?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Recently, nanoparticles have been demonstrated to have tremendous merit in terms of improving the treatment specificity and thermal ablation effect on tumors. However, the potential toxicity and long-term side effects caused by the introduced nanoparticles and by expelling them out of the body following surgery remain a significant challenge. Here, we propose for the first time to directly adopt magnesium nanoparticles as the heating enhancer in laser thermal ablation to avoid these problems by making full use of the perfect biodegradable properties of this specific material.
Methods
To better understand the new nano “green” hyperthermia modality, we evaluated the effects of magnesium nanoparticles on the temperature transients inside the human body subject to laser interstitial heating. Further, we experimentally investigated the heating enhancement effects of magnesium nanoparticles on a group of biological samples: oil, egg white, egg yolk, in vitro pig tissues, and the in vivo hind leg of rabbit when subjected to laser irradiation.
Results
Both the theoretical simulations and experimental measurements demonstrated that the target tissues injected with magnesium nanoparticles reached much higher temperatures than tissues without magnesium nanoparticles. This revealed the enhancing behavior of the new nanohyperthermia method.
Conclusion
Given the unique features of magnesium nanoparticles – their complete biological safety and ability to enhance heating – which most other advanced metal nanoparticles do not possess, the use of magnesium nanoparticles in hyperthermia therapy offers an important “green” nanomedicine modality for treating tumors. This method has the potential to be used in clinics in the near future.
Introduction
Cancer is a major public health problem all over the world. Currently, one in four deaths in the USA are caused by cancer, and the incidence of cancer continues to increase.Citation1 Among the many endeavors developed to combat cancer, nanotechnology offers new hope to fight cancer.Citation2,Citation3 In 2003, Hirsch et al applied gold nanoshells, which comprise a gold layer over a silica core, to treat human breast carcinoma cells in vitro.Citation4 The carcinoma cells were found to have undergone photothermally induced morbidity on exposure to near infrared (NIR) light. They also used human epidermal growth factor receptor-2 (HER2)-targeted gold nanoshells to treat the breast cancer cell line overexpressing HER2.Citation5 Following exposure to NIR laser light, the dual imaging/therapy immuno-targeted nanoshells can selectively induce cells that overexpress HER2 to die. Huang and colleagues have demonstrated that gold nanorods have a longitudinal absorption band in NIR light on account of their surface plasmon resonance oscillations and are effective as photothermal agents.Citation6 Further, Huang et al have demonstrated that gold nanorods conjugated to antiepidermal growth factor receptor (anti-EGFR) antibodies could selectively target cell lines that overexpress EGFR. Subsequent continuous laser exposure of nanoparticle-treated cells resulted in photothermal destruction of the EGFR positive cells at half the energy required to kill EGFR negative cells.Citation7 Chen et al have investigated the photothermal destruction of cancer using gold nanocages in vitro and in vivo.Citation8 The results revealed that the strong absorption of light by gold nanocages could generate enough heat to kill cancer cells. Carbon nanotubes have also been considered for applications in various biological systems.Citation9 The combination of nanotechnology with medicine has thus yielded important advances in the fight against cancer. Overall, nanoparticles have shown high promise for application in hyperthermic cancer therapy. However, most of these nanoparticles are not degradable in the human body and thus are still required to be less cytotoxic to the surrounding normal cells. In fact, important evidence has shown that many of the tested nanoparticles, including the well-known gold nanoparticles, unfortunately have varying degrees of toxicity.Citation10
As is well-known, Mg2+ is one of the most abundant divalent cations in living cells and it plays a vital role in many cellular processes. The recommended dietary allowance of magnesium is 300 mg/day for a nonpregnant adult woman and 350 mg/day for an adult man. An adult human body contains about 25 g of magnesium and it is the second most abundant intracellular element in the body. About 1% of the total body magnesium is found in blood plasma, about a third of which is bound to proteins.Citation11,Citation12 In fact, magnesium has recently become a promising material of tremendous interest as a biocompatible and biodegradable implant metal, with its alloys continuing to be proven excellent candidate materials for biodegradable orthopedic implantsCitation13 and vascular stents.Citation14 Overall, the safety of the magnesium nanoparticle lies in its degradation into magnesium ions, which are completely absorbable by the human body. Magnesium nanocomposites have also been used for hydrogen storage.Citation15 However, as far as the authors are aware, until now, no research has been undertaken on introducing such an interesting material in nanoscale into tumor therapy, especially into the area of laser nanohyperthermia. In this study, due to its high heat conductivity and uniquely perfect biodegradability,Citation16 we proposed for the first time to adopt magnesium nanoparticles for enhancing cancer hyperthermia therapy with the assistance of an NIR laser. To investigate the basic features of this new nanohyper thermia modality, theoretical and experimental evaluations were carried out. The same strategy can probably be extended to other areas in nanomedicine.
Principles, materials, and methods
Theoretical model and calculation method
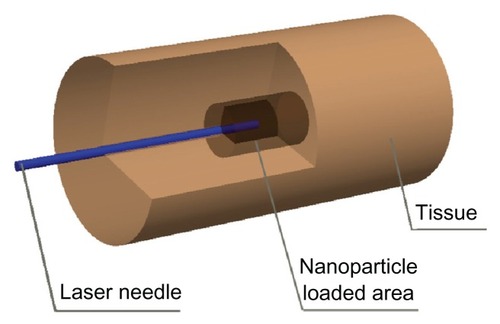
To understand the basic behavior of magnesium nanoparticle-enhanced laser heating, a computational model was set up, as shown in . Briefly, we considered that the nanoparticles were distributed uniformly. The laser needle was inserted into the center of the simulated model to emit energy. Theoretically, the transmission of photons from the laser beam to the surrounding tissues can be expressed as follows:
where r is the position vector; ŝ,ŝ′ are direction vectors; I(r,ŝ) is the intensity of laser light at position r in ŝ direction; w ′ is the solid angle; μt is the total attenuation coefficient defined as the sum of the absorption coefficient, μa, and the scattering coefficient, μ, p(ŝ,ŝ′), is the phase function. This equation as well as its initial and boundary conditions was simulated by the Monte-Carlo method to obtain the optical energy distribution in the model.
To predict the effects of the magnesium nanoparticle-enhanced laser heating on the target tissues, bioheat transfer equations were used to characterize the temperature field. For tissue area, the classic Pennes equation was adopted, which generally reads as:
where ρ is the density, c is the heat capacity, λ is the thermal conductivity, Wb is the blood perfusion rate, Qm is metabolic heat generation, Qr is the volumetric heat source due to spatial heat generation, and t and b denote tissue and blood, respectively.
To safely irradiate using a laser, the surface of the laser beam must be cooled. In this study, water was often adopted as the cooling medium. Therefore, the heat transfer equation to characterize the cooling water domain can be expressed as:
where V is the velocity for the cooling water and w denotes water.
Clearly, the addition of nanoparticles would change the optical properties of the tissues. The absorption and scattering coefficient of tissue equations following nanoparticle loading are therefore prescribed as:
where η is the volumetric concentration of the nanoparticles, which depends on the number of particles per unit volume n and particle radius Rnp; that is:
Similarly, other tissue properties would also change, such as the values of density, specific heat, and thermal conductivity for the tissues loaded with nanoparticles. These can be modified thus:
A similar calculation method has been previously demonstrated in Wang et al’s work, in which gold nanoshells were used to enhance laser heating; readers are referred to their study for more details.Citation17
Experimental set up
The magnesium nanoparticles used for the nano laser heating experiments in this study are commercially available and were directly purchased from Beijing Nachen S&T Ltd (Beijing, China).Citation18 For conceptual experiments, only the commercially available magnesium nanoparticles with a specified average diameter of 80 nm were tested in this study. A detailed comparison of the heating enhancement effects resulting from variable sizes and structures of these particles therefore needs to be researched in the future. It is worth noting that in this study only pure magnesium was used, which is often black in color. Such pure (99.9%) metal particle material is stored in vacuum packaging to avoid oxidation. Because the properties of magnesium nanoparticles, such as high heat conductivity and uniquely perfect biodegradability,Citation16 have been well addressed previously, in this study we focused on evaluating their heating enhancement effects. In contrast to pure magnesium, the oxidized magnesium nanoparticle – that is, the MgO nanoparticle – is generally white in color, thus almost does not absorb laser light. It is therefore of limited use for heating enhancement and was not our current concern. Magnesium nanoparticles are not very reactive with water below a certain temperature, such as the body core temperature of 37°C. Therefore, a suspension fluid containing the nanoparticles with an initial temperature around room temperature, 25°C, can easily be injected into the target sample or tissues.
A diode laser system (KS3-11311-106; BWT Beijing Ltd, Beijing, China) was used to administer laser radiation as the heating source. An infrared camera (ThermoVision® A40; FLIR Systems, Wilsonville, OR) was used to record the dynamic thermal images of the heated materials. The internal temperature of the tested objects was monitored by a thermocouple and data acquisition system (Agilent 34970; Agilent Technologies, Santa Clara, CA).
In this study, we designed four cases to measure the temperature changes of the materials administered magnesium nanoparticles then subjected to laser heating. Each case comprised several experimental samples (). In addition, each experiment was performed twice and the result was the average value from these two tests.
Table 1 Experimental groups
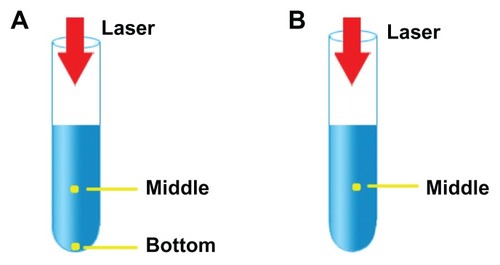
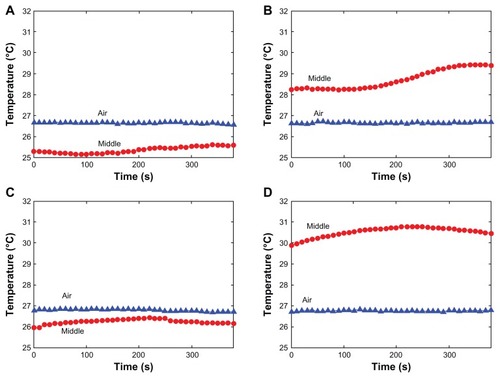
illustrates the experimental setup of Cases A and B. The temperature of 10 mL of liquid in a beaker was measured with thermocouples. For Case A, three thermocouples were used, one each to measure the room temperature and the temperatures at the bottom and the middle of the liquid. As water may react to magnesium nanoparticles under high temperature, oil was used here to reveal the basic features of the new nanohyperthermia modality, in which the heating effect was caused mainly by the laser absorbance of the magnesium nanoparticles; in the future, for the practical injection of magnesium nanoparticles into biological tissues containing water, a nanosuspension with a temperature below that of the body core will fulfill this requirement.
For Case B, using egg white and egg yolk, two thermocouples were used to measure temperature, one for the room temperature and the second for the temperature at the middle of the liquid. Egg was used because egg white and egg yolk are simple biological tissues that were suitable for testing the new nanohyperthermia modality. In particular, the heating phenomenon is easy to observe in these two materials. Egg white is mostly composed of protein, while egg yolk consists of protein, fat, and water.
Case C used the muscle tissue of pig as a test model. The size of the muscle tissue was 3 cm3 and the injection depths for samples 9, 10, 11 were all 5 mm. Water (0.1 mL), 0.1 mL of 0.01 g/mL magnesium nanoparticle aqueous solution, and 0.1 mL of 0.02 g/mL magnesium nanoparticle aqueous solution were injected intramuscularly into samples 9, 10, 11, respectively. Once the pre-prepared nanoparticle solutions were injected into the samples, an infrared camera was turned on to monitor the surface temperatures.
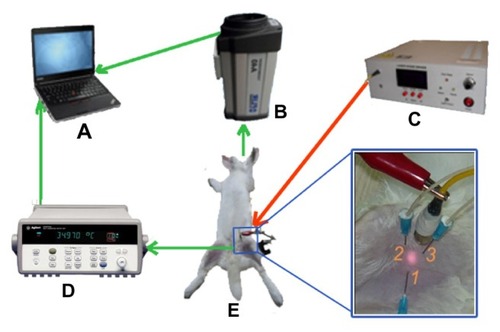
Cases A–C were in vitro experiments, while Case D was an in vivo experiment. For this latter case, the in vivo experiment was conducted on one healthy male New Zealand white rabbit with weight about 3 kg, which was bought from Fang Yuanyuan Farm, Beijing, China. The animal experiment was approved by the Ethical Committee of Tsinghua University under contract, Beijing, China. presents the schematic for the Case D experiment. Firstly, the rabbit was anesthetized with 25% (w/w) isoflurane solution via intraperitoneal injection. Then, we used hair-removal cream to carefully remove hair from the rabbit leg. Once the 0.02 g/mL magnesium nanoparticle aqueous solution was prepared and dispersed via ultrasound, 1 mL of the solution was injected into the target tissue of the left hind leg of the rabbit. The right hind leg was loaded with 1 mL water as the comparison experiment. The infrared camera and the thermocouple thermometers were used to monitor the surface and internal temperatures, respectively, of the rabbit. The infrared camera was placed in front of the rabbit’s leg at a distance of about 60 cm and the thermocouples were inserted into the femoral muscle to measure the specific tissue sites. The depth of thermocouple 1 was 3 mm in the center of the laser spot, and the depths of thermocouples 2 and 3 were 10 mm and 5 mm, respectively, beside the site of the laser irradiation. The room temperature was also monitored with a thermocouple thermometer.
Figure 3 Schematic representation of the in vivo animal experiment. The rabbit (E) was anesthetized with isoflurane and hair was removed from its leg. The diode laser system (C) was used to emit laser radiation as a heating source. The infrared camera (B) recorded the thermal images of the heated materials in real time and the internal temperature was monitored by thermocouple thermometer (D). The depth of thermocouple 1 was 3 mm in the center of the laser pot and the depths of thermocouples 2 and 3 were 1 cm and 0.5 cm beside the pot of the laser, respectively. The thermal images and internal temperature data were shown on the laptop computer (A).
Results
Theoretical results
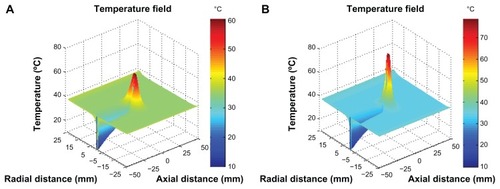
lists the thermal and optical properties of the tissues, water, and nanoparticles.Citation17,Citation19,Citation20 To analyze the influence of the nanoparticles on the heating effect, we simulated the temperature distribution of tissues loaded with nanoparticles as well as without nanoparticles for comparison. The results for the tissues loaded with and without nanoparticles are shown in and , respectively. During the simulation, the diameter of the particles and the number of particles per unit volume were prescribed as 80 nm and 1016/m3, respectively. shows the temperature field of the simulated tissues with and without nanoparticles during hyperthermia. It was found that the nanoparticle-loaded tissues reached a maximum temperature of 78.7°C, while the tissues without nanoparticles only reached 60.4°C. Obviously, such evident temperature differences were caused by the addition of the nanoparticles. depicts the transient maximum temperature during the irradiation process for the two cases. This value is appropriate for the safety of the biological tissues.Citation17 If the simulation conditions were different, the temperature distributions would also differ. Thus, it was clear that the nanoparticles enhanced the laser heat disposition. All the simulated results demonstrate that the magnesium nanoparticles could serve as a highly promising heating enhancer for a targeted hyperthermia treatment.
Table 2 Parameters for tissue, water, and nanoparticles (NPs)
Experimental results
Mixing magnesium nanoparticles with oil
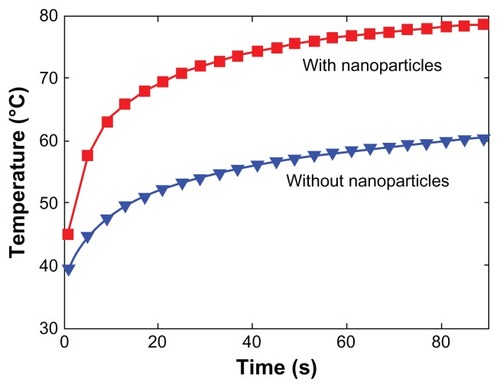
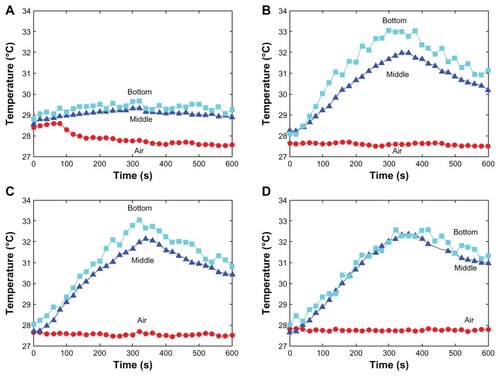
In this experiment, the laser irradiation time was set as 360 seconds and the temperature was recorded for a total of 600 seconds. The temperature of the surrounding environment was stabilized at 27.5°C. As shown in , the temperature of the oil without nanoparticles under laser irradiation remained almost unaltered. However, the temperature of the magnesium nanoparticle-loaded oil increased from 27.5°C to nearly 33°C. The temperatures at the bottom were almost the same among the different concentrations of the magnesium nanoparticles. It appears that the temperature increase showed a concentration-saturated effect. The temperature difference between the middle and the bottom of the liquid decreased when the concentration of magnesium nanoparticles increased, which was due to the good thermal conductivity of magnesium nanoparticles.
Mixing magnesium nanoparticles with egg white and egg yolk
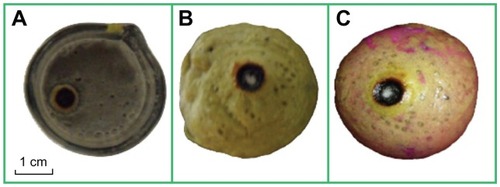
In these experiments, the laser irradiation time was set as 240 seconds and the temperature was recorded for a total of 380 seconds. The room temperature was about 26.7°C. presents the temperature change of the egg white under laser irradiation with and without nanoparticles. It was interesting to note that the temperature immediately increased by 3.5°C for the egg white mixed with magnesium nanoparticles. As reflected in , the temperature of the mixture increased to 29.5°C under laser irradiation. This homogeneous mixture had a combustive property, as indicated in . A similar phenomenon occurred with the egg yolk. When subjected to laser irradiation, the temperature of the egg yolk hardly changed, as shown in . However, the temperature would quickly increase by 4°C when the egg yolk was mixed with the magnesium nanoparticles in advance, and the temperature increased to 31°C under laser irradiation, as shown in . It can be seen from that the egg yolk mixed with magnesium nanoparticles burned at the laser irradiation spot. During the experiment, when we dropped two drops of phenolphthalein on the surface of the egg yolk mixture, it grew red (). A reaction between water and Mg occurs. It is beneficial for treating tumors because this reaction releases amounts of heat.
Figure 7 Plots of temperature increase for the egg white (A), the egg white with magnesium nanoparticles at 0.02 g/mL (B), egg yolk (C), and egg yolk with magnesium nanoparticles at 0.02 g/mL (D) in 10 mL beakers, as a function of irradiation time, under irradiation by diode laser at power of 1.5 W.
Pig tissues in vitro
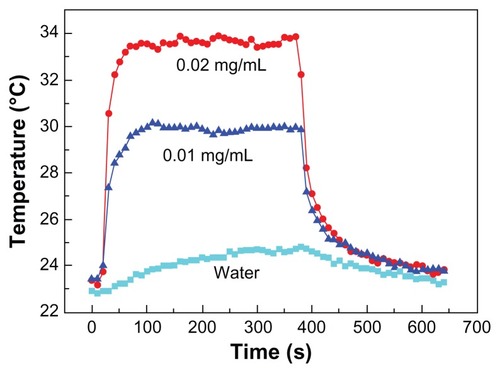
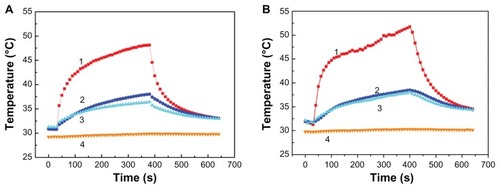
For this case, the laser irradiation time was set as 360 seconds and the temperature was recorded for a total of 640 seconds. The temperature curves derived from the infrared camera images for samples 9, 10, and 11 of Case C are shown in . The temperature changes that occurred in the tissues injected with magnesium nanoparticles appear greater than those that occurred in the tissues injected with water. presents the infrared images after 60 seconds of laser irradiation for the three samples. It is clear that the pig tissues injected with the 0.02 g/mL magnesium nanoparticle aqueous solution produced the most significant temperature change.
Hind leg of rabbit in vivo
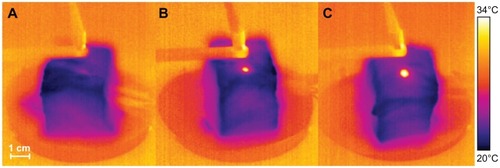
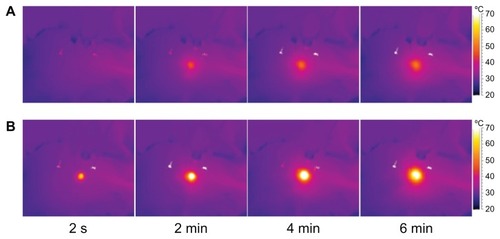
The room temperature during this experiment was 29.8°C (labeled “4” in ). present the temperatures of the hind leg of the rabbit with 1 mL water and with 1 mL of 0.02 g/mL magnesium nanoparticle aqueous solution, respectively, under the same level of laser irradiation. Clearly, the thermal effect with the magnesium nanoparticle aqueous solution appears much greater. shows infrared temperature images with and without magnesium nanoparticle aqueous solution. The temperature of the hind leg of the rabbit injected with magnesium nanoparticle aqueous solution was much higher than that of the leg not injected with magnesium nanoparticle aqueous solution.
Figure 11 Temperature measurements of the rabbit leg injected with 1 mL water (A) and with 1 mL magnesium nanoparticle aqueous solution (B) irradiated with the laser at 1.5 W.
Notes: The depth of thermocouple 1 was 3 mm in the center of the laser pot and the depths of thermocouples 2 and 3 were 10 mm and 5 mm, respectively, beside the pot of the laser. Curve 4 indicates the room temperature.
Discussion
Overall, according to the theoretical evaluation and experimental tests, magnesium nanoparticles are a highly promising material for use in hyperthermia. As far as the authors are aware, this is the first time the heating enhancement effect of such a material has been reported in nanomedicine. Compared with many existing popular nanomaterials, such as gold, magnesium has many unique advantages. Firstly, the density of magnesium is much lower than that of gold and therefore more particles can be loaded into the target tissues. Importantly, the density of magnesium is approximately 1.7 g/cm3, which is very similar to that of human bone (1.75 g/cm3). Besides this, magnesium is highly abundant in nature and will thus be cost-effective for clinical utilization.Citation21 Secondly, magnesium is beneficial to human health. In content, it is second only to potassium in the human body. As a kind of “positive ion” in human cells, it plays an important role in the synthesis of protein, activation of multiple enzymes, regulation of the activities of the neuromuscular and central nervous systems, and in ensuring normal myocardial contraction and temperature regulation.
Li et al’s preliminary cytotoxicity study showed that magnesium has no inhibitory effects on bone marrow cell growth and no signs of cellular lysis were observed.Citation22 Further, Abumaria et al’s latest work suggests that elevation of brain magnesium might even be a novel approach for enhancing synaptic plasticity in a regional-specific manner leading to enhancement of extinction efficacy without enhancing or impairing fear memory formation.Citation23 Importantly, it is a kind of biodegradable material in the human body second to none compared with any other metal material. The standard electrode potential of magnesium is low, and it can be corrosively degraded and absorbed into the body in the presence of chloride ions in the physiological environment.
Qiao et al’s work reported some results of testing the cytotoxicity, hemolysis, and acute toxicity on magnesium samples, and quite attractive features were obtained.Citation24 In fact, magnesium and its alloys have been increasingly used as body stents because of the advantages already mentioned.Citation24 Clearly, the use of magnesium nanoparticles proposed in the present study opens new pathways toward the full use of such material in medical fields.
However, it should be pointed out that there are still many problems that need to be solved before a hyperthermia modality such as the one described here is applied in clinics. We found that an amount of heat is released at the moment the nanoparticles were injected, which may result in tissues being burned. The heat energy comes from the chemical reaction between the magnesium nanoparticles and water contained in the tissue and this phenomenon would bring about a certain level of corrosion of the material. Further, if the rate of material degradation is too fast, the heating enhancement effect may not be maintained for long or fully occur. Moreover, the increased temperature would encourage the magnesium nanoparticles to react with the water in the tissues. Such a reaction could cause further tissue degeneration, although this would clearly be of benefit in the ablation of target tissues.Citation25
Therefore, it is important that the degradation rate of the magnesium nanoparticles used in hyperthermia is controlled. In this regard, a protective coating on the nanoparticles might be a feasible way to avoid their reacting with water as well as to control the degradation rate and further improve biocompatibility.Citation26 Another possibility is alkali-heat-treated magnesium (the magnesium is first immersed in super-saturated NaHCO3–MgCO3 for 24 hours and then heat treated at 773 K for 10 hours), which may have an improved corrosion resistance.Citation23 In Qiao et al’s study, alkali-treated magnesium caused distinct morphological changes in cells and a small hemolytic effect (2.2%), which is less than the allowable 5%.Citation24 Other useful surface modification methods to control the corrosion rate include micro arc oxidation, phosphating treatment, electrodepositing, and polymer coating.Citation16
Some studies have looked at the biodegradation of surface- modified magnesium and its alloys. In Geng et al’s study, magnesium with β-tricalcium phosphate coating showed good biodegradation behavior in vitro, thus may slow down the biodegradation rate of magnesium.Citation27 Grillo et al used fluoride conversion coatings to reduce corrosion rates and found that fluoride release from conversion coatings did not show cytotoxic effects.Citation28 Further, Hannover Medical School in Germany has developed a biodegradable magnesium-hydroxyapatite metal matrix composite material that may adjust the degradation rate through the size and distribution of the hydroxyapatite particles.Citation29
There are also a number of other studies that have reported less than ideal biocompatibility of pure magnesium. Citation22,Citation30 However, generally, the samples used in these experiments were cultured cells, which are sensitive to the environment. As already discussed, pure magnesium reacts easily with water mediums, which then results in an increase in pH value and release of ions. However, this situation is different from that described here for hyperthermia using magnesium nanoparticles. Firstly, as the magnesium nanoparticles are injected into the center of the tumor, environmental changes – including to pH and the amount of ions released – in the surrounding normal tissues are less than in the site of the tumor. Secondly, the increase in pH value and release of ions in the tumor region may contribute to tumor death; this needs to be investigated further.
The present nanohyperthermia modality has a generalized purpose. The same strategy can also be extended to other hyperthermia fields, such as electrical, magnetic, or focused acoustic nanoparticle hyperthermia, and thermochemical heating. One of the reasons is that the thermal conductivity of magnesium and its alloys is quite high, so they can effectively enhance heat transfer, which is transformed from other forms of energy. Targeted thermochemical ablation therapy, which is based on the release of heat from chemical reactions into the target tissues, involves two typical methods: acid–alkali reaction-enabled thermal ablation and alkali metal-enabled thermal ablation.Citation25 On account of their better thermal conductivity, magnesium nanoparticles could not only be used as a kind of alkali metal involved in chemical reactions to produce heat but also to improve the thermal conductivity of the target tissues.
Although iron is more magnetic than more common materials such as magnetite and maghemite, it is easily oxidized in vivo and its magnetic properties are reduced, which restricts its use in magnetic nanohyperthermia. The general solution is to cover the iron nanoparticle with a shell of gold,Citation31 silver,Citation32 or one of their hybrids to protect the magnetic core against oxidation. However, these materials are not biodegradable. Compared with them, magnesium is much cheaper and safer for use in the human body.
Finally, particularly of note for the emerging role of the presently proposed magnesium nanoparticles in nanomedicine is their potential for implementation as a new and completely safe nanocarrier drug-delivery system. In fact, finding a safe and highly efficient delivery system for delivering drugs or even genes and proteins (growth factors) to the target tissue site for specific medical purpose has become an exciting frontier. Important discoveries continue to be made in this area of research.Citation33–Citation38 Clearly, with perfect biodegradability and medical safety, magnesium nanoparticles may contribute significantly to this important endeavor. More effort, either fundamental or practical, is thus necessary to extend the medical applications of these nanoparticles in the future.
Conclusion
In this study, biodegradable magnesium nanoparticles have been proposed for the first time as a heating enhancer targeted to high-performance hyperthermia, especially laser thermal ablation. The new strategy would help to resolve the problems facing current tumor nanomedicine such as side effects and toxicity. In addition, expulsion of the nanoparticles out of the human body, which carries potential dangers, may no longer be necessary. This will significantly simplify the surgical process and reduce treatment costs. Both theoretical simulations and conceptual experiments demonstrate the heating enhancement effect of the magnesium nanoparticles for laser thermal ablation. Some new thermal phenomena were observed and exciting insights into biodegradable nanoparticle-enhanced laser thermal therapy for future practice revealed. Compared with many existing metal agents used in nanomedicine, magnesium nanoparticles have excellent degradability in vivo. Further, the magnesium nanoparticle-enhanced laser hyperthermia therapy discussed has a generalized purpose that can be extended to other areas of nanomedicine. In this sense, magnesium nanoparticle-enhanced hyperthermia opens a “green” way for future tumor therapy.
Acknowledgment
This work is supported by the National Natural Science Foundation of China under Grant 81071255, the Specialized Research Fund for the Doctoral Program of Higher Education, and the Research Fund from Tsinghua University under Grant 523003001.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- FerrariMCancer nanotechnology: opportunities and challengesNat Rev Cancer20055316117115738981
- JainKKRole of nanobiotechnology in developing personalized medicine for cancerTechnol Cancer Res Treat20054664565016292884
- HirschLRStaffordRJBanksonJANanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidanceProc Natl Acad Sci U S A200310023135491355414597719
- LooCLoweryAHalasNWestJDrezekRImmunotargeted nanoshells for integrated cancer imaging and therapyNano Lett20055470971115826113
- HuangXEl-SayedIHQianWEI-SayedMACancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorodsJ Am Chem Soc200612862115212016464114
- ChenJWangDXiJImmuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cellsNano Lett2007751318132217430005
- ChenJGlausCLaforestRGold nanocages as photothermal transducers for cancer treatmentSmall20106781181720225187
- LiuZCaiWHeLIn vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in miceNat Nanotechnol200721475218654207
- GoodmanCMMcCuskerCDYilmazTRotelloVMToxicity of gold nanoparticles functionalized with cationic and anionic side chainsBioconjug Chem200415489790015264879
- LiaoFFolsomARBrancatiFLIs low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) StudyAm Heart J199813634804909736141
- HamptonEMWhangDDWhangRIntravenous magnesium therapy in acute myocardial infarctionAnn Pharmacother19942822122198173140
- StaigerMPPietakAMHuadmaiJDiasGMagnesium and its alloys as orthopedic biomaterials: a reviewBiomaterials20062791728173416246414
- HeubleinBRohdeRKaeseVNiemeyerMHartungWHaverichABiocorrosion of magnesium alloys: a new principle in cardiovascular implant technology?Heart200389665165612748224
- JeonKJMoonHRRuminskiAMAir-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalystsNat Mater201110428629021399630
- GuXNZhengYFA review on magnesium alloys as biodegradable materialsFront Mater Sci China201042111115
- WangXGaoXLiuJMonte-Carlo simulation on gold nanoshells enhanced laser interstitial thermal therapy on target tumorJ Comput Theor Nanosci20107610251031
- SINONANO, N&C Beijing Nachen S&T Ltd Available from: http://www.nanoinchina.comAccessed August 6, 2012
- WangQDengZSLiuJEffects of nonuniform tissue properties on temperature prediction in magnetic nanohyperthermiaJ Nanotechnol Eng Med201122021012
- The Engineering ToolboxThermal conductivity of some common materials and gases [web page on the Internet] Available from: http://www.engineeringtoolbox.com/thermal-conductivity-d_429.htmlAccessed December 29, 2011
- MakerGLKrugerJCorrosion of magnesiumInt Mater Rev1993383138153
- LiLGaoJWangYEvaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluidSurf Coat Technol20041859298
- AbumariaNYinBZhangLEffects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdalaJ Neurosci20113142148711488122016520
- QiaoLYGaoJCWangYWangSLWuSXueYBiocompatibility evaluation of magnesium-based materialsMater Sci Forum2007546–549459462
- RaoWLiuJZhouYXYangYZhangHAnti-tumor effect of sodium-induced thermochemical ablation therapyInt J Hyperthermia200824867568118608585
- GrayJELuanBProtective coatings on magnesium and its alloys – a critical reviewJ Alloys Compd20023361–288113
- GengFTanLLJinXXYangJYYangKThe preparation, cytocompatibility, and in vitro biodegradation study of pure beta-TCP on magnesiumJ Mater Sci Mater Med20092051149115719132512
- GrilloCAAlvarezFde MeleMABiological effects of magnesium particles degradation on UMR-106 cell line: influence of fluoride treatmentsColloids Surf B Biointerfaces201188147147621839622
- WitteFFeyerabendFMaierPBiodegradable magnesium-hydroxyapatite metal matrix compositesBiomaterials200728132163217417276507
- LorenzCBrunnerJGKollmannsbergerPJaafarLFabryBVirtanenSEffect of surface pre-treatments on biocompatibility of magnesiumActa Biomater2009572783278919427423
- RavelBCarpenterEEHarrisVGOxidation of iron in iron/gold core/shell nanoparticlesJ Appl Phys2002911081958197
- ChoiJYKimKShinKSSurface-enhanced raman scattering Inducible by recyclable Ag-coated magnetic particlesVib Spectrosc2010531117120
- HosseinkhaniHDNA nanoparticles for gene delivery to cells and tissueInt J Nanotechnol200634416461
- SubramaniKAPathakSAHosseinkhaniHRecent trends in diabetes treatment using nanotechnologyDig J Nanomat Biostructures2012718595
- HosseinkhaniHTabataYSelf assembly of DNA nanoparticles with polycations for the delivery of genetic materials into cellsJ Nanosci Nanotechnol2006682320232817037837
- HosseinkhaniHHosseinkhaniMBiodegradable polymer-metal complexes for gene and drug deliveryCurr Drug Saf200941798319149528
- MahmoudiMHosseinkhaniHHosseinkhaniMMagnetic resonance imaging tracking of stem cells in vivo using iron oxide nanoparticles as a tool for the advancement of clinical regenerative medicineChem Rev2011111225328021077606
- SubramaniKHosseinkhaniHKhraisatAHosseinkhaniMPathakYTargeting nanoparticles as drug delivery systems for cancer treatmentCurr Nanosci200952135140