?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and methods
A dual (immediate/sustained-release) oral amoxicillin suspension was developed as a new dosage form to eradicate Helicobacter pylori. Carbopol®-loaded amoxi-cillin nanospheres could bind with the mucosa after delivery to the stomach and could increase the efficiency of the drug, providing both an immediate and a sustained action.
Results
The objective of this research was to develop amoxicillin nanospheres using a spray-drying technique and to investigate such features as their particle size, drug content, percentage yield, surface morphology, in vitro release, and stability. The nanospheres had a particle size range of 280–320 nm after optimizing the preparation method using a central composite design. The drug content and percentage yield was 85.3% ± 0.7% and 92.8% ± 0.9%, respectively. The in vitro release profile of the amoxicillin nanospheres was consistent with a Korsmeyer-Peppas pattern, and the release after one hour was 19%, while for the original drug, amoxicillin, under the same conditions, 90% was released in the first 30 minutes.
Conclusion
The nanospheres used in this study enabled controlled release of amoxicillin over an extended period of time for up to 12 hours and the formulation was stable for 12 months.
Introduction
Helicobacter pylori is a bacterium commonly found in the stomach.Citation1 Infection with H. pylori is universally accepted as the main threat for chronic gastritis and gastric carcino-genesis at present, and was classified as a carcinogen by the World Health Organization in 1994.Citation2 H. pylori resides primarily in the gastrointestinal mucosa or at the interface between the mucus layer and the epithelial cells of the pyloric antrum region of the stomach.Citation3
Amoxicillin, a semisynthetic penicillin, is an effective antibiotic for H. pylori infection.Citation4 It is stable in acid, inhibits synthesis of the bacterial cell wall, and has good sensitivity to H. pylori in vitro.Citation5 These triple attributes contribute to the efficacy of amoxicillin in medical applications. Nevertheless, there are some reports indicating that treatment with amoxicillin cannot completely eliminate H. pylori, suggesting that additional research is needed to improve its therapeutic effects.Citation6,Citation7
One of the reasons for only partial elimination of H. pylori using amoxicillin may be degradation of the drug in gastric acid and also a short drug residence time in the stomach, such that an effective drug concentration cannot be achieved in the gastric mucosal layer or epithelial cell surface where H. pylori resides.Citation8–Citation10
Hence, administration of large doses of amoxicillin on a daily basis is necessary for eradication of H. pylori, and poor patient compliance due to adverse effects such as diarrhea, nausea, and vomiting is not unusual. Consequently, it is expected that if local delivery of antimicrobial agents from the gastric lumen into the mucus layer can be achieved, the H. pylori eradication rate would be increased.Citation11
Several formulations have been suggested to help retain amoxicillin in the stomach, including gellan beads,Citation12 nanocapsules,Citation13 hydrogel nanoparticles,Citation14 floating microballoons,Citation15 and mucoadhesive microspheres,Citation16 which are able to remain in the gastrointestinal tract for a longer time, and therefore be more effective in H. pylori eradication.
Various methods can be used to prepare polymer nano-spheres, such as micro-emulsion, solvent evaporation, supercritical fluid technology, salting-out, dialysis, surfactant-free emulsion, mini-emulsion, and interfacial polymerization.Citation17 Spraydrying is a well recognized, rapid, one-step process frequently used in pharmaceutical engineering to produce a dry powder from a liquid phase.Citation18
In light of the above issues, there is a pressing need to develop a new formulation that delivers amoxicillin into the stomach to bind with the gastric mucosa, increase the efficiency of the drug, and provide a sustained action.
To date, no research has been published on the preparation of a suspension containing a dual-release formulation of amoxicillin. The purpose of this study was to design Carbopol®-loaded amoxicillin nanospheres as a new dual-release suspension containing pure amoxicillin as well as a granular nanosphere form of amoxicillin using a Buchi Nanosprayer B-90 (Buchi Labortechnik AG, Flawil, Switzerland) for H. pylori eradication therapy.
Materials and methods
Amoxicillin trihydrate was kindly gifted for this research by Karnataka Antibiotics and Pharmaceuticals Ltd, Bangalore, India. Carbopol-934P was purchased from Sigma-Aldrich (St Louis, MO). All other reagents and chemicals used were of analytical grade.
Formulation of nanospheres by spraydrying
Carbopol-loaded amoxicillin nanospheres were prepared using a spraydrying method.Citation19–Citation21 The experiments were designed to develop an efficient spraydrying technique. Response surface methodology (specifically, a randomized rotatable central composite design) was used because it allows clarification of the relationship between cause and effect of variables used in the procedure and output. Nanospheres were prepared by varying parameters such as the concentration of Carbopol-934P (0.5–2.0 g), inlet temperature (80°C–120°C), and flow rate (120–180 mL/hour). A solution of Carbopol-934P (Carbopol-934P soaked for one hour in 100 mL of water) containing a predetermined quantity of amoxicillin was sprayed using a spray nozzle through a 5.5 μm diameter. All samples were filtered prior to the spraydrying process to avoid blockage of the nozzle. Flow of heated air aspirated by a pump rapidly removed the solvent from the solution, leading to formation of solid nanospheres. The dried powder was removed from the particle collector using a particle scraper and stored in a desiccator at 25°C for further characterization.
Surface morphology
The surface morphology of the nanospheres was observed using scanning electron microscopy (FEI Quanta 200, Eindhoven, The Netherlands). The nanospheres were placed in graphite casing and coated with gold using an ion sputter machine (JEOL, Tokyo, Japan) and detected at 12 kV.Citation22,Citation23
Particle size
Analysis of amoxicillin nanosphere particle size was done using laser light diffraction and ultrasonic techniques. The nanospheres were diluted 10-fold with deionized water using a Malvern Zetasizer Nano Series Nano-ZS (Malvern Instruments Inc, Westborough, MA).Citation24
Drug content
A known quantity of amoxicillin nanosphere particles was dissolved in 0.1 M HCl. The amoxicillin content was assayed using spectrometry at 272 nm by constructing a calibration curve (UV-1601 spectrophotometer, Shimadzu, Tokyo, Japan). The best fitting straight line equation on the calibration curve was found to be A = 0.294x + 0.0071.Citation25,Citation26 The experiments were performed in triplicate. The drug content was calculated as the ratio between the actual and theoretical amount of amoxicillin loaded for an optimized formulation.
Product recovery
The efficiency of nanosphere recovery was determined as the percentage weight of the nanospheres obtained compared with the entire amount of drug and polymer initially used in the preparation process.Citation26
In vitro release and kinetics
Amoxicillin release studies were carried out for both the nanospheres and dry powder suspensions using the paddle method specified in United State Pharmacopeia (USP) XXVII. The in vitro release studies were carried out using the USP I basket method (50 rpm, 500 mL, and 37°C ±1°C). Amoxicillin-loaded nanosphere suspensions equivalent to 250 mg of drug were placed in a dialysis cellulose membrane bag (D9402-100FT, Sigma-Aldrich) with a molecular weight cutoff of 12,400, tangled, placed into the baskets, and transferred to 500 mL of 0.05 M phosphate buffer. Aliquots were withdrawn at prespecified time intervals and an equal volume of dissolution medium was replaced into the flask to maintain a constant volume. The aliquots drawn were analyzed at 272 nm, and the data were used to determine a cumulative drug release profile for the nanospheres. Sink conditions were retained throughout. Analysis of the suspension containing only amoxicillin was carried out in a similar manner.Citation27
Formulation of dry mixtures for reconstitution
Xanthan gum was used as the suspending agent in the suspensions. D-sorbitol was used to impart a sweet taste. Sodium citrate and citric acid were used to vary the pH of the study formulations. To produce a dry mixture for reconstitution, the powder components were shrunk to approximately the same particle size. Ingredients present in small quantities (buffer components, sodium benzoate 0.2% and sodium lauryl sulfate 0.05%) were mixed homogeneously. The same procedure was followed for xanthan gum (0.6%) and amoxicillin-loaded nanospheres (containing the equivalent to amoxicillin 250 mg). The constituents were mixed with a portion of D-sorbitol (20%) according to the geometric dilution principle. To formulate the reconstituted suspension, 10 mL of water was added to the dry suspension powder in two stages and stirred with a spoon until a uniform product was attained.Citation28
Stability
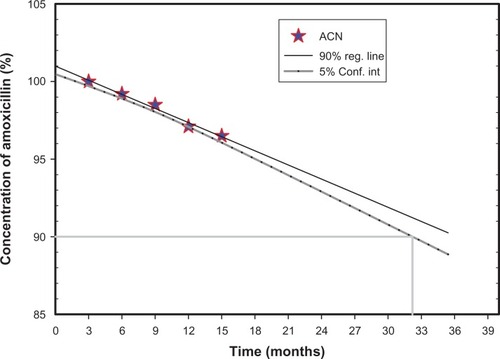
The powdered nanospheres were put into a bottle and stored for 12 months at 3°C–5°C, 15°C–25°C, and 37°C. Surface morphology and amoxicillin content were examined periodically.Citation26
Results and discussion
Appearance of nanospheres
The amoxicillin nanospheres formed under most of the experimental conditions selected were smooth, almost spherical, and free flowing (). Other investigators have reported that spraydried particles become shriveled, which may be the case if the polymer concentration is not optimal, and the shriveling may be due to rapid drying in the spraying funnel.Citation29–Citation31 A logical assumption is that when the solution is sprayed at the most favorable temperature and concentration in a spraying funnel, the smaller particles would dry out more rapidly and the critical point of crust formation would be reached soon after atomization. However, for larger particles, it takes longer to shape a shell, resulting in shriveled nanospheres, and spherical particles would release the drug in a controlled fashion at the target site.
Optimization of pharmaceutical preparations with respect to one or more features is important in formulation studies. In a typical pharmaceutical formulation experiment, the focus is on determining which process variables have the greatest effect on the results. In pharmaceutical manufacturing, there may be numerous potentially significant parameters due to the large number of ingredients typically used in a pharmaceutical formulation. If all these variables were included in the experimental design, an excessive number of experiments would need to be performed. The formulation scientist must first identify the parameters most likely to be important in the formulation process in order to limit the number of experiments needed to a reasonable extent. A delicate equilibrium is established between what is worth investigating and what is not worth investigating. The formulator’s skill, knowledge, and experience play a crucial role in selecting the appropriate variables.
Response surface methodology is the most appropriate method for modeling and analysis of trials in which the response of interest is nonlinear and influenced by numerous variables. In this experiment, a full central composite design was used to develop the particle size model. Analysis using this mathematical model were carried out with Design-expert 6.0.5© Statease Inc (Minneapolis, MN). The fit and summary experimental specifies that the quadratic central composite design model was more significant than the linear model and that the linear model had a significant lack of fit. Therefore, the quadratic model was preferred in order to develop the central composite design.
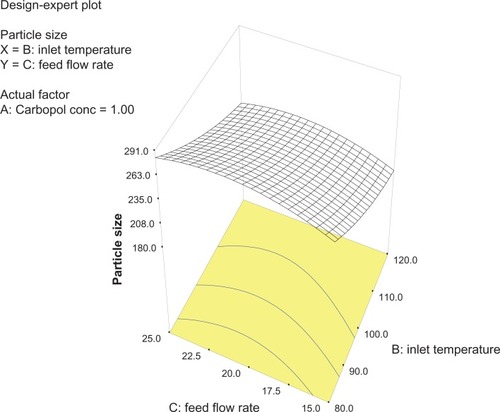
A central composite design was generated to study the key effects and interactions of three factors, ie, Carbopol (g), inlet temperature (°C), and feed flow rate (mL/hour) on particle size (). Twenty preparations of nanospheres were formulated as per to interpret the effect of these three independent variables. Experiments were conducted in accordance with our earlier studyCitation24 in random sequence in a face-centered manner in order to evaluate the interaction. The particle size data are shown in . The experimental results show that the particle size was between 200 nm and 404 nm, and was not affected by transformations, and this trailed a quadratic arrangement indicating that the response measured clenches good over a range of other factors, ie, Carbopol concentration, inlet temperature, and feed flow rate. This information would, in turn, help in setting the optimal particle size. The polynomial equation giving the final equation in terms of actual factors is:
Table 1 Design summary for DOE runs of amoxicillin microsphere construction with variable and response constraints
Table 2 Investigational design by central composite design of amoxicillin nanospheres
Process optimization
The objective of this study was to ensure a narrow particle size range of 280–320 nm, so results obtained between 200 nm and 404 nm were considered to be unacceptable. Another goal was to identify any constraints on design space and the vulnerability of the investigational model. This is important because it indicates the factors, responses, and the goal for each variable with respect to the measured response. In this study, 10 optimum solutions were created in quantitative terms (), ie, the probable behavior of the measured response in terms of analyzed factors in a random manner within the design space. The optimized methods were selected based on the numerical optimization data () for which the predicted results were closest to the observed results.
Table 3 Numerical optimization solutions for amoxicillin nanospheres
Table 4 Optimized formulae and formulation codes for amoxicillin nanospheres
From the numerical optimization solutions, three formulations (ACN-2, ACN-5, ACN-8) were randomly selected and further evaluated for particle size (), and the observed result was very similar to the response predicted by the expert design software.
As shown in , P ≤ 0.05 for any factor in analysis of variance indicates an important effect of a variable on particle size. From the F ratios given in analysis of variance, it can be concluded that inlet temperature and Carbopol concentration had a significant effect on particle size.
Table 5 Summary of analysis of variance results for quadratic model of particle size for amoxicillin nanospheres
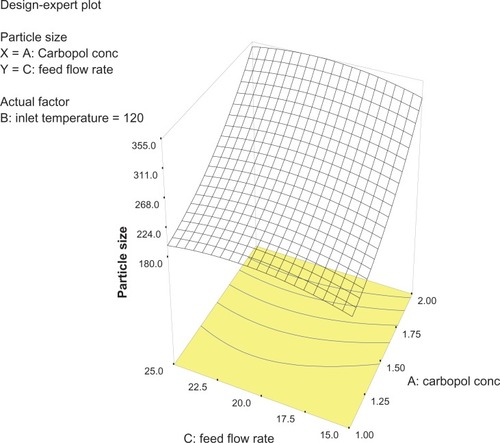
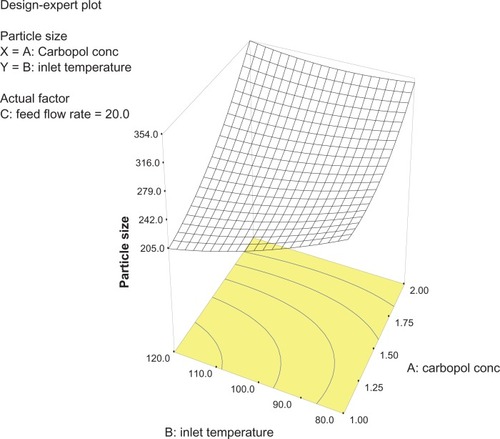
Analysis of variance for the response surface model revealed that the concentration of the polymer is the most important parameter for particle size (). In general, a higher concentration of the polymer led to larger particle size, which is in agreement with earlier reports.Citation32 The relationship between the dependent and independent variables was further elucidated using response surface plots. However, the three-dimensional plot () shows that increasing the inlet temperature led to a decreased particle size. The correlation coefficient observed in the current investigation was found to be low (15.11). From and it can be seen that lower and higher levels of feed flow rate did not vary to a noticeable extent. The drug content and percentage yield of the optimized formulations were found to be 85.3% ± 0.7% and 92.8% ± 0.9%, respectively. It seems logical that loss of product occurred mainly because of deposition of residual nanoparticles in the spraying compartment or by loss during manual removal from the collecting electrode.
Figure 2 Three-dimensional plot showing the effect of inlet temperature and Carbopol concentration, and their mutual interaction on particle size.
Size distribution of nanoparticles
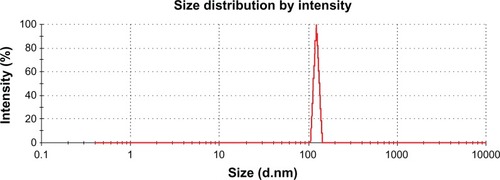
The size distribution of nanoparticles prepared by the spraydrying method showed a narrow distribution pattern with a size range of 200–404 nm (). Particle size uniformity is very important because the target area of the nanoparticle containing the drug depends on the size of the particle, and bioavailability and plasma drug levels will fluctuate if the size deviation is wide. It has been reported elsewhere that nanoparticles prepared by spraydrying have advantages over those prepared by mechanical stirring or ultrasonification procedures. Also, the particle size distribution range is broad when nanopar-ticles are prepared by methods other than spraydrying.Citation33 Spraydrying technology is very attractive due to the one-step procedure involved in preparation of drug-loaded nanoparticles and achieving a uniform particle size.Citation34,Citation35 A narrow particle size range could be useful for amoxicillin nanospheres, which would adhere when given orally to the mucosal membrane, providing a high concentration gradient of the drug towards the absorption membrane for H. pylori eradication.
In vitro drug release pattern
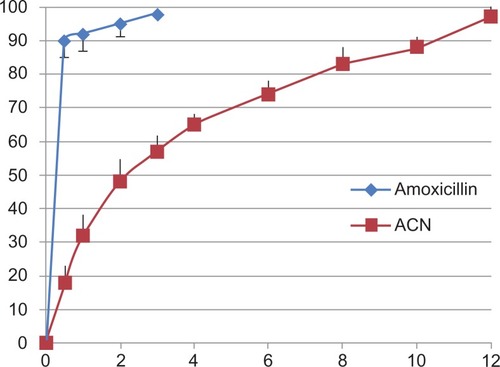
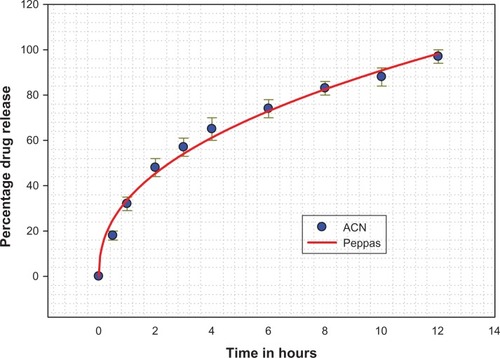
shows in vitro drug release from the amoxicillin nanospheres and from amoxicillin suspension. Approximately 18% of the total amount of amoxicillin in the nanospheres was released in the first hour, reflecting the significant amount of amoxicillin adsorbed onto or incorporated near the surface of the nanospheres. In clinical practice, this would lead to a “burst effect”, enabling the preparation to have a rapid onset of action in patients. However, complete drug release from the amoxicillin nanospheres in this study occurred in 12 hours. During this time, the amount released was 97.3%. In comparison with amoxicillin nanospheres, the amoxicillin suspension released amoxicillin very rapidly, with 90% of the amoxicillin released in 30 minutes. The results indicate that amoxicillin nanospheres had well controlled release efficacy.
The data obtained from our in vitro release studies were fitted to various kinetic equations (ie, first-order, Baker and Lonsdale, Hixson and Crowell, Korsmeyer-Peppas, and Higuchi)Citation21 to determine the mechanism of drug release and the release rate, using a macro written for SigmaPlot version 9.01 (). The correlation coefficient value, R2, was taken into account to decide upon the relevance of the model/curve fit which best described the extent of fit. According to the R2 values given by the different data fits for amoxicillin nanospheres, the Korsmeyer-Peppas model was identified as having the ideal fit (R2 = 0.9968). According to the Korsmeyer-Peppas fit, drug release is determined by diffusion of the polymeric matrix and is governed by Fick’s law. The release pattern showed classical Fickian diffusion, aided by initial swelling of the nanospheres and release of drug particles adsorbed on the surface, leading to a “burst” release.Citation25 Later on, the release pattern was more controlled and was sustained for a longer period of time due to diffusion.
Reconstitutable amoxicillin nanosphere suspension
Because of the risk of medication flooding to the suspending medium during storage, the suspension formulations were stored as dry suspensions for reconstitution before use. Xantham gum was chosen as the suspending agent and stabilizer in the dispersing medium due to its safety and unobjectionable toxicological properties for food and pharmaceutical applications. It is water-soluble and imparts high viscosity at a small concentration with thixotropic flow characteristics. Amoxicillin showed no notable changes in external morphology or drug content during storage at 3°C–5°C or at room temperature (15°C–25°C) for 12 monthsCitation26 (). However, characteristic clumping was observed at 37°C and a relative humidity of 75%.
Conclusion
The major conclusion of this work is that the dual-release suspension is a useful means of targeting drugs to the stomach and can be used for delivery of effective antibiotic therapy. Our findings can also be used to develop an effective blueprint for targeted stomach-specific drug delivery, and can be investigated further in vivo with other drugs, using a slight modification in the techniques used here for preparation of the nanospheres.
The amoxicillin nanosphere suspension developed for this study was found to have suitable physicochemical properties and to have a favorable particle size range. The nanospheres were found to release the drug to a maximum extent in vitro. This novel dual-release formulation adds to the already important domain of targeted drug delivery systems, and holds promise as an alternative to conventional drug delivery, enabling bacteria such as H. pylori which are notoriously difficult to treat to be managed effectively.
Acknowledgements
This work was supported by the Deanship of Scientific Research, King Faisal University, Saudi Arabia (research project number 130012, 2012).
Disclosure
The author reports no conflict of interest in this work.
References
- HejaziRAmijiMStomach-specific anti-H. pylori therapy. I: preparation and characterization of tetracycline-loaded chitosan microspheresInt J Pharm20022351–2879411879743
- KruegerSRoessnerAKuesterDMurine models of H. pylori-induced gastritis and gastric adenocarcinomaPathol Res Pract20112071059960722014883
- PetersonWLHelicobacter pylori and peptic ulcer diseaseN Engl J Med199132415104310482005942
- MeurerLNBowerDJManagement of Helicobacter pylori infectionAm Fam Physician20026571327133611996414
- RomanoMCuomoAEradication of Helicobacter pylori: a clinical updateMed Gen Med200417619
- LinCKHsuPILaiKHOne-week quadruple therapy is an effective salvage regimen for Helicobacter pylori infection in patients after failure of standard triple therapyJ Clin Gastroenterol200234554755111960067
- LiuZLuWQianLZhangXZengPPanJIn vitro and in vivo studies on mucoadhesive microspheres of amoxicillinJ Control Release2005102113514415653140
- AxonATThe role of acid inhibition in the treatment of Helicobacter pylori infectionScand J Gastroenterol Suppl200420116238047818
- FontanaGLicciardiMMansuetoSSchillaciDGiammonaGAmoxicillin-loaded polyethylcyanoacrylate nanoparticles: influence of PEG coating on the particle size, drug release rate and phagocytic uptakeBiomaterials200122212857286511561891
- PatelJKChavdaJRFormulation and evaluation of stomach-specific amoxicillin-loaded Carbopol-934P mucoadhesive microspheres for anti-Helicobacter pylori therapyJ Microencapsul200926436537618720199
- NaokiNYohkoAMasafumiNMayumiTMegumiKYasuyukiOMucoadhesive microspheres containing amoxicillin for clearance of Helicobacter pyloriAntimicrob Agents Chemother19984210249224949756746
- NarkarMSherPPawarAStomach-specific controlled release gellan beads of acid-soluble drug prepared by ionotropic gelation methodAAPS Pharm Sci Tech2010111267277
- JainPJainSPrasadKNJainSKVyasSPPolyelectrolyte coated multilayered liposomes (nanocapsules) for the treatment of Helicobacter pylori infectionMol Pharm20096259360319718807
- MoogooeeMRamezanzadehHJasooriSOmidiYDavaranSSynthesis and in vitro studies of cross-linked hydrogel nanoparticles containing amoxicillinJ Pharm Sci201110031057106621280053
- AwasthiRKulkarniGTPawarVKGargGOptimization studies on gastroretentive floating system using response surface methodologyAAPS Pharm Sci Tech20121318593
- SambathkumarRVenkateswaramurthyNVijayabaskaranMPerumalPFormulation of clarithromycin loaded mucoadhesive microspheres by emulsification-internal gelation technique for anti-Helicobacter pylori therapyInt J Pharm Pharm Sci20113173177
- RaoJPGeckelerKEPolymer nanoparticles: preparation techniques and size-control parametersProg Polym Sci20113670887913
- LeeSHHengDKiongWNGChanHKTanRBHNano spray drying: A novel method for preparing protein nanoparticles for protein therapyInt J Pharm20114031–219220020951781
- ShamJOHZhangYFinlayWHRoaWHLöbenbergRFormulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lungInt J Pharm2004269245746714706257
- PilcerGVanderbistFAmighiKPreparation and characterization of spray-dried tobramycin powders containing nanoparticles for pulmonary deliveryInt J Pharm20093651–216216918782609
- LiXAntonNArpagausCBelleteixFVandammeTFNanoparticles by spray drying using innovative new technology: The Buchi Nano Spray Dryer B-90J Control Release2010147230431020659510
- DhanarajuMDRajKannanRSelvarajDJayakumarRVamsadharaCBiodegradation and biocompatibility of contraceptive-steroid-loaded poly (dl-lactide-co-glycolide) injectable microspheres: in vitro and in vivo studyContraception200674214815616860053
- HarshaSShobhaROfloxacin targeting to lungs by way of microspheresInt J Pharm20093801–212713219646516
- NowacekASBalkundiSMcMillanJAnalyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophagesJ Control Release2011150220421121108978
- LuBZhangJQYangHLung-targeting microspheres of carboplatinInt J Pharm20032651–211114522113
- HarshaNSRaniSHDrug targeting to lungs by way of microspheresArch Pharm Res200629759860416903082
- SinghSKChidrawarVRUshirYVVadaliaKRShethNRSinghSPharmaceutical characterization of amoxicillin trihydrate as mucoadhesive microspheres in management of H. pyloriPharm Tech201062348358
- Gomez-EstacaJBalaguerMPGavaraRHernandez-MunozPFormation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcuminFood Hydrocolloids2011288291
- DevrimBBozkirACanefeKFormulation and evaluation of reconstitutable suspensions containing ibuprofen-loaded Eudragit microspheresActa Pol Pharm201184459359921796942
- RaulaJEerikinenHKauppinenEIInfluence of the solvent composition on the aerosol synthesis of pharmaceutical polymer nanoparticlesInt J Pharm20042841–2132115454292
- TononRVGrossoCRFHubingerMDInfluence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray dryingFood Res Int2011441282289
- BurkiKJeonIArpagausCBetzGNew insights into respirable protein powder preparation using a nano spray dryerInt J Pharm20114081–224825621335078
- WangLYGuYHSuZGMaGHPreparation and improvement of release behavior of chitosan microspheres containing insulinInt J Pharm20063111–218719516436319
- JoscelyneSMTrägårdhGMembrane emulsification a literature reviewJ Memb Sci20001691107117
- MizoeTBeppuSOzekiTOkadaHOne-step preparation of drug-containing microparticles to enhance the dissolution and absorption of poorly water-soluble drugs using a 4-fluid nozzle spray drierJ Control Release2007120320521017582644