Abstract
Malignant gliomas are primary brain tumors with high rates of morbidity and mortality; they are the fourth most common cause of cancer death. Novel diagnostic and therapeutic techniques based on nanomaterials provide promising options in the treatment of malignant gliomas. In order to evaluate the potential of FePt nanoparticles (NPs) for malignant glioma therapy, FePt NPs with different surface coatings and components were tunably synthesized using oleic acid/oleylamine (OA/OA) and cysteines (Cys) as the capping agents, respectively. The samples were characterized using X-ray diffraction, transmission electron microscopy (TEM), X-ray photon spectroscopy, Fourier transform infrared spectroscopy, atomic absorption spectrum, and zeta potential. The influence of the surface coatings and components of the FePt NPs on the proliferation of glioma cells was assessed through MTT assay and TEM observation using three typical glioma cell lines (glioma U251 cells, astrocytoma U87 cells, and neuroglioma H4 cells) as in vitro models. The results showed that the proliferation of glioma cells was significantly suppressed by lipophilic FePt-OA/OA NPs in a time- and/or dose-dependent manner, while no or low cytotoxic effects were detected in the case of hydrophilic FePt-Cys NPs. The IC50 value of FePt-OA/OA NPs on the three glioma cell lines was approximately 5–10 μg mL−1 after 24 hours’ incubation. Although the cellular uptake of FePt NPs was confirmed regardless of the surface coatings and components of the FePt NPs, the suppression of FePt NPs on glioma cell proliferation was dominantly determined by their surface coatings rather than their components. Therefore, these results demonstrate that, through engineering of the surface coating, FePt NPs can potentially be developed as novel therapeutic agents for malignant gliomas.
Introduction
Malignant gliomas are primary brain tumors with high rates of morbidity and mortality; they are the fourth most common cause of cancer death.Citation1–Citation3 However, progress in the clinical diagnosis of and therapy for malignant gliomas has lagged behind that of other cancers due to their complicated pathogenesis and the obstacles posed by the blood–brain barrier. Novel diagnosis and therapeutic techniques based on nanomaterials provide promising options for treatment of malignant gliomas.Citation4–Citation6 In addition to various nanocarriers for drug delivery, noble metal and oxide nanoparticles (NPs) (such as Au, Ag, and Fe2O3 NPs) have displayed promising potential in the diagnosis and therapy of malignant gliomas.Citation7–Citation9 Magnetothermal-and photothermal-mediated hyperthermia has proved to be efficient in the promotion of glioma cell death, the reduction of gliomal masses, and an increase in survival rate in most preclinical and clinical experiments.Citation10 For example, using a supramolecular self-assembly approach, Au NPs (~2 nm), functionalized with a polymer shell and target-specific ligand, significantly enhance photothermal treatment on U87 and MCF7 cells.Citation11 TiO2 NPs, covalently modified with an antibody (antihuman-IL13α2R), can bind exclusively to glioma cells and initiate the production of intracellular reactive oxygen species under visible light irradiation, which subsequently results in oxidative damage to organelles and cell apoptosis.Citation12 To date, exploration of multifunctional nanomaterials for targeted diagnosis of and therapy for malignant gliomas is one of the most emergent challenges.
Superparamagnetic FePt NPs have attracted considerable attention due to their tempting potential in biomedical fields. For example, a sensitive and quick assay capturing both Gram-negative and Gram-positive bacteria was developed by combining vancomycin with the surface of FePt NPs.Citation13–Citation15 Similarly, FePt NPs have been used to magnetically separate and detect proteins and DNA.Citation16–Citation18 The magnetization of FePt NPs up to ~1000 emu cc−1 is higher than that of commonly used iron oxide (approximately 300–400 emu cc−1) and comparable to that of Co (~1400 emu cc−1) and Fe (~1700 emu cc−1), making them valuable candidates for magnetic resonance imaging.Citation19–Citation22 It has been reported that FePt NPs display stronger contrast enhancement when compared with several commercial magnetic resonance imaging contrast agents (Feridex, MION, and Sinerem; AMAG Pharmaceuticals Inc., Lexington, MA, MGH Center of Molecular Imaging Research, Boston, MA, and Guerbet Group, Villepinte, France, respectively) according to their apparent transverse relaxivity values.Citation19,Citation20 Therefore, FePt NPs can serve as dual modality contrast agents for molecular CT (computed tomography molecular imaging or MRI (magnetic resonance imaging) in vitro and in vivo after engineering their surfaces with functional molecules.Citation21 Of particular interest, superparamagnetic FePt NPs have been derived from the anticancer activity of FePt NPs. Both FePt@CoS2 and FePt@Fe2O3 yolk-shell NPs display a much lower IC50 on HeLa cells than that of cisplatin, according to MTT assay.Citation19,Citation23 Additionally, FePt NPs significantly suppress the proliferation of various tumor cells, such as human ovarian cancer cells (A2780), human epithelial carcinoma cells (A431), human breast cancer cells (Sk-Br3), and human embryonic kidney cells (HEK-293).Citation24 Thus, superparamagnetic FePt NPs could be considered good candidates for developing multi-functional nanomedicines for the diagnosis and therapy of malignant gliomas.
In the present study, FePt NPs with different surface coatings and components were tunably synthesized using oleic acid/oleylamine (OA/OA) and amino acid (cysteine [Cys]) as capping agents, respectively. The samples were characterized using X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photon spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), and atomic absorption spectroscopy (AAS). In particular, the suppression of FePt NPs on the proliferation of glioma cells was evaluated by MTT assay using different glioma cell lines (human glioma U251 cells, human astrocytoma U87 cells, and human neuroglioma H4 cells) as models. The influence of the surface coatings and the components of FePt NPs on both suppression and cellular uptake were collectively investigated.
Materials and methods
Materials
Platinum acetylacetonate (Pt[acac]2, 97%), potassium hexachloroplatinate (K2PtCl6, 98%), iron acetylacetonate (Fe(acac)3, 99.9%), 1,2-hexadecanediol (90%), and oleylamine (≥70%) were used (all from Sigma-Aldrich, St Louis, MO). Other chemicals were of analytical grade and used without further purification. Deionized water (16 MΩ cm) was supplied by Nanopure Water Systems UV (Thomas Scientific, Swedesboro, NJ).
Synthesis of FePt NPs coated by oleic acid and oleylamine (FePt-OA/OA NPs)
FePt-OA/OA NPs were synthesized according to a modified method.Citation25 Briefly, Fe(acac)3 (0.5 mmol), oleic acid (400 μL) and ethylene glycol (100 mL) were mixed and heated to 150°C in a three-necked round-bottom flask in a nitrogen atmosphere. Pt(acac)2 (0.5 mmol) and oleylamine (400 μL) were dissolved in another 100 mL of ethylene glycol at 100°C, and then transferred to a three-necked round-bottom flask. After both of the solutions containing the iron and platinum salts were mixed completely at 150°C, 1,2-hexadecanediol (1.5 mmol) was added and dissolved in this mixture. Subsequently, the mixture was heated to 197°C at a heating rate of 10°C min−1 and refluxed at this temperature for 3 hours. Finally, the heat source was removed and the product was cooled down to room temperature. The black product was precipitated out and washed with ethanol (200 mL) by centrifugation (10,000 rpm × 5 minutes).
Synthesis FePt NPs coated by L-cysteine (FePt-Cys NPs)
The synthetic experiments were carried out using standard airless procedures. In a typical synthesis, L-Cys (0.1 mmol) and K2PtCl6 (0.5 mmol) were dissolved in 100 mL deionized water with nitrogen flow (~5 mL/min) in a 250 mL three-neck flask while being magnetically stirred. The mixture was heated to 50°C to ensure the dissolution of K2PtCl6 and then cooled down to room temperature. FeCl2 · 4H2O (0.5 mmol) was introduced and dissolved completely in this mixture. Then, NaBH4 (5 mmol) dissolved in 10 mL of deionized water was added dropwise to the mixture containing the iron and platinum salts, which was then incubated at 25°C for 2 hours. The resulting precipitate was centrifuged (10,000 rpm × 3 minutes), washed several times – alternately using deionized water and ethanol – and dried in a vacuum for 12 hours.
FePt NPs characterization
The phase structures of the samples were identified by powder XRD on an X’Pert PRO diffractometer (PANalytical B.V., Almelo, The Netherlands) using Cu Kα radiation (λ = 1.5406 Å). FTIR spectra were recorded with a NEXUS FTIR spectrometer (Thermo Nicolet, Madison, WI). AAS (GBC Avanta M; GBC Scientific Equipment Pty Ltd, Braeside, Australia) was used to analyze the Fe/Pt molar ratios in the samples. TEM (Tecnai G2 20; FEI Company, Eindhoven, The Netherlands) and high-resolution transmission electron microscopy (HRTEM) (JEM-2100F; JEOL, Tokyo, Japan) were used to observe the morphology, particle size, and detailed information about the lattice of the samples. XPS were measured on a VG Multilab 2000 (Thermo Electron Corporation, Waltham, MA) with an Al Kα excitation source, where the binding energies were calibrated by referencing the C1s peak (285.1 eV). Zeta potential was measured on a zeta and size analyzer (Zetasizer 3000; Malvern Instruments Ltd, Malvern, UK).
Cell culture and treatment
Brain tumor cells (human glioma U251 cells, human astrocytoma U87 cells, and human neuroglioma H4 cells) were purchased from the China Center for Type Culture Collection (CCTCC; Wuhan, China). All cell lines were maintained in MEM (Hyclone Corp., Logan, UT) supplemented with 10% fetal bovine serum (FBS) (Gibco®; Life Technologies, Carlsbad, CA), 100 units penicillin mL−1, and 100 μg streptomycin/mL at 37°C with 5% CO2. Cells were harvested by trypsinizing for 5 minutes at 37°C with 0.25% trypsin (AMRESCO, Solon, OH).
Suppression of FePt NPs in glioma cell proliferation
In the initial experiments, the possible cytotoxic effects of FePt NPs on U251, U87, and H4 cells were evaluated. We examined the time- and dose-dependent effects induced by FePt NPs on cell viability through MTT assay. According to the results of the pre-trials, different exposed doses and incubation intervals were designed for systematically evaluating the cytotoxic effects of FePt NPs. Cells were seeded at a density of 3 × 104 cells/well in the culture plates and grown for 14 hours before FePt NPs were added. In the case of FePt-OA/OA NPs, cells were treated at doses of approximately 0.1, 1, 5, and 25 μg/mL for 6, 12, 24, and 48 hours, respectively; in the case of FePt-Cys NPs, cells were treated at doses of approximately 25, 50, 100, and 200 μg/mL for 24, 48, and 72 hours, respectively. After the cells were treated with FePt NPs at the designed doses and times, 20 μL MTT (5 mg/mL) were added to each well, and then the plates were incubated in minimum essential medium (MEM) at 37°C for 4 hours. Finally, all of the medium was removed; 150 μL dimethyl sofoxide (DMSO) was added to each well, and the plates were shaken for 10 min. The absorbance of each well was measured at a wavelength of 550 nm using a microplate spectrophotometer 1500 (Thermo Labsystems, Finland). Data were expressed as means ± standard deviation (SD) and oneway analysis of variance (ANOVA) was carried out.
Cellular uptake of FePt NPs
Cells were treated with different FePt NPs at 25 μg/mL for 24 hours. Then the culture medium was removed. Cells were fixed successively by 4% glutaraldehyde phosphate buffer at 4°C for 4 hours and 1% osmic acid for 2 hours. Subsequently, the samples were desiccated through a graded ethanol series (30%, 50%, 60%, 70%, 80%, 90%), which were further embedded in EPON812 and polymerized in the oven at 60°C for 24 hours. Ultrathin sections of ~70 nm thick were cut with a diamond knife on a Leica ultramicrotome (Leica Microsystems, Wertzlar, Germany) and transferred to a copper grid. The images were observed by TEM.
Results
Synthesis and characterization of FePt NPs
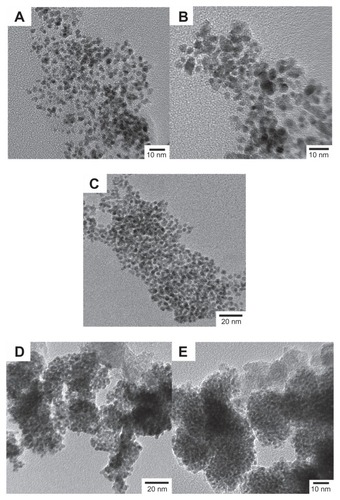
shows the TEM images of FePt NPs synthesized at different surface coatings and the Fe/Pt molar ratios. As shown in , the uniform and well-dispersed FePt NPs obtained had a nearly spherical shape and no aggregations were observed due to the surface coating of OA/OA molecules. In the TEM images, the sizes of the FePt NPs corresponding to the initial Fe/Pt molar ratios of 3:1, 1:1, and 1:3 were approximately 4.3 ± 0.5, 7.1 ± 0.9 and 3.6 ± 0.4 nm, respectively. Similarly, few aggregates of FePt NPs were observed when OA/OA was replaced with Cys, which could be attributed to an interparticle repulsive force derived from the highly negatively charged surfaces (as will be discussed later). In the TEM images, the sizes of the FePt NPs synthesized with different Fe/Pt molar ratios and surface coatings of 1:1 and 1:3 were approximately. 3.1 ± 0.4 and 3.4 ± 0.3 nm, respectively ().
Figure 1 TEM images of FePt NPs synthesized with different Fe/Pt molar ratios and surface coatings.
Notes: (A) Fe:Pt = 3:1, OA/OA; (B) Fe:Pt = 1:1, OA/OA; (C) Fe:Pt = 1:3, OA/OA; (D) Fe:Pt = 1:1, Cys; (E) Fe:Pt = 1:3, Cys.
Abbreviations: TEM, transmission electron microscopy; NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
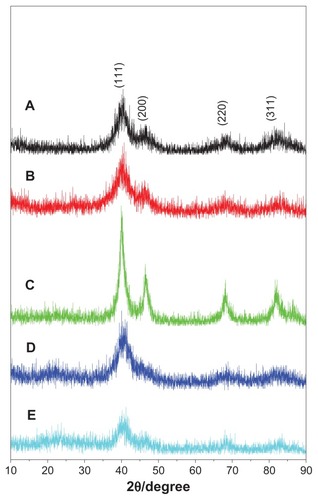
The phase structures of the as-synthesized samples were identified by powder XRD. All peaks in could be assigned to planes of (111), (200), (220), and (311) of the chemically disordered face-centered cubic structure of FePt.Citation26–Citation29 No additional peak attributed to iron oxide or pure Pt was observed in the XRD patterns. It is apparent that only the main peak assigned to the (111) plane was distinguished when OA/OA molecules were replaced with Cys molecules (), suggesting poor crystal structure due to the low synthesis temperature (room temperature). As shown in , the sizes of the FePt NPs calculated according to the Debye–Scherrer formula were slightly larger than those obtained from the TEM images.
Table 1 Compositions and sizes of FePt NPs synthesized at different Fe/Pt ratios and surface coatings
Figure 2 XRD patterns of FePt NPs synthesized at different Fe/Pt molar ratios and with different surface coatings.
Notes: (A) Fe:Pt = 3:1, OA/OA; (B) Fe:Pt = 1:1, OA/OA; (C) Fe:Pt = 1:3, OA/OA; (D) Fe:Pt = 1:1, Cys; (E) Fe:Pt = 1:3, Cys.
Abbreviations: XRD, X-ray diffraction; NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
The Fe/Pt atomic ratios in the FePt NPs were measured by AAS. summarizes the Fe/Pt molar ratios in the precursors and FePt NPs. The Fe/Pt molar ratios of the FePt NPs were slightly different from those of the precursors. This could be attributed to the different chemical activities of Fe and Pt ions in the formation and growth of alloy nuclei.Citation26,Citation30 In context, FePt NPs with different surface coatings were denoted as FexPt100−x-OA/OA NPs or FexPt100−x-Cys NPs.
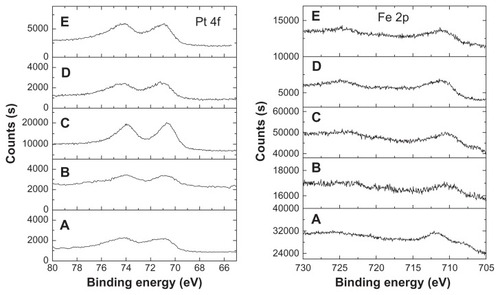
The chemical states of Fe and Pt elements in the FePt NPs were detected by XPS. As shown in , the Pt 4f signal was split into two spin-orbit doublets (4f7/2 and 4f5/2) with maxima at approximately 71.0–71.5 eV and 74.1~74.8 eV, corresponding to the samples synthesized at different surface coatings and Fe/Pt molar ratios. The slight shift of Pt 4f binding energy compared with that of bulk Pt was attributed to the adsorption of capping molecules through –NH2 in oleylamine molecules or –SH in Cys molecules.Citation26,Citation31–Citation33 Similarly, the Fe 2p signal was split into two (2p3/2 and 2p1/2) with maxima at approximately. 710.4–711.5 eV and 724.5–725 eV due to spin-orbit coupling. The formation of Fe-O bonds due to the surface coating of OA/OA or Cys molecules resulted in a slight shift of binding energy when compared with that of neutral Fe.Citation26,Citation31–Citation33
Figure 3 XPS spectra of Pt and Fe elements in the FePt NPs synthesized at different Fe/Pt molar ratios and with different surface coatings.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) Fe60Pt40-Cys; (E) Fe24Pt76-Cys.
Abbreviations: XPS, X-ray photon spectroscopy; OA/OA, oleic acid/oleylamine; Cys, cysteine.
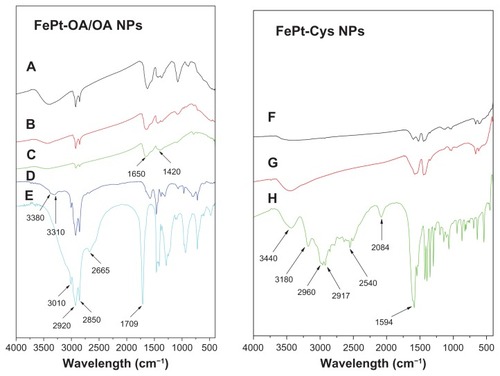
The existence of capping molecules on the surface of the FePt NPs was further confirmed by FT-IR spectra. The absorption at ~3400 cm−1 in the FTIR spectra of the FePt-OA/OA NPs could be attributed to the N–H stretching of oleylamine, while absorption at ~2920 and ~2850 cm−1 could be assigned to the symmetric and asymmetric −CH2 stretching modes in the OA/OA molecules (). It is worthy of note that the absorption at ~1709 cm−1 derived from the C=O stretching mode of oleic acid () was shifted to ~1650 cm−1 in the FTIR spectra of FePt-OA/OA NPs (), suggesting the chemical adsorption of oleic acid as a carboxylate on the surface of FePt NPs.Citation26,Citation34,Citation35 The absorption at ~2540 cm−1 corresponding to the S–H stretching vibration of Cys molecules () disappeared in the FTIR spectra of the FePt-Cys NPs (), implying that Cys molecules were anchored on the surface of the FePt NPs through mercapto groups, with which Pt atoms have a high affinity.Citation36
Figure 4 FTIR spectra of FePt NPs synthesized with different surface coatings and components.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) oleylamine; (E) oleic acid; (F) Fe60Pt40-Cys; (G) Fe24Pt76-Cys; (H) cysteine.
Abbreviations: FTIR, Fourier transform infrared spectroscopy; NPs, nanoparticles; OA/OA, oleic acid/oleylamine.
Suppression of FePt NPs in glioma cell proliferation
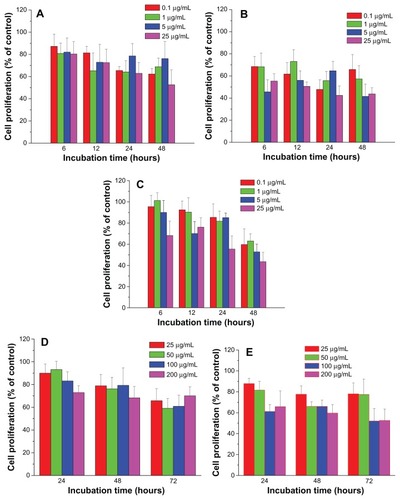
In order to evaluate the cytotoxicity of FePt NPs on glioma cells, three typical glioma cell lines (human glioma U251 cells, human astrocytoma U87 cells, and human neuroglioma H4 cells) were employed as in vitro models. Glioma cells were incubated with FePt NPs at designed doses and time intervals and cell viability was assessed by MTT assay. As shown in , human glioma U251 cells were exposed to lipophilic FePt-OA/OA NPs at doses ranging from 0.1 to 25 μg/mL for up to 48 hours and hydrophilic FePt-Cys NPs at doses ranging from 25 to 200 μg/mL for up to 72 hours. When U251 cells were treated with Fe60Pt40-OA/OA NPs at a dose of 0.1 μg/mL, cell proliferation was slightly decreased to approximately 88.6% ± 3.4%, 88.0 % ± 10.0%, 74.3% ± 6.4%, and 78.0% ± 5.1% compared with that of the control group at incubation times of 6, 12, 24, and 48 hours (). Increasing the exposed dose to 25 μg/mL, cell proliferation was dramatically decreased to approximately 58.4% ± 5.0%, 55.2% ± 3.5%, 51.7% ± 4.4%, and 43.3% ± 3.6% compared to that of the control group at the same incubation intervals. When U251 cells were treated with Fe60Pt40-OA/OA NPs at the exposed doses of 0.1, 1, 5, and 25 μg/mL for 6 hours, cell proliferation was sharply decreased to 88.6% ± 3.4%, 78.6% ± 6.2%, 60.8% ± 9.0%, and 58.4% ± 5.0% compared to that of the control group. These results show that the proliferation of U251 cells was significantly suppressed by Fe60Pt40-OA/OA NPs in a time-and dose-dependent manner. Similarly, the proliferation of U251 cells was significantly suppressed by both Fe45Pt55-OA/OA NPs and Fe27Pt73-OA/OA NPs in a dose-dependent manner (). It seems that the proliferation of U251 cells was more sensitive to changes in the doses than to changes in the incubation times in the case of both Fe45Pt55-OA/OA NPs and Fe27Pt73-OA/OA NPs, suggesting acute cytotoxic effects on U251 cells induced by these two FePt NPs. On the other hand, both Fe45Pt55-OA/OA NPs and Fe27Pt73-OA/OA NPs displayed slightly stronger cytotoxic effects on U251 cells than Fe60Pt40-OA/OA NPs under the same incubation conditions. For example, when U251 cells were incubated with FePt-OA/OA NPs for 12 hours at doses ranging from 0.1 to 25 μg/mL, cell proliferation was decreased to approximately 88.0% ± 10.0%, 67.7% ± 7.4%, 57.7% ± 6.1%, and 55.2% ± 3.5% in the case of Fe60Pt40-OA/OA NPs; approximately 67.5% ± 11.1%, 51.1% ± 8.8%, 53.3% ± 4.7%, and 47.6% ± 4.0% in the case of Fe45Pt55-OA/OA NPs; and approximately 59.3% ± 7.7%, 52.9% ± 3.8%, 47.2% ± 5.0%, and 46.7% ± 8.2% in the case of Fe27Pt73-OA/OA NPs. On the contrary, no or low cytotoxic effects were observed when U251 cells were incubated with hydrophilic FePt-Cys NPs at doses lower than 25 μg/mL for up to 48 hours (data not shown). Significant suppression of cell proliferation by FePt-Cys NPs was observed at exposed doses higher than 25 μg/mL. As shown in , cell proliferation was significantly suppressed in a dose-dependent manner when the exposed doses ranged from 25 μg/mL to 200 μg/mL. Therefore, these results indicate that the surface coatings of FePt NPs play a more crucial role in the suppression of U251 cell proliferation than their components.
Figure 5 Cell proliferation of human glioma U251 cells treated with FePt NPs with different components and surface coatings.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) Fe60Pt40-Cys; (E) Fe24Pt76-Cys.
Abbreviations: NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
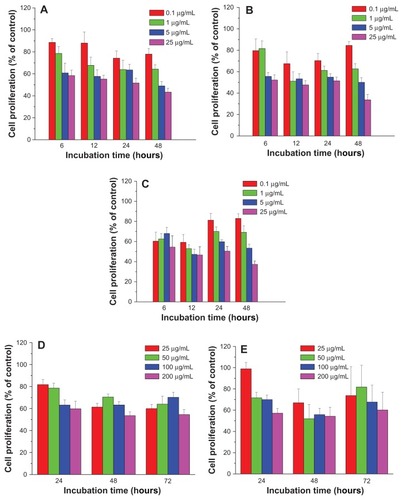
The suppression of FePt NPs on glioma cell proliferation was further confirmed using human astrocytoma U87 cells and human neuroglioma H4 cells as models. As shown in , the proliferation of U87 cells was significantly suppressed by both Fe60Pt40-OA/OA NPs and Fe27Pt73-OA/OA NPs in a time-dependent manner at exposed doses higher than 5 μg/mL, while dose-dependent suppression on cell proliferation was observed after 12 hours’ incubation. By comparison, Fe45Pt55-OA/OA NPs induced a time-dependent suppression of U87 cell proliferation and seemed to be more toxic than the other two FePt-OA/OA NPs. For example, cell proliferation was decreased to approximately 72.8% ± 5.4%, 67.8% ± 4.5%, 67.9% ± 1.3%, and 64.4% ± 4.2% compared to that of the control group when cells were incubated with Fe45Pt55-OA/OA NPs for 6 hours at exposed doses from 0.1 to 25 μg/mL. Under the same incubation conditions, cell proliferation decreased to approximately 81.2% ± 9.7%, 80.1% ± 12.9%, 80.0% ± 9.4%, and 64.6% ± 7.6% in the case of the Fe60Pt40-OA/OA NPs, and approximately 86.3% ± 5.7%, 84.9% ± 4.9%, 87.8% ± 11.4% and 72.1% ± 7.9% in the case of the Fe27Pt73-OA/OA NPs.
Figure 6 Cell proliferation of human astrocytoma U87 cells treated with FePt NPs with different components and surface coatings.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) Fe60Pt40-Cys; (E) Fe24Pt76-Cys.
Abbreviations: NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
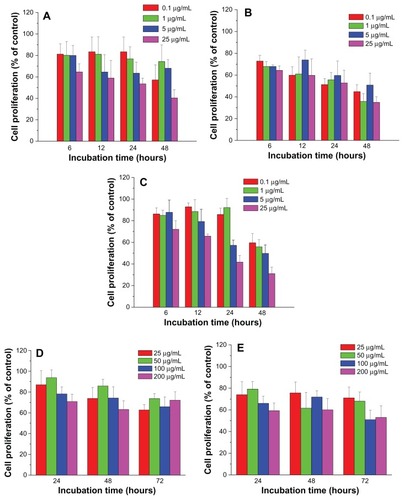
Similarly, the proliferation of H4 cells was significantly suppressed by lipophilic FePt-OA/OA NPs in a time-dependent manner (). Under the same incubation conditions, Fe45Pt55-OA/OA NPs were more toxic to H4 cells than the other two FePt-OA/OA NPs. For example, when H4 cells were incubated with FePt-OA/OA NPs at different doses ranging from 0.1 to 25 μg/mL for 12 hours, cell proliferation was decreased to approximately 81.4% ± 6.0%, 65.3% ± 16.1%, 72.8% ± 16.1%, and 72.6% ± 12.1% in the case of Fe60Pt40-OA/OA NPs; approximately 61.7% ± 10.2%, 73.1% ± 10.7%, 56.0% ± 8.6%, and 50.6% ± 4.0% in the case of Fe45Pt55-OA/OA NPs; and approximately 92.5% ± 8.3%, 90.4% ± 13.6%, 70.1% ± 11.2%, and 76.1% ± 8.8% in the case of Fe27Pt73-OA/OA NPs. On the other hand, the slight suppression on the proliferation of both U87 cells and H4 cells was induced by hydrophilic FePt-Cys NPs at the exposed dose of 25 μg/mL after 24 hours’ incubation (; ). The remarkable decreases of cell viability were observed either at prolonged incubation times (up to 72 hours) or at higher exposed doses (>100 μg/mL). Therefore, these results demonstrated that the suppression of FePt NPs on glioma cell proliferation was dominantly determined by their surface coatings rather than their components.
Figure 7 Cell proliferation of human neuroglioma H4 cells treated by FePt NPs with different components and surface coatings.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) Fe60Pt40-Cys; (E) Fe24Pt76-Cys.
Abbreviations: NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
Cellular uptake of FePt NPs by glioma cells
The cellular uptake of FePt NPs with different surface coatings and components by glioma cells was studied using TEM. Glioma cells were incubated with FePt NPs at a dose of 25 μg/mL for 24 hours. As shown in , numerous vesicles containing FePt NPs were visible in the interior space of the U251 cells, providing direct evidence for the internalization of FePt NPs. The insets in show that the aggregates of FePt NPs contained in the vesicles were released and diffused into the cytoplasm. The initial phase involved the caveolae, and the formation of the vesicles enwrapping the aggregates of FePt NPs is shown in the inset of . Although the proliferation of U251 cells was significantly suppressed by FePt NPs dominantly dependent on their surface coatings, cellular uptake of FePt NPs by U251 cells seemed to be independent of both their surface coatings and components.
Figure 8 Representative TEM images showing the uptake of FePt NPs with different components and surface coatings by human glioma U251 cells at the exposed dose of 25 μg/mL after 24 hours’ incubation.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) Fe60Pt40-Cys; (E) Fe24Pt76-Cys. Numerous vesicles containing the aggregative FePt NPs were visible intracellularly and were dispersed randomly in the cytoplasm. The insets in panels (A and B) are enlargements of the areas marked in white, showing the aggregative FePt NPs contained in the vesicles. The inset in panel (E) is the enlargement of the area marked in white, showing the formation of a vesicle containing an aggregate of FePt NPs.
Abbreviations: TEM, transmission electron microscopy; NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
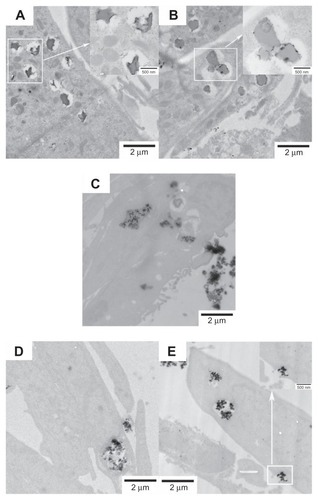
The internalizations of FePt NPs with different surface coatings and components were further confirmed by human astrocytoma U87 cells and human neuroglioma H4 cells, as shown in and . The adsorption of FePt NPs on the surface of cellular membranes is shown in (marked with a white arrow); the release and diffusion of FePt NPs from the vesicles into the cytoplasm are shown in , (marked with white arrows); while shows that an aggregate of FePt NPs penetrated through the nuclear membrane into the cell nucleus (marked with a white arrow).
Figure 9 Representative TEM images showing the uptake of FePt NPs with different components and surface coatings by human astrocytoma U87 cells at the exposed dose of 25 μg/mL after 24 hours’ incubation.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) Fe60Pt40- Cys; (E) Fe24Pt76-Cys. The release and diffusion of FePt NPs from the vesicles into the cytoplasm were observed and are marked with white arrows in panel (A); the penetration of FePt NPs through the nuclear membrane was shown and is marked with white arrows in panel (B). The adsorption of FePt NPs on the surface of cellular membrane was shown and is marked with a white arrow in panel (D).
Abbreviations: TEM, transmission electron microscopy; NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
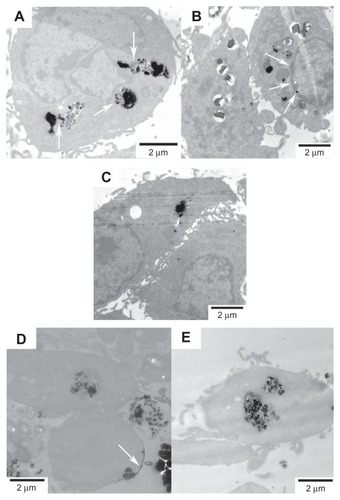
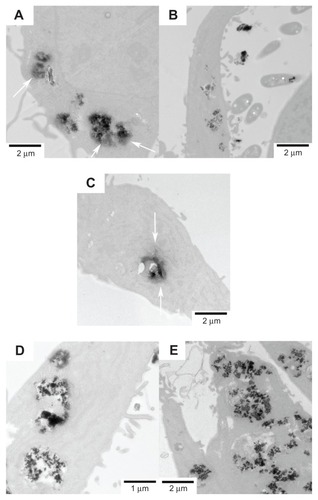
Figure 10 Representative TEM images showing the uptake of FePt NPs with different components and surface coatings by human neuroglioma H4 cells at the exposed dose of 25 μg/mL after 24 hours’ incubation.
Notes: (A) Fe60Pt40-OA/OA; (B) Fe45Pt55-OA/OA; (C) Fe27Pt73-OA/OA; (D) Fe60Pt40- Cys; (E) Fe24Pt76-Cys. The release and diffusion of FePt NPs from the vesicles into the cytoplasm were observed and are marked with white arrows in panels (A and B).
Abbreviations: TEM, transmission electron microscopy; NPs, nanoparticles; OA/OA, oleic acid/oleylamine; Cys, cysteine.
Zeta potential of FePt NPs
The zeta potential of the FePt NPs was measured in phosphate buffer solution and cell culture medium, respectively. When the FePt NPs were dispersed in phosphate buffer solution with a pH value of ~7.4, the measured zeta potential was approximately −20.5 ± 6.2, −12.9 ± 1.4, and −9.0 ± 3.0 mV corresponding to lipophilic Fe60Pt40-OA/OA, Fe45Pt55-OA/OA, and Fe27Pt73-OA/OA NPs, respectively, which was much higher than that of hydrophilic Fe60Pt40-Cys NPs (approximately −42.5 ± 4.1 mV) and Fe24Pt76-Cys NPs (−22.8 ± 3.2 mV). The differences in zeta potential could be attributed to different functional groups contained in the surface coating molecules. Each oleic acid and oleylamine molecule only contains one carboxyl group (−COOH) and one amine group (−NH2), which was consumed for adsorbing on the surface of FePt NPs, while each Cys molecule contains one carboxyl group (−COOH), one mercapto group (—SH), and one amine group (−NH2). This means that at least one of the negatively charged groups (−COOH or −SH) was free after the adsorption of Cys molecules on FePt NPs. Subsequently, when FePt NPs were dispersed in cell culture medium with a pH value of ~7.25, the zeta potentials of lipophilic Fe60Pt40-OA/OA, Fe45Pt55-OA/OA and Fe27Pt73-OA/OA NPs were dramatically increased to approximately −6.4 ± 1.6, −8.6 ± 1.0, and −3.6 ± 1.5 mV, respectively, and those of hydrophilic Fe60Pt40-Cys and Fe24Pt76-Cys NPs were increased to approximately −16.3 ± 3.0 mV and −12.1 ± 1.6 mV. The dramatic increases of zeta potential in cell culture medium could be attributed to different ionic strengths, the presence of proteins, and other factors in culture media. Therefore, the negative zeta potential of FePt NPs confirms that few aggregates of FePt NPs could be attributed to the repulsive forces of the negatively charged surfaces of FePt NPs.
Discussion
It has been reported that both FePt@CoS2 and FePt@Fe2O3 yolk-shell NPs significantly suppress the proliferation of HeLa cells with the IC50 value of approximately 35.5 ± 4.7 and 238 ± 9 ng of Pt/mL, which is much lower or approximate to that of cisplatin (230 ng of Pt/mL).Citation19,Citation23 Additionally, FePt NPs coated with a phospholipid bilayer or polymer shell have been shown to suppress the proliferation of various tumor cells (such as A2780, A431, Sk-Br3, and HEK-293) and induce a proinflammatory response on monocyte-derived macrophages and monocyte-derived dendritic cells.Citation24,Citation37 In the present study, monodispersed FePt NPs were tunably synthesized with different surface coatings and components. The potential application of FePt NPs for malignant glioma therapy was evaluated in vitro using three typical brain glioma cell lines (human glioma U251 cells, human astrocytoma U87 cells, and human neuroglioma H4 cells) as models. The results demonstrated that the proliferation of glioma cells was significantly suppressed by lipophilic FePt-OA/OA NPs in a time- and/or dose-dependent manner. The suppression of the proliferation of glioma cells was dominantly determined by their surface coatings rather than their components. Among these samples, Fe45Pt55-OA/OA NPs displayed the greatest suppression of the proliferation of the three glioma cell lines under the same incubation conditions. The IC50 value of Fe45Pt55-OA/OA NPs in the three glioma cell lines was approximately 5–10 μg/mL after 24 hours’ incubation. Therefore, these results demonstrate that, by engineering the surface coating, FePt NPs could be utilized as a novel and promising therapeutic nanomedicine for malignant gliomas.
In order to investigate the interactions between FePt NPs and glioma cells, the cellular uptake of FePt NPs was observed by TEM. Generally, cellular uptake of the NPs by the cells has been associated with the size, shape, and surface properties of the NPs.Citation38–Citation41 For example, gold and silver NPs coated with antibodies have been demonstrated to regulate membrane receptor-mediated internalization.Citation39 The cellular uptake of gold nanostructures has been shown to be influenced by their size and shape, while their dependency on the factors could be varied by engineering the surface coatings.Citation40 In this study, the cellular uptake of FePt NPs with different surface coatings and components was confirmed in different glioma cell lines. The zeta potential of FePt NPs was remarkably increased in cell culture medium, which decreased the repulsive force between negatively charged FePt NPs and the cell membrane and promoted the adsorption of FePt NPs on the cell membrane. The cellular uptake of FePt NPs by glioma cells may involve the adsorption of FePt NPs on the cell membrane (, , and ), the caveolae (inset of ) and the formation of the vesicles containing the aggregates of FePt NPs, and the release of FePt NPs from the vesicles and their diffusion into cytoplasm (, ) and various organelles. It is very interesting that the cellular uptake of FePt NPs was independent of their surface coatings and components. Recent work suggests that, in the absence of particle aggregation, cellular uptake of gold NPs is dependent on the ratio of sedimentation to diffusion velocities while independent of size, shape, density, surface coating, and initial concentration.Citation42 Further investigation into the precise uptake mechanisms of FePt NPs by glioma cells are currently in progress.
The major cytotoxicities of inorganic NPs on tumor cells were attributed to the intracellular degradation of NPs, the subsequent release of toxic metal ions and the enhancement of intracellular reactive oxygen species levels.Citation43–Citation46 The excessive oxidative stress triggered a series of oxidative damages to cellular organelles and finally induced cell apoptosis and necrosis. The cytotoxic mechanisms of FePt NPs on glioma cells were still amphibolous. In previous studies, Gao et alCitation19,Citation23 suggest that FePt cores contained in FePt@CoS2 and FePt@ Fe2O3 yolk-shell NPs were oxidized and disintegrated under intracellular acidic environments. Subsequently, the released Pt2+ ions diffused out of the shells and entered into the nucleus and mitochondria. The coordination between Pt2+ ions and 5′-GG-3′ bases of DNA finally resulted in damage to DNA and the apoptosis of HeLa cells. However, Sun et alCitation24 indicate that FePt NPs coated with phospholipid bilayers are etched in low pH solution (pH = 4.8), which results in the controlled release of Fe ions but without the detectable release of Pt2+ ions. Fe ion-induced enhancement of intracellular reactive oxygen species levels initiates a series of oxidative damages and cell apoptosis. In this study, FePt NPs significantly suppressed the proliferation of glioma cells dominantly dependent on their surface coatings rather than their components. Although the zeta potentials of the FePt NPs were dependent on their surface coatings, the cellular uptake of FePt NPs was confirmed regardless of the surface the coatings and components. Therefore, we speculate that the surface coatings play a crucial role in the intracellular disintegration of FePt NPs. For example, the high affinity between the mercapto group of Cys molecules and Pt/Pt2+ may play a crucial role in the intracellular disintegration of FePt NPs and DNA damage. Further investigation into the relationship between the surface coatings of FePt NPs and their intracellular disintegration are to be carried out by our group; these studies will be of great benefit to the elucidation of the primary cytotoxic mechanisms of FePt NPs in glioma cells.
Conclusion
We reported the tunable synthesis of monodispersed FePt NPs with different surface coatings and components and their characterization through XRD, TEM, XPS, FT-IR, AAS, and zeta potential. The potential application of FePt NPs for malignant glioma therapy was evaluated using three typical glioma cell lines (human glioma U251 cells, human astrocytoma U87 cells, and human neuroglioma H4 cells) as in vitro models. The results indicated that the proliferation of glioma cells was significantly suppressed by lipophilic FePt-OA/OA NPs instead of hydrophilic FePt-Cys NPs in a time-and/or dose-dependent manner. Although the cellular uptake of FePt NPs was confirmed by TEM observation regardless of the surface coatings and components of the FePt NPs, the suppression of FePt NPs on the proliferation of glioma cells was dominantly determined by their surface coatings rather than their components. The different surface coatings of FePt NPs were shown to impart different surface charges in both phosphate buffered saline and cell culture medium. These results suggest attractive potential for the use of FePt NPs in novel nanomedicines for malignant glioma therapy.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (No 30800256) and the Major Program of National Natural Science Foundation of China (No 81190133), the Research Fund for the Doctoral Program of Higher Education of China (No 200804971065), the Natural Science Foundation of Hubei Province of China (No 2008CDB035), the Self-Determined and Innovative Research Funds of WUT (No 2010la012), and the Program for Changjiang Scholars and Innovative Research Team in University (No IRT1169) at Wuhan University of Technology.
Disclosure
The authors report no conflicts of interest in this work.
References
- BarbuEMolnàrETsibouklisJGóreckiDCThe potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrierExpert Opin Drug Deliv2009655356519435406
- PardridgeWMDrug and gene delivery to the brain: the vascular routeNeuron20023655555812441045
- Juillerat-JeanneretLThe targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug-nanoparticles?Drug Discov Today2008131099110618848640
- FerrariMCancer nanotechnology: opportunities and challengesNat Rev Cancer2005516117115738981
- RiehemannKSchneiderSWLugerTAGodinBFerrariMFuchsHNanomedicine – challenge and perspectivesAngew Chem Int Ed Engl20094887289719142939
- KooYLReddyGRBhojaniMBrain cancer diagnosis and therapy with nanoplatformsAdv Drug Deliv Rev2006581556157717107738
- CabadaTFde PabloCSSerranoAMdel GuerreroFPOlmedoJJGomezMRInduction of cell death in a glioblastoma line by hyperthermic therapy based on gold nanorodsInt J Nanomed2012715111523
- ChertokBDavidAEYangVCPolyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administrationBiomaterials2010316317632420494439
- VeisehOSunCGunnJOptical and MRI multifunctional nanoprobe for targeting gliomasNano Lett200551003100815943433
- SilvaACOliveiraTRMamaniJBApplication of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatmentInt J Nanomed20116591603
- WangSTChenKJWuTHPhotothermal effects of supramolecularly assembled gold nanoparticles for the targeted treatment of cancer cellsAngew Chem Int Ed Engl2010493777378120391446
- RozhkovaEAUlasovILaiBDimitrijevicNMLesniakMSRajhTA high-performance nanobio photocatalyst for targeted brain cancer therapyNano Lett200993337334219640002
- GaoJHGuHWXuBMultifunctional magnetic nanoparticles: design, synthesis, and biomedical applicationsAcc Chem Res2009421097110719476332
- GuHWHoPTsangKWTYuCWXuBUsing biofunctional magnetic nanoparticles to capture gram-negative bacteria at an ultra-low concentrationChem Commun20031519661967
- GuHWHoPTsangKWWangLXuBUsing biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentrationJ Am Chem Soc2003125157021570314677934
- XuCXuKGuHNitrilotriacetic acid-modified magnetic nanoparticles as a general agent to bind histidine-tagged proteinsJ Am Chem Soc20041263392339315025444
- HongRFischerNOEmrickTRotelloVMSurface PEGylation and ligand exchange chemistry of FePt nanoparticles for biological applicationsChem Mater20051746174621
- ChiangPCHungDSWangJWHoCSYaoYDEngineering water-dispersible FePt nanoparticles for biomedical applicationsIEEE Trans Magn20074324452447
- GaoJLiangGCheungJSMultifunctional yolk-shell nanoparticles: a potential MRI contrast and anticancer agentJ Am Chem Soc2008130118281183318681432
- ChenSWangLJDuceSLEngineering biocompatible nanoparticles for in vivo imaging applicationsJ Am Chem Soc2010132150221502920919679
- ChouSWShauYHWuPCYangYSShiehDBChenCCIn vitro and in vivo studies of FePt nanoparticles for dual modal CT/MRI molecular imagingJ Am Chem Soc2010132132701327820572667
- YangHZhangJJTianQWOne-pot synthesis of amphiphilic superparamagnetic FePt nanoparticles and magnetic resonance imaging in vitroJ Magn Magn Mater2010322973977
- GaoJLiangLZhangBKuangYZhangXXuBFePt@CoS2 yolk-shell nanocrystals as a potent agent to kill HeLa cellsJ Am Chem Soc20071291428143317263428
- XuCYuanZKohlerNKimJChungMASunSFePt nanoparticles as an Fe reservoir for controlled Fe release and tumor inhibitionJ Am Chem Soc2009131153461535119795861
- ElkinsKEVedantamTSLiuJPUltrafine FePt nanoparticles prepared by the chemical reduction methodNano Lett2003316471649
- SaitaSMaenosonoSFormation mechanism of FePt nanoparticles synthesized via pyrolysis of iron (III) ethoxide and platinum (II) acetylacetonateChem Mater20051766246634
- TengXYangHSynthesis of magnetic nanocomposites and alloys from platinum-iron oxide core-shell nanoparticlesNanotechnology200516S55456121727477
- Loc NguyenHHowardLEMStintonGWSynthesis of size-Controlled fcc and fct FePt nanoparticlesChem Mater20061864146424
- MonnierVDelalandeMBayle GuillemaudPSamsonYReissPSynthesis of homogeneous FePt nanoparticles using a nitrile ligandSmall200841139114218623297
- SrivastavaCBalasubramanianJTurnerCHWiestJMBagariaHGThompsonGBFormation mechanism and composition distribution of FePt nanoparticlesJ Appl Phys2007102104310-1104310-8
- StahlBGajbhiyeNSWildeGElectronic and magnetic properties of monodispersed FePt nanoparticlesAdv Mater2002142427
- SunSAndersSThomsonTControlled synthesis and assembly of FePt nanoparticlesJ Phys Chem B200310754195425
- SudaMNakagawaMLyodaTEinagaYReversible photoswitching of ferromagnetic FePt nanoparticles at room temperatureJ Am Chem Soc20071295538554317411039
- Salgueirino-MaceiraVLiz-MarzánLMFarleMWater-based ferrofluids from FexPt1−x nanoparticles synthesized in organic mediaLangmuir2004206946695015274608
- LaiCWWangYHUttamBPOne-pot solvothermal synthesis of FePt/Fe3O4 core-shell nanoparticlesChem Commun (Camb)2008145342534418985204
- WuQCaoHLuanQBiomolecule-assisted synthesis of water-soluble silver nanoparticles and their biomedical applicationsInorg Chem2008475882588818498157
- LehmannADParakSJZhangFFluorescent-magnetic hybrid nanoparticles induce a dose-dependent increase in proinflammary response in lung cells in vitro correlated with intracellular localizationSmall2010675376220205203
- VermaAStellacciFEffect of surface properties on nanoparticle-cell IinteractionsSmall20106122119844908
- JiangWKimBYRutkaJTChanWCNanoparticle-mediated cellular response is size-dependentNat Nanotechnol2008314515018654486
- ChoECAuLZhangQXiaYThe effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cellsSmall2010651752220029850
- VermaAUzunOHuYSurface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticlesNat Mater2008758859518500347
- ChoECZhangQXiaYThe effect of sedimentation and diffusion on cellular uptake of gold nanoparticlesNat Nanotechnol2011638539121516092
- LewinskiNColvinVDrezekRCytotoxicity of nanoparticlesSmall20084264918165959
- BhabraGSoodAFisherBNanoparticles can cause DNA damage across a cellular barrierNat Nanotechnol2009487688319893513
- AuffanMRoseJBotteroJYLowryGVJolivetJPWiesnerMRTowards a definition of inorganic nanoparticles from an environmental, health and safety perspectiveNat Nanotechnol2009463464119809453
- PuzynTRasulevBGajewiczAUsing nano-QSAR to predict the cytotoxicity of metal oxide nanoparticlesNat Nanotechnol2011617517821317892