Abstract
Cancer is a highly complex disease to understand, because it entails multiple cellular physiological systems. The most common cancer treatments are restricted to chemotherapy, radiation and surgery. Moreover, the early recognition and treatment of cancer remains a technological bottleneck. There is an urgent need to develop new and innovative technologies that could help to delineate tumor margins, identify residual tumor cells and micrometastases, and determine whether a tumor has been completely removed or not. Nanotechnology has witnessed significant progress in the past few decades, and its effect is widespread nowadays in every field. Nanoparticles can be modified in numerous ways to prolong circulation, enhance drug localization, increase drug efficacy, and potentially decrease chances of multidrug resistance by the use of nanotechnology. Recently, research in the field of cancer nanotechnology has made remarkable advances. The present review summarizes the application of various nanotechnology-based approaches towards the diagnostics and therapeutics of cancer.
Introduction
Cancer is a highly heterogeneous complex disease that encompasses a group of disorders characterized by continuous indefinite growth.Citation1 Through the annals of history, the malaise of cancer has ailed humans. Despite impressive advances in cancer biology, it is the leading cause of death worldwide and remains a challenge. There are over 200 different types of cancer reported all over the globe.Citation2 In 2008, approximately 12.7 million cancer cases were reported, causing approximately 7.6 million cancer deaths, out of which 64% of the deaths were reported from economically developing countries.Citation3 The complexity of this disease at genetic and phenotypic levels clarifies its clinical diversity and therapeutic resistance. There is a 5-year relative survival rate of cancer patients,Citation4 which provides potential opportunities for early diagnosis and improved treatment, which in turn is highly desirable because of widespread occurrence, high death rate, and recurrence after treatment.Citation5
Nanotechnology is an interdisciplinary research field developed with an amalgamation of chemistry, engineering, biology, and medicine, and has various useful applications in cancer biology, such as early detection of tumors, discovery of cancer biomarkers, and development of novel treatments.Citation6 It is a rapidly evolving and expanding discipline that has gained public and media interest worldwide. Use of nanotechnology in cancer biology has provided hope within scientific communities of developing novel cancer therapeutic strategies. Nanotechnology involves the creation and/or manipulation of materials at nanometer scale, either by scaling up from single groups of atoms or by refining or reducing bulk materials into nanoparticles (NPs).Citation7 NPs are typically several hundred nanometers in size and can offer unprecedented interactions with biomolecules present both on the cell surface as well as inside the cell.Citation8 NPs can be engineered as nanoplatforms for effective and targeted delivery of drugs, and imaging labels by overcoming many biological, biophysical, and biomedical barriers. For in vitro and ex vivo applications, the advantages of state-of-the-art nanodevices such as nanochips and nanosensors over traditional methods are quite obvious.Citation9,Citation10 A variety of NPs are used for diagnosis-cum-therapy of different cancer types, by visualizing tumors and carrying out targeted delivery of drugs with reduced toxic side effects. Cancer related examples of nanodevices include quantum dots (QDs), carbon nanotubes (CNTs), paramagnetic NPs, liposomes, gold NPs (GNPs), magnetic resonance imaging (MRI) contrast agents for intraoperative imaging, and a novel NP-based method for high-specificity detection of DNA and protein.Citation6,Citation11–Citation15
Recent advances have led to development of bioaffinity NP probes for molecular and cellular imaging, targeted NP drugs for cancer therapy, and integrated nanodevices for early screening and detection of cancer. These developments raise exciting opportunities for personalized oncology in which genetic and protein biomarkers are used to diagnose and treat cancer, based on the molecular profiles of individual patients. However, several barriers do exist for in vivo applications of nanodevices in preclinical and clinical use of nanotechnology. Amongst them are biocompatibility, in vivo kinetics, tumor-targeting efficacy, acute and chronic toxicity, ability to escape the reticuloendothelial system, and cost-effectiveness.Citation6,Citation16 The development of novel nanotechnology-based approaches towards cancer treatment provides a new ray of hope in the cancer research field. The present review article summarizes the application of various nanotechnology-based approaches towards the diagnostics and therapeutics of cancer.
Use of nanotechnology in cancer treatment
Current therapies and their drawbacks
In the past decade, remarkable progress has been made towards understanding the proposed hallmarks of cancer progression and treatment. With time, the cancer burden is changing for combined types as well as individual types of cancer. However, with ever-increasing incidence, the clinical management of cancer continues to be a grim challenge for the twenty-first century. Over the past couple of decades, a huge amount of detailed data have been amassed regarding the basic biological processes that become perturbed in cancer, such as disturbances in growth-factor binding, signal transduction, gene transcription control, cell-cycle checkpoints, apoptosis, and angiogenesis.Citation17 These in turn have prompted the search for rational anticancer drugs and produced a record number of novel compounds, currently being used in cancer treatment trials.
A number of targeted drugs are licensed for routine clinical use, including rituximab, trastuzumab, imatinib, gefitinib, bevacizumab, lapatinib, and cetuximab.Citation17 Present therapeutic approaches are based on rectifying the damaging mechanism of the genes or by stopping the blood supply to the cancer cells or by destroying the cancer cells itself.Citation18 Conventional treatment options like surgical excision to remove cancerous parts, radiation therapy, and chemotherapy have their own limitations. Surgery cannot be applied for all types of cancers and might result in loss of an organ, coupled with the risk of cancer recurrence. The radiation approach kills cancerous cells, but it also damages the surrounding healthy cells.Citation18 The most common therapeutic approach, chemotherapy, is used either alone or in combination with other therapeutic approaches that kill cancer cells by drug toxicity or by preventing cell division, either by stopping the nutrient uptake or by inhibiting the mechanism responsible for cell division.Citation19 However, this approach is gross and rarely successful for advanced stages of cancer, as pharmacologically active cancer drugs reach the tumor site with poor specificity and dose-limiting toxicity.Citation20 Currently available chemotherapeutic agents are time-tested and confer good disease-free survival only for a limited period of time. Nevertheless, nontarget tissue toxicity and drug resistance curtails the utility of these agents. There is scope to develop newer agents or site-specific delivery systems to transfer these chemotherapeutic agents, which can nullify the major obstacles of toxicity and drug resistance.Citation21 The destruction of cancer cells with minimum harm to healthy tissues and delivery of high doses of drug molecules to tumor sites for maximum treatment efficacy are the needs of the hour.
Will nanotechnology-based approaches help in cancer treatment?
Cancer nanotechnology is a rapidly growing field and has made a remarkable contribution to treatment strategies by enabling site-specific release of chemotherapeutic agents, based on their physicochemical characteristics and biological attributes.Citation21,Citation22 Several stability and drug-payload studies on NP formulations have shown that they are quite stable with high carrier capacity, and are suitable for administration of both hydrophilic and hydrophobic substances by various routes.Citation23 They also have the ability to carry loaded active drugs to cancer cells by selectively utilizing the unique pathophysiology of tumors.Citation22 Due to the advances in synthetic chemistry over the last few decades, different biological nanomaterials have been developed, which can be used for a variety of biological therapies, such as drug delivery, cancer diagnosis, treatment, and imaging.Citation24
The nanotechnology-based drug-delivery system (NDDS) targeted specifically towards cancer cells has several advantages over conventional therapies, such as longer shelf life, improvement in biodistribution of cancer drugs, and administration of both hydrophilic and hydrophobic substances, through oral, nasal, parenteral, and intraocular routes.Citation19
Current NP systems for cancer therapeutics
There are a variety of NP systems currently being explored for cancer therapeutics.Citation25 The material properties of some NP systems have been developed to enhance their delivery to the tumor site directly.Citation26 Functionalization of NPs by the incorporation of a hydrophilic polymer creates a stealth surface from opsonization.Citation26 Currently used NPs in cancer therapeutics include dendrimers, liposomes, lipid NPs (LNPs), polymeric NPs (PNPs), micelles, protein NPs, ceramic NPs, viral NPs, metallic NPs, and CNTs.Citation26–Citation28 Despite extensive research on NP systems for cancer therapeutics, there are only a few NDDSs approved by the US Federal Drug Administration and European Medicines Agency. Specifically, the NDDSs that have been approved include liposomal doxorubicin (Myocet; Elan Pharmaceuticals, Cedar Knolls, NJ), PEGylated liposomal doxorubicin (Doxil; Ortho Biotech, and Caelyx; Schering-Plough), PEGylated liposomal daunorubicin (DaunoXome; Diatos), and the recently approved albumin-bound paclitaxel-loaded NPs (Abraxane; Abraxis Bioscience).Citation26
Various approaches towards cancer treatment with different nanomaterials
Targeted drug delivery via nanocarriers
The development of targeted therapy represents an exciting approach towards cancer treatment.Citation21 Different targeting strategies suggest the potential effect of NP systems and will possibly revolutionize current methods in cancer treatment. Different significant events in cancer mechanisms like angiogenesis, uncontrolled cell proliferation, and tumor mass are the targets for NP systems.Citation26 The effectiveness of NP carrier systems reflects their ability to reduce the tumor or related events without damaging healthy tissues. Moreover, the major improvements offered by NP systems include higher efficacy, lesser side effects, site specificity, efficient delivery, and overcoming multidrug resistance (MDR).Citation29
Targeting tumor cells
The most common targeting strategy is ligand-mediated specific interactions between NPs and cancer cell surface. Longer circulation times and easier endocytosis are the significant factors that need to be considered while choosing target moieties for effective delivery of NPs.Citation30 These ligand-targeted NPs are expected to deliver cytotoxic agents selectively and specifically to tumor cells via receptor-mediated endocytosis, thereby enhancing intracellular drug accumulation. A variety of tumor-targeting ligands, such as antibodies, folate or growth factors, and cytokines have been used to facilitate the uptake of carriers into target cells.Citation31 Moreover, monoclonal antibodies and antibody fragments can reduce immunogenicity and improve pharmacokinetics.Citation32 Artificially engineered antibodies have also been reported as a conjugate to thermosensitive liposomes (affisomes) and to poly-(d,l-lactic acid)-polyethylene glycol (PLA-PEG) maleimide copolymer for the delivery of paclitaxel.Citation33,Citation34
Internalization in cancer cells is an important step, because it reduces the dispersal of the drug outside the cancerous cell and can enhance the therapeutic potential of the said drug.Citation35–Citation37 Passive targeting approaches have also been reported for the delivery of NPs in angiogenesis as a compensatory mechanism of diffusion. It depends upon the properties of NPs, such as, size, surface nature, and circulation half-life.Citation19,Citation38 Passive targeting also involves the use of other innate characteristics of NPs (viz charge) capable of inducing tumor targeting. Cationic liposomes are reported to bind by electrostatic interactions to negatively charged phospholipid head groups, preferentially expressed on tumor endothelial cells.Citation19,Citation39 Human cervical carcinoma cells devoid of folate receptor are incapable of cellular uptake of folate-conjugated NPs.Citation40 Similarly, enhanced antiproliferative potential of epirubicin going from folate receptor (−) to folate receptor (++) cells was also observed.Citation41 The uptake of folic acid–conjugated doxorubicin by HeLa cells has also been reported.Citation40 The anticancer activity of transferring-conjugated solid-lipid NPs of curcumin on the MCF-7 cell line is also evident.Citation42 Several such studies suggested the potential of targeted therapeutic nanoparticles (TNPs) as effective anti-cancer drug-delivery systems.Citation2,Citation22,Citation25,Citation40,Citation43 In an in vivo animal study, targeted TNP-delivered paclitaxel was mainly located in tumor cells, while nontargeted TNP-delivered paclitaxel was detected at intercellular level.Citation44
Targeting the tumor microenvironment
It has been debated that the tumor microenvironment enhanced permeability and retention (EPR) effect might be a key rationale for the development of nanoscale carriers to solid tumors. As an outcome of EPR, nanotherapeutics are expected to improve drug and detection probe delivery, have less adverse effects, and thus result in improved detection and treatment of tumors.Citation45 Exploiting the abnormal tumor microenvironment for selective and homogeneous delivery of nanomedicines to tumor sites is another way of cancer treatment.Citation46
The caveolae (endothelium transcytosis vesicles)-induced accumulation of an albumin-bound nanomedicine (Abraxane) is initiated by the binding of albumin to a cell surface glycoprotein receptor, suggesting that caveolae targeting might provide a universal portal to pump drugs out of the blood into nearby tissue.Citation47 Moreover, arginine–glycine–aspartic acid (RGD) motif has been found to exhibit a strong affinity and selectivity for cell surface in many proteins, thus proclaiming it to be a suitable ligand for tumor targeting by therapeutic NPs.Citation48
Targeting recurrent and drug-resistant cancers
Due to the lack of specific ligands, the specific killing of recurrent cancer cells by using targeted TNPs is still unexplored. Although many studies have illustrated the potential use of TNPs to minimize drug resistance, the lack of specific ligands for drug-resistant cancer cells limits the application of targeted TNPs to these aggressive populations. However, targeting of well-progressed cancer environments like recurrence and metastasis is still a challenge. Advanced knowledge of the molecular mechanisms of late-stage cancer has given new leads to target metastatic cancer with NP systems. Moreover, PEGylated liposome modified with a fibronectin-mimetic peptide has been reported to target metastatic colon cancer cells (which overexpress integrins α5β1).Citation49
Nanotechnology-based drug-delivery systems
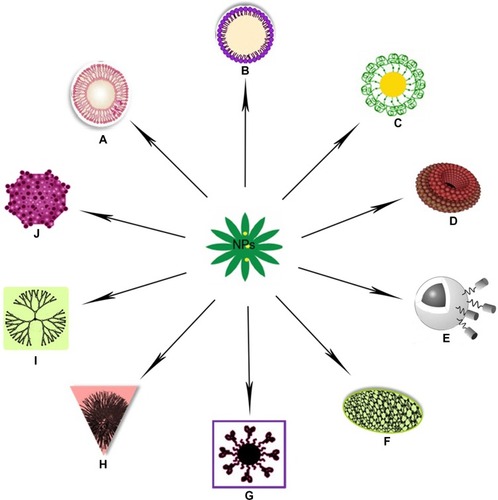
Nanotechnology-based systems can be used to deliver therapeutic entities such as small-molecule drugs, peptides, proteins, and nucleic acids, either alone or in combinations.Citation48,Citation50 Their properties could be attributed to their small sizes, lower drug toxicity, maximized bioavailability, cell death, and modification of drug pharmacokinetics.Citation51–Citation53 depicts several nanotechnology based drug delivery systems used in recent years. Several anticancer drugs, such as paclitaxel, doxorubicin, 5-fluorouracil, and dexamethasone, have been successfully formulated using nanomaterials.Citation51 Polylactic/glycolic acid (PLGA)-based NPs have been formulated to encapsulate dexamethasone.Citation51 It has also been reported that NPs can escape from the vasculature through the leaky endothelial tissue that surrounds the tumorCitation54 and can accumulate in certain solid tumors via the EPR effect.Citation55 In contrast, tumor-targeted NPs can enter tumor cells from the extracellular space via receptor-mediated endocytosis.Citation56 Significant progress has been made in tumor-targeted nanotherapeutics, and some are already in clinical trials or have been approved by the FDA.Citation37 Kang et al studied doxorubicin-loaded solid-lipid NPs on a doxorubicin-resistant breast cancer cell line (MCF-7/ADR).Citation57 Their results showed that doxorubicin-loaded solid-lipid NPs efficiently enhanced apoptotic cell death through higher accumulation of doxorubicin in MCF-7/ ADR cells compared with free doxorubicin.
Figure 1 (A–J) Illustration to clarify the described drug-delivery systems of various nanoparticles (NPs). (A) Liposomal nanoparticle; (B) solid lipid nanoparticle; (C) gold nanoparticle; (D) nanodiamond; (E) magnetic nanovector;Citation220 (F) carbon nanotube; (G) quantum dot nanocarrier; (H) polymeric nanoparticle; (I) dendrimer nanoparticle; (J) virus-mediated nanocarrier.Citation219
Advantages of NP drug-delivery system
To overcome lack of selectivity of anticancer drugs: The major goal of targeted therapies is to target the chemotherapeutics to cancer cells, which ultimately reduces the side effects. To decrease the toxicity and to enhance the selectivity of existing drugs, many NDDSs have been developed in recent years that target specific sites either actively or passively.Citation19
To overcome MDR: Cancers such as non-small-cell lung cancer, and rectal cancer may not respond to standard chemotherapy from the beginning, which is known as primary resistance, while some sensitive tumors respond well to chemotherapy drugs initially but develop acquired resistance later. MDR is mostly due to increased efflux pumps in the cell membrane, such as P-glycoprotein (Pgp). The adenosine triphosphate–binding cassette superfamily includes MDR-associated proteins and breast cancer-resistant proteins. Several studies reported NP-based drug-delivery systems to overcome MDR.Citation19,Citation58,Citation59 It was reported that paclitaxel-loaded NP-based drugs could be introduced inside the cells without triggering the Pgp pump in human colorectal tumor.Citation60 Also, paclitaxel entrapped in emulsifying wax NPs was shown to overcome drug resistance in a human colon adenocarcinoma cell line (HCT-15).Citation51 The PLGA NPs were reported to moderately reverse MDR activity in MCF-7/ADR cells.Citation59 The activity of doxorubicin and Bcl-2-targeted siRNA on multidrug-resistant A2780/AD human ovarian cancer cells was studied by Chen et al, using mesoporous silica NPs.Citation58 They showed that by delivering doxorubicin and Bcl-2 siRNA simultaneously into cancer cells, the Bcl-2 siRNA can effectively silence the Bcl-2 mRNA, significantly suppress non-pump resistance, and substantially enhance the anticancer potential of doxorubicin. Moreover, poloxamer 188 NPs have been reported to overcome MDR in human breast cancer cells.Citation61
To overcome low aqueous solubility of anticancer drugs: Most of the anticancer drugs exhibit poor solubility, which results in reduced bioavailability, increased chances of food effect, frequent incomplete release in dosage form, and higher interpatient variability. Paclitaxel bound to biocompatible proteins is an injectable nanosuspension, that has been approved by the FDA, and is in phase trials for a variety of cancers.Citation62,Citation63
Liposomal nanoparticles as a targeted drug-delivery system
Liposomes are colloidal carriers, formed spontaneously when certain lipids are hydrated in aqueous media. They consist of an aqueous volume entrapped by one or more bilayers of natural and/or synthetic lipids. They are comparatively stable, biocompatible, biodegradable, self-assembled phospholipid membranes with an inner core where hydrophilic drugs could be encapsulated. Liposomes have been suggested as carriers of antineoplastic and antimicrobial drugs, chelating agents, steroids, vaccines, and genetic materials.Citation64 They are efficient vehicles for targeted delivery of hydrophobic drugs without eliciting an immune response.Citation21 Drugs with widely varying lipophilicities can be encapsulated in liposomes, either in the phospholipid bilayer, in the entrapped aqueous volume, or at the bilayer interface. Drugs encapsulated within this lipid bilayer are, therefore, protected from extra-liposomal reactions that could alter the effectiveness of the drug.Citation65 The lipid bilayer could have a marked effect on the pharmacokinetics and biodistribution of LNPs. In one study, Johnston et al reported significant increase in drug-retention properties in LNPs containing sphingomyelin lipid.Citation66 Generally, liposomes have advantages over polymer-based NPs for the formulation of cancer therapeutics.Citation67 In most of the cases, the lipid membrane structure mimics the most common structure, which provides a remarkable permeability barrier. Moreover, liposome-encapsulated drugs are protected from extraliposomal reactions, which enhance the effectiveness of the said drug.
LNPs encapsulating therapeutic agents or liposomal nanomedicines represent one of the most advanced classes of drug-delivery systems, with several currently in the market and many more in clinical trials.Citation68 There are several examples of LNPs for conventional small-molecule drugs.Citation65,Citation69 Curcumin’s efficacy as a chemotherapeutic agent against several cancers of the stomach, prostate, breast, and lung has been well documented.Citation70,Citation71 Cheng et al reported the development of liposomal curcumin encompassing the anticancer curcumin within liposomal formulations.Citation72 In another study, liposome preparations of curcumin exhibited superior antiproliferative and apoptotic effects on six pancreatic cancer cells, and also inhibited pancreatic tumor growth in mouse models.Citation73 Use of the ionophore method of drug loading allows much higher drug:lipid ratios than the conventional citrate method.Citation74 This formulation has given excellent results in studies comparing the efficacy of conventional CHOP (chemotherapy treatment composed of cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP in which the vincristine has been replaced by LNP-vincristine.Citation75,Citation76 Moreover, increased drug retention of vincristine has been reported to be related to increased drug:lipid ratios.Citation75 Lipid-based nanocapsules have given a novel approach to scientific communities for encapsulation of poorly soluble drugs like cisplatin.Citation21
Drug delivery via carbon nanotubes
CNTs have shown great promise as nanoscale vehicles for targeted drug delivery.Citation2 In the past, there have been numerous experimental studies performed in vitro and in vivo using antibody-functionalized CNTs loaded with chemotherapeutic agents.Citation2,Citation77–Citation79 It has been suggested that CNTs could be used as nanocarriers for delivering drugs into the body via intravenous routes.Citation80 Drugs can either attach to the outer surface of CNTs via functional groups or be loaded inside the CNTs.Citation2 Nanoscale size and ease of cellular uptake makes single-walled carbon nanotubes (SWCNTs) useful for drug delivery, and their photothermal effects make them potentially useful in a wide range of applications. The poor solubility of SWCNTs could be overcome by functionalization of the surface of the tubes. Multiwalled carbon nanotubes (MWCNTs) have been reported to possess shorter incubation time and relatively higher cytotoxic potential. MWCNT-g-PCA-PTX compared with free drug suggests improved cell penetration of MWCNT-g-PCA-PTX.Citation81 The grafting of drugs to SWCNTs can be used as a new tool and useful method for potential drug delivery in cancer patients.Citation82
Drug delivery via nanodiamonds
Nanodiamonds (NDs) are attractive agents for use in medicobiological applications, largely due to their greater biocompatibility than other carbon nanomaterials, stable photoluminescence, commercial availability, minimal cytotoxicity, and ease of purification.Citation83,Citation84 NDs could be functionalized and conjugated to a variety of molecules for the purpose of cell labeling and drug delivery that can improve their solubility, direct them to specific binding sites on target cells and tissues, and reduce their effects on normal tissues.Citation83 The diversity of ND functionalization broadens the scope of their potential diagnostic and therapeutic applications. They are suitable for controlled drug-delivery applications because of their capability to release drugs slowly and consistently and have abundant capacity for drug loading due to their large surface area:volume ratio.Citation85,Citation86 A thin film made of ND clusters loaded with doxorubicin was found to effectively release the drug and kill the target cells.Citation85 They could also be used to solubilize and efficiently deliver water-insoluble chemotherapeutic agents to breast and liver tumor cells.Citation58,Citation86 NDs can be used for cell labeling and tracing because they do not interrupt cell division or differentiation and have less cytotoxicity as well. They have successfully been used as biomarkers or tracers to label or trace HeLa cells, lung cancer cells, and murine fibroblasts.Citation84,Citation87
Virus-mediated nanocarriers for drug delivery
Different virus-based nanocarriers for drug delivery have also been reported in scientific literature as an emerging nano-carrier platform.Citation43,Citation88–Citation90 The versatile hierarchical assembly of viral coat protein subunits provides a natural and easy way of drug packaging. Virus-like particles (VLPs) can easily meet the requirements needed for a drug nanocarrier system, such as biocompatibility, water solubility, and high uptake efficiency. Moreover, VLPs can be modified with polymers such as PEG to improve their half-life in the host by moderating their immunogenicity.Citation43 Different viruses such as cowpea chlorotic mottle virus (CCMV), a member of the bromovirus group of the Bromoviridae,Citation43 cowpea mosaic virus, and red clover necrotic mosaic virusCitation88 have been reported as viral nanocarriers. Recently, nanocarriers based on CCMV have also been suggested.Citation89 The incorporation of CdSe/ZnS semiconductor QDs into brome mosaic virus for the design of intracellular microscopic probes and vectors have also been reported.Citation90 GNPs could also be encapsulated in VLPs like MS2 bacteriophage in a similar way.Citation91
Magnetic nanovectors for drug delivery
Use of NP platforms holds the promise of novel and more effective site-specific delivery of therapeutic agents to tumors by magnetic vectoring of magnetically responsive NPs.Citation92 Nanovectors based on the three ferromagnetic elements Co, Ni, and Fe can be used either to mediate a hyperthermic effect (predominantly causing tumor necrosis) or to deliver drugs to achieve intratumoral levels. Nickel-based nanomaterials have also been reported for tumor targeting by serving either as inducers of hyperthermia in response to an externally applied magnetic field (as drug-delivery platforms)Citation93 or directly as proapoptotic mediators.Citation94 Liu et al synthesized a Co- and Cu-based nonviral carrier for DNA transfer in gene therapy.Citation95 Strong binding to DNA involves electrostatic attractions as well as intercalation of the ligands between DNA base pairs, and these complexes efficiently condensed free DNA into globular NPs. Magnetite NPs have reportedly been loaded with daunorubicin to overcome MDR of K562-n/VCR human erythroid leukemia cells.Citation96
Enzyme-responsive nanoparticles for drug release
Enzyme-responsive NPs (ENPs), such as polymer-based NPs, liposomes, GNPs, and QDs have been introduced recently and the modulation of their physicochemical properties by the enzymes has been accentuated. Unique physical properties of nanomaterials combined with ENPs are designed with high specificity for triggering the stimulus in drug-delivery schemes.Citation97 Hydrolases are the most widely used enzymes for drug delivery, probably due to their facile design.Citation98 The attachment of bioactive moieties to carrier through enzyme-cleavable units triggers drug delivery from NP-based carriers.Citation99 Similarly, the dispersion of inorganic NPs can be triggered by a hydrolase when the NPs are assembled by biomolecules presenting cleavable units. The rational design and development of a novel enzyme-responsive, doxorubicin peptide–coated magnetic silica NP was conjugated for selectively triggering intracellular delivery of doxorubicin into the tumor cells with specific protease enzyme expression.Citation100 The highly efficient doxorubicin is released by the said ENP conjugate upon the specific enzyme interactions in vitro. Some hydrolase-responsive nanomaterials are already under clinical trials.Citation98,Citation101 The utilization of oxidoreductase is still in the proof-of-concept stage, and some pioneering examples of their utilization for drug delivery and diagnostics are being debated.Citation99 Other enzymes such as kinases and acetyltransferase have also been explored recently in this field.Citation99
Dendrimers as nanoparticle drug-carrier system
Dendrimers are a unique class of repeatedly branched polymeric macromolecules with numerous arms extending from the center, resulting in a nearly perfect 3-D geometric pattern.Citation102 They comprise a series of branches around an inner core, the size and shape of which can be modulated as per the requirement. NPs based on dendrimers and oligonucleotide-linked dendrimers are expected to improve the therapeutic index of cytotoxic drugs by direct delivery to cancerous cells.Citation103 There is also hope that delivering drugs by this approach could overcome drug resistance in tumor cells via bypassing Pgp pumps,Citation103 which could serve as an attractive modality for drug delivery.Citation104–Citation106
The following proper ties are associated with dendrimers:Citation102,Citation107
Nanoscale sizes that have similar dimensions to important biobuilding blocks.
Numbers of terminal surface groups suitable for bioconjugation of drugs, signaling groups, targeting moieties, or biocompatibility groups.
Surfaces designed with functional groups to augment or resist transcellular, epithelial, or vascular biopermeability.
An interior void space may be used to encapsulate small-molecule drugs, metals, or imaging moieties and facilitates controlled release.
Positive biocompatibility patterns that are associated with lower generation of anionic or neutral polar terminal surface groups compared with higher-generation neutral apolar and cationic surface groups.
Non- or low immunogenicity associated with most den-drimer surfaces modified with small functional groups or PEG.
Surface groups that can be modified to optimize biodistribution, receptor-mediated targeting, therapy dosage, or controlled release of drug from the interior space.
Ability to arrange excretion mode from body.
The possibility of a 2,2-bis(hydroxymethyl) propanoic acid-based dendritic scaffold as a delivery carrier for doxorubicin in vitro and in vivo has been explored.Citation108 In an attempt to improve the efficacy of doxorubicin, photochemical internalization technology was utilized for site-specific delivery of membrane-impermeable macromolecules from endocytic vesicles into cytosol.Citation109 Many researchers have also explored the feasibility of cisplatin incorporation in dendrimers.Citation102,Citation110,Citation111 Polyamidoamine dendrimer complexes are reported to be used for DNA delivery to cell nucleus due to their high transfection efficiency and very low toxicity.Citation102 They are favorite candidates to be used as the backbone of multitasking therapeutics because of their well-defined surface functionality, good water solubility, low polydispersity, and lack of immunogenicity.Citation102 One of the early examples of polyamidoamine dendrimer having a fairly water-soluble nanoformulation has the ability to release cisplatin slowly in vitro.Citation112 PEG-dendrimers are generally synthesized by the conjugation of PEG or polyethylene oxide chains to a multifunctional dendritic chain.Citation113 They constitute a subclass of dendrimers that has attracted numerous researchers because of their prolonged blood circulation time, lower level of toxicity, and relatively lower accumulation in different organs.Citation114
Targeted drug delivery using gold nanoparticles
GNPs are nanometer-sized colloidal suspensions. Multifunctional GNPs have been demonstrated to be highly stable and versatile scaffolds for drug delivery, due to their unique size coupled with their chemical and physical properties.Citation115 The multiple receptor targeting, multimodality imaging, and multiple therapeutic entities holds the promise for a “magic gold bullet” against cancer.Citation6 Different subtypes of GNPs such as gold nanospheres, nanorods, nanoshells, nanocages, and SERS NPs have been reported in literature based on their size, shape, and physical properties.Citation6,Citation116,Citation117 Colloidal GNPs have great potential to overcome delivery limitations because of their biocompatibility, low toxicity, small size, and tunable surface functionalities.Citation118 In cancer cell cultures, surface properties have been shown to regulate cellular uptake, intracellular release, and distribution in subcellular compartments.Citation118 Modification of the surface properties of GNPs has potential to control accumulation in tumors, where drug payloads are released. GNPs functionalized with a thiolated PEG monolayer capped with a carboxylate group showed an improvement in platinum-based anticancer drugs, such as cisplatin, carboplatin, and oxaliplatin efficacy.Citation119 Recently, GNPs conjugated with bovine serum albumin have been reported as one of the best vehicles for drug release.Citation120 A modification of GNPs was reported, in which 11-mercapto-undecanoic acid-modified GNPs conjugated with chloroquine act as an efficient cancer therapy.Citation121
Quantum-dot nanocarrier systems for drug delivery
QDs are spherical light-emitting NPs composed of a semiconductor material. They are a promising class of fluorescent probes for cancer screening from biological fluids, classification of tumor from biopsies, and for high-resolution biomolecular and cellular imaging.Citation122,Citation123 Initial results displayed by QDs synthesized by using reduced glutathione and TGA co-capped CdTe with good biological compatibility and high fluorescence intensity are quite encouraging for the treatment of colorectal cancer.Citation124 Novel folate-conjugated carboxy methyl chitosan–ferroferric oxide-doped cadmium telluride QD NPs were developed with high drug-loading efficiency, low cytotoxicity, and favorable cell compatibility, and are promising candidates for carboxymethyl chitosan-based targeted drug delivery and cellular imaging.Citation125 Codelivery of siRNA and chemotherapeutic agents has been developed recently to combat MDR in cancer therapy. Several QDs functionalized by β-cyclodextrin coupled to amino acids have been reported recently, some of which can be used to facilitate the delivery of siRNA. The intrinsic fluorescence of the QDs provides an opportunity to track the system by laser confocal microscopy. These multifunctional QDs are promising vehicles for the codelivery of nucleic acids and chemotherapeutics, and for real-time tracking during cancer treatment.Citation126 Recent advances have led to multifunctional NP probes that are highly bright and stable under complex in vivo conditions. Polymer-encapsulated QDs are essentially nontoxic to cells and small animals, but their long term in vivo toxicity and degradation needs to be carefully studied. Nonetheless, bioconjugated QDs have raised new possibilities for ultrasensitive and multiplexed imaging of molecular targets in living cells and animal models.Citation123 Moreover, magnetic NPs and QDs have been used effectively to achieve early diagnosis and recurrence prevention of lung cancer micrometastases in peripheral blood.Citation127
Solid-lipid nanoparticles as targeted drug-delivery system
SLNs are physiological lipid-based NP systems that offer physical stability, protection of labile drugs from degradation, easy preparation, and low toxicity.Citation57 SLNs have been developed as an alternative system to the existing traditional carriers, such as liposomes and polymeric NPs. They are new-generation submicron-sized lipid emulsions where the liquid lipid has been substituted by a solid lipid. SLNs offer unique properties, such as small size, large surface area, high drug loading, and the interaction of phases at the interfaces, and are attractive for their potential to improve performance of pharmaceuticals, neutraceuticals, and other materials.Citation128 Recently, several studies reported the use of SLNs as tumor-targeted drug-delivery systems.Citation42,Citation57,Citation129,Citation130 SLNs loaded with doxorubicin were studied in Pgp-over-expressing MCF-7/ADR cell lines.Citation57 Efficiently enhanced apoptotic cell death via higher accumulation of doxorubicin by SLNs in cancer cells was also reported.Citation57 Targeted delivery of a potent anticancer compound, namely curcumin, incorporated with transferrin-mediated SLNs has also been reported in MCF-7 breast cancer cells.Citation42 More recently, high-pressure homogenized emodin-loaded SLNs gave positive antitumor activity in vitro.Citation129 Moreover, cationic lipid-bound oligonucleotide-loaded SLNs are also reported as a potential new approach for carrying anti-miRNA inhibitors for cancer therapy.Citation130
Polymeric nanoparticles as targeted drug-delivery system
PNPs offer a promising means of targeted drug delivery of chemotherapeutic drugs with enhanced efficacy, reduced toxicity, controlled and long-term release rates, prolonged bioactivity, increased patient compliance due to less administration frequency, and the ability to codeliver multiple drugs with synergistic effects at the same site.Citation131 A protective covering provided by the PNPs to chemotherapeutic drugs limits their interaction with healthy cells.Citation131 PNPs are available in different types, such as nanospheres, in which the particles’ entire mass is solid and molecules may be adsorbed at the sphere surface or encapsulated within the particle matrix, and nanocapsules, in which the entrapped substances are confined to a cavity consisting of a liquid core (either oil or water) surrounded by a solid material shell.Citation132 The properties of PNPs need to be optimized depending on the particular application. In order to achieve the properties of interest, the mode of preparation plays a vital role.Citation132
There is scope for many variations in polymer chemistry where PNPs can be easily manipulated without loss of their desired physical, chemical, and biological properties.Citation133 PNPs are often produced by pairing PEG with the polymer of NPs. PEG inhibits binding of plasma proteins to the surface of PNPs, which provides prolonged systemic circulation time, prevents recognition by the reticuloendothelial system,Citation134 and an EPR effect in different types of tumors.Citation131 Hu et al reported prolonged circulation and enhanced anticancer potential of one anticancer agent, endostar, a novel recombinant human endostatin, which was approved by the Chinese State Food and Drug Administration in 2005 with PNPs (PEG-PLGA nanoparticles).Citation135
PNPs coupled with ligands and aptamers have been reported as a way to actively target cancerous cells that further induce receptor-mediated endocytosis for intracellular delivery. A novel injectable formulation of an anticancer drug, sirolimus, using PNP has been reported as an attractive new therapeutic approach for cancer therapy that offers improved pharmacokinetic features and gets readily dispersed in physiological media without any surfactants or cosolvents. PNP-sirolimus effectively inhibited tumor cell proliferation and tumor mass growth and enhanced radiation-induced cell death by inhibition of the mammalian target of rapamycin (mTOR), mTOR pathway and activation of autophagy.Citation136
Anticancer drugs loaded in PLGA-lecithin-PEG NPs and functionalized with AS1411 antinucleolin aptamers have been reported as having high encapsulation efficiency and superior sustained drug release for differential targeted drug delivery in cancer treatment.Citation137 Aptamer-functionalized PEG-PLGA nanoparticles have been reported with enhanced delivery of paclitaxel as a therapeutic application in the treatment of gliomas.Citation138 Targeted delivery of an anticancer agent, cisplatin, against prostate cancer cells by aptamer-functionalized Pt(IV) prodrug-PLGA-PEG NPs have been also reported with higher efficiency compared with free cisplatin.Citation139 Enhanced cellular uptake of folic acid–conjugated PLGA-PEG nanoparticles loaded with vincristine sulfate have been reported to contain significantly higher cellular uptake in the folic acid receptor over expressed MCF-7 cells, compared to PLGA-mPEG NPs without folic acid modification.Citation140 A novel redox-sensitive biodegradable polymer with “trimethyl-locked” benzoquinone has been reported for the preparation of paclitaxel-incorporated NPs to release paclitaxel in response to chemically triggered reduction.Citation141 With further development in these technologies and other methods of triggering and targeting, polymer nanomaterials will provide improved cancer treatment methods.
Use of NPs in cancer treatment via thermal ablation
There is a great demand for minimally invasive options to conventional surgery like thermal ablative technologies for the treatment of cancer.Citation142 Radio-frequency, microwave, and laser-based hyperthermia allow less invasive treatments but still require insertion of a probe into the lesion to be treated.Citation143 Recent advances in nanoscale materials have provided a potentially noninvasive means of heating cells to cytotoxic levels. Temperatures above 42°C induce cell death in some tissues. Cells of any nature (cancerous or normal) heated to temperatures in the range of 41°C–47°C begin to show signs of apoptosis,Citation144 while temperatures above 50°C are associated with less apoptosis and more necrosis.Citation145 Hyperthermic adjuvant chemotherapy plays an increasing role in multimodality cancer treatment.Citation146 It has been suggested that minimal tissue hyperthermia increases the blood flow and yields higher concentrations of chemotherapeutic agents, which results in enhanced therapeutic effect. Similar to chemotherapy, radiotherapy is also more effective using hyperthermia as an adjunct treatment.Citation145 Targeted hyperthermia is achieved by using nanoscale metallic particles that convert electromagnetic energy into heat. Metal NPs exemplify the potential for application in targeted hyperthermic therapy, specifically iron oxide NPs, gold-silica nanoshells, solid-gold NPs and CNTs.Citation145,Citation147 Iron oxide NPs have been used as both diagnostic and therapeutic nanoscale materials to treat deep tissue tumors. With certain drawbacks, iron NPs continue to be actively investigated because of their minimal toxicity and potential for rapid heating.Citation148 GNPs could be heated with shortwave radio-frequency fields. By labeling GNPs with antibodies against particular cancer cells, higher concentrations of GNPs could be achieved. Once the particles are internalized, radio-frequency fields applied to cells result in localized heat and killing of cancer cells.Citation145
CNTs are capable of being targeted and delivered to specific cells either through direct covalent functionalization or through noncovalent wrapping of targeting moieties. The absorption characteristics of CNTs have been utilized as hyperthermic enhancers using near-infrared absorptions.Citation149 CNTs significantly absorb intense heat release under capacitively coupled radio-frequency fields, similar to GNPs.Citation150 QDs also have great potential in photodynamic therapy, where they act as either photosensitizers themselves or as a carrier.Citation151,Citation152 Although nanotechnology is a relatively new field of investigation, using targeted NPs in the hyperthermic treatment of cancer cells is expected to be a viable option for cancer treatment.Citation145 Moreover, these days application of nanotechnology for laser thermal-based killing of cancerous cells, targeted with absorbing NPs, is becoming an extensive area of research.Citation153
Use of nanotechnology in cancer treatment via gene therapy
Gene therapy can be defined as the transfer of genetic material to the cells of an individual, in order to ensure a targeted molecular intervention and achieve a higher level of specific action than conventional cytotoxic chemotherapy. Recently, the gene-silencing technique has gained much popularity. There are three major nucleic acid-based gene-silencing molecules viz antisense oligodeoxyribonucleic acids, siRNA, and micro RNA. Despite so much promise of gene therapy, a number of difficulties remain to be conquered; the most important is the need for more efficient gene-delivery systems. The success of a gene therapy largely depends upon the activity induced by the target genes and the efficiency of gene delivery resulting from the combined effects of the delivery vector and the applied delivery route.Citation154 Various NPs have been investigated for gene delivery, including GNPs,Citation155 silver NPs,Citation156 CNTs,Citation157 liposomes,Citation158 polymersomes,Citation159 and polyplexes. Use of NPs enables targeting of tumor tissue through the EPR effect in gene therapy. Kim et al reported a mannosylated chitosan NP-mediated cytokine gene-delivery system for cancer immunotherapy.Citation160 Recently, thiolated chitosan NPs as a delivery system for antisense therapy has also been reported in T47D breast cancer cells.Citation161 A nanosized immune liposome-based delivery complex was also reported, which preferentially targets and delivers molecules useful in gene medicine, including plasmid DNA and antisense oligonucleotides to tumor cells.Citation162 This tumor-targeting NP delivery vehicle can also deliver siRNA to both primary and metastatic cells.
A gene-delivery vehicle, nonviral vector DOTAP: cholesterol has been suggested as an effective systemic gene-delivery vector that efficiently delivers tumor-suppressor genes to disseminated lung tumors.Citation163 A promising role of novel nonviral gene carrier heparin-polyethyleneimine (HPEI) NP-mediated ms-T34 A in C-26 colon carcinoma therapy was also reported.Citation164 PEI-derived NPs such as poly (ε-caprolactone)-pluronic-poly(ε-caprolactone)-grafted PEI (PCFC-g-PEI), folic acid–PCFC-isophorone diidocyanate-PEI and HPEI are reported as gene-therapy delivery systems for renal cell carcinoma.Citation165 It has been reported in the literature that QDs may also function as gene-delivery vehicles if modified with lipofectamine,Citation166,Citation167 encapsulated in poly(maleic-anhydride-alt-1-decene), surface-modified with dimethylamino propylamine and poly(maleic-anhydride-alt-1-decene) coated. QDs have been reported as siRNA delivery vehicles and exhibit up to a 20-fold increase in silencing effect and a sixfold decrease in toxicity compared with other common delivery agents.Citation167
Gene delivery could also be achieved by the use of CNTs.Citation2 They are a good nonviral vector for gene therapy, and can cross the cell membrane by an endocytosis process.Citation168 CNTs are promising vectors for gene and protein, for which they have shown much greater fluorescent activity of protein and DNA when conjugated to single-walled NTs.Citation169 The delivery of siRNA for treatment of tumor cells using functionalized MWCNTs and liposomes showed that the siRNA delivered via MWCNTs achieved significant inhibition of tumor growth.Citation170 Dendrimers have also been reported to enter tumors and carry either chemotherapeutic agents or genetic therapeutics. They could be conjugated to fluorochromes and possess the ability to enter into cells. They can then be detected within the cell in a manner compatible with sensing apparatus for evaluation of physiologic changes.Citation171 DNA-assembled polyamidoamine dendrimer clusters for cancer cell-specific targeting were also reported. Dendrimer-5-fluorouracil (5FU) conjugates were prepared by acetylation, and upon hydrolysis release free 5FU, thus minimizing the toxicity of 5FU.Citation172 In earlier studies, researchers have shown that cisplatin encapsulated in dendrimer polymers has increased efficacy and is comparatively less toxic.Citation173 Novel gene-silencing strategy in gene therapy involving the use of siRNA can be conjugated to phospholipid-functionalized SWNTs using a cleavable disulfide linker, resulting in efficient gene silencing and subsequent death of the targeted cell.Citation149 Amino-functionalized MWNT–siRNA complexes have shown successful suppression of tumor and prolonged survival in lung tumor in animal model.Citation170 CNTs cationically functionalized with PEI have been shown to be capable of complexing with siRNA and significantly increasing silencing activity and cytotoxicity.Citation174 A highly efficient, low-toxic, and specifically targeting gene-delivery vector (H1) was also reported.Citation175 Peritumoral injection of H1/pIL-2 NPs displayed effective antitumor potential. Researchers have derived PEI-coated virus-like particles from an adeno-associated virus type 2 delivery system for potential therapy of breast cancer.Citation176
Nanotechnology-based approaches in cancer diagnostics
One of the major reasons behind low survival rates among cancer patients is the failure to detect the disease at an early stage. Modern imaging technologies have made enormous advances. However, the effective early detection of precancerous and neoplastic lesions remains an elusive goal. The challenge is to find a test that can detect clinically apparent cancer cases at an early stage, long before the symptoms become visible. A variety of highly integrated nanotechnology platforms to diagnose cancer hold considerable promise. NP probes, nanocantilever, nanowire, and nanotube arrays are expected to solve the problem of early detection of different types of cancer.Citation13 Nanomaterials possess long-term stability and enable the design of powerful bioassays for simultaneous measurements of multiple markers of a disease.Citation177 Nanomaterial-based probes involve the use of nanotechnology to meet the demands of in vitro diagnostics for increased sensitivity and rapid detection from complex environmental systems.Citation178 NPs labeled in vivo signaling moiety offer better molecularly targeted specificity than conventional imaging techniques. Various combinations of multifunctional NPs have been established with imaging agents like radioisotopes, lanthanides, or fluorophores.Citation179 Hopefully, nanotechnology could lead to a paradigm shift in cancer detection, diagnosis, and therapy.
Some of the existing technologies for cancer imaging include noninvasive imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), ultrasound scans, single-photon emission CT, optical imaging, and macroscopically visualizing tumors.Citation180 Unfortunately, most of these imaging techniques are somewhat limited to detecting abnormalities at the microscopic level. It is possible, however, to combine existing optical imaging technologies with sophisticated NP-based optical contrast agents for high-resolution in vivo cancer imaging. Recently, this strategy has been successfully demonstrated for detecting tumors.Citation181 However, developing reliable early detection approaches from serum, other biological fluids, or any sample obtained through minimal or noninvasive procedures remains of supreme importance.
Various nanotechnology platforms for the detection of cancer
Approaches for the in vivo detection and monitoring of cancer biomarkers
To identify malignancies based on their molecular expression profiles, all imaging technologies require contrast agents, comprising a signal-amplifying material conjugated to a molecular recognition and targeting agent such as an antibody.Citation13
In vitro detection
Various novel assemblies of NP systems have been reported as nanoprobes for the early detection of cancer. Nanomaterials such as NPs, nanowires, nanotubes and nanodevices have been explored as ultrasensitive probes to detect cancer biomarkers.Citation178 NP probes with molecularly targeted recognition agents might provide information on the presence, relative abundance, and distribution of cancer signatures and markers associated with the tumor microenvironment.Citation13 Because of their unique properties, QDs are one of the well-established NPs that cover applications such as cellular imaging, immunoassays, in vivo imaging, and sensing.Citation182,Citation183 QD-based microfluidic protein chip detection and optically addressed fluorescence resonance energy transfer (FRET) probes are being used in signal transduction to design the detection systems for nucleic acids, proteins, peptides, and small molecules.Citation184,Citation185 The increased number of acceptors linked to QDs can significantly amplify the signal of the target through enhanced energy-transfer efficiency. This ultrasensitive nanoprobe based on the FRET system had the capability to detect low concentrations of DNA, which have great potential to diagnose DNA mutations of tumor cells in clinical samples.Citation178 Methylation-specific QD-FRET offers great promise for its translational use in early cancer diagnosis, prognostic assessment of tumor behavior, and monitoring response to therapeutic agents in clinical samples.Citation186 QD-based bioluminescence resonance energy transfer (BRET) conjugated to bioluminescent proteins uses biochemical energy to excite fluorophores and offers additional advantages over FRET system for various bioanalyses.Citation187 A nanoplatform of protease probing based on the BRET system was designed to detect protease activity in complex biological samples.Citation188 The sensitivity of this technology has great clinical value to cancer diagnosis and monitoring.
Nanophotonic methods also possess important applications in bioanalyses and spectroscopy using plasmonic NPs and can monitor the molecular binding events and changes in molecular conformation with exquisite sensitivity.Citation178 The said nanoprobe could be used to follow cell-signaling pathways at the single-molecule level in the living cells, which was not possible by conventional single-molecule-imaging techniques.Citation189,Citation190 The multiplex protein detection of potential cancer markers was achieved using highly sensitive giant magnetoresistive sensors capable of detecting even ten magnetic nanotags.Citation191 Recently, a magnetic NP-based method has been reported, which is an alternate to PCR methods and enzyme-linked immunosorbent assays for in vitro detection of viruses and circulating tumor cells in biological samples.Citation178 Although this technique is fast, sensitive, and suitable in complex biological media, it could be broadly applied to detect different biomarkers and biological species with increased sensitivity and specificity, and might become a truly portable, easy-to-use and low-cost device for point-of-care in vitro diagnostics of different types of cancer in future.Citation178
Ex vivo detection
NPs have also shown promise for the ex vivo detection of cancer biomarkers.Citation13 The ex vivo detection technique offers the potential advantages of readily identifying the conjugate markers, yielding specific information on their tissue distribution, introducing new protocols that include cell surface, endocellular, and microenvironmental antigens in the same test. Gold-nanoshell-based immunoassays have been developed for the detection of various analytes of subnanogram-per-milliliter quantities.Citation192 Fluorescent NPs have been used for an ultrasensitive DNA-detection system,Citation193 and QD bioconjugates with targeting antibodies have been used to recognize molecular signatures, including ERBB2.Citation194 In one study, fluorophore-laden silica beads were used for the optical identification of leukemia cells in blood samples.Citation195
Nanotubes, nanowires, and nanodevices
Nanocantilevers, nanowires, and nanotubes also have many applications in biology and medicine because of their unique structures and properties. Use of these devices facilitates the transition from single-biomarker to multiple-biomarker cancer diagnostics, prognostics, and treatment. Nanocantilevers are in the class of nanotechnology-based methods that deflect and change resonant frequencies as a result of affinity-binding of biomarker proteins or nucleic acid hybridization events occurring on their free surfaces.Citation196 The breakthrough potential afforded by nanocantilevers resides in their extraordinary multiplexing capability.Citation197 In one study, Majumdar used microcantilevers to detect SNPs in a 10-mer DNA target oligonucleotide without using extrinsic fluorescent or radioactive labeling.Citation198
Devices based on nanowires are emerging as a powerful and general platform for ultrasensitive, direct electrical detection of biological and chemical species. Nanowire and nanotube arrays might contain several thousand sensors on a single chip, therefore offering even greater multiplexing advantages.Citation13 A silicon (Si) nanowire field-effect device was developed, in which distinct nanowires and surface receptors are incorporated into arrays.Citation199 The capability of Si-nanowire probes for multiplexed real-time monitoring of protein markers with high sensitivity and selectivity in clinically relevant samples opens up substantial possibilities for the diagnosis and treatment of cancer.Citation200 SWCNTs exhibit distinct electrical and spectroscopic properties such as photo luminance in the near-infrared range and strong resonant Raman scattering.Citation201 They promise great potential applications in biological detection and imaging because of their unique Raman properties.Citation202
Multifunctional nanomaterials
Combined uses of multiple-platform diagnostic nanotechnologies are emerging day by day. Multifunctional nanomaterials are highly sensitive, stable, detectable, biocompatible, and targetable.Citation178 The development of multiple integrated nanoprobe systems includes metals, oxides, polymers, enzymes, or other components to give the system, required functionality, and specificity.Citation115,Citation178 The combination of QDs and iron oxide NPs can create a single NP probe that may lead to clinically useful measurements and images of cancer at molecular levels both in vitro and in vivo.Citation100 The combination of metallic NPs and magnetic NPs is another type of multifunctional NP, which is likely to lead to new applications in biomedicine because metallic NPs possess intrinsic properties and functions as optical-contrast agents and probes.Citation178 Park et al reported the simultaneous dual-mode imaging (MRI and fluorescence imaging) in vitro and in vivo, using hybrid nanostructures containing magnetic NPs and QDs.Citation203 A polymeric multifunctional optical nanoprobe platform was developed and successfully demonstrated the applications of multifunctional nanomaterials, such as real-time oxygen sensors, in situ optical probes, magnetic pH sensors, and stimuli-responsive magnetic optical probes.Citation204
Recent developments in nanotechnology-based cancer detection
A cyclic RGD peptide-conjugated type II CdTe/CdS QD formulation was reported for targeting and imaging of pancreatic tumor vasculature in live animals.Citation205 Lanthanoid trivalent ion-based luminescence agents have been reported as optical probes that have more potential than QDs.Citation206,Citation207 The efficacy of europium-catalyzed SWCNT was reported as a novel excellent visible candidate probe for targeted cellular imaging.Citation207 Lewis et al reported noble metal NPs coated with cerium and luminescent europium complexes for bioimaging and potential biodelivery applications.Citation208
Theranostic MRI is now receiving growing interest in imaging-guided drug delivery, monitoring treatment, and personalized administration.Citation209 Recently, an SWCNT-Au-PEG nanocomposite was reported as an interesting optical theranostic probe for cancer imaging and therapy.Citation210 Luminescence-quenched shell cross-linked NPs as photonic nanoprobes are reported to detect a protease, which is a viable marker for cancer imaging in vivo.Citation211 Polysorbate 80-coated temozolomide-loaded PLGA-based superparamagnetic NPs have been characterized as drug carriers and good MRI contrast agents for diagnosing malignant brain glioma.Citation212 A multifunctional nanoprobe is prepared by functionalizing SnO2 NPs with both folic acid as targeting moiety and a gene probe to inhibit or recognize intracellular miRNA levels for target cell-specific imaging and in situ detection of intracellular miRNA.Citation213 A sensitive and selective sensor for detecting colon cancer based on NP covalent modified antihuman epithelial cell adhesion–molecule antibody was also developed.Citation214
Recently, a highly efficient multifunctional nanoplatform, multicolor luminescent NaYF4:Yb3+,Er3+ upconversion NPs were reported by Liu et al,Citation215 which could be utilized in image-guided cancer photodynamic therapy. For the first time, Wang et al reported a synthesis of biocompatible triplex Ag@SiO2 @mTiO2 core–shell NPs that possessed a combined capacity for fast and multiplexed fluorescence-surface-enhanced Raman scattering F-SERS labeling for imaging as well as drug loading for cancer therapy.Citation210 In one study, optimized magnetic-particle imaging tracers using iron oxide NPs traced significant signal intensity and better spatial resolution compared with commercial NPs developed for MRI.Citation216 High-contrast imaging can be achieved by gold nanorods with targeted tumor-growth-factor receptors as compared to nontargeted gold nanorods. This can facilitate imaging and demarcating tumor margins during surgical resection.Citation217 In one study, hyaluronan-coated superpara-magnetic iron oxide NPs with high magnetic relaxivity were reported as a promising system with enhanced uptake and enabled MRI of cancer cells for targeted drug delivery.Citation218
Future perspectives
Nanotechnology is a rapidly developing field that has given new hope in the treatment of various diseases. Early detection and treatment of cancer remains a challenge to the scientific community. Moreover, different strategies have been explored in recent years for cancer detection and therapy. Application of nanotechnology in cancer treatment seems to solve these limitations, which has given new hope to humanity. Specific targeting of cancer cells was also the major challenge faced by conventional therapeutic approaches of cancer treatment. Recently, various NP-based drug-delivery systems such as liposomes, dendrimers, diamondoids, QDs, viral NPs, and CNTs have shown encouraging results in cancer therapy. Properties like prolonged existence in systemic circulation, enhanced drug localization, and their efficacy make the NP-based model an excellent one. One of the major challenges in cancer treatment, ie, MDR, can also be overcome by these NP formulations. In the light of our review, we expect that in future, different NP formulations would serve as “Trojan horses” in the field of cancer diagnostics and its treatment. Hereby, we find it pertinent to highlight that toxicity and immune-system induction should be given due consideration before finalizing the use of any NP formulation for diagnostic and treatment purposes.
Acknowledgements
The authors gratefully acknowledge King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia, for providing the research platform and IT facility.
Disclosure
The authors report no conflicts of interest in this work.
References
- FeinbergAPOhlssonRHenikoffSThe epigenetic progenitor origin of human cancerNat Rev Genet200671213316369569
- MadaniSYNaderiNDissanayakeOTanASeifalianAMA new era of cancer treatment: carbon nanotubes as drug delivery toolsInt J Nanomedicine201162963297922162655
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- American Cancer SocietyCancer Facts and Figures 2011Atlanta, GAAmerican Cancer Society2011
- ChoiYEKwakJWParkJWNanotechnology for early cancer detectionSensors201010142845522315549
- CaiWGaoTHongHSunJApplications of gold nanoparticles in cancer nanotechnologyNanotechnol Sci Appl2008111
- Jean-MaryFSpectroscopic and Microscopic Studies of Aggregated Molecules Coated onto NanomaterialsAnn ArborProQuest2006
- ElsersawiAWorld of Nanobioengineering: Potential Big Ideas for the FutureBloomingtonAuthorHouse2010
- GrodzinskiPSilverMMolnarLKNanotechnology for cancer diagnostics: promises and challengesExpert Rev Mol Diagn20066330731816706735
- SahooSKParveenSPandaJJThe present and future of nanotechnology in human health careNanomedicine200731203117379166
- KircherMFMahmoodUKingRSWeisslederRJosephsonLA multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineationCancer Res200363238122812514678964
- NamJMStoevaSIMirkinCABio-bar-code-based DNA detection with PCR-like sensitivityJ Am Chem Soc2004126195932593315137735
- FerrariMCancer nanotechnology: opportunities and challengesNat Rev Cancer20055316117115738981
- NeuweltEAVárallyayPBagóAGMuldoonLLNesbitGNixonRImaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumoursNeuropathol Appl Neurobiol200430545647115488022
- JamiesonTBakhshiRPetrovaDPocockRImaniMSeifalianAMBiological applications of quantum dotsBiomaterials200728314717473217686516
- CaiWChenXMultimodality molecular imaging of tumor angiogenesisJ Nucl Med200849Suppl 2113S128S18523069
- SikoraKThe impact of future technology on cancer careClin Med20022656056812528971
- SinghOPNehruRMNanotechnology and cancer treatmentAsian J Exp Sci20082226
- ChidambaramMManavalanRKathiresanKNanotherapeutics to overcome conventional cancer chemotherapy limitationsJ Pharm Pharm Sci2011141677721501554
- SinhaRKimGJNieSShinDMNanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug deliveryMol Cancer Ther2006581909191716928810
- RanganathanRMadanmohanSKesavanANanomedicine: towards development of patient-friendly drug-delivery systems for oncological applicationsInt J Nanomedicine201271043106022403487
- ChoKWangXNieSChenZGShinDMTherapeutic nanoparticles for drug delivery in cancerClin Cancer Res20081451310131618316549
- GelperinaSKisichKIsemanMDHeifetsLThe potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosisAm J Respir Crit Care Med2005172121487149016151040
- GhanbariHde MelASeifalianAMCardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: a glimpse into prospective horizonsInt J Nanomedicine2011677578621589645
- HaleyBFrenkelENanoparticles for drug delivery in cancer treatmentUrol Oncol2008261576418190833
- ByrneJDBetancourtTBrannon-PeppasLActive targeting schemes for nanoparticle systems in cancer therapeuticsAdv Drug Deliv Rev200860151615162618840489
- HahnMASinghAKSharmaPBrownSCMoudgilBMNanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectivesAnal Bioanal Chem2011399132720924568
- NagaAPSiddiquiANanomedical platform for drug deliveryJ Nanomed Nanotechnol20112122
- Brannon-PeppasLBlanchetteJONanoparticle and targeted systems for cancer therapyAdv Drug Deliv Rev200456111649165915350294
- YuMKParkJJonSTargeting strategies for multifunctional nanoparticles in cancer imaging and therapyTheranostics20122134422272217
- DongXMumperRJNanomedicinal strategies to treat multidrug-resistant tumors: current progressNanomedicine (Lond)20105459761520528455
- KontermannREImmunoliposomes for cancer therapyCurr Opin Mol Ther200681394516506524
- AlexisFBastoPLevy-NissenbaumEHER-2-targeted nanoparticle-affibody bioconjugates for cancer therapyChem Med Chem20083121839184319012296
- PuriAKramer-MarekGCampbell-MassaRHER2-specific affibody-conjugated thermosensitive liposomes (Affisomes) for improved delivery of anticancer agentsJ Liposome Res200818429330718937120
- LavasanifarASamuelJKwonGSPoly(ethylene oxide)-block-poly (l-amino acid) micelles for drug deliveryAdv Drug Deliv Rev200254216919011897144
- GullottiEYeoYExtracellularly activated nanocarriers: a new paradigm of tumor targeted drug deliveryMol Pharm2009641041105119366234
- LammersTHenninkWEStormGTumour-targeted nanomedicines: principles and practiceBr J Cancer200899339239718648371
- AllenTMCullisPRDrug delivery systems: entering the mainstreamScience200430356651818182215031496
- PauwelsEKJKairemoKErbaPBergstromKNanoparticles in cancerCurr Radiopharm2010113036
- ZhangCZhaoLDongYZhangXLinJChenZFolate-mediated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug deliveryEur J Pharm Biopharm2010761101620472060
- CanalFVicentMJPasutGSchiavonORelevance of folic acid/ polymer ratio in targeted PEG-epirubicin conjugatesJ Control Release2010146338839920621587
- MulikRSMönkkönenJJuvonenROMahadikKRParadkarARTransferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosisInt J Pharm20103981–219020320655375
- MaYNolteRJCornelissenJJVirus-based nanocarriers for drug deliveryAdv Drug Deliv Rev201264981182522285585
- DuncanRVicentMJGrecoFNicholsonRIPolymer-drug conjugates: towards a novel approach for the treatment of endrocine-related cancerEndocr Relat Cancer200512Suppl 1S189S19916113096
- FangJNakamuraHMaedaHThe EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effectAdv Drug Deliv Rev201163313615120441782
- StylianopoulosTWongCBawendiMGJainRKFukumuraDMultistage nanoparticles for improved delivery into tumor tissueMethods Enzymol201250810913022449923
- PetrelliFBorgonovoKBarniSTargeted delivery for breast cancer therapy: the history of nanoparticle-albumin-bound paclitaxelExpert Opin Pharmacother20101181413143220446855
- GindyMEPrud’hommeRKMultifunctional nanoparticles for imaging, delivery and targeting in cancer therapyExpert Opin Drug Deliv20096886587819637974
- GargATisdaleAWHaidariEKokkoliETargeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptideInt J Pharm20093661–220121018835580
- RuoslahtiEBhatiaSNSailorMJTargeting of drugs and nanoparticles to tumorsJ Cell Biol2010188675976820231381
- SuriSSFenniriHSinghBNanotechnology-based drug delivery systemsJ Occup Med Toxicol20072161618053152
- PengXHQianXMaoHTargeted magnetic iron oxide nanoparticles for tumor imaging and therapyInt J Nanomedicine20083331132118990940
- MacEwanSRCallahanDJChilkotiAStimulus-responsive macromolecules and nanoparticles for cancer drug deliveryNanomedicine (Lond)20105579380620662649
- JinCBaiLWuHLiuJGuoGChenJPaclitaxel-loaded poly(D,L-lactide-co-glycolide) nanoparticles for radiotherapy in hypoxic human tumor cells in vitroCancer Biol Ther20087691191618367873
- TorchilinVTumor delivery of macromolecular drugs based on the EPR effectAdv Drug Deliv Rev201163313113520304019
- ChoiCHJAlabiCAWebsterPDavisMEMechanism of active targeting in solid tumors with transferrin-containing gold nanoparticlesProc Natl Acad Sci U S A201010731235124020080552
- KangKWChunMKKimODoxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapyNanomedicine20106221021320060074
- ChenAMZhangMWeiDCo-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cellsSmall20095232673267719780069
- SongXRCaiZZhengYReversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticlesEur J Pharm Sci2009373–430030519491019
- KoziaraJMWhismanTRTsengMTMumperRJIn-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumorsJ Control Release2006112331231916626835
- ZhangYTangLSunLA novel paclitaxel-loaded poly(epsilon-caprolactone)/Poloxamer 188 blend nanoparticle overcoming multidrug resistance for cancer treatmentActa Biomater2010662045205219969111
- MieleESpinelliGPMieleETomaoFTomaoSAlbumin-bound formulation of paclitaxel (Abraxane ABI–007) in the treatment of breast cancerInt J Nanomedicine200949910519516888
- ZhaoDZhaoXZuYPreparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticlesInt J Nanomedicine2010566967720957218
- SharmaASharmaUSLiposomes in drug delivery: progress and limitationsInt J Pharm19971542123140
- FenskeDBChonnACullisPRLiposomal nanomedicines: an emerging fieldToxicol Pathol2008361212918337218
- JohnstonMJWSempleSCKlimukSKAnsellSMaurerNCullisPRCharacterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelinBiochim Biophys Acta2007176851121112717321495
- FenskeDBMacLachlanICullisPRLong-circulating vectors for the systemic delivery of genesCurr Opin Mol Ther20013215315811338928
- FenskeDBCullisPRLiposomal nanomedicinesExpert Opin Drug Deliv200851254418095927
- SempleSCLeoneRWangJOptimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activityJ Pharm Sci20059451024103815793796
- ChoudhuriTPalSDasTSaGCurcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent mannerJ Biol Chem200528020200592006815738001
- StrijkersGJKluzaEVan TilborgGAFParamagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesisAngiogenesis201013216117320390447
- ChengALHsuCHLinJKPhase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesionsAnticancer Res2001214B2895290011712783
- ThangapazhamRLPuriATeleSBlumenthalRMaheshwariRKEvaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cellsInt J Oncol20083251119112318425340
- FenskeDBWongKFMaurerEIonophore-mediated uptake of ciprofloxacin and vincristine into large unilamellar vesicles exhibiting transmembrane ion gradientsBiochim Biophys Acta199814141–21882049804953
- JohnstonMJWSempleSCKlimukSKTherapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulationsBiochim Biophys Acta200617581556416487476
- RodriguezMADangNHFayadLSphingosomal vincristine in CHOP is a promising new treatment for elderly, as well as poor prognosis patients with aggressive non-Hodgkin’s lymphoma (NHL): follow-up results of a phase II studyJ Clin Oncol20042214S8080
- BhirdeAAPatelVGavardJTargeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug deliveryACS Nano20093230731619236065
- LiuZChenKDavisCDrug delivery with carbon nanotubes for in vivo cancer treatmentCancer Res200868166652666018701489
- ChakravartyPMarchesRZimmermanNSThermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubesProc Natl Acad Sci U S A2008105258697870218559847
- BegSRizwanMSheikhAMHasnainMSAnwerKKohliKAdvancement in carbon nanotubes: basics, biomedical applications and toxicityJ Pharm Pharmacol201163214116321235578
- SobhaniZDinarvandRAtyabiFGhahremaniMAdeliMIncreased paclitaxel cytotoxicity against cancer cell lines using a novel functionalized carbon nanotubeInt J Nanomedicine2011670571921556345
- KhazaeiARadMNSBorazjaniMKOrganic functionalization of single-walled carbon nanotubes (SWCNTs) with some chemotherapeutic agents as a potential method for drug deliveryInt J Nanomedicine2010563964520856839
- KruegerANew carbon materials: biological applications of functionalized nanodiamond materialsChemistry20081451382139018033700
- KatebBChiuKBlackKLNanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy?Neuroimage201154Suppl 1S106S12420149882
- HuangHPierstorffEOsawaEHoDActive nanodiamond hydrogels for chemotherapeutic deliveryNano Lett20077113305331417918903
- LamRChenMPierstorffEHuangHOsawaEHoDNanodiamond-embedded microfilm devices for localized chemotherapeutic elutionACS Nano20082102095210219206456
- LiuKKWangCCChengCLChaoJIEndocytic carboxylated nano-diamond for the labeling and tracking of cell division and differentiation in cancer and stem cellsBiomaterials200930264249425919500835
- ShermanMBGuentherRHTamaFRemoval of divalent cations induces structural transitions in red clover necrotic mosaic virus, revealing a potential mechanism for RNA releaseJ Virol20068021103951040616920821
- KwakMMintenIJAnayaDMVirus–like particles templated by DNA micelles: a general method for loading virus nanocarriersJ Am Chem Soc2010132237834783520481536
- DixitSKGoicocheaNLDanielMCQuantum dot encapsulation in viral capsidsNano Lett2006691993199916968014
- GoicocheaNLDeMRotelloVMMukhopadhyaySDragneaBCore-like particles of an enveloped animal virus can self-assemble efficiently on artificial templatesNano Lett2007782281229017645363
- KlostergaardJSeeneyCEMagnetic nanovectors for drug deliveryMaturitas2012
- KaleSNJadhavADVermaSCharacterization of biocompatible NiCo2O4 nanoparticles for applications in hyperthermia and drug deliveryNanomedicine20128445245921839056
- AhamedMAkhtarMJSiddiquiMAOxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cellsToxicology20112832–310110821382431
- LiuLZhangHMengXYinJLiDLiuCDinuclear metal(II) complexes of polybenzimidazole ligands as carriers for DNA deliveryBiomaterials20103161380139119906419
- LaiBBChenBAChengJDaunorubicin-loaded magnetic nanoparticles of Fe3O4 greatly enhance the responses of multidrug-resistant K562 leukemic cells in a nude mouse xenograft model to chemotherapyZhongguo Shi Yan Xue Ye Xue Za Zh2009172345351
- AndresenTLThompsonDHKaasgaardTEnzyme-triggered nanomedicine: drug release strategies in cancer therapyMol Membr Biol201027735336320939771
- GuptaSAndresenHGhadialiJEStevensMMKinase-actuated immunoaggregation of Peptide-conjugated gold nanoparticlesSmall20106141509151320578112
- de la RicaRAiliDStevensMMEnzyme-responsive nanoparticles for drug release and diagnosticsAdv Drug Deliv Rev Epub January 14, 2012.
- YangYAwJChenKEnzyme-responsive multifunctional magnetic nanoparticles for tumor intracellular drug delivery and imagingChem Asian J2011661381138921548100
- GuptaSAndresenHStevensMMSingle-step kinase inhibitor screening using a peptide-modified gold nanoparticle platformChem Commun (Camb)20114782249225121246106
- BharaliDJKhalilMGurbuzMSimoneTMMousaSANanoparticles and cancer therapy: a concise review with emphasis on dendrimersInt J Nanomedicine200941719421366
- BakerJRDendrimer-based nanoparticles for cancer therapyHematology Am Soc Hematol Educ Program20092009170871920008257
- SvensonSTomaliaDADendrimers in biomedical applications – reflections on the fieldAdv Drug Deliv Rev200557152106212916305813
- TekadeRKKumarPVJainNKDendrimers in oncology: an expanding horizonChem Rev20091091498719099452
- MenjogeARKannanRMTomaliaDADendrimer-based drug and imaging conjugates: design considerations for nanomedical applicationsDrug Discov Today2010155–617118520116448
- TomaliaDAReynaLASvensonSDendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imagingBiochem Soc Trans200735Pt 1616717233602
- Padilla De JesúsOLIhreHRGagneLFréchetJMJSzokaFCJrPolyester dendritic systems for drug delivery applications: in vitro and in vivo evaluationBioconjug Chem200213345346112009933
- LaiPSLouPJPengCLDoxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapyJ Control Release20071221394617628166
- KirkpatrickGJPlumbJASutcliffeOBFlintDJWheateNJEvaluation of anionic half generation 3.5–6.5 poly(amidoamine) dendrimers as delivery vehicles for the active component of the anticancer drug cisplatinJ Inorg Biochem201110591115112221704583
- GilliesERFréchetJMJDendrimers and dendritic polymers in drug deliveryDrug Discov Today2005101354315676297
- MalikNEvagorouEGDuncanRDendrimer-platinate: a novel approach to cancer chemotherapyAnticancer Drugs199910876777610573209
- LeeCCGilliesERFoxMEA single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomasProc Natl Acad Sci U S A200610345166491665417075050
- MaedaHWuJSawaTMatsumuraYHoriKTumor vascular permeability and the EPR effect in macromolecular therapeutics: a reviewJ Control Release2000651–227128410699287
- HanGGhoshPRotelloVMMulti-functional gold nanoparticles for drug deliveryAdv Exp Med Biol2007620485618217334
- ChenJMcLellanJMSiekkinenAXiongYLiZYXiaYFacile synthesis of gold-silver nanocages with controllable pores on the surfaceJ Am Chem Soc200612846147761477717105266
- AlkilanyAMMurphyCJToxicity and cellular uptake of gold nanoparticles: what we have learned so far?J Nanopart Res20101272313233321170131
- KimBHanGToleyBJKimCKRotelloVMForbesNSTuning payload delivery in tumour cylindroids using gold nanoparticlesNat Nanotechnol20105646547220383126
- BrownSDNativoPSmithJAGold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatinJ Am Chem Soc2010132134678468420225865
- KhullarPSinghVMahalABovine serum albumin bioconjugated gold nanoparticles: synthesis, hemolysis, and cytotoxicity toward cancer cell linesJ Phys Chem C20121161588348843
- JoshiPChakrabortySDeySBinding of chloroquine-conjugated gold nanoparticles with bovine serum albuminJ Colloid Interface Sci2011355240240921216410
- SmithAMDaveSNieSTrueLGaoXMulticolor quantum dots for molecular diagnostics of cancerExpert Rev Mol Diagn20066223124416512782
- GaoXDaveSRQuantum dots for cancer molecular imagingAdv Exp Med Biol2007620577318217335
- YuYXuLChenJHydrothermal synthesis of GSH-TGA co-capped CdTe quantum dots and their application in labeling colorectal cancer cellsColloids Surf B Biointerfaces20129524725322494668
- ShenJMTangWJZhangXLChenTZhangHXA novel carboxymethyl chitosan-based folate/Fe3O4/CdTe nanoparticle for targeted drug delivery and cell imagingCarbohydr Polym2012881239249
- LiJMWangYYZhaoMXMultifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time trackingBiomaterials20123392780279022243797
- WangYZhangYDuZWuMZhangGDetection of micrometastases in lung cancer with magnetic nanoparticles and quantum dotsInt J Nanomedicine201272315232422661888
- UnerMYenerGImportance of solid lipid nanoparticles (SLN) in various administration routes and future perspectivesInt J Nanomedicine20072328930018019829
- WangSChenTChenRHuYChenMWangYEmodin loaded solid lipid nanoparticles: preparation, characterization and antitumor activity studiesInt J Pharm20124301–223824622465546
- ShiSJZhongZRLiuJZhangZRSunXGongTSolid lipid nanoparticles loaded with anti-microRNA oligonucleotides (AMOs) for suppression of microRNA-21 functions in human lung cancer cellsPharm Res20122919710921732152
- BrewerEColemanJLowmanAEmerging technologies of polymeric nanoparticles in cancer drug deliveryJ Nanomater2011201111021808638
- RaoJPGeckelerKEPolymer nanoparticles: preparation techniques and size-control parametersProg Polym Sci2011367887913
- van VlerkenLEAmijiMMMulti-functional polymeric nanoparticles for tumour-targeted drug deliveryExpert Opin Drug Deliv20063220521616506948
- OwensDE3rdPeppasNAOpsonization, biodistribution, and pharmacokinetics of polymeric nanoparticlesInt J Pharm200630719310216303268
- HuSZhangYEndostar-loaded PEG-PLGA nanoparticles: in vitro and in vivo evaluationInt J Nanomedicine201051039104821170352
- WooHNChungHKJuEJPreclinical evaluation of injectable sirolimus formulated with polymeric nanoparticle for cancer therapyInt J Nanomedicine201272197220822619555
- AravindAJeyamohanPNairRAS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug deliveryBiotechnol Bioeng Epub May 21, 2012.
- GuoJGaoXSuLAptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug deliveryBiomaterials201132318010802021788069
- DharSGuFXLangerRFarokhzadOCLippardSJTargeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticlesProc Natl Acad Sci U S A200810545173561736118978032
- ChenJLiSShenQHeHZhangYEnhanced cellular uptake of folic acid-conjugated PLGA-PEG nanoparticles loaded with vincristine sulfate in human breast cancerDrug Dev Ind Pharm201137111339134621524153
- ChoHBaeJGarripelliVKAndersonJMJunHWJoSRedox-sensitive polymeric nanoparticles for drug deliveryChem Commun (Camb)2012
- PrajapatiBGNanoparticles as platforms for targeted drug delivery system in cancer therapyInternet J Nanotechnol20093118
- SternJMCadedduJAEmerging use of nanoparticles for the therapeutic ablation of urologic malignanciesUrol Oncol2008261939618190837
- MilleronRSBrattonSB‘Heated’ debates in apoptosisCell Mol Life Sci200764182329233317572850
- CherukuriPGlazerESCurleySATargeted hyperthermia using metal nanoparticlesAdv Drug Deliv Rev201062333934519909777
- EllisLMCurleySATanabeKKRadiofrequency Ablation for Cancer: Current Indications, Techniques, and OutcomesNew YorkSpringer-Verlag2004
- ModyVVSiwaleRSinghAModyHRIntroduction to metallic nanoparticlesJ Pharm Bioallied Sci20102428228921180459
- SamantaBYanHFischerNOShiJJerryDJRotelloVMProtein-passivated Fe3O4 nanoparticles: low toxicity and rapid heating for thermal therapyJ Mater Chem200818111204120819122852
- KamNWSLiuZDaiHFunctionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencingJ Am Chem Soc200512736124921249316144388
- GannonCJCherukuriPYakobsonBICarbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency fieldCancer2007110122654266517960610
- SamiaACSChenXBurdaCSemiconductor quantum dots for photodynamic therapyJ Am Chem Soc200312551157361573714677951
- BakalovaROhbaHZhelevZIshikawaMBabaYQuantum dots as photosensitizers?Nat Biotechnol200422111360136115529155
- LetfullinRRIversenCBGeorgeTFModeling nanophotothermal therapy: kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticlesNanomedicine20117213714520732456
- FillatCJoseABofill-DeRosXMato-BercianoAMaliandiMVSobrevalsLPancreatic cancer gene therapy: from molecular targets to delivery systemsCancers201131368395
- SongWJDuJZSunTMZhangPZWangJGold nanoparticles capped with polyethyleneimine for enhanced siRNA deliverySmall20106223924619924738
- LiuJSonshineDAShervaniSHurtRHControlled release of biologically active silver from nanosilver surfacesACS Nano20104116903691320968290
- NunesAAmsharovNGuoCHybrid polymer-grafted multiwalled carbon nanotubes for in vitro gene deliverySmall20106202281229120878655
- EwertKKZidovskaAAhmadACationic liposome-nucleic acid complexes for gene delivery and silencing: pathways and mechanisms for plasmid DNA and siRNATop Curr Chem201029619122621504103
- TannerPBaumannPEneaROnacaOPalivanCMeierWPolymeric vesicles: from drug carriers to nanoreactors and artificial organellesAcc Chem Res201144101039104921608994
- KimTHJinHKimHWChoMHChoCSMannosylated chitosan nanoparticle-based cytokine gene therapy suppressed cancer growth in BALB/c mice bearing CT-26 carcinoma cellsMol Cancer Ther2006571723173216891458
- TalaeiFAziziEDinarvandRAtyabiFThiolated chitosan nanoparticles as a delivery system for antisense therapy: evaluation against EGFR in T47D breast cancer cellsInt J Nanomedicine201161963197521976973
- PirolloKFRaitAZhouQMaterializing the potential of small interfering RNA via a tumor-targeting nanodelivery systemCancer Res20076772938294317409398
- GopalanBItoIBranchCDStephensCRothJARameshRNanoparticle based systemic gene therapy for lung cancer: molecular mechanisms and strategies to suppress nanoparticle-mediated inflammatory responseTechnol Cancer Res Treat20043664765715560723
- ZhangLGaoXMenKGene therapy for C-26 colon cancer using heparin-polyethyleneimine nanoparticle-mediated survivin T34AInt J Nanomedicine201162419242722072877
- XuZShenGXiaXComparisons of three polyethyleneimine-derived nanoparticles as a gene therapy delivery system for renal cell carcinomaJ Transl Med201191464621513541
- ChenAADerfusAMKhetaniSRBhatiaSNQuantum dots to monitor RNAi delivery and improve gene silencingNucleic Acids Res20053322e19016352864
- XieJLeeSChenXNanoparticle-based theranostic agentsAdv Drug Deliv Rev201062111064107920691229
- CaiDMatarazaJMQinZHHighly efficient molecular delivery into mammalian cells using carbon nanotube spearingNat Methods20052644945415908924
- ElhissiAMAAhmedWHassanIUDhanakVRD’EmanueleACarbon nanotubes in cancer therapy and drug deliveryJ Drug Deliv2012 2012:Article ID837327
- PodestaJEAl-JamalKTHerreroMAAntitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft modelSmall20095101176118519306454
- BarkerSLShortreedMRKopelmanRUtilization of lipophilic ionic additives in liquid polymer film optodes for selective anion activity measurementsAnal Chem19976969909959075401
- ChoiYThomasTKotlyarAIslamMTBakerJRJrSynthesis and functional evaluation of DNA-assembled polyamidoamine dendrimer clusters for cancer cell-specific targetingChem Biol2005121354315664513
- DuncanRMNDendrimers: biocompatibility and potential for delivery of anticancer agentsProc Int Symp Control Release Bioact Mater199623105106
- VarkouhiAKFoillardSLammersTSiRNA delivery with functionalized carbon nanotubesInt J Pharm2011416241942521320582
- YaoHNgSSHuoLFEffective melanoma immunotherapy with interleukin-2 delivered by a novel polymeric nanoparticleMol Cancer Ther20111061082109221518728
- PrakashSWei ShaoMPaulAA novel polyethyleneimine-coated adeno-associated virus-like particle formulation for efficient siRNA delivery in breast cancer therapy: preparation and in vitro analysisInt J Nanomedicine20121575158622619514
- FortinaPKrickaLJNanotechnology: improving clinical testing?Clin Chem20105691384138920802097
- ChiXHuangDZhaoZZhouZYinZGaoJNanoprobes for in vitro diagnostics of cancer and infectious diseasesBiomaterials201233118920621959007
- SullivanDCFerrariMNanotechnology and tumor imaging: seizing an opportunityMol Imaging20043436436915802054
- MedintzILUyedaHTGoldmanERMattoussiHQuantum dot bio-conjugates for imaging, labelling and sensingNat Mater20054643544615928695
- PericleousPGazouliMLyberopoulouARizosSNikiteasNEfstathopoulosEPQuantum dots hold promise for early cancer imaging and detectionInt J Cancer2012131351952822411309
- SmithAMDuanHMohsAMNieSBioconjugated quantum dots for in vivo molecular and cellular imagingAdv Drug Deliv Rev200860111226124018495291
- GaoJChenXChengZNear-infrared quantum dots as optical probes for tumor imagingCurr Top Med Chem201010121147115720388111
- FrascoMFChaniotakisNSemiconductor quantum dots in chemical sensors and biosensorsSensors (Basel)2009997266728622423206
- HuMYanJHeYUltrasensitive, multiplexed detection of cancer biomarkers directly in serum by using a quantum dot-based microfluidic protein chipACS Nano20104148849420041634
- BaileyVJEaswaranHZhangYMS-qFRET: a quantum dot-based method for analysis of DNA methylationGenome Res20091981455146119443857
- SoMKXuCLoeningAMGambhirSSRaoJSelf-illuminating quantum dot conjugates for in vivo imagingNat Biotechnol200624333934316501578
- XiaZXingYSoMKKohALSinclairRRaoJMultiplex detection of protease activity with quantum dot nanosensors prepared by intein-mediated specific bioconjugationAnal Chem200880228649865518922019
- JunYWSheikholeslamiSHostetterDRTajonCCraikCSAlivisatosAPContinuous imaging of plasmon rulers in live cells reveals early-stage caspase-3 activation at the single-molecule levelProc Natl Acad Sci U S A200910642177351774019805121
- LeeSELeeLPBiomolecular plasmonics for quantitative biology and nanomedicineCurr Opin Biotechnol201021448949720801636
- OsterfeldSJYuHGasterRSMultiplex protein assays based on real-time magnetic nanotag sensingProc Natl Acad Sci U S A200810552206372064019074273
- HirschLRJacksonJBLeeAHalasNJWestJLA whole blood immunoassay using gold nanoshellsAnal Chem200375102377238112918980
- ZhaoXTapec-DytiocoRTanWUltrasensitive DNA detection using highly fluorescent bioconjugated nanoparticlesJ Am Chem Soc200312538114741147513129331
- WuXLiuHLiuJImmunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dotsNat Biotechnol2003211414612459735
- SantraSZhangPWangKTapecRTanWConjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkersAnal Chem200173204988499311681477
- RiehemannKSchneiderSWLugerTAGodinBFerrariMFuchsHNanomedicine – challenge and perspectivesAngew Chem Int Ed Engl200948587289719142939
- YueMLinHDedrickDEA 2-D microcantilever array for multiplexed biomolecular analysisJ Microelectromech Syst2004132290299
- MajumdarABioassays based on molecular nanomechanicsDis Markers200218416717412590170
- ZhengGPatolskyFCuiYWangWULieberCMMultiplexed electrical detection of cancer markers with nanowire sensor arraysNat Biotechnol200523101294130116170313
- SternEVacicARajanNKLabel-free biomarker detection from whole bloodNat Nanotechnol20105213814220010825
- SunYPFuKLinYHuangWFunctionalized carbon nanotubes: properties and applicationsAcc Chem Res200235121096110412484798
- LiuZTabakmanSWelsherKDaiHCarbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug deliveryNano Res2009228512020174481
- ParkJHvon MaltzahnGRuoslahtiEBhatiaSNSailorMJMicellar hybrid nanoparticles for simultaneous magnetofluorescent imaging and drug deliveryAngew Chem Int Ed Engl200847387284728818696519
- MistlbergerGKorenKScheucherEMultifunctional magnetic optical sensor particles with tunable sizes for monitoring metabolic parameters and as a basis for nanotherapeuticsAdv Funct Mater2010201118421851
- YongKTBiophotonics and biotechnology in pancreatic cancer: cyclic RGD-peptide-conjugated type II quantum dots for in vivo imagingPancreatology201010555356420975319
- BünzliJCLanthanide luminescence for biomedical analyses and imagingChem Rev201011052729275520151630
- SitharamanBAvtiLuminescent single-walled carbon nanotube-sensitized europium nanoprobes for cellular imagingInt J Nanomedicine20121953195322619533
- LewisDJBruceCBohicSIntracellular synchrotron nanoimaging and DNA damage/genotoxicity screening of novel lanthanide-coated nanovectorsNanomedicine (Lond)20105101547155720879836
- LiuYZhangNGadolinium loaded nanoparticles in theranostic magnetic resonance imagingBiomaterials201233215363537522521487
- WangXWangCChengLLeeSTLiuZNoble metal coated single-walled carbon nanotubes for applications in surface enhanced Raman scattering imaging and photothermal therapyJ Am Chem Soc2012134177414742222486413
- CordovillaCSwagerTMStrain release in organic photonic nanoparticles for protease sensingJ Am Chem Soc2012134166932693522489929
- LingYWeiKZouFZhongSTemozolomide loaded PLGA-based super paramagnetic nanoparticles for magnetic resonance imaging and treatment of malignant gliomaInt J Pharm20124301–226627522486964
- DongHLeiJJuHTarget-cell-specific delivery, imaging, and detection of intracellular MicroRNA with a multifunctional SnO2 nanoprobeAngew Chem Int Ed Engl201251194607461222473624
- TaoLZhangKSunYJinBZhangZYangKAnti-epithelial cell adhesion molecule monoclonal antibody conjugated fluorescent nanoparticle biosensor for sensitive detection of colon cancer cellsBiosens Bioelectron201235118619222464249
- LiuKLiuXZengQCovalently assembled NIR Nanoplatform for simultaneous fluorescence imaging and photodynamic therapy of cancer cellsACS Nano2012654054406222463487
- FergusonRMKhandharAPKrishnanKMTracer design for magnetic particle imaging (invited)J Appl Phys201211177B318317B3185
- PuvanakrishnanPDiagaradjanePKazmiSMSDunnAKKrishnanSTunnellJWNarrow band imaging of squamous cell carcinoma tumors using topically delivered anti-EGFR antibody conjugated gold nanorodsLasers Surg Med201244431031722415634
- El-DakdoukiMHZhuDCEl-BoubbouKDevelopment of multifunctional hyaluronan-coated nanoparticles for imaging and drug delivery to cancer cellsBiomacromolecules20121341144115122372739
- ChoKWangXNieSChenZShinDMTherapeutic Nanoparticles for Drug Delivery in CancerClin Cancer Res2008141310131618316549
- McBainSCYiuHHPDobsonJMagnetic nanoparticles for gene and drug deliveryInt J Nanomedicine20083216918018686777