Recent advancements in non-invasive formulations for protein drug delivery. Comput Struct Biotechnol J. 2019;17:1290–1308. doi:10.1016/j.csbj.2019.09.004
- Cheng Z, Zaki AA, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338(6109):903–910. doi:10.1126/science.1226338
- Stearns LJ, Narang S, Albright RE, et al. Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain. JAMA Network Open. 2019;2(4):1–14. doi:10.1001/jamanetworkopen.2019.1549
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):1–33. doi:10.1186/s12951-018-0392-8
- Muthu MS, Wilson B. Challenges posed by the scale-up of nanomedicines. Nanomedicine (Lond). 2012;7(3):307–309. doi:10.2217/nnm.12.3
- Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. Pharm Ther. 2017;42(12):742–755.
- Landesman-Milo D, Peer D. Transforming nanomedicines from lab scale production to novel clinical modality. Bioconjug Chem. 2016;27(4):855–862. doi:10.1021/acs.bioconjchem.5b00607
- Fernández-García R, Lalatsa A, Statts L, Bolás-Fernández F, Ballesteros MP, Serrano DR. Transferosomes as nanocarriers for drugs across the skin: quality by design from lab to industrial scale. Int J Pharm. 2020;573:1–44. doi:10.1016/j.ijpharm.2019.118817
- Çalış S, Öztürk K, Fatma A, Arslan B, Eroğlu H, Çapan Y. Chapter 4 - Nanopharmaceuticals as drug delivery systems: for, against, and current applications. In: Mohapatra S, Ranjan S, Dasgupta N, Mishra R, Thomas S, editors. Nanocarriers for Drug Delivery Nanoscience and Nanotechnology in Drug Deliver. Elsevier.
- Wu LP, Wang D, Li Z. Grand challenges in nanomedicine. Mater Sci Eng C. 2020;106:1–7. doi:10.1016/j.msec.2019.110302
- Dormont F, Rouquette M, Mahatsekake C, et al. Translation of nanomedicines from lab to industrial scale synthesis: the case of squalene-adenosine nanoparticles. J Control Release. 2019;307:302–314. doi:10.1016/j.jconrel.2019.06.040
- Alvarez MM, Aizenberg J, Analoui M, et al. Emerging trends in micro- and nanoscale technologies in medicine: from basic discoveries to translation. ACS Nano. 2017;11(6):5195–5214. doi:10.1021/acsnano.7b01493
- Colombo AP, Briançon S, Lieto J, Fessi H. Project, design, and use of a pilot plant for nanocapsule production. Drug Dev Ind Pharm. 2001;27(10):1063–1072. doi:10.1081/ddc-100108369
- Flegler A, Wintzheimer S, Schneider M, Gellermann C, Mandel K. Chapter 7 - Tailored nanoparticles by wet chemical particle technology: from Lab to Pilot Scale. In: Hussain CM, editor. Handbook of Nanomaterials for Industrial Applications Micro and Nano Technologies. Elsevier; 2018. doi:10.1016/B978-0-12-813351-4.00007-9
- Venditto VJ, Szoka FC. Cancer nanomedicines: so many papers and so few drugs! Adv Drug Deliv Rev. 2013;65(1):80–88. doi:10.1016/j.addr.2012.09.038
- Katouzian I, Jafari SM. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci Technol. 2016;53:34–48. doi:10.1016/j.tifs.2016.05.002
- Sun Q, Barz M, De Geest BG, et al. Nanomedicine and macroscale materials in immuno-oncology. Chem Soc Rev. 2019;48(1):351–381. doi:10.1039/c8cs00473k
- Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014;15(6):1527–1534. doi:10.1208/s12249-014-0177-9
- Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomedicine. 2017;12:2957–2978. doi:10.2147/IJN.S127683
- Kanwar R, Rathee J, Salunke DB, Mehta SK. Green nanotechnology-driven drug delivery assemblies. ACS Omega. 2019;4(5):8804–8815. doi:10.1021/acsomega.9b00304
- Zhong D, Wu H, Wu Y, et al. Rational design and facile fabrication of biocompatible triple responsive dendrimeric nanocages for targeted drug delivery. Nanoscale. 2019;11(32):15091–15103. doi:10.1039/c9nr04631c
- Palai PK, Mondal A, Chakraborti CK, Banerjee I, Pal K, Rathnam VSS. Doxorubicin loaded green synthesized Nanoceria decorated functionalized graphene nanocomposite for cancer-specific drug release. J Cluster Sci. 2019;30:1565–1582. doi:10.1007/s10876-019-01599-4
- Hasan I, Quais FA, Husain FM, et al. Eco-friendly green synthesis of dextrin based poly (methyl methacrylate) grafted silver nanocomposites and their antibacterial and antibiofilm efficacy against multi-drug resistance pathogens. J Clean Prod. 2019;230:1148–1155. doi:10.1016/j.jclepro.2019.05.157
- Huang W, Wu X, Qi J, et al. Ionic liquids: green and tailor-made solvents in drug delivery. Drug Discov Today. 2020;25(5):901–908. doi:10.1016/j.drudis.2019.09.018
- Thakur N, Sharma B, Bishnoi S, Jain S, Nayak D, Sarma TK. Biocompatible Fe3+ and Ca2+ dual cross-linked G-quadruplex hydrogels as effective drug delivery system for pH-responsive sustained zero-order release of doxorubicin. ACS Appl Bio Mater. 2019;2(8):3300–3311. doi:10.1021/acsabm.9b00334
- Di L, Kerns EH, Carter GT. Drug-like property concepts in pharmaceutical design. Curr Pharm Des. 2009;15(19):2184–2194. doi:10.2174/138161209788682479
- Gali-Muhtasib H, Chouaib C. Nanoparticle Drug Delivery Systems for Cancer Treatment. 1st ed. Jenny Stanford Publishing; 2020:342.
- Petschauer JS, Madden AJ, Kirschbrown WP, Song G, Zamboni WC. The effects of nanoparticle drug loading on the pharmacokinetics of anticancer agents. Nanomedicine (Lond). 2015;10(3):447–463. doi:10.2217/nnm.14.179
- Dogra P, Butner JD, Ruiz Ramírez J, et al. A mathematical model to predict nanomedicine pharmacokinetics and tumor delivery. Comput Struct Biotechnol J. 2020;18:518–531. doi:10.1016/j.csbj.2020.02.014
- Garofalo M, Grazioso G, Cavalli A, Sgrignani J. How computational chemistry and drug delivery techniques can support the development of new anticancer drugs. Molecules. 2020;25(7):1756. doi:10.3390/molecules25071756
- Zhang T, Zhu G, Lu B, Qian Z, Peng Q. Protein corona formed in the gastrointestinal tract and its impacts on oral delivery of nanoparticles. Med Res Rev. 2021;41(3):1835–1850. doi:10.1002/med.21767
- Peng Q, Liu J, Zhang T, Zhang TX, Zhang CL, Mu H. Digestive enzyme corona formed in the gastrointestinal tract and its impact on epithelial cell uptake of nanoparticles. Biomacromolecules. 2019;20(4):1789–1797. doi:10.1021/acs.biomac.9b00175
- Peng Q, Mu H. The potential of protein-nanomaterial interaction for advanced drug delivery. J Control Release. 2016;225:121–132. doi:10.1016/j.jconrel.2016.01.041
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi:10.1016/j.arabjc.2017.05.011
- Lauterwasser C. Small sizes that matter: Opportunities and risks of Nanotechnologies. Report in co-operation with the OECD International Futures Programme. Allianz Center for Technology. Available from: https://www.oecd.org/science/nanosafety/44108334.pdf. Accessed February 06, 2021.
- Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72(21):5566–5575. doi:10.1158/0008-5472.CAN-12-1683
- Lamberti M, Zappavigna S, Sannolo N, Porto S, Caraglia M. Advantages and risks of nanotechnologies in cancer patients and occupationally exposed workers. Expert Opin Drug Deliv. 2014;11(7):1087–1101. doi:10.1517/17425247.2014.913568
- De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3(2):133–149. doi:10.2147/ijn.s596
- Hua S, de Matos MBC, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. doi:10.3389/fphar.2018.00790
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822. doi:10.1126/science.1095833
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi:10.1038/sj.clpt.6100400
- Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14(2):303–315. doi:10.1208/s12248-012-9330-0
- Narang AS, Chang RK, Hussain MA. Pharmaceutical development and regulatory considerations for nanoparticles and nanoparticulate drug delivery systems. J Pharm Sci. 2013;102(11):3867–3882. doi:10.1002/jps.23691