Abstract
Carbon nanotubes (CNTs) are emerging as novel nanomaterials for various biomedical applications. CNTs can be used to deliver a variety of therapeutic agents, including biomolecules, to the target disease sites. In addition, their unparalleled optical and electrical properties make them excellent candidates for bioimaging and other biomedical applications. However, the high cytotoxicity of CNTs limits their use in humans and many biological systems. The biocompatibility and low cytotoxicity of CNTs are attributed to size, dose, duration, testing systems, and surface functionalization. The functionalization of CNTs improves their solubility and biocompatibility and alters their cellular interaction pathways, resulting in much-reduced cytotoxic effects. Functionalized CNTs are promising novel materials for a variety of biomedical applications. These potential applications are particularly enhanced by their ability to penetrate biological membranes with relatively low cytotoxicity. This review is directed towards the overview of CNTs and their functionalization for biomedical applications with minimal cytotoxicity.
Introduction
The greatest advantage of nanotechnology lies in its potential to create novel structures with enhanced abilities to translocate through cell membranes, and increased solubilization, stability, and bioavailability of biomolecules, thereby enhancing their delivery efficiency. Nanotechnology offers intriguing opportunities for various applications in biomedical fields, including bioimagingCitation1 and targeted delivery of biomacromolecules into cells.Citation2 Many strategies have been proposed to functionalize carbon nanotubes (CNTs) with increased solubility for effective use in biomedical applications.Citation3 CNTs, hollow cylinders composed of rolled sheets of graphene built from a hexagonal arrangement of sp2-hybridized carbon atoms in nanoscale dimensions, were first introduced by Iijima.Citation4 CNTs have unique structures and extravagant mechanical, thermal, magnetic, optical, electrical, surface, and chemical properties, and the combination of these characteristics bestows them with extensive biomedical applications.Citation5,Citation6 CNTs are relatively flexible and interact with the cell membranes and penetrate various biological tissuesCitation7–Citation9 due to a “snaking effect,”Citation10 hence both the pharmacologicalCitation11 and toxicologicalCitation12 profiles of CNTs have gathered much attention recently.Citation13,Citation14 In this review, we have focused on functionalized CNTs (fCNTs) with low/ no cytotoxicity using functionalization processes, which is the fundamental prerequisite for applications of CNTs in biomedicine. The review also focuses on in vitro and in vivo toxic effects of various fCNTs as compared to CNTs. Advantages and applications of CNT functionalization methods in reducing the cytotoxicity followed by their in vivo applications as biomaterials in tissue, cells, bone, and blood are also discussed.
Toxicity of carbon nanotubes
The physicochemical properties of CNTs make them unique and capable of changing the biological or toxicological behavior of living organisms or the environment. CNTs have a highly hydrophobic surface and a nonbiodegradable nature that contributes to their reduced biocompatibility, limiting their biomedical applications, with growing concerns about their chronic toxicity.Citation15 With several years of research, CNTs have been shown to have adverse effects. The toxicity of CNTs is attributed to their physicochemical properties, including structure, length and aspect ratio, surface area, degree of aggregation, extent of oxidation, surface topology, bound functional group(s), manufacturing method, concentration, and dose offered to cells or organisms.Citation16–Citation19 CNTs can elicit toxicity through membrane damage, DNA damage, oxidative stress, changes in mitochondrial activities, altered intracellular metabolic routes, and protein synthesis.Citation10,Citation20–Citation24 The most common mechanisms of CNT cytotoxicity also encompass apoptosis and necrosis.Citation25–Citation27 However, CNT cytotoxicity is significantly controversial, with a large number of studies reporting altered toxic responses to CNTs both in vitro and in vivo.Citation10,Citation15,Citation28,Citation29
In vitro toxicity studies
One of the first studies investigating the toxicity of pristine single-walled CNTs (SWCNTs) in human epidermal keratinocytes revealed that exposure to SWCNTs could elevate oxidative stress and reduce cell viability.Citation30 Even purified CNTs in pristine form showed cellular toxicity.Citation26,Citation27 The cytotoxicity of CNTs has also been related to their structure.Citation15 It is reported that multiwalled CNTs (MWCNTs) with larger diameters are more cytotoxic compared to ones with lesser diameters.Citation31 At the same time, the cytotoxicity of unmodified SWCNTs was found to be dose- and time-dependent. Citation27,Citation32,Citation33 Surface modification led to reduced cell death, with non–surface-modified CNTs being more cytotoxic compared to surface-modified CNTs at concentrations of 0.1 mg/mL and 5 × 10−5 μg/mL, respectively.Citation17 Similarly, enhanced interaction times resulted in higher amounts of apoptosis in both fCNTs and CNTs, though fCNTs were less apoptotic.Citation34 Another study involving HeLa cells treated with increased doses of functionalized SWCNTs and MWCNTs showed a 50% reduction in cell number.Citation35 In a similar study, human epidermal keratinocytes when treated with 0.00000005–0.05 mg/mL of 6-aminohexanoic acid–derivatized (AHA)-SWCNTs resulted in diminished cell viability and escalation in the expression of cytokines, demonstrating that greater concentrations of AHA-SWCNTs were cytotoxic.Citation25 Similarly, macrophages showed a higher half maximal effective concentration for MWCNTsCitation36 compared to human lung epithelial cells (A549).Citation37 It has also been shown that the functional group significantly affects cellular toxicity.Citation38 SWCNTs functionalized with phenyl-SO3H and phenyl-SO3 Na had no crucial mutilation to the cells in vitro even at high concentrations (>2 mg/mL), whereas phenyl-(COOH)2-SWCNTs manifested toxicity even at low concentrations of 80 μg/mL. Thus, there have been numerous reports on in vitro effects of CNTs in various cellular models that have demonstrated that the adverse effects of CNTs are dependent on their size, structure, and the functionalization modules.
In vivo toxicity studies
In order to investigate further the effects of CNTs in vivo, several studies have been conducted. In one such study, mice intratracheally infused with SWCNT implants developed epithelioid granulomas with interstitial inflammation.Citation39 Similarly, undoped MWCNTs induced severe inflammatory responses when compared to nitrogen-doped MWCNTs upon intratracheal administration in mice.Citation40 There have been several indications that CNTs cause oral, dermal, pulmonary, and systemic toxicities.Citation41 Inhaled SWCNTs have been shown to cause pulmonary toxicity in rats.Citation42 Lavaged fluids from CNT-treated mice have shown a dose-dependent increase in inflammation and oxidative stress.Citation43 It has also been observed that pristine SWCNTs show increased oxidative stress in liver and lung in a dose-dependent manner; instead relatively persistent stress has been recorded in the spleen even at higher concentration of CNTs. In vitro testing demonstrated that the size and shape of the CNTs affect their entry into the macrophages, resulting in various immune responses.Citation44 In a similar study, shorter CNTs showed low toxicity with increased penetration ability for macrophages and phagocytes compared to their longer (>0.8 μm) counterparts.Citation45 This result was validated in another study, wherein short CNTs were injected subcutaneously in rats and detected in the cytosol of macrophages after 4 weeks; however, the longer CNTs were moving, freely resulting in inflammation.Citation41 Intraperitoneal injection of both long and short CNTs in mice has produced similar results.Citation46 The nontoxic length affirmed for CNTs was ~10 μm.Citation47 It has been revealed that when CNTs surpass 20 μm length, they cannot be phagocytized and could thus exhibit destruction of the plasma membranes in cells, further eliciting greater inflammatory responses.
Thus, with the progress in the field of CNT research, it can be asserted that the biocompatibility of CNTs towards cells relies on various manageable properties, including the size, morphology, the conjugates, and surface modifications of CNTs, which would be able to address the key issue of biosafety of CNTs. Functionalization is a process allowing the conjugation of various molecules of choice onto the surface of CNTs, leading to reduced toxicity.Citation47 Functionalization of CNTs has several advantages, including enhanced solubility in water, increased dispersion, and a lower tendency to form agglomerates, resulting in reduced cytotoxicity.Citation48
Advantages and applications of functionalized CNTs
The smooth surface of carbon nanomaterials lacking any overhanging bonds renders them chemically inert and incompatible with almost all organic and inorganic solvents, which further makes them less amenable for manipulation and downstream applications. In order to address this problem, surface modifications or functionalization of nanoparticles could play a crucial role in improving their physicochemical and surface properties.Citation49 The overall objective of functionalizing CNTs for biomedical applications is to increase their solubility or dispersion in biocompatible (aqueous) media, thereby reducing toxic effects. It has been reported that after modifications, fCNT solubility increased significantly.Citation50 Many studies have shown that increased solubility (or dispersion) of fCNTs improves their performance and lowers their toxicity ().Citation16,Citation51–Citation53 These fCNTs have excellent electro-optical properties, high tensile strength, and a high surface-area-to-volume ratio that facilitates surface functionalization.Citation54 The addition of a layer of biocompatible material can be used to annihilate the toxicity of pristine CNT aggregates by making them more dispersible in aqueous solutions.Citation55 Functionalization of CNTs can be achieved by using oligonucleotides, biomolecules, surfactants, and polymers, () thus increasing the dispersibility of CNTs and decreasing their cytotoxicity.Citation56–Citation59 In a study comparing CNTs dispersed either through functionalization or with the help of a surfactant, it was revealed that fCNTs had low cytotoxicity, whereas the surfactant-dispersed CNTs in turn showed less toxicity than pristine CNTs.Citation17 Reports have also shown that the highly water-soluble modified SWCNTs had low agglomeration and were taken up into the cells without distressing cell viability.Citation37 Studies have also shown that the cytotoxicity on lung mesothelic cells (MSTO-211H) is linked to the extent of agglomeration, and also that the suspended CNTs had less toxicity.Citation60 Subsequently, it has been shown that using fCNTs permits testing with living cells through miscibility in cell culture with satisfactory distribution, with low aggregation and reduced cytotoxicity.Citation47
Table 1 Functionalized carbon nanotubes and reduced cytotoxic effects
Figure 1 Overview of functionalization of carbon nanotubes (CNTs) using different molecules and their biomedical applications.
Abbreviations: SWCNT, single-walled carbon nanotube; siRNA, small interfering RNA; PEG, polyethylene glycol.
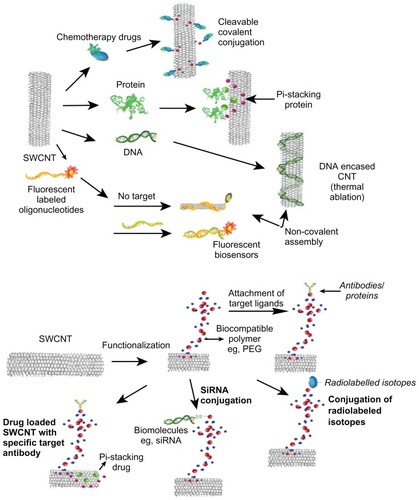
fCNTs display distinctive characteristics that make them more biocompatible with physiological systems, thus decreasing their toxicity compared to CNTs. fCNTs have the capability to infiltrate cell membranes with fairly low toxicity.Citation8,Citation9,Citation25 Recent studies have suggested that CNTs could be translocated into cells through insertion and diffusion into the lipid bilayer of the cell membrane and also that water-soluble nanotubes displayed no significant cytotoxicity to living cells.Citation61 Surface modifications of the CNTs could alter the surface chemistry, thus changing the interactions with the lipid bilayer and enhancing uptake into the cells.Citation62 As reported in several studies carried out in cell cultures, water-soluble fCNTs exhibit noCitation63,Citation64 or abridged cytotoxicity and oxidative stress,Citation26,Citation33,Citation42,Citation65 compared to CNTs by themselves. CNTs have shown a decrease in toxicity with higher functionalization on their sidewalls.Citation42 Cytotoxicity studies on the J774A MOs cell line involving unpurified CNTs (UP-CNTs), purified CNTs (P-CNTs), fluorescein isothiocyanate–conjugated CNTs (FITC-CNTs), and Entamoeba histolytica L220-CNTs showed cytotoxic effects in the order of UP-CNTs > P-CNTs > FITC-CNTs, with a reduction in cell viability and an escalation in apoptosis compared to MOs that were allowed to interact with L220- CNTs, with a rise in cell viability without any significant manifestation of apoptosis. UP-CNTs and P-CNTs displayed induction of cyclooxygenase-2 with 6.0 mg/L. However, fCNTs were able to induce cyclooxygenase-2 at 0.06-mg/L concentrations. It is evident from such studies that regardless of the extent of proteins conjugated to CNTs, cytotoxicity has been lowered. Moreover, the expression of cytotoxic behavior is a measure of the purification process as well as the functional groups attached to the CNTs, thereby enabling them to establish cross talk with the cell-surface receptors.Citation34 CNTs coated with mucin-like polymers were able to interact with the carbohydrate receptors on the cell surface, offering them a way to interact with the cell surface minus any toxic effect.Citation66 Glucosamine-functionalized SWCNTs were able to improve the interactions of the cells with SWCNTs.Citation67 A study conducted on HL60 cells using two types of fluorescent CNTs with FITC-CNTs and biotin conjugates resulted in enhanced membrane translocation with reduced cytotoxicity,Citation67 and another study also indicated the internalization of fluorescently labeled nanotubes into cells with no apparent toxicity.Citation68 Similarly, a study conducted on immune-system cells using two classes of fCNTs – one with 1,3-dipolar cycloaddition and another with oxidation/ amidation – showed that both types of fCNTs were taken up by B and T lymphocytes as well as by macrophages in vitro without affecting cell viability.Citation63 Interestingly, cationic fCNTs have been known to cause much reduced cytotoxicity in vitro, and also functionalized SWCNTs can traverse both nonadherent and adherent cell lines (CHO, 3T3 fibroblast, Jurkat, HL60) with no toxic effects.Citation69 Moreover, when functionalized SWCNTs were injected into the bloodstream of mice, no indication of toxicity was revealed with respect to clinical and laboratory parameters.Citation70 In a biodistribution study on mice, functionalized SWCNTs were found in the bone, kidney, and stomach of mice, which would finally be excreted via the renal route, whereas unmodified CNTs were hoarded in the liver, lungs, and spleen, exhibiting toxic effects.Citation63 At the same time, functionalized nanorods have discrete effects on cell survival through killing cancer cells and having trivial effect on normal cells and mesenchymal stem cells.Citation5,Citation71,Citation72 Thus, the extent of cytotoxicity can directly be correlated with their pristine or functionalized nature, and hence it becomes necessary to establish comparatively simpler and more applicable methods for the functionalization of CNTs, making them more water-soluble, biocompatible, noncytotoxic, and optimally biodegradable compounds.
With recent advances in the field of tissue engineering, various biocompatible materials are being devised for various biomedical applications in different tissues, including bone and the cardiovascular system. In order to exploit CNTs as biomaterials for such tissue-regeneration purposes, it is a prerequisite to understand their biocompatibility. CNTs have been reported to be used in preservation of cells, delivery of growth factors or genes, and as scaffolding matrices in order to promote integration with the host tissue.Citation73 Furthermore, the functionalization of CNTs can greatly expand their potential applications without causing any side effects.Citation74 For tissue regeneration, collagen and polymer fCNT–based matrices (collagen–CNT and polymer–CNT) were used as scaffolds.Citation75,Citation76 In another such study, it was found that human mesenchymal stem cells when seeded onto polylactic acid–MWCNT composites could survive and proliferate.Citation77 Cell adhesion, viability, and proliferation were also studied on the surface of scaffolds consisting of MWCNTs with chitosan (CS; a biocompatible and biodegradable material) that supported cell growth in vitro.Citation78 A novel nanomaterial – nonwoven SWCNTs – was used as cell-growing scaffold in order to study growth behaviors such as adhesion, proliferation, and cytoskeletal development.Citation79 It was observed that nonwoven SWCNTs increased cell adhesion and proliferation substantially. Nanocomposites made from MWCNTs and poly(l-lactide) (PLLA) were reported to inhibit the growth of fibroblast cells.Citation80 Similarly nanocomposite films based on SWCNTs and poly-d,l-lactide- co-glycolide copolymer were processed, and it was found that the biodegradation behavior of the nanocomposites depends on the amount and type of functionalization of CNTs used.Citation81 At the same time, MWCNTs modified with poly-d, l-lactide were shown to have enhanced polymer stability as compared to poly-d,l-lactide alone, signifying that the implants made from such composites could disperse in vivo relatively slowly.Citation82 There have been reports indicating that fCNTs promote the proliferation of osteoblastic cells,Citation74 which is a useful characteristic of CNTs when used in biomaterials placed in contact with bone. In vivo studies showing bone-tissue compatibility of CNTs and their influence on bone formation showed that MWCNTs were effective in periosteal tissue refurbishment, resulting in slight inflammation in the subperiosteal pocket. It was also reported that the functionalized MWCNTs were unable to elicit a strong inflammatory reactions, with noticeable effects on tissue restoration and bone formation even when placed in contact with it, showing decent tissue and bone compatibility.Citation73,Citation83 CNTs have been studied with respect to their biocompatibility for bones, tissues, and blood for various in vivo applications.Citation84 MWCNTs were functionalized with polyurethane for use in cardiovascular applications.Citation85 CNTs with oxygen-containing functional groups on the surface enhanced adhesion to the platelets and amended anticoagulation activity, making them better biomaterials for implants for blood-related environments. CNTs functionalized with poly(ethylene glycol) (PEG) have been injected intravenously, and were found to be distributed in various organs with relatively low uptake by the reticuloendothelial system and almost completely cleared from the organs in nearly 2 months with no toxic effects.Citation86 Thus, it becomes quintessential in order to exploit the capabilities of CNTs devoid of toxic effect to devise a CNT-based regimen with respect to the functional groups, the process used for it to reduce their cytotoxic effects and improve their biocompatibility.
CNT functionalization methods and their biomedical applications
CNTs when produced initially are insoluble and less dispersible substances; therefore, it becomes essential to improve their surface properties for enhanced dispersion, solubilization, biocompatibility, and reduced cytotoxicity. Modification of CNT surfaces could elevate their solubility in water, serum, and various solvents for enhancing their biocompatibility, reducing their cytotoxicity in biological systems for biomedical applications.Citation15 Biological activities and cytotoxic effects of CNTs are highly dependent on their surface chemistry and the process of their purification and functionalization. We have reviewed various methods that are used for surface modifications of CNTs and their applications in the following sections.
Covalent functionalization
Solubility and biocompatibility of CNTs are the most imperative factors for their effective use in biomedical applications. Enhanced solubility and reduced toxicity of CNTs could be achieved by purifying the CNTs by covalent functionalization through multistep acid treatment.Citation87 The solubility of CNTs can be increased through various methods of purification, which could also expose certain charged groups thereby, reducing cytotoxicity.Citation51 Short, acid-oxidized, carboxylated CNTs with hydrophilic surfaces and high aqueous dispersions were found to be less toxic and more biocompatible than pristine CNTs in mice.Citation88 The uptake of acid-treated, water-soluble SWCNTs was studied using human monocyte–derived macrophage cells, and P-CNTs were found to be inside the lysosomes and cytoplasm without any effects on cell viability or structure compared to UP-CNTs. Similarly, functionalized SWCNTs have been used on cultured mammalian cells, signifying that removal of toxic contaminants related to carboxylated SWCNTs is crucial for the development of carboxylated SWCNTs for pharmacological applications.Citation71 Introduction of chemical groups such as COOH, OH, and CO increases the O2 content of CNTs, which can also decrease the cytotoxicity of P-CNTs.Citation34,Citation89 Oxidized ultrashort SWCNTs have been used as nonviral vectors for the intracellular delivery of oligonucleotide molecules to human macrophages without any cytotoxic effects.Citation90 Recently, it was also reported that dispersed SWCNTs are quite benign in terms of cytotoxicity, and also that purified and isolated SWCNTs were unable to cause acute cell death.Citation91 It was reported that covalent functionalization of CNTs is a superior method that depends on the degree of functionalizationCitation42 that augments the biocompatibility of CNTs with lessened cytotoxic effects.
Noncovalent functionalization
Another method of surface modification of CNTs includes noncovalent functionalization. CNTs are known to noncovalently interact with various molecules through weak interactions such as surface adsorption onto the side walls of CNTs, π–π stacking, electrostatic interactions, hydrogen bonding, and van der Waals force. Such noncovalent methods increase water miscibility of CNTs, making them less toxic.Citation15 Many biomolecules, polymers, and surfactants have been used for the noncovalent functionalization of CNTs to obtain biocompatibility. Porphyrin derivatives and FITC-terminated PEG chains have been also coated onto the CNT surface with π–π interaction between pyrene and the graphitic surface of CNTs, which led to enhanced biocompatibility and reduced toxicity.Citation15
Functionalization using protein
Interactions between proteins and CNTs could play a key role in the biological effects of CNTs.Citation92,Citation93 A π–π stacking occurs between CNTs and aromatic residues (Trp, Phe, Tyr) of proteins, enhancing their adsorptivity and biocompatibility, which renders them less toxic as compared to pristine CNTs ()Citation13,Citation94,Citation95 The CNT–protein nanoconjugates have been found very beneficial in biosensor fabrication,Citation96 drug delivery,Citation97 and cancer therapy.Citation98 For example, streptavidin was adsorbed onto the graphitic surface and formed CNT–protein conjugates that were used for cancer therapy, with no cytotoxic effects to the cells in the proximity.Citation99 Similarly, CNT–polycarbonate urethane adsorbed with protein fibronectin was reported to have improved cellular activities and tissue growth by demarcating their physical nanoroughness.Citation100 A competitive binding of human serum proteins to CNTs was also observed.Citation13 Studies on human acute monocytic leukemia cell lines and human umbilical vein endothelial cells have revealed that these blood proteins bind to SWCNT surface, which significantly changes the cellular interaction pathways of the cells, with substantially decreased cytotoxicity. Thus, comparatively safer CNT-based nanomaterials could be devised after understanding their association with the serum protein.Citation13
Figure 2 π–π stacking interaction between single-walled carbon nanotube (SWCNT) and protein molecules.
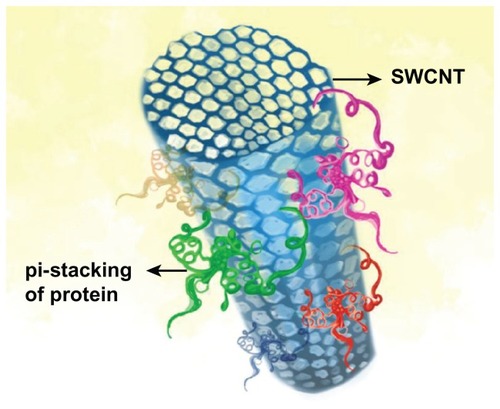
Bovine serum albumin (BSA) is another water-soluble globular protein that adsorbs onto the CNT surfaceCitation101 and gives excellent dispersing capability in vitro.Citation14 BSA-dispersed SWCNTs can be uptaken by human mesenchymal stem cells and HeLa cells without significant acute harmful cellular effects.Citation102 Similarly, albumin adsorbed onto the surface of SWCNTs can induce cyclooxygenase-2 in the RAW 264.7 macrophage cell lines, moderating the uptake and cytotoxicity of SWCNTs.Citation103 These studies have contributed significantly to the knowledge of biological effects of CNTs at the cellular level. These proteins helped the nanoparticles attain their biological identity, either by diminishing the interactions or altering the cellular machinery.Citation94,Citation104 The interaction of proteins (BSA, Tf, BFG, Ig, etc) with CNTs has been shown to affect their uptake, clearance, distribution, and delivery to the intended target sites, thus potentially lowering their toxicity.Citation13
Functionalization using DNA
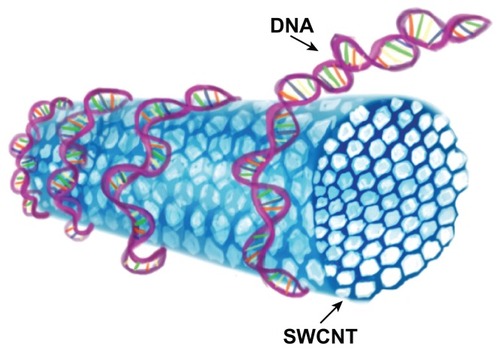
CNTs functionalized with DNA have actually been shown to enhance stability.Citation105,Citation106 DNA can bind to SWCNTs, forming tight helices around them,Citation107 or can form noncovalent conjugates with CNTs ().Citation108 CNTs wrapped with flavin mononucleotide and DNA were found to enhance dispersion of these nanotubes.Citation59,Citation109 DNA-functionalized CNTs can be used as biological transporters and also as biosensors.Citation110 DNA-encased MWCNTs were more effective than plain MWCNTs against malignant tissues when tested in vivo for their thermal ablation capability.Citation111 It was further found that DNA–CNTs could penetrate lymphocytes instantaneously with a needle-like mechanism, thus reducing cytotoxic effects.Citation112 fCNTs were found to be similar to cell-penetrating proteins, as they can penetrate cells without endocytosis;Citation47 however, the internalization of nanomaterials depends on the type of functionalization process.
Functionalization using poly(ethylene glycol)
Polymers including PEG and PEGylated phospholipids are known for their high biocompatibility and dispersibility, thus making them some of the most efficient surface enhancers for CNTs through noncovalent bonding.Citation3,Citation113,Citation114 Several recent studies have found adsorbing phospholipid (PL)-PEG–functionalized CNTs to be noncytotoxic.Citation16,Citation86,Citation115 SWCNT-PEGs have displayed relatively lower cytotoxicity in neuronal PC12 cells than uncoated SWCNTs and had decreased reactive oxygen species–mediated toxicological response in vitro, as they have been shown to have less interaction with cell membranes compared to uncoated SWCNTs.Citation10 Thus, SWCNT-PEGs are estimated to have impending applications in nanomedicine.Citation15,Citation116 SWCNT-PEGs are well suited for generation of multifunctional drugs and as imaging tools.Citation117 When loaded onto PEG-modified SWCNTs, doxorubicin (DOX), an anticancer drug, exhibited improved therapeutic capability and substantial reduction in cytotoxicity effects compared to the free drug.Citation98 Intravenously injected noncovalently functionalized SWCNT-PEGs in mice have revealed no evidence of toxicity.Citation70 PL-PEGs having an amine group or a methyl group could make stable aqueous suspensionsCitation69 and were found to stimulate primary macrophage immune cellsCitation118 and proinflammatory cytokines in cultures.Citation63
Functionalization using chitosan
CNTs were functionalized using CS, a copolymer of 2-amino- 2-deoxy-β-d-glucopyranose and 2-acetamido-2-deoxy-β-d-glucopyranose, through surface adsorption. CS has been the material of choice for CNT functionalization due to its striking water solubility, biocompatibility, biodegradability, nontoxicity, and good complexing ability. Therefore, CS has been widely studied for biomedical and pharmaceutical applications such as drug delivery, cancer therapy, and biosensors.Citation56,Citation119,Citation120 CNTs modified with CS are being used for the removal of heavy metals from aqueous solution,Citation122 as biomaterials for tissue engineering,Citation123 and in delivery of molecules.Citation119 A novel biomaterial – MWCNT–CS– phycocyanin (PC) – prepared by functionalizing MWCNTs with CS and conjugated to PC (photodynamic therapy and photothermal therapy agent) () for photodynamic and photothermal cancer therapy were tested on breast and liver cancer cell lines (MCF-7 and HepG2) and a normal liver cell line (L-O2). The results revealed that MWCNT–CS–PC showed specific photo-induced toxicity to MCF-7 and HepG2, and the introduction of CS increased solubility. PC reduced the cytotoxicity of the CNT complex on normal cells as well.Citation56 SWCNTs modified with biocompatible CS and conjugated to folic acid (FA) (CS–SWCNT–FA) for targeting tumor cells showed that CS could effectively disperse the SWCNTs and provide a suitable biological interface for immobilization of biomolecules.Citation123
Functionalization using other polymers
Block copolymers like poly(l-amino acid), poly(ester), and pluronics have been used for noncovalent functionalization of nanomaterials, having increased dispersibility during drug delivery.Citation124 Pluronic F68, a biocompatible linear copolymer of isopropylene glycol repeating units, was found to stabilize aqueous dispersion of SWCNTs.Citation125 Moreover, CNT suspensions in two biocompatible dispersants (pluronic F108 and hydroxypropylcellulose), showed no signs of agglomeration and remained dispersed when used in vitro.Citation126 Similarly, SWCNTs functionalized with polymers like hexamethylenediamine and poly(diallyldimethylammonium chloride) facilitated noncovalent conjugation for intracellular delivery of negatively charged biomolecules with few cytotoxic effects.Citation127 Polymer-functionalized CNTs did not cause cytotoxicity either.Citation63,Citation128
Functionalization using surfactants
Surfactants can enhance the stability and dispersibility of CNTs in the culture medium by absorbing onto the surface of CNTs, thereby reducing cytotoxicity.Citation25 Surfactants like sodium dodecyl sulfate,Citation129 sodium dodecylbenzenesulfonate,Citation130 cetyltrimethylammonium bromide (CTAB),Citation131 and the Triton-X seriesCitation132 have been shown to disperse CNTs effectively. Cytotoxicity studies in human umbilical endothelial cells using CTAB-SWCNTs showed that SWCNTs in deionized water had higher cytotoxicity than the SWCNTs in CTAB solution, signifying that the surfactant rendered the CNTs more dispersible in the culture medium and less cytotoxic.Citation25 The dispersion of SWCNTs has a significant role in diminishing SWCNT cytotoxicity.Citation15 Studies with polyoxylethylene sorbitanmonooleate, a surfactant, also enhanced the dispersibility of CNTs and showed no toxicity to human lung mesothelial (MSTO-211-H) cells.Citation60
Multifunctionalization of CNTs
The physical properties of SWCNTs make them suitable candidates for several biological applications.Citation133–Citation136 The delivery of DNA, proteins, or drug molecules into living cells is important for therapeutic purposes.Citation137 SWCNTs have been shown to transport various biomolecular cargoes across cellular membrane without cytotoxicity.Citation138–Citation140 Several recent studies have demonstrated that CNTs can prove excellent biological vehicles due to their physical properties, without any substantial toxic effects.Citation69 SWCNTs functionalized with a folate led to their selective uptake by tumor cells having folate-receptor markers and induced near-infrared radiation– triggered cell death, but not in the normal cells.Citation69 PEGylated SWCNTs were attached to FA, which was linked to a Pt (IV) prodrug compound yielding an SWCNT–Pt(IV)–FA conjugate that showed higher toxicity to folate receptor– positive cells but not to folate receptor–negative cells.Citation141 SWCNTs functionalized with an arginine–glycine–aspartic acid (RGD) peptide were found to target integrin-positive tumors in mice.Citation142
SWCNTs have been known to offer higher surface area when prefunctionalized noncovalently or covalently using general surfactants, polymers or acid-oxidation routes, allowing the attachment of various aromatic molecules, such as anticancer drug (DOX), a fluorescence molecule (fluorescein), and combinations of molecules with very high loading efficiency.Citation145 DOX-loaded SWCNTs caused much higher apoptosis and death in U87 cancer cells than free DOX, clearly demonstrating that DOX-loaded SWCNTs were transported inside the cells by nanotube transporters via endocytosis.Citation144 SWCNTs have been shown to target drug delivery to specific cell types without killing the nontargeted cells.Citation142 Prefunctionalized SWCNTs carrying DOX were coupled to a target molecule recognizing target-associated antigens for enhanced selectivity and reduced lethal side effects.Citation145 When DOX-loaded SWCNTs were conjugated to cyclic RGD peptide attached to the terminal groups of PEG, the functionalized CNTs were shown to recognize integrin αυβ3 receptors overexpressed in solid tumors.Citation147 RGD-conjugated SWCNTs showed increased DOX delivery and fluorescence signal and caused enhanced cellular uptake and killing in integrin αυβ3–positive U87MG cells as compared to DOX-loaded SWCNTs without RGD of the SWCNT drug.Citation142 PEGylated SWCNTs with RGD peptide and radiolabels (64Cu-DOTA) when intravenously injected into glioblastoma U87MG tumor-bearing mice and examined by micro–positron emission tomography showed elevated tumor uptake when compared to plain SWCNTs without RGD (SWCNT-PEG5400).Citation147 In another similar study, integrin αυβ3 monoclonal antibody conjugated and PL-PEG functionalized SWCNTs were found to have extraordinary targeting efficiency on U87MG cells with reduced cellular toxicity.Citation145 Functionalized SWCNTs have shown promising effects in tumor-targeted accretion in mice and demonstrated biocompatibility, excretion, and negligible toxicity.Citation148 In vivo SWCNT drug delivery for tumor suppression in mice was performed using paclitaxel (PTX, a cancer chemotherapy drug), conjugated to PEG chains on SWCNTs via an ester bond, resulting in a water-soluble SWCNT–PTX conjugate. SWCNT–PTX showed greater efficacy in subduing the tumor growth compared to taxol (the commercial PTX) in a murine 4T1 breast cancer model, leading to longer blood circulation and higher permeability and retention. Thus, several other similar studies clearly indicate that surface functionalization of SWCNTs is imperative for tumor targeting in vivoCitation147 and drug delivery with enhanced efficacy and slightest side effects in cancer therapy with low drug doses.Citation148
CNTs have been functionalized with drugs as well as fluorescence labels for in vitro delivery. One such bioconjugate of CNTs conjugated to an anticancer drugCitation97 or an antifungal drugCitation149 was used for drug delivery into cells. Noncovalently PEGylated SWCNTs were used as a delivery regime to internalize a platinum (IV) complex, a prodrug, against cancer cells.Citation97 Targeted intracellular delivery of therapeutic biomolecules is significant, as they do not diffuse through cell membranes easily.Citation147 Thus, research has recently been focused on conjugation of these biomolecules, eg, proteins to CNTs through either covalent or noncovalent bonding for intracellular delivery.Citation69,Citation139 The hydrophobic surface of partially functionalized SWCNTs (eg, oxidized SWNTs) permits nonspecific binding of proteins. These biomolecules can become active after being internalized and released from endosomes.Citation69 CNTs were also modified with positive charges to conjugate plasmids for gene transfection.Citation35,Citation150 Polyethylenimine modified MWCNTs were exploited for DNA conjugation and delivery with competitive transfection efficiency to that of standard polyethylenimine transfection with abridged cytotoxicity.Citation106 In recent years, knowledge has grown immensely in the field of small interfering RNA (siRNA) technology, and so have their applications in both basic and applied biology.Citation2 siRNA linked to PL–PEG–SWCNTs through disulfide bonds effectively brought gene-silencing effects.Citation147 Researchers have also been using the functionalization approach, ie, multifunctionalization or using multiple groups such as PEG, drug molecules, proteins, antibodies, or DNA, either simultaneously or sequentially,Citation115 making them apt for various biomedical applications.
These studies suggest that functionalization of CNTs elevates their dispersibility, biostability, and reduction in aggregate formation, and reduces cytotoxicity.Citation16 Covalent and noncovalent functionalization of CNTs with biomolecules, polymers, copolymers and surfactants are significant practices, leading to the solubility of the CNTs. Moreover, conjugated CNTs can also be used to enhance the biocompatibility and biosafety of CNTs.Citation15
Conclusions
In conclusion, CNTs have been used for various biomedical applications for targeted delivery, anticancer activities, imaging, etc. The cytotoxicity of CNTs has been well addressed through various methods of surface functionalization of CNTs, thereby improving their interaction within biological systems. Given CNTs’ relatively lower toxicity, their surface functionalization is a promising strategy for delivering different biological molecules. They are important biomaterials due to their superior characteristics over conventional biomaterials. It is generally agreed that fCNTs constitute a major improvement over unmodified CNTs, since unmodified CNTs often cause adverse reactions to living cells and tissues, whereas fCNTs are less toxic due to more biocompatible functional groups. The most efficient way to transform the surface of CNTs from hydrophobic to hydrophilic is by attaching different water-soluble and functional moieties. Functionalization of CNTs results in highly soluble materials that are further derivatized with active molecules, making them compatible with biological systems. Thus, fCNTs possess wider biological applications compared to nonfunctionalized CNTs.
Acknowledgments
The authors would like to acknowledge grant support from NSF-CREST (HRD-0734232). We also acknowledge Ms Eva Dennis for her assistance in preparing the figures used in this review article.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiuZTabakmanSLiXLMultiplexed five-color molecular imaging of cancer cells and tumor tissues with carbon nanotube Raman tags in the near-infraredNano Res2010322223321442006
- LiuZWintersMHolodniyMDaiHsiRNA delivery into human T cells and primary cells with carbon-nanotube transportersAngew Chem Int Ed Engl2007462023202717290476
- HadidiNKobarfardFNafissi-VarchehNAboofazeliROptimization of single-walled carbon nanotube solubility by noncovalent PEGylation using experimental design methodsInt J Nanomed20116737746
- IijimaSHelical microtubules of graphitic carbonNature19913545658
- LiangFChenBA review on biomedical applications of single-walled carbon nanotubesCurr Med Chem201017102419941481
- KhazaeiARadMNBorazjaniMKOrganic functionalization of single-walled carbon nanotubes (SWCNTs) with some chemotherapeutic agents as a potential method for drug deliveryInt J Nanomedicine2010563964520856839
- KhodakovskayaMVde SilvaKNedosekinDAComplex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactionsProc Natl Acad Sci U S A20111081028103321189303
- MahmoodMCascianoDAMocanTCytotoxicity and biological effects of functional nanomaterials delivered to various cell linesJ Appl Toxicol201030748319760634
- ZhangYAliSFDervishiECytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cellsACS Nano201043181318620481456
- ZhangYXuYLiZMechanistic toxicity evaluation of uncoated and PEGylated single-walled carbon nanotubes in neuronal PC12 CellsACS Nano201157020703321866971
- HigginsPDawsonJWaltersMNanomedicine: nanotubes reduce stroke damageNat Nanotechnol201126121125
- Ali-BoucettaHAl-JamalKTKostarelosKCytotoxic assessment of carbon nanotube interaction with cell culturesMethods Mol Biol201172629931221424457
- GeCDuJZhaoLBinding of blood proteins to carbon nanotubes reduces cytotoxicityProc Natl Acad Sci U S A2011108169681697321969544
- WangXXiaTNtimSAQuantitative techniques for assessing and controlling the dispersion and biological effects of multiwalled carbon nanotubes in mammalian tissue culture cellsACS Nano201047241725221067152
- YanLZhaoFLiSHuZZhaoYLow-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenesNanoscale2011336238221157592
- BhirdeAAPatelSSousaAADistribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in miceNanomedicine201051535154621143032
- FoldvariMBagonluriMCarbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issuesNanomedicine2008418320018550450
- MaynardADBaronPAFoleyMExposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube materialJ Toxicol Environ Health A2004678710714668113
- TejralGPanyalaNRHavelJCarbon nanotubes: toxicological impact on human health and environmentJ Appl Biomed20097113
- PacurariMYinXJZhaoJRaw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-κB, and Akt in normal and malignant human mesothelial CellsEnviron Health Perspect20081161211121718795165
- ShvedovaAAPietroiustiAFadeelBKaganVEMechanisms of carbon nanotube-induced toxicity: focus on oxidative stressToxicol Appl Pharmacol201226112113322513272
- YuanJGaoHSuiJDuanHChenWNChingCBCytotoxicity evaluation of oxidized single-walled carbon nanotubes and graphene oxide on human hepatoma HepG2 cells: an iTRAQ-coupled 2D LC-MS/ MS proteome analysisToxicol Sci201212614916122157353
- YuanJGaoHChingCBComparative protein profile of human hepatoma HepG2 cells treated with graphene and single-walled carbon nanotubes: an iTRAQ-coupled 2D LC-MS/MS proteome analysisToxicol Lett201120721322121963432
- MurrayARKisinELeonardSSOxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubesToxicology200925716117119150385
- ZhangLWZengLBarronARMonteiro-RiviereNABiological interaction of functionalized single-wall carbon nanotubes in human epidermal keratinocytesInt J Toxicol20072610311317454250
- BottiniMBrucknerSNikaKMulti-walled carbon nanotubes induce T lymphocyte apoptosisToxicol Lett200616012112616125885
- DingLStilwellJZhangTMolecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblastNano Lett200552448246416351195
- YanXBGuYHHuangDBinding tendency with oligonucleotides and cell toxicity of cetyltrimethyl ammonium bromide-coated single-walled carbon nanotubesTrans Nonferrous Met Soc China20112110851091
- ZhaoYLNalwaHSNanotoxicology: Interactions of Nanomaterials with Biological SystemsLos AngelesAmerican Scientific2006
- ShvedovaACastranovaVKisinEExposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cellsJ Toxicol Environ Health20036619091926
- WangXJiaGWangHDiameter effects on cytotoxicity of multi-walled carbon nanotubesJ Nanosci Nanotechnol200993025303319452965
- CuiDTianFOzkanCSWangMGaoHEffect of single wall carbon nanotubes on human HEK293 cellsToxicol Lett2005155738515585362
- Monteiro-RiviereNANemanichRJInmanAOWangYYRiviereJEMulti-walled carbon nanotube interactions with human epidermal keratinocytesToxicol Lett200515537738415649621
- Montes-FonsecaSLOrrantia-BorundaEAguilar-ElguezabalAGonzález HortaCTalamás-RohanaPSánchez-RamírezBCytotoxicity of functionalized carbon nanotubes in J774A macrophagesNanomedicine2012885385922033080
- PantarottoDSinghRMcCarthyDFunctionalized carbon nanotubes for plasmid DNA gene deliveryAngew Chem Int Ed Engl2004435242524615455428
- SotoKGarzaKMMurrLECytotoxic effects of aggregated nanomaterialsActa Biomater2007335135817275430
- UoMAkasakaTWatariFSatoYTohjiKToxicity evaluations of various carbon nanomaterialsDent Mater J20113024526321597228
- SayesCMLiangFHudsonJLFunctionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitroToxicol Lett200616113514216229976
- LamCWJamesJTMcCluskeyRHunterRLPulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillationToxicol Sci20047712613414514958
- Carrero-SánchezJCEliasALMancillaRBiocompatibility and toxicological studies of carbon nanotubes doped with nitrogenNano Lett200661609161616895344
- SatoYYokoyamaAShibataKiInfluence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivoMol Biosyst2006117618616880981
- WarheitDBLaurenceBRReedKLComparative pulmonary toxicity assessment of single-wall carbon nanotubes in ratsToxicol Sci20047711712514514968
- ShvedovaAAKisinERMercerRUnusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in miceAm J Physiol Lung Cell Mol Physiol2005289L698L70815951334
- NelAXiaTMädlerLLiNToxic potential of materials at the nanolevelScience200631162262716456071
- Kolosnjaj-TabiJHartmanKBBoudjemaaSIn vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss miceACS Nano201041481149220175510
- PolandCADuffinRKinlochICarbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot studyNat Nanotechnol2008342342818654567
- FirmeCPBandaruPRToxicity issues in the application of carbon nanotubes to biological systemsNanomedicine2010624525619699321
- CocciniTRodaESarigiannisDAEffects of water-soluble functionalized multi-walled carbon nanotubes examined by different cytotoxicity methods in human astrocyte D384 and lung A549 cellsToxicology2010269415320079395
- WuHCChangXLiuLZhaoFZhaoYChemistry of carbon nanotubes in biomedical applicationsJ Mater Chem20102010361052
- ZhangYBaiYYanBFunctionalized carbon nanotubes for potential medicinal applicationsDrug Discov Today20101542843520451656
- CuiHFVashistSKAl-RubeaanKLuongJHTSheuFSInterfacing carbon nanotubes with living mammalian cells and cytotoxicity issuesChem Res Toxicol2010231131114720402485
- JiangWMardyaniSFischerHChanWCWDesign and characterization of lysine cross-linked mercapto-acid biocompatible quantum dotsChem Mater20064872881
- VeroneseFMPasutGPEGylation, successful approach to drug deliveryDrug Discov Today2005101451145816243265
- KostarelosKBiancoAPratoMPromises, facts and challenges for carbon nanotubes in imaging and therapeuticsNat Nanotechnol2009462763319809452
- BhirdeAALiuGJinACombining portable Raman probes with nanotubes for theranostic applicationsTheranostics2011131032121769298
- LiaoXZhangXPreparation, characterization and cytotoxicity of carbon nanotube–chitosan–phycocyanin complexNanotechnology201223113
- HaggenmuellerRRahatekarSSFaganJAComparison of the quality of aqueous dispersions of single wall carbon nanotubes using surfactants and biomoleculesLangmuir2008245070507818442227
- NishAHwangJYDoigJNicholasRJ Highly selective dispersion of single-walled carbon nanotubes using aromatic polymersNat Nanotechnol2007264064618654390
- ZhengMJagotaASemkeEDDNA-assisted dispersion and separation of carbon nanotubesNat Mater2003233834212692536
- WickPManserPLimbachLKThe degree and kind of agglomeration affect carbon nanotube cytotoxicityToxicol Lett200716812113117169512
- HirschAThe era of carbon allotropesNat Mater2010986887120966925
- LopezCFNielsenSOMoorePBKleinMLUnderstanding nature’s design for a nanosyringeProc Nat Acad Sci U S A200410144314434
- DumortierHLacotteSPastorinGFunctionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cellsNano Lett200661522152816834443
- PantarottoDPartidosCDGraffRSynthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptidesJ Am Chem Soc20031256160616412785847
- MagrezAKasasSSalicioVCellular toxicity of carbon-based nanomaterialsNano Lett200661121112516771565
- ChenXTamUCCzlapinskiJLInterfacing carbon nanotubes with cellsJ Am Chem Soc20061286292629316683774
- NimmagaddaAThurstonKNollertMUMcFetridgePSChemical modification of SWNT alters in vitro cell-SWNT interactionsJ Biomed Mater Res A20067661462516315191
- PantarottoDBriandJPPratoMBiancoATranslocation of bioactive peptides across cell membranes by carbon nanotubesChem Commun200471617
- KamNWSLiuZDaiHFunctionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencingJ Am Chem Soc2005127124921249316144388
- SchipperMLNakayama-RatchfordNDavisCRA pilot toxicology study of single-walled carbon nanotubes in a small sample of miceNat Nanotechnol2008321622118654506
- WangRMikoryakCLiSCytotoxicity screening of single-walled carbon nanotubes: detection and removal of cytotoxic contaminants from carboxylated carbon nanotubesMol Pharm201181351136121688794
- YangDZhaoYGuoH[Gd@C82(OH)22]n nanoparticles induce dendritic cell maturation and activate Th1 immune responsesACS Nano201041178118620121217
- SaitoNUsuiYAokiKCarbon nanotubes: biomaterial applicationsChem Soc Rev2009381897190319551170
- SaitoNUsuiYAokiKCarbon nanotubes for biomaterials in contact with boneCurr Med Chem20081552352718289008
- MacDonaldRALaurenziBFViswanathanGAjayanPMStegemannJPCollagen–carbon nanotube composite materials as scaffolds in tissue engineeringJ Biomed Mater Res A20057448949615973695
- CaoYZhouYMShanYJuHXXueXJPreparation and characterization of grafted collagen-multiwalled carbon nanotubes compositesJ Nanosci Nanotechnol2007744745117450777
- McCullenSDStevensDRRobertsWACharacterization of electrospun nanocomposite scaffolds and biocompatibility with adipose-derived human mesenchymal stem cellsInt J Nanomed20072253263
- AbarrategiAGutiérrezMCMoreno-VicenteCMultiwall carbon nanotube scaffolds for tissue engineering purposesBiomaterials2008299410217928048
- MengJSongLKongHUsing single-walled carbon nanotubes nonwoven films as scaffolds to enhance long-term cell proliferation in vitroJ Biomed Mater Res A20067929830616817220
- ZhangDKandadaiMACechJRothSCurranSAPoly (L-lactide) (PLLA)/multiwalled carbon nanotube (MWCNT) composite: characterization and biocompatibility evaluationJ Phys Chem20061101291012915
- ArmentanoIDottoriMPugliaDKennyJEffects of carbon nanotubes (CNTs) on the processing and in-vitro degradation of poly(dl-lactide-coglycolide)/ CNT filmsJ Mater Sci Mater Med2008192377238718158616
- KrulLPVolozhynAIBelovDANanocomposites based on poly-d,l-lactide and multiwall carbon nanotubesBiomol Eng200724939516908214
- UsuiYAokiKNaritaNCarbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effectsSmall2008424024618205152
- MurugesanSParkTJYangHMousaSLinhardtRJBlood compatible carbon nanotubes – nano-based neoproteoglycansLangmuir2006223461346316584210
- MengJKongHXuHYImproving the blood compatibility of polyurethane using carbon nanotubes as fillers and its implications to cardiovascular surgeryJ Biomed Mater Res A20057420821415962271
- LiuZDavisCCaiWCirculation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopyProc Natl Acad Sci U S A20081051410141518230737
- PorterAEGassMBendallJSUptake of noncytotoxic acidtreated single-walled carbon nanotubes into the cytoplasm of human macrophage cellsACS Nano200931485149219459622
- JainSThakareVSDasMToxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization densityChem Res Toxicol2011242028203921978239
- ChengCMüllerKHKoziolKKKToxicity and imaging of multi-walled carbon nanotubes in human macrophage cellsBiomaterials2009304152416019473699
- CrinelliRCarloniEMenottaMOxidized ultrashort nanotubes as carbon scaffolds for the construction of cell-penetrating NF-κB decoy moleculesACS Nano201042791280320411956
- HoltBDShortPARapeADCarbon nanotubes reorganize actin structures in cells and ex vivoACS Nano201044872487820669976
- LundqvistMStiglerJEliaGNanoparticle size and surface properties determine the protein corona with possible implications for biological impactsProc Natl Acad Sci U S A2008105142651427018809927
- CedervallTLynchILindmanSUnderstanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticlesProc Natl Acad Sci U S A20071042050205517267609
- NelAEMadlerLVelegolDUnderstanding biophysicochemical interactions at the nano-bio interfaceNat Mater2009854355719525947
- ShimMShi KamNWChenRJLiYDaiHFunctionalization of carbon nanotubes for biocompatibility and biomolecular recognitionNano Lett20022285288
- ZhangLZhaoGCWeiXWYangZSA nitric oxide biosensor based on myoglobin adsorbed on multi-walled carbon nanotubesElectroanalysis200517630634
- FeazellRPNakayama-RatchfordNDaiHLippardSJSoluble single-walled carbon nanotubes as longboat delivery systems for platinum (IV) anticancer drug designJ Am Chem Soc20071298438843917569542
- LiuZFanACRakhraKSupramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapyAngew Chem Int Ed Engl2009487668767219760685
- BalavoineFSchultzPRichardCHelical crystallization of proteins on carbon nanotubes: a first step towards the development of new biosensorsAngew Chem Int Ed Engl19993819121915
- KhangDKimSYLiu-SnyderPEnhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: independent role of surface nano-roughness and associated surface energyBiomaterials2007284756476817706277
- ZhaoXLiuRChiZTengYQinPNew insights into the behavior of bovine serum albumin adsorbed onto carbon nanotubes: comprehensive spectroscopic studiesJ Phys Chem B20101145625563120373820
- HoltBDDahlKNIslamMFQuantification of uptake and localization of bovine serum albumin-stabilized single-wall carbon nanotubes in different human cell typesSmall2011723482355
- DuttaDSundaramSKTeeguardenJGAdsorbed proteins influence the biological activity and molecular targeting of nanomaterialsToxicol Sci200710030331517709331
- LynchIDawsonKAProtein-nanoparticle interactionsNano Today200834047
- SinghRPantarottoDMcCarthyDBinding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectorsJ Am Chem Soc20051274388439615783221
- LiuYWuDCZhangWDPolyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNAAngew Chem Int Ed Engl2005444782478515995988
- ZangmeisterRAMaslarJEOpdahlATarlovMJAdsorption behavior of DNA-wrapped carbon nanotubes on self-assembled monolayer surfacesLangmuir2007236252625617455960
- TasisDTagmatarchisNBiancoAPratoMChemistry of carbon nanotubesChem Rev20061061105113616522018
- JuSDollJSharmaIPapadimitrakopoulosFSelection of carbon nanotubes with specific chiralities using helical assemblies of flavin mononucleotideNat Nanotechnol2008335636218654547
- Sánchez-PomalesGSantiago-RodríguezLCabreraCDNA-functionalized carbon nanotubes for biosensing applicationsJ Nanosci Nanotechnol200992175218819437957
- GhoshSDuttaSGomesEIncreased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubesACS Nano200932667267319655728
- CheungWPontorieroFTaratulaOChenAMHeHDNA and carbon nanotubes as medicineAdv Drug Delivery Rev201062633649
- PrencipeGTabakmanSMWelsherKPEG branched polymer for functionalization of nanomaterials with ultra long blood circulationJ Am Chem Soc20091314783478719173646
- Nakayama-RatchfordNBangsaruntipSSunXWelsherKDaiHNoncovalent functionalization of carbon nanotubes by fluorescein–polyethylene glycol: supramolecular conjugates with pH-dependent absorbance and fluorescenceJ Am Chem Soc20071292448244917284037
- LiuZTabakmanSMChenZDaiHPreparation of carbon nanotube bioconjugates for biomedical applicationsNat Protoc200941372138119730421
- LiXMFanYBWatariFCurrent investigations into carbon nanotubes for biomedical applicationBiomed Mater20105022001022012
- BottiniMRosatoNBottiniNPEG-modified carbon nanotubes in biomedicine: current status and challenges aheadBiomacromolecules2011123381339321916410
- PortneyNOzkanMNano-oncology: drug delivery, imaging, and sensingAnal Bioanal Chem200638462063016440195
- LiaoXXZhangBCWangXQYanHDZhangXWPurification of C-phycocyanin from Spirulina platensis by single-step ion-exchange chromatographyChromatographia201173291296
- KimDGJangMJChoiCYKimTHJangMKNahJWEnhance of tumor targeting by receptor-mediated endocytosis using low molecular water-soluble chitosan nanoparticles loaded with anticancer agentKey Eng Mater2007342–343469
- SalamMAMakkiMSIAbdelaalMYPreparation and characterization of multi-walled carbon nanotubes/chitosan nanocomposite and its application for the removal of heavy metals from aqueous solutionJ Alloys Compd201150925822587
- VenkatesanJQianZJRyuBKumarNAKimSKPreparation and characterization of carbon nanotube-grafted-chitosan – natural hydroxyapatite composite for bone tissue engineeringCarbohydr Polym201183569577
- WuBOuZXingDFunctional single-walled carbon nanotubes/ chitosan conjugate for tumor cells targetingProc SPIE2009751975190K175190K8
- AdamsMLLavasanifarAKwonGSAmphiphilic block copolymers for drug deliveryJ Pharm Sci2003921343135512820139
- Anson-CasaosAGonzalez-DominguezJMMartinezMTSeparation of single-walled carbon nanotubes from graphite by centrifugation in a surfactant or in polymer solutionsCarbon20104829172924
- PiretJPDetricheSVigneronRDispersion of multi-walled carbon nanotubes in biocompatible dispersantsJ Nanopart Res2010127582
- KrajcikRJungAHirschANeuhuberWZolkOFunctionalization of carbon nanotubes enables non-covalent binding and intracellular delivery of small interfering RNA for efficient knock-down of genesBiochem Biophys Res Commun200836959560218298946
- SmartSCassadyALuGMartinDThe biocompatibility of carbon nanotubesCarbon20064410341047
- YuJRGrossiordNKoningCELoosJControlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solutionCarbon200745618623
- WhitsitteEAAndrewRBSilica coated single walled carbon nanotubesNano Lett20033775778
- RyabenkoAGDorofeevaTVZverevaGIUV-VIS-NIR spectroscopy study of sensitivity of single-wall carbon nanotubes to chemical processing and Van-der-Waals SWNT/SWNT interaction. Verification of the SWNT content measurements by absorption spectroscopyCarbon20044215231535
- BaiYLinDWuFWangZXingBAdsorption of Triton X-series surfactants and its role in stabilizing multi-walled carbon nanotube suspensionsChemosphere20107936236720206374
- AlivisatosPThe use of nanocrystals in biological detectionNat Biotechnol200422475214704706
- KimSLimYTSolteszEGNear-infrared fluorescent type II quantum dots for sentinel lymph node mappingNat Biotechnol200422939714661026
- CuiYWeiQParkHLieberCMNanowire nanosensors for highly sensitive and selective detection of biological and chemical speciesScience20012931289129211509722
- ChenRJBangsaruntipSDrouvalakisKANoncovalent functionalization of carbon nanotubes for highly specific electronic biosensorsProc Natl Acad Sci U S A20031004984498912697899
- HenryCMNew wrinkles in drug deliveryChem Eng News2004823742
- BiancoAKostarelosKPartidosCDPratoMBiomedical applications of functionalized carbon nanotubesChem Commun20055571577
- Shi KamNWJessopTCWenderPADaiHNanotube molecular transporters: internalization of carbon nanotube-protein conjugates into mammalian cellsJ Am Chem Soc20041266850685115174838
- CherukuriPBachiloSMLitovskySHWeisman near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cellsJ Am Chem Soc2004126156381563915571374
- DharSLiuZThomaleJDaiHLippardSJTargeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing deviceJ Am Chem Soc2008130114671147618661990
- LiuZCaiWHeLIn vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in miceNat Nanotechnol20072475218654207
- LiuZSunXNakayama-RatchfordNDaiHSupramolecular chemistry on water-soluble carbon nanotubes for drug loading and deliveryACS Nano20071505619203129
- KamNWSDaiHCarbon nanotubes as intracellular protein transporters: generality and biological functionalityJ Am Chem Soc20051276021602615839702
- OuZWuBXingDFunctional single-walled carbon nanotubes based on an integrin αvβ3 monoclonal antibody for highly efficient cancer cell targetingNanotechnology200910510217
- MizejewskiGJRole of integrins in cancer: survey of expression patternsProc Soc Exp Biol Med199922212413810564536
- LiuZTabakmanSWelsherKDaiHCarbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug deliveryNano Res200928512020174481
- LiuZChenKDavisCDrug delivery with carbon nanotubes for in vivo cancer treatmentCancer Res2008686652666018701489
- GaoLZNieLWangTHCarbon nanotube delivery of the GFP gene into mammalian cellsChembiochem2006723924216370018
- MelloCCConteDRevealing the world of RNA interferenceNature200443133834215372040
![Figure 4 Functionalization of multiwalled carbon nanotube (MWCNT) with chitosan (CS) conjugated to phycocyanin (PC) (photodynamic therapy [PDT] and photothermal therapy [PTT] agent) for PDT and PTT cancer therapy.](/cms/asset/822a8b79-8672-4ab1-b81a-4739082acdfd/dijn_a_35832_f0004_c.jpg)