?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chitosan (CS) nanoparticles of thymoquinone (TQ) were prepared by the ionic gelation method and are characterized on the basis of surface morphology, in vitro or ex vivo release, dynamic light scattering, and X-ray diffractometry (XRD) studies. Dynamic laser light scattering and transmission electron microscopy confirmed the particle diameter was between 150 to 200 nm. The results showed that the particle size of the formulation was significantly affected by the drug:CS ratio, whereas it was least significantly affected by the tripolyphosphate:CS ratio. The entrapment efficiency and loading capacity of TQ was found to be 63.3% ± 3.5% and 31.23% ± 3.14%, respectively. The drug-entrapment efficiency and drug-loading capacity of the nanoparticles appears to be inversely proportional to the drug:CS ratio. An XRD study proves that TQ dispersed in the nanoparticles changes its form from crystalline to amorphous. This was further confirmed by differential scanning calorimetry thermography. The flat thermogram of the nanoparticle data indicated that TQ formed a molecular dispersion within the nanoparticles. Optimized nanoparticles were evaluated further with the help of scintigraphy imaging, which ascertains the uptake of drug into the brain. Based on maximum concentration, time-to-maximum concentration, area-under-curve over 24 hours, and elimination rate constant, intranasal TQ-loaded nanoparticles (TQ-NP1) proved more effective in brain targeting compared to intravenous and intranasal TQ solution. The high drug-targeting potential and efficiency demonstrates the significant role of the mucoadhesive properties of TQ-NP1.
Introduction
Alzheimer’s disease (AD) is the most common form of progressive neurodegenerative disorder and primarily affects the elderly population (50%–60% of the >65-year-old age group).Citation1 More than 18 million of the global population currently suffers from AD and this is expected to double by 2025. AD is a major medical and social problem for developing societies. The etiology of AD involves cognitive dysfunction, primarily memory loss,Citation2,Citation3 and in later stages it causes language deficits, depression, and behavioral problems including agitation, mood disturbances, and psychosis.Citation4,Citation5
Moreover, senile plaques, neurofibrillary tangles, oxidative stress, inflammatory processes, and neurotransmitter disturbances are common diagnostic features found in the brain of an AD patient. Many promising agents have failed in clinical trials because of their therapeutic limitations in providing symptomatic relief from cognitive deficits. An agent that not only improves cognitive functions, but also blocks neuronal loss in the brain, is urgently needed.Citation6 Alternatively, the use of medicinal plants has been another new therapeutic approach in the treatment of AD.Citation7 Most herbal drugs, especially those with a hydrophobic nature, although possessing excellent potential, fail in clinical trials because of a lack of safety or poor efficacy. Although natural products have served as sources for the majority of drugs, poor oral bioavailability has hindered their development.Citation7 In traditional and folk medicines, herbal drugs have been used extensively to enhance cognition and to alleviate other symptoms associated with AD.Citation8 This approach has been used in various practices of traditional medicine, including Ayurveda and Unani, where herbal medication is frequently prescribed. An ethnopharmacologic approach for the treatment of AD is expected to be useful in providing leads to identify plants and potential new drugs.
Recently, thymoquinone (TQ) a major active lipophilic component of Nigella Sativa (Ranunculaceae) was reported to have many pharmacological qualities such as its immunomodulation, anticancer, anti-inflammatory, antiasthmatic, and antioxidant effects. In many reports, the antioxidant and anti-inflammatory effects showed amelioration of cognitive deficits and neurodegeneration.Citation9–Citation11
Hydrophobic drugs delivered orally encounter permeability problems and hence poor bioavailability. They undergo chemical and enzymatic degradation in the gastrointestinal tract and show extensive hepatic first-pass metabolism. Similarly, various additional factors hinder drug discovery and the development of an effective delivery of different therapeutic molecules for the treatment and prevention of AD. The inability to deliver drugs effectively to the brain is due to the numerous protective natural barriers surrounding the central nervous system (CNS) such as the blood–brain barrier (BBB). These natural barriers also limit the effectiveness of various potential drug-delivery systems (DDS) based on transdermal, buccal, and intravenous routes.Citation12,Citation13
Many strategies that include development of DDS, magnetic drug targeting, and drug carrier systems such as antibodies, liposomes, or nanoparticles (NPs) have been developed to overcome these problems. Among the various DDS, polymeric NPs have attracted great attention as potential DDS for the CNS because they can efficiently deliver a wide range of therapeutic molecules to the targeting area. These NP carrier molecules also fulfill the criteria for controlled and site-specific delivery of a variety of hydrophilic, hydrophobic natural and synthetic drugs, proteins, vaccines, and biological macromolecules.Citation14,Citation15
Despite various added advantages, CNS drug-delivery strategies through the intranasal route have received relatively little attention. Intranasal drugs are transported along olfactory sensory neurons to yield significant concentrations in the cerebrospinal fluid (CSF) and olfactory bulb. Recent evidence of direct nose-to-brain transport and direct access to CSF of neuropeptides bypassing the bloodstream has been shown in human trials, despite the inherent difficulties of delivery.Citation16,Citation17
Intranasal delivery is noninvasive and essentially painless, does not require sterility regulations, and is readily administered by the patient or health professionals. DDS are designed to promote the localized therapeutic effect and minimize toxic side effects. This may be achieved by optimizing the amount and duration of the drug in the vicinity of the target cells while reducing the drug exposure to nontarget cells.Citation18
Owing to the success of the intranasal DDS, especially for brain targeting, the present investigation aimed to formulate a nanoparticulate delivery system for TQ targeted to the brain through a nasal route to avoid first-pass metabolism and its distribution to a nontargeted site with sustained action. This may lead to a decrease in peripheral side effects. The mucoadhesive polymeric NPs of TQ that we developed are expected to offer many advantages over conventional nasal dosage forms, such as increased nasal residence and possibility of drug release at a slow and constant rate to the brain.Citation17,Citation19,Citation20
As part of the development studies for TQ delivery into the brain, the objective of the present study was to simultaneously investigate the plasma pharmacokinetics and brain distribution profiles of the TQ-loaded NPs in Wistar rats after intravenous and intranasal administration and to assess whether there is a direct nose-to-brain transport pathway.
Materials and methods
CS with medium molecular weight and degree of deacetylation about 96% and sodium tripolyphosphate (TPP) was purchased from Sigma-Aldrich (St Louis, MO). Potassium dihydrogen phosphate, methanol, sodium hydroxide (NaOH), and 1-octanol were all purchased from SD Fine Chemicals, Ltd (Mumbai, India). Glacial acetic acid was purchased from IOL Chemical Ltd (Mumbai, India). Methanol high-pressure liquid chromatography (HPLC) grade, acetonitrile HPLC grade, and ammonia solution analytical reagent (AR) grade were also procured from SD Fine Chemicals, Ltd. A cellophane tube (mol wt cut-off: 12,000 Da, flat with 25 mm, diameter of 16 mm, capacity 60 mL/ft) was obtained from Sigma-Aldrich. All reagents were of analytical grade.
Preparation of chitosan NPs using ionic gelation method
CS NPs were prepared according to the ionic gelation process.Citation21,Citation22 Placebo NPs were obtained upon dropwise addition of TPP aqueous solution (2 mg/mL) to a CS solution (1.5 mg/mL) with continuous stirring at room temperature. The formation of NPs was a result of the ionic interaction between the positively charged amino groups of CS and negative groups of TPP. The ratio of CS/TPP was established according to the preliminary studies. TQ-loaded NPs were obtained according to the same procedure and the ratio of CS/TPP remained unchanged. A variable ratio of TQ was incorporated in the CS solution prior to the formation of NPs in order to investigate the effect of the initial TQ concentration on the NP characteristics and in vitro release profiles. NPs were collected by centrifugation (Remi, Delhi, India) at 15,000 rpm for 30 minutes at 4°C and the supernatant was discarded.
Loading capacity, entrapment efficiency, and process yields
Process yield (Y) of NPs with desired particle size range and polydispersity index (PDI) was calculated from the weight of dried NPs recovered (W1) and the sum of the initial dry weight of starting materials (W2) as free drug:
Similarly, the entrapment efficiency (EE) and loading capacity (LC) of NPs were determined by ultracentrifugation at 15,000 rpm, 4°C for 30 minutes. The amount of free TQ in the supernatant was measured by the reverse phase-HPLC method (water:methanol:2-propanol::50:45:5% [v:v]; 2 mL min−1) at 254 nm reported previously.Citation23 The EE and LC of NPs were calculated by the following equations and all measurements were performed in triplicate (n = 3).
Dynamic light scattering (DLS) measurements
The particle size, particle size distribution, PDI, and zeta potential were determined by Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, UK). The sample volume used for the analysis was kept constant (1 mL). The particles exhibit Brownian motion, which causes the intensity of light to scatter from particles, which is then detected as a change in intensity with suitable optics and a photo multiplier. All the data analyses were performed in automatic mode with triplicate measurement within each run. The instrument is well equipped with appropriate software for particle size analysis and PDI.
Differential scanning calorimetry (DSC) study
DSC analysis of pure TQ, pure CS, physical mixture (CS + TQ), and freeze-dried TQ-loaded CS NPs was carried out using a PerkinElmer DSC-7® (PerkinElmer, Inc., Waltham, MA) calibrated with indium. A 5 mg sample was placed onto a standard aluminum pan, crimped and heated from 20°C −350°C at a heating rate of 5°C/minute under continuous purging of nitrogen (20 mL/minute). An empty sealed pan was used as reference. All samples were run in triplicate.
X-ray diffractometry (XRD)
X-ray diffractometry was used to investigate the physical form (crystalline or amorphous) of drug dispersion within the matrix of the CS NPs. The XRD experiments were performed over the range 2θ from 5 to 50°C, using an XRD (PANalytical X’pert PRO; PANalytical, Almelo, The Netherlands), with Cu Kα radiation at a scanning speed of 5°/minute.
Transmission electron microscopy (TEM)
The surface morphology of the prepared NPs was determined using TEM. A drop of nanosuspension was placed on a paraffin sheet and a copper grid was placed on the sample and left for 1 minute to allow the NPs to adhere. The grid was placed on a drop of phosphotungstate for more than 5 seconds. The remaining solution was removed by absorbing the liquid with a piece of filter paper and samples were air dried. The samples were further examined by TEM (Morgagni 268D; Fei Company, Hillsboro, OR).
Scanning electron microscopy (SEM)
The surface texture of the optimized NPs was further confirmed by SEM (Zeiss EVO40; Carl Zeiss, Cambridge, UK). Samples were spread over a double-sided conductive tape fixed on to a metallic stud and coated under vacuum with gold in a Blazers SCD020 sputter coater unit (BAL-TEC GmbH, Witten, Germany) in an argon atmosphere at 50 mA for 100 seconds.
In vitro release modeling
The in vitro release profile of the TQ suspension and TQ-loaded CS NPs was performed using dialysis sacs (MWCO 12,000 g/mole; Sigma-Aldrich). Equivalent volume of drug-loaded CS-TQ NPs (TQ was 4.275 mg) was filled in cellulose membrane dialysis sacs and study was performed using dissolution apparatus 2 (Veego, Mumbai, India) containing 500 mL of PBS (pH 6.5) at 37°C ± 0.5°C. At predetermined time intervals, aliquots were withdrawn from the released medium and replaced with the phosphate buffer. The samples were analyzed in triplicate using reported HPLC. The data obtained from in vitro drug release were fitted to various release models (zero order, first order, Higuchi, and Korsemeyer Peppas) to understand the possible mechanism of drug release from the NPs.Citation24
Ex vivo permeation studies on nasal mucosa
Fresh nasal tissues were carefully removed from the nasal cavities of goats obtained from the local slaughterhouse. Tissue samples were fixed in cells displaying a permeation area of 0.785 cm2 (Logan Instrument Corporation, Piscataway, NJ). Twenty milliliters of phosphate-buffered saline (PBS; pH 7.4) maintained at 37°C was added to the receptor chamber. After a preincubation time of 20 minutes, pure drug solution and formulation equivalent to 5 mg of TQ was placed in the donor chamber (2 mL) in each case. At predetermined time points, 0.5 mL samples were withdrawn from the receptor chamber, replacing the sampled volume with PBS over a period of 24 hours. The withdrawn samples were passed through a membrane filter before analysis. Blank samples (without TQ) were run simultaneously throughout the experiment to check for any interference. The amount of permeated drug was determined using reverse-phase HPLC.
Radiolabeling protocol
The TQ solution and TQ-NP1 were radiolabeled using technetium (99mTc) by a direct labeling method.Citation17,Citation25 One milliliter of the TQ solution and TQ-NP1 (5 mg/mL) was taken separately and stannous chloride dihydrate solution (100 mg in 100 mL of 0.10 N HCl) was added. The pH was adjusted to 7.0 ± 0.50 using 50 mM sodium bicarbonate solution. To the resultant mixture, 1 mL of sterile 99mTc-pertechnetate (75 to 400 MBq) was added gradually over a period of 1 minute with continuous mixing. The resultant mixture was incubated (30°C ± 0.5°C) for 30 minutes in an inert environment. The final volume was made up using isotonic (0.90% w/v) saline solution. The radiochemical purity of 99mTc-TQ solution (99mTc-labeled TQ) and 99mTc-TQ-NP1 (99mTc-labeled TQ-loaded CS NP) were determined by instant thin-layer chromatography (ITLC; Gelman Sciences, Inc, Ann Arbor, MI) using a previously optimized mobile phase consisting of acetone (100% v/v). The effect of incubation time, pH, and stannous chloride concentration on radiolabeling efficiency were studied to achieve optimum reaction conditions. The optimized radiolabeled formulations were assessed for in vitro stability in normal saline solution, rat plasma and in rat brain homogenate. Finally, the optimized stable radiolabeled formulations were used to study biodistribution in rats.
Biodistribution and pharmacokinetics
All experiments conducted on animals were approved by the animal ethical committee of Jamia Hamdard, New Delhi (Proposal no 635/173/CPCSEA for the purpose of control and supervision on animals and experiments). Male Wistar rats aged 5–6 months, weighing between 200–250 g (average weight 200 g), were selected for the study. Three rats were used for each formulation per time point (0.25, 0.5, 2, 4, 6, 24 hours). Prior to nasal administration of the formulations, the rats were anesthetized using chloral hydrate (400 mg/kg, intraperitoneally) and the formulations were instilled into the nostrils with the help of a micropipette (100 μL) attached to a low-density polyethylene tube (0.1 mm internal diameter). The radiolabeled complex of 99mTc-TQ (100 mCi/100 mL) containing 500 μg of TQ/25 μL (equivalent to 0.5 mg/200 g body weight) was administered intranasally in each nostril.Citation10,Citation11,Citation26 The rats were held by their backs in a slanted position during the intranasal administration of the formulations. The rats were sacrificed at predetermined time intervals (0.25, 0.5, 2, 4, 6, 24 hours) and blood was collected by cardiac puncture. Subsequently, different tissues/organs including the brain were dissected, washed twice using normal saline solution, made free from adhering tissue/fluid, and weighed. The radioactivity present in each tissue/organ was measured using a shielded well-type gamma scintillation counter. The radiopharmaceutical uptake per gram in each tissue/organ was calculated as a fraction of the administered dose.Citation17
The pharmacokinetic parameters were derived from using Kinetica (version 4.10; Innaphase, Philadelphia, PA) and recorded in . To evaluate the nose-to-brain targeting of different formulations, two indices, ie, brain-targeting efficiency (% DTE) and brain drug-targeting potential (% DTP) were adopted as mentioned below:Citation25,Citation27
Table 1 Pharmacokinetic profile of different formulations
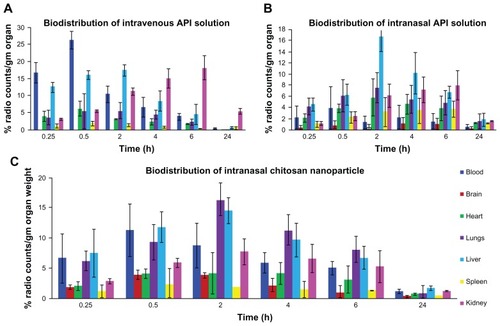
Figure 1 Biodistribution study of (A) TQ solution (intravenous), (B) TQ solution (intranasal), and (C) chitosan nanoparticles encapsulating TQ (intranasal).
In order to more clearly define nose-to-brain direct transport, the brain drug direct transport percentage (DTP%), was derived from the equation given below:
where Bx = (Biv/Piv) × Pin, Bx is the brain area-under-the-curve (AUC) fraction contributed by systemic circulation through the BBB following an intranasal administration; Biv is the AUC0–24 (brain) following intravenous administration; Piv is the AUC0–24 (blood) following intravenous administration; Bin is the AUC0–24 (brain) following intranasal administration; and Pin is the AUC0–24 (blood) following intranasal administration.
Statistical analysis
All data are reported as mean ± standard error of mean and the differences between the groups were tested using Student’s t-test at a significance level of P < 0.05. More than two groups were compared using analysis of variance and the difference of P < 0.05 was considered significant.
Results and discussion
Formulation selection
Based on previously published literature, different trial compositions were performed to obtain an optimized formulation primarily on the basis of clarity and system aggregation () and later with improved performance of minimum particle size and PDI, high process yield, EE, and LC (). High EE may be the consequence of an ionic interaction between negatively charged TQ with positively charged CS (). Among various trials, the selected formulation S-3C (103.7 nm, 0.404, and 54.43%) had a smaller minimum particle, optimum PDI, and higher process yields than S-3A (368 nm, 0.215, and 44.86%) and S-3B (227 nm, 0.382, and 47.71%), respectively. Although S-4C, S-5C, and S-6B had a greater process yield than S-3C, the particle size was above 200 nm. Some formulations have low process yield whereas some have a higher yield due to low and high concentrations of CS, respectively. Therefore, S-3C was chosen as the optimized formulation of TQ-NPs. The above findings conclude that a unique ratio of CS:TPP (1.25–1.87) showed a particle size below 200 nm with a percentage yield greater than 25% whereas CS:TPP ratios exceeding the above limit showed a larger variability in particle size and percentage yield (). These findings are also in agreement with earlier reports that the ratio between CS and TPP is an important factor controlling the size distribution and process yield of NPs.Citation28 Although the minimum particle size obtained was 84.03 nm, containing 0.5 mg/mL CS and 1.5 mg/mL TPP (), the low percent yield (18.78%) limits its applicability (). The mean particle size of optimized placebo NPs containing 1.5 mg/mL CS and 2 mg/mL TPP was found to be 103.6 nm. A comparative evaluation of particle size, PDI, and percentage yields of different preliminary formulations is listed in . On the basis of the above findings, S-3C was considered an optimized formulation.
Table 2 Results showing effects of different concentration of CS and TPP
Table 3 Particle size and particle size distribution of placebo formulations
Figure 3 Dynamic light scattering technique for determining the particle size distribution of placebo nanoparticles (A and B) and TQ-encapsulated nanoparticles (C), and zeta potential of TQ-encapsulated nanoparticles (D).
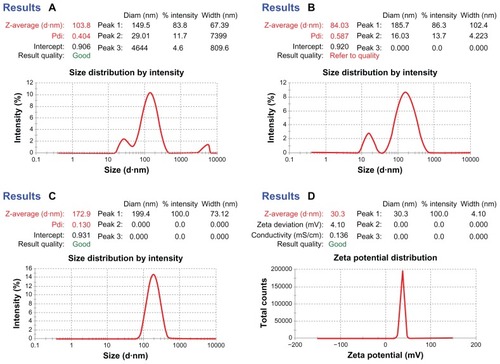
The effects of drug concentration on particle size, PDI, EE, and LC of optimized TQ-NPs are summarized in and . It was observed that, upon increasing the drug: polymer ratio from 1 to 3, the average size of TQ-loaded NPs increased from 172.4 ± 7.4 (TQ-NP1) to 281.3 ± 4.7 nm (TQ-NP3). Increasing drug proportions in solution caused a reduction of CS and TPP interaction, which leads to an increased NP size. The increase in drug concentration also slightly increases the PDI value (0.24 [TQ-NP3] > 0.22 [TQ-NP2] > 0.130 [TQ-NP1]) with a decrease in percentage yield (42.12 [TQ-NP3] < 47.82 [TQ-NP2] < 53.42 [TQ-NP1]) (). The surface charge of optimized TQ-loaded NPs was found to be positive, indicating the partial stabilization of cationic charge of CS by anionic charged TPP.Citation22 The positive surface charge will also support better interactions with the negatively charged biomembrane.Citation29 This will be discussed in detail in the section on surface morphology.
Table 4 Particle size and particle size distribution of drug-loaded formulation
Table 5 Effect of TQ concentration on EE and LC
LC and EE of TQ-loaded chitosan NPs
The EE and LC increased from 28.1% to 63.3% and 19.23% to 31.23%, respectively, depending upon the drug:polymer ratio (). The above data clearly shows that the 1:1 drug:polymer ratio shows better entrapment and LC. With the increase in initial CS concentration during the entrapment process, more protonized CS (–NH3+) were available in the system, as shown by increased surface charge, which leads to a stronger electrostatic attraction between TQ (negative charge) and CS (positive charge) (). This high polymer concentration leads to an increase in binding sites for TPP with high EE. When the drug:polymer ratio increased, the ionic interaction between CS and TPP was hindered by drug molecules and hence led to lower entrapment and larger particle size ( and ). This finding seems to be in agreement with Mohanraj and Chen: the higher the drug concentration, the lower the entrapment and LC.Citation30
Dynamic light scattering (DLS) measurements
show the particle size distribution of placebo-optimized CS NPs, whereas shows the TQ-loaded NPs. The size of the CS NPs could be influenced by factors such as TPP:CS ratio and concentration of CS. These trends show that the NP size was directly dependent on concentration and drug loading. The droplet size of the CS-based NPs was the smallest when the TQ:CS ratio was 1:1, whereas the droplet size was maximized by increasing the ratio to 3:1. Their droplet sizes at this concentration ratio were 172 and 281 nm, respectively (). The NPs showed a positive surface electric charge (measured by zeta potential), which varied depending on the proportion of CS and TQ (). Because of enough protonated amine groups remaining, the process of the ionic crosslinking occurs more easily for CS with a high degree of deacetylation. The data of mean particle size and zeta potential are listed in . The surface charge is the critical parameter on the stability of the nanosuspension and bioadhesion of particulate systems on biological surfaces. CS NPs are all positively charged, which is a typical characteristic of CS:TPP particles. This quality can be explained by the particle formation mechanism since the cationic charged amine groups are neutralized by their interaction with the anionic charge of TPP molecules. The residual amino groups are responsible for the positive potential.
A higher zeta potential in a certain range (24–30 mV) signifies the stability of CS NPs ( and ). It also signifies a hindrance imposed by long-chain amino groups and an anion adsorption to keep the high value of the electrical double layer thickness, thus preventing the aggregation. The zeta potentials in three batches of TQ-NPs are over +20 mV. The positive surface charge of NPs will improve the interaction, especially with the mucosal surfaces, which carry negative surface charge. This way, the biologically active molecule will act favorably on the target tissues.
Surface morphology (TEM and SEM study)
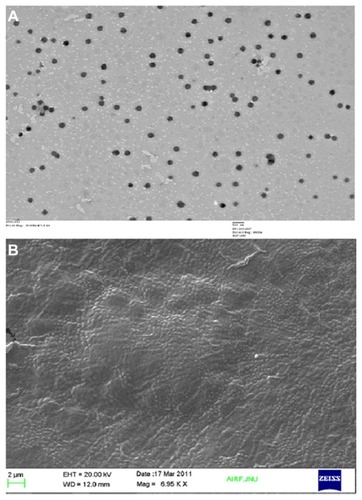
The shape and surface texture of the NPs could be detected using a number of sophisticated techniques such as TEM or SEM, respectively. NPs showed a round and smooth surface in TEM. The morphology of TQ-loaded CS-NPs as prepared is shown in . NP size was determined by TEM, which proved its sphericity. The particle size ranged between 150 and 200 nm (). The SEM of NPs proved their smooth surface texture (). Electron microscopy and DLS studies () further corroborated the NP size.
Differential scanning calorimetry (DSC)
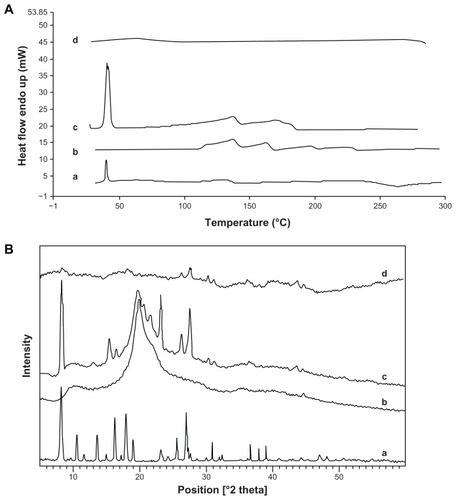
The DSC thermograms of TQ, physical mixture of TQ-CS, and CS- and TQ-encapsulated TQ-NPs, respectively, are shown in . An experimental study showed a sharp and well-defined endothermic peak at ~46.59°C equivalent to the melting point of TQ followed by an endothermic broad band at 146.7°C corresponding to the decomposition process, and ending at 160°C. Similarly, the physical mixture of TQ-CS showed the characteristic peaks of CS and TQ, which was absent in TQ-encapsulated CS NPs. Drug-loaded NPs showed a very small exothermic peak, whereas the polymer showed a predominant endothermic peak at 132.41°C, the drug had an endothermic peak at 126.053°C, and the physical mixture showed both drug and polymer peaks. No peak of TQ and CS was visible in the TQ-loaded NPs. This finding suggests that TQ is molecularly dispersed within the CS NPs showing the amorphous nature that further authenticates the entrapment of TQ.Citation28,Citation31
X-ray diffractometry (XRD)
In order to identify the physical state of the drugs incorporated in CS NPs, XRD was performed and the patterns of TQ, CS, and the physical admixture of TQ-CS as well as TQ encapsulated CS NPs are shown in . Powder diffraction data were collected at room temperature in the 2θ range 5.5° to 57.058° (d = 11.451–1.495 Å). represents the characteristic diffraction pattern of TQ at 6.7 Å. In the XRD patterns of the TQ-CS NPs, the characteristic peaks at 2θ = 12.09°, 18.65°, and 24.26° can be attributed to the crystalline structure of CS which is missing in TQ-encapsulated NPs (). TQ probably formed a molecular dispersion or an amorphous nanodispersion within the CS matrix of the NPs.Citation32
In vitro release modeling
The release profile of TQ from optimized CS NPs showed a sustained release pattern. It was observed that the released TQ primarily showed a rapid initial release (burst release) followed by a characteristic slow-release pattern. The initial rapid release of drug may be due to release of TQ from the NP surface, while at a later stage, TQ may be constantly released from the core of NPs as a consequence of CS hydration and swelling.Citation33 The release pattern was further confirmed by applying the release kinetic to ascertain the release order (). Among various models tried, the coefficient of correlation (R2) for the Higuchi model was near to unity (ie, 0.981), therefore the best-fit model for TQ-NPs was the Higuchi model. When the release data were analyzed using the Korsmeyer–Peppas equation, the value of the release exponent n was between 0.43 and 0.85 (), which is an indication of both diffusion-controlled and swelling-controlled drug release, ie, anomalous transport.Citation33
Table 6 Coefficient of correlation for optimized CS NPs
Ex vivo permeation studies on nasal mucosa
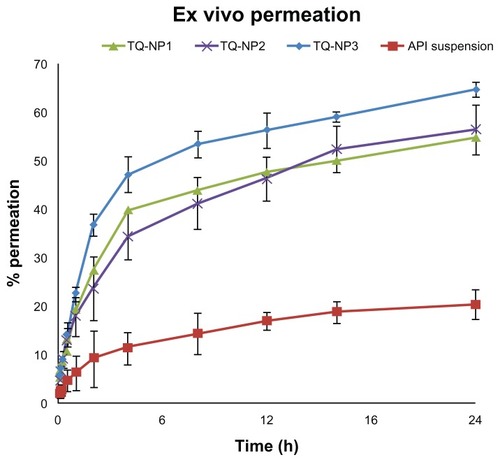
The TQ-loaded CS NPs showed more permeation compared to the pure drug solution (). The significant difference in permeation profile (P > 0.001) of the optimized formulation TQ-NP1 might be due to the permeation-enhancing activity of CS. The maximum permeation in 24 hours was found to be >60% whereas API was only 20.39%. The increase in permeation of TQ could be attributed to an interaction of a positively charged amino group on the carbon two position of CS with negatively charged sites on the cell membranes. Similarly, one possible mechanism may be related to the tight junction permeability of the mucosal epithelial cells.Citation34 A justification for the least permeability of pure drug solution might be its hydrophobicity and possessing negative surface charge. Similarly, Richter and Keipert suggested that the drug should be lipophilic for better permeation through nasal mucosa.Citation35 The smaller size (<200 nm) and surface hydrophobicity of TQ-loaded CS NPs may support better partitioning through the biological membrane. Finally, on the basis of smaller particle size, higher percentage yield, better EE as well as LC, and relatively enhanced permeation profile, TQ-NP1 was selected as the final optimized formulation.
Radiolabeling stability study
TQ-NP1 effectively radiolabeled with 99mTc was optimized for maximum labeling efficiency and stability in normal saline, rat plasma, and in rat brain homogenate for 24 hours. The optimal SnCl2·2H2O concentration was found to be 100 mg/mL at pH 7.0 with an incubation time of 30 minutes. The radiolabeling stability achieved was 97.39 ± 4.26, 95.07 ± 4.28, and 96.12 ± 3.52 in normal saline, rat plasma, and rat brain homogenate, respectively (). The results suggested a high bonding strength and stability of 99mTc-TQ-NP1. Therefore, 99mTc-TQ-NP1 were found suitable for biodistribution studies in rats. The results obtained are also in agreement with the earlier findings.Citation17,Citation25,Citation27
Table 7 In vitro radiolabeling stability in normal saline, rat plasma, and in rat brain homogenate
Biodistribution and pharmacokinetic study
The biodistribution pattern and different pharmacokinetic properties of intranasal administered NPs was evaluated using scintigraphic imaging. Scintigraphic imaging was performed using a gamma camera and the activity counts (TQ-TC99m) in different organs such as brain, liver, kidney, spleen, heart, and lungs were performed with a gamma counter. shows the concentration of 99mTC in different organs after the administration of intravenous 99mTC-TQ solutions, intranasal 99mTC-TQ solution, and intranasal 99mTC-TQ-NP. The present investigation observed that the tissue concentration in the form of counts (99mTC) was higher in the brain with the intranasal administration of TQ-NP in comparison to the TQ solution after intravenous and intranasal administration. The above finding might be due to existence of direct nose-to-brain transport bypassing the BBB.Citation36,Citation37 Similar to systemic organs, the concentration of TQ-NP was higher in the brain compared to the TQ intranasal solution. This finding might be the combined upshot of the nanometric size range and mucoadhesive nature of the formulation. The special mucoadhesive property of CS will decrease mucociliary clearance, whereas the conventional intranasal formulation rapidly exits the nasal tract. The concentrations of 99mTC-loaded TQ-NPs in the liver when administered intravenously was higher compared to intranasal 99mTC-loaded TQ-NPs and 99mTC solution because of the presence of the reticuloendothelial system (). A similar pattern of 99mTC-loaded TQ-NP distribution was also obtained in the lungs and in kidney.Citation17,Citation25,Citation27 The higher concentrations of 99mTC achieved in the highly perfused organs, such as liver, lungs, and kidney are probably due to the combined activity of the circulating blood passing through the organs as well as particle uptake by reticuloendothelial system cells. The above results further support the earlier finding by Wang et al after intranasal administration of CS NPs.Citation18,Citation38
The highest bioavailability in the brain might be the consequences of drug uptake from the nasal mucosa via three proposed pathways (). One is the systemic pathway by which some of the drug is absorbed into the systemic circulation and subsequently reaches the brain by crossing the BBB. The others are the olfactory pathway and the trigeminal neural pathway by which partly the drug travels directly from the nasal cavity to the CSF and brain tissue.Citation39 We can conclude that the amount of drug reaching the brain tissue after intranasal administration is attributed to these three pathways.Citation20,Citation40–Citation42
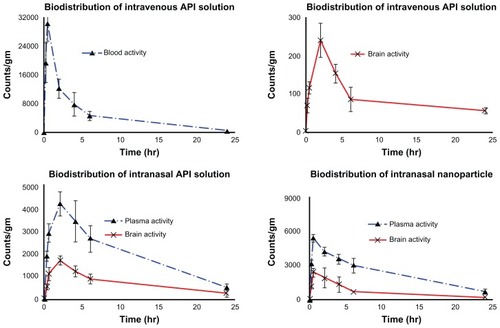
The pharmacokinetic parameters ( and ) were also calculated from a time-to-99mTC-activity graph (). Intranasal administration of TQ-NP1 showed lower Tmax for brain (0.5 hours) compared to blood (2 hours). This may be attributed to preferential nose-to-brain transport following intranasal administration, which correlates with reports in the literature.Citation16,Citation39–Citation42 The brain:blood ratio of the drug was found to be higher for the TQ-NP1 formulation over the intranasal TQ solution ( and ). Similarly, the brain:blood ratio of the drug were higher for the intranasal TQ solution compared to the intravenous TQ solution. This finding further proved the significant role of the olfactory lobe in direct nose-to-brain transport. The concentrations of the drug in the brain following intranasal administration of TQ solution and TQ-NP1 were significantly higher (P > 0.005) at all sampling time points (24 hours) compared to the intravenous TQ solution. Moreover, following intranasal TQ-NP1, the drug concentrations in the brain were sustained for 2–3 hours, which was lacking in TQ solution (intranasal and intravenous). The substantially higher uptake in the brain after intranasal administration suggests a larger extent of selective transport of TQ-NP1 from nose-to brain. The formulations showed a significant difference in Tmax (0.5 and 2 hours), Cmax (242.88, 1717.74, and 2417.17 counts) and Kel (0.101, 0.086, and 0.0696 counts/hour) for intravenous TQ solution, intranasal TQ solution, and intranasal TQ-NP1, respectively. Significantly lower Cmax (P > 0.01) and AUC (P > 0.005) for the intranasal TQ solution may be due to the mucociliary clearance under normal circumstances, which rapidly clears the instilled formulation. On the other hand, TQ-NP1 which shares an intrinsic mucoadhesive property showed a significant improvement in Cmax and AUC. This demonstrates the value of the mucoadhesive agent in prolonging the contact time of the formulation with the nasal mucosa. The significantly higher AUC and Cmax for TQ-NP1 compared to the TQ solution is attributed to the importance of nanoparticulate carriers.
Table 8 Nose-to-brain drug-targeting parameters of different formulations
Figure 7 Concentration–time profile of thymoquinone (TQ) in plasma and brain after intravenous administration of TQ solution and intranasal administration of TQ solution and TQ nanoparticles, respectively (anti-clockwise).
Abbreviation: API, active pharmaceutical ingredient.
Similarly, different nose-to-brain targeting parameters () were calculated with the help of pharmacokinetics parameters as shown in . DTP (%) represents the percentage of drug directly transported to the brain via the olfactory pathway and the trigeminal neural pathway. The TQ-NP1 showed significantly high (P > 0.001) DTE (%) and DTP (%) values among all the other formulations. The almost 15-fold higher DTE (%) and twofold higher DTP (%) for TQ-NP1 compared to the intranasal TQ solution shows the benefit of the mucoadhesive formulation (). The higher DTE (%) and DTP (%) suggest that TQ-NP1 has better brain targeting efficiency mainly because of substantial and direct nose-to-brain transport. The possible mechanism may be that the cationic TQ-CS systems showed a higher targeting efficiency in brain, which is consistent with previous studies.Citation43,Citation44 These findings are in congruence with the observations reported by Zhang et al, who also proved the potential role of nanocarriers in nose-to-brain targeting.Citation45
Conclusion
In the present investigation, TQ-encapsulated CS NPs were prepared successfully. A physical evaluation and electron microscope screening supported the suitability for intranasal administration. The scintigraphic study in rats demonstrated that intranasal administration delivers TQ to the brain rapidly and more effectively than previous methods. The accumulation of TQ-NP1 formulation within interstitial spaces and transport of the drug to the brain may be due the nanometric size range and the stretching of tight junctions within the nasal mucosa. The finding also supported the formulation’s CSF-penetrating potential. The studies suggest intranasal delivery of TQ to be a promising approach for brain targeting as well as in reducing the systemic exposure. However, benefit-to-risk ratio and clinical intricacies need to be established scientifically for its suitability in clinical practice in the management of Alzheimer symptoms.
Acknowledgments
The authors are grateful to University Grant Commission (UGC), Government of India for providing fellowship to Sanjar Alam. Authors are also thankful for the support provided by Advanced Instrumentation Research Facility (AIRF), Jawaharlal Nehru University, and Institute of Nuclear Medicine and Allied Sciences (INMAS), New Delhi in this research activity.
Disclosure
The authors report no conflicts of interest in or financial benefit from this work.
References
- FrancisPTPalmerAMSnapeMWilcockGKThe cholinergic hypothesis of Alzheimer’s disease: a review of progressJ Neurol Neurosurg Psychiatry19996613714710071091
- DesgrangesBBaronJCde la SayetteVThe neural substrates of memory systems impairment in Alzheimer’s disease: A PET study of resting brain glucose utilizationBrain19981216116319577389
- ForstlHHentschelFSattelHAge-associated memory impairment and early Alzheimer’s diseaseDrug Res1995451394397
- KumarVDuraiNBJobeTPharmacologic management of Alzheimer’s diseaseClin Geriatr Med19981411291469456339
- McGeerPLSchulzerMMcGeerEGArthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studiesNeurology1996474254328757015
- IshratTHodaMNKhanMBAmelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT)Eur Neuropsychopharmacol20091963664719329286
- AkhondzadehSAbbasiSHHerbal medicine in the treatment of Alzheimer’s diseaseAm J Alzheimers Dis Other Demen200621211311816634467
- HowesaMRHoughtonPJPlants used in Chinese and Indian traditional medicine for improvement of memory and cognitive functionPharmacol Biochem Behav20037551352712895669
- Al-MajedAAAl-OmarFANagiMNNeuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampusEur J Pharmacol2006543404716828080
- Al-GhamdiMSThe anti-inflammatory, analgesic and antipyretic activity of Nigella sativaJ Ethnopharmacol200176454811378280
- MansourMANagiMNEl-KhatibASAl-BekairiAMEffects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of actionCell Biochem Funct20022014315111979510
- LockmanPRMumperRJKhanMAAllenDDNanoparticle technology for drug delivery across blood–brain barrierDrug Dev Ind Pharm20022811211858519
- WittKADavisTPCNS drug delivery: Opioid peptides and the blood-brain barrierAAPS J2006817688
- GabrielASNanotechnology approaches for drug and small molecule delivery across the blood brain barrierSurg Neurol20076711311617254859
- HansMLLowmanAMBiodegradable nanoparticles for drug delivery and targetingCurr Opin Solid State Mater Sci200264319327
- IllumLTransport of drugs from the nasal cavity to central nervous systemEur J Pharm Sci20001111810913748
- VyasTKShahiwalaAMaratheSMisraAIntranasal drug delivery for brain targetingCurr Drug Del20052164175
- WangXChiNTangXPreparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targetingEuro J Pharm Biopharm200870735740
- UgwokeMIVerbekeNKingetRThe biopharmaceutical aspects of nasal mucoadhesive drug deliveryJ Pharm Pharmacol20015332111206189
- FazilMMdSHaqueSDevelopment and evaluation of rivastigmine loaded chitosan nanoparticles for brain targetingEur J Pharm Sci201247161522561106
- CalvoPRemunan-LopezCVila-JataJLAlonsoMJChitosan and chitosan: ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccinesPharm Res199714143114369358557
- AktasYAndrieuxKAlonsoMJPreparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitorInt J Pharm200529837838315893439
- GhoshehOAHoudiAACrooksPAHigh performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.)J Pharm Biomed Anal19991975776210698539
- GeHHuYJiangXPreparation, characterization, and drug release behaviors of drug nimodipine-loaded poly(e-caprolactone)-poly(ethylene oxide)-poly(e-caprolactone) amphiphilic triblock copolymer micellesJ Pharm Sci2002911463147312115846
- BabbarAKSinghAKGoelHCChauhanUPSSharmaRKEvaluation of 99mTc labelled Photosan-3, a haematoporphyrin derivative, as a potential radiopharmaceutical for tumor scintigraphyNucl Med Biol20002741942610938479
- BuritsMBucarFAntioxidant activity of Nigella sativa essential oilPhytother Res200014532332810925395
- KumarMMisraABabbarAKMishraAKMishraPPathakKIntranasal nanoemulsion based brain targeting drug delivery system of risperidoneInt J Pharm200835828529118455333
- PapadimitriouSBikiarisDAvgoustakisKKaravasEGeorgarakisMChitosan nanoparticles loaded with dorzolamide and pramipexoleCarbohydr Polym2008734454
- QuellecPGrefRPerrinLProtein entrapment within polyethylene glycol-coated nanospheres. I. Physicochemical characterizationJ Biomed Mater Res19984245549740006
- MohanrajVJChenYNanoparticles – A reviewTrop J Pharm Res20065561573
- JoshiSAChavhanSSSawantKKRivastigmine-loaded PLGA and PBCA nanoparticles: Preparation, optimization, characterization, in vitro and pharmacodynamic studiesEur J Pharm Biopharm20107618919920637869
- PagolaSBenaventeARaschiARomanoEMolinaMAAStephensPWCrystal structure determination of thymoquinone by high-resolution X-ray powder diffractionAAPS PharmSciTech200352e2815760086
- RitgerPLPeppasNAA simple equation for description of solute release II. Fickian and anomalous release from swellable devicesJ Control Release198753744
- VllasaliuDExposito-HarrisRHerasATight junction modulation by chitosan nanoparticles: comparison with chitosan solutionInt J Pharm20104001–218319320727955
- RichterTKeipertSIn vitro permeation studies comparing bovine nasal mucosa, porcine cornea and artificial membrane: androstenedione in microemulsions and their componentsEur J Pharm Biopharm20045813714315207547
- TosiGCostantinoLRivasiFTargeting the central nervous system: In vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123J Control Release20071221917651855
- VergoniAVTosiGTacchiRVandelliMABertoliniACostantinoLNanoparticles as drug delivery agents specific for CNS: in vivo biodistributionNanomed Nanotech Biol Med20095369377
- WangXHeHLengWTangXEvaluation of brain-targeting for the nasal delivery of estradiol by the microdialysis methodInt J Pharm2006317404616631329
- ThorneRGPronkGJPadmanabhanVFreyWH2ndDelivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administrationNeuroscience200412748149615262337
- Bhavna SharmaVAliMBabootaSAliJPreparation and characterization of chitosan nanoparticles for nose to brain delivery of a cholinesterase inhibitorInd J Pharm Sci2007695712723
- Al-GhananeemAMSaeedHFlorenceRYokelRAMalkawiAHIntranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by AIDS virusesJ Drug Target201018538138820001275
- SoniSKumarBAKumarSRBanerjeeTMaitraAPharmacoscintigraphic evaluation of polysorbate 80-coated chitosan nanoparticles for brain targetingAm J Drug Del200533205212
- HuoMRZhouJPWeiYLuLPreparation of paclitaxel-loaded chitosan polymeric micelles and influence of surface charges on their tissue biodistribution in miceActa Pharm Sin200641867872
- MahatoRIKawabataKNomuraTTakakuraYHashidaMPhysicochemical and pharmacokinetic characteristics of plasmid DNA/cationic liposome complexesJ Pharm Sci199584126712718587040
- ZhangQJiangXXiangWLuWSuLShiZPreparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation of the targeting efficiency to brainInt J Pharm2004275859615081140