Abstract
Background
The objective of this study was to evaluate the synthesis and biocompatibility of Fe3O4 nanoparticles and investigate their therapeutic effects when combined with magnetic fluid hyperthermia on cultured MCF-7 cancer cells.
Methods
Magnetic Fe3O4 nanoparticles were prepared using a coprecipitation method. The appearance, structure, phase composition, functional groups, surface charge, magnetic susceptibility, and release in vitro were characterized by transmission electron microscopy, x-ray diffraction, scanning electron microscopy-energy dispersive x-ray spectroscopy, and a vibrating sample magnetometer. Blood toxicity, in vitro toxicity, and genotoxicity were investigated. Therapeutic effects were evaluated by MTT [3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide] and flow cytometry assays.
Results
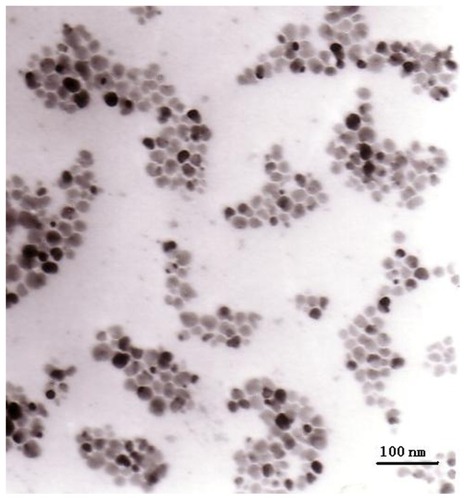
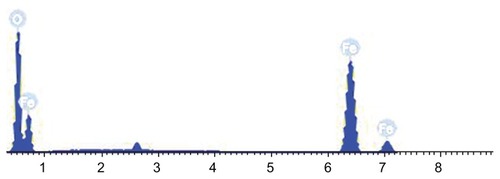
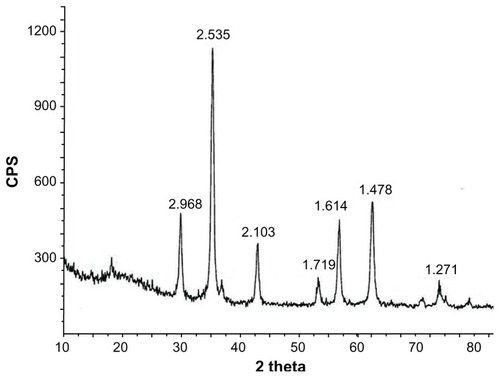
Transmission electron microscopy revealed that the shapes of the Fe3O4 nanoparticles were approximately spherical, with diameters of about 26.1 ± 5.2 nm. Only the spinel phase was indicated in a comparison of the x-ray diffraction data with Joint Corporation of Powder Diffraction Standards (JCPDS) X-ray powder diffraction files. The O-to-Fe ratio of the Fe3O4 was determined by scanning electron microscopy-energy dispersive x-ray spectroscopy elemental analysis, and approximated pure Fe3O4. The vibrating sample magnetometer hysteresis loop suggested that the Fe3O4 nanoparticles were superparamagnetic at room temperature. MTT experiments showed that the toxicity of the material in mouse fibroblast (L-929) cell lines was between Grade 0 to Grade 1, and that the material lacked hemolysis activity. The acute toxicity (LD50) was 8.39 g/kg. Micronucleus testing showed no genotoxic effects. Pathomorphology and blood biochemistry testing demonstrated that the Fe3O4 nanoparticles had no effect on the main organs and blood biochemistry in a rabbit model. MTT and flow cytometry assays revealed that Fe3O4 nano magnetofluid thermotherapy inhibited MCF-7 cell proliferation, and its inhibitory effect was dose-dependent according to the Fe3O4 nano magnetofluid concentration.
Conclusion
The Fe3O4 nanoparticles prepared in this study have good biocompatibility and are suitable for further application in tumor hyperthermia.
Introduction
A nanomaterial is a substance that has at least one dimension at the nanolevel (usually 0.1–100 nm). Because of their size distribution, nanomaterials have special surface, size, and macroscopic quantum tunneling effects, and have been used in numerous fields, such as mechanics, electricity, optics, magnetics, and catalysis. Magnetic nanomaterials are of particular interest, and have permeated into various fields of biological medicine, including as drug carriers and biological molecular probes. They have played important roles in the treatment of malignant tumors,Citation1 in which an alternating magnetic field is used to heat up magnetic nanomaterials and thereby kill targeted tumor cells. Jordan first proposed magnetic fluid hyperthermia technology based on this principle.Citation2,Citation3 The advent of magnetic fluid hyperthermia technology and the continuous innovation and development of related equipment and technologies has brought new vitality to traditional tumor thermotherapy. Some limitations of conventional heating technology have been overcome by magnetic fluid hyperthermia, ie, the heating temperature can be controlled, and the surfaces of the magnetic nanomaterials can be modified to avoid killing normal cells while selectively destroying tumor cells.
Currently, superparamagnetic ferric oxide nanoparticles represented by Fe3O4, γ-Fe2O3, and CO-Fe2O4 Citation4,Citation5 are the most promising magnetic nanomaterials. The research described herein used a modified chemical coprecipitation method to prepare high-purity superparamagnetic ferric oxide (Fe3O4) nanoparticles, characterize them, and evaluate their biocompatibility and suitability for the treatment of tumors.
Materials and methods
Instruments and materials
L-929 cells were provided by the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. MTT [3-(4, 5-dimethyl-2- thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide] was purchased from Sigma (St Louis, MO), and diluted to 5 mg/mL using D-Hanks liquid. RPMI 1640 medium and fetal calf serum were obtained from Gibco (Grand Island, NY). Dimethyl sulfoxide and Giemsa stain were purchased from Sigma. Cytoxan was acquired from Hen-Rui Co, Ltd (Jiangsu, China).
Elemental analysis was performed using scanning electron microscopy-energy dispersive x-ray spectroscopy (SEM-EDS, EDAX 9100; EDAX International, Prairie View, IL). X-ray diffraction data were collected using an XD-D1 x-ray diffractometer (Shimadzu, Kyoto, Japan) with Cu-Kα radiation (λ = 0.154 nm) at 40 kV and 30 mA. Transmission electron microscopy (TEM, model JSM- 6360LV; JEOL, Tokyo, Japan) was operated at 200 kV to observe the morphology and sizes of the particle samples. A Thermo Noran Vantage (Thermo Scientific, Waltham, MA) energy-dispersive spectrometer was used. Temperatures were measured with a digital thermometer (TM902C, Shenzhen Jingtengwei Industrial Co, Ltd, Shenzhen, China). A high-frequency magnetic induction heating device (SPG- 06A, 230 kHz frequency, 5 to 30 A output alternating heating current) was provided by Shenzhen Shuangping High- Frequency Heater Factory (Shenzhen, China).
Experimental animals
New Zealand rabbits and Kunming mice were purchased from Shanghai Laboratory Animal Inc (Shanghai, China). The laboratory animal facility was maintained on a 12-hour light/dark cycle at a temperature of 20°C–22°C with a relative humidity of 20%–50%. All animal experiments were carried out in compliance with the national laws related to the conduct of animal experimentation.
Preparation of magnetic Fe3O4 nanoparticles
A modified chemical coprecipitation was used, based on the method described by Yan et al.Citation6 The basic steps were as follows. FeCl3 · 6H2O and FeCl2 · 4H2O were precisely weighed and dissolved in distilled water. Ammonia water (1 mol/L, 100 mL) was added. The solutions were then transferred into wide-necked flasks equipped with breather plugs and magnetic stirring bars, and the flasks were filled with nitrogen gas. The reaction temperature was raised to 50°C and the contents were stirred at 3750 g for 30 minutes, after which the precipitate was filtered, washed three times with deionized water, and then dried in vacuo.
Characterization of magnetic Fe3O4 nanoparticles
Morphological evaluations using electron microscopy
A small sample of the magnetic Fe3O4 nanoparticles was dispersed in anhydrous alcohol using ultrasound for 15 minutes and then dripped onto membrane-supported copper gauze to prepare the sample for observation by H-600 TEM.
X-ray diffraction analysis
X-ray diffraction conditions were as follows: Cu target Kα (λ = 0.154 nm), tube voltage 40 kV, and tube current 30 mA.
Characterization by SEM-EDS
A small amount of the powder was dispersed in anhydrous alcohol and dripped onto copper gauze. The composition of the Fe3O4 was determined by scanning electron microscopy-energy dispersive x-ray spectroscopy (SEM-EDS, testing conditions: accelerating voltage, 15 kV; takeoff angle, 25.6934°; live time, 210 seconds; dead time, 13,658 seconds).
Magnetic performance testing
A vibrating sample magnetometer was used to measure the magnetic hysteresis of the Fe3O4 nanoparticles at 300 K under an applied field of ±5000 g.
In vitro heating of Fe3O4
The procedure used by Tang et alCitation7 was followed, according to which Fe3O4 nanoparticles are dispersed in 150 mM NaCl and diluted to various concentrations (0.5, 1.0, 1.5, 2.0, and 2.5 mg/mL). A 5 mL sample of fluid was placed in a 60 mm diameter dish and then exposed to a high-frequency alternating electromagnetic field (SPG-06A). The temperature of the fluid was measured at 10-minute intervals over an irradiation time of 60 minutes. The distance between the bottom of the dish and the center of the hyperthermia coil of the high- frequency electromagnetic field was 5 mm. The magnetic field frequency and intensity were 230 kHz and 28 A, respectively. Each experiment was carried out in triplicate.
Biocompatibility of Fe3O4 nanoparticles
Culture of L-929 cells
L-929 cells (mouse fibroblasts, ATCC CCLl, NCTC clone 929; American Tissue Culture Collection, Manassas, VA) were cultured in RPMI 1640 medium containing 10% fetal calf serum at 37°C in a 5% CO2/95% air incubator at 95% humidity.
In vitro evaluation of cytotoxicity
MTT assays were performed to evaluate whether the Fe3O4 nanoparticles were biocompatible and suitable for biomedical application. The Fe3O4 was treated with low temperature intermittent sterilization, and RPMI 1640 culture medium containing 10% calf serum was then added to it in a sterile closed vessel (final concentration 0.1 g/mL). Leaching conditions were 37°C over 72 hours. The samples were then centrifuged at 1250 g for 5 minutes, the supernatant was removed by suction and filtered through a microporous filter, and its pH was adjusted to 7.0–7.2 using 1 M HCl to provide a 100% leach solution. During the test, the RPMI 1640 culture medium was diluted to the required concentration. L-929 cells were removed during their logarithmic growth phase and digested in the cell suspension; the cell concentration was next adjusted to 5 × 104/mL and inoculated on a 96-well culture plate at 100 μL/well. The sample was cultured in a culture box at 37°C under saturated humidity and 5% CO2 conditions. The primary solution was discarded after 24 hours, at which time the leaching solution was added until the final concentration was 100%, 75%, 50%, and 25%. The RPMI 1640 culture inoculum was used as the negative control, and a 0.7% polyacrylamide monomer solution was used as the positive control. Each group comprising eight wells was cultured for 72 hours, and 20 μL of MTT was then added into each well and vibrated for 10 minutes. The absorbance value was measured at 493 nm using an immunoenzyme labeler (note that the literature cites various values for the wavelength of maximum absorbance; when dimethyl sulfoxide was used as a solvent to dissolve MTT crystals and form a purple solution, the maximum absorbance occurred at about 490–515 nm, and the test reported herein used 493 nm). The relative cell growth rate was calculated as follows: relative growth rate % = OD (Optical Density) mean value of test group/OD mean value of negative control group × 100%. The relative growth rate value was converted into a six-level reaction scheme, as indicated in . A level 0 or 1 reaction is acceptable, whereas a level 2 reaction should be assessed comprehensively considering cell morphology, and a level 3–5 reaction is unacceptable.
Table 1 Results of MTT test
Hemolysis testing
A 10 mL blood sample was taken from a New Zealand rabbit (male, 2.1 kg), to which 0.5 mL of 20 g/L potassium oxalate was added. Fresh rabbit blood was diluted (dilution ratio 8 mL blood to 10 mL normal saline). Fe3O4 was washed twice with distilled water, dried, and suspended using normal saline (final concentration 0.1 g/mL). Normal saline was used as the negative control and distilled water as the positive control. Each group consisted of three test tubes. A suspension of the materials to be tested (10 mL), 10 mL normal saline, and 10 mL distilled water were added to each tube, which was then placed in a 37°C water bath for 30 minutes. Diluted fresh rabbit blood (0.2 mL) was added to each tube, which was returned to the 37°C water bath for 60 minutes. Each tube was then centrifuged in a dry centrifuge trunnion for 5 minutes at 2500 rpm; the supernatant was then removed and OD values were measured at 545 nm with a spectrophotometer. The absorbance of the positive control group should be 0.8 ± 0.3 and that of the negative control group should be less than 0.03. The hemolytic rate, (HR, %) = (mean OD of sample to be tested − mean OD of negative control)/(mean OD of positive control − mean OD of negative control) × 100% was calculated. If the hemolytic rate is less than 5%, the material will have no hemolytic effect and conform to the requirements of the hemolysis test for medical materials.
Micronucleus assay
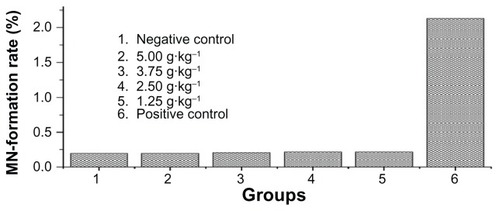
Kunming mice (n = 60) aged 4 weeks and weighing 20–22 g were divided into six groups, with 10 mice per group (half male and half female). After intermittent low temperature sterilization of the Fe3O4, sterile normal saline was added to prepare mixed suspensions of different concentrations. The dosage groups were 5, 2.5, 1.25, and 0.625 g/kg. The positive control group used cyclophosphamide (40 mg/kg) for abdominal injection and the negative control group used normal saline for abdominal injection. The common 30-hour injection method was used, ie, a 24-hour interval between two injections with a 6-hour wait after the second injection, after which time the mice were killed by cervical vertebra dislocation. Bone marrow was taken from the femur and a smear was made. The smear was dried, fixed with methanol, stained with Giemsa, and observed. Polychromatic erythrocytes and micronuclei were counted for each mouse, and the result was expressed as a percentage. Statistical analysis using Poisson’s distribution was used to establish if any differences existed between the three groups.
Determination of half-lethal dose
Kunming mice (n = 80) were randomly divided into eight groups, with 10 mice in each group (half being male and half being female). After fasting overnight, a suspension of Fe3O4 nanoparticles was injected abdominally at dosages of 1.77, 2.51, 3.54, 5.00, 7.06, 9.98, and 14.09 g/kg. NaCl 0.9% in a volume equivalent to that of the maximum dosage served as the negative control group. The animals in each group died during the 15 days following injection. After the experiment, the LD50 for the mice injected with Fe3O4 nanoparticles was calculated using Karber’s method, ie, LD50 = Lg−1[Xk − I (∑p − 0.5)].
Influence of Fe3O4 nanoparticles and magnetofluid thermotherapy on MCF-7 cell growth
MCF-7 cell culture
Cells were inoculated in RPMI 1640 medium with 10% calf serum and incubated at 37°C with saturated humidity and 5% CO2. One generation formed every 2–3 days. Logarithmic growth phase cells were used for the experiments.
Cell proliferation rate
The following groups were used: a negative control group (RPMI 1640 medium containing 10% fetal calf serum), a heating group comprising different concentrations of Fe3O4 nano magnetofluid (0.5, 1.0, 1.5, and 2.0 g/L Fe3O4), and a simple magnetic field irradiation group (a negative control group plus magnetic field irradiation). Each group consisted of six wells. Each well of the simple magnetic field radiation group and the magnetic fluid hyperthermia group was placed on the coils of a SPG-06A high-frequency heater plate (Shenzhen Shuangping High-Frequency Heater Factory). The wells were irradiated for one hour using an alternating magnetic field operating at 200 kHz and 4 kW with an output current of 300 A. After irradiation, the 96-well plate was placed in a culture box at 37°C and 5% CO2 with saturated humidity, and cultured for 48 hours. MTT (20 μL/well) was added and the samples were cultured for 4 hours under the same conditions. The fluid was then discarded, dimethyl sulfoxide (160 μL) was added to each well, and the samples were vibrated to mix them evenly for 10 minutes. They were then centrifuged for 10 minutes in a 96-well centrifuge, and the fluid in each cell was then moved to the corresponding well of another 96-well plate. The OD values of the 96 wells were measured at 493 nm using an automatic enzyme-linked immunosorbent assay. The inhibition rates were calculated as follows: inhibition rate (%) = (1 − OD value of test group/ OD value of control group) × 100%.
Flow cytometry
The groups and treatments were the same as those described above. The cells were inoculated in a 50 mL flask, collected after culturing for 48 hours, washed twice with phosphate-buffered saline, and finally fixed with 70% alcohol at 4°C for more than 24 hours. The cells were suspended in 0.5 mL propidium iodide stain (0.05, 0.1 g/L RNase A), stained at room temperature without exposure to light for 30 minutes, filtered, and then measured using the flow cytometer. All data were collected and analyzed using Lysis II software.
Results
Morphological observations
TEM showed that the electron density of the Fe3O4 particles was relatively high, and that the particles, which were spherical and partially agglomerated, had a primary diameter of 26.1 ± 5.2 nm ().
Energy-dispersive spectrometer surface analysis
SEM-EDS analysis () showed that the Fe3O4 nanoparticles only contained oxygen and iron, with Fe = 73.77 wt% and O = 26.23 wt%. The O-to-Fe ratio was similar to that predicted for pure Fe3O4, which further demonstrated that the ferrite prepared in the present study was Fe3O4.
Phase analysis
Sharp diffraction peaks were seen in the x-ray diffraction patterns of the magnetic Fe3O4 nanoparticles prepared (), indicating good crystallization. The face spacings (d values) of the diffraction peaks were 2.968 (220), 2.535 (311), 2.103 (400), 1.719 (422), 1.614 (511), 1.478 (440), and 1.271 (533). These matched the characteristic peaks for Fe3O4 in the standard PDF card in the X-ray diffraction atlas (JCPDS card 19-0629) issued by the International Powder Union. No extraneous peaks were observed, demonstrating that the prepared magnetic nanoparticles were Fe3O4 of high purity.
Magnetic performance
A vibrating sample magnetometer was used to measure the magnetic hysteresis of the Fe3O4 nanoparticles at 300 K in an applied field of ±5000 g. The results show that the saturated magnetic intensity of the Fe3O4 nanoparticles was 38.5 emu/g. The two lines of the hysteresis loop overlapped, ie, no coercivity was noted, indicating that the Fe3O4 nanoparticles prepared were superparamagnetic.
Thermodynamic testing of various doses of Fe3O4 nanoparticles in vitro
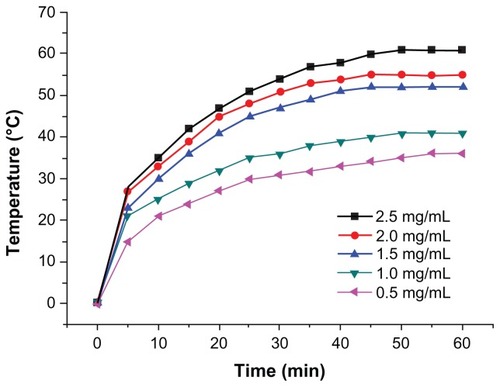
shows the results of in vitro thermodynamic testing of various doses of Fe3O4 nanoparticles (0.5, 1.0, 1.5, 2.0, and 2.5 mg/mL). The nanoparticles were dispersed in 150 mM NaCl before exposure to a high-frequency alternating electromagnetic field (28 A, 230 kHz) for 60 minutes. The temperature of the magnetic fluid rose to 36.5°C, 40.8°C, 43.1°C, 46.4°C, and 48.5°C, respectively, and the temperature remained unchanged after 30 minutes of exposure to the magnetic field.
Biocompatibility of Fe3O4 nanoparticles
Material toxicity testing of Fe3O4 nanoparticle leach solution
Inverted microscope observations revealed no significant difference in L-929 cell growth compared with that in the negative control group after a Fe3O4 nanoparticle leaching solution (100%, 75%, 50%, and 25%) was added. However, the positive control group behaved completely differently, ie, 24 hours after dosing, the cells became round and split, and almost no surviving cells were observed 72 hours later. The relative growth rate is shown in . The results indicate that the toxicity of this material toward L-929 cells was grade 0–1, and that of the positive control group was grade 4. Considering the morphological observations, the Fe3O4 nanoparticles can be considered nontoxic.
Hemolytic tests
The absorbance values at 545 nm for each test group are listed in . The hemolytic rate was calculated using the formula: hemolytic rate (%) = ODexperimental group − Dnegative control/ Dpositive control − Dnegative control. The 0.49% value calculated was markedly less than 5%.
Table 2 Results of hemolytic testing of magnetic Fe3O4 nanoparticle extract
Determination of half-lethal dose
Some mice developed lassitude, poor appetite, and watery stools after abdominal injection of the different doses of Fe3O4 nanoparticle suspension in each test group. The experimental animals died one after another in each group within 15 days of injection. The deaths of the tested animals from each group at the various doses are summarized in . Karter’s method was used to calculate the median lethal dose (LD50) of the mice as 8.39 g/kg with a wide safe range, ie, a 95% confidence interval of 10.14–6.95 g/kg.
Table 3 Results of acute toxicity testing of magnetic Fe3O4 nanoparticles
Testing of mouse bone marrow micronuclei
The rate of appearance of micronuclei in the polychromatic erythrocytes was recorded. No significant difference was found between the test animals and the negative controls in this regard. The results shown in demonstrate that the Fe3O4 nanoparticles did not cause any deformations or mutations.
Fe3O4 magnetic nanoparticles + magnetofluid thermotherapy and MCF-7 cell growth inhibition in vitro
MTT test results
The cell inhibition rate for each group was calculated as follows: inhibition rate (%) = (1 − OD value of test group/OD value of control group) × 100%. The results are shown in . Fe3O4 nano magnetofluid thermotherapy inhibited MCF-7 cell proliferation, and the inhibition effect was dose-dependent according to the concentration of the Fe3O4 nano magnetofluid. The maximum inhibition rate was 78%. A significant difference was noted between the thermotherapy, control, and simple magnetic field irradiation groups (P < 0.05).
Table 4 Growth inhibitory rate achieved by Fe3O4 nano magnetofluid thermotherapy to MCF-7 cells
Flow cytometry results
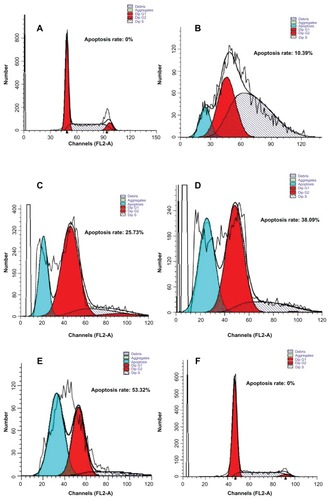
After 48 hours of treatment with the Fe3O4 nano magnetofluid, flow cytometry clearly showed a typical subdiploid apoptosis peak before the ambiguous (G0)/pre-DNA-synthetic (G1) phase. No obvious apoptosis peak was seen in the simple magnetic field irradiation and control groups. Increasing the concentration of the Fe3O4 nano magnetofluid significantly increased the apoptosis rate in the thermotherapy group. Cell cycles were retarded at the post-DNA-synthetic (G2)/mitotic (M) phase to different degrees ().
Figure 6 Flow cytometry showing apoptosis of MCF-7 cells induced by Fe3O4 nano magnetofluid thermotherapy after 48 hours. (A) Negative control, (B) heating group (0.5 g/L Fe3O4), (C) heating group (1.0 g/L Fe3O4), (D) heating group (1.5 g/L Fe3O4), (E) heating group (2.0 g/L Fe3O4), and (F) simple magnetic field irradiation group.
Abbreviation: Dip, diploid.
Discussion
Magnetic nanomaterials have great potential, and their preparation, performance, and applications have become very active research topics.Citation8 With the development of nanotechnology, many methods of preparing magnetofluids have appeared, both physical and chemical.Citation9 Each method has its own set of advantages and disadvantages, and the objectives of a study will influence which method is used.Citation10 The present study used a modified chemical coprecipitation technique to prepare magnetofluids. The advantages of this approach include ease of preparation, good control of conditions, and repeatable experimental results.Citation11 Morphological observations using various electron microscopic methods confirmed that we successfully prepared Fe3O4 nanoparticles with uniform electron density, regular morphology, and homogeneous particle size, which are all important factors for subsequent research. In vitro thermodynamic testing demonstrated that the magnetofluids prepared were readily heated by magnetic induction. At a fixed magnetic field intensity, the heating ability was positively correlated with the concentration of the magnetofluid, ie, the higher the concentration, the stronger the heating ability and the greater the temperature rise. The temperature plateaued after 50 minutes, suggesting potential application in magnetic fluid hyperthermia for treatment of tumors.
Biocompatibility is the most fundamental prerequisite for the clinical application of any biomaterial.Citation12 Governments and academic circles are attaching increasing importance to the safety of medical materials. Before any clinical study of a new biomaterial can take place, its compatibility must be evaluated by in vivo and in vitro testing. GBPT 16886-1997 (equivalent to ISO 10993)Citation13,Citation14 is a biological assessment standard for medical instruments and is based on cell toxicity assays. The present study also carried out cell toxicity tests, ie, acute systemic toxicity, pyrogen, hemolytic, and intradermal reactions. We also performed an in vitro cytotoxicity test using a leaching solution, a hemolytic test, an acute toxicity test in mice, and a micronucleus test to evaluate the biocompatibility and biosafety of the Fe3O4 nanoparticles prepared in this study.
MTT is a basic method of evaluating the biocompatibility of biomaterials when studying cytotoxicity.Citation15 It is a simple, fast, flexible, and repetitive technique. The principle is that amber mitochondrial dehydrogenase reduces methyl thiazolyl tetrazolium to purple crystals. The amount of purple crystals positively correlates with the quantity and function of living cells. Hence, the absorbance value indirectly reflects the quantity and activity of cells. Our results show that Fe3O4 magnetic nanoparticle leaching solutions of different concentrations have no obvious influence on cell proliferation; all cell proliferation rates were greater than 75%, and the cytotoxicity rating was grade 1. Thus, it can be regarded as a nontoxic biomaterial.
The hemolytic test is considered to be a supplementary test for assessment of cytotoxicity. It is used to evaluate if erythrocytes will dissolve and release hemoglobin after direct contact of the biomaterial with blood. It can be a sensitive measure of the influence of a biomaterial on erythrocytes and plays an important role in evaluation of biosafety.Citation16 In the present study, sedimentation of blood cells was observed in both the magnetic particle leaching solution group and the normal saline group. No significant difference in OD values was observed between the two groups (P > 0.05). The final hemolytic rate of the leaching solution was 0.49%, which conformed to the standard requiring less than 5%,Citation17 further demonstrating the good biocompatibility of this material.
The micronucleus test is a quick method for assessing if biomaterials can cause chromosomal damage and interfere with mitosis.Citation18 This test found no significant difference in the rate of micronucleus formation in bone marrow between mice of the material and of the negative control group (P > 0.05). However, a significant difference from that of the positive control group was noted (P < 0.05). Based on these results, the experimental material does not induce deformations or mutations.
During the acute toxicity test, some of the experimental animals had adverse reactions such as diarrhea, reduced mobility, and weight loss, and died in rapid succession. However, the volume of the test material reached 5 g/kg and its LD50 was 5.748 g/kg, meeting the requirements of the bioevaluation standards for medical instruments.
Next, we carried out an in vitro cytotoxicity test. Previous tests have shown that thermotherapy could induce apoptosis of normal cells and tumor cells.Citation19 Flow cytometry analysis with fluorescein isothiocyanate protein V and propidium iodide staining showed that death of L-929 cells 3 hours after thermotherapy at 43°C was mainly due to apoptosis.Citation20 In vitro tests showed that apoptosis of osteosarcoma cells occurred rapidly 6 hours after one hour of thermotherapy at 43.5°C.Citation21 Apoptosis is a process of active suicide of cells and is a form of planned cell death. Our flow cytometry analysis showed the same phenomenon. Apoptosis occurred in cells treated with Fe3O4 nano magnetofluid thermotherapy of different Fe3O4 concentrations. This magnetofluid thermotherapy could induce cell apoptosis in a dose-dependent way, and the maximum apoptosis rate reached 69.33%. Compared with cells in the control group, a hypodiploid peak was seen in the pre-G1 phase in cells of the thermotherapy group, which can induce hysteresis in the G2/M phase of the cell cycle.Citation22
In summary, in the present study, we successfully prepared Fe3O4 paramagnetic nanoparticles. In vitro heating tests confirmed their good heating ability. Evaluation of biocompatibility using a series of in vivo and in vitro tests demonstrated that this magnetic nanomaterial has low toxicity, its performance conforms to clinical requirements, and its biocompatibility conforms to the standard for medical polymeric materials. We observed a therapeutic effect of Fe3O4 nano magnetofluids at different concentrations under combined alternating magnetic field/magnetic fluid hyperthermia radiation in MCF-7 cell cultures. The hope is that the findings will provide a reliable laboratory basis for future clinical applications.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81071881, 30872999), the Jiangsu Province Natural Science Foundation of China (BK2007023), and the Six Talents Peak of Jiangsu Province (2010-WS-062), as well as a grant (RC2011033) from the Revitalize and Defend the Key Talents Subsidy Project in Science and Education of Department of Public Health of Jiangsu Province, China.
Disclosure
The authors report no conflicts of interest in this work.
References
- IslamTJosephsonLCurrent state and future applications of active targeting in malignancies using superparamagnetic iron oxide nanoparticlesCancer Biomark200959910719414927
- JordanAWestPScholzRCellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitroInt J Hyperthermia1996127057228950152
- JordanAScholzRWustPEffects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivoInt J Hyperthermia199735876059421741
- XinBJSiSFXingGWProteaseimmobilization on gamma-Fe2O3/Fe3O4 magnetic nanoparticlesfor the synthesis of oligopeptides in organic solventsChem Asian J2010561389139420446336
- MouliKCJosephTRamamKSynthesis and magnetic studies of Co-Ni-Zn ferrite nano crystalsJ Nanosci Nanotechnol2009995596559919928271
- YanSYZhangDSZhengJStudy on the therapeutic effect of Fe2O3 nanometer magnetic fluid hyperthermia on liver cancerChinese Journal of Experimental Surgery20042114431446
- TangQZhangDCongXWanMJinLUsing thermal energy produced by irradiation of Mn-Zn ferrite magnetic nanoparticles (MZF-NPs) for heat-inducible gene expressionBiomaterials2008292673267918396332
- KawataYAdachiYHagaSAnalysis of the temperature and pressure dependence of the 129Xe NMR chemical shift and signal intensity for the derivation of basic parameters of adsorption as applied to zeolite ZSM-5Anal Sci2007231397140218071225
- AbrahamSNarineSSA facile synthesis of lipid stabilized gold nanoparticles: a step towards biodegradable biosensorsJ Nanosci Nanotechnol2011117027703222103117
- SunZHZhangXGuoMStrong magnetic field effects on solid-liquid and particle-particle interactions during the processing of a conducting liquid containing non-conducting particlesJ Colloid Interface Sci201237520321222443967
- MenHFLiuHQZhangZLSynthesis, properties and application research of atrazine Fe(3)O (4)@SiO (2) magnetic molecularly imprinted polymerEnviron Sci Pollut Res Int2012192271228022246642
- ThoratNDShindeKPPawarSHBarickKCBettyCANingthoujamRSPolyvinyl alcohol: an efficient fuel for synthesis of superparamagnetic LSMO nanoparticles for biomedical applicationDalton Trans2012413060307122277953
- ISO-10993-1Biological evaluation of medical devices1992 [S]. Available from: http://iccvam.niehs.nih.gov/docs/pyrogen/regulatory/iso10993.pdfAccessed December 16, 2011
- GBPT 16886-1997Biological evaluation of medical devices1997 [S]. Available from: http://www.cn-standard.net/ebzdetail/6C8/F98AE78D.shtml?LangType=e&UrlQianZhui=/&keyword=GB/T16886-1-1997Accessed December 16, 2011
- RiggioCCalatayudMPHoskinsCPoly-l-lysine-coated magnetic nanoparticles as intracellular actuators for neural guidanceInt J Nanomedicine201273155316622811603
- XuLChenYHeJWMagnetic resonance imaging and spectroscopy of the bone marrow in children with common hematological diseasesZhonghua Yi Xue Za Zhi201292587591 Chinese22800944
- ZhouCRBiomateriallogyBeijing, ChinaMedicine Technology Press of China2004
- MagdolenovaZLorenzoYCollinsADusinskaMCan standard genotoxicity tests be applied to nanoparticles?J Toxicol Environ Health A20127580080622788367
- TronovVAKonstantinovEMKramarenkoIIHyperthermia induced signal for apoptosis and pathways of its transduction in the cellTsitologiia20024410791088 Russian12561728
- YuenWFFungKPLeeCYHyperthermia and tumour necrosis factor-alpha induced apoptosis via mitochondrial damageLife Sci20006772573212659178
- ManygoatsKRYazzieMStearnsDMUltrastructural damage in chromium picolinate-treated cells: a TEM study. Transmission electron microscopyJ Biol Inorg Chem2002779179812203015
- YuguchiTSaitoMYokoyamaYCombined use of hyperthermia and irradiation cause antiproliferative activity and cell death to human esophageal cell carcinoma cells – mainly cell cycle examinationHum Cell200215334212126062