 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Novel panax notoginsenoside-loaded core-shell hybrid liposomal vesicles (PNS-HLV) were developed to resolve the restricted bioavailability of PNS and to enhance its protective effects in vivo on oral administration.
Methods
Physicochemical characterizations of PNS-HLV included assessment of morphology, particle size and zeta potential, encapsulation efficiency (EE%), stability and in vitro release study. In addition, to evaluate its oral treatment potential, we compared the effect of PNS-HLV on global cerebral ischemia/reperfusion and acute myocardial ischemia injury with those of PNS solution, conventional PNS-loaded nanoparticles, and liposomes.
Results
In comparison with PNS solution, conventional PNS-loaded nanoparticles and liposomes, PNS-HLV was stable for at least 12 months at 4°C. Satisfactory improvements in the EE% of notoginsenoside R1, ginsenoside Rb1, and ginsenoside Rg1 were shown with the differences in EE% shortened and the greater controlled drug release profiles were exhibited from PNS-HLV. The improvements in the physicochemical properties of HLV contributed to the results that PNS-HLV was able to significantly inhibit the edema of brain and reduce the infarct volume, while it could markedly inhibit H2O2, modified Dixon agar, and serum lactate dehydrogenase, and increase superoxide dismutase (P < 0.05).
Conclusion
The results of the present study imply that HLV has promising prospects for improving free drug bioactivity on oral administration.
Introduction
The herbal formulation Panax notoginseng (PN) is derived from the root of the traditional Chinese herb P. notoginseng, which exhibits activities in modulating vascular tone such as promotion of blood circulation, removal of blood stasis, and alleviation of pain.Citation1 Furthermore, PN also decreased the plasma lipid level and fibrinogen in high-fat rats.Citation2 Panax notoginsenoside (PNS) is the major active component of PN, including notoginsenoside R1 (R1, 2.74%), ginsenoside Rb1 (Rb1, 29.86%), ginsenoside Rg1 (Rg1, 20.46%), and ginsenoside Rd (Rd, 7.96%).Citation3 Several studies on the physiological effects of PNS have been reported, such as anti-inflammatory action, vasodilator effect, protective effect on microcirculatory disturbance induced by lipopolysaccharides, hepatic fibrosis, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity and antioxidant effect by increasing the superoxidase activity in the blood.Citation4–Citation7 PNS, in addition, can promote the synthesis of protein and DNA, modulate emotional responses in rats, and their metabolites show anticancer action.Citation8,Citation9 The antioxidant properties of the PN extract are one reason why they are pharmacologically useful. It was reported that the extract of PN was highly effective in chelating ferrous iron and scavenging hydroxyl radicals and hydrogen peroxide.Citation10,Citation11
However, PNS is poorly absorbed when administrated orally. Little R1 and Rb1 were absorbed from the digestive tract by oral administration to rats.Citation12,Citation13 It was also reported that the amount of Rg1 absorbed via oral administration was within 1.9%–20.0% of the dose.Citation14 The low bioavailability of PNS could be explained by the following reasons: (1) the decomposition in the stomach, metabolism in the intestine, and elimination in the liver;Citation15,Citation16 (2) PNS are highly water-soluble substances with high molecular weight, which lead to low membrane permeability; (3) Rg1 and Rb1 can aggregate into micelles in PNS aqueous solution and be salted out in gastrointestinal tract fluid which contains electrolytes. Such an aggregation limits the permeation of PNS through the cell membrane of the gastrointestinal tract.Citation17 To overcome these obstacles, PNS delivery using novel drug carriers will likely yield more promising clinical applications.
Accordingly, several alternative formulations were investigated for the sustained release, stability, bioavailability of PNS, such as phospholipids complex, microspheres, liposomes, osmotic pump tablets, and microemulsion.Citation17,Citation18 Most of the work focused on the individual components of PNS such as Rg3, Rg1, and Rb1. To our knowledge, there are few reports on designing optimal drug carriers with high entrapment efficiency and controlled release profile for the principal components of PNS, which should be able to be entrapped at the original ratio in herbal medicine and exhibit synergistic effect for therapy.
Liposomes have been extensively considered as useful carriers for the delivery of therapeutic agents because of their unique properties such as biocompatibility, biodegradability, variable particle size, surface charge, and membrane fluidity.Citation19 However, liposomes are generally rather unstable and tend to degrade or aggregate and fuse, which leads to leakage of entrapped drug during storage or after administration. Therefore, modifying the liposomal surface with a single layer of hydrophilic polymers or polyelectrolytes or varying the size of liposomes has been investigated.Citation20–Citation22
Nanoparticle systems have already been successfully used to improve the physicochemical profiles of drug compounds and increase their bioavailability.Citation23 It was also reported that nanoparticles can significantly increase and sustain cellular levels of the entrapped drug, which results in enhanced therapeutic efficacy of the nanoparticle-encapsulated drug.Citation24 Coating the nanoparticle surface with a hydrophilic polymer such as poly(ethylene glycol) (PEG), in particular, has been shown to exhibit long circulation properties when PEG was covalently bound on nanoparticle surface rather than adsorbed.Citation25 However, the use of nanoparticles for delivery of water-soluble drugs has been limited because of their poor drug encapsulation efficiency and rapid release of the encapsulated drug.Citation26,Citation27 For manufacturing of nanosized nanoparticles, the incorporation efficiency of drugs with relatively high aqueous solubility is low, because of the drug’s rapid partitioning to the external aqueous phase.Citation28,Citation29
To minimize these disruptive influences, we investigate for the first time a potential technology capable of delivering the drug efficiently. Combining the advantages of liposomes with those of nanoparticles, we report on a novel liposomal system encapsulating methyl ether poly(ethylene glycol)-poly(lactide-co-glycolide) (mPEG-PLGA)-based nanoparticles. We demonstrated that the novel system, core-shell hybrid liposomal vesicles (HLV), could elevate the entrapment efficiency and retard the release of all the major components of PNS to a similar degree and resulting vesicles have architecture and properties suitable for oral application. Finally, a further attempt was made to determine the protective effects of PNS-loaded vesicles in two experimental in vivo settings.
Materials and methods
Materials
Panax notoginsenoside (PNS) was purchased from Nanjing Zelang Medical Technological Co, Ltd (Nanjing, China). Soy phosphatidylcholine (SPC) was from Shanghai Taiwei Pharmaceutical Industry Co, Ltd (Shanghai, China). Cholesterol was purchased from Taizhou Hisound Chemical Co, Ltd (Zhejiang, China). Polyvinylalcohol (PVA; MW 30,000–70,000) was from Sigma-Aldrich (St Louis, MO). mPEG-PLGA (MW 20,000) having 50/50 molar ratio of lactic to glycolic acid moieties and mPEG with 5 k molecular weight was supplied by Jinan Daigang Biomaterial Co, Ltd (Jinan, China). Pituitrin was purchased from Shanghai No1 Biochemical and Pharmaceutical Co, Ltd (Shanghai, China). 2,3,5-triphenyltetrazolium chloride (TTC) was from Sigma-Aldrich. Xuesaitong Tablets® were purchased from Yunnan Daphne Pharmaceutical (Group) Co, Ltd (Yunnan, China).
Animals
Male Sprague Dawley rats (200 g ± 20 g) were from the Jiangxi University of Traditional Chinese Medicine Animal Institution (No JZDW 2011-0006). The rats were housed under standard conditions and were fed on a commercial diet and had access to water ad libitum. For each study, animals were randomized into six groups of ten animals each and allowed to acclimatize for 1 week before the experiments. All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council. The protocol was approved by the local animal ethics committee.
Preparation of PNS-loaded core-shell hybrid liposomal vesicles (PNS-HLV)
mPEG-PLGA nanoparticles loaded with PNS (PNS-NP) were prepared using a water-in-oil-in-water double emulsion solvent evaporation method as described previously.Citation30 0.2 mL of 150 mg · mL−1 PNS solution was emulsified in 2.5 mL of dichloromethane containing 5% mPEG-PLGA by using a probe sonicator (JY98-IIIN ultrasonic processor; Xinyi Ultrasonic Equipment Co, Ltd, Jinan, China) over an ice bath at 300 W for 2 minutes. This W/O emulsion was added to 30 mL phosphate-buffered saline (PBS; pH = 7.4) and the mixture was probe sonicated at 200 W for 1 minute over an ice bath. A stabilizer, polyvinylalcohol was dissolved in both inner and external aqueous phases at 1% w/v and 1.5% w/v, respectively. The obtained double emulsion was gently stirred at room temperature until the evaporation of the organic phase was complete.
For preparation of PNS-NP, the thin-film hydration method was applied. In a typical procedure, the lipid fraction composed of SPC, cholesterol (83:17, w/w), and α-tocopherol was dissolved in an appropriate amount of chloroform. The mixture was dried to a thin film under vacuum. The film was then hydrated with the obtained PNS-NP (pH = 7.4) to make a 30 mL PNS-encapsulated lipid coarse suspension. Sonication was carried out over an ice bath to obtain a homogenous dispersion of liposomes.
Formulations were prepared similarly for PNS-loaded liposomes (PNS-LP). The thin-layer evaporation technique used was based on a previously described method using PNS solution as the aqueous phase instead of PNS-NP.Citation31
Morphology analysis
The morphological examination of HLV was performed by a JEM-1200 EX electron microscope (JEOL, Tokyo, Japan) by conventional negative staining methods using 0.3% phosphotungstic acid buffer (pH = 6.0) as a staining agent.
Particle size and zeta potentials analysis
The size and zeta potentials of the liposomes were measured by dynamic light scattering and electrophoretic light scattering, respectively, both using a PSA NANO2590 apparatus (Malvern Instruments, Malvern, UK) at 25°C. For size measurements, the liposomal dispersions were diluted about 100 times in the same buffer used for their preparation. Dust particles were eliminated by filtration (0.45 μm) from the vesicle preparation as well as from the buffer solution. The dispersion medium for the evaluation of zeta potential of liposomes was Millipore-filtered water (pH = 7.0; Millipore, Billerica, MA) and ions were eliminated to avoid influence on the surface electric charge of liposomes. The equations used for converting the electrophoretic mobility to the zeta potential are:Citation32
ζ is the zeta potential, μ the electrophoretic mobility, η the viscosity of the solvent, and ε the dielectric constant.
Determination of encapsulation efficiency
Liposome encapsulation efficiency (EE%) was determined using the ultrafiltration technique for separating the nonentrapped drug from liposomes.Citation33 For this, 500 μL drug-loaded liposomal dispersion was placed in an ultrafiltration tube (Nanosep MF; Pall Corporation, Port Washington, NY) which was fitted with a filter membrane (molecular weight cut off: 10,000). The free drug in the underlayer solution was collected by centrifugation at 8000 rpm for 15 minutes (3–18K high-speed refrigerated centrifuge; Sigma, Germany) and the drug content (R1, Rb1, and Rg1) in the ultrafiltrate (Cfree) was determined by high-pressure liquid chromatography (HPLC) on a C18 Hypersil column (Thermo, Finnigan, UK) (250 × 4.6 mm, 5 μm) at 203 nm. Gradient elution was employed using solvent A (acetonitrile) and solvent B (water) at 25°C; the gradient program used was as follows: initial 0–15 minutes, linear change from A–B (20:80, v/v) to A–B (21.5:78.5, v/v); 15–36 minutes, linear change to A–B (40:60, v/v). The flow rate was kept at 1.0 mL · min−1 and the sample injection volume was 20 μL. Then 0.5 mL of liposomal suspension was diluted with 2.0 mL of a mixture (acetone:chloroform = 2:1, v/v) to determine the total drug (ctotal) by HPLC. The EE% was calculated by:
Stability
The physical stability of the products protected from light at 4°C was assessed by evaluation of the suspensions at predetermined time points.Citation31
In vitro release
The releases of R1, Rb1, and Rg1 from different PNS preparations, PNS solution, PNS-NP, PNS-LP, and PNS-HLV, were all evaluated. PNS-loaded nanoparticles were suspended in 10 mL of 0.01 M PBS (pH = 7.4, 6.8, and 2, respectively). The samples were placed in a thermostatic water bath at 37°C, at scheduled times, collected, and centrifuged for 5 minutes at 10,000 rpm.
On release of PNS either from liposomes or PNS-HLV, the solutions were each placed in a dialysis cellulose membrane bag (Sigma-Aldrich) corresponding to 2 mg of drug, having a molecular weight cut-off of 10,000. The bag was incubated in 200 mL of PBS (pH = 7.4, 6.8 and 2, respectively) at 37°C and agitated moderately. At predetermined time intervals, in vitro medium (1.5 mL) was withdrawn and replaced with fresh PBS buffer.
A control experiment to determine the release behavior of the free drug, PNS dissolved in 0.01 M PBS (1 mg · mL−1), was also performed. All the samples were analyzed for the amount of PNS by HPLC as mentioned above, and for each determination, three release samples were tested.
Experimental protocol for global cerebral ischemia/reperfusion
Six groups of rats were fed with drug or vehicles by gavage for 10 days prior to the experiment and were treated as follows, where the water solution of Xuesaitong Tablets® was used as the positive control (PNS solution): Group 1: normal saline (6 mL · kg−1, orally), no ischemia-reperfusion, control group; Group 2: normal saline (6 mL · kg−1, orally), bilateral common carotid artery occlusion (BCCAO) for 2 hours, followed by removal of clamps (reperfusion) for 4 hours, model group; Group 3: PNS solution (30 mg · kg−1), BCCAO for 2 hours, followed by reperfusion for 4 hours; Group 4: PNS-NP (30 mg · kg−1), BCCAO for 2 hours, followed by reperfusion for 4 hours; Group 5: PNS-LP (30 mg · kg−1), BCCAO for 2 hours, followed by reperfusion for 4 hours; Group 6: PNS-HLV (30 mg · kg−1), BCCAO for 2 hours, followed by reperfusion for 4 hours.
Induction of ischemia/reperfusion
Before the operation, all animals were deeply anesthetized with chloral hydrate (350 mg · kg−1, intraperitoneal) and groups 2–6 were subjected to BCCAO. Animals were placed on their back, both carotid arteries were exposed over a midline incision, and a dissection was made between the sternocleidomastoid and the sternohyoid muscles, which are parallel to the trachea. Each carotid artery was separated from its adventitial sheath and vagus nerve. The induction of ischemia was performed by using atraumatic microclamps for 2 hours followed by 4 hours reperfusion. The skin was stitched with waxed silk suture. After the completion of the occlusion period, rats that had not fully lost their righting reflex were excluded from the study. Sham control animals received the same surgical procedures without carotid ligation.Citation34 Rectal temperature was maintained at (37°C ± 0.5°C) with a heating pad placed under the rats. After the completion of the reperfusion period, the animals were sacrificed for tissue collection and analysis.
Brain weight and water content
Brains from six groups were removed and immediately weighed in preweighed glass vials and the wet weights were recorded. The brains were dried to constant weight in an oven (DHG-9101-1SA; Guangdu Instrument and Equipment Co, Ltd, Shanghai, China) at 105°C for 12 hours. The percentage water content of each brain was calculated.
Infarct volume
In another study involving the same protocol and treatment, in order to determine the ischemia infarct area of the brain, the cross-sectional infarction area on the surfaces of every brain slice was observed by the 2,3,5-triphenyltetrazolium chloride (TTC) staining method. Two millimeter serial slices were obtained, starting 1 mm from the frontal pole by cutting the weighed brain in each group. The coronal slices were immersed in a 1% phosphate-buffered triphenyltetrazolim chloride (TTC) solution (pH = 7.4) for 20 minutes at 37°C. After TTC staining, the slices were fixed in 10% phosphate-buffered formalin and the infarction area was then calculated by an image analyzer using Osiris 4.19 software (University of Geneva, Geneva, Switzerland). The infarct area (mm2) from each slice was determined and the percent of infarct volume (mm3) was calculated from sum of the slice area (7 slices in all) × thickness (2 mm).Citation35
Experimental protocol for acute myocardial ischemia
Six groups of rats were fed with drug or vehicles for 10 days prior to the experiment and were treated as follows, where the water solution of Xuesaitong Tablets® was used as the positive control (PNS solution): Group 1: normal saline (6 mL · kg−1, orally), no pituitrin-induced myocardial ischemia, control group; Group 2: normal saline (6 mL · kg−1, orally), pituitrin-induced myocardial ischemia, model group; Group 3: PNS solution (30 mg · kg−1, orally), pituitrin-induced myocardial ischemia; Group 4: PNS-NP (30 mg · kg−1, orally), pituitrin-induced myocardial ischemia; Group 5: PNS-LP (30 mg · kg−1, orally), pituitrin-induced myocardial ischemia; Group 6: PNS-HLV (30 mg · kg−1, orally), pituitrin-induced myocardial ischemia.
Induction of acute myocardial ischemia
After being anesthetized with chloral hydrate (350 mg · kg−1, intraperitoneal), the rats were placed on an electric heating pad to keep their body temperature at 37°C. Lead-II of the electrocardiogram (ECG) was monitored with subcutaneous stainless steel electrodes. Electrocardiogram was monitored using a computer-based BL-420F system (Chengdu Technology and Market Co, Ltd, Taimen, China). Pituitrin (PIT) sensitive rats were screened by observing the changes of ECG after tail intravenous injection of PIT (0.5 U · kg−1). Then the PIT-sensitive rats were randomized into six groups as aforementioned. Myocardial ischemia model was made in rats by injecting PIT (1 U · kg−1) intravenously.Citation36 A successful myocardial ischemia model was confirmed by changes of ST segment elevation (>0.1 mv) and heart rate decrease. The hearts were harvested at the end of the 20-minute ischemic period. Rats in control group were subjected to the same procedures without the administration of PIT.
Determination of serum lactate dehydrogenase (LDH)
Blood samples were collected from the abdominal aorta and immediately centrifuged at 400 rpm to separate serum (3–18K high-speed refrigerated centrifuge; Sigma, Germany). Serum LDH levels were detected spectrophotometrically at 440 nm (UV-2550 UV-VIS spectrophotometer; Shimadzu, Tokyo, Japan) using a LDH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the supplier’s instructions.
Determination of superoxide dismutase (SOD), hydrogen peroxide (H2O2) and malondialdehyde (MDA)
After harvesting, the heart from each group was homogenized in 10 times their mass of saline solution and a clear supernatant was obtained by centrifugation at 10,000 rpm for 15 minutes at 4°C (3–18K high-speed refrigerated centrifuge; Sigma, Germany). SOD, H2O2, and MDA levels were measured spectrophotometrically using diagnostic kits (Nanjing Jiancheng Bioengineering Institute), according to the manufacturer’s instructions.
Statistical analysis
The mean value of each parameter in each individual group of rats was calculated from at least eight individual values. All data were expressed as means ± standard deviation (SD). Statistical analysis was using a one-way analysis of variance (ANOVA) with SPSS software (SPSS Inc, Chicago, IL). Probability values < 0.05 were considered significant.
Results and discussion
Characterization of PNS preparations
The features of PNS-loaded nanoparticles and HLV with PVA as the surfactant, and PNS-loaded liposomes are summarized in and . In the morphological characterization, spherical-shaped particles were found. The presence of phospholipid vesicles surrounding the inner nanoparticles in PNS-HLV led to the appearance of larger vesicles.
Table 1 Physicochemical characterizations of PNS formulations (n = 3)
Figure 1 Transmission electron microscopy images of (A) PNS-NP, (B) PNS-LP, and (C) PNS-HLV.
Note: Bar is 500 nm.
Abbreviations: PNS-HLV, panax notoginsenoside-loaded core-shell hybrid liposomal vesicles; PNS-LP, PNS-loaded liposomes; PNS-NP, PNS-loaded nanoparticles.
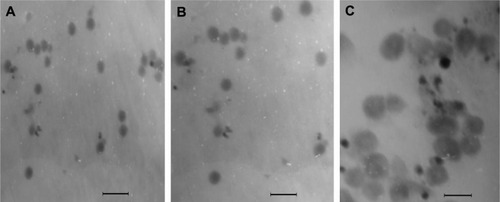
Higher surface negativity of the PNS-HLV is due to not only the end of carboxyl acid groups of the PLGA chains being capped with PEG, but also the incorporation of SPC in the formulation which is determined to contain unsaturated fatty acids. EE% of all the three components of PNS was increased, especially for Rg1, increasing from 15.2% to 40.5%, dependent on the applied method. The preparation methods for HLV, a liposomal system encapsulating mPEG-PLGA-based nanoparticles, were applied on the basis of the polarity of the PNS components. The water-in-oil-in-water double emulsion solvent evaporation method for the nanoparticle preparation was designed for the components with relative higher polarity and the thin film hydration method was applied for the components with relative lower polarity. Since the logP values of major active components of PNS, Rb1, R1, and Rg1, are −0.5618, 0.034, and 0.8, respectively, the EE% of Rb1 after first encapsulation in nanoparticles was the highest. As for Rg1 with the lowest polarity, its EE% was significantly elevated when entrapped in HLV, being 1.7- and 0.4-fold superior to PNS-NP and PNS-LP, respectively. Furthermore, the differences among the EE% of these three components of PNS, R1, Rb1, and Rg1, were shortened with the relative standard deviation (RSD) decreasing from 74.0% to 35.5%, indicating the synchronously elevated EE% for PNS in HLV nanocarriers.
Stability of PNS-HLV
For the conventional nanoparticle suspension in the stability study, the EE% of R1 decreased by 7.3% and the EE% of Rb1 and Rg1 decreased by 6% and 23.0% in 3 months, respectively. The EE% of Rb1 was only 93.75% of the original level and EE% of R1 was decreased by 10% at the 12th month for PNS-LP. The mean particle sizes of both PNS-NP and PNS-LP were increased significantly at the end of 12 months. No significant change in vesicle size or drug EE% was observed for PNS suspensions formed by embedding nanoparticles within liposomes during the long-term stability study (), and they had a homogeneous, semitransparent, and ivory appearance. Hence, it could be assumed that the PNS-HLV were stable.
Table 2 Storage stability of PNS formulations at 4°C
In vitro release
– display the cumulative percentage release profiles of notoginsenoside R1, ginsenoside Rb1 and Rg1 from PNS solution, nanoparticles, liposomes, and HLV at every time point in PBS (pH = 7.4, 6.8, and 2, respectively). It appears that the release rate from HLV was remarkably slower than those of other PNS preparations.
Figure 2 Cumulative percentage release profiles of R1, Rb1, and Rg1 from PNS preparations at pH = 7.4 (n = 3).
Abbreviations: PNS, panax notoginsenoside; PNS-HLV, panax notoginsenoside-loaded core-shell hybrid liposomal vesicles; PNS-LP, PNS-loaded liposomes; PNS-NP, PNS-loaded nanoparticles; R1, notoginsenoside R1; Rb1, ginsenoside Rb1; Rg1, ginsenoside Rg1.
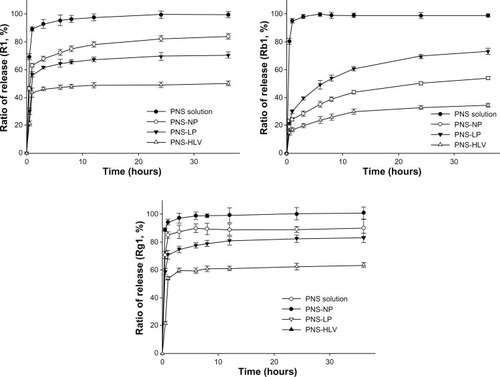
As for the release profiles of R1, Rb1, and Rg1 from different preparations at pH = 7.4 (), liposomes and nanoparticles exhibited a typical initial rapid burst effect of around 60% R1 in the first 1 hour while HLV displayed a restrained burst effect along with a slowed release of R1 (<50%) over the extended period of 36 hours.
As to Rb1, 98.4% Rb1 was released from PNS solution within 3 hours, while less than 34% of Rb1 was released from HLV with in 24 hours. Almost 28.7% of Rb1 released from PNS-NP within 3 hours. The release of Rb1 from PNS-LP displays similar tendency with higher rates.
Nearly 89.5% and 79.1% of Rg1 were released from nanoparticles and liposomes within 8 hours, respectively. Furthermore, less than 62% Rg1 was released from HLV within 12 hours.
Even though there were still burst release of drugs for up to 3 hours, a prolonged release phenomenon was shown in all PNS nanocarriers. Nanoparticle-embedded liposomes released drugs significantly more slowly than conventional liposomes and nanoparticles, providing a greater control of the release rate of the encapsulated solute. Moreover, the ratios of release for R1, Rb1, and Rg1 from PNS-HLV were retarded to 50.1%, 34.6%, and 63.4%, while the release ratios of R1, Rb1, and Rg1 from PNS-LP were 83.9%, 54.2%, and 90.2%, respectively.
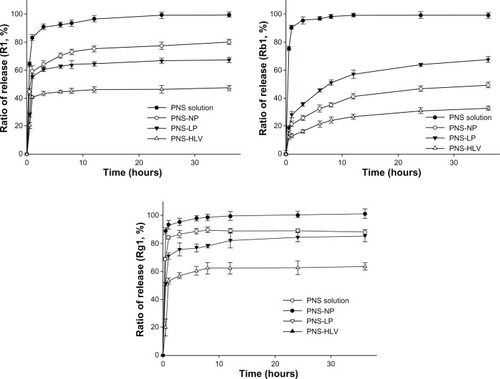
As for the release profiles of R1, Rb1, and Rg1 from different preparations at pH = 6.8 (), R1 and Rg1 can be easily released from nanoparticles and liposomes. The drug release rates were 73% and 89.5% after releasing from nanoparticles for 8 hours, respectively. With the similar trend in drug-releasing profiles to that at pH = 7.4, the drug release curves of R1, Rb1, and Rg1 from HLV include two stages: an immediate release (burst) and a sustained release profile. The first stage is probably due to the free drugs and the drugs adhering to the surface of vesicles. Then the second stage of release profile might be attributed to the diffusion of the encapsulated drugs within the vesicles. The structure of HLV lead to the attenuated burst release of drugs, in comparison with nanoparticles and liposomes.
Figure 3 Cumulative percentage release profiles of R1, Rb1, and Rg1 from PNS preparations at pH = 6.8 (n = 3).
Abbreviations: PNS, panax notoginsenoside; PNS-HLV, panax notoginsenoside-loaded core-shell hybrid liposomal vesicles; PNS-LP, PNS-loaded liposomes; PNS-NP, PNS-loaded nanoparticles; R1, notoginsenoside R1; Rb1, ginsenoside Rb1; Rg1, ginsenoside Rg1.
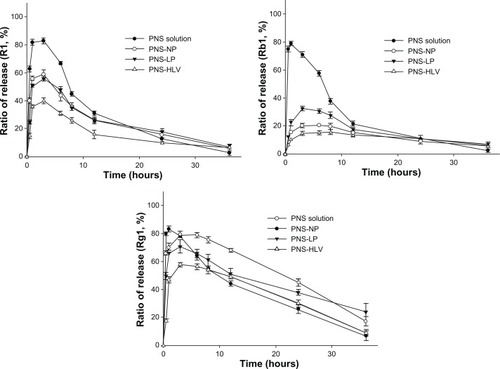
As for the release profiles of R1, Rb1, and Rg1 from different preparations at pH = 2 (), the amount of drug which could be detected dropped significantly in 3 hours. It could be explained by the fact that the saponins are unstable and could be degraded quickly under acid condition, especially for Rb1 and Rg1, whose percentage of prototype drug were both less than 30% under acid condition after 2 hours.Citation37 After 60 minutes, there was still burst release of drugs. Almost 21% and 33% Rb1 were released from NP and LP within 3 hours, respectively. However, less than 16% Rb1 was released from HLV within 8 hours. Even under the acid condition and with the degradation of drugs, less amount of drugs were degraded from HLV, compared with that from nanoparticles and liposomes, which indicate that HLV could possibly possess better protection of PNS in the gastrointestinal tract and therefore increase the bioavailability of PNS.
Figure 4 Cumulative percentage release profiles of R1, Rb1, and Rg1 from PNS preparations at pH = 2 (n = 3).
Abbreviations: PNS, panax notoginsenoside; PNS-HLV, panax notoginsenoside-loaded core-shell hybrid liposomal vesicles; PNS-LP, PNS-loaded liposomes; PNS-NP, PNS-loaded nanoparticles; R1, notoginsenoside R1; Rb1, ginsenoside Rb1; Rg1, ginsenoside Rg1.
This can be used to provide synchronous encapsulation and retarded release formulations. However, more importantly the HLV suspension may be especially suitable for incorporating traditional Chinese medicine. The synergistic effect of each component in herbal medicines could be exhibited by the synchronously elevated EE% of components and their retarded percent of release (%) in the form of HLV.Citation38
Brain water content and infarct volume
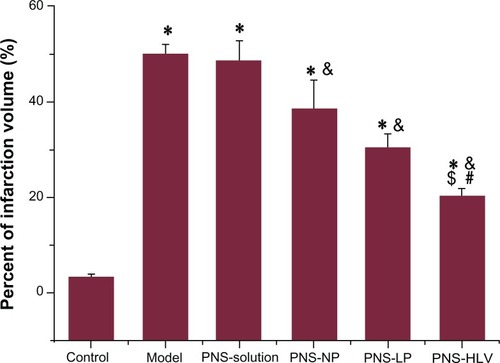
The effect of the occlusion and reperfusion time on brain infarct was examined initially. Different degrees of ischemia infarction volume were created by varying duration of occlusion and reperfusion (data not shown). The highest infarct volume was induced in the animals after 2 hours of ischemia and 4 hours of reperfusion. Brain water content revealed significant differences between groups (P < 0.05) in . Infarction caused elevation in water content in the global model. However, there was significant decrease of brain water content observed in the PNS-HLV-treated group, which was also lower than that of groups 3, 4, and 5 (P < 0.05). Treatment with PNS-HLV for 10 days prior to the induction of ischemia attenuated the ischemia/reperfusion-induced brain infarction significantly, in comparison with PNS-solution, PNS-NP, and PNS-Liposomes-treated groups ().
Figure 5 Effect of different PNS preparations on brain water content of rats with induction of ischemia/reperfusion.
Notes: *P < 0.05 vs control; &P < 0.05 vs model; #P < 0.05 vs PNS-NP; $P < 0.05 vs PNS-LP.
Abbreviations: PNS, panax notoginsenoside; PNS-HLV, panax notoginsenoside-loaded core-shell hybrid liposomal vesicles; PNS-LP, PNS-loaded liposomes; PNS-NP, PNS-loaded nanoparticles; R1, notoginsenoside R1; Rb1, ginsenoside Rb1; Rg1, ginsenoside Rg1.
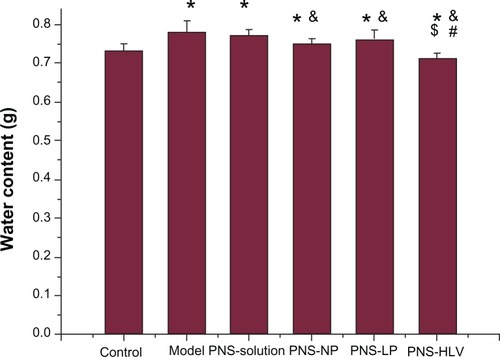
Figure 6 Effect of different PNS preparations on percent of infarction volume of rats with induction of ischemia/reperfusion.
Notes: *P < 0.05 vs control; &P < 0.05 vs model; #P < 0.05 vs PNS-NP; $P < 0.05 vs PNS-LP.
Abbreviations: PNS, panax notoginsenoside; PNS-HLV, panax notoginsenoside-loaded core-shell hybrid liposomal vesicles; PNS-LP, PNS-loaded liposomes; PNS-NP, PNS-loaded nanoparticles; R1, notoginsenoside R1; Rb1, ginsenoside Rb1; Rg1, ginsenoside Rg1.
LDH level and antioxidant enzyme activities
The results of different preparations of PNS biochemical parameters are shown in . ANOVA revealed significant (P < 0.05) differences between various treatments. LDH in the serum, H2O2, and MDA exhibited significant increase and SOD showed a significant decrease in the acute myocardial ischemic group versus the control group. In the PNS-treated group, except for group 3 (PNS solution), there were significant restorations of the enzymatic level compared with the model group (P <0.05), especially for the group 6 (PNS-HLV). Treatment with PNS-HLV markedly reversed the alterations in enzymatic parameters brought about by ischemia. The values were almost restored to near-normal levels.
Table 3 Effect of different PNS preparations on the biochemical parameters (n = 10)
In the present study, we observed the therapeutic potential of PNS-HLV on the model of global cerebral ischemia/reperfusion and model of acute myocardial ischemia in rats. We selected two different models because the formulation and accumulation of reactive oxygen species is believed to be one of the mechanisms of ischemic brain and myocardial damage.Citation39
It was reported that antioxidant supplements may be an excellent prevention strategy for many diseases, such as liver injury, liver fibrosis, cancer, aging, and diabetes.Citation40 Research on the mechanisms of actions of natural products from traditional Chinese medicine has been focused on their antioxidant activities. As the chief pharmacological active components in the total saponins of Panax notoginseng root, ginsenoside Rg1, ginsenoside Rb1, and notoginsenoside R1 had the wider effects of angiocarpy such as inhibiting thrombosis, protecting ischemic myocardial cells, expanding peripheral vessels, anti-ischemic brain damage, etc.Citation41 Various studies indicated that their biological functions are due to, at least partially, their protective effects against oxidation and inhibitive effects on lipid peroxidation. The oxidant chain reaction is initiated by hydroxyl radicals attacking lipids and extended by the generated lipid hydroperoxide free radicals.Citation10,Citation42 It was reported that PNS significantly inhibited hemolysis induced by 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) free radicals and its strong scavenging activity towards hydroxyl free radicals and hydrogen peroxide might suppress the initiation of lipid oxidation by hydroxyl radicals, leading to inhibitive effects on lipid peroxidation.Citation10
Compared with PNS, PNS-NP, and PNS-LP, PNS-HLV exhibited greater effects on attenuation on brain damage and bioactivities and achieved higher potency than free drug, conventional nanoparticles and liposomes in vivo upon oral treatment to the experimental animals. The formation of HLV and its preparation method, the application of W/O/W double emulsion solvent evaporation method and thin-film hydration method, were designed according to the polarities of the main components of PNS. The entrapment efficiency is a crucial factor for delivering drug into pathologic tissue. The encapsulated drugs in HLV were increased synchronously compared with other PNS preparations and a high concentration of drugs could be delivered into the circulation. Moreover, due to the greater control release of drugs and the narrowed gap among the release profiles of R1, Rb1, and Rg1, we assumed there was a synchronous elevated EE% and control release profile of components with restrained burst release from PNS, which contributed to the synergetic pharmacological effect of R1, Rb1, and Rg1.
Conclusion
The novel PNS-loaded core-shell hybrid liposomal vesicles delivery system in this study helped synchronously enhance the EE% and retard the release of chief components of PNS. In the two animal models, global cerebral ischemia/reperfusion and acute myocardial ischemia in rats, the decrement of brain water content and infarction volume and improvement of biochemical parameters were exhibited, which might be attributed to the improvement of physiochemical properties of PNS-HLV. These applications will be explored in further studies.
Acknowledgements
The study was supported by the Science and Technology Support Program of Jiangxi Province (20111BBG70004-1), the Scientific Research Foundation of Traditional Chinese Medicine of Jiangxi Provincial Health Department (2011A132) and the Technology Platform for Novel Drug Delivery System of TCM (2009ZX09310-005).
Disclosure
The authors report no conflicts of interest in this work.
References
- LeiXLChiouGCCardiovascular pharmacology of Panax notoginseng (Burk) FH. Chen and Salvia miltiorrhizaAm J Chin Med1986143–41451523799531
- CiceroAFVitaleGSavinoGArlettiRPanax notoginseng (Burk) effects on fibrinogen and lipid plasma level in rats fed on a high-fat dietPhytother Res200317217417812601683
- ChenZHLiJLiuJSaponins isolated from the root of Panax notoginseng showed significant anti-diabetic effects in KK-Ay miceAm J Chin Med200836593995119051359
- PengXDDaiLLHuangCQHeCMYangBChenLJRelationship between anti-fibrotic effect of Panax notoginseng saponins and serum cytokines in rat hepatic fibrosisBiochem Biophys Res Commun20093881313419632202
- LuoFCWangSDQiLSongJYLvTBaiJProtective effect of panaxatriol saponins extracted from Panax notoginseng against MPTP-induced neurotoxicity in vivoJ Ethnopharmacol2011133244845320951784
- SunKWangCSGuoJProtective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesenteryLife Sci200781650951817655881
- ChenXCZhuYGZhuLAGinsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stressEur J Pharmacol200347311712877931
- CiceroAFGBandieriEArlettiROrally administered Panax notoginseng influence on rat spontaneous behaviorJ Ethnopharmacol200073338739111090991
- SunSWangCZTongREffects of steaming the root of Panax notoginseng on chemical composition and anticancer activitiesFood Chem20101182307314
- ZhaoGRXiangZJYeTXYuanYJGuoZXAntioxidant activities of Salvia miltiorrhiza and Panax notoginsengFood Chem2006994767774
- KwokHHNgWYYangMSMakNKWongRNYuePYThe ginsenoside protopanaxatriol protects endothelial cells from hydrogen peroxide-induced cell injury and cell death by modulating intracellular redox statusFree Radic Biol Med201048343744519932166
- FengLJiangXHStudy on the pharmacokinetic behavior of Panax notoginseng saponins in ratWest China J Pharm Sci20102514649
- TakinoYOdaniTTanizawaHHayashiTStudies on the absorption, distribution, excretion and metabolism of ginseng saponins. I. Quantitative analysis of Ginsenoside Rg1 in ratsChem Pharm Bull1982306219622017127606
- OdaniTTanizawaHTakinoYStudies on the absorption, distribution, excretion and metabolism of ginseng saponins. II. The absorption, distribution and excretion of ginsenoside Rg1 in the ratChem Pharm Bull19833112922986850945
- KarikuraMMiyaseTTanizawaHTaniyamaTTakinoYStudies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of ratsChem Pharm Bull1991399235723611804549
- LeeJLeeEKimDLeeJYooJKohBStudies on absorption, distribution and metabolism of ginseng in humans after oral administrationJ Ethnopharmacol2009122114314819146939
- XiongJGuoJXHuangLSMengBYPingQNSelf-micelle formation and the incorporation of lipid in the formulation affect the intestinal absorption of Panax notoginsengInt J Pharm20083601–219119618502060
- ZouLLengJLiuYZhaoGProgress of new preparations of Panax notoginseng saponinsJ Sichuan Trad Chin Med20102832325
- LeeCMLeeHCLeeKYO-Palmitoylcurdlan sulfate (OPCurS)-coated liposomes for oral drug deliveryJ Biosci Bioeng2005100325525916243273
- WangSLZhangJJiangTYProtective effect of coenzyme Q10 against oxidative damage in human lens epithelial cells by novel ocular drug carriersInt J Pharm20114031–221922920971176
- HaidarZSHamdyRCTabrizianMProtein release kinetics for core-shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomesBiomaterials20082991207121518076987
- TakeuchiHYamamotoHToyodaTToyobukuHHinoTKawashimaYPhysical stability of size controlled small unilamellar liposomes coated with a modified polyvinyl alcoholInt J Pharm19981641–2103111
- DaiJDNagaiTWangXQZhangTMengMZhangQpH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine AInt J Pharm20042801–222924015265562
- PanyamJLabhasetwarVSustained cytoplasmic delivery of drugs with intracellular receptors using biodegradable nanoparticlesMol Pharm200411778415832503
- AvgoustakisKBeletsiAPanagiZEffect of copolymer composition on the physicochemical characteristics, in vitro stability, and biodistribution of PLGA-mPEG nanoparticlesInt J Pharm20032591–211512712787641
- UenoYFutagawaHTakagiYUenoAMizushimaYDrug- incorporating calcium carbonate nanoparticles for a new delivery systemJ Control Release20051031939815710503
- CasconeMGLazzeriLCarmignaniCZhuZGelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexateJ Mater Sci Mater Med200213552352615348607
- TewesFMunnierEAntoonBComparative study of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles prepared by single and double emulsion methodsEur J Pharm Biopharm200766348849217433641
- GovenderTRileyTEhtezaziTDefining the drug incorporation properties of PLA-PEG nanoparticlesInt J Pharm200019919511010794931
- AvgoustakisKBeletsiAPanagiZKlepetsanisPKarydasAGIthakissiosDSPLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood propertiesJ Control Release2002791–312313511853924
- ZhangJGuanPPWangTYChangDJiangTYWangSLFreeze-dried liposomes as potential carriers for ocular administration of cytochrome c against selenite cataract formationJ Pharm Pharmacol20096191171117819703366
- ZhangJWangSLTopical use of Coenzyme Q10-loaded liposomes coated with trimethyl chitosan: Tolerance, precorneal retention and anti-cataract effectInt J Pharm20093721–2667519437594
- XiangQXiaoJZhangHBPreparation and characterisation of bFGF-encapsulated liposomes and evaluation of wound-healing activities in the ratBurns201137588689521377274
- MukherjeePKAhamedKFHKumarVMukherjeeKHoughtonPJProtective effect of biflavones from Araucaria bidwillii Hook in rat cerebral ischemia/reperfusion induced oxidative stressBehav Brain Res2007178222122817250903
- HongJTRyuSRKimHJProtective effect of green tea extract on ischemia/reperfusion-induced brain injury in Mongolian gerbilsBrain Res20018881111811146047
- ChenQExperimental Methodology of TCM Pharmacology2nd edBeijing, ChinaPeople’s Medical Publishing House2006
- HanMHanLMWangQSMechanism of oral absorption of panaxnotoginseng saponinsActa Pharmaceutica Sin2006416498505
- HeCYLiXHHeXFEffect of different combinations of sanchinoside monomers on monocyte-endotheliocyte adhesionJ Traditional Chin Med2007484354361
- SiesjőBKPathophysiology and treatment of focal cerebral ischemia. Part II: Mechanisms of damage and treatmentJ Neurosurg19927733373541506880
- RodrigoRGuichardCCharlesRClinical pharmacology and therapeutic use of antioxidant vitaminsFundam Clin Pharmacol200721211112717391284
- DongTTCuiXMSongZHChemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituentsJ Agric Food Chem200351164617462314705886
- WuLZhangWTangYHEffect of total saponins of “panax notoginseng root” on aortic intimal hyperplasia and the expressions of cell cycle protein and extracellular matrix in ratsPhytomedicine2010173–423324019748258