Abstract
Background
Magnetic resonance imaging (MRI) is widely used in modern clinical medicine as a diagnostic tool, and provides noninvasive and three-dimensional visualization of biological phenomena in living organisms with high spatial and temporal resolution. Therefore, considerable attention has been paid to magnetic nanoparticles as MRI contrast agents with efficient targeting ability and cellular internalization ability, which make it possible to offer higher contrast and information-rich images for detection of disease.
Methods
LTVSPWY peptide-modified PEGylated chitosan (LTVSPWY-PEG-CS) was synthesized by chemical reaction, and the chemical structure was confirmed by 1H-NMR. LTVSPWY-PEG-CS-modified magnetic nanoparticles were prepared successfully using the solvent diffusion method. Their particle size, size distribution, and zeta potential were measured by dynamic light scattering and electrophoretic mobility, and their surface morphology was investigated by transmission electron microscopy. To investigate their selective targeting ability, the cellular uptake of the LTVSPWY-PEG-CS-modified magnetic nanoparticles was observed in a cocultured system of SKOV-3 cells which overexpress HER2 and A549 cells which are HER2-negative. The in vitro cytotoxicity of these nanoparticles in SKOV-3 and A549 cells was measured using the MTT method. The SKOV-3-bearing nude mouse model was used to investigate the tumor targeting ability of the magnetic nanoparticles in vivo.
Results
The average diameter and zeta potential of the LTVSPWY-PEG-CS-modified magnetic nanoparticles was 267.3 ± 23.4 nm and 30.5 ± 7.0 mV, respectively, with a narrow size distribution and spherical morphology. In vitro cytotoxicity tests demonstrated that these magnetic nanoparticles were carriers suitable for use in cancer diagnostics with low toxicity. With modification of the LTVSPWY homing peptide, magnetic nanoparticles could be selectively taken up by SKOV-3 cells overexpressing HER2 when cocultured with HER2-negative A549 cells. In vivo biodistribution results suggest that treatment with LTVSPWY-PEG-CS-modified magnetic nanoparticles/DiR enabled tumors to be identified and diagnosed more rapidly and efficiently in vivo.
Conclusion
LTVSPWY-PEG-CS-modified magnetic nanoparticles are a promising contrast agent for early detection of tumors overexpressing HER2 and further diagnostic application.
Introduction
During recent decades, use of magnetic nanoparticles in biomedical applications, such as magnetic drug delivery, magnetic resonance imaging (MRI), and cell and tissue targeting, has drawn considerable attention due to their unique magnetic properties and relatively small size as biological entities.Citation1 The responsiveness of magnetic nanoparticles to external magnetic fields is fully exploited when they are used as drug delivery systems, whereby chemotherapeutic drugs can be delivered to specific locations in the body with the aid of external magnetic fields, whilst minimizing their side effects in healthy cells or tissues.Citation2–Citation5
MRI is widely used in modern clinical medicine as a diagnostic tool, and enables noninvasive and three-dimensional visualization of biological phenomena in living organisms with high spatial and temporal resolution.Citation6,Citation7 As a consequence, considerable attention has been paid to multifunctional magnetic nanoparticles as potentially attractive stimuli-responsive MRI contrast agents,Citation8,Citation9 because their magnetism can be modulated by their dispersion state.Citation10 However, sometimes the contrast difference between the biological tissues under investigation is trivial due to poor cellular uptake and failure to provide a high contrast signal.Citation11 Therefore, a contrast agent with efficient targeting ability and cellular internalization ability is needed to provide higher contrast and information-rich images for disease detection.
Solid lipid nanoparticles, developed in the early 1990s, are composed of physiologically compatible lipid components which decrease the risk of acute and chronic toxicity. Compared with traditional drug carriers, solid lipid nanoparticles combine the advantages of polymeric nanoparticles and emulsions for drug delivery, including having low toxicity, good biocompatibility, and tumor targeting ability.Citation12 Prolonged presence of the contrast agent at the target site is the primary requisite of any effective delivery system used for tumor imaging and diagnosis. In this regard, the effects of coating solid lipid nanoparticles with hydrophilic and flexible macromolecules such as poly(ethylene glycol) (PEG) to achieve a longer circulation time has been investigated.Citation13–Citation15 Moreover, for enhancing solid lipid nanoparticles as an imaging probe, it has been necessary to modify the surfaces of solid lipid nanoparticles with a ligand to facilitate selective targeting of cancer cells more efficiently without loss of affinity and specificity. Human epidermal growth factor receptor 2 (HER2, also known as ErbB2, c-erbB2 or HER2/neu), plays an essential role in proliferation and antiapoptosis mechanisms in HER2-positive breast cancer.Citation16 LTVSPWY, a homing peptide which was identified using a biopanning procedure, has been reported to be able to deliver an antisense oligonucleotide selectively to HER2-positive tumor cells by conjugation with the oligonucleotide.Citation17
Herein, we report the use of a lipid-modified magnetic nanoparticle as a model carrier to improve the sensitivity and selectivity of Fe3O4-based MRI contrast agents. To achieve this, we synthesized LTVSPWY-modified PEGy-lated chitosan (LTVSPWY-PEG-CS), and then prepared and characterized LTVSPWY-PEG-CS-modified magnetic nanoparticles. Their average diameter, zeta potential, and surface morphology were investigated in detail. The tumor targeting ability of the modified magnetic nanoparticles when used as an MRI contrast agent was studied in vitro in a coculture system containing SKOV-3 cells which overexpress the cell surface marker HER-2 and A549 cells which are HER2-negative, and in vivo in an SKOV-3 tumor cell-bearing nude mouse model.
Materials and methods
Chitosan with a molecular weight of 18.0 kDa was prepared by enzymatic degradation of chitosan (95% deacetylate, molecular weight 450 kDa) sourced from Yuhuan Marine Biochemistry Co, Ltd, Zhejiang, China, as in our previous work.Citation18 N,N′-disuccinimidyl carbonate (DSC) was purchased from Bio Basic Inc, Amherst NY. Di-tert-butyl dicarbonate [(Boc)2O], 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), and monostearin were purchased from Shanghai Medpep Co, Ltd, Shanghai, China. Oleic acid was purchased from Shuanglin Co, Ltd, Hangzhou, China. Trypsin and RPMI 1640 medium were purchased from Gibco BRL, Gaithersburg, MD. Fetal bovine serum was obtained from Hangzhou Sijiqing Biology Engineering Materials Co, Ltd, Hangzhou, China. Iron II, III oxide powder (Fe3O4, < 50 nm) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co, St Louis, MO. Rhodamine B isothiocyanate (RITC) was also purchased from Sigma. Hoechst 33342 was obtained from Acros Organics, Fair Lawn, NJ. PKH67 fluorescent cell linker was sourced from Sigma. 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indotricarbocyanine iodide (DiR) was purchased from Invitrogen, Grand Island, NY. All other reagents were analytical or chromatographic grade.
Synthesis of LTVSPWY-PEG-CS copolymers
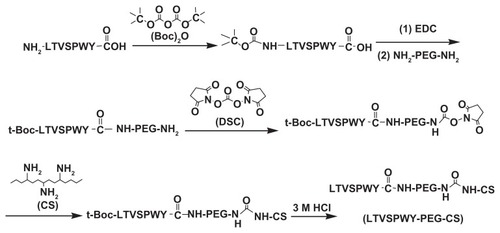
Briefly, 10 mg of LTVSPWY peptide was dissolved in dry dimethylformamide, and 3.1 μL (BOC)2O was added into the solution. The reaction was carried out for 24 hours at 40°C to protect the amine group in the LTVSPWY peptide. Next, 19.8 mg of EDC and 20.8 mg of NH2-PEG-NH2 were added, followed by stirring at 40°C for 24 hours. After that, the reaction solution was reacted with N,N′-disuccinimidyl carbonate (NH2-PEG-NH2:DSC 1:1, mol/mol) for 12 hours at 40°C to obtain a succinimidyl t-Boc-LTVSPWY-PEG-NH2 solution.
To obtain LTVSPWY-PEG-CS, chitosan was added into the reaction solution (chitosan:succinimidyl t-Boc-RGD-PEG-NH2 1:1, mol/mol) and the reaction was allowed to continue at 40°C for 24 hours. To stop protection of the LTVSPWY peptide by (BOC)2O, 3 M HCl was added and allowed to react for 2 hours. The pH of the reaction solution was then adjusted to 7.0 using 3 M NaOH. Finally, the reaction solution was dialyzed using a dialysis membrane (molecular weight cutoff 14 kDa; Spectrum Laboratories, Laguna Hills, CA) against distilled water for 24 hours, followed by lyophilization.
To obtain PEG-CS as a control, 200 mg of chitosan were dissolved in 30 mL of distilled water. Then, 55 mg of mPEG2000 with an aldehyde side group (PEG:chitosan 1:1, mol/mol) was added into the chitosan-SA solution. The solution was stirred overnight at room temperature and then dialyzed against distilled water using a dialysis membrane (molecular weight cutoff 7 kDa, Spectrum Laboratories) for 24 hours. The final product was lyophilized.
Preparation of magnetic nanoparticles
Magnetic nanoparticles were prepared using the solvent diffusion method. Briefly, 5 mg of Fe3O4 were added into 47 mL of 0.1% Poloxamer 188 in deionized water solution followed by sonication for 30 minutes using a probe sonicator (600 W, Sonicator JY92-II DN, Zhejiang, China) to form a dispersion of magnetic nanoparticles. To stabilize the nanoparticles, 1 mL of oleic acid in ethanol solution (5 mg/mL) was added and sonicated for 5 minutes. Next, 35 mg of monostearin was dissolved in 2 mL of dimethyl sulfoxide at 70°C and immediately injected into the aqueous dispersion of magnetic nanoparticles in an ultrasound waterbath at 70°C. The pre-emulsion was then cooled down to room temperature until magnetic nanoparticles were obtained.
To modify the magnetic nanoparticles further, an aqueous solution of LTVSPWY-PEG-CS (100 μg/mL) was added dropwise into the magnetic nanoparticle solution obtained under sonication using an ultrasound waterbath for 5 minutes to form LTVSPWY-PEG-CS-modified magnetic nanoparticles. As a control, PEG-CS or chitosan-modified magnetic nanoparticles were prepared as described above.
Characteristics of magnetic nanoparticles
1H NMR analysis of LTVSPWY-modified PEGylated chitosan
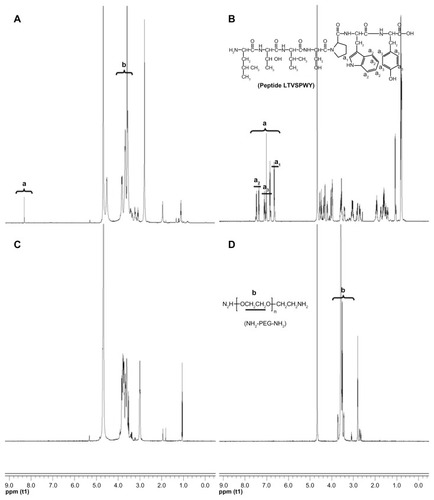
1H NMR spectra were used to analyze the synthesized LTVSPWY-PEG-CS. LTVSPWY, PEG2000, chitosan, PEG-CS, and LTVSPWY-PEG-CS were dissolved in D2O, and measured using an NMR spectrometer (AC-80, Bruker Biospin, Germany).
Determination of particle size, zeta potential, and TEM
The particle size and zeta potential of the magnetic nanoparticles were measured by dynamic light scattering using a Zetasizer (3000HS, Malvern Instruments Ltd, Worcestershire, UK). Morphological examination of the magnetic nanoparticles was performed using transmission electron microscopy (TEM, JEOL JEM-1230, Tokyo, Japan). The samples were stained with 2% (w/v) phosphotungstic acid and placed on copper grids with films for viewing.
Cell culture
A SKOV-3 human ovarian carcinoma cell line and an A549 human lung carcinoma (alveolar type 2) cell line were obtained from the Cell Resource Center of the China Science Academy. The cells were cultured in RMPI 1640 medium at 37°C with 5% CO2 under fully humidified conditions. All media were supplemented with 10% (v/v) fetal bovine serum, penicillin 100 U/mL, and streptomycin 100 U/mL. The cells were subcultured regularly using trypsin-ethylenediamine tetra-acetic acid.
Cytotoxicity assay in vitro
A comparison of cytotoxicity was performed on the test cells with in vitro proliferation using the MTT method.Citation19 Briefly, SKOV-3 and A549 cells were plated in 96-well plates at a density of 1 × 104 cells/well in 200 μL of complete medium, and incubated for 24 hours to allow the cells to attach. The cells were then exposed to serial concentrations of magnetic nanoparticles at 37°C for 48 hours. At the end of incubation, 20 μL of the MTT solution was added, with incubation at 37°C for another 4 hours, and the medium was then replaced with 100 μL of dimethyl sulfoxide to dissolve the MTT formazan crystals. The plates were shaken for 10 minutes and absorbance was measured at 570 nm using a microplate reader (BioRad, Model 680, Hercules, CA).
Competitive cellular uptake in vitro
Before incubation with the magnetic nanoparticles, the A549 cells were stained using a PKH67 fluorescent cell linker kit (Sigma), with the fluorescent cells becoming incorporated into the cell membrane with no modification of biological activity,Citation20 following the manufacturer’s protocol with some modification. Briefly, the cells were resuspended in 200 μL diluent C, and then 200 μL of PHK67 dye (4 μM) was added, followed by incubation for 10 minutes at room temperature. To stop the staining reaction, 1 mL of serum was added and incubated for 2 minutes, followed by centrifugation at 400 × g for 10 minutes. The cell pellet was washed twice more with 10 mL of complete medium to ensure removal of unbound dye and resuspended to the desired concentration.
The PKH67-labeled HER2-negative A549 cells were then cocultured with the HER2-overexpressing SKOV-3 cellsCitation21 in the same well of a 24-well plate and incubated for 24 hours to allow attachment. The cells were then incubated with RITC-labeled magnetic nanoparticles (2:1, mol/mol) in growth medium for 12 hours. After washing the cells with phosphate-buffered solution three times, cellular uptake was observed using fluorescence microscopy (Olympus DP70, Melville, NY).
Biodistribution of magnetic nanoparticles in vivo
Far-red or near-infrared light (spectral range 650–900 nm) provides a “clear” window for in vivo optical imaging because it is separated from the major absorption peaks of blood and water.Citation22 After optical calculations, we estimated that use of near-infrared-emitting DiR would improve tumor imaging sensitivity by at least ten-fold. DiR-loaded magnetic nanoparticles were prepared as described earlier, with some modifications. Briefly, a mixture of DiR and monostearin in dimethyl sulfoxide was injected into the aqueous dispersion of magnetic nanoparticles.
Tumor xenograft models were established by subcutaneous injection of approximately 5 × 106 SKOV-3 cells in 100 μL of serum-free RMPI 1640 medium into the flank of male BALB/C+nu/F1 nude mice. The mice were then subjected to imaging studies when their tumors had reached an acceptable size. After tail vein injection of DiR-loaded magnetic nanoparticles (with Fe3O4, oleic acid, monostearin, chitosan, and DiR concentrations of 50, 50, 350, 50, and 200 μg/mL, respectively), the mice were anesthetized and imaged at multiple time points (1, 3, 6, 12, 24, 48, 72, and 96 hours) using Maestro in vivo imaging system (CRI Inc, Woburn, MA). The tunable filter was automatically stepped up in 10 nm increments from 580 nm to 700 nm while the camera captured images at each wavelength with constant exposure. Overall acquisition time was about 0.4 seconds. For ex vivo imaging, the tissues were subjected to fluorescence imaging using the Spectral system immediately after the tumors and organs were harvested.
Statistical analysis
The data are expressed as the mean of three separate experiments. The statistical significance of the differences were determined using the Student’s t-test for each paired experiment. A P value < 0.05 was considered to be significant in all cases.
Results and discussion
Synthesis and characterization of LTVSPWY-PEG-CS
As shown in , t-Boc-LTVSPWY-PEG-NH2 was prepared by chemical reaction between the –COOH of LTVSPWY (its amino terminus preprotected by (Boc)2O) and the –NH2 of NH2-PEG-NH2 in the presence of EDC. t-Boc-LTVSPWY-PEG-CS was synthesized by conjugating the remaining amino groups of NH2-PEG-NH2 and the primary amino groups of chitosan in the presence of DSC, which has been recognized as a versatile reagent for active synthesis of esters.Citation23 In addition, PEG-CS was formed by reaction between the amino groups of chitosan and the aldehyde group of PEG. The chemical structure of the LTVSPWY-PEG-CS obtained was confirmed by 1H NMR spectroscopy, and is shown in . The peaks of the methylene protons in the ethylene glycol units (CH2CH2O) were at 3.58 ppm, and the benzene protons in the tryptophan and tyrosine units were at 8.32 ppm.
Characteristics of magnetic nanoparticles
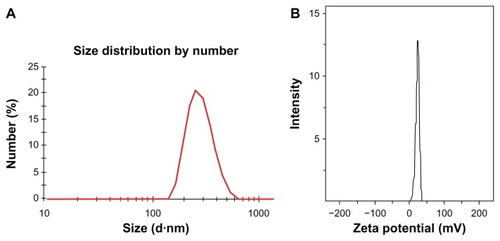
As previous reported, oleic acid is used as a dispersant to obtain a stable aqueous dispersion of Fe3O4 nanoparticles.Citation24 The oleic acid adsorbed onto the surface of Fe3O4 nanoparticles was then favored for the coating of solid lipid material monostearin by the solvent diffusion method due to the compatibility between oleic acid and monostearin. Furthermore, LTVSPWY-PEG-CS-modified magnetic nanoparticles were formed successfully by the charge interaction between LTVSPWY-PEG-CS with its positive charge and the magnetic nanoparticles with their negative charge. The average diameter and zeta potential of the magnetic nanoparticles are shown in . Modification with LTVSPWY-PEG-CS, PEG-CS, or chitosan increased the particles significantly. However, there was no significant difference in particle size between the three types of modified magnetic nanoparticles. With further modification of LTVSPWY-PEG-CS, the zeta potential of the magnetic nanoparticles as well as the PEG-CS and chitosan-modified nanoparticles was reversed in the range of −10.4 ± 3.7 mV to 30.5 ± 7.0 mV. This charge reversal indicates that the LTVSPWY-PEG-CS-modified magnetic nanoparticles had been prepared successfully. shows the size distribution and zeta potential of the LTVSPWY-PEG-CS-modified magnetic nanoparticles obtained by dynamic light scattering, suggesting that they had a narrow size distribution.
Table 1 Size and zeta potential of magnetic nanoparticles
Figure 3 Characteristics of magnetic nanoparticles. Size distribution (A) and zeta potential (B) of LTVSPWY-PEG-CS-modified magnetic nanoparticles obtained by dynamic light scattering.
Abbreviations: PEG, poly(ethylene glycol); CS, chitosan.
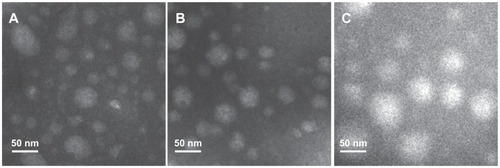
TEM is a powerful tool for detecting particle size and surface morphology in nanoparticles. The spherical morphology of our magnetic nanoparticles was confirmed by TEM (). It was found that the particle size observed under TEM was smaller than that obtained by dynamic light scattering, which may be due to shrinkage of the inner core of the magnetic nanoparticles during the drying process needed when preparing samples for TEM.
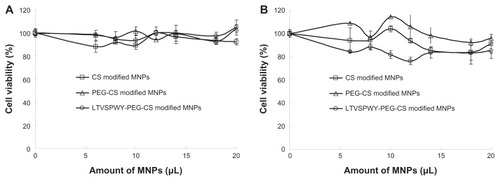
In vitro cytotoxicity assay
The cytotoxic effects of the magnetic nanoparticles against SKOV-3 and A549 cells was evaluated using the MTT test. Variations in cell viability according to nanoparticle concentration are shown in . Regardless of the increasing magnetic nanoparticle concentration, the viability of SKOV-3 and A549 cells remained above 80%, indicating that these nanoparticles would have low toxicity when used as carriers for cancer diagnostics.
Figure 5 Cytotoxicity of magnetic nanoparticles against A549 (A) and SKOV-3 (B) cells. The magnetic nanoparticles prepared consisted of 50 μg/mL Fe3O4, 50 μg/mL oleic acid, 350 μg/mL monostearin, and 50 μg/mL chitosan.
Note: Data represent the mean ± standard deviation (n = 3).
Abbreviations: PEG, poly(ethylene glycol); CS, chitosan.
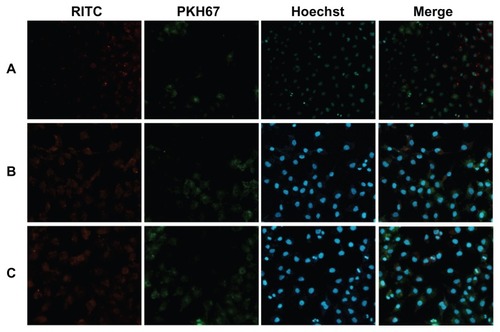
Competitive cellular uptake in vitro
Using a coculture system containing SKOV-3 and A549 cells, the competitive cellular uptake of magnetic nanoparticles was investigated to determine the targeting ability of LTVSPWY-PEG-CS-modified magnetic nanoparticles toward SKOV-3 cells in vitro. The competitive cellular uptake was observed by fluorescence microscopy after the cells were incubated with LTVSPWY-PEG-CS-modified magnetic nanoparticles, PEG-CS-modified magnetic nanoparticles, or chitosan-modified magnetic nanoparticles for 12 hours.
shows the fluorescence images of the effects of RITC-labeled magnetic nanoparticles in a cocultured system containing SKOV-3 and A549 cells. shows clearly that there was a significant difference in uptake of LTVSPWY-PEG-CS-modified magnetic nanoparticles between SKOV-3 and A549 cells in the coculture system. Uptake of LTVSPWY-PEG-CS-modified magnetic nanoparticles by SKOV-3 cells was more efficient than by A549 cells, due to endocytosis mediated by the LTVSPWY homing peptide. However, the PEG-CS-modified magnetic nanoparticles showed similar uptake between SKOV-3 and A549 cells (), as did the chitosan-modified magnetic nanoparticles (). The competitive cellular uptake data confirm strong and specific binding of LTVSPWY-PEG-CS-modified magnetic nanoparticles with SKOV-3 cells, due to the abundant presence of HER-2, a cell surface marker that is highly expressed by SKOV-3 cells.Citation21 These results confirm that LTVSPWY-PEG-CS-modified magnetic nanoparticles retain the binding activity and specificity of the LTVSPWY peptide, and may be used as an active targeting carrier via LTVSPWY peptide mediation.
Figure 6 Fluorescence images of competitive cellular uptake of RITC-labeled magnetic nanoparticles for 12 hours. A549 cells (HER2-negative, cytoplasmic membrane-labeled with PKH67 fluorescent linker, green) cocultured with HER2-overexpressing SKOV-3 cells were incubated with RITC-labeled magnetic nanoparticles (red). (A) LTVSPWY-PEG-CS-modified magnetic nanoparticles, (B) PEG-CS-modified magnetic nanoparticles, and (C) chitosan-modified magnetic nanoparticles.
Note: The cells were all stained with Hoechst 33342.
Abbreviations: RITC, rhodamine B isothiocyanate; PEG, poly(ethylene glycol); CS, chitosan.
Biodistribution of magnetic nanoparticles in vivo
The in vivo behavior of the magnetic nanoparticles was then assessed following tail vein injection in a nude mouse model of a SKOV-3 xenograft in the left flank. The fluorescent images obtained at 1, 6, 24, and 48 hours after injection of DiR-loaded magnetic nanoparticles are shown in . The uptake and retention of magnetic nanoparticles took place primarily in the liver, with little accumulation in tumor tissue. The maximum fluorescence signal is achieved at 48 hours after injection, and the tumor tissue is clearly delineated. Similar to the results obtained for cell uptake in vitro, more active accumulation of LTVSPWY-PEG-CS-modified magnetic nanoparticles was observed in a short time (one hour) within the area of the tumor (), in comparison with the PEG-CS-modified and chitosan-modified magnetic nanoparticles (). For PEG-CS-modified magnetic nanoparticles, the circulation time was increased, leading to slower accumulation of the magnetic nanoparticles in the tumors compared with the chitosan-modified magnetic nanoparticles.
Figure 7 In vivo and ex vivo biodistribution of DiR-loaded magnetic nanoparticles after intravenous tail injection in tumor-bearing nude mice. The fluorescent images of in vivo tumor-bearing nude mice (A–B) and ex vivo image of major organs (A′–C′) using DiR probes with three different magnetic nanoparticles, ie, (A and A′) LTVSPWY-PEG-CS-modified magnetic nanoparticles, (B and B′) PEG-CS-modified magnetic nanoparticles, and (C and C′) chitosan-modified magnetic nanoparticles.
Notes: The images were spectrally resolved. The tumor area is emphasized by a red arrow.
Abbreviations: PEG, poly(ethylene glycol); CS, chitosan. for the development of novel molecular imaging probes in the early detection and diagnosis of cancer.
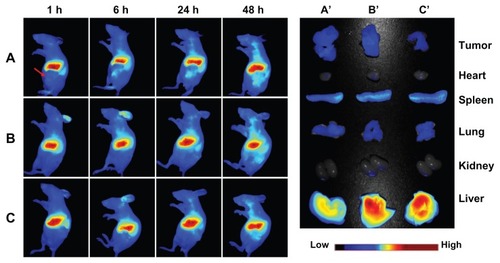
Observation of the organs 96 hours after injection and dissection of the animals confirmed similar accumulation of LTVSPWY-PEG-CS-modified and PEG-CS-modified magnetic nanoparticles in the tumors. However, accumulation of LTVSPWY-PEG-CS-modified magnetic nanoparticles in the liver was reduced compared with that of the PEG-CS-modified and chitosan-modified ones. The biodistribution results obtained in this study indicate that modification by PEG, which prevents protein binding, can prolong the blood circulation time of the magnetic nanoparticles in vivo. More importantly, active tumor targeting using a tumor-specific ligand is much faster and more efficient than passive targeting based on tumor permeation, uptake, and retention.Citation25 Therefore, LTVSPWY-PEG-CS-modified magnetic nanoparticles can be delivered to tumors by both passive and active targeting mechanisms under in vivo conditions.
Conclusion
In summary, LTVSPWY-PEG-CS-modified magnetic nanoparticles were successfully prepared using the solvent diffusion method. With modification by the LTVSPWY homing peptide, these magnetic nanoparticles could be taken up selectively by HER2-overexpressing SKOV-3 cells when cocultured with HER2-negative A549 cells, and with low toxicity. Treatment using LTVSPWY-PEG-CS-modified DiR-loaded magnetic nanoparticles enabled the tumors to be identified and diagnosed more rapidly and efficiently in vivo. Peptide-based nanoprobes may open up new opportunities
Acknowledgment
We are grateful for the financial support of the National Nature Science Foundation of China under contract 81171334 and Medical and health research funding schemes of Zhejiang Province under contract 2012KYB098.
Disclosure
The authors report no conflicts of interest in this work.
References
- MahmoudiMSimchiAImaniMA new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticlesColloids Surf B2010751300309
- PolyakBFriedmanGMagnetic targeting for site-specific drug delivery: applications and clinical potentialExpert Opin Drug Deliv200961537019236208
- LubbeASAlexiouCBergemannCClinical applications of magnetic drug targetingJ Surg Res200195220020611162046
- GuptaAKGuptaMSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials200526183995402115626447
- SunderlandCJSteiertMTalmadgeJEDerfusAMBarrySETargeted nanoparticles for detecting and treating cancerDrug Dev Res20066717093
- OkadaSMizukamiSKikuchiKSwitchable MRI contrast agents based on morphological changes of pH-responsive polymersBioorgan Med Chem2012202769774
- KaurSVenktaramanGJainMSenapatiSGargPKBatraSKRecent trends in antibody-based oncologic imagingCancer Lett201231529711122104729
- Briley-SaeboKBjørnerudAGrantDAhlstromHBergTKindbergGMHepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications of magnetic resonance imagingCell Tissue Res2004316331532315103550
- SinghADilnawazFMewarSSharmaUJagannathanNRSahooSKComposite polymeric magnetic nanoparticles for co-delivery of hydrophobic and hydrophilic anticancer drugs and MRI imaging for cancer therapACS Appl Mater Interfaces20113384285621370886
- TanakaKKitamuraNMoritaMInubushiTChujoYAssembly system of direct modified superparamagnetic iron oxide nanoparticles for target-specific MRI contrast agentsBioorg Med Chem Lett200818205463546518829309
- CorotCRobertPIdeeJMPortMRecent advances in iron oxide nanocrystal technology for medical imagingAdv Drug Deliv Rev200658141471150417116343
- SarmentoBMazzagliaDBonferoniMCNetoAPMonteiroMCSeabraVEffect of chitosan coating in overcoming the phagocytosis of insulin loaded solid lipid nanoparticles by mononuclear phagocyte systemCarbohydr Polym2011843919925
- GaoJQEtoYYoshiokaYEffective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administrationJ Control Release2007122110211017628160
- InadaYFurukawaMSasakiHBiomedical and biotechnological applications of PEG- and PM-modified proteinsTrends Biotechnol199513386917766222
- HongJWParkJHHuhKMChungHKwonICJeongSYPEGylated polyethylenimine for in vivo local gene delivery based on lipiodolized emulsion systemJ Control Release200499116717615342189
- TaiWMahatoRChengKThe role of HER2 in cancer therapy and targeted drug deliveryJ Control Release2010146326427520385184
- ShadidiMSioudMIdentification of novel carrier peptides for the specific delivery of therapeutics into cancer cellsFASEB J200317225625812490548
- HuFQRenGFYuanHDuYZZengSShell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxelColloids Surf B200550297103
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- MartinaMSWilhelmCLesieurSThe effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cellsBiomaterials200829304137414518667235
- Lewis PhillipsGDLiGDuggerDLTargeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugateCancer Res200868229280929019010901
- TziachristosVBremerCWeisslederRFluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imagingEur Radiol200313119520812541130
- BodenNBushbyRJLiuQN, N′-Disuccinimidyl carbonate as a coupling agent in the synthesis of thiophospholipids used for anchoring biomembranes to gold surfacesTetrahedron199854381153711548
- YingXYDuYZHongLHYuanHHuFQMagnetic lipid nanoparticles loading doxorubicin for intracellular delivery: preparation and characteristicsJ Magn Magn Mater2011323810881093
- GaoXCuiYLevensonRMChungLWKNieSIn vivo cancer targeting and imaging with semiconductor quantum dotsNat Biotechnol200422896997615258594