?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Icariin is a bioactive herbal ingredient isolated from Herba epimedii, which has been widely used for the treatment of osteoporosis and male sexual dysfunction in traditional Chinese medicine. The major objective of this work is to investigate the different enhancing effects of β-cyclodextrin (β-CD) and hydroxypropyl-β-cyclodextrin (HP-β-CD) on the intestinal absorption of icariin, and to identify the molecular mechanisms of this action. Host–guest-type interactions of icariin with cyclodextrins nanocavities were unambiguously demonstrated by the phase-solubility diagram, ultraviolet spectroscopy, Fourier transform infrared spectroscopy, differential scanning calorimetry, X-ray powder diffractometry, and two dimensional proton nuclear magnetic resonance rotating-frame Overhauser effect spectroscopy. These results were further supported using molecular modeling studies. The rat single-pass intestinal perfusion model showed that the absorption of icariin was affected by P-glycoprotein (Pgp). The icariin/HP-β-CD inclusion complex provided greater enhancement in the intestinal absorption than the icariin/β-CD inclusion complex. Therefore, the enhancing effect was involved in a solubilizing effect and/or Pgp inhibitory effect. Finally, fluorescence anisotropy measurements and Pgp adenosine triphosphatase (ATPase) assay demonstrated that β-CD exhibited no effect on Pgp, while HP-β-CD showed inhibition by restraining the Pgp ATPase activity rather than changing the fluidity of cell membrane.
Introduction
Icariin () is a major constituent of flavonoids isolated from Epimedium brevicornum Maxim, which has been used in traditional Chinese medicine to enhance male sexual function for over 2000 years. Herba epimedii and its complex formulations, such as Xianling Gubao capsules, are widely used in China for the treatment of osteoporosis, coronary artery disease, male sexual dysfunction, acute myocardial ischemia, and asthmatic cough.Citation1–Citation4 Modern pharmacology shows that icariin performs a lot of pharmacological and biological activities, which include preventing osteoporosis, protecting neurons, facilitating penile erection, and performing anti-cancer, anti-depression, and hypouricemic action.Citation5–Citation10 However, poor bioavailability of icariin is a significant barrier to its clinical application.Citation11–Citation13 It should be noted that possible interactions of icariin with P-glycoprotein (Pgp) that are closely involved in the absorption and exsorption of structurally and pharmacologically unrelated drugs in the intestine are taken into consideration.
Figure 1 Structure and proton labeling of (A) icariin, (B) β-CD and (C) HP-β-CD.
Abbreviations: β-CD, beta-cyclodextrin; HP-β-CD, hydroxypropyl-beta-cyclodextrin.
Cyclodextrins (CDs) are cyclic polysaccharides mainly composed of six to eight D-glucose monomers linked by α-1, 4-glucosidic bonds. They have hydrophobic nanocavities and hydrophilic outer surfaces, and can encapsulate hydrophobic guest molecules to form host-guest complexes or super-molecular species. This usually enhances drug solubility in water and affects the physicochemical properties of the drug.Citation14,Citation15 The most widely used natural cyclodextrin, β-CD (), is limited in its pharmaceutical applications due to its limited aqueous solubility (1.85 g/100 mL).Citation16,Citation17 Therefore, chemically modified CDs have been synthesized to overcome this problem (eg, methylated, hydroxypropylated, and sulfobutyl ether CD derivatives).Citation18,Citation19 Hydroxypropyl-β-cyclodextrin (HP-β-CD, ) has been extensively investigated for its relatively high water solubility, low toxicity, and satisfactory inclusion ability.Citation20–Citation22 Several commercial formulations are composed of cyclodextrin inclusion complexes, illustrating the usefulness of this approach.Citation23–Citation26 Additionally, many references have reported that the inhibitory mechanisms of Pgp by methylated β-CD were releasing transporters,Citation27 altering cholesterol levels,Citation28,Citation29 increasing plasma membrane fluidity,Citation30 and so on. We therefore speculate that HP-β-CD might inhibit Pgp activity.
In a more general attempt to optimize the pharmaceutical properties and the absorption of icariin, this work aimed to investigate the effectiveness of β-CD and HP-β-CD in improving the intestinal absorption of icariin. The phase solubility technique and two-dimensional (2D) proton nuclear magnetic resonance (1H NMR) spectroscopy (rotating-frame Overhauser effect spectroscopy, ROESY) were used to investigate the interactions of icariin with β-CD or HP-β-CD in an aqueous solution. Solid inclusion complexes were prepared by a freeze-drying method and were characterized by Fourier transform infrared (FTIR) spectroscopy, differential scanning calorimetry (DSC), and powder X-ray diffractometry (XRD). The main purpose of this study was to investigate the different enhancing effects between β-CD and HP-β-CD on the intestinal absorption of icariin, to identify both β-CD and HP-β-CD as solubility enhancers, and to identify HP-β-CD as a Pgp inhibitor. Moreover, the mechanism by which HP-β-CD affects Pgp inhibition is investigated by membrane anisotropy measurements and Pgp ATPase assay.
Materials and methods
Materials
Icariin was bought from Nanjing Chongyuan biotechnology Co (Nanjing, China). β-CD (molecular mass, 1135) was kindly donated by Maxdragon International Co (Guangzhou, China). HP-β-CD (molecular mass, 1540) was purchased from Wacker Chemie AG (Munich, Germany). Pgp-Glo Assay kit was from Promega Co (Madison, WI). 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC; molecular mass, 734.04, batch number 78K5203), and 1,6-diphenyl-1,3,5-hexatriene (DPH; molecular mass, 232.32, batch number MKBD1354V) were purchased from Sigma-Aldrich Co (St Louis, MO). Acetonitrile and methanol for high-performance liquid chromatography (HPLC) analysis were obtained from Merck Co (Darmstadt, Germany). Deionized water was obtained from a Milli-Q water purification system (Molsheim, France). Other chemicals were commercially available and used as received.
Phase solubility studies
Phase solubility studies were carried out in water according to the method previously reported by Calabrò et al.Citation31 Briefly, excessive amounts of icariin were added to a series of capped tubes containing increasing amounts of β-CD or HP-β-CD (0–18 mM). The suspensions were shaken at room temperature for 2 days. After equilibrium was attained, the samples were filtered through 0.45 μm pore size Millipore syringe filters and assayed by HPLC method. The HPLC analysis was performed on a Waters 2695 system (Waters, Milford, MA) equipped with a 2487 dual λ absorbance detector (Waters). The samples of 10 μL were injected onto an ODS-2 Hypersil reversed-phase C18 column (250 mm × 4.6 mm, 5 μm) at 25°C. All samples were detected with an ultraviolet (UV) detector at 268 nm. The mobile phase consisted of a mixture of acetonitrile/water (30:70, v/v). The flow rate was 1.0 mL/minute. Each test group was performed in triplicate. Phase solubility profiles were obtained by plotting the solubility of icariin versus the concentration of β-CD or HP-β-CD.
Preparation of the inclusion complexes
The inclusion complexes were prepared by way of a freeze-drying method at a 1:1 molar ratio based on the results of phase solubility studies. In short, icariin was dissolved with small amounts of methanol at 45°C. The solution was added to a β-CD or HP-β-CD water solution, and the resulting suspension kept on stirring for 8 hours before cooling slowly to room temperature. The prepared suspension was quickly filtered through 0.45 μm pore size Millipore syringe filters to remove any free icariin. The suspension was then frozen and lyophilized in a Thermo Savant ModulyoD Freeze Dryer (Thermo Scientific, Waltham, MA) at −45°C and 100 mbar to obtain the inclusion complexes. In parallel, physical mixtures containing the same ratios of icariin and β-CD or HP-β-CD were prepared in a mortar.
Characterization of the inclusion complexes
UV spectroscopic analysis of icariin (0.01 mmol/L) with increasing amounts of β-CD and HP-β-CD in 50% DMSO (v/v) was done using a Flexstation 3 microplate reader (Molecular Devices, Sunnyvale, CA) at room temperature.
FTIR spectra were recorded on KBr pellets in the 3900–400 cm−1 wave number range using a Bio-Rad FTS 3000 spectrophotometer (Bio-Rad, Richmond, CA).
DSC was recorded on a Perkin-Elmer DSC 7 (Perkin-Elmer, Norwalk, CT). Samples ranging between 5–10 mg were sealed in aluminum pans and heated at a rate of 10°C/minute from room temperature to 260°C and under N2 flow (100 mL/minute).
XRD was measured in the range of 2θ = 4–54° by step scanning on the Rigaku D/MAX-2500 diffractometer (Rigaku Co, Tokyo, Japan) with Cu Kα radiation operating at 40 kV and 100 mA, and a scanning rate of 2°/minute.
ROESY experiments were obtained on Bruker Avance DRX500 spectrometer (Bruker Analytik, Rheinstetten, Germany) at 298 K in D2O and DMSO-d6 (50:50, v/v). Samples were equilibrated for at least 24 hours before measurement.
Absorption studies
Animals
Male Sprague Dawley rats, weighing 200–220 g, were obtained from Tianjin Shanchuanhong Laboratory Animal Technology Co, Ltd (SCXK 2009-0001; Tianjin, China). The rats were housed under standard conditions and fasted overnight with free access to water until the experiment. Animal experiments were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and the protocol was approved by the Animal Ethics Committee at the Tianjin University of Traditional Chinese Medicine.
Perfusion preparation
The Krebs–Ringer’s buffer, containing 10 μg/mL of phenol red, was used as the perfusion solution. Icariin (8 μg/mL) in the absence or presence of Pgp inhibitor, verapamil (16 μg/mL), or an equivalent amount of the inclusion complexes were dissolved in 1000 mL of perfusion solution.
Rat experiment
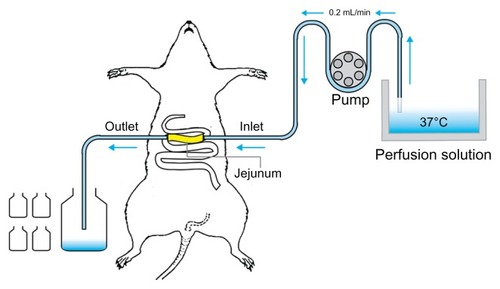
The rat single-pass intestinal perfusion model suggested in this paper is illustrated in . The experiment was performed according to the reported method.Citation32,Citation33 After fasting overnight, male Sprague Dawley rats were randomly assigned to different experimental groups (n = 4). Rats were anesthetized with urethane (1 g/kg, intraperitoneally) and affixed supine on a surface under suitable lighting to maintain body temperature. The jejunum was exposed by a midline abdominal incision, and two polyethylene cannulae (outer diameter, 5 mm; inner diameter, 3 mm) were inserted through small slits at the proximal and distal ends (about 10 cm). To clear the gut, saline solution at 37°C was passed slowly through it until the effluent was clear. The perfusion solutions were incubated in a 37°C water bath. The perfusion flow rate used was 0.2 mL/min, which was 10 times lower than that used in humans (2–3 mL/min). The outflow solution was collected every 15 min for 120 min, starting 15 min after the initiation of the perfusion when steady state had been achieved (as assessed by the inlet/outlet concentration ratio of icariin, which approached 1 at a steady state). The perfusate samples were immediately assayed by HPLC. The length of the perfused intestinal segment was measured accurately at the termination of the experiment.
HPLC assay
The HPLC method described above was used. Linearity was demonstrated over a concentration range of 0.82–6.59 μg/mL (R = 0.9998) for icariin in a Krebs–Ringer’s buffer with a retention time of 12.4 minutes, and 1–25 μg/mL (R = 0.9997) for phenol red with a retention time of 4.4 minutes. The method was precise in the sample analysis with coefficient of variations of 0.74%, 0.55%, and 1.20% at 1.65, 3.30, and 6.59 μg/mL for icariin, respectively; and coefficient of variations of 2.07%, 0.59%, and 0.71% at 2.5, 10, and 25 μg/mL for phenol red, respectively. The method was also reproducible with within-day and between-day variations of 4.13% and 4.20% for icariin and 3.39% and 3.61% for phenol red, respectively.
Data analysis
The netwater flux, resulting from both water absorption and efflux in the intestinal segment, was determined by measurement of phenol red, a nonabsorbed, nonmetabolized marker. The measured Cout′/Cin′ ratio was corrected for water transport according to the following equation:
where Cin phenol red is equal to the concentration of phenol red in the inlet sample, and Cout phenol red is equal to the concentration of phenol red in the outlet sample.
The drug absorption rate constant (Ka) and the effective permeability coefficient (Peff) were calculated using the following equations:
where Q is the perfusion solution flow rate (0.2 mL/minute), V, r, and l are the volume, radius, and length of the intestine segment, respectively.
Mechanisms of Pgp inhibition
Measurement of fluorescence anisotropy
The membrane fluidity was evaluated by anisotropy measurements with the fluorescent probe DPH.Citation34 Around 1 mg of DPPC was dissolved in 500 μL of chloroform and dried under a stream of nitrogen. After the lipids had been dried under a high vacuum for at least 2 hours, they were hydrated by adding 100 μL of phosphate buffered saline of β-CD, HP-β-CD (6.8 mmol/L), or an equivalent of the inclusion complexes. Each sample was vortexed for 2 hours at 60°C to uniformly disperse the lipids. It was then subjected to five cycles of freeze and thaw in liquid nitrogen and a 40°C water bath to ensure solute equilibration between trapped and bulk solutions. The resulting liposomes (0.15 mg of lipids/mL) were incubated for 30 min at 37°C in phosphate buffered saline at a pH of 7.4 with 10 mM DPH which was added as a 100-fold concentrated stock solution in tetrahydrofuran. The anisotropy values were determined with a Flexstation 3 microplate reader (Molecular Devices). The wavelengths of excitation and emission were 360 and 430 nm, respectively.
Determination of Pgp ATPase activity
The ATPase activity of Pgp was determined using a luminescent ATP detection kit (Pgp-Glo Assay kit; Promega) according to the manufacturer’s instructions. Briefly, 0.5 mg/mL of Pgp membranes and 5 mM MgATP were incubated in the absence or presence of 100 μM sodium orthovanadate at 37°C for 40 minutes, and the remaining ATP was detected as a luciferase-generated luminescent signal. Basal Pgp ATPase activity was determined as the difference between ATP hydrolysis in the presence or absence of vanadate. Verapamil-stimulated Pgp ATPase activity was measured in the presence of 200 μM verapamil.
Statistical analysis
Results are presented as mean ± standard deviation. Statistical differences were calculated by one-way analysis of variance with Tukey’s post hoc test using Origin 7.5 (OriginLab Corporation, Northampton, MA). P < 0.05 was considered to be statistically significant.
Results and discussion
Phase solubility studies
The phase solubility diagrams for the icariin/β-CD and the icariin/HP-β-CD systems () showed drug solubility increased linearly with increasing β-CD or HP-β-CD concentration. The solubility increased significantly from 10.1 × 10−3 mM at 0 mM β-CD and HP-β-CD to 1.7 × 10−2 mM and 2.2 × 10−2 mM at 18 mM β-CD and HP-β-CD. The diagrams can be classified as AL type according to the model proposed by Higuchi and Connors,Citation35 and can be related to the formulation of inclusion complexes. The apparent 1:1 stability constant, Kc, was calculated from the phase solubility diagrams based on the following equation:
Figure 3 Phase solubility diagrams of icariin with β-CD or HP-β-CD.
Abbreviations: β-CD, beta-cyclodextrin; HP-β-CD, hydroxypropyl-beta-cyclodextrin.
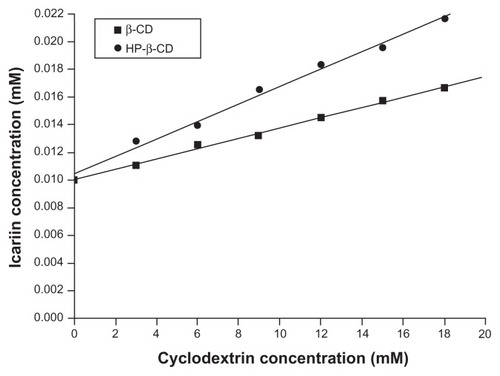
where slope is the value found in the linear regression and S0 (10.1 × 10−3 mM) is the water solubility of icariin in the absence of β-CD or HP-β-CD (intercept). This gave a Kc of 39.62 M−1 and 57.73 M−1 for icariin/β-CD and icariin/HP-β-CD systems, respectively.
UV spectroscopy
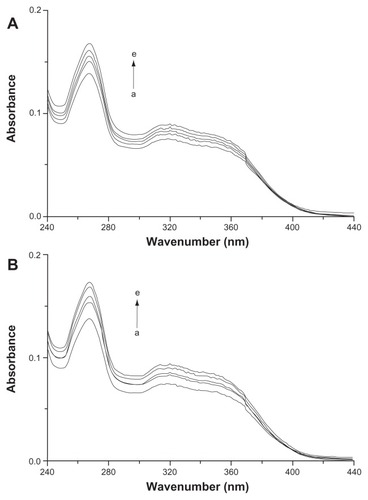
UV spectroscopy was used to further confirm the complexation of icariin with β-CD and HP-β-CD in the solution. The absorption of icariin (0.01 mmol/L) at 268 nm gradually increased upon the addition of varying the concentration (from 0–4 mM) of β-CD and HP-β-CD (). This indicates the formation of inclusion complexes and suggests that this complexation involves the icariin chromophore entering the cyclodextrin cavity.Citation36,Citation37
FTIR spectroscopy
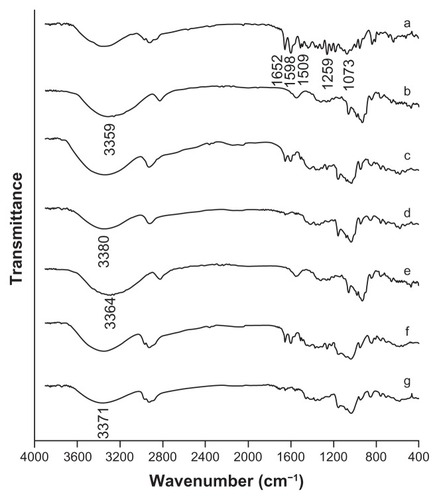
FTIR spectrograms are given in . The free icariin had intense absorptions peaks at 1598 cm−1, 1509 cm−1, 1073 cm−1, 1259 cm−1, and 1652 cm−1, which were the characteristic peaks for identification. The spectrums of β-CD and HP-β-CD presented characteristic peaks at 3359 cm−1 and 3364 cm−1, respectively. The absorption peaks of icariin appeared unchanged in the physical mixtures. This indicated that no interaction occurred during simply physical mixing of the free drug and β-CD or HP-β-CD. The spectrums of the icariin/β-CD and icariin/HP-β-CD inclusion complexes showed the broad peak at around 3380 cm−1 and 3371 cm−1, and less-intense absorptions at around 1598 cm−1, 1509 cm−1, 1073 cm−1, 1259 cm−1, and 1652 cm−1. These results indicated that some functional groups of icariin might be included in the cavity of β-CD or HP-β-CD molecules to form the inclusion complexes. No new peaks were observed in the FTIR spectra of the icariin/β-CD inclusion complex or the icariin/HP-β-CD inclusion complex, indicating that no chemical bonds were created in the formation of this complex.
Figure 5 FTIR spectrograms of the inclusion complexes. (a) icariin, (b) β-CD, (c) the icariin/β-CD physical mixture, (d) the icariin/β-CD inclusion complex. (e) HP-β-CD, (f) the icariin/HP-β-CD physical mixture, and (g) the icariin/HP-β-CD inclusion complex.
Abbreviations: FTIR, Fourier transform infrared; β-CD, beta-cyclodextrin; HP-β-CD, hydroxypropyl-beta-cyclodextrin.
Differential scanning calorimetry
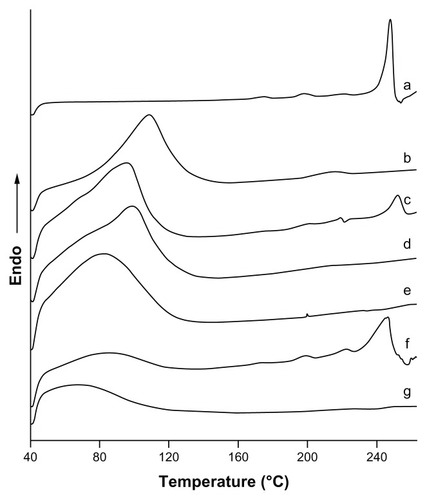
DSC spectrograms are given in . The thermogram of icariin was typical of that of a highly crystalline compound, showing a fusion endothermic peak at 247.57°C (ΔH = 103.25 J/g). The thermograms of β-CD and HP-β-CD exhibited a very broad endothermic peak between 40°C and 140°C because of the loss of water molecules from the cyclodextrin cavity. In the thermal curve of the physical mixtures, the fusion endothermic peak of icariin was much lower than that of the crystalline substance and shifted to a lower temperature as a consequence of the interaction between icariin and β-CD or HP-β-CD. However, this interaction could be induced by the thermal energy supplied to the sample in the DSC scan.Citation38 In contrast, there were no sharp peaks attributable to the crystalline form of icariin in the inclusion complexes, indicating that icariin was no longer present as a crystalline form, but was converted into an amorphous state.
Figure 6 DSC thermograms for the inclusion complexes. (a) icariin, (b) β-CD, (c) the icariin/β-CD physical mixture, (d) the icariin/β-CD inclusion complex. (e) HP-β-CD, (f) the icariin/HP-β-CD physical mixture, and (g) the icariin/HP-β-CD inclusion complex.
Abbreviations: DSC, differential scanning calorimetry; β-CD, beta-cyclodextrin; HP-β-CD, hydroxypropyl-beta-cyclodextrin.
Powder X-ray diffractometry
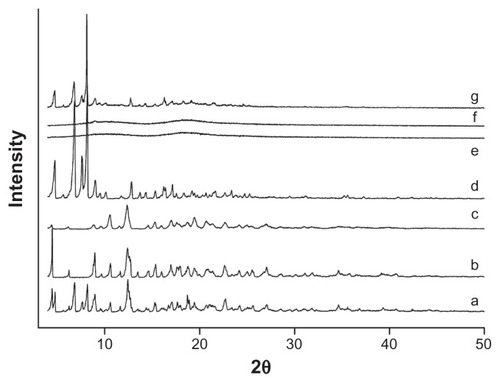
XRD was another valid strategy used to confirm the cyclodextrin complexation in powder or microcrystalline states. The diffraction pattern of the drug/CD complex should be clearly distinct from that containing the individual components of the inclusion complex. As shown in , XRD patterns clearly confirmed the crystalline nature of both icariin and β-CD, as well as the amorphous state of HP-β-CD. The XRD patterns of the physical mixture confirmed the presence of both compounds as isolated solids, because the diffractograms were simply the superposition of each component. The peaks in the diffractogram of the icariin/β-CD inclusion complex reduced in number and intensity when compared to icariin and β-CD. The diffractograms of the icariin/HP-β-CD inclusion complex showed a typical diffuse pattern, indicating a loss of crystallinity of icariin. Moreover, the intensity differences observed on the diffractograms of HP-β-CD and the inclusion complexes in the 3–50° (2θ) range, strengthen the theory that the inclusion complexes had been formed given that an approximately 1.5-fold intensity decrease was observed after complexation. After taking into account the results of the DSC and FTIR spectroscopy, we can assume that the formation of new solid phases might be due to the formation of the icariin/β-CD inclusion complex and the icariin/HP-β-CD inclusion complex.
Figure 7 X-ray diffractograms for the inclusion complexes. (a) icariin, (b) β-CD, (c) the icariin/β-CD inclusion complex, (d) the icariin/β-CD physical mixture, (e) HP-β-CD, (f) the icariin/HP-β-CD inclusion complex, and (g) the icariin/HP-β-CD physical mixture.
Abbreviations: β-CD, beta-cyclodextrin; HP-β-CD, hydroxypropyl-beta-cyclodextrin.
Two-dimensional 1H NMR spectroscopy
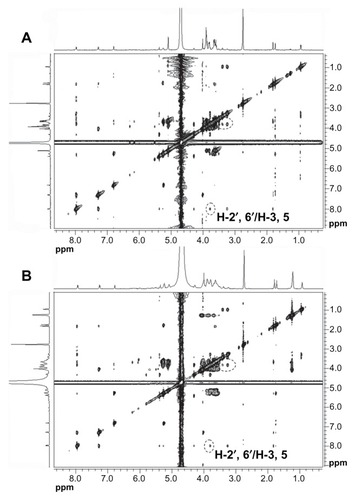
The 2D 1H NMR is a powerful tool used for investigating inter- and intramolecular interactions, because the presence of nuclear Overhauser effect cross-peaks between the protons from two different species indicate spatial contact within 0.4 nm.Citation39 Furthermore, it is an effective method to study spatial conformations of CD inclusion complexes.Citation40,Citation41 To gain more confirmatory information, we obtained 2D ROESY of the icariin inclusion complex with β-CD or HP-β-CD. These studies were conducted in D2O and DMSO-d6 (50:50, v/v) due to solubility constraints. The essential feature of the interaction of icariin with CD in DMSO should be similar to that in water due to the similar relative magnitude of the dielectric constants of DMSO and H2O (ɛ = 46.8 and 80, respectively).Citation36 The 1H resonance of icariin was assigned as follows (): 1H-NMR (500 MHz, D2O:DMSO-d6 [50:50, v/v]) δ: 7.87 (2H, d, J = 8.5 Hz, H-2′, 6′), 7.20 (2H, d, J = 8.5 Hz, H-3′, 5′), 6.73 (1H, s, H-6), 5.29 (1H, brs, rha- H-1), 5.19 (1H, m, H-2″), 3.94 (3H, s, OCH3), 1.73 (1H, s, H-5″), 1.66 (1H, s, H-4″), 0.86 (3H, d, J = 6.0 Hz, rha-H-6). The 2D ROESY spectrums of the inclusion complexes are shown in . Cross-peaks were observed between the H-2′ and H-6′ protons of icariin and the H-3 and H-5 protons of β-CD or HP-β-CD. The cross-peaks between 7-glucosyl group and 3-rhamnose group protons of icariin and β-CD or HP-β-CD were not definite due to problems associated with overlap of the cross-peaks. Regarding the solubility of the 7-glucosyl group and the 3-rhamnose group of icariin, we propose that it is the aromatic ring of icariin, not the 7-glucosyl group or the 3-rhamnose group of icariin, that are included in the β-CD or HP-β-CD nanocavity.
Figure 8 1H NMR spectra of icariin in D2O and DMSO-d6 (50:50, v/v) at 25°C.
Abbreviations: 1H NMR, proton nuclear magnetic resonance; DMSO-d6, deuterated dimethyl sulfoxide; v/v, volume-to-volume; H, hydrogen.
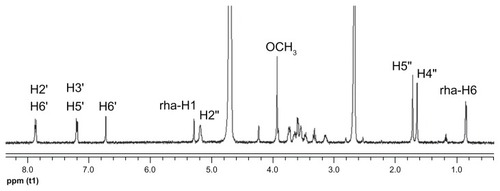
Figure 9 ROESY spectrums of (A) the icariin/β-CD inclusion complex and (B) the icariin/HP-β-CD inclusion complex.
Abbreviations: ROESY, rotating-frame Overhauser effect spectroscopy; β-CD, beta-cyclodextrin; HP-β-CD, hydroxypropyl-beta-cyclodextrin.
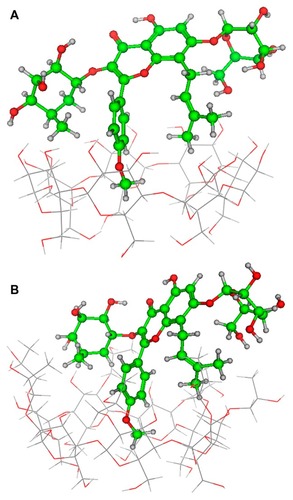
Some insights into the inclusion mode were gained by molecular dynamics simulations using the Amber force field and the semiempirical method Parameterized Model 3. The optimized structure of the icariin/β-CD inclusion complex and the icariin/HP-β-CD inclusion complex are shown in . The icariin/HP-β-CD inclusion complex and the icariin/β-CD inclusion complex have a similar inclusion structure (ie, the phenoxymethyl fragment of icariin is included in both the β-CD and HP-β-CD cavity). However, the phenoxymethyl fragment of icariin penetrates deeper into the cavity of HP-β-CD than β-CD. The energy of the icariin/HP-β-CD inclusion complex (−30180 kcal/mol) is smaller than that of the icariin/β-CD inclusion complex (−23681 kcal/mol), suggesting that the icariin/HP-β-CD inclusion complex is more stable than icariin/β-CD inclusion complex. This result is consistent with the results of phase solubility studies.
In situ absorption studies
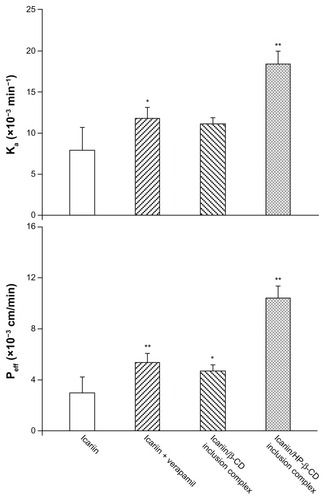
In situ perfusion technique was performed to evaluate the effect of the inclusion complexes on the absorption of icariin as it provides a greater correlation with intestinal absorption in humans than Caco-2 and MDCK cell lines.Citation42 During the perfusion experiment, icariin was stable in the perfusion solution at 37°C and no absorption was observed either to the inlet and outlet tubes, or to the plastic vials. As shown in , the presence of verapamil significantly increased the absorption rate and the permeability of icariin (Ka = 7.92 × 10−3 min−1, Peff = 3.01 × 10−3 cm/min). This improvement indicated that intestinal Pgp was involved in icariin intestinal absorption. When compared to icariin alone, the icariin/β-CD inclusion complex gave a 1.49- and 1.55-fold absorption rate and permeability rate, respectively. For the icariin/HP-β-CD inclusion complex, the absorption rate was further improved and was 2.32-fold higher than icariin alone. Similarly, the permeability rate showed a 3.46-fold increase when compared to icariin alone. A more substantial increase in intestinal absorption was seen with the icariin/HP-β-CD inclusion complex. This enhancement may be attributed to both the solubility enhancement and/or the Pgp inhibitory effect of both β-CD and HP-β-CD.
Mechanisms of Pgp inhibition
The mechanisms by which β-CD and HP-β-CD inhibit Pgp activity may be different from that of other Pgp inhibitors, such as cyclosporine A, quinidine, and verapamil.Citation43 Pgp recognizes many compounds as substrates, and tends to have a high affinity with hydrophobic and positively charged compounds at a certain physiological pH.Citation44 β-CD and HP-β-CD do not appear to be substrates of Pgp because they are both hydrophilic and are electrically neutral cyclic oligosaccharides with a relatively high molecular weight.Citation45 Furthermore, β-CD and HP-β-CD should not compete with Pgp substrates because of their lack of cell permeability.Citation27 Thus, β-CD and HP-β-CD must have an alternative inhibitory effect on Pgp activity that differs from the other Pgp inhibitors described above.
To mimic the interactions between β-CD or HP-β-CD and the cell membrane, a DPPC bilayer membrane was formed, and fluorescence polarization measurements were used to detect any change in the dynamic properties of DPPC induced by β-CD or HP-β-CD.Citation46 High anisotropy values indicate high membrane rigidity and low bilayer fluidity. As shown in , β-CD and HP-β-CD did not change the membrane fluidity when compared to the DPPC bilayer.
Figure 12 (A) DPH anisotropy values of the DPPC bilayer membrane, containing either β-CD or HP-β-CD. (B) Effects of β-CD and HP-β-CD on Pgp ATPase activity.
Notes: *P < 0.05, **P < 0.001, significantly different from the basal Pgp ATPase activity.
Abbreviations: DPH, 6-diphenyl-1,3,5-hexatriene; DPPC, 1,2-dipalmitoyl-sn-glycero- 3-phosphocholine; Pgp ATPase, P-glycoprotein adenosine triphosphatase; β-CD, beta-cyclodextrin; HP-β-CD, hydroxypropyl-beta-cyclodextrin.
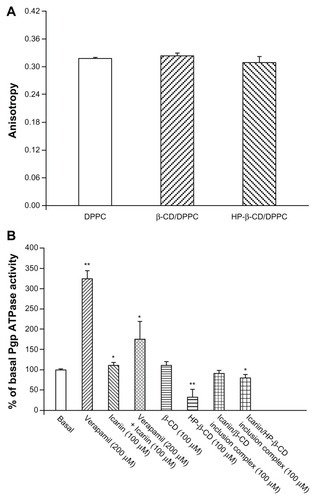
Pgp is an ATP-dependent efflux pump; therefore, Pgp ATPase activity plays an important role in the mechanisms of Pgp inhibition.Citation47 The influences of β-CD and HP-β-CD on the ATPase activity of Pgp were measured using recombinant human Pgp membranes (). Verapamil, a known substrate of Pgp, stimulated Pgp ATPase activity. Icariin alone stimulated the basal Pgp ATPase activity and inhibited the verapamil-stimulated Pgp ATPase activity. These results suggest that icariin is a substrate of Pgp, and competitively interacts at the drug-binding site of Pgp. β-CD and the icariin/β-CD inclusion complex did not alter the Pgp ATPase activity, while HP-β-CD and the icariin/HP-β-CD inclusion complex significantly inhibited the Pgp ATPase activity. HP-β-CD might reduce the use of ATP by Van der Waals forces or hydrophobic interactions between HP-β-CD chains and the ATP binding site, or through steric perturbation, which blocks an allosteric site relevant for Pgp activity.
Conclusion
The molecular association of icariin with β-CD and HP-β-CD was determined. The phase-solubility profile indicated that the icariin/HP-β-CD inclusion complex showed the highest solubility, followed by the icariin/β-CD inclusion complex, and then icariin alone. The 1H NMR spectroscopy and molecular modeling studies revealed that the phenoxymethyl fragment of icariin was held within the lipophilic nanocavity of β-CD or HP-β-CD. Results obtained by DSC, XRD, and SEM supported the complex formation in solid phase. The intestinal transport of icariin was Pgp-dependent. The icariin/β-CD inclusion complex improved the intestinal absorption of icariin by enhancing solubility, while the icariin/HP-β-CD inclusion complex both enhanced solubility and inhibited the Pgp efflux pump. Unlike other Pgp inhibitors, the icariin/HP-β-CD inclusion complex does not alter membrane fluidity; rather, its inhibitory action is due to its effect on Pgp ATPase activity.
Acknowledgments
This work was financed by the National Natural Science Foundation of China (No 30570495 and 81173469), and by the Program for New Century Excellent Talents in University (No NCET-09-0899). We thank the Institute of Materia Medica, the Chinese Academy of Medical Sciences, and the Peking Union Medical College for performing the 2D ROESY NMR spectroscopies.
Disclosure
The authors report no conflicts of interest in this work.
References
- FrewSESammutSMShoreAFChinese health biotech and the billion-patient marketNature Biotechnol2008261375318183014
- ZhangGQinLShiYEpimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trialJ Bone Miner Res20072271072107917419678
- MakarovaMNPozharitskayaONShikovANTesakovaSVMakarovVGTikhonovVPEffect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in ratsJ Ethnopharmacol2007114341241617890032
- MengFHLiYBXiongZLJiangZMLiFMOsteoblastic proliferative activity of Epimedium brevicornum MaximPhytomedicine200512318919315830840
- PanYZhangWYXiaXKongLDEffects of icariin on hypothalamic-pituitary-adrenal axis action and cytokine levels in stressed Sprague-Dawley ratsBiol Pharm Bull200629122399240317142971
- PanYKongLXiaXZhangWXiaZJiangFAntidepressant-like effect of icariin and its possible mechanism in micePharmacol Biochem Behav200582468669416380159
- HuangXZhuDLouYA novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cellsEur J Pharmacol20075641–3263617382317
- MoSFZhouFLvYZHuQHZhangDMKongLDHypouricemic action of selected flavonoids in mice: structure-activity relationshipsBiol Pharm Bull20073081551155617666819
- LiFGongQHWuQLuYFShiJSIcariin isolated from Epimedium brevicornum Maxim attenuates learning and memory deficits induced by d-galactose in ratsPharmacol Biochem Behav201096330130520566405
- XinZCKimEKLinCSEffects of icariin on cGMP-specific PDE5 and cAMP-specific PDE4 activitiesAsian J Androl200351151812646997
- ZhangYPXuWLiNLC-MS-MS method for simultaneous determination of icariin and its active metabolite icariside II in human plasmaChromatographia2008683–4245250
- XuWZhangYYangMLC-MS/MS method for the simultaneous determination of icariin and its major metabolites in rat plasmaJ Pharm Biomed Anal200745466767217706393
- ChengSQiuFWangSHeJHPLC analysis and pharmacokinetics of icariin in ratsJ Sep Sci20073091307131217623472
- ChaoJWangHZhaoWZhangMZhangLInvestigation of the inclusion behavior of chlorogenic acid with hydroxypropyl-β-cyclodextrinInt J Biol Macromol201250127728222115718
- LoftssonTHreinsdottirDMassonMEvaluation of cyclodextrin solubilization of drugsInt J Pharm20053021–2182816099118
- RajewskiRAStellaVJPharmaceutical applications of cyclodextrins. 2. In vivo drug deliveryJ Pharm Sci19968511114211698923319
- LiJXiaoHLiJZhongYDrug carrier systems based on water-soluble cationic beta-cyclodextrin polymersInt J Pharm2004278232934215196638
- LiuJQiuLGaoJJinYPreparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-beta-cyclodextrinInt J Pharm20063121–213714316459034
- KanoKKitagishiHTanakaSCharacterization of an inclusion complex of 5,10,15,20-tetrakis(4-sulfonatophenyl)-porphinato iron and an O-methylated β-cyclodextrin dimer having a pyridine linker and its related complexes in aqueous solutionJ Incl Phenom Macrocycl Chem2006561–26974
- GouldSScottRC2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology reviewFood Chem Toxicol200543101451145916018907
- CastronuovoGNiccoliMThermodynamics of inclusion complexes of natural and modified cyclodextrins with propranolol in aqueous solution at 298 KBioorg Med Chem200614113883388716480882
- ChallaRAhujaAAliJKharRKCyclodextrins in drug delivery: an updated reviewAAPS Pharm Sci Tech200562E329357
- BrewsterMELoftssonTThe use of chemically modified cyclodextrins in the development of formulations for chemical delivery systemsPharmazie20025729410111878196
- DavisMEBrewsterMECyclodextrin-based pharmaceutics: past, present and futureNat Rev Drug Discov20043121023103515573101
- DucheneDWouessidjeweDPonchelGCyclodextrins and carrier systemsJ Control Release1999621–226326810518659
- IrieTUekamaKPharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluationJ Pharm Sci19978621471629040088
- ArimaHYunomaeKHirayamaFUekamaKContribution of P-glycoprotein to the enhancing effects of dimethyl-beta-cyclodextrin on oral bioavailability of tacrolimusJ Pharmacol Exp Ther2001297254755511303042
- Meyer dos SantosSWeberCCFrankeCMullerWEEckertGPCholesterol: Coupling between membrane microenvironment and ABC transporter activityBiochem Biophys Res Commun2007354121622117223079
- FenyvesiFFenyvesiESzenteLP-glycoprotein inhibition by membrane cholesterol modulationEur J Pharm Sci2008344–523624218539442
- CaiCZhuHChenJOverexpression of caveolin-1 increases plasma membrane fluidity and reduces P-glycoprotein function in Hs578T/DoxBiochem Biophys Res Commun2004320386887415240128
- CalabroMLTommasiniSDonatoPThe rutin/beta-cyclodextrin interactions in fully aqueous solution: spectroscopic studies and biological assaysJ Pharm Biomed Anal20053651019102715620528
- KunesMSvobodaZKvetinaJHeroutVHerinkJBajgarJIntestinal single-pass in situ perfusion technique in rat: the influence of L-carnitine on absorption of 7-methoxytacrineBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2005149243343516601805
- NornooAOZhengHLopesLBJohnson-RestrepoBKannanKReedROral microemulsions of paclitaxel: in situ and pharmacokinetic studiesEur J Pharm Biopharm200971231031718793723
- BelliSElsenerPMWunderli-AllenspachHKramerSDCholesterol- mediated activation of P-glycoprotein: distinct effects on basal and drug-induced ATPase activitiesJ Pharm Sci20099851905191818937360
- HiguchiTConnorsKPhase solubility techniquesAdvances in Analytical Chemistry and Instrumentation19657117212
- YangHParniakMAIsaacsCEHillierSLRohanLCCharacterization of cyclodextrin inclusion complexes of the anti-HIV non-nucleoside reverse transcriptase inhibitor UC781AAPS J200810460661319089644
- Badr-EldinSMElkheshenSAGhorabMMInclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: preparation and in-vitro evaluationEur J Pharm Biopharm200870381982718655829
- CappelloBDi MaioCIervolinoMMiroAImprovement of solubility and stability of valsartan by hydroxypropyl-beta-cyclodextrinJ Incl Phenom Macrocycl Chem2006543–4289294
- SchneiderHJHacketFRudigerVIkedaHNMR studies of cyclodextrins and cyclodextrin complexesChem Rev19989851755178611848948
- de AraujoMVVieiraEKSilva LazaroGSulfadiazine/hydroxypropyl-beta-cyclodextrin host-guest system: Characterization, phase-solubility and molecular modelingBioorg Med Chem200816105788579418434167
- JullianCOrosteguisTPerez-CruzFSanchezPMendizabalFOlea-AzarCComplexation of morin with three kinds of cyclodextrin. A thermodynamic and reactivity studySpectrochim Acta A Mol Biomol Spectrosc200871126927518262835
- SalphatiLChildersKPanLTsutsuiKTakahashiLEvaluation of a single-pass intestinal-perfusion method in rat for the prediction of absorption in manJ Pharm Pharmacol20015371007101311480535
- EckfordPDSharomFJABC efflux pump-based resistance to chemotherapy drugsChem Rev200910972989301119583429
- ZhouSLimLYChowbayBHerbal modulation of P-glycoproteinDrug Metab Rev20043615710415072439
- TeodoriEDeiSScapecchiSGualtieriFThe medicinal chemistry of multidrug resistance (MDR) reversing drugsFarmaco200257538541512058813
- DaiFLiuWEnhanced gene transfection and serum stability of polyplexes by PDMAEMA-polysulfobetaine diblock copolymersBiomaterials201132262863820888634
- CollnotEMBaldesCWempeMFMechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidityMol Pharm20074346547417367162