 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cisplatin (CDDP) is one of the most effective and potent anticancer drugs used as first-line chemotherapy against several solid tumors. However, the severe side effects and its tendency to provoke chemoresistance often limit CDDP therapy. To avoid these inconveniences, the present study’s research group developed long-circulating and pH-sensitive liposomes containing CDDP (SpHL-CDDP). The present study aimed to evaluate the antitumor effect and toxicity of SpHL-CDDP, as compared with that of free CDDP, and long-circulating and non- pH-sensitive liposomes containing CDDP (NSpHL-CDDP), after their intravenous administration in solid Ehrlich tumor-bearing mice. Antitumor activity was evaluated by analysis of tumor volume and growth inhibition ratio, serum vascular endothelial growth factor (VEGF) levels, and histomorphometric and immunohistochemical studies. Body weight variation and the histological examination of bone marrow and kidneys were used as toxicity indicators. A significant reduction in the tumor volume and a higher tumor growth inhibition ratio was observed after SpHL-CDDP treatment, compared with free CDDP and NSpHL-CDDP treatments. In addition, complete remission of the tumor was detected in 18.2% of the mice treated with SpHL- CDDP (16 mg/kg). As such, the administration of SpHL-CDDP, as compared with free CDDP and NSpHL-CDDP, led to a decrease in the area of necrosis and in the percentage of positive CDC 47 tumor cells. A significant reduction in the VEGF serum level was also observed after SpHL-CDDP treatment, as compared with free-CDDP treatment. SpHL-CDDP administered in a two-fold higher dose than that of free CDDP presented a loss in body weight and changes in the hematopoietic tissue morphology, which proved to be similar to that of free CDDP. No changes could be verified in the renal tissue after any formulations containing CDDP had been administered. These findings showed that SpHL-CDDP allowed for the administration of higher doses of CDDP, significantly improving its antitumor effect.
Introduction
Cis-diamminedichloroplatinum (II) or cisplatin (CDDP) is one of the most widely used chemotherapeutic agents in the treatment of solid tumors. CDDP covers a broad spectrum and has been used to treat breast, lung, neck, ovary, and testicular cancers. However, broader therapeutic applications of CDDP are limited by drawbacks, such as the presence of intrinsic or acquired resistance, rapid inactivation, and severe toxic side effects (myelotoxicity, neurotoxicity, and particularly nephrotoxicity).Citation1,Citation2 Among the various resistance mechanisms involved, the decreased cellular accumulation of CDDP has been demonstrated in most cases.Citation3 Therefore, to overcome these CDDP-related disadvantages, efforts have been made to develop CDDP delivery systems, including the use of liposomes.Citation4 Liposomes are able to reduce unwanted drug actions that can cause toxicity, improve drug bioavailability, and provide targeted drug delivery.Citation5 In the case of cancer treatments, passive targeting can be accomplished by using liposomes as carriers of anticancer agents. Solid tumors present peculiar pathophysiological characteristics, such as hyperplasia and hyperpermeability of the tumor vasculature, which facilitates the extravasation of colloidal systems.Citation6
The present study’s research group has developed long-circulating and pH-sensitive liposomes containing CDDP (SpHL-CDDP) which previously showed both two- and three-fold higher values of median lethal doses than those obtained for free CDDP after their intravenous (IV) and intraperitoneal administration in healthy mice, respectively.Citation7,Citation8 At the same time, hematological investigations revealed no alteration in red and white blood cell counts upon SpHL-CDDP administration in mice. In addition, SpHL-CDDP treatment caused no pronounced alterations in the blood urea and creatinine levels, nor did it induce morphological alterations in the kidneys of the mice. These liposomes are made up of dioleoylphosphatidilethanolamine (DOPE), cholesteryl hemisuccinate (CHEMS), and distearoylphosphatidylethanolamine-polyethyleneglycol 2000 (DSPE-PEG2000). In an acidic medium, such as that found in tumor sites, CHEMS undergoes protonation, followed by the destabilization of liposomes, and the release of CDDP.Citation9 Thus, it is expected that the released CDDP in tumor sites can improve the antitumor effect and reduce, or even eliminate, the side effects. It is well known that the antitumor efficacy of the liposomes depends on their ability to release the drug into the tumor’s extracellular fluid.Citation10 Previous study of tissue distribution showed a higher concentration and affinity of CDDP toward tumor tissues resulting from the administration of SpHL-CDDP, as compared to the injection of free CDDP.Citation4 Therefore, the aim of the present study was to investigate whether these findings can in fact contribute SpHL-CDDP’s enhanced antitumor efficiency against Ehrlich solid tumors in Swiss mice, after its administration by IV route.
Materials and methods
Materials
CDDP was purchased from Quiral Quimica do Brasil S.A (Juiz de Fora, Brazil). Dioleoylphosphatidylethanolamine (DOPE), dioleoylphosphatidylcholine, and distearoylphosphatidylethanolamine- polyethyleneglycol2000 (DSPE-PEG2000) were purchased from Lipoid GmbH (Ludwigshafen, Germany). Cholesteryl hemisuccinate (CHEMS) was supplied by Sigma–Aldrich (St Louis, MO). Sodium chloride was obtained from Merck (Merck and Co, Inc, Whitehouse Station, NJ). All other chemicals and reagents used were of analytical grade. The reagents used in the immunohistochemical analysis were purchased from NeoMarkers Inc (CDC47 [clone 47DC141]; Fremont, CA) and Dako North America Inc (K0690-HRP; Carpinteria, CA).
Liposome preparation
The SpHL-CDDP were prepared by reverse-phase evaporation, as described by Junior et al.Citation4 DOPE, CHEMS, and DSPE-PEG2000 (in a 5.7:3.8:0.5 molar ratio, respectively) were dissolved in chloroform at a 40 mmol L−1 total lipid concentration and submitted to evaporation under reduced pressure until a thin lipid film had been obtained. The resulting film was dissolved in ethyl ether, and a CDDP solution (2 mg/mL), prepared in a 0.9% (weight/volume) NaCl solution, was added. The mixture was stirred with a vortex, and the ethyl ether was completely removed under reduced pressure. The liposomes were then sequentially extruded (Lipex™ Biomembranes Extruder; Northern Lipids Inc, Burnaby, Canada) through a series of polycarbonate membranes with pore sizes of 0.4, 0.2, and 0.1 μm (5 cycles for each). Nonentrapped CDDP was eliminated by ultracentrifugation (Optima® L-80XP; Beckman Coulter, Indianapolis, IN) at 150,000 × g at 10°C for 90 minutes. Long-circulating and non-pH-sensitive liposomes containing CDDP (NSpHL-CDDP) consisting of DOPC, CHEMS, DSPE-PEG2000 (in a 5.7:3.8:0.5 molar ratio, respectively), as well as empty (without drug) liposomes, SpHL, and NSpHL, were also prepared as described above.
Liposome characterization
The liposomes were characterized by their encapsulation percentage, size, zeta potential and polydispersity index. The encapsulation percentage of CDDP in liposomes was determined by high-performance liquid chromatography. The chromatographic apparatus consisted of a pump (model 515; Waters Instruments, Inc, Rochester, MN), an autoinjector (model 717 Plus; Waters Instruments, Inc), and a variable wavelength UV detector (model 2487; Waters Instruments, Inc) connected to the Empower2 software (Waters Instruments, Inc). Separations were performed using a, 4 × 4 mm, 5 μm guard column (Lichrospher® 100 NH2; Merck Millipore, Darmstadt, Germany) connected to a 25 cm × 4 mm, 10 μm column (LiChroCART® 25-4 LiChrospher® 100 NH2; Merck Millipore). The eluent system consisted of methanol/ ethylacetate/N,N-dimethylformamide/water (in a ratio of 4:4:1:1, respectively), and the flow rate was 1.0 mL/min. Samples (20 μL) were injected, and the eluate absorbance was monitored at 310 nm. The amount of CDDP was determined in the liposome before ultracentrifugation (non-purified liposomes) and after ultracentrifugation (purified liposomes). The liposomes were disrupted using isopropanol in a volume ratio of 1:3. The encapsulation percentage was calculated using the following equation:
The average diameter of the vesicles was determined by quasielastic light scattering at 25°C and at a 90° angle, using the unimodal analysis. The zeta potential was evaluated by determining the electrophoretic mobility at an angle of 90°. All samples were diluted in a 0.9% (weight/volume) NaCl solution (1000 fold), and the measurements were performed in triplicate using a Zetasizer analyzer (Zetasizer 3000HS; Malvern Instruments Ltd, Worcestershire, UK). Data related to the SpHL-CDDP physico-chemical characterization were expressed as mean ± standard deviation (SD).
Animals
Female Swiss mice, 4–6 weeks old, weighing 22.5 ± 2.5 g, were obtained from the Federal University of Minas Gerais School of Pharmacy, Belo Horizonte, Brazil. The animals were kept in plastic cages with free access to food and water and were maintained in an area with a standardized light/ dark cycle. All protocols were approved by the Ethics Committee for Animal Experiments at the Federal University of Minas Gerais, and are in compliance with the guidelines for the care and use of laboratory animals recommended by the Institute of Laboratory Animal Resources (Protocol number 165/2007).
Ehrlich solid tumor model
Ehrlich ascites tumor cells, derived from a spontaneous murine mammary adenocarcinoma, were inoculated by intraperitoneal route in female mice, and the tumors were collected after 8 days. Ascitic tumor cell counts were performed in a Neubauer hemocitometer (Bright-line −0.025 mm2; Normax, Marinha Grande, Portugal) using a Trypan blue dye exclusion method. A viable tumor cell suspension was then prepared at a density of 1 × 107 cells/mL. Tumors cells were transplanted subcutaneously into the right flank of the female Swiss mice (0.2 mL/mouse). Tumors were allowed to grow for 15 days until all inoculated mice had palpable tumors measuring approximately 9–10 mm.
Antitumor activity
When the tumor volume reached approximately 9 to 10 mm, the animals were randomly divided into eight experimental groups (15 animals per group). Different groups of Ehrlich solid tumor-bearing Swiss mice received, by IV route, free CDDP at a dose of 8 mg/kg, and SpHL-CDDP and NSpHL-CDDP at doses of 8 and 16 mg/kg, whereas the control groups were treated with a saline solution of SpHL, or NSpHL (the lipid dose administered was equal to that for SpHL-CDDP or NSpHL-CDDP treatment at doses of 16 mg/kg). The first day in which the formulations were administered was considered day 0 (D0) of the study. As such, the formulations were administered on D0, D7, and D14. Antitumor activity was evaluated over a 23-day period by determining the tumor volume and calculating the tumor growth inhibition ratio. The solid tumor volume (V) was measured by three orthogonal diameters with a slide caliper, and calculated according to the formula:
where d1, d2, and d3 represent the length, width, and height, respectively.Citation11 The relative tumor volume (RTV), and inhibition ratio (IR) were calculated on D23, as follows:
and
In addition, the tumor response was classified as follows: progressive disease occurred where there was an increase in tumor volume (>25%) over a 23-day period; no change occurred where the tumor volume was equal to the volume at the beginning of treatment (within the range of −25% to +25%); partial remission occurred where there was a decrease in tumor volume (from −25% to −90%); and complete remission occurred where the tumor volume was less than 10% of the initial volume, as previously described.Citation12
Histomorphometric and immunohistochemical analyses of the tumor
Tumor-bearing mice were anesthetized with a mixture of xylazine (7.5 mg/kg) and ketamine (60 mg/kg) on D23, at which time the tumor was removed for histopathological evaluation. The tumor tissue was set in 10% (volume/volume) buffered formalin, embedded in paraffin blocks, sectioned into a 4 μm thickness, placed onto glass slides, and hematoxylin-eosin stained. The images of cross-sections were obtained for morphometric evaluation, and 15 fields per slide were examined using a 40× magnification in an optical microscope (final magnification = × 1000). The images were captured with a microcamera (Spot Insight Color; SPOT Imaging Solutions, Sterling Heights, MI) attached to a microscope (Olympus BX-40; Olympus, Tokyo, Japan) and Spot software, version 3.4.5. Image analysis was performed using Corel DRAW® (version 7.468; Microsoft Corporation, Redmond, WA). The percentage of the necrotic area, viable neoplastic tissue, and inflammation was determined using a 25-point graticule. For the immunohistochemical study, tumor cell proliferative activity was evaluated using the cell proliferation marker CDC47 – clone 47DC141 (by Neomarkers Inc, Fremont, CA). Histological sections (4 μm) were stained by applying an avidin-biotinylated peroxidase complex (Dako K0690; K0690-HRP). CDC 47 diluted 1:300, was used as the primary antibody, and the incubation period was 60 minutes. Next, peroxidase activity was revealed using diaminobenzidine (DAB-Dako). For negative control, either nonimmune mouse or rabbit serum was used in place of the primary antibody. The images were also taken with the Spot Insight Color microcamera attached to the Olympus BX-40 Microscope. The proliferative index was obtained by calculating the percentage of positive cells in 500 tumor cells.
Determination of the effect of formulations containing CDDP on serum vascular endothelial growth factor
Blood was collected from the Ehrlich solid tumor-bearing Swiss mice on D23 after the first administration of free CDDP (8 mg/kg), SpHL-CDDP (16 mg/kg), NSpHL-CDDP (16 mg/kg), and SpHL and NSpHL controls. The serum was collected by centrifuging blood at 2500 × g for 10 minutes, and the serum vascular endothelial growth factor (VEGF) levels were measured using a Quantikine VEGF ELISA kit (mouse VEGF; R&D Systems, Minneapolis, MN), according to manufacturer’s protocols.
Toxicity evaluation
The body weight of the mice was monitored simultaneously as an indicator of systemic toxicity at D0, D7, D14, and D23 after application, and their weights were expressed as the percentage of the initial body weight. In addition, the kidneys and bone marrow were also removed. These organs were washed with 0.9% (weight/volume) NaCl solution and set in 10% (volume/volume) buffered formalin. All tissues were embedded in paraffin blocks, sectioned in 5 μm thickness, using a microtome (Leica RM2245; Leica Biosystems, Wetzlar, Germany), and placed onto glass slides. After hematoxylin-eosin staining, the slides were observed, and the photos were taken using an optical microscope. The pathologist was blinded to the treatment type during the analysis of the pathology slides.
Statistical analysis
The normality and homogeneity of the variance analysis were performed using the Lilliefors’ and Bartlett’s tests, respectively. The difference among experimental groups was tested using the one-way analysis of variance (ANOVA), followed by the Tukey’s test. To analyze the body weight variation parameter, the value before drug treatment was used as the covariate. Data relative to the tumor volume were transformed by using the following equation:
The regression model estimates were used at time intervals for tumor growth investigations. Differences were considered statistically significant when P values were <0.05.
Results
Physicochemical characterization of SpHL-CDDP
shows the physicochemical characteristics of different liposomal formulations containing CDDP. Size measurements demonstrated monodisperse populations (polydispersity index < 0.3) of liposomes with mean diameters varying between 120 and 135 nm. All formulations exhibited a zeta potential value near neutrality, varying between −1.6 and −2.8 mV. The encapsulation of CDDP in liposomes did not affect the size or the zeta potential of the liposomes, as we previously reported.Citation7,Citation8 The encapsulation percentage of CDDP into liposomes proved to be similar in both SpHL-CDDP and NSpHL-CDDP. All formulations offered an appropriate size and homogeneity for IV administration.
Table 1 Physicochemical characteristics of CDDP-liposomal formulations
Antitumor activity
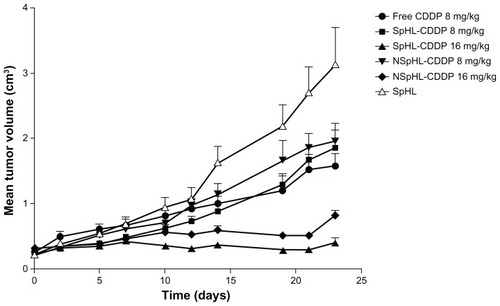
The antitumor activity of SpHL-CDDP, NSpHL-CDDP, and free-CDDP treatments was evaluated at doses of 8 mg/kg and 16 mg/kg, administered once weekly over a 3-week period in Ehrlich solid tumor-bearing Swiss mice, by assessing the tumor volume variation over time (). Regression analysis was performed to detect the changes in the growth delay after treatment with different doses of liposomal CDDP and free CDDP. The models that best fit, together with their respective determination coefficients, are shown in . As shown in , the tumor volume in the SpHL control group increased rapidly over time. The same profile of tumor growth was also observed for NSpHL and saline treatment groups (data not shown). Since no significant difference among these control groups could be observed, only the data from SpHL treatments in the control groups were shown in . By contrast, the tumor volume was significantly lower in mice treated with SpHL-CDDP, NSpHL-CDDP, and free CDDP at doses of 8 mg/kg, than in mice from the control group. No significant difference (P > 0.05) was observed in the mean tumor volume of mice treated with SpHL-CDDP (8 mg/kg) and free CDDP (8 mg/kg) for up to 23 days after the first injection of each treatment (). However, the regression analysis did show significant differences in the growth rate of the Ehrlich solid tumor after SpHL-CDDP (quadratic regression model) and free-CDDP (linear regression model) treatments. The tumor growth in mice treated with SpHL- CDDP proved to be slower during the period up to 14 days after the first administration than in mice that had received free CDDP. On the other hand, the tumor volume was significantly suppressed by the SpHL-CDDP treatment at doses of 16 mg/kg, as compared with free CDDP at doses of 8 mg/kg. Practically no change could be observed in the tumor volume for up to 23 days after the first injection of SpHL-CDDP at doses of 16 mg/kg. This finding justifies the absence of the equation from the regression analysis for the SpHL-CDDP treatment at doses of 16 mg/kg (). NSpHL-CDDP administered at doses of 16 mg/kg showed the same tumor growth inhibition profile as observed for SpHL-CDDP when administered at the same dose (). However, it is worth noting that the tumor growth inhibition ratio proved to be higher in the mice treated with SpHL-CDDP, as compared with those treated with NSpHL-CDDP, at the same dose (). This finding suggests that the lipid composition of the liposomal formulation that contains CDDP plays a role in the antitumor efficacy. No significant difference was observed in the relative tumor volume in mice treated with SpHL-CDDP or NSpHL-CDDP at doses of 8 mg/kg, as compared with free-CDDP treatments at doses of 8 mg/kg. The increase in the administered dose of SpHL-CDDP or NSpHL-CDDP to 16 mg/kg led to a significantly lower relative tumor volume and tumor growth inhibition ratio than was observed after free-CDDP treatments at doses of 8 mg/kg ( and ). In addition, after 23 days of treatment with free CDDP (8 mg/kg), SpHL-CDDP (8 mg/kg), NSpHL-CDDP (8 or 16 mg/kg), and SpHL, all surviving mice presented a progressive disease with an increase in tumor volume of >25%. On the other hand, in surviving mice that received SpHL-CDDP at doses of 16 mg/kg, the tumor response was classified as progressive disease (36.3%), no change (9.2%), partial remission (36.3%), and complete remission (18.2%).
Table 2 Regression analysis of the data of antitumor activity
Table 3 Relative tumor volume and tumor growth inhibition ratio after administration of free CDDP, SpHL-CDDP and NSpHL-CDDP by IV route
Figure 1 Antitumor effect of free CDDP, SpHL-CDDP, NSpHL-CDDP, and SpHL control group administered by IV route, in Ehrlich solid tumor-bearing Swiss mice.
Note: The values represent the mean ± SD.
Abbreviations: CDDP, cisplatin; SpHL-CDDP, long-circulating and pH-sensitive liposomes containing CDDP; NSpHL-CDDP, long-circulating and non-pH-sensitive liposomes containing CDDP; SpHL, long-circulating and pH-sensitive liposomes; IV, intravenous.
Histomorphometric and immunohistochemical analyses of the tumor
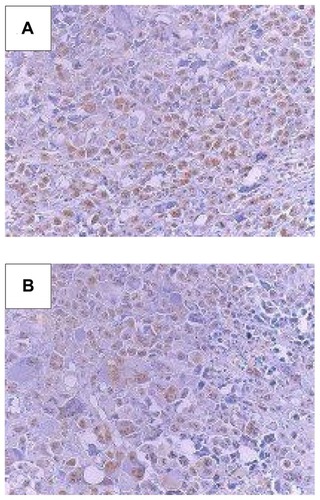
Histopathological and morphometric analyses of the Ehrlich tumor were carried out in the mice treated with free CDDP (8 mg/kg), SpHL-CDDP (16 mg/kg), NSpHL-CDDP (16 mg/kg), and their respective control groups (saline, SpHL, and NSpHL). In all evaluated tumors, neoplastia, necrosis, and inflammation could be observed. The percentage variation of these parameters among the different treatment groups is shown in . All parameters evaluated after treatment with free CDDP (8 mg/kg) showed values similar to those of the control groups. The administration of SpHL-CDDP (16 mg/kg) led to a significant decrease in necrosis in the Ehrlich tumor, as compared with the free CDDP and the SpHL control groups. No significant difference in the percentage of the inflammation area was detected. After NSpHL-CDDP treatment (16 mg/kg), no changes in any of the parameters could be observed, especially when compared with the free CDDP and the control treatment groups (). Immunohistochemical studies found no significant difference in the percentage of CDC 47 tumor cells after SpHL-CDDP and NSpHL-CDDP treatments, as compared with the control treatment groups. However, a significant decrease was observed in the percentage of positive CDC 47 tumor cells in the mice treated with SpHL-CDDP and NSpHL-CDDP, as compared with those treated with free CDDP, could be observed (). Representative immunohistochemistry sections of CDC 47 are illustrated in .
Table 4 Morphometric variables of the Ehrlich tumor and percentage of positive CDC 47 tumor cells in 500 tumor cells evaluated 23 days after the administration of free CDDP, SpHL-CDDP, NSpHL-CDDP and control groups
Figure 2 Immunohistochemistry sections of CDC 47 tumor cells in Ehrlich solid tumor-bearing Swiss mice treated by IV route at doses of 16 mg/kg with (A) SpHL, and (B) SpHL-CDDP.
Abbreviations: IV, intravenous; SpHL, long-circulating and pH-sensitive liposomes; SpHL-CDDP, long-circulating and pH-sensitive liposomes containing CDDP (cisplatin).
Effect of formulations containing CDDP on serum VEGF levels
A significant reduction was observed in the serum VEGF levels after SpHL-CDDP treatment, as compared with free- CDDP treatment. Mean serum VEGF levels in the free-CDDP treatment group was 92 ± 9 pg/mL, while the value obtained in the SpHL-CDDP-treated group was 40 ± 6 pg/mL. No significant difference in serum VEGF levels was observed between the SpHL-CDDP and NSpHL-CDDP-treated groups (48 ± 4 pg/mL). In the control groups (SpHL and NSpHL), the mean VEGF values were 46 ± 5 pg/mL and 34 ± 3 pg/mL, respectively.
Toxicity evaluation
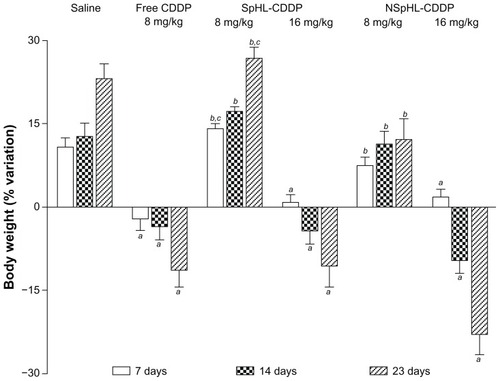
The toxicity of each treatment regimen was monitored using the loss of animal body weight, local skin reactions, and the histological examination of the bone marrow and kidneys as indicators. The body weight changes in the mice after free CDDP (8 mg/kg), SpHL-CDDP (8 and 16 mg/kg), NSpHL-CDDP (8 and 16 mg/kg), and saline treatments are illustrated in . Only the saline control group is shown, given that SpHL and NSpHL treatments presented the same weight variation profile. In the group of mice that received an IV injection of free CDDP (8 mg/kg), body weight decreased gradually, reaching a maximum loss on D23 of 11.3%. By contrast, neither the SpHL-CDDP (8 mg/kg) nor the NSpHL-CDDP (8 mg/kg) treatment groups presented any body weight loss at the investigated time intervals, and no significant difference, when compared to the saline control group, could be observed. At a dose of 16 mg/kg for SpHL-CDDP and NSpHL-CDDP treatments, a loss of body weight was observed at D14 and D23 after the administration of both formulations. However, no significant difference in body weight could be observed after the administration of these treatments when compared with the free-CDDP treatment. It is important to note that the dose of SpHL-CDDP and NSpHL-CDDP administered was two-fold higher than that of free CDDP. The only significant difference between the SpHL-CDDP and NSpHL-CDDP treatments was observed at doses of 8 mg/kg (D7 and D23), where a high decrease in body weight was found after NSpHL-CDDP administration. A severe skin reaction, followed by necrosis at the injection site, was only observed in the mice that received the free-CDDP treatment.
Figure 3 Changes in body weight in Ehrlich solid tumor-bearing Swiss mice treated with free CDDP, SpHL-CDDP, NSpHL-CDDP, and saline solution by IV route.
Notes: aRepresents a significant difference between free CDDP, SpHL-CDDP, or NSpHL-CDDP treatments and the saline solution; brepresents a significant difference between SpHL-CDDP and NSpHL-CDDP treatments, and the free CDDP-treatment; crepresents a significant difference between SpHL-CDDP and NSpHL-CDDP when administered at the same dose. P-values of less than 0.05 were set as the significance level. Data were expressed as mean ± SD.
Abbreviations: CDDP, cisplatin; SpHL-CDDP, long-circulating and pH-sensitive liposomes containing CDDP; NSpHL-CDDP, long-circulating and non-pH-sensitive liposomes containing CDDP; IV, intravenous.
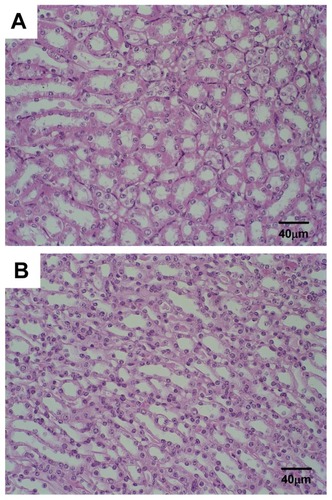
In the present study, only histological photomicrographs of the mice treated with SpHL-CDDP (16 mg/kg) are illustrated, given that all groups treated with the formulation containing CDDP presented the same histological profile. No morphological alteration in the bone marrow was observed for Ehrlich solid tumor-bearing Swiss mice treated with the SpHL control (). On the other hand, the microscopic examination of the bone marrow of female mice treated with free CDDP (8 mg/kg), SpHL-CDDP (16 mg/kg), or NSpHL-CDDP (16 mg/kg), as compared with the control groups, revealed a slight hypocellularity characterized by increased amounts of intercellular material and decreased blood cell precursors (). No histological alteration was observed in the renal tissue of the mice treated with different formulations containing CDDP ().
Figure 4 Photomicrographs of hematopoietic tissue from Ehrlich solid tumorbearing Swiss mice treated by IV route with (A) SpHL and (B) SpHL-CDDP, at doses of 16 mg/kg, evaluated 23 days after treatment.
Note: Hematoxylin and eosin staining.
Abbreviations: IV, intravenous; SpHL, long-circulating and pH-sensitive liposomes; SpHL-CDDP, long-circulating and pH-sensitive liposomes containing CDDP (cisplatin).
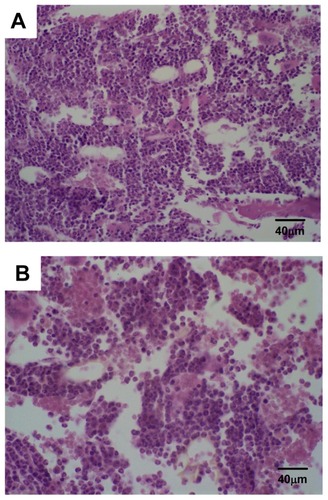
Figure 5 Photomicrographs of renal tissue from Ehrlich solid tumor-bearing Swiss mice treated by IV route with (A) SpHL and (B) SpHL-CDDP, at doses of 16 mg/kg, evaluated 23 days after treatment.
Note: Hematoxylin and eosin staining.
Abbreviations: IV, intravenous; SpHL, long-circulating and pH-sensitive liposomes; SpHL-CDDP, long-circulating and pH-sensitive liposomes containing CDDP (cisplatin).
Discussion
Prior research carried out by the present study’s research group demonstrated the ability of SpHL-CDDP to promote a high concentration of CDDP in Ehrlich solid tumors, as well as a lower renal perfusion of the anticancer agent, after IV administration.Citation4 In addition, SpHL-CDDP administered through the IV route, as compared with the free CDDP-treatment, significantly reduced nephrotoxicity.Citation8 These findings indicate that the use of SpHL-CDDP as a drug delivery system may well improve the therapeutic efficacy of the CDDP-based treatment. SpHL-CDDP administered by the intraperitoneal route in Ehrlich ascitic tumor-bearing female Swiss mice provided a satisfactory approach to improving the bioavailability and antitumor efficacy of CDDP in tumors located in the abdominal cavity.Citation13 The present study carried out investigations related to the antitumor activity as well as to the toxicity of SpHL-CDDP in the Ehrlich solid tumor animal model after IV administration. It is well known that long-circulating carriers, such as SpHL-CDDP, are able to increase the drug accumulation in tumors due to the enhanced permeability and retention effect (a consequence of the formation of leaky capillaries around the tumor, coupled with a lack of a lymphatic system for the transport of drugs to the bloodstream).Citation14 However, the therapeutic efficacy of a drug delivery system also involves the release of anticancer agents in the tumor site. SpHL-CDDP consists of DOPE, CHEMS, and DSPE-PEG2000, and in an acidic medium, such as found in tumor sites, the CHEMS molecules undergo protonation, followed by the destabilization of liposomes and the release of CDDP. Therefore, it can be suggested that the release of CDDP in this specific site may well contribute to the improvement of the antitumor effect.
According to this study’s results (), the encapsulation of CDDP in SpHL did not alter the antitumor activity of the drug. A significant difference in the solid tumor growth profile of the mice that received SpHL-CDDP at doses of 8 mg/kg, as compared with those that received free CDDP at doses of 8 mg/kg, could be verified. However, 23 days after the first administration of either SpHL-CDDP or free CDDP, the relative tumor volume and tumor growth inhibition ratio presented similar values. Considering that the main limitation of increasing doses of free CDDP is related to its toxicity, improvements in cancer therapy through the use of SpHL-CDDP will almost certainly be observed at high doses of this drug delivery system. In previous studies, the maximum tolerated dose obtained after the administration of SpHL-CDDP (20 mg/kg) was approximately three times higher than that obtained using free CDDP (7.5 mg/kg).Citation8 This reduction in toxicity may well allow for a two-fold increase in the administered dose with no adverse side effects. A significant increase in antitumor efficacy was observed after the administration of a high dose of SpHL-CDDP (16 mg/kg/week, for 3 weeks). The inhibition of tumor growth reached approximately three- and five-fold higher levels after SpHL-CDDP (16 mg/kg) treatment than after SpHL-CDDP (8 mg/kg) and free-CDDP (8 mg/kg) treatments, respectively ( and ). According to Kurihara et al,Citation15 the antitumor activity of CDDP is dependent on the total administered dose as well as on the total exposure of free and total platinum in the plasma over the time period. Prior studies from the present study’s research group demonstrated an increase of 2.6-fold in plasma drug exposure after SpHL-CDDP administration in mice, as compared with free-CDDP injections.Citation4 This finding may explain the great antitumor effect obtained with SpHL-CDDP treatment. It is worth noting that 18.2% of the mice treated with SpHL-CDDP at doses of 16 mg/kg presented complete remission of the tumor. These results demonstrated that the encapsulation of CDDP in SpHL allowed for the administered dose to be increased, significantly improving the therapeutic efficacy of the drug.
As tumors are heterogeneous in nature and consist of areas of edema and necrosis, measurements of tumor volume alone are not always the most accurate markers of efficacy, and consequently, the microscopic evaluation of tumors is additionally required to improve the assessment of therapeutic efficacy.Citation16 In this context, the histomorphometric and immunohistochemical analyses of the tumor were performed on the groups of mice that received free CDDP at doses of 8 mg/kg (corresponding to the maximum tolerated dose), administered once a week for three weeks, as well as on those that received SpHL-CDDP at doses of 16 mg/kg once a week for three weeks. The administration of free CDDP, as compared with the control group, caused no changes in the percentage of neoplastia, necrosis, and inflammation observed. By contrast, SpHL-CDDP treatment, as compared with free CDDP and SpHL treatments, induced a significant increase in areas of neoplasia and a reduction in areas of necrosis. Furthermore, the immunohistochemical evaluation demonstrated a reduction in the count of CDC 47 positive cells (a cellular proliferation marker) after SpHL-CDDP treatment, as compared with free-CDDP treatment. These findings, associated with a reduced tumor volume, suggest an impairment of cell proliferation induced by CDDP. The lower proliferative rate may well be due to an increased CDDP concentration in the tumor region when administered as SpHL-CDDP, as compared with the free-CDDP administration.
As regards serum VEGF levels, the obtained values proved to be consistent with those obtained for tumor volume growth curves, showing the antiangiogenic activity of the SpHL-CDDP treatment. VEGF is a potent and specific mitogen for endothelial cells, which activates the angiogenic switch in vivo, and enhances vascular permeability in tumor areas.Citation17,Citation18 Angiogenesis is critical for tumor growth and is a prerequisite for the development of metastases.Citation19 Therefore, the higher serum VEGF levels obtained in the free CDDP-treated group compared with those obtained after liposomal CDDP (16 mg/kg) treatments, may be explained by the continued tumor volume growth. On one hand, the stimulation of angiogenesis is required for tumor growth. On the other hand, the low serum VEGF values obtained after SpHL-CDDP treatment demonstrated the antiangiogenic activity of this formulation, which certainly contributed to the great antitumor response obtained in the SpHL-CDDP treated group. In the control group, the tumor volume was too large and presented a large area of necrosis. These findings indicate a reduced vascularization of the tumor region, which may well explain the low values of VEGF found for both SpHL and NSpHL treatment groups. In addition, it is well known that VEGF is essential for initial, but not for continued, in vivo growth of Ehrlich tumor cells.Citation19
From the present study’s findings, it is possible to suggest that the lipidic composition of SpHL-CDDP appears to play a role in the antitumor effect. The higher antitumor efficacy of the SpHL-CDDP treatment, as compared with the NSpHL-CDDP treatment, was demonstrated by its ability to promote a greater tumor growth inhibition rate, in some cases leading to the complete remission of the tumor. The construction of pH-sensitive liposomes takes advantage of the polymorphic phase behavior of DOPE, which forms inverted hexagonal phases rather than bilayers. Liposome stabilization within bilayers is achieved by the presence of CHEMS molecules, which are negatively charged at neutral pH. This lipid, homogenously distributed among DOPE molecules, provides electrostatic repulsions which decrease DOPE intermolecular interactions, thus preventing hexagonal phase formation under physiological conditions. The protonation of CHEMS molecules in acidic medium, such as that found in the tumor site, neutralizes their negative charges. As such, the liposomes undergo destabilization and release the CDDP.Citation9
Concerning toxicity, the analysis of changes in body weight is defined as an adverse effect of a therapeutic regimen. In this study, the body weight decreased gradually after the injection of free CDDP (8 mg/kg) but no loss in body weight was observed after SpHL-CDDP (8 mg/kg) treatment. On the other hand, the increase in the administered dose of SpHL-CDDP to 16 mg/kg provoked a significant reduction in body weight. However, SpHL-CDDP administered at a two-fold higher dose than that of free CDDP induced a similar weight loss in mice, showing that SpHL-CDDP treatment induced lower systemic toxicity than did the free-CDDP treatment. The decrease in the body weight of the animals treated with free CDDP can be attributed to the CDDP toxicity, whereas the increase in body weight of the mice from the control groups resulted from the remarkable increase in tumor size. It is well known that the CDDP treatment induces a myelotoxicity through the alteration of the bone marrow, which was confirmed in the present study.Citation20,Citation21 By contrast, no alteration in the renal tissue after the administration of all formulations containing CDDP, could be verified. These results are similar to those obtained from other studies, which demonstrated alterations in the hematological parameters and the absence of renal toxicity due to the repeated administration of a liposomal formulation containing CDDP in experimental models.Citation22
Conclusion
In conclusion, the results of the present study demonstrated that the SpHL-CDDP administration at higher doses than those used for the administration of free CDDP in Ehrlich solid tumor-bearing mice proved to be a potential approach to improving the antitumor efficacy of CDDP in solid tumors. Thus, this CDDP carrier is a promising candidate for IV chemotherapy in patients with malignant tumors, given that SpHL-CDDP can overcome therapy limitations, such as the difficulty in adjusting doses and the toxicity induced by free CDDP.
Acknowledgments
The authors would like to thank FAPEMIG, CNPq, and FINEP for their financial support. EA Leite wishes to thank FAPEMIG for her research grant. The authors also wish to thank Pró-Reitoria de Pesquisa of UFMG for their funding to review this manuscript and Dr Jorge Luiz Pesquero (Department of Physiology and Biophysics, Biological Sciences Institute, UFMG, Belo Horizonte, MG, Brazil) for the donation of Ehrlich cells.
Disclosure
The authors report no conflicts of interest in this work.
References
- RabikCADolanMEMolecular mechanisms of resistance and toxicity associated with platinating agentsCancer Treat Rev200733192317084534
- WangLRLiuJHuangMZXuNComparison of pharmacokinetics, efficacy and toxicity profile of gemcitabine using two different administration regimens in Chinese patients with non-small-cell lung cancerJ Zhejiang Univ Sci B20078530731317542057
- Carvalho JúniorADVieiraFPMeloVJPreparation and cytotoxicity of cisplatin-containing liposomesBraz J Med Biol Res20074081149115717665053
- JúniorADMotaLGNunanEATissue distribution evaluation of stealth pH-sensitive liposomal cisplatin versus free cisplatin in Ehrlich tumor-bearing miceLife Sci200780765966417141809
- AllenTMNewmanMSWoodleMCMayhewEUsterPSPharmacokinetics and anti-tumor activity of vincristine encapsulated in sterically stabilized liposomesInt J Cancer19956221992047622296
- UchinoHMatsumuraYNegishiTCisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in ratsBr J Cancer200593667868716222314
- LeiteEAGiubertiCSWainsteinAJAcute toxicity of long-circulating and pH-sensitive liposomes containing cisplatin in mice after intraperitoneal administrationLife Sci20098419–2064164919302806
- LeiteEALanaAMJuniorADCoelhoLGDe OliveiraMCAcute toxicity study of cisplatin loaded long-circulating and pH-sensitive liposomes administered in miceJ Biomed Nanotechnol20128222923922515074
- De OliveiraMCFattalECouvreurPpH-sensitive liposomes as a carrier for oligonucleotides: a physico-chemical study of the interaction between DOPE and a 15-mer oligonucleotide in quasi-anhydrous samplesBiochim Biophys Acta1998137223013109675320
- ZamboniWCJungLLEgorinMJPhase I and pharmacologic study of intermittently administered 9-nitrocamptothecin in patients with advanced solid tumorsClin Cancer Res200410155058506415297407
- LeeCMTanakaTMuraiTNovel chondroitin sulfate-binding cationic liposomes loaded with cisplatin efficiently suppress the local growth and liver metastasis of tumor cells in vivoCancer Res200262154282428812154030
- HovingSvan TielSTEggermontAMten HagenTLEffect of low-dose tumor necrosis factor-alpha in combination with STEALTH liposomal cisplatin (SPI-077) on soft-tissue- and osteosarcoma-bearing ratsAnticancer Res2005252A74375015868905
- AraújoJGMotaLGLeiteEABiodistribution and antitumoral effect of long-circulating and pH-sensitive liposomal cisplatin administered in Ehrlich tumor-bearing miceExp Biol Med (Maywood)2011236780881521685237
- GryparisECHatziapostolouMPapadimitriouEAvgoustakisKAnticancer activity of cisplatin-loaded PLGA-mPEG nanoparticles on LNCaP prostate cancer cellsEur J Pharm Biopharm20076711817303395
- KuriharaNKubotaTHoshiyaYPharmacokinetics of cis-diamminedichloroplatinum (II) given as low-dose and high-dose infusionsJ Surg Oncol19966221351388649040
- VassilevaVGrantJDe SouzaRAllenCPiquette-MillerMNovel biocompatible intraperitoneal drug delivery system increases tolerability and therapeutic efficacy of paclitaxel in a human ovarian cancer xenograft modelCancer Chemother Pharmacol200760690791417375303
- FerraraNRole of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implicationsSemin Oncol2002296 Suppl 16S10S14
- MorenoDZalbaSNavarroITros de IlarduyaCGarridoMJPharmacodynamics of cisplatin-loaded PLGA nanoparticles administered to tumor-bearing miceEur J Pharm Biopharm201074226527419883755
- SuddekGMSunitinib improves chemotherapeutic efficacy and ameliorates cisplatin-induced nephrotoxicity in experimental animalsCancer Chemother Pharmacol20116751035104420652569
- WorkingPKNewmanMSSullivanTComparative intravenous toxicity of cisplatin solution and cisplatin encapsulated in longcirculating, pegylated liposomes in cynomolgus monkeysToxicol Sci19984611551659928679
- AsnaNLewyHAshkenaziIETime dependent protection of amifostine from renal and hematopoietic cisplatin induced toxicityLife Sci200576161825183415698860
- BoulikasTLow toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenograftsOncol Rep200412131215201951