Abstract
Despite several recent advances, current therapy and prevention strategies for myocardial infarction are far from satisfactory, owing to limitations in their applicability and treatment effects. Nanoparticles (NPs) enable the targeted and stable delivery of therapeutic compounds, enhance tissue engineering processes, and regulate the behaviour of transplants such as stem cells. Thus, NPs may be more effective than other mechanisms, and may minimize potential adverse effects. This review provides evidence for the view that function-oriented systems are more practical than traditional material-based systems; it also summarizes the latest advances in NP-based strategies for the treatment and prevention of myocardial infarction.
Introduction
The growing burden of ischemic heart disease (IHD) is a major public health issue. The most harmful type of IHD is acute myocardial infarction (MI), which leads to loss of tissue and impaired cardiac performance, accounting for two in five deaths in China.Citation1 Timely revascularization after MI, including percutaneous coronary intervention, thrombolytic treatment and bypass surgery, is key to improving cardiac function and preventing post-infarction pathophysiological remodeling.Citation2 However, these effective but invasive approaches cannot be used in all patients owing to their applicability, which is limited based on specific clinical characteristics, and the possibility of severe complications such as bleeding and reperfusion injury.Citation2,Citation3 Attempts to limit infarct size and improve prognosis using pharmacotherapy (including antiplatelet and antiarrhythmic drugs and angiotensin-converting enzyme inhibitors) without reperfusion has been proven generally inefficient, due to non-targeted drug distribution and side effects, and short half-life of some drugs.Citation1,Citation3,Citation4 Consequently, many patients in which this approach is used still progress to cardiac hypertrophy and heart failure.Citation1 Growth and rupture of atherosclerotic plaques and the ensuing thrombosis are the major causes of acute MI.Citation4 Currently available interventions for atherosclerosis (AS) including statins can reduce acute MI, but the effects vary between individuals, and leave significant residual risks.Citation5–Citation8 Some chemotherapies, such as docetaxelCitation9 and methotrexate,Citation10,Citation11 also seem to have beneficial effects in AS; however, systemic administration of these drugs is limited because of their adverse effects.Citation12 The demand for safer and more efficient therapies and prevention strategies for MI is therefore increasing.
Several optimized strategies have so far been explored, one of which is the application of nanoparticles (NPs). These nanoscale particles have been widely used in the treatment of tumors and neural diseases.Citation13,Citation14 NPs enable delivery of therapeutic compounds to target sites with high spatial and temporal resolution, enhancement of tissue engineering processes and regulation of the behaviour of transplants such as stem cells. The application of NPs improves the therapeutic effects and minimizes the adverse effects of traditional or novel therapies, increasing the likelihood that they can be successfully translated to clinical settings.Citation15–Citation18 However, research on NPs in this field is still in its infancy.Citation5,Citation19–Citation21 This review summarizes the latest NP-based strategies for managing acute MI, mostly published within the past 7 years, with a particular focus on effects and mechanisms rather than particle types, which have been extensively covered in other reviews (). In addition, we offer an initial viewpoint on the value of function-based systems over those based on materials, and discuss future prospects in this field.
Figure 1 Overview of nanoparticle-based strategies for the treatment and prevention of myocardial infarction. Nanoparticles are capable of delivering therapeutic agents and nucleic acids in a stable and targeted manner, improving the properties of tissue engineering scaffolds, labeling transplanted cells and regulating cell behaviors, thus promoting the cardioprotective effects of traditional or novel therapies.
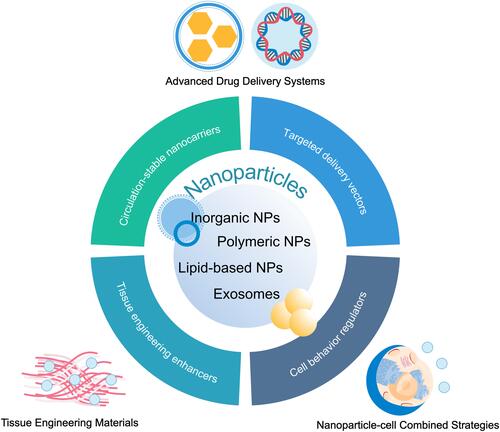
The Types and Properties of Nanoparticles
The Types of Nanoparticles
A multitude of NP types are currently under investigation, including lipid-based NPs, polymeric NPs, micelles, inorganic NPs, and exosomes. Virus can also be considered as NPs; however they will not be discussed in this review.Citation22 NPs made from different materials show similar in vivo metabolic kinetic characteristics and protective effects on infarcted heart.Citation19,Citation20 Function-based NP types, oriented towards a specific purpose, may be preferable compared with traditional types, on account of their practicality in basic research and clinical translation. In this review, we discuss NPs used in the treatment and prevention of MI that fall into the following four categories: 1) circulation-stable nanocarriers (polymeric, lipid or inorganic particles); 2) targeted delivery vectors (magnetic or particles modified to improve target specificity); 3) enhancers of tissue engineering; and 4) regulators of cell behavior (). We propose that the choice of each NP for any given application should be primarily based on the roles or mechanisms they perform.
Many NPs, whether composed of either naturally occurring or synthetic materials, act as nanocarriers to improve the circulating stability of therapeutic agents.Citation15,Citation16 Polymeric NPs comprise one of the most widely employed types, with excellent biocompatibility, tunable mechanical properties, and the ability be easily modified with therapeutic agents using a broad range of chemical techniques.Citation23,Citation24 The most commonly used polymer for these NPs is polylactide-co-glycolide (PLGA), which has Food and Drug Administration approval.Citation25,Citation26 Recently, there has been a therapeutic emphasis on polydopamine (PDA), from which several related nanomaterials have been created, including PDA NPs and PDA NP-knotted hydrogels.Citation27,Citation28 NPs made from polylactic acid (PLA),Citation29,Citation30 poly-ε-caprolactone (PCL),Citation31 polyoxalates,Citation32 polyacrylonitrile,Citation33 chitosanCitation29,Citation34 and hollow mesoporous organosilicaCitation35 have also been constructed and administered in vitro in cells and in vivo in animal models.
Lipid NPs or liposomes are also considered promising candidates for the delivery of therapeutic agents, due to their morphology, which is similar to that of cellular membranes and ability to carry both lipophilic and hydrophilic drugs. These non-toxic, non-immunogenic and biodegradable amphipathic nanocarriers can be designed to reduce capture by reticuloendothelial cells, increase circulation time, and achieve satisfactory targeting.Citation36,Citation37 Solid lipid NPs (SLNs) combine the advantages of polymeric NPs, fat emulsions, and liposomes, remaining in a solid state at room temperature. Active key components of SLNs are mainly physiological lipids, dispersed in aqueous solution containing a stabilizer (surfactant).Citation38 Micelles are made by colloidal aggregation in a solution through self-assembly of amphiphilic polymers, or a simple lipidic layer of transfer vehicles;Citation39 these have been used in cellular and molecular imagingCitation40 and treatmentCitation41 for a long time.
Inorganic NPs used in basic IHD research are classified as metal, metal compounds, carbon,Citation42 or silicon NPs;Citation43 these are relatively inert, stable, and biocompatible. Gold (Au),Citation44 silver (Ag)Citation45 and copper (Cu)Citation46 are commonly used materials in their production. These NPs can be delivered orally,Citation47 or injected intravenouslyCitation48 or intraperitoneally.Citation56 However, they are more widely used to construct electrically conductive myocardial scaffolds in tissue engineering.Citation49,Citation50 Myocardial patches and scaffolds are promising therapeutic approaches to repairing heart tissue after IHD; incorporating conductive NPs can further improve functionality, introducing beneficial physical properties and electroconductivity. Some organic particles, such as liposomes anchored with poly(N-isopropylacrylamide)-based copolymer groups, are also suitable for the production of effective nanogels or patches for this purpose.Citation37
Several metal compounds have been used for treatment of IHD.Citation51–Citation54 The application of magnetic particles made from iron oxide has been of particular interest in recent research. These NPs are more prone to manipulation with an external magnetic field, and thus serve as powerful tools for targeted delivery of therapeutics. In addition, modification with targeted peptides or antibodies is another approach to the construction of targeted delivery systems.
Another strategy to protect cardiac performance after MI is the transplantation of cells; however, the beneficial effects of this are currently limited.Citation58 Many NPs can improve the behavior of cells; in this context, they may stimulate cardioprotective potential. In particular, exosomes – a major subgroup of extracellular vesicles (EVs) with a diameter of 30–150 nm, which are secreted via exocytosisCitation55 – represent novel, heterogeneous, biological NPs with an endogenous origin. They are able to carry a variety of proteins, lipids, nucleic acids, and other bioactive substances.Citation55–Citation57 Mechanistic studies have confirmed that exosomes offer a cell-free strategy to rescue ischemic cardiomyocytes (CMs).Citation59,Citation60
The Physical Properties and Modifications of Nanoparticles
The physical properties of NPs, including size, shape, and surface charge, impact on how biological processes behave, and consequently, responses in the body.Citation61 The recommended definition of NPs in pharmaceutical technology and biomedicine includes a limitation that more than 50% of particles should be in a size distribution range of 10–100 nm.Citation39 However, this is not strictly distinguished in studies, so for the purposes of this review, we have relaxed this definition. Small NPs have a faster uptake and processing speed and longer blood circulation half-lives than larger ones; a decreased surface area results in increased reactivity to the microenvironment and greater speed of release of the compounds they carry.Citation61–Citation63 However, an exception to this principle is that, among particles of less than 50 nm diameter, larger NPs have longer circulatory half-lives.Citation64,Citation65 NPs can be spherical, discoidal, tubular or dendritic.Citation61,Citation63 The impact of NP shape on uptake and clearance has also been revealed;Citation66,Citation67 for instance, spheres endocytose more easily,Citation20 while micelles and filomicelles target aortic macrophages, B cells, and natural killer (NK) cells in the immune system more effectively than polymersomes.Citation68 In terms of charge, cationic NPs are more likely to interact with cells than negatively charged or neutral particles because the mammalian cell membrane is negatively charged.Citation62 As a result, positively charged particles are reported to be more likely to destabilize blood cell membranes and cause cell lysis.Citation61 Additionally, the rate of drug release is largely determined by the diameter of the pore. Motivated by the idea, Palma-Chavez et al developed a multistage delivery system by encapsulating PLGA NPs in micron-sized PLGA outer shells.Citation69
Some types of NPs, such as micelles, possess core–shell morphological structures: a core composed of hydrophobic block segments is surrounded by hydrophilic polymer blocks in a shell that stabilizes the entire micelle. The core provides enough space to accommodate compounds, while the shell protects drug molecules from hydrolysis and enzymatic degradation.Citation36 Surface chemical composition largely governs the chemical interactions between NPs and molecules in the body. Appropriate surface coatings can create a defensive layer, protect encapsulated cargo, and affect biological behaviors. Coating with inert polymers like polyethylene glycol (PEG) is the most commonly used method, which hinders interactions with proteins, alters the composition of the protein corona, attenuate NP recognition by opsonins which tag particles for phagocytosis, and extend the half-life of particles.Citation36,Citation70 Additionally, PEG coating helps the therapeutic agents reach ischemic sites, because PEGylated macromolecules tend to diffuse in the interstitial space of the heart.Citation71 Functionalization of gangliosides can further attenuate the immunogenicity of PEGylated liposomes without damaging therapeutic efficacy.Citation72 Removal of detachable PEG conjugates in the microenvironment of the target sites improves capture by cells. Wang and colleagues synthesized PDA-coated tanshinone IIA NPs by spontaneous hydrophobic self-assembly.Citation73 Polyethyleneimine (PEI) is capable of condensing nucleic acid and overcoming hamper of cell membrane. Therefore, modification with PEI is mainly used for the transport of DNA and RNA.Citation74 Of note, despite their inertness, novel NPs composed of metals can also be modified with compounds such as PEG, thiols, and disulfides.Citation48,Citation75 Hydrogels mixed with peptide-coated Au NPs attain greater viscosity than hydrogels mixed with Au NPs.Citation24
Targeted delivery is a primary goal in the development of nanocarriers. Passive targeting is based on enhanced permeability in ischemic heart tissue, which does not meet the needs of clinical application.Citation76 This fact has prompted work on targeting agent modification and magnetic guidance. Conjugation with specific monoclonal antibodies is a feasible method for delivering drug payloads targeted to ischemic lesions. Copper sulfide (CuS) NPs coupled to antibodies targeting transient receptor potential vanilloid subfamily 1 (TRPV1), permit specific binding to vascular smooth muscle cells (SMCs), and can also act as a switch for photothermal activation of TRPV1 signaling.Citation52 In another study conducted by Liu and colleagues, two types of antibodies, binding CD63 (expressed on the surface of exosomes) or myosin light chain (MLC, expressed on injured CMs) are utilized to allow NPs to capture exosomes and accumulate in ischemic heart tissue. These NPs have a unique structure comprising an ferroferric oxide core and PEG-decorated silica shell, which simultaneously enables magnetic manipulation and molecule conjugation via hydrazone bonds.Citation21 Targeted peptides such as atrial natriuretic peptide (ANP),Citation43 S2P peptide (plague-targeting peptide),Citation77 and stearyl mannose (type 2 macrophage-targeting ligand)Citation16 allow NPs to precisely target atherosclerotic tissue and ischemic heart lesions. Modification with EMMPRIN-binding peptide (AP9) has been shown to enable more rapid uptake of micelles by H9C2 myoblasts and primary CMs and to deliver drug payloads targeted to lesions in vivo.Citation78,Citation79 Another strategy for targeted nanocarriers is to produce cell mimetic carriers. Using the inflammatory response as a marker after MI,Citation76 Boada and colleagues synthesized biomimetic NPs (leukosomes) by integrating membrane proteins purified from activated J774 macrophages into the phospholipid bilayer of NPs. Local chronic inflammatory lesions demonstrated overexpression of adhesion molecules, which bound leukosomes efficiently.Citation80
The Biocompatibility of Nanoparticles
The biocompatibility of NPs is difficult to predict because any interaction with molecules or cells can cause toxic effects. Generally, NPs remain in blood, but can also extravasate from vasculature with enhanced permeability, or accumulate in the mononuclear phagocyte system.Citation81 Important causes of NP-associated toxicity include: oxidative stress injury and cell apoptosis secondary to the production of free radicals, lack of anti-oxidants, phagocytic cell responses, and the composition of some types of particles.Citation61 Hepatotoxicity, nephrotoxicity and any other potential off-target organ damage caused by accumulation of particles, especially those with poor degradability and slow clearance, are also essential to explore in toxicity tests.Citation82 Additionally, the evaluation of evoked immune responses according to the expression of inflammatory factors and stimulation of leukocytes in cell lines and animal models is also important.Citation83
A few studies have reported NP-associated acute and chronic hazards in pharmacological applications, although some of these observations may be contentious. Specifically, aggregation of non-functionalized carbon nanotubes (CNTs) has been observed owing to inherent hydrophobicity of these particles.Citation61 Aside from inflammation and T lymphocyte apoptosis, multi-walled CNTs can rupture cell membranes, resulting in macrophage cytotoxic effects.Citation84,Citation85 Silica NPs induce vascular endothelial dysfunction and promoted the release of proinflammatory and procoagulant factors, mediated by miR-451a negative regulation of the interleukin 6 receptor/signal transducer and activator of transcription/transcription factor (IL6R/STAT/TF) signaling pathway.Citation86–Citation88 Metal NPs, such as Au and Ag, can also penetrate the cell membrane, increase oxidative stress and decrease cell viability.Citation89,Citation90 Consequently, exposure to Au may cause nephrotoxicityCitation91 and reversible cardiac hypertrophy.Citation92 El-Hussainy and colleagues observed myocardial dysfunction in rats given alumina NPs.Citation93,Citation94 Nemmar and colleagues investigated the toxicity of ultrasmall superparamagnetic iron oxide nanoparticles (SPIONs) administered intravenously, which resulted in cardiac oxidative stress and DNA damage as well as thrombosis.Citation95 Cell-derived exosomes and a majority of natural polymers are considered relatively safe;Citation83 however, Babiker and colleagues demonstrated that dendritic polyamidoamine NPs compromise recovery from ischemia/reperfusion (I/R) injury in isolated rat hearts.Citation96 The effects of degradation byproducts are also of concern.Citation83 An advantage of the nanoscale size of NPs is that their injection is unlikely to block the microvascular system; however, it remains controversial whether NPs give rise to arrhythmias.Citation97 These factors highlight that examining the biocompatibility of NPs both in vitro and in vivo is a vital component of preclinical or clinical research.
NP toxicity depends on many parameters, including material composition, coating, size, shape, surface charges and concentration.Citation39 For instance, larger particles seem to be more favorable from a toxicology standpoint.Citation83 However, single-walled CNTs are considered more harmful than multi-walled CNTs, due to their smaller size resulting in less aggregation and increased uptake by macrophages.Citation61 Cationic AuNPs are more toxic compared with anionic AuNPs, which appear to be nontoxic.Citation98 Generally speaking, NP-associated toxicity can be lowered by functionalization with nontoxic surface molecules, stabilization and localization in the region of interest by using scaffolds.Citation24,Citation99 The toxicity of CNTs mediated by oxidative stress and inflammation was reduced using these strategies in several studies.Citation24,Citation100 Local application and targeted delivery also enabled dose reduction and concurrently decreased the incidence of adverse effects. Administration of therapeutic agents directly into the infarcted or peri-infarcted myocardium is a conventional approach with a low risk of inducing embolization.
The Advanced Nanotherapeutic Strategies for Myocardial Infarction
The Advanced Drug Delivery Nanocarriers
NP is a suitable method for the administration of therapeutic agents in terms of the minimization of side effects, enhanced stability of cargo, and possibility of controlled delivery and release.Citation76 Detailed information on the experimental design and results of the latest studies on the use of NPs as therapeutic vectors are provided in . Recently, several drugs approved for clinical use as immunosuppressants have been suggested as potentially effective cardioprotective agents. For example, NPs containing cyclosporine A inhibited apoptosis and inflammation in ischemic myocardium by improving mitochondrial function.Citation25,Citation101 Commercial methotrexate also showed minor cardioprotective effects; additionally, when loaded into lipid core NPs, adenosine bioavailability and echocardiographic and morphometric results were all improved a rats model of MI.Citation102 Margulis and colleagues developed a method to fabricate NPs via a supercritical fluids setup, which loaded and transferred celecoxib, a lipophilic nonsteroidal anti-inflammatory drug, into the NPs. These celecoxib-containing NPs alleviated ejection function damage and ventricular dilation by inducing significant levels of neovascularization.Citation103 Furthermore, a series of investigations indicated that drugs used for hypoglycemia (eg pioglitazone, exenatide and liraglutide)Citation104–Citation106 and lipid lowering (statins)Citation107 attenuate the progression of post-MI heart failure, and are therefore also potential therapeutic cargoes for NPs in the treatment of MI.
Table 1 NPs-Based Drug Delivery Systems for Treatment for MI Reported in the Last 7 Years
NP systems also offer an alternative method for delivering plant-derived therapeutic agents, most of which belong to traditional Chinese medicine. It’s of vital importance because of the criticization on adverse reactions caused by direct injection of such complexes. Cheng and colleagues designed a dual-shell polymeric NP as a multistage, continuous, targeted vehicle of resveratrol, a reactive oxygen species (ROS) scavenger. Due to the severe oxide stress in areas of infarction, the proposed antioxidant-delivery NPs represent a new method to effectively treat MI. These NPs are modified with two peptides, targeting ischemic myocardium and mitochondria, respectively; cardioprotective effects have been confirmed in both hypoxia/reoxygenated (H/R) H9C2 cells and I/R rats.Citation108 In addition, Dong and colleagues also demonstrated that puerarin-SLNs produced smaller areas of infarction in a MI rat model, evaluated by 2,3,5-triphenyltetrazolium chloride (TTC) staining. These particles were modified with cyclic arginyl-glycyl-aspartic acid peptide, a specific targeting moiety to αvβ3 integrin receptors, which are highly expressed on endothelial cells (ECs) during angiogenesis.Citation109 In a recent study, quercetin was loaded into mesoporous silica NPs, which enhanced the inhibition of cell apoptosis and oxidative stress, improving ventricular remodeling and promoting the recovery of cardiac function by activating the janus kinase 2 (JAK2)/STAT3 pathway.Citation110 Similarly, curcumin–polymer NPs, administered by gavage, improved serum inflammatory cytokine levels compared with direct administration of curcumin.Citation111
Translation of novel bioactive agents into clinical practice has been limited, owing to lack of sufficient bioavailability and systemic toxicity.Citation76 Encapsulating small molecules such as 3i-1000 (an inhibitor of the GATA4–NKX2-5 interaction),Citation43 TAK-242 (inhibitor of toll-like receptor 4, TLR4)Citation112 and C143 (inhibitor of ERK1/2)Citation113 in NPs promotes myocardial repair after MI without the risk of uncontrolled and off-target adverse effects. Administration of vascular endothelial growth factor (VEGF) causes elevated vascular permeability and tissue edema. The cardioprotective effects of VEGF-loaded polymeric NPs injected either intravenouslyCitation114 or intramyocardiallyCitation115 eliminated vascular leakage due to promotion of lymphangiogenesis. Further studies have confirmed these results and add to the evidence that combined delivery of VEGF with other growth factors is recommended, since VEGF primarily drives the formation of new capillaries.Citation116 Furthermore, in line with previous research, similar therapeutic effects have been demonstrated in studies using polymeric NPs loaded with stromal cell derived factor 1 (SDF-1) and insulin-like growth factor 1 (IGF-1).Citation117,Citation118
We also notice that some novel payloads in NPs-based therapy for MI have been studied. For example, deoxyribozyme-AuNP can silence tumor necrosis factor-α (TNF-α).Citation119 A target that is implicated in irreversible heart damage after MI; its effects are mediated by free radical production, downregulation of contractile proteins, and initiation of pro-inflammatory cytokine cascades. Mesoporous iron oxide NPs containing the hydrogen sulfide donor compound diallyl trisulfide act as a platform for the controlled and sustained release of this therapeutic gas molecule. The application of these NPs at appropriate concentrations, resulted in the preservation of cardiac systolic performance without any observable detrimental effects on homeostasis in vivo.Citation15
With increasing insight into the molecular mechanisms of MI, a particular emphasis on gene therapy has emerged. Gene expression can be modulated by DNA fragments, messenger RNA (mRNA), microRNA (miRNA) and small interfering RNA (siRNA), which thus represent new approaches for treating ischemia. Currently available nucleic acid delivery systems are mainly divided into viral and non-viral systems. However, virus-based approaches are limited by their potential for uncontrollable mutagenesis.Citation36 From a clinical point of view, NP represents a suitable choice as novel non-viral nucleic acid vector, which could feasibly transfect in a stable, targeted, and sustained manner (as shown in ).
Table 2 NPs-Based Nucleic Acid Delivery Systems for Treatment for MI Reported in the Last 7 Years
As a common gene vehicle, plasmids face the risk of being destroyed by DNase and immunoreactivity in the serum, and transduction in non-target organs.Citation120 A recent study by Kim and colleagues aligns with current research trends focused on virus-free therapies, in which carboxymethylcellulose NPs were designed to transfer 5-azacytidine to halt proliferation, and deliver plasmid DNA containing GATA4, myocyte enhancer factor 2C (MEF2C), and TBX5 to induce reprogramming and cardiogenesis of mature normal human dermal fibroblasts.Citation121 In a methodological study, lipidoid NPs were used to successfully deliver pseudouridine-modified mRNA, encoding enhanced green fluorescent protein.Citation122
MiRNAs act as essential regulators of cellular processes through post-transcriptional suppression; increasing evidence reveals miRNAs play critical roles in cardiovascular diseases. An miRNA-transferring platform with self-accelerating nucleic acid release, containing a heparin core and an ethanolamine-modified poly(glycidyl methacrylate) shell, has been constructed and used as an efficient vector of miR-499, which inhibits cardiomyocyte apoptosis.Citation123 Intravenous administration of anionic hyaluronan-sulfate NPs (mean diameter 130 nm) enable the stable delivery of miR-21 mimics, thus modulating the expression of TNFα, transforming growth factor (TGF)β, and suppressor of cytokine signaling 1 (SOCS1). Consequently, these NPs switch the phenotype of macrophages from pro-inflammatory to reparative, promote neovascularization and reduced collagen deposition.Citation124 Interestingly, silencing miR-21 using antagomiR-21a-5p in a nanoparticle formulation has also been shown to reduce expression of pro-inflammatory cytokines in vitro, and attenuate inflammation and fibrosis in mice with autoimmune myocarditis.Citation125 A number of other potentially therapeutic miRNAs have also been successfully transferred to CMs in recent works, including miR-146a, miR-146b-5p, miR-181b, miR-199-3p, miR-214-3p, miR-194-5p and miR-122-5p.Citation126–Citation128 Evaluation of angiogenesis, cardiac function, and scar size in these studies indicated that injectable miRNA–NPs can deliver miRNA to restore injured myocardium efficiently and safely. Yang and colleagues developed an in vivo miRNA delivery system incorporating a shear-thinning hydrogel and NPs characterized by surface presence of miRNA and cell-penetrating peptide (CPP).Citation126 Additionally, angiotensin II type 1 receptor-targeting peptide-modified NPs serve as targeted carriers for anti-miR-1 antisense oligonucleotide, significantly reducing apoptosis and infarct size.Citation129
SiRNAs inhibit gene expression by mediating mRNA cleavage in a sequence-specific manner, highlighting NP-based RNA interference as another viable approach to modulate cellular phenotype and attenuate cardiac failure. Dosta and colleagues demonstrated that poly(β-amino ester) particles modified by adding lysine-/histidine-oligopeptides could represent a system for the transfer of siRNA.Citation130 Studies have now revealed that chemokine C–C motif ligand 2 (CCL2) and its cognate receptor C–C chemokine receptor 2 (CCR2) promoted excessive Ly6Chigh inflammatory monocyte infiltration in infarcted area and aggravate myocardial injury.Citation131 Photoluminescent mesoporous silicon nanoparticles (MSNPs) carrying siCCR2 have been reported to improve the effectiveness of transplanted mesenchymal stem cells (MSCs) in reducing myocardial remodeling after acute MI.Citation131 Targeted transportation and enhanced uptake with minimum leakage improved the efficiency of delivery via NPs, significantly outperforming the control group. Taken together, these studies demonstrate that NPs act as promising drug delivery systems in the treatment of MI.
Enhancement of Cardiac Engineering Biomaterials by Nanoparticles
Myocardial patches and scaffolds, consisting of either bioactive hydrogels or nanofibers, are minimally invasive, relatively localized, and targeted approaches to repair the heart after IHD. Those biomaterials must have an anisotropic structure, mechanical elasticity, electrical conductivity, and the ability to promote ischemic heart repair.Citation132 A variety of NPs have been applied in this field, among which inorganic NPs have been the focus of most research efforts.Citation42 These investigations of inorganic NPs can be divided into four categories based on their effects and the mechanisms involved, which are described in this section.
NPs enhance physical properties and electroconductivity, which is essential for the biomaterials to properly accommodate cardiac cells and subsequently resulted in cell retention, cell-cell coupling and robust synchronized beating behavior. CNTs are able to increase the required physical properties of scaffolds, such as maximum load, elastic modulus, and toughness.Citation133,Citation134 Gelatin methacrylate (GelMA) also has decreased impedance, hydrogel swelling ratio, and pore diameter, as well as increased Young’s modulus when combined with gold nanorods (AuNRs).Citation135 Given this insight, highly electroconductive NPs have been increasingly investigated.Citation34,Citation99 Specifically, Ahadian and colleagues revealed that a higher integrated CNT concentration in gels resulted in greater conductivity.Citation136 Zhou and colleagues verified the therapeutic effects of patches incorporating single-walled CNT for myocardial ischemia, which halted progressive cardiac dysfunction and regenerated the infarcted myocardium.Citation137 Spherical AuNPs have also been shown to increase the conductivity of chitosan hydrogels in a concentration-dependent manner.Citation138 Interestingly, silicon NPs mimic the effects of AuNRs without affecting conductivity or stiffness, as reported by Navaei and colleagues.Citation139
Several studies demonstrate the effects of CNT on CM functions. When CMs are cultured on multi-walled CNT substrates or treated with CNT-integrated patches, these cells show spontaneous electrical activity.Citation34,Citation99,Citation140 Brisa and colleagues functionalized reverse thermal gels with AuNPs, investigating the phenotype of CMs in vitro; the growth of cells with a CM phenotype was observed, along with gap junction formation.Citation141 CMs exposed to AuNR-containing GelMa show higher affinity, leading to packed and uniform tissue structure.Citation135 These conductive scaffolds also facilitate the robustness and synchrony of spontaneous beating in CMs without damaging their viability and metabolic activity.
Combined incorporation of inorganic NPs and cells represents a feasible strategy to promote therapeutic effects. Despite some reports on the cytotoxicity of Au,Citation89,Citation90 no significant loss of viability, metabolism, migration, or proliferation of MSCs in scaffolds containing AuNP is reported. A CNT-embedded, electrospun chitosan/polyvinyl alcohol mesh is reported to promote the differentiation of MSCs to CMs.Citation142 In another approach, Baei and colleagues added AuNPs to chitosan thermosensitive hydrogels seeded with MSCs.Citation138 There was a significant increase in expression of early and mature cardiac markers, indicating enhanced cardiomyogenic differentiation of MSCs compared to the matrix alone, while no difference in growth was observed. Gao et al created a fibrin scaffold, in which cells and AuNPs were suspended simultaneously; these bioactive patches were shown to promote left ventricular function and decrease infarct size and apoptosis in the periscar boarder zone myocardium in swine models of acute MI.Citation97 These studies of AuNP-containing scaffolds demonstrated reduced infarct and fibrotic size, as well as facilitated angiogenesis and cardiac function, which can be attributed at least in part to the enhanced expression of connexin 43 and atrial natriuretic peptide, and activation of the integrin-linked kinase(ILK)/serine-threonine kinase (p-AKT)/GATA4 pathway.Citation49,Citation143,Citation144 Scaffolds containing Ag NPs evoke M2 polarization of macrophages in vitro;Citation145 which may also play a role in cardioprotective action because M2 macrophages are capable of promoting cardiac recovery via the secretion of anti-inflammatory cytokines, collagen deposition, and neovascularization.Citation146
Similarly, CNT also act synergically with poly(N-isopropylacrylamide) scaffolds containing adipose-derived stem cells;Citation147 significant improvement of cardiac function and increased implantation and proliferation of stem cells has been observed with these scaffolds, compared with scaffolds without CNT.Citation147 Selenium NPsCitation148 and titania NPsCitation53 have been shown to improve the mechanical and conductive properties of chitosan patches, promoting their ability to support proliferation and the synchronous activity of cells growing on these patches.
Mounting evidence demonstrates the unique benefits of using cardiac scaffolds with magnetic NPs such as SPIONs; these benefits include, but are not limited to, significant improvements in cell proliferationCitation149 and assembly of electrochemical junctions.Citation150 Given that magnetic manipulation enhances the therapeutic efficacy of iron oxide NPs in cardiac scaffolds, Chouhan and colleagues designed a magnetic actuator device by incorporating magnetic iron oxide NPs (MIONs) in silk nanofibers; this resulted in more controlled drug release properties, as well as the promotion of proliferation and maturation in CMs.Citation151 Magnetic NPs can be used to label induced pluripotent stem cell (iPSC)-derived CMs via conjugation with antibodies against signal-regulatory protein α. Zwi-Dantsis and colleagues reported the construction of tailored cardiac tissue microstructures, achieved by orienting MION-labelled cells along the applied field to impart different shapes without any mechanical support.Citation152 However, the interactions between and effects of NPs and cells in scaffolds, and the cardioprotective efficacy of patches in which NP-labelled cells are suspended, require further elucidation.
Polymeric nanomaterials have also been investigated in the context of cardiac bioengineering materials; for instance, water-swollen polymer NPs have been used to prepare nanogels. With a 3D structure containing cross-linked biopolymer networks, nanogels can encapsulate, protect, and deliver various agents.Citation83,Citation153 PDA-coated tanshinone IIA NPs suspended in a ROS-sensitive, injectable hydrogel via PDA-thiol bonds significantly improved cardiac performance, accompanied by inhibition of the expression of inflammation factors in rat model.Citation73 After implanting cryogel patches consisting of GelMa and linked conductive polypyrrole NPsCitation154 or scaffolds of electrospun GelMA/polycaprolactone with GelMA-polypyrrole NPs,Citation155 left ventricular (LV) ejection fraction (EF) has been shown to increase, with a concurrent decrease in infarct size, in MI animal models.
Combined Nanoparticle–Cell Strategies
Progenitor or stem cell-based therapy in the form of injections and engineered cardiac patches, discussed in the previous section, has been recognized as a promising strategy to improve the cardiac niche and ameliorate adverse remodeling processes and fibrosis after acute MI.Citation56,Citation156,Citation157 However, poor survival and low engraftment rates for transplanted cells are still major challenges in this field.Citation157 Among possible optimization strategies, combining NPs with stem cell therapy is of great interest ().
Table 3 Studies Combining NPs and Cell Therapy Reported in the Last 7 Years
Accumulating evidence has shown two main mechanisms for NP-loaded cell therapy in the context of MI treatment. Firstly, various NP types could efficiently improve survival and cell proliferation, modulating differentiation of implanted cells in the ischemic microenvironment.Citation62,Citation158 Specifically, electrically driven nanomanipulators could guide cardiomyogenic differentiation of MSCs: in a previous study, electroactuated gold NPs were administrated with pulsed electric field stimulation, and tube-like morphological alterations were observed, along with upregulation of cardiac specific markers.Citation143 Adipose-derived stem cells that load PLGA-simvastatin NPs promoted differentiation of these cells into SMCs and ECs, and had cardioprotective effects in a mouse model of MI induced by left anterior descending ligation.Citation17 Secondly, engraftment rate is another important factor affecting treatment efficacy in this context.Citation159 Zhang and colleagues designed silica-coated, MION-labelled endothelial progenitor cells; intravenous administration of these cells in a rat model of MI significantly improved cardiac performance, as indicated by echocardiogram, morphological, and histological evidence, and neovascularization. This indicates magnetic guidance may potentially address the problem of low levels of stem cell retention, which has typically been observed.Citation51 In particular, NPs can link the therapeutic cells to injured CMs, thereby promoting cell anchorage and engraftment. To this end, Cheng and colleagues established a magnetic, bifunctional cell connector by conjugating NPs with two antibodies: one against cell determinant (CD)45, which is expressed on bone marrow-derived stem cells, and one against MLC. The magnetic core of this NP also enabled physical enrichment in ischemic heart tissue using external magnets.Citation160 More than one mechanism may be involved in a study. Chen and colleagues fabricated a sustained release carrier of insulin-like growth factor (IGF), a pro-survival agent, via in situ growth of Fe3O4 NPs on MSNPs. In this study, the NPs promoted both the survival and retention of MSCs, and intramyocardial injection of the NP-labeled MSCs was able to ameliorate functional and histological damage without any obvious toxicity in vivo.Citation161 However, SPION labeling does not seem to improve therapeutic efficiency, as demonstrated by Wang and colleagues in a study using hypoxia-preconditioned SPION-labeled adipose-derived stem cells (ASCs).Citation162
Application of Exosomes in MI Treatment
Primary criticisms of cell-based therapies include their potential immunogenicity, arrhythmogenicity and tumorigenicity. It is widely accepted that the beneficial effects of cell-based therapy are mainly attributable to paracrine effects rather than directly replenishing lost CMs;Citation56 researchers are therefore investigating of cell-free approaches. Exosomes have attractive properties including stable transport, homing to target tissues or cells, and penetration of biological barriers, as well as being more biocompatible with lower immunogenicity than cell-based approaches. Interestingly, post-MI circulating exosomes serve as important cardioprotective messengers.Citation163,Citation164 Manipulating their biodistribution has proven to be a viable strategy to reduce infarct size, promoting angiogenesis and ejection functions.Citation21 However, from a therapeutic standpoint, the lack of control over endogenous exosome production and cargo encapsulation limits the use of this naturally-present mechanism for therapeutic enhancement. The low purity and weak targeting of natural exosomes are two further obstacles to overcome before clinical application. Strategies to address these include finding robust sources; optimized isolation methods for higher yields, efficiency and purity; and improving therapeutic payloads. These have been systematically summarized in other reviews.Citation165–Citation167
Nanoparticle-Based Prevention Strategies for Myocardial Infarction
AS is considered a low-grade, chronic inflammatory disease, characterized by accumulation and deposition of cholesterol in arteries, as well as remodeling of the extracellular matrix in the intima and inner media.Citation12,Citation168 Inflammation of ECs, proliferation of SMCs, and recruitment of monocytes and macrophages play a critical role in the development of AS. NPs allow for the packaging of large amounts of therapeutic compounds in a compact nanostructure, specifically targeting pathological mechanisms and attenuating atherogenesis. Optimization of the loaded drug and NP target together lead to enhanced efficacy while minimizing side effects.Citation169 In this section, we summarize recent breakthroughs in the order of pathological progression, as shown in .
Table 4 NPs-Based Preventive Strategies for MI Reported in the Last 7 Years
Primary prevention refers to control of the risk factors of AS, one of which is hypertension.Citation170 PLA NPs have been shown to improve the efficacy of aliskiren, the first oral direct renin inhibitor and the first in a new class of antihypertensive agents.Citation29 Encapsulation in nanocarriers also renders the application of anandamide viable, which was once limited; recent research revealed that this new therapy could lower blood pressure and LV mass index in rats.Citation171 Similar results were observed in a study in which angiotensinogen was silenced using small hairpin RNA.Citation172 NPs may also help to make more anti-hypertensive drugs available, reduce side effects such as asthma, and lessen the effective dosage by providing sustained drug release over time. The link between AS and diabetes mellitus, which describes a group of metabolic disorders, has also been investigated in numerous studies.Citation173 Possible mechanisms include oxidative stress, altered protein kinase signaling, and epigenetic modifications. Cetin and colleagues successfully constructed NP-based drug delivery systems for the administration of metformin, an oral antihyperglycemic agent with low oral bioavailability and short biological half-life.Citation174 NPs are also promising tools for improving the oral bioavailability of insulin, which is of great interest because oral insulin will significantly increase patients’ compliance.Citation175,Citation176
The inflammatory hypothesis of AS is now widely established, making selective targeting and accumulation of NPs in inflammatory lesions attractive therapeutic strategies. Targeting macrophages in apoE-/- mice has been shown to result in decreased phagocytosis and suppression of inflammatory genes in lesional macrophages, thus lessening burden of atherosclerotic plaques.Citation177 Tom and colleagues used NPs consisting of high-density lipoprotein (HDL), a known atheroprotective bionanomaterial, as carriers for TNF receptor-associated factor in mice, and observed reductions in both leukocyte recruitment and macrophage activation.Citation178 Both single-walled CNT and HDL-NPs have a favorable safety profile. In a pathological context, activated endothelial tissue expresses more adhesion molecules, such as selectins, than usual. These molecules are thus potential targets for cardiovascular nanomedicine. Glycoprotein Ib (GPIb)Citation179 and biotinylated Sialyl Lewis A (sLeA)Citation69 specifically bind to selectins, leading to the accumulation of conjugated NPs in injured vessels; an in vitro study demonstrated that GPIb-conjugated NPs could bind to target surfaces, where they were taken up by activated ECs under shear stress conditions. In another study, Sager and colleagues simultaneously inhibited five adhesion molecules associated with leukocyte recruitment in post-MI apoE-/- mice. Inflammation in plaque and ischemic heart, rendering acute coronary events and post-MI complications less likely to occur.Citation180 However, targeting inflammatory process may have heterogeneous effects in humans because the targeting moieties and target receptors may be overexpressed in several different pathologic conditions in addition to AS. Oxidation is another factor involved in the development of AS. Upregulation of endothelial nitric oxide synthase (eNOS) leads to vascular construction and other AS-promoting effects. Pechanova and colleagues observed that the application of PLA NPs resulted in larger decreases in NOS than direct administration.Citation29
Aside from these processes, avoiding plaque rupture and thrombosis could be another therapeutic aim. Nakashiro and colleagues showed that delivering pioglitazone via NPs inhibited plaque rupture in apoE-/- mice.Citation181 The integrin ανβ3 is upregulated in angiogenic vasculature, which is ubiquitous in plaque ruptures, which may lead to MI.Citation182 ανβ3 integrin-targeted NPs provide a site-specific drug delivery platform that has been shown to successfully stabilize plaques in rabbits.Citation182 Ji and colleagues used NPs composed of albumin with an average diameter of 225.6 nm to deliver a plasmid containing the tissue-type plasminogen activator gene (t-PA); this system plays a role in preventing thrombosis in addition to attenuating intimal thickness and proliferation of vascular SMCs.Citation183 NPs consisting of engineered amphipathic cationic peptide and serine/threonine protein kinase JNK2 siRNA also reduces thrombotic risk, plaque necrotic area, and vascular barrier disorder in mice given the equivalent of a 14-week western diet.Citation184
The Future Prospects
Innovation and development of therapies based on NPs in recent years has led to significant advances towards complete repair of the injured myocardium following acute MI. Nevertheless, developing clinically relevant solutions remains difficult for several reasons. Firstly, as shown in tables, there is little consistency among studies regarding the characteristics of NPs, their payloads, and their methods of administration, as well as methods used for evaluating cardiac repair. It can be difficult to control characteristics such as the size of the synthesized particles in a narrow range, even within single studies. Such significant heterogeneity can lead to differences in observed results in repeated experiments, or under different conditions. Secondly, although many studies have focused on the health effects of unintentional exposure to NPs by inhalation or ingestion,Citation185,Citation186 most of the studies on medical applications of NPs have not reported on toxicity of NP systems until recently.Citation73 Remarkably, there has not been a consensus on NP-associated adverse effects in existing reports, making assessments of biocompatibility a priority for NP characterization.
NPs have emerged as a powerful tool for controlling cell signaling pathways in regenerative strategies using novel therapeutics and drugs that are unsuitable for direct administration. One advantage of the application of NP systems is the ability to release the drug payload or regulate gene expression in a stable and controlled manner. Therefore, many otherwise serious side effects, such as sudden arrhythmic deaths resulting from persistent and uncontrolled expression of miRNA by viral vectors, may be completely avoided.Citation187 More research is required to develop stable and efficient methods of NP production, improve encapsulation efficiency of drugs, and achieve satisfactory targeting. In particular, a greater focus on investigating NP-based switches, including optical, electrical and magnetic methods, has enabled the regulation of cell signaling, exemplified by the development of a CuS NP-based photothermal switch.Citation52 Optimizing tissue engineering scaffolds containing conductive NPs is a promising strategy for the protection of the myocardium after ischemia by mimicking the myocardial extracellular matrix. Improvements in understanding of cardiac repair mechanisms, and how these biomaterials may interfere with them, is therefore urgently needed. Furthermore, heart repair is complex and involves many processes, including apoptosis, angiogenesis, inflammatory infiltration, and fibrosis. Therefore, novel treatments should be designed using NP-based integrative strategies based on these multiple different mechanisms. However, it’s important to highlight that synergistic effects of different drug payloads, NPs, and NP–cell combined strategies should be addressed, as not all may be compatible with one another. Future research should focus on these aspects to translate NP-based therapeutic strategies for MI into practical and effective clinical use.
Disclosure
The authors report no conflicts of interest in this work.
References
- Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389(10065):197–210. doi:10.1016/s0140-6736(16)30677-8
- Marín-Juez R, El-Sammak H, Helker CSM, et al. Coronary revascularization during heart regeneration is regulated by epicardial and endocardial cues and forms a scaffold for cardiomyocyte repopulation. Dev Cell. 2019;51(4):503–515.e4. doi:10.1016/j.devcel.2019.10.019
- Rentrop KP, Feit F. Reperfusion therapy for acute myocardial infarction: concepts and controversies from inception to acceptance. Am Heart J. 2015;170(5):971–980. doi:10.1016/j.ahj.2015.08.005
- Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125(9):1147–1156. doi:10.1161/circulationaha.111.047431
- Ou LC, Zhong S, Ou JS, Tian JW. Application of targeted therapy strategies with nanomedicine delivery for atherosclerosis. Acta Pharmacol Sin. 2021;42(1):10–17. doi:10.1038/s41401-020-0436-0
- Gorabi AM, Kiaie N, Reiner Ž, Carbone F, Montecucco F, Sahebkar A. The therapeutic potential of nanoparticles to reduce inflammation in atherosclerosis. Biomolecules. 2019;9(9):2545. doi:10.3390/biom9090416
- Young DR, Hivert MF, Alhassan S, et al. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134(13):e262–79. doi:10.1161/cir.0000000000000440
- Ekroos K, Jänis M, Tarasov K, Hurme R, Laaksonen R. Lipidomics: a tool for studies of atherosclerosis. Curr Atheroscler Rep. 2010;12(4):273–281. doi:10.1007/s11883-010-0110-y
- Meneghini BC, Tavares ER, Guido MC, et al. Lipid core nanoparticles as vehicle for docetaxel reduces atherosclerotic lesion, inflammation, cell death and proliferation in an atherosclerosis rabbit model. Vascul Pharmacol. 2019;115:46–54. doi:10.1016/j.vph.2019.02.003
- Bulgarelli A, Martins Dias AA, Caramelli B, Maranhão RC. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 2012;59(4):308–314. doi:10.1097/FJC.0b013e318241c385
- Cervadoro A, Palomba R, Vergaro G, et al. Targeting inflammation with nanosized drug delivery platforms in cardiovascular diseases: immune cell modulation in atherosclerosis. Front Bioengineering Biotechnol. 2018;6:177. doi:10.3389/fbioe.2018.00177
- Zhang J, Zu Y, Dhanasekara CS, et al. Detection and treatment of atherosclerosis using nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1). doi:10.1002/wnan.1412
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(22):2108–2121. doi:10.1056/NEJMoa1809615
- Bahadur S, Sachan N, Harwansh RK, Deshmukh R. Nanoparticlized system: promising approach for the management of alzheimer’s disease through intranasal delivery. Curr Pharm Des. 2020;26(12):1331–1344. doi:10.2174/1381612826666200311131658
- Wang W, Liu H, Lu Y, et al. Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia-reperfusion injury. Int J Nanomedicine. 2019;14:875–888. doi:10.2147/ijn.S186225
- Banik B, Surnar B, Askins BW, Banerjee M, Dhar S. Dual-targeted synthetic nanoparticles for cardiovascular diseases. ACS Appl Mater Interfaces. 2020;12(6):6852–6862. doi:10.1021/acsami.9b19036
- Yokoyama R, Ii M, Masuda M, et al. Cardiac regeneration by statin-polymer nanoparticle-loaded adipose-derived stem cell therapy in myocardial infarction. Stem Cells Transl Med. 2019;8(10):1055–1067. doi:10.1002/sctm.18-0244
- Wang J, Xiang B, Deng J, et al. Externally applied static magnetic field enhances cardiac retention and functional benefit of magnetically iron-labeled adipose-derived stem cells in infarcted hearts. Stem Cells Transl Med. 2016;5(10):1380–1393. doi:10.5966/sctm.2015-0220
- Gadde S, Rayner KJ. Nanomedicine Meets microRNA: current Advances in RNA-Based Nanotherapies for Atherosclerosis. ACS Nano. 2016;36(9):e73–9. doi:10.1161/atvbaha.116.307481
- Sun Y, Lu Y, Yin L, Liu Z. The roles of nanoparticles in stem cell-based therapy for cardiovascular disease. Int J Nanomedicine. 2020;8:947. doi:10.3389/fbioe.2020.00947
- Liu S, Chen X, Bao L, et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng. 2020;4(11):1063–1075. doi:10.1038/s41551-020-00637-1
- Pitek AS, Wang Y, Gulati S, et al. Elongated plant virus-based nanoparticles for enhanced delivery of thrombolytic therapies. Mol Pharm. 2017;14(11):3815–3823. doi:10.1021/acs.molpharmaceut.7b00559
- Won YW, McGinn AN, Lee M, Bull DA, Kim SW. Targeted gene delivery to ischemic myocardium by homing peptide-guided polymeric carrier. Article. Mol Pharm. 2013;10(1):378–385. doi:10.1021/mp300500y
- Sink E, Narayan SP, Abdel-Hafiz M, Mestroni L, Peña B. Nanomaterials for cardiac tissue engineering. Molecules. 2020;25(21). doi:10.3390/molecules25215189
- Zhang CX, Cheng Y, Liu DZ, et al. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J Nanobiotechnology. 2019;17(1):18. doi:10.1186/s12951-019-0451-9
- Oduk Y, Zhu W, Kannappan R, et al. VEGF nanoparticles repair the heart after myocardial infarction. Am J Physiol Heart Circ Physiol. 2018;314(2):H278–h284. doi:10.1152/ajpheart.00471.2017
- Caldas M, Santos AC, Veiga F, Rebelo R, Reis RL, Correlo VM. Melanin nanoparticles as a promising tool for biomedical applications - a review. Acta biomaterialia. 2020;105:26–43. doi:10.1016/j.actbio.2020.01.044
- Wang X, Wang C, Wang X, Wang Y, Zhang Q, Cheng Y. A polydopamine nanoparticle-knotted poly(ethylene glycol) hydrogel for on-demand drug delivery and chemo-photothermal therapy. Chem Mater. 2017;29(3):1370–1376. doi:10.1021/acs.chemmater.6b05192
- Pechanova O, Barta A, Koneracka M, et al. Protective effects of nanoparticle-loaded aliskiren on cardiovascular system in spontaneously hypertensive rats. Molecules. 2019;24(15):2710. doi:10.3390/molecules24152710
- Mao S, Wang L, Chen P, Lan Y, Guo R, Zhang M. Nanoparticle-mediated delivery of Tanshinone IIA reduces adverse cardiac remodeling following myocardial infarctions in a mice model: role of NF-κB pathway. Artif Cells, Nanomed Biotechnol. 2018;46(sup3):S707–s716. doi:10.1080/21691401.2018.1508028
- Guex AG, Kocher FM, Fortunato G, et al. Fine-tuning of substrate architecture and surface chemistry promotes muscle tissue development. Acta biomaterialia. 2012;8(4):1481–1489. doi:10.1016/j.actbio.2011.12.033
- Bae S, Park M, Kang C, et al. Hydrogen peroxide-responsive nanoparticle reduces myocardial ischemia/reperfusion injury. J Am Heart Assoc. 2016;5(11). doi:10.1161/jaha.116.003697
- Wu S, Duan B, Qin X, Butcher JT. Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering. Acta biomaterialia. 2017;51:89–100. doi:10.1016/j.actbio.2017.01.051
- Pok S, Vitale F, Eichmann SL, Benavides OM, Pasquali M, Jacot JG. Biocompatible carbon nanotube-chitosan scaffold matching the electrical conductivity of the heart. ACS Nano. 2014;8(10):9822–9832. doi:10.1021/nn503693h
- Zhu K, Wu M, Lai H, et al. Nanoparticle-enhanced generation of gene-transfected mesenchymal stem cells for in vivo cardiac repair. Biomaterials. 2016;74:188–199. doi:10.1016/j.biomaterials.2015.10.010
- Singh B, Garg T, Goyal AK, Rath G. Recent advancements in the cardiovascular drug carriers. Artif Cells, Nanomed Biotechnol. 2016;44(1):216–225. doi:10.3109/21691401.2014.937868
- Jung H, Jang MK, Nah JW, Kim YB. Synthesis and Characterization of Thermosensitive Nanoparticles Based on PNIPAAm Core and Chitosan Shell Structure. Article. Macromol Res. 2009;17(4):265–270. doi:10.1007/bf03218690
- Paliwal R, Paliwal SR, Kenwat R, Kurmi BD, Sahu MK. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin Ther Pat. 2020;30(3):179–194. doi:10.1080/13543776.2020.1720649
- Piperigkou Z, Karamanou K, Engin AB, et al. Emerging aspects of nanotoxicology in health and disease: from agriculture and food sector to cancer therapeutics. Food Chem Toxicol. 2016;91:42–57. doi:10.1016/j.fct.2016.03.003
- Chen W, Jarzyna PA, van Tilborg GA, et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB j. 2010;24(6):1689–1699. doi:10.1096/fj.09-139865
- Nguyen J, Sievers R, Motion JP, Kivimäe S, Fang Q, Lee RJ. Delivery of lipid micelles into infarcted myocardium using a lipid-linked matrix metalloproteinase targeting peptide. Mol Pharm. 2015;12(4):1150–1157. doi:10.1021/mp500653y
- Ashtari K, Nazari H, Ko H, et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv Drug Deliv Rev. 2019;144:162–179. doi:10.1016/j.addr.2019.06.001
- Kinnunen SM, Tölli M, Välimäki MJ, et al. Cardiac Actions of a Small Molecule Inhibitor Targeting GATA4-NKX2-5 Interaction. Sci Rep. 2018;8(1):4611. doi:10.1038/s41598-018-22830-8
- Dong Y, Hong M, Dai R, Wu H, Zhu P. Engineered bioactive nanoparticles incorporated biofunctionalized ECM/silk proteins based cardiac patches combined with MSCs for the repair of myocardial infarction: in vitro and in vivo evaluations. Sci Total Environ. 2020;707:135976. doi:10.1016/j.scitotenv.2019.135976
- Ramirez-Lee MA, Aguirre-Bañuelos P, Martinez-Cuevas PP, et al. Evaluation of cardiovascular responses to silver nanoparticles (AgNPs) in spontaneously hypertensive rats. Nanomedicine. 2018;14(2):385–395. doi:10.1016/j.nano.2017.11.013
- Sharma AK, Kumar A, Sahu M, Sharma G, Datusalia AK, Rajput SK. Exercise preconditioning and low dose copper nanoparticles exhibits cardioprotection through targeting GSK-3β phosphorylation in ischemia/reperfusion induced myocardial infarction. Microvasc Res. 2018;120:59–66. doi:10.1016/j.mvr.2018.06.003
- Zhang T, Dang M, Zhang W, Lin X. Gold nanoparticles synthesized from Euphorbia fischeriana root by green route method alleviates the isoprenaline hydrochloride induced myocardial infarction in rats. J Photochem Photobiol B. 2020;202:111705. doi:10.1016/j.jphotobiol.2019.111705
- Tian A, Yang C, Zhu B, et al. Polyethylene-glycol-coated gold nanoparticles improve cardiac function after myocardial infarction in mice. Can J Physiol Pharmacol. 2018;96(12):1318–1327. doi:10.1139/cjpp-2018-0227
- Hosoyama K, Ahumada M, Variola F, et al. Nanoengineered Electroconductive Collagen-Based Cardiac Patch for Infarcted Myocardium Repair. ACS Appl Mater Interfaces. 2018;10(51):44668–44677. doi:10.1021/acsami.8b18844
- You JO, Rafat M, Ye GJ, Auguste DT. Nanoengineering the heart: conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011;11(9):3643–3648. doi:10.1021/nl201514a
- Zhang BF, Jiang H, Chen J, Hu Q, Yang S, Liu XP. Silica-coated magnetic nanoparticles labeled endothelial progenitor cells alleviate ischemic myocardial injury and improve long-term cardiac function with magnetic field guidance in rats with myocardial infarction. J Cell Physiol. 2019;234(10):18544–18559. doi:10.1002/jcp.28492
- Gao W, Sun Y, Cai M, et al. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat Commun. 2018;9(1):231. doi:10.1038/s41467-017-02657-z
- Liu N, Chen J, Zhuang J, Zhu P. Fabrication of engineered nanoparticles on biological macromolecular (PEGylated chitosan) composite for bio-active hydrogel system in cardiac repair applications. Int J Biol Macromol. 2018;117:553–558. doi:10.1016/j.ijbiomac.2018.04.196
- Zheng Y, Zhang H, Hu Y, Bai L, Xue J. MnO nanoparticles with potential application in magnetic resonance imaging and drug delivery for myocardial infarction. Int J Nanomedicine. 2018;13:6177–6188. doi:10.2147/ijn.s176404
- Xiong YY, Gong ZT, Tang RJ, Yang YJ. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics. 2021;11(3):1046–1058. doi:10.7150/thno.53326
- Huang P, Wang L, Li Q, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116(2):353–367. doi:10.1093/cvr/cvz139
- Zhang LL, Xiong YY, Yang YJ. The vital roles of mesenchymal stem cells and the derived extracellular vesicles in promoting angiogenesis after acute myocardial infarction. Stem Cells Dev. 2021;30(11):561–577. doi:10.1089/scd.2021.0006
- Rodrigo SF, van Ramshorst J, Hoogslag GE, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6(5):816–825. doi:10.1007/s12265-013-9507-7
- Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69. doi:10.1016/j.ijcard.2015.05.020
- Huang P, Tian X, Li Q, Yang Y. New strategies for improving stem cell therapy in ischemic heart disease. Heart Fail Rev. 2016;21(6):737–752. doi:10.1007/s10741-016-9576-1
- Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev. 2009;61(6):457–466. doi:10.1016/j.addr.2009.03.010
- Sun Y, Lu Y, Yin L, Liu Z. The roles of nanoparticles in stem cell-based therapy for cardiovascular disease. Front Bioengineering Biotechnol. 2020;8:947. doi:10.3389/fbioe.2020.00947
- Chan CKW, Zhang L, Cheng CK, et al. Recent advances in managing atherosclerosis via nanomedicine. Small. 2018;14(4):1702793. doi:10.1002/smll.201702793
- Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114(14):1504–1511. doi:10.1161/circulationaha.106.646380
- Pérez-Medina C, Binderup T, Lobatto ME, et al. In Vivo PET Imaging of HDL in Multiple Atherosclerosis Models. JACC Cardiovasc Imaging. 2016;9(8):950–961. doi:10.1016/j.jcmg.2016.01.020
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4934. doi:10.1073/pnas.0600997103
- Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2010;6(1):12–21. doi:10.1002/smll.200901158
- Yi S, Allen SD, Liu YG, et al. Tailoring nanostructure morphology for enhanced targeting of dendritic cells in atherosclerosis. ACS Nano. 2016;10(12):11290–11303. doi:10.1021/acsnano.6b06451
- Palma-Chavez JA, Fuentes K, Applegate BE, Jo JA, Charoenphol P. Development and characterization of PLGA-based multistage delivery system for enhanced payload delivery to targeted vascular endothelium. Macromol Biosci. 2021;21(3):e2000377. doi:10.1002/mabi.202000377
- Schöttler S, Becker G, Winzen S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11(4):372–377. doi:10.1038/nnano.2015.330
- Zheng Y, Lu L, Yan Z, et al. mPEG-icariin nanoparticles for treating myocardial ischaemia. Artif Cells, Nanomed Biotechnol. 2019;47(1):801–811. doi:10.1080/21691401.2018.1554579
- Mima Y, Abu Lila AS, Shimizu T, et al. Ganglioside inserted into PEGylated liposome attenuates anti-PEG immunity. J Controlled Release. 2017;250:20–26. doi:10.1016/j.jconrel.2017.01.040
- Wang W, Chen J, Li M, et al. Rebuilding Postinfarcted Cardiac Functions by Injecting TIIA@PDA Nanoparticle-Cross-linked ROS-Sensitive Hydrogels. ACS Appl Mater Interfaces. 2019;11(3):2880–2890. doi:10.1021/acsami.8b20158
- Thomas TJ, Tajmir-Riahi HA, Pillai CKS. Biodegradable polymers for gene delivery. Molecules. 2019;24(20):3744. doi:10.3390/molecules24203744
- Yang X, Yang M, Pang B, Vara M, Xia Y. Gold nanomaterials at work in biomedicine. Chem Rev. 2015;115(19):10410–10488. doi:10.1021/acs.chemrev.5b00193
- Saludas L, Pascual-Gil S, Roli F, Garbayo E, Blanco-Prieto MJ. Heart tissue repair and cardioprotection using drug delivery systems. Maturitas. 2018;110:1–9. doi:10.1016/j.maturitas.2018.01.011
- Esfandyari-Manesh M, Abdi M, Talasaz AH, Ebrahimi SM, Atyabi F, Dinarvand R. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. DARU J Pharmaceutical Sci. 2020;28(1):131–138. doi:10.1007/s40199-019-00324-w
- Cuadrado I, Piedras MJ, Herruzo I, et al. EMMPRIN-targeted magnetic nanoparticles for in vivo visualization and regression of acute myocardial infarction. Theranostics. 2016;6(4):545–557. doi:10.7150/thno.13352
- Ferreira MP, Ranjan S, Correia AM, et al. In vitro and in vivo assessment of heart-homing porous silicon nanoparticles. Biomaterials. 2016;94:93–104. doi:10.1016/j.biomaterials.2016.03.046
- Boada C, Zinger A, Tsao C, et al. Rapamycin-loaded biomimetic nanoparticles reverse vascular inflammation. Circ Res. 2020;126(1):25–37. doi:10.1161/circresaha.119.315185
- Matoba T, Koga JI, Nakano K, Egashira K, Tsutsui H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J Cardiol. 2017;70(3):206–211. doi:10.1016/j.jjcc.2017.03.005
- Markovsky E, Baabur-Cohen H, Eldar-Boock A, et al. Administration, distribution, metabolism and elimination of polymer therapeutics. J Control Release. 2012;161(2):446–460. doi:10.1016/j.jconrel.2011.12.021
- Yao Y, Liao W, Yu R, Du Y, Zhang T, Peng Q. Potentials of combining nanomaterials and stem cell therapy in myocardial repair. Nanomedicine. 2018;13(13):1623–1638. doi:10.2217/nnm-2018-0013
- Bottini M, Bruckner S, Nika K, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160(2):121–126. doi:10.1016/j.toxlet.2005.06.020
- Hirano S, Kanno S, Furuyama A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol. 2008;232(2):244–251. doi:10.1016/j.taap.2008.06.016
- Duan J, Yu Y, Yu Y, et al. Silica nanoparticles enhance autophagic activity, disturb endothelial cell homeostasis and impair angiogenesis. Part Fibre Toxicol. 2014;11:50. doi:10.1186/s12989-014-0050-8
- Duan J, Liang S, Yu Y, et al. Inflammation-coagulation response and thrombotic effects induced by silica nanoparticles in zebrafish embryos. Nanotoxicology. 2018;12(5):470–484. doi:10.1080/17435390.2018.1461267
- Feng L, Yang X, Liang S, et al. Silica nanoparticles trigger the vascular endothelial dysfunction and prethrombotic state via miR-451 directly regulating the IL6R signaling pathway. Part Fibre Toxicol. 2019;16(1):16. doi:10.1186/s12989-019-0300-x
- Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11:11. doi:10.1186/1743-8977-11-11
- Asharani PV, Lian Y, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19(25):255102. doi:10.1088/0957-4484/19/25/255102
- Sereemaspun A, Rojanathanes R, Wiwanitkit V. Effect of gold nanoparticle on renal cell: an implication for exposure risk. Ren Fail. 2008;30(3):323–325. doi:10.1080/08860220701860914
- Yang C, Tian A, Li Z. Reversible cardiac hypertrophy induced by PEG-coated gold nanoparticles in mice. Sci Rep. 2016;6:20203. doi:10.1038/srep20203
- El-Hussainy HM, Hussein AM, Abdel-Aziz A, El-Mehasseb I. Effects of aluminum oxide (Al2O3) nanoparticles on ECG, myocardial inflammatory cytokines, redox state, and connexin 43 and lipid profile in rats: possible cardioprotective effect of gallic acid. Can J Physiol Pharmacol. 2016;94(8):868–878. doi:10.1139/cjpp-2015-0446
- Keyoumu Y, Huo Q, Cheng L, et al. The detailed biological investigations about combined effects of novel polyphenolic and photo-plasmonic nanoparticles loaded graphene nanosheets on coronary endothelial cells and isolated rat aortic rings. J Photochem Photobiol B. 2020;202:111666. doi:10.1016/j.jphotobiol.2019.111666
- Nemmar A, Beegam S, Yuvaraju P, et al. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part Fibre Toxicol. 2016;13(1):22. doi:10.1186/s12989-016-0132-x
- Babiker F, Benter IF, Akhtar S. Nanotoxicology of Dendrimers in the Mammalian Heart: ex vivo and in vivo Administration of G6 PAMAM Nanoparticles Impairs Recovery of Cardiac Function Following Ischemia-Reperfusion Injury. Int J Nanomedicine. 2020;15:4393–4405. doi:10.2147/ijn.s255202
- Gao L, Gregorich ZR, Zhu W, et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018;137(16):1712–1730. doi:10.1161/circulationaha.117.030785
- Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15(4):897–900. doi:10.1021/bc049951i
- Shin SR, Jung SM, Zalabany M, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7(3):2369–2380. doi:10.1021/nn305559j
- Costa PM, Bourgognon M, Wang JT, Al-Jamal KT. Functionalised carbon nanotubes: from intracellular uptake and cell-related toxicity to systemic brain delivery. J Controlled Release. 2016;241:200–219. doi:10.1016/j.jconrel.2016.09.033
- Ikeda G, Matoba T, Nakano Y, et al. Nanoparticle-mediated targeting of cyclosporine a enhances cardioprotection against ischemia-reperfusion injury through inhibition of mitochondrial permeability transition pore opening. Sci Rep. 2016;6:20467. doi:10.1038/srep20467
- Maranhão RC, Guido MC, de Lima AD, et al. Methotrexate carried in lipid core nanoparticles reduces myocardial infarction size and improves cardiac function in rats. Int J Nanomedicine. 2017;12:3767–3784. doi:10.2147/ijn.S129324
- Margulis K, Neofytou EA, Beygui RE, Zare RN. Celecoxib Nanoparticles for Therapeutic Angiogenesis. ACS Nano. 2015;9(9):9416–9426. doi:10.1021/acsnano.5b04137
- Tokutome M, Matoba T, Nakano Y, et al. Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc Res. 2019;115(2):419–431. doi:10.1093/cvr/cvy200
- Qi Q, Lu L, Li H, et al. Spatiotemporal delivery of nanoformulated liraglutide for cardiac regeneration after myocardial infarction. Int J Nanomedicine. 2017;12:4835–4848. doi:10.2147/ijn.s132064
- Zhang Y, Qian P, Zhou H, et al. Pharmacological signatures of the exenatide nanoparticles complex against myocardial ischemia reperfusion injury. Kidney Blood Press Res. 2018;43(4):1273–1284. doi:10.1159/000492409
- Ichimura K, Matoba T, Nakano K, et al. A translational study of a new therapeutic approach for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin into reperfused myocardium reduces ischemia-reperfusion injury in a preclinical porcine model. PLoS One. 2016;11(9):e0162425. doi:10.1371/journal.pone.0162425
- Cheng Y, Liu DZ, Zhang CX, et al. Mitochondria-targeted antioxidant delivery for precise treatment of myocardial ischemia-reperfusion injury through a multistage continuous targeted strategy. Nanomedicine. 2019;16:236–249. doi:10.1016/j.nano.2018.12.014
- Dong Z, Guo J, Xing X, Zhang X, Du Y, Lu Q. RGD modified and PEGylated lipid nanoparticles loaded with puerarin: formulation, characterization and protective effects on acute myocardial ischemia model. Biomed Pharmacother. 2017;89:297–304. doi:10.1016/j.biopha.2017.02.029
- Liu CJ, Yao L, Hu YM, Zhao BT. Effect of quercetin-loaded mesoporous silica nanoparticles on myocardial ischemia-reperfusion injury in rats and its mechanism. Int J Nanomedicine. 2021;16:741–752. doi:10.2147/ijn.s277377
- Boarescu PM, Chirilă I, Bulboacă AE, et al. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid Med Cell Longev. 2019;2019:7847142. doi:10.1155/2019/7847142
- Fujiwara M, Matoba T, Koga JI, et al. Nanoparticle incorporating Toll-like receptor 4 inhibitor attenuates myocardial ischaemia-reperfusion injury by inhibiting monocyte-mediated inflammation in mice. Cardiovasc Res. 2019;115(7):1244–1255. doi:10.1093/cvr/cvz066
- Ferreira MPA, Ranjan S, Kinnunen S, et al. Drug-loaded multifunctional nanoparticles targeted to the endocardial layer of the injured heart modulate hypertrophic signaling. Small. 2017;13(33):548. doi:10.1002/smll.201701276
- Qiao B, Nie JJ. Functional nanocomplexes with vascular endothelial growth factor a/c isoforms improve collateral circulation and cardiac function. Small. 2020;16(4):e1905925. doi:10.1002/smll.201905925
- Ding Y, Zhao AS, Liu T, et al. An Injectable Nanocomposite Hydrogel for Potential Application of Vascularization and Tissue Repair. Ann Biomed Eng. 2020;48(5):1511–1523. doi:10.1007/s10439-020-02471-7
- Garbayo E, Pascual-Gil S. Nanomedicine and drug delivery systems in cancer and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(5):e1637. doi:10.1002/wnan.1637
- Fang R, Qiao S, Liu Y, et al. Sustained co-delivery of BIO and IGF-1 by a novel hybrid hydrogel system to stimulate endogenous cardiac repair in myocardial infarcted rat hearts. Int J Nanomedicine. 2015;10:4691–4703. doi:10.2147/ijn.s81451
- Awada HK, Long DW, Wang Z, Hwang MP, Kim K, Wang Y. A single injection of protein-loaded coacervate-gel significantly improves cardiac function post infarction. Biomaterials. 2017;125:65–80. doi:10.1016/j.biomaterials.2017.02.020
- Somasuntharam I, Yehl K, Carroll SL, et al. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials. 2016;83:12–22. doi:10.1016/j.biomaterials.2015.12.022
- Kataria K, Sharma A, Garg T, K. goyal A, Rath G. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 2014;4(1):79–86. doi:10.2174/22103031113036660018
- Kim HJ, Oh HJ, Park JS, Lee JS, Kim JH, Park KH. Direct conversion of human dermal fibroblasts into cardiomyocyte-like cells using CiCMC Nanogels Coupled with Cardiac Transcription Factors and a Nucleoside Drug. Advanced Science (Weinheim, Baden-Wurttemberg, Germany). 2020;7(7):1901818. doi:10.1002/advs.201901818
- Turnbull IC, Eltoukhy AA, Fish KM, et al. Myocardial delivery of lipidoid nanoparticle carrying modrna induces rapid and transient expression. Mol Therapy. 2016;24(1):66–75. doi:10.1038/mt.2015.193
- Nie JJ, Qiao B, Duan S, et al. Unlockable nanocomplexes with self-accelerating nucleic acid release for effective staged gene therapy of cardiovascular diseases. Adv Materials. 2018;30(31):e1801570. doi:10.1002/adma.201801570
- Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S. Nanoparticle Delivery of miRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018;18(9):5885–5891. doi:10.1021/acs.nanolett.8b02578
- Mirna M, Paar V, Topf A, et al. A new player in the game: treatment with antagomiR-21a-5p significantly attenuates histological and echocardiographic effects of experimental autoimmune myocarditis. Cardiovasc Res. 2021. doi:10.1093/cvr/cvab015
- Yang H, Qin X, Wang H, et al. An in Vivo miRNA Delivery System for Restoring Infarcted Myocardium. ACS Nano. 2019;13(9):9880–9894. doi:10.1021/acsnano.9b03343
- Ma S, Tian XY, Zhang Y, et al. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep. 2016;6:22910. doi:10.1038/srep22910
- Sayed N, Tambe P, Kumar P, Jadhav S, Paknikar KM, Gajbhiye V. miRNA transfection via poly(amidoamine)-based delivery vector prevents hypoxia/reperfusion-induced cardiomyocyte apoptosis. Nanomedicine. 2020;15(2):163–181. doi:10.2217/nnm-2019-0363
- Xue X, Shi X, Dong H, et al. Delivery of microRNA-1 inhibitor by dendrimer-based nanovector: an early targeting therapy for myocardial infarction in mice. Nanomedicine. 2018;14(2):619–631. doi:10.1016/j.nano.2017.12.004
- Dosta P, Demos C, Ramos V, et al. Delivery of siRNA to Endothelial Cells In Vivo Using Lysine/Histidine Oligopeptide-Modified Poly(β-amino ester) Nanoparticles. Cardiovasc Eng Technol. 2021;12(1):114–125. doi:10.1007/s13239-021-00518-x
- Lu W, Xie Z, Tang Y, et al. Photoluminescent mesoporous silicon nanoparticles with siCCR2 improve the effects of mesenchymal stromal cell transplantation after acute myocardial infarction. Theranostics. 2015;5(10):1068–1082. doi:10.7150/thno.11517
- Izadifar M, Chapman D, Babyn P, Chen X. UV-Assisted 3d bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods. 2018;24(2):74–88. doi:10.1089/ten.TEC.2017.0346
- Ho CM, Mishra A, Lin PT, et al. 3D Printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromol Biosci. 2017;17(4):1600250. doi:10.1002/mabi.201600250
- Kharaziha M, Shin SR, Nikkhah M, et al. Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials. 2014;35(26):7346–7354. doi:10.1016/j.biomaterials.2014.05.014
- Navaei A, Saini H, Christenson W, Sullivan RT, Ros R, Nikkhah M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta biomaterialia. 2016;41:133–146. doi:10.1016/j.actbio.2016.05.027
- Ahadian S, Davenport Huyer L, Estili M, et al. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta biomaterialia. 2017;52:81–91. doi:10.1016/j.actbio.2016.12.009
- Zhou J, Chen J, Sun H, et al. Engineering the heart: evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci Rep. 2014;4:3733. doi:10.1038/srep03733
- Baei P, Jalili-Firoozinezhad S, Rajabi-Zeleti S, Tafazzoli-Shadpour M, Baharvand H, Aghdami N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater Sci Eng C Mater Biol Appl. 2016;63:131–141. doi:10.1016/j.msec.2016.02.056
- Navaei A, Rahmani Eliato K, Ros R, Migrino RQ, Willis BC, Nikkhah M. The influence of electrically conductive and non-conductive nanocomposite scaffolds on the maturation and excitability of engineered cardiac tissues. Biomaterials sci. 2019;7(2):585–595. doi:10.1039/c8bm01050a
- Martinelli V, Cellot G, Toma FM, et al. Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 2012;12(4):1831–1838. doi:10.1021/nl204064s
- Peña B, Maldonado M, Bonham AJ, et al. Gold Nanoparticle-Functionalized Reverse Thermal Gel for Tissue Engineering Applications. ACS Appl Mater Interfaces. 2019;11(20):18671–18680. doi:10.1021/acsami.9b00666
- Mombini S, Mohammadnejad J, Bakhshandeh B, et al. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int J Biol Macromol. 2019;140:278–287. doi:10.1016/j.ijbiomac.2019.08.046
- Thrivikraman G, Madras G, Basu B. Electrically driven intracellular and extracellular nanomanipulators evoke neurogenic/cardiomyogenic differentiation in human mesenchymal stem cells. Biomaterials. 2016;77:26–43. doi:10.1016/j.biomaterials.2015.10.078
- Li Y, Shi X, Tian L, et al. AuNP-collagen matrix with localized stiffness for cardiac-tissue engineering: enhancing the assembly of intercalated discs by β1-integrin-mediated signaling. Adv Materials. 2016;28(46):10230–10235. doi:10.1002/adma.201603027
- Allison S, Ahumada M, Andronic C, et al. Electroconductive nanoengineered biomimetic hybrid fibers for cardiac tissue engineering. J Materials Chem B. 2017;5(13):2402–2406. doi:10.1039/c7tb00405b
- Xu JY, Xiong YY, Lu XT, Yang YJ. Regulation of Type 2 Immunity in Myocardial Infarction. Front Immunol. 2019;10:62. doi:10.3389/fimmu.2019.00062
- Li X, Zhou J, Liu Z, et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials. 2014;35(22):5679–5688. doi:10.1016/j.biomaterials.2014.03.067
- Kalishwaralal K, Jeyabharathi S, Sundar K, Selvamani S, Prasanna M, Muthukumaran A. A novel biocompatible chitosan-Selenium nanoparticles (SeNPs) film with electrical conductivity for cardiac tissue engineering application. Mater Sci Eng C Mater Biol Appl. 2018;92:151–160. doi:10.1016/j.msec.2018.06.036
- Nazari H, Heirani-Tabasi A, Hajiabbas M, et al. Incorporation of SPION-casein core-shells into silk-fibroin nanofibers for cardiac tissue engineering. J Cell Biochem. 2020;121(4):2981–2993. doi:10.1002/jcb.29553
- Mou Y, Lv S, Xiong F, et al. Effects of different doses of 2,3-dimercaptosuccinic acid-modified Fe(2) O(3) nanoparticles on intercalated discs in engineered cardiac tissues. J Biomed Mater Res B Appl Biomater. 2018;106(1):121–130. doi:10.1002/jbm.b.33757
- Chouhan D, Mehrotra S, Majumder O. Magnetic actuator device assisted modulation of cellular behavior and tuning of drug release on silk platform. ACS Biomater Sci Eng. 2019;5:92–105. doi:10.1021/acsbiomaterials.8b00240
- Zwi-Dantsis L, Wang B, Marijon C, et al. Remote magnetic nanoparticle manipulation enables the dynamic patterning of cardiac tissues. Adv Materials. 2020;32(6):e1904598. doi:10.1002/adma.201904598
- McClements DJ. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: physicochemical aspects. Adv Colloid Interface Sci. 2017;240:31–59. doi:10.1016/j.cis.2016.12.005
- Wang L, Jiang J, Hua W. Mussel-inspired conductive cryogel as cardiac tissue patch to repair myocardial infarction by migration of conductive nanoparticles. Adv Funct Mater. 2016;26:4293–4305. doi:10.1002/adfm.201505372
- He Y, Ye G, Song C, et al. Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization. Theranostics. 2018;8(18):5159–5177. doi:10.7150/thno.27760
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi:10.1038/35070587
- Xu J, Xiong YY, Li Q, et al. Optimization of timing and times for administration of atorvastatin-pretreated mesenchymal stem cells in a preclinical model of acute myocardial infarction. Stem Cells Transl Med. 2019;8(10):1068–1083. doi:10.1002/sctm.19-0013
- Chistiakov DA, Melnichenko AA, Orekhov AN, Bobryshev YV. Engineered nanoparticles: their properties and putative applications for therapeutic approaches utilizing stem cells for the repair of atherosclerotic disease. Nat Biomed Eng. 2018;19(14):1639–1648. doi:10.2174/1389450118666171027111528
- Vandergriff AC, Hensley TM, Henry ET, et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials. 2014;35(30):8528–8539. doi:10.1016/j.biomaterials.2014.06.031
- Cheng K, Shen D, Hensley MT, et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat Commun. 2014;5:4880. doi:10.1038/ncomms5880
- Chen F, Zhao ER, Hableel G, et al. Increasing the Efficacy of Stem Cell Therapy via Triple-Function Inorganic Nanoparticles. ACS Nano. 2019;13(6):6605–6617. doi:10.1021/acsnano.9b00653
- Wang J, Xiang B, Deng JX, et al. Hypoxia enhances the therapeutic potential of superparamagnetic iron oxide-labeled adipose-derived stem cells for myocardial infarction. J Huazhong Univ Sci Technol Med Sci. 2017;37(4):516–522. doi:10.1007/s11596-017-1766-0
- Li H, Liao Y, Gao L, et al. Coronary serum exosomes derived from patients with myocardial ischemia regulate angiogenesis through the mir-939-mediated nitric oxide signaling pathway. Theranostics. 2018;8(8):2079–2093. doi:10.7150/thno.21895
- Cheng M, Yang J, Zhao X, et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat Commun. 2019;10(1):959. doi:10.1038/s41467-019-08895-7
- Chen GH, Xu J, Yang YJ. Exosomes: promising sacks for treating ischemic heart disease? Am J Physiol Heart Circ Physiol. 2017;313(3):H508–h523. doi:10.1152/ajpheart.00213.2017
- Xu JY, Chen GH, Yang YJ. Exosomes: a rising star in falling hearts. Front Physiol. 2017;8:494. doi:10.3389/fphys.2017.00494
- Tan SJO, Floriano JF, Nicastro L, Emanueli C, Catapano F. Novel applications of mesenchymal stem cell-derived exosomes for myocardial infarction therapeutics. Biomolecules. 2020;10(5). doi:10.3390/biom10050707
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi:10.1161/atvbaha.108.179705
- Allen S, Liu YG, Scott E. Engineering nanomaterials to address cell-mediated inflammation in atherosclerosis. Regenerative Eng Translational Med. 2016;2(1):37–50. doi:10.1007/s40883-016-0012-9
- Ning B, Chen Y, Waqar AB, et al. Hypertension enhances advanced atherosclerosis and induces cardiac death in Watanabe heritable hyperlipidemic rabbits. Am J Pathol. 2018;188(12):2936–2947. doi:10.1016/j.ajpath.2018.08.007
- Martín Giménez VM, Díaz-Rodríguez P, Sanz RL, et al. Anandamide-nanoformulation obtained by electrospraying for cardiovascular therapy. Int J Pharm. 2019;566:1–10. doi:10.1016/j.ijpharm.2019.05.047
- Yuan LF, Sheng J, Lu P, Wang YQ, Jin T, Du Q. Nanoparticle-mediated RNA interference of angiotensinogen decreases blood pressure and improves myocardial remodeling in spontaneously hypertensive rats. Mol Med Rep. 2015;12(3):4657–4663. doi:10.3892/mmr.2015.3909
- Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835. doi:10.3390/ijms21051835
- Cetin M, Sahin S. Microparticulate and nanoparticulate drug delivery systems for metformin hydrochloride. Drug Deliv. 2016;23(8):2796–2805. doi:10.3109/10717544.2015.1089957
- Li P, Nielsen HM, Fano M, Müllertz A. Preparation and characterization of insulin-surfactant complexes for loading into lipid-based drug delivery systems. J Pharm Sci. 2013;102(8):2689–2698. doi:10.1002/jps.23640
- Ahn S, Lee IH, Lee E, Kim H, Kim YC, Jon S. Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J Controlled Release. 2013;170(2):226–232. doi:10.1016/j.jconrel.2013.05.031
- Flores AM, Hosseini-Nassab N, Jarr KU, et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat Nanotechnol. 2020;15(2):154–161. doi:10.1038/s41565-019-0619-3
- Seijkens TTP, van Tiel CM, Kusters PJH, et al. Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J Am Coll Cardiol. 2018;71(5):527–542. doi:10.1016/j.jacc.2017.11.055
- Kona S, Dong JF, Liu Y, Tan J, Nguyen KT. Biodegradable nanoparticles mimicking platelet binding as a targeted and controlled drug delivery system. Int J Pharm. 2012;423(2):516–524. doi:10.1016/j.ijpharm.2011.11.043
- Sager HB, Dutta P, Dahlman JE, et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med. 2016;8(342):342ra80. doi:10.1126/scitranslmed.aaf1435
- Nakashiro S, Matoba T, Umezu R, et al. Pioglitazone-Incorporated Nanoparticles Prevent Plaque Destabilization and Rupture by Regulating Monocyte/Macrophage Differentiation in ApoE-/- Mice. Arterioscler Thromb Vasc Biol. 2016;36(3):491–500. doi:10.1161/atvbaha.115.307057
- Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1(5):624–634. doi:10.1016/j.jcmg.2008.06.003
- Ji J, Yang JA, He X, Ling WP, Chen XL. Cardiac-targeting transfection of tissue-type plasminogen activator gene to prevent the graft thrombosis and vascular anastomotic restenosis after coronary bypass. Thromb Res. 2014;134(2):440–448. doi:10.1016/j.thromres.2014.04.018
- Pan H, Palekar RU, Hou KK, et al. Anti-JNK2 peptide-siRNA nanostructures improve plaque endothelium and reduce thrombotic risk in atherosclerotic mice. Int J Nanomedicine. 2018;13:5187–5205. doi:10.2147/ijn.S168556
- Ferdous Z, Al-Salam S, Greish YE, Ali BH, Nemmar A. Pulmonary exposure to silver nanoparticles impairs cardiovascular homeostasis: effects of coating, dose and time. Toxicol Appl Pharmacol. 2019;367:36–50. doi:10.1016/j.taap.2019.01.006
- Rossi S, Savi M, Mazzola M, et al. Subchronic exposure to titanium dioxide nanoparticles modifies cardiac structure and performance in spontaneously hypertensive rats. Particle Fibre Toxicol. 2019;16(1):25. doi:10.1186/s12989-019-0311-7
- Nakano Y, Matoba T, Tokutome M, et al. Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci Rep. 2016;6:29601. doi:10.1038/srep29601
- Tong F, Liu S, Yan B, Li X, Ruan S, Yang S. Endogenous ornithine decarboxylase/polyamine system mediated the antagonist role of insulin/PEG-CMCS preconditioning against heart ischemia/reperfusion injury in diabetes mellitus. Int J Nanomedicine. 2018;13:2507–2520. doi:10.2147/ijn.s160848
- Huang Z, Song Y, Pang Z, et al. Targeted delivery of thymosin beta 4 to the injured myocardium using CREKA-conjugated nanoparticles. Int J Nanomedicine. 2017;12:3023–3036. doi:10.2147/ijn.S131949
- Bei W, Jing L, Chen N. Cardio protective role of wogonin loaded nanoparticle against isoproterenol induced myocardial infarction by moderating oxidative stress and inflammation. Colloids Surf B Biointerfaces. 2020;185:110635. doi:10.1016/j.colsurfb.2019.110635
- Zhang S, Wang J, Pan J. Baicalin-loaded PEGylated lipid nanoparticles: characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv. 2016;23(9):3696–3703. doi:10.2147/ijn.s131893
- Qiu J, Cai G, Liu X, Ma D. α(v)β(3) integrin receptor specific peptide modified, salvianolic acid B and panax notoginsenoside loaded nanomedicine for the combination therapy of acute myocardial ischemia. Biomed Pharmacother. 2017;96:1418–1426. doi:10.1016/j.biopha.2017.10.086
- Shao M, Yang W, Han G. Protective effects on myocardial infarction model: delivery of schisandrin B using matrix metalloproteinase-sensitive peptide-modified, PEGylated lipid nanoparticles. Int J Nanomedicine. 2017;12:7121–7130. doi:10.2147/ijn.S141549
- Tan ME, He CH, Jiang W, et al. Development of solid lipid nanoparticles containing total flavonoid extract from Dracocephalum moldavica L. and their therapeutic effect against myocardial ischemia-reperfusion injury in rats. Int J Nanomedicine. 2017;12:3253–3265. doi:10.2147/ijn.s131893
- Vinodhini A, Govindaraju K, Singaravelu G, Sadiq AM, Kumar VG. Cardioprotective potential of biobased gold nanoparticles. Colloids Surf B Biointerfaces. 2014;117:480–486. doi:10.1016/j.colsurfb.2014.01.006
- Mihalko E, Huang K, Sproul E, Cheng K, Brown AC. Targeted Treatment of Ischemic and Fibrotic Complications of Myocardial Infarction Using a Dual-Delivery Microgel Therapeutic. ACS Nano. 2018;12(8):7826–7837. doi:10.1021/acsnano.8b01977
- Ishikita A, Matoba T, Ikeda G, et al. Nanoparticle-Mediated Delivery of Mitochondrial Division Inhibitor 1 to the Myocardium Protects the Heart From Ischemia-Reperfusion Injury Through Inhibition of Mitochondria Outer Membrane Permeabilization: a New Therapeutic Modality for Acute Myocardial Infarction. J Am Heart Assoc. 2016;5(7). doi:10.1161/jaha.116.003872
- Asanuma H, Sanada S, Yoshitomi T, et al. Novel Synthesized Radical-Containing Nanoparticles Limit Infarct Size Following Ischemia and Reperfusion in Canine Hearts. Cardiovascular Drugs and Therapy. 2017;31(5–6):501–510. doi:10.1007/s10557-017-6758-6
- Swyer TW, Strom J, Larson DF. Nanoparticle oxygen delivery to the ischemic heart. Perfusion. 2014;29(6):539–543. doi:10.1177/0267659114534290
- Yu J, Li W, Yu D. Atrial natriuretic peptide modified oleate adenosine prodrug lipid nanocarriers for the treatment of myocardial infarction: in vitro and in vivo evaluation. Drug Des Devel Ther. 2018;12:1697–1706. doi:10.2147/dddt.s166749
- Lee YS, Choi JW, Oh JE, Yun CO, Kim SW. Human relaxin gene expression delivered by bioreducible dendrimer polymer for post-infarct cardiac remodeling in rats. Biomaterials. 2016;97:164–175. doi:10.1016/j.biomaterials.2016.04.025
- Krohn-Grimberghe M, Mitchell MJ. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nat Biomed Eng. 2020;4(11):1076–1089. doi:10.1038/s41551-020-00623-7
- Zhou LS, Zhao GL, Liu Q, Jiang SC, Wang Y, Zhang DM. Silencing collapsin response mediator protein-2 reprograms macrophage phenotype and improves infarct healing in experimental myocardial infarction model. J Inflammation. 2015;12:11. doi:10.1186/s12950-015-0053-8
- Courties G, Heidt T, Sebas M, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63(15):1556–1566. doi:10.1016/j.jacc.2013.11.023
- Zhu K, Lai H, Guo C, et al. Nanovector-based prolyl hydroxylase domain 2 silencing system enhances the efficiency of stem cell transplantation for infarcted myocardium repair. Int J Nanomedicine. 2014;9:5203–5215. doi:10.2147/ijn.s71586
- Hao T, Li J, Yao F, et al. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano. 2017;11(6):5474–5488. doi:10.1021/acsnano.7b00221
- Yin Q, Pei Z, Wang H, Zhao Y. Cyclosporine A-nanoparticles enhance the therapeutic benefit of adipose tissue-derived stem cell transplantation in a swine myocardial infarction model. Int J Nanomedicine. 2014;9:17–26. doi:10.2147/ijn.S52005
- Ma Q, Yang J, Huang X, et al. Poly(Lactide-Co-Glycolide)-Monomethoxy-Poly-(Polyethylene Glycol) Nanoparticles Loaded with Melatonin Protect Adipose-Derived Stem Cells Transplanted in Infarcted Heart Tissue. Stem Cells. 2018;36(4):540–550. doi:10.1002/stem.2777
- Ishii M, Shibata R, Shimizu Y, et al. Multilayered adipose-derived regenerative cell sheets created by a novel magnetite tissue engineering method for myocardial infarction. Int J Cardiol. 2014;175(3):545–553. doi:10.1016/j.ijcard.2014.06.034
- Mao Y, Hu Y, Feng W, et al. Effects and mechanisms of PSS-loaded nanoparticles on coronary microcirculation dysfunction in streptozotocin-induced diabetic cardiomyopathy rats. Biomed Pharmacother. 2020;121:109280. doi:10.1016/j.biopha.2019.109280
- Czyzynska-Cichon I, Janik-Hazuka M, Szafraniec-Szczęsny J, et al. Low Dose Curcumin Administered in Hyaluronic Acid-Based Nanocapsules Induces Hypotensive Effect in Hypertensive Rats. Int J Nanomedicine. 2021;16:1377–1390. doi:10.2147/ijn.S291945
- Tadin-Strapps M, Robinson M, Le Voci L, et al. Development of lipoprotein(a) siRNAs for mechanism of action studies in non-human primate models of atherosclerosis. J Cardiovasc Transl Res. 2015;8(1):44–53. doi:10.1007/s12265-014-9605-1
- Dudhipala N, Veerabrahma K. Candesartan cilexetil loaded solid lipid nanoparticles for oral delivery: characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Deliv. 2016;23(2):395–404. doi:10.3109/10717544.2014.914986
- Chen Y, Zeng Y, Zhu X, et al. Significant difference between sirolimus and paclitaxel nanoparticles in anti-proliferation effect in normoxia and hypoxia: the basis of better selection of atherosclerosis treatment. Bioactive Materials. 2021;6(3):880–889. doi:10.1016/j.bioactmat.2020.09.005
- Akhlaghi S, Rabbani S, Alavi S, et al. Green formulation of curcumin loaded lipid-based nanoparticles as a novel carrier for inhibition of post-angioplasty restenosis. Mater Sci Eng C Mater Biol Appl. 2019;105:110037. doi:10.1016/j.msec.2019.110037
- Di Francesco V, Gurgone D, Palomba R, et al. Modulating Lipoprotein Transcellular Transport and Atherosclerotic Plaque Formation in ApoE(-/-) Mice via Nanoformulated Lipid-Methotrexate Conjugates. ACS Appl Mater Interfaces. 2020;12(34):37943–37956. doi:10.1021/acsami.0c12202
- Mishra S, Bedja D, Amuzie C, et al. Improved intervention of atherosclerosis and cardiac hypertrophy through biodegradable polymer-encapsulated delivery of glycosphingolipid inhibitor. Biomaterials. 2015;64:125–135. doi:10.1016/j.biomaterials.2015.06.001
- Barbieri LR, Lourenço-Filho DD, Tavares ER, et al. Influence of Drugs Carried in Lipid Nanoparticles in Coronary Disease of Rabbit Transplanted Heart. Ann Thorac Surg. 2017;104(2):577–583. doi:10.1016/j.athoracsur.2016.12.044
- Gomes FLT, Maranhão RC, Tavares ER, et al. Regression of Atherosclerotic Plaques of Cholesterol-Fed Rabbits by Combined Chemotherapy With Paclitaxel and Methotrexate Carried in Lipid Core Nanoparticles. J Cardiovasc Pharmacol Ther. 2018;23(6):561–569. doi:10.1177/1074248418778836
- Daminelli EN, Martinelli AE, Bulgarelli A, Freitas FR, Maranhão RC. Reduction of Atherosclerotic Lesions by the Chemotherapeutic Agent Carmustine Associated to Lipid Nanoparticles. Cardiovascular Drugs and Therapy. 2016;30(5):433–443. doi:10.1007/s10557-016-6675-0
- Huang Y, Yu L, Ren J, et al. An activated-platelet-sensitive nanocarrier enables targeted delivery of tissue plasminogen activator for effective thrombolytic therapy. J Controlled Release. 2019;300:1–12. doi:10.1016/j.jconrel.2019.02.033
