 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
It has been reported that the tumor suppressor gene, PTEN, which is inactivated in many malignant tumors, plays an important role in apoptosis, cell cycle arrest, cell migration, and cell spread. For cancer gene therapy, one of the most important problems is low gene transfection efficiency.
Methods
In the present study, to take full advantage of adenovirus in gene expression, we prepared mannan-modified recombinant adenovirus using the PTEN gene (Man-Ad5-PTEN) and investigated the effect of Man-Ad5-PTEN combined with docetaxel (Man-Ad5-PTEN-docetaxel) on tumor growth in a murine model of hepatocellular carcinoma.
Results
Man-Ad5-PTEN effectively suppressed tumor growth and induced significant apoptosis of murine H22 hepatoma in vivo. Apoptosis levels in tumor-bearing mice treated with Man-Ad5-PTEN-docetaxel were significantly higher than those in tumor-bearing mice treated with naked Ad5-PTEN, Man-Ad5-PTEN, or docetaxel alone. Treatment with Man-Ad5-PTEN-docetaxel resulted in a significant inhibitory effect in this tumor model. Compared with the controls treated with phosphate-buffered solution, the tumor inhibition rate with naked Ad5-PTEN, docetaxel, Man-Ad5-PTEN, and Man-Ad5-PTEN-docetaxel was 48.69%, 49.98%, 75.88%, and 96.93%, respectively.
Conclusion
These results suggest that combined treatment with Man-Ad5-PTEN and other chemotherapeutic agents may be a potent adjuvant therapeutic approach for the treatment of hepatocellular carcinoma.
Introduction
Viral and nonviral methods of gene delivery have been used in clinical trials, in which viral vectors such as retroviruses, adenoassociated viruses, herpes simplex viruses, and adenovirusesCitation1 are the most promising vectors for high transfection efficiency. Among these viral vectors, the replication-deficient adenovirus has become one of the most promising gene delivery vectors due to its many advantages, including being easily produced in high titers, an ability to transfect nonproliferating cells readily, no integration into the host genome, and broad tissue or cell tropism. However, the broad host range of the adenovirus is also a disadvantage and can lead to lack of tissue specificity and poor safety. Therefore, it is necessary to improve this therapeutic strategy by improving the cancer-targeting ability of the adenovirus vector. Previous studies have developed strategies using heterologous retargeting complexes, such as chimeric fusion proteins and bispecific antibodies, or genetic capsid modification.Citation2–Citation5
Hepatocellular carcinoma is an aggressive and rapidly fatal malignancy of the liver and is the third leading cause of cancer-related death worldwide. Conventional treatment for hepatocellular carcinoma includes surgery, radiotherapy, and chemotherapy. Although remarkable advances have been made in the clinical study of hepatocellular carcinoma, only a definitive subset of cases is cured, and the overall dismal outcome of patients with hepatocellular carcinoma has not changed, so it is essential that we find a more effective way to treat this disease. Gene therapy represents an exciting biotechnological advance that may revolutionize conventional cancer treatment, and combination treatment using gene therapy and chemotherapy is being paid much attention.
The phosphatase and tensin homolog deleted on chromosome ten (PTEN) gene is a tumor-suppressor gene located on human chromosome 10q23.3, originally identified by two research groups in 1997.Citation6,Citation7 Since then, a significant number of PTEN deletions and mutations have been reported in many malignant tumors, including glioblastoma, prostate cancer, melanoma, endometrial carcinoma, lung carcinoma,Citation8 and hepatocellular carcinoma.Citation9 PTEN has been reported to play a role in apoptosis, cell cycle arrest, proliferation, cell migration, angiogenesis, and overall survival.Citation10–Citation13 Overexpression of PTEN in cancer cells carrying mutant-type or deletion-type PTEN can inhibit cell proliferation and tumorigenicity via induction of cell cycle arrest at the G1 phase and apoptosis.Citation14–Citation16 Loss of PTEN expression has been reported to be strongly correlated with aggressive tumor behavior, eg, loss of nuclear PTEN expression was found to be associated with liver metastasis.Citation17,Citation18 Restoring PTEN expression in PTEN-deficient tumor cells has been shown to increase radiosensitivity in vivo.Citation19 Furthermore, previous studies have shown that PTEN is involved in drug resistance in several types of cancer,Citation20,Citation21 and cell lines with a high expression of PTEN are sensitive to chemotherapy with 5-fluorouracil and oxaliplatin.Citation18 Therefore, in this work, we investigated whether the PTEN gene transfected by adenovirus could effectively suppress hepatocellular carcinoma in vivo.
On the other hand, among those targeting strategies mentioned above, conjugating the ligand of the receptor to vectors of interest is an attractive approach. Moreover, it was reported that there is considerably high expression of the mannose receptor in liver nonparenchymal cells and endothelial sinusoidal cells, and that mannose receptors are involved in the binding and subsequent endocytosis of mannose-containing glycoproteins to and into cells.Citation22–Citation24 One study showed that plasmid DNA complexed with mannosylated liposomes had high transfection activity due to recognition by mannose receptors both in vitro and in vivo.Citation25 Another study showed that conjugation of mannan with a recombinant antigen, mucin-1, gives rise to a high cellular immune response, leading to total resistance of experimental tumors in mice.Citation26
Putting all this information together, in the present study, to test whether the combination strategy of cancer gene therapy and chemotherapy could enhance antitumor effects, conjugates of mannan-modified Ad5-PTEN (Man-Ad5-PTEN) were prepared and used to treat H22 tumor-bearing mice, together with docetaxel, a well known chemotherapeutic agent.Citation27 After treatment, tumor volume and weight, along with apoptosis level, were investigated.
Materials and methods
Mice and cell culture
Male Kunming mice, aged 6–7 weeks, were purchased from the Laboratory Animal Center of Luzhou Medical College, Luzhou, China. The study was approved by the animal ethics committee of Luzhou Medical College, and all procedures with the animals were conducted according to the guidelines of the local animal use and care committees of Luzhou and performed according to the National Animal Welfare Law of China.
Murine H22 hepatoma cells (syngeneic with the Kunming mouse strain)Citation28 and human embryonic kidney (HEK) 293 cells were sourced from the Chinese Academy of Sciences, Shanghai, China, and maintained in our laboratory. The H22 and HEK 293 cells were, respectively, cultured in RPMI 1640 medium and Dulbecco’s Modified Eagle’s medium (Hyclone Corporation, Logan, UH) supplemented with 10% fetal bovine serum (Hyclone Corporation), 100 units/mL penicillin, and 100 μg/mL streptomycin, and cultured at 37°C in air containing 5% CO2.
Adenoviral vectors
Replication-deficient human type 5 adenovirus with the expressing reporter gene for β-galactosidase (Ad5-LacZ) was purchased from the Vector Gene Technology Company Limited (Beijing, China), and another adenovirus carrying the PTEN gene (Ad5-PTEN) was a gift from the National Key Laboratory of Biotherapy, Sichuan University, China. Ad5-PTEN was constructed according to a report published elsewhere.Citation29 Briefly, it was constructed by subcloning full-length PTEN cDNA from a plasmid construct (pCDNA3-PTEN) into a shuttle plasmid containing CMV promoter and SV40 poly (A) elements. The resulted PTEN-containing plasmid was cotransfected with pJM17 into HEK 293 cells to obtain the Ad-PTEN virus. The resulting Ad5-PTEN virus was propagated in HEK 293 cells and purified by two rounds of CsCl (Amresco Inc, Solon, OH) density gradient centrifugation. The genome copy number of adenovirus stock was determined by Taqman real-time polymerase chain reaction, and the adenovirus titer was detected using a plaque-forming assay on HEK 293 cells.
Mannan and adenovirus conjugation
Mannan was conjugated to adenovirus in the following manner. Briefly, mannan (Sigma, St Louis, MO), at 25 mg/mL in 0.1 M phosphate buffer (pH 6.0) optimized by periodic acid-Schiff (PAS) stain, was oxidized with sodium periodate (0.01 M, Sigma) for 60 minutes at 4°C. The mixture was desalted by dialysis at 4°C for 5 hours against bicarbonate buffer (pH 9.0) and replaced with fresh buffer every other 30 minutes. The resulting oxidized mannan was mixed with Ad5-PTEN, incubated overnight at room temperature, and used without further purification.
In vivo animal tumor model experiment
H22 tumor cells were obtained for inoculation from in vivo passage. Briefly, the stocked murine hepatoma H22 cells were first subcultured in RPMI 1640 containing 10% fetal bovine serum and collected in blank RPMI 1640 culture medium (1 × 108/mL). About 0.2 mL of preformed cell suspension (including 2 × 107 cells) was injected into the abdominal cavity of the mouse. After 7 days, the mouse had developed an ascites tumor and was sacrificed by nitrogen inhalation and disinfected by immersing the body in 75% ethanol. Intraperitoneal tumor cells were collected, washed twice, and resuspended in phosphate-buffered solution. Viable cells were counted by Trypan blue staining; next, 1 × 107 murine H22 hepatoma cells in 0.1 mL of phosphate-buffered saline were injected subcutaneously into the right flank of each mouse on day 0. All tumor-bearing mice were divided randomly into five groups (at least eight mice per group), and the treatments were carried out on day 6 when the tumors had grown to 100–150 mm3. At that time, 100 μL of solution were injected intratumorally into each mouse from each group. One of the five groups was selected to be injected with phosphate-buffered saline as the control group, and the others were treated with naked Ad5-PTEN, docetaxel (Credit Pharma Co Ltd, Chengdu, China), Man-Ad5-PTEN, or Man-Ad5-PTEN-docetaxel, in which the particle number of Ad5 was 1 × 109 and the amount of docetaxel was about 8 mg/kg body weight.Citation30
All the mice were monitored daily after treatment for tumor size. The tumor measurements were converted to tumor volume (V) by the formula (L × W2 × 0.52) and expressed as the mean ± standard deviation where L and W are the length and width, respectively. The tumors were measured with a vernier caliper every day until day 19 when the mice treated with phosphate-buffered saline began to die, and then all the mice were sacrificed by nitrogen inhalation. The tumor burden of each mouse was weighed.
H&E staining and TUNEL assay
After being treated for 2 weeks, the tumor-bearing mice were sacrificed and the tumor burdens from each mouse were weighed and immediately fixed in 4% buffered paraformaldehyde overnight for hematoxylin and eosin (H&E) staining and the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay.
The fixed tumor tissues were dehydrated with gradient ethanol, and embedded in paraffin. Tissue sections (5 μm) were then dewaxed and rehydrated according to the standard protocol. The sections were stained with H&E for observation. For TUNEL assay, an in situ apoptosis detection kit (Roche Applied Science, Penzberg, Germany) was used to detect apoptotic cells in the tumor tissue. Briefly, the sections were treated with 20 μg/mL of proteinase K in distilled water for 10 minutes at room temperature. To block endogenous peroxidase, the slides were incubated in methanol containing 3% hydrogen peroxide for 20 minutes and sections were incubated with equilibration buffer and terminal deoxynucleotidyl transferase. Finally, the sections were incubated with anti-digoxigenin-peroxidase conjugate. Peroxidase activity in each tissue section was shown by the application of diaminobenzidine. Sections were counterstained with hematoxylin.
Apoptosis assay by flow cytometric analysis
The murine hepatoma H22 cells were collected for apoptosis detection by flow cytometric analysis (n = 3) from tumor tissues belonging to the different groups and subjected to FACS detection using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide kit (Nanjing KeyGen Biotech Co, Ltd, Nanjing, China) according to the manufacturer’s instructions. Briefly, the tumors were isolated by surgery, washed with phosphate-buffered saline, cut into pieces, and squeezed through a 200-mesh sieve using a 5 mL syringe core to obtain a single cell suspension. Next, 1 × 106 single cells were collected, washed with phosphate-buffered saline, and resuspended in 500 μL of binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4). After incubation, 5 μL of Annexin V-FITC was added into each tube. The tubes were then incubated for 30 minutes at room temperature in the dark. The cells were washed with binding buffer and resuspended in 500 μL of binding buffer, with 5 μL propidium iodide added. Finally, the tube was gently vortexed and incubated for another 30 minutes in the dark, and the stained cells were analyzed by FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) with CellQuest software within one hour.
Statistical analysis
The data are shown as the mean ± standard deviation. The significance of the difference between the groups was assessed by the Student’s t-test using SPSS version 16.0 (SPSS Inc, Chicago, IL). In all cases, there was a statistically significant difference (P < 0.05, P < 0.01, P < 0.001).
Results
Characterization assay on Man-Ad5-PTEN by PAS staining
Man-Ad5-PTEN was prepared under the appropriate oxidizing conditions as described earlier (). First, mannan was oxidized by sodium periodate to form oxidized mannan (Ox-Man). The Ox-Man with its aldehyde functional groups was able to bind the amino groups anchored on the surface of the adenovirus by Schiff ’s base reaction, which led to formation of Man-Ad5-PTEN. The efficacy of conjugation was confirmed by PAS staining. The PAS stain is a histochemical reaction commonly used to detect glycogen and other polysaccharides in biological specimens. The reaction of periodic acid oxidizes the diol functional groups in glucose and other sugars, creating aldehydes that react with the Schiff reagent to give a purple-magenta color. Therefore, after the conjugation reaction, both the Man-Ad5-PTEN and the redundant Ox-Man will react with the Schiff reagent to give positive staining, and the more the Ox-Man, the darker the color. Further, according to the result of PAS staining, we could determine the optimal concentration of mannan used to modify the adenovirus. As shown in , both the free mannan and the conjugation groups stained positive for PAS, except for the low mannan-modified Ad5, but stained negative for the unconjugated control. The well containing a medium concentration of free mannan () was a darker magenta color, whereas the well containing the product resulting from a medium concentration of mannan conjugated with adenovirus showed an impalpable clear color when stained with PAS (). In contrast, the well containing a high concentration of mannan-modified Ad5 showed a moderate color (). The well containing free Ad5 () and the well containing a low concentration of mannan-modified Ad5 () showed no color. The inconspicuous light color observed in the well shown in indicates that the redundant Ox-Man is relatively less likely to react with the PAS reagent. Meanwhile, in our preliminary experiment (data not shown), we used mannan at different concentrations to react with Ad5, and the resulting products were subjected to PAS staining. The absorbance of the mixture was read at 544 nm using a microplate reader (model-550, BioRad, Hercules, CA) after PAS staining. The results indicate that when the concentration of mannan was less than 25 mg/mL, the PAS stain () was negative and the absorbance in each mixture showed no differences compared with the negative control of PAS reagent alone. Increasing the concentration of mannan to 25, 30, 35, 40, 45, and 50 mg/mL, the absorbance of the mixture also increased and is a representative image. Therefore, we took the corresponding amount as the optimal concentration of mannan conjugated with adenovirus. For visualization, the wells shown in were also observed under light microscopy (AL and FL). All the results confirmed the formation of mannan-adenovirus conjugates.
Figure 1 Synthesis scheme of conjugation strategy for mannan to adenovirus under the appropriate oxidizing conditions.
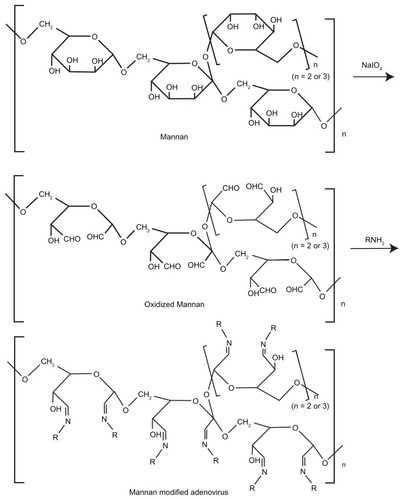
Figure 2 PAS staining to confirm the formation of mannan-modified adenovirus. The different groups subjected to PAS staining are medium-free mannan (A), PAS reagent (B), free Ad5 (C), low mannan-modified Ad5 (D), medium mannanmodified Ad5 (E), and high mannan-modified Ad5 (F), respectively. Mannan of different concentrations was conjugated to adenovirus as described in the Materials and methods section, and then the conjugates were subjected to PAS assay. At the same time, PAS reagent alone was used as the negative control. Both well (A) and well (F) were further observed under light microscopy (100×).
Abbreviations: PAS, periodic acid Schiff; Man, mannan; Ad5, recombinant adenovirus using the PTEN gene; PTEN, phosphatase and tensin homolog deleted on chromosome ten.
Combination treatment significantly decreased tumor volume
To determine the effects of naked Ad5-PTEN, docetaxel, Man-Ad5-PTEN, and Man-Ad5-PTEN-docetaxel on tumor development in mice, we constructed a mouse tumor model and a single injection of each formulation was administered intratumorally. Among them, one control group was treated with 0.1 mL of phosphate-buffered saline, and the other four groups of mice were treated with Man-Ad5-PTEN, docetaxel, or Man-Ad5-PTEN-docetaxel in a total amount of 0.1 mL.
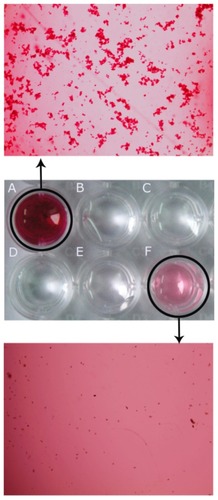
As shown in , there was no therapeutic effect in the control group receiving phosphate-buffered saline, which was consistent with the untreated group (data not shown). Tumor growth in mice treated with Man-Ad5-PTEN-docetaxel was significantly inhibited compared with the other groups of animals treated with phosphate-buffered saline, naked Ad5-PTEN, or docetaxel alone, respectively (P < 0.01). Tumor volume was suppressed by both Man-Ad5-PTEN and Man-Ad5-PTEN-docetaxel. Compared with the group treated with Man-Ad5-PTEN-docetaxel, the mice treated with Ad5-PTEN or docetaxel appeared to be moderately inhibited in tumor growth (P < 0.05). There was no difference between the groups treated with docetaxel or Ad5-PTEN (P > 0.05). A clear difference was observed between the Man-Ad5-PTEN-docetaxel and Man-Ad5-PTEN treated groups from day 14 post-injection. When the Ad5-PTEN or docetaxel groups were compared with the combination group, significant differences were also observed (P < 0.05).
Figure 3 Antitumor effect of combined therapy on suppression of pre-existing subcutaneous mice tumors.
Notes: The tumor model were established by subcutaneous injection of murine H22 hepatoma cells (1.0 × 107) into the right mouse flank. When the mean tumor size reached 100–150 mm3, the mice were divided into five groups (at least n = 8) and injected intratumorally with phosphate-buffered solution, naked Ad5-PTEN, docetaxel alone, Man-Ad5-PTEN, or Man-Ad5-PTEN-docetaxel. Arrow indicates the time point of single injection. Tumor volumes were monitored every day and were estimated as: tumor volume V (mm3) = 0.52 × length (mm) × width (mm)2, which was compared in tumor-bearing mice after treatment with either phosphate-buffered solution, naked Ad5-PTEN, docetaxel alone, Man-Ad5-PTEN, or Man-Ad5-PTEN-docetaxel. The significance of the differences was evaluated using the Student’s t-test. Data are shown as the mean ± standard deviation for five mice in each group.
Abbreviations: Man, mannan; Ad5, recombinant adenovirus using the PTEN gene; PTEN, phosphatase and tensin homolog deleted on chromosome ten.
These results indicate that combination treatment of Man-Ad5-PTEN-docetaxel was the most effective of these formulations, suggesting that Man-Ad5-PTEN can synergize with docetaxel to improve the antitumor effect in H22 tumor-bearing mice after intratumoral administration.
Combination strategy significantly decreases tumor weight
To demonstrate the tumor growth inhibition effect more clearly, the H22 tumor-bearing mice were first given the formulation of Ad5-PTEN, docetaxel, PBS, Man-Ad5-PTEN, or Man-Ad5-PTEN-docetaxel. Then, after two weeks, the mice were euthanized by nitrogen inhalation, the tumors were removed and weighed, and the mean tumor weight was calculated. The tumor inhibition rate was calculated using the following formula:
The results are shown in .
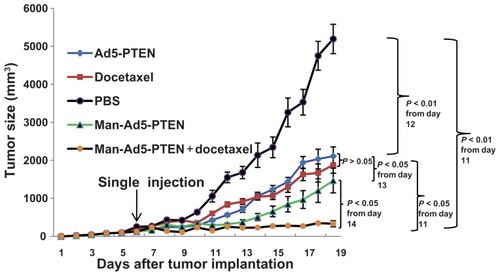
Figure 4 Effect of combination treatment on tumor weight. The H22-tumor bearing mice in each group were given the different formulations, respectively. Two weeks later, the mice were euthanized by nitrogen inhalation. Tumor images were taken by Nikon D70 digital camera (I) and the weight was also examined (II). The tumor weight was compared between phosphate-buffered solution (A) and docetaxel (B), Ad5-PTEN (C), Man-Ad5-PTEN (D), and Man-Ad5-PTEN-docetaxel (E).
Notes: The significance of the differences was evaluated using the Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.001). The tumor inhibition rate was calculated and normalized to the phosphate-buffered solution group. Data are shown as the mean ± standard deviation in each group.
Abbreviations: Man, mannan; Ad5, recombinant adenovirus using the PTEN gene; PTEN, phosphatase and tensin homolog deleted on chromosome ten.
indicates that the average tumor weight of the mice treated with phosphate-buffered saline (), docetaxel (), Ad5-PTEN (), Man-Ad5-PTEN (), and Man-Ad5-PTEN-docetaxel () was 4.8827 ± 0.3942 g, 2.4424 ± 0.2860 g, 2.5053 ± 0.2274 g, 1.1779 ± 0.2008 g, and 0.1499 ± 0.0123 g, respectively. There is a significant difference between (P < 0.05), (P < 0.05), (P < 0.01), and (P < 0.001). Compared with the control group receiving phosphate-buffered saline, the tumor inhibition rate in was 49.98%, 48.69%, 75.88%, and 96.93%, respectively. These results demonstrate that combination treatment with Man-Ad5-PTEN and docetaxel significantly decreased the tumor weight in H22-tumor bearing mice.
Effect of combination treatment on tumor issues analyzed by FACS
We found that all four treatments could suppress tumor growth to varying degrees compared with the control group receiving phosphate-buffered saline, so we wanted to study the likely mechanism of suppression. To investigate quantitatively whether the treatments induced apoptosis in H22 tumor cells, we also performed double-staining flow cytometry with Annexin V-FITC and propidium iodide. A single-cell suspension obtained from tumor tissue was prepared as described earlier.
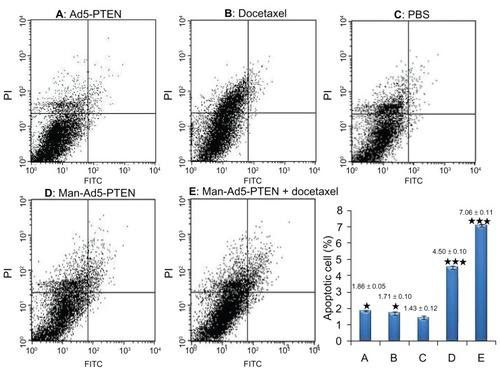
As shown in , compared with the control group treated with phosphate-buffered solution, all the other treatment groups, including Ad5-PTEN (, P < 0.05), docetaxel (, P < 0.05), Man-Ad5-PTEN (, P < 0.001), and Man-Ad5-PTEN-docetaxel (, P < 0.001) showed elevated levels of apoptosis, as measured by the percentage of Annexin V-positive cells. Of note, combined treatment with Man-Ad5-PTEN and docetaxel led to more obvious apoptosis of tumor cells from tumor-bearing mice compared with treatment using Man-Ad5-PTEN or docetaxel alone.
Figure 5 Flow cytometry analysis of cell apoptosis in murine hepatoma H22 cells obtained from tumor-bearing mice. The different groups of mice were treated with Ad5-PTEN, docetaxel, phosphate-buffered solution, Man-Ad5-PTEN, and Man-Ad5-PTEN-docetaxel, respectively. The single cell solution was prepared as described in the Materials and methods section and then stained with Annexin V and propidium iodide. The samples were analyzed by FACSCalibur within one hour. The percent of apoptotic cells was compared between the phosphate-buffered solution group (C) and Ad5-PTEN (A), docetaxel (B), Man-Ad5-PTEN (D), and Man-Ad5-PTEN-docetaxel (E).
Notes: The significance of the differences was evaluated using the Student’s t-test (*P < 0.05; ***P < 0.001). Data are expressed as the mean ± standard deviation (n = 3) in each group.
Abbreviations: FITC, fluorescein isothiocyanate; Man, mannan; Ad5, recombinant adenovirus using the PTEN gene; PTEN, phosphatase and tensin homolog deleted on chromosome ten.
Effect of combination on tumor tissues analyzed by H&E and Tunel assay
To investigate qualitatively whether the treatments induced cell necrosis and apoptosis in H22 tumor cells, all the groups of tumor-bearing mice were treated intratumorally on day 6. Two weeks later, mice were euthanized by nitrogen inhalation, and the tumors were removed and cut into 5 μm slices. The slices were then prepared for subsequent H&E staining and Tunel assay. The H&E staining results () indicate that tumor cells from the treated mice showed the typical morphology of apoptosis, ie, nuclear fragmentation, chromatin condensation, and loss of membrane asymmetry. They also showed that the treated groups had increased apoptotic and necrotic cells compared with the control group treated with phosphate-buffered solution. Both the apoptotic and necrotic cell populations in the Ad5-PTEN and docetaxel groups were much smaller than those in the Man-Ad5-PTEN and Man-Ad5-docetaxel groups. Moreover, there were fewer apoptotic and necrotic cells in the Man-Ad5-PTEN group than in the group receiving Man-Ad5-PTEN-docetaxel.
Figure 6 In vivo tumor cell death study of Man-Ad5-PTEN in combination with docetaxel. The H22 tumor-bearing mice were treated as mentioned earlier with Ad5-PTEN, docetaxel, phosphate-buffered solution, Man-Ad5-PTEN, or Man-Ad5-PTEN-docetaxel. Tumor sections were excised, fixed, dewaxed, and followed by standard H&E staining for necrosis (A, 200×) and apoptosis (B, 400×). As the arrow indicates, condensation and fragmentation of nuclei were observed, which are the hallmarks of apoptosis. Detection of apoptotic cells were also carried out by TUNEL assay (C, 400×).
Abbreviations: Man, mannan; Ad5, recombinant adenovirus using the PTEN gene; PTEN, phosphatase and tensin homolog deleted on chromosome ten; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
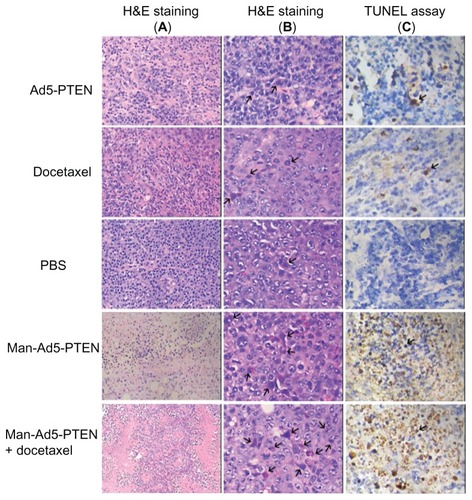
Cellular apoptosis in tumor samples derived from the control and treated mice was also examined using the in situ TUNEL method. The results are shown in , and indicate that the number of apoptotic cells was dramatically increased in tumors treated with Man-Ad5-PTEN and Man- Ad5-PTEN-docetaxel when compared with those in tumors treated with the control phosphate-buffered solution. The number of TUNEL-positive cells was not different between tumors treated with naked Ad5-PTEN and those treated with docetaxel. These results were consistent with those from H&E staining. All the results were consistent with the tumor growth effect in the different groups, suggesting that the apoptotic effect led to tumor growth inhibition.
Discussion
The success of gene therapy in vivo relies on development of a vector that achieves targeted cell-specific, efficient, and prolonged transgene expression after its application. Currently, recombinant adenovirus vectors represent one of the most efficient delivery vehicles for gene therapy in cancer. Their translocation from the cell surface to the nucleus is extremely rapid and far more efficient than nonviral gene delivery vectors. However, adenoviral vectors show strong antigenicity as a result of their capsid domains, leading to decreased expression of the target gene constructed in the adenoviral vector, along with a need for increasing frequency of administration. Moreover, transfer of adenovirus has limited tissue specificity.
Numerous strategies have been developed to address the abovementioned issues, including second-generation and third-generation adenoviral vectors, fiber-mutated or pentone-mutated recombinant adenoviral vectors, conjugated adenovirus, and some ligands to modify adenoviral tropism and reduce vector-related toxicity. On the other hand, active targeting delivery systems have become a focus in cancer therapeutics, and some targeting ligands, such as aptamer, transferrin, antibody, and folate, are used to modify the vectors of interest for binding to particular receptors overexpressed by tumor cells or tumor vasculature.Citation31 In this work, in order to block native tropism, extend blood residence time, and enhance tumor targeting, we conjugated adenovirus with mannan under appropriate oxidizing conditions to enable formation of a mannan-modified adenovirus based on the fact that mannose receptors are extensively expressed in nonparenchymal liver tissue. Thus, introduction of mannose residues may lead to enhancement of endocytosis mediated by these receptors. Compared with naked Ad5-PTEN, Man-Ad5-PTEN significantly suppressed growth of H22 tumors, indicating that the mannan and adenovirus conjugation strategy indeed played an important role. The cytotoxicity of Ad5-PTEN or preformed Man-Ad5-PTEN at a variable multiplicity of infection (80, 40 and 20 plaque-forming units/cell)Citation32 was assessed by MTT viability assay in normal Chang hepatocytes (data not shown). No cytotoxicity was observed in the group treated with naked Ad5-PTEN or in the group treated with Man-Ad5-PTEN, even at the highest multiplicity of infection (80 plaque-forming units/cell), taking the phosphate-buffered solution group as the control. This result suggests that both Ad5-PTEN and Man-Ad5-PTEN may not be cytotoxic to normal cells in vivo. To ensure that chemical modification did not damage the gene expression mediated by Ad5, we investigated transgene expression in vitro using the LacZ reporter gene. The experiment was carried out according to our previous reportCitation33 whereby murine H22 hepatoma cells were treated with naked Ad5-LacZ, Man-Ad5-LacZ, and Man-Ad5-LacZ-docetaxel, respectively. The results show that the average amounts of β-galactosidase in murine H22 hepatoma cells transduced by Man-Ad5-LacZ or Man-Ad5-LacZ-docetaxel were (3.80 ± 0.12) × 105 and (3.83 ± 0.25) × 105 pg β-galactosidase/μg protein, respectively. Their expression levels were about 8-fold higher than those of β-galactosidase in cells transduced by naked Ad5-LacZ, ie, (4.12 ± 0.05) × 104 pg β-galactosidase/μg protein. There was no difference in gene expression between Man-Ad5-LacZ and Man-Ad5-LacZ-docetaxel, indicating that docetaxel 10 μM did not affect gene expression when combined with Man-Ad5-LacZ. These result suggest that neither mannan modification nor the presence of docetaxel affected gene expression mediated by Ad5. Moreover, mannan-modified Ad5 could enhance LacZ gene expression in murine H22 hepatoma cells. The results of this in vitro experiment laid a good foundation for the subsequent in vivo experiments.
Hepatocellular carcinoma is the fifth most common cancer worldwide and the third most common cause of cancer mortality. The conventional therapeutic strategy for hepatocellular carcinoma is surgery, chemotherapy, and radiotherapy. However, the cancer recurs and metastasizes in more than one third of patients after conventional therapy. Currently, combined traditional treatment such as chemotherapy and radiation with vaccines has attracted increasing attention. Vaccination can be quite effective in protecting naive mice from tumor challenge and for delaying tumor growth in mice bearing minimal residual disease, but is less effective when given to mice with established tumors. On the other hand, it has been reported that the cancer-suppressing gene, PTEN, plays a critical role in controlling tumorigenesis and regulating cell spread and migration,Citation34 and adenovirus-mediated PTEN gene therapy is an attractive strategy. However, the ability of single gene therapy alone to suppress tumor growth is limited. To achieve an improved chemotherapeutic effect, a combination of two or more different agents according to their individual mechanisms of action has already been demonstrated to be a popular and effective therapeutic strategy. In a previous study, we reported that combining carmustine chemotherapy with adenovirus-mediated stromal cell-derived factor-1α gene therapy could be successful in the treatment of the murine B16 melanoma.Citation33 Thus, in this study, we developed a mannose receptor-mediated chemoadenovirus-PTEN therapy strategy and combined this with docetaxel, a conventional chemotherapy agent, and PTEN, a tumor suppressor gene.
To investigate the inhibitory effect on tumor growth directly in an empirical manner, we developed a tumor model in vivo and administered Ad5-PTEN, docetaxel, Man-Ad5-PTEN, or Man-Ad5-PTEN-docetaxel by the intratumoral route. To ensure uniform distribution of the formulation in tumor tissue, a single intratumoral injection was carried out in a multipoint manner, whereby pinpoint with fluid does not quit the tumor tissue during one single injection. The results indicate that treatment with Ad5-PTEN and with docetaxel alone could only moderately inhibit tumor growth in H22-bearing mice. However, a combination of Man-Ad5-PTEN and docetaxel dramatically suppressed tumor growth and induced high levels of apoptosis.
The present study is, to our knowledge, the first to combine adenovirus-mediated PTEN gene therapy with docetaxel for cancer treatment. However, the results we obtained are still far from our goal of complete inhibition of tumor growth. Even now, the antitumor mechanism of this combination treatment is still not very clear and requires further investigation. In future studies, we will further optimize the ratio of Ad5-PTEN particle numbers and docetaxel, and also investigate the use of combination strategies in hepatocellular carcinoma and other tumors by intravenous administration and elucidate clearly the mechanism of combination therapy. Meanwhile, in the present study, Man-Ad5-PTEN was injected intratumorally and showed obvious tumor growth inhibition. In the next study, we plan to use this approach for in situ liver cancer by intravenous administration. High targeting efficiency mediated by the mannose receptor and strong antitumor activity are expected.
Conclusion
Overall, this study demonstrated that Man-Ad5-PTEN significantly enhanced the antitumor effect in a murine model of hepatocellular carcinoma in vivo. Combination treatment of Man-Ad5-PTEN and docetaxel had a better antitumor effect than either strategy used alone. Because of its simplicity, this method may be used to target therapy in other kinds of tumors by intratumoral or intravenous administration. It can be concluded that the combined use of Man-Ad5-PTEN and docetaxel may be a powerful and effective tool for future exploration in tumor therapy.
Acknowledgments
We are thankful for the financial supports of the National Natural and Science Foundation of China (No. 81202479), the Key Project of Chinese Ministry of Education (No. 212148), the Educational Commission of Sichuan Province of China (No. 10ZA039), the Scientific Research Project of Sichuan Provincial Health Department (No. 100225), and the Key Project of Natural Science Foundation of Luzhou Medical College.
Disclosure
The authors report no conflicts of interest in this work.
References
- HatefiACappelloJGhandehariHAdenoviral gene delivery to solid tumors by recombinant silk-elastin-like protein polymersPharm Res200724477377917308969
- YaoXLNakagawaSGaoJQCurrent targeting strategies for adenovirus vectors in cancer gene therapyCurr Cancer Drug Targets201111781082521762081
- YaoXYoshiokaYMorishigeTTumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapyMol Ther20111991619162521673661
- KorokhovNMikheevaGKrendelshchikovATargeting of adenovirus via genetic modification of the viral capsid combined with a protein bridgeJ Virol20037724129311294014645549
- ZengQHanJZhaoDGongTZhangZSunXProtection of adenovirus from neutralizing antibody by cationic PEG derivative ionically linked to adenovirusInt J Nanomedicine2012798599722412299
- LiJYenCLiawDPTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancerScience19972755308194319479072974
- SteckPAPershouseMAJasserSAIdentification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancersNat Genet19971543563629090379
- ZhangCKangCWangPMicroRNA-221 and −222 regulate radiation sensitivity by targeting the PTEN pathwayInt J Radiat Oncol Biol Phys201180124024821481725
- VillanuevaANewellPChiangDYFriedmanSLLlovetJMGenomics and signaling pathways in hepatocellular carcinomaSemin Liver Dis2007271557617295177
- WengLPBrownJLEngCPTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer modelHum Mol Genet200110659960411230179
- WengLBrownJEngCPTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathwaysHum Mol Genet200110323724211159942
- TamuraMGuJMatsumotoKAotaSParsonsRYamadaKMInhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTENScience19982805369161416179616126
- DahiaPLPTEN, a unique tumor suppressor geneEndocr Relat Cancer20007211512910903528
- LiDMSunHPTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cellsProc Natl Acad Sci U S A1998952615406154119860981
- FurnariFBHuangHJCaveneeWKThe phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cellsCancer Res19985822500250089823298
- DaviesMALuYSanoTAdenoviral transgene expression of MMAC/PTEN in human glioma cells inhibits Akt activation and induces anoikisCancer Res19985823528552909850049
- SawaiHYasudaAOchiNLoss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survivalBMC Gastroenterol200885619036165
- HsuCPKaoTYChangWLNiehSWangHLChungYCClinical significance of tumor suppressor PTEN in colorectal carcinomaEur J Surg Oncol201137214014721194879
- PappasGZumsteinLAMunshiAHobbsMMeynREAdenoviral-mediated PTEN expression radiosensitizes non-small cell lung cancer cells by suppressing DNA repair capacityCancer Gene Ther200714654354917431403
- SteelmanLSNavolanicPMSokoloskyMLSuppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitorsOncogene200827294086409518332865
- MellinghoffIKCloughesyTFMischelPSPTEN-mediated resistance to epidermal growth factor receptor kinase inhibitorsClin Cancer Res2007132 Pt 137838117255257
- MagnussonSBergTExtremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cellsBiochem J198925736516562930475
- YeepraeWKawakamiSYamashitaFHashidaMEffect of mannose density on mannose receptor-mediated cellular uptake of mannosylated O/W emulsions by macrophagesJ Control Release2006114219320116876282
- MagnussonSBergTEndocytosis of ricin by rat liver cells in vivo and in vitro is mainly mediated by mannose receptors on sinusoidal endothelial cellsBiochem J1993291Pt 37497558489503
- KawakamiSSatoANishikawaMYamashitaFHashidaMMannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomesGene Ther20007429229910694809
- ApostolopoulosVPieterszGALovelandBESandrinMSMcKenzieIFOxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responsesProc Natl Acad Sci U S A1995922210128101327479739
- LiuQLiRZhuZEnhanced antitumor efficacy, biodistribution and penetration of docetaxel-loaded biodegradable nanoparticlesInt J Pharm20124301–235035822525076
- LinCWeiWZhangJLiuSLiuYZhengDFormyl peptide receptor-like 1 mediated endogenous TRAIL gene expression with tumoricidal activityMol Cancer Ther20076102618262517938258
- StewartALMhashilkarAMYangXHPI3 kinase blockade by Ad-PTEN inhibits invasion and induces apoptosis in RGP and metastatic melanoma cellsMol Med20028845146112435856
- LiuQLiRTQianHQGelatinase-stimuli strategy enhances the tumor delivery and therapeutic efficacy of docetaxel-loaded poly(ethylene glycol)-poly(ɛ-caprolactone) nanoparticlesInt J Nanomedicine2012728129522287839
- ZhongZWanYHanJShiSZhangZSunXImprovement of adenoviral vector-mediated gene transfer to airway epithelia by folate-modified anionic liposomesInt J Nanomedicine201161083109321698075
- ZhongZShiSJHanJFZhongZRSunXAnionic liposomes increase the efficiency of adenovirus-mediated gene transfer to coxsackie-adenovirus receptor deficient cellsMol Pharm20107110511519968324
- ZhongZWanYShiSHanJZhangZSunXCo-delivery of adenovirus and carmustine by anionic liposomes with synergistic anti-tumor effectsPharm Res201229114515721789727
- CantleyLCNeelBGNew insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathwayProc Natl Acad Sci U S A19999684240424510200246