?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The stability of protein drugs remains one of the key hurdles to their success in the market. The aim of the present study was to design a novel nanoemulsion drug-delivery system (NEDDS) that would encapsulate a standard-model protein drug – bovine serum albumin (BSA) – to improve drug stability.
Methods
The BSA NEDDS was prepared using a phase-inversion method and pseudoternary phase diagrams. The following characteristics were studied: morphology, size, zeta potential, drug loading, and encapsulation efficiency. We also investigated the stability of the BSA NEDDS, bioactivity of BSA encapsulated within the NEDDS, the integrity of the primary, secondary, and tertiary structures, and specificity.
Results
The BSA NEDDS consisted of Cremophor EL-35, propylene glycol, isopropyl myristate, and normal saline. The average particle diameter of the BSA NEDDS was about 21.8 nm, and the system showed a high encapsulation efficiency (>90%) and an adequate drug-loading capacity (45 mg/mL). The thermodynamic stability of the system was investigated at different temperatures and pH levels and in room-temperature conditions for 180 days. BSA NEDDS showed good structural integrity and specificity for the primary, secondary, and tertiary structures, and good bioactivity of the loaded BSA.
Conclusions
BSA NEDDS showed the properties of a good nanoemulsion-delivery system. NEDDS can greatly enhance the stability of the protein drug BSA while maintaining high levels of drug bioactivity, good specificity, and integrity of the primary, secondary, and tertiary protein structures. These findings indicate that the nanoemulsion is a potential formulation for oral administration of protein drugs.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Many protein targets and protein drugs have recently been discovered, with approximately a dozen therapeutic protein drugs receiving regulatory approval each year around the world.Citation1,Citation2 Over 125 protein drugs are already in the US market, and the value of advanced drug-delivery systems in this market had reached $121 billion by 2010.Citation3 Although many varieties of protein drugs have been designed for therapeutic purposes, several challenges remain, including enzymatic susceptibility, stability during storage period, and efficacy after administration.Citation4 Thus, protein stability is one of the major hurdles for a drug’s successful entry into the market.Citation5 At present, many of the marketed protein drugs are mainly designed for injective delivery. Because of the thermal instability of protein drugs, they need to be stored and transported at cold temperatures in order to ensure long shelf life.Citation5,Citation6
To overcome the crucial problem of protein instability, stabilizers such as nonionic surfactants, cyclodextrin derivatives, poloxamers, glycerol, ethylene glycol, glucose, sucrose, and trehalose have been used.Citation7 However, the effects of these stabilizers vary among different proteins. With a view to enhancing the stability of therapeutic peptides and proteins, many studies have focused on the use of such drug-delivery systems as microspheres, nanoparticles, liposomal systems, and traditional emulsions.Citation8 However, these drug-delivery systems have several drawbacks, such as low encapsulation efficiency and drug loading, use of inorganic solvents such as methylene chloride and acetone (which induce toxicity), low yield and high production costs.Citation8 A nanoemulsion-drug delivery is a novel, thermodynamically stable delivery system that can enhance the stability of a protein drug by protecting it from oxidative and enzymatic degradation. Bovine serum albumin (BSA) has often been used as a standard and model protein drug in drug development and delivery, using carriers such as microparticles, nanospheres, nanoparticles, and gels.Citation9–Citation13
The nanoemulsion drug-delivery system (NEDDS) is an isotropic system in which two immiscible liquids are combined to form a single phase by application of appropriate surfactants or by mixing to form a droplet with a diameter range of approximately 0.1–100 nm.Citation14 Such systems can also be formed by heterogeneous dispersion of oil droplets in an aqueous medium stabilized using surfactant molecules.Citation15 In recent years, the focus on NEDDS has increased because of its distinctive structure and properties, including a small droplet size, possibility of fabrication with biocompatible materials, increased dissolution rate and solubility, improved diffusion across an unstirred aqueous layer, and enhanced mucosal permeability.Citation16 NEDDS possesses high kinetic and long-term thermodynamic stability and transparency. Therefore, it has been widely used to improve the stability of drugs such as ramipril,Citation17 celecoxib,Citation18 hyaluronic acid,Citation19,Citation20 β-cypermethrin,Citation21 β-carotene,Citation22,Citation23 β-cyclodextrinCitation24 and Bacillus anthracis protective antigen.Citation25
NEDDS possesses a good storage capacity and thermodynamic stability for drug delivery. In this study, we chose BSA as our model drug. And also, we prepared and optimized BSA NEDDS by using a phase-inversion method and pseudoternary phase diagrams. The delivery system was evaluated using transmission electron microscopy, atomic force microscopy, Nano ZS90 (Malvern Instruments, Malvern, UK). We also tested the following characteristics of the system stored at room temperature for 180 days: thermodynamic stability, specificity, bioactivity and stability of this system and model protein drug, BSA, including integrity of the primary, secondary, and tertiary structures.
Materials and methods
Materials
BSA (MW 66 kDa; fat, purity 98.3%; lot 071K7602) was purchased from Sigma-Aldrich Chemicals (St Louis, MO) and used without further purification. Isopropyl myristate (IPM) was purchased from Croda (Goole, UK). Cremophor EL 35 (polyethoxylated castor oil) was obtained from BASF (Mumbai, India). Propylene glycol and Tween 20 were obtained from Shanghai Chemical Reagent Corporation (Shanghai, China). Low molecular protein ladder marker (14.4–97.4 kDa) and predye protein ladder marker (17–170 kDa) were purchased from Fermentas International (Burlington, Canada). A micro-BCA protein assay kit was purchased from Wuhan Boster Bioengineering, Wuhan, China. Rabbit anti-mouse BSA monoclonal antibody was purchased from Beijing Saichi Shengwu Keji (Beijing, China). Horseradish peroxidase (HRP) goat anti-mouse immunoglobulin G (IgG) was purchased from Beijing Zhongshan Golden Bridge Biotechnology (Beijing, China). High-purity Milli-Q water (Millipore, Billerica, MA) was used for all solutions, with a resistance value of > 18.2 MV/cm. All other chemicals used in the study were of analytical reagent grade.
Pseudoternary phase diagram construction
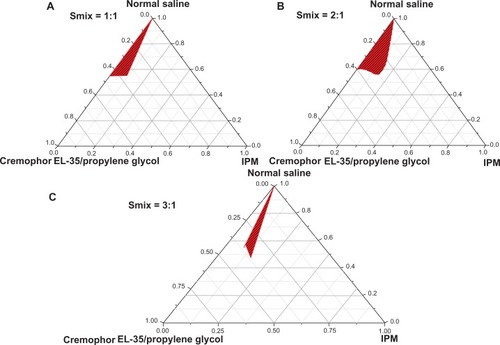
On the basis of the results of preliminary experiments, we chose IPM as the oil phase, Cremophor EL-35 as the surfactant, propylene glycol as the cosurfactant, and normal saline as the aqueous phase. The surfactant and the cosurfactant were mixed (Smix) in different mass ratios (1:1, 2:1, and 3:1). The pseudoternary phase diagrams for these mixtures were created using titration at room temperature to ascertain the ideal concentration ranges of the components. Nine different combinations of oil and Smix (1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1) were added drop-wise to the aqueous phase by using gentle agitation. Transparent and easily flowing nanoemulsion was observed. Using Origin 7.0 software (OriginLab, Northampton, MA), we generated the physical state of the nanoemulsion by creating a pseudo-three-component phase diagram, with the first axis representing the aqueous phase, the second axis representing the oil, and the third axis representing the mixture of the surfactant and cosurfactant at a fixed mass ratio.
Formulation of the BSA NEDDS
From the pseudoternary diagram and visual observations, a precisely defined composition was selected for the nanoemulsion-drug delivery system. Briefly, Smix was prepared by mixing Cremophor EL 35, propylene glycol in the ratio 2:1 (w/w) and the mixture was stirred at 600 rpm at 25°C. Next, IPM was added to Smix to obtain the desired ratio of 9:1 (Smix–IPM) and stirred for 5 minutes, after which the mixture was cooled to room temperature. This was followed by the addition of aqueous phase (normal saline) to the Smix–IPM mixture while stirring, such that the final concentration of aqueous phase in the mixture was 20% w/w. The contents were stirred for 15 minutes to ensure nanoemulsion formation, which was monitored by checking the optical clarity and physical properties of the system. To prepare the novel BSA NEDDS, a blank formula and different concentrations of BSA power and aqueous solution, being 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 mg/mL, were used following the same protocol and method to the pseudoternary phase diagram construction, with the exception of water being replaced by BSA as the aqueous phase.
Characterization of the BSA NEDDS
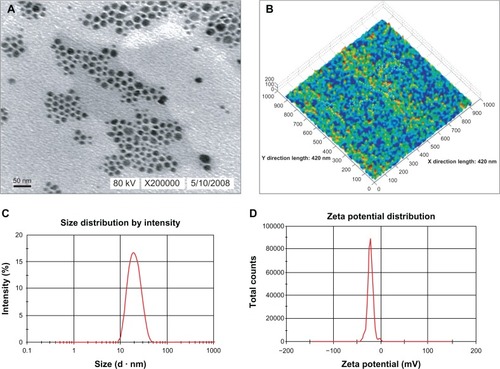
Sample solutions were imaged using transmission electron microscopy (Tecnai-10 TEM; Hillsboro, OR). A drop of the particle suspension was placed onto a carbon-coated copper grid obtained from Ted Pella (Redding, CA) to yield a thin liquid film. The films were negatively stained with 1% phosphotungstic acid solution (w/w; pH 7.4) for 1 minute. A high-resolution atomic-force microscope (IPC-208B; Chongqing University, Chongqing, China) was used to characterize the molecular morphology of the BSA NEDDS. Approximately 0.05 mL of the sample solution was dropped onto microscopic glass slides with gold coating to fracture the film, which was then dried and tested. The measurement was performed under the following conditions: tungsten probes (force constant, 0.06 N · m); scan range, 10.5 nm × 10.5 nm; imaging mode, tapping mode; and scanning method, point by point scanning at room temperature. The G3DR software (Changqing, China) was used to process the data. The excess phosphotungstic solution was removed using filter paper, and stained samples were characterized at 80 kV. The average size and zeta potential of the BSA NEDDS were tested at 20°C by using a dynamic light-scattering instrument (Zetasizer) equipped with an argon ion laser.
Drug-loading and encapsulation efficiency
For sample preparation, triplicate aliquots of the blank and novel BSA NEDDS (1 g, approximately, 1 mL) were placed in sterile 2-mL eppendorf centrifuge tubes and diluted to a protein concentration of 250–500 mg/mL with normal saline. Two volumes of absolute ethanol were added to approximately 0.3 mL of blank or sample, and the mixture was shaken to ensure complete emulsification, then centrifuged at 13,000 × g for 30 minutes. The supernatant was removed, and the precipitate was mixed with 0.3 mL of the above solutions. The amount of BSA incorporated in the nanoemulsion was determined by centrifuging the BSA-loaded nanoemulsion and separating the supernatant. The precipitate was discarded, and the BSA content of the solution was then analyzed by using the commercially available bicinchoninic acid kit. The following equations were used in calculating for the percentage of drug loading and encapsulation efficiency:
Stability study of the BSA NEDDS
Thermodynamic stability
Thermodynamic stability was tested and revised according to the methods described by Shafiq et al.Citation26 Briefly, selected formulations were centrifuged at 13,000 × g for 30 minutes at 4°C and 25°C, maintain for 48 hours. Six cycles of centrifugation were performed, and the appearance of these formulations was investigated by using indexes including turbidity, phase separation, precipitation, drug separation, demulsification, and creaming.
Effects of different temperatures and pH values
Effects of the temperatures and pH on particle size and zeta potential of the BSA NEDDS were measured directly. Effects of the increasing temperature in the range 20°C–80°C on particle size were determined by Nano ZS90 at 20°C. Effects of increasing pH in the range 1–14 on particle size and zeta potential were measured by Nano ZS90 at 20°C.
Short- and long-term stability
To examine the physical stability of this system, short- and long-term stability studies were carried out on the selected formulations. Samples were divided into three vials after production and stored at room temperature for 180 days. On days 0, 30, 60, 90, and 180, all samples were examined and centrifuged for 30 minutes at 13,000 × g. Turbidity, phase separation, precipitation, drug separation, breaking, and creaming were evaluated, and also the primary structural integrity was again assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Furthermore, the main physical characteristics, including particle size, polydispersity index, zeta potential, electrophoresis mobility, and conductivity were determined using photon correlation spectroscopy on days 0, 30, 60, 90, and 180 at 20°C.
Stability study of the BSA enclosed in the BSA NEDDS
Relative content and bioactivity assay
The relative content and bioactivity of four samples stored at room temperature for 0, 30, 90, and 180 days were determined. BSA was extracted from the nanoemulsion by using the drug-loading and encapsulation efficiency protocol described previously. The BSA content of the BSA NEDDS, which were stored at room temperature for 0, 30, 90, and 180 days, were detected using microbicinchoninic acid methods. Relative bioactivity was determined by enzyme-linked immunosorbent assay. The methods were performed as follows: 96-well flat-bottom plates (Costar, WA) were coated overnight at 4°C with 50 μg/mL of theoretical protein drug above four samples, blocked with a blocking buffer (10 mM PBS, pH 7.4, with 0.05% Tween 20), and kept at overnight at 4°C. Goat anti-mouse BSA monoclonal antibody (1:300) was used as the primary antibody. And HRP-conjugated goat anti-mouse IgG (1:10,000) was used as the secondary antibody. O-phenylenediamine was used as a substrate. The absorbance of the solutions was then determined using Bio-Rad model 680 microplate reader (Bio-Rad, Hercules, CA) set at a wavelength of 490 nm.
Evaluation of primary structural integrity and specificity
To determine the primary structural integrity and specificity of the BSA NEDDS, sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting were performed. The supernatant and precipitate of a blank nanoemulsion, the supernatant and precipitate of the BSA nanoemulsion, the supernatant and precipitate of a BSA emulsion, and native BSA aqueous solution were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The gels were electroblotted onto polyvinylidene fluoride filter membranes at 25 mV for 15 minutes by using a TransBlot semidry blotter (Bio-Multiphor II electrophoresis unit; GE Healthcare, Amersham, UK), after which they were blocked using a buffer (10 mM PBS, pH 7.4 with 0.05% Tween 20) and kept overnight at 4°C. Subsequently, the membranes were hybridized with goat anti-mouse BSA monoclonal antibody (1:300) for 1 hour at 37°C. HRP-conjugated goat anti-mouse IgG was used as the secondary antibody (1:10,000). Binding reaction was detected using diaminobenzidine color-development reagent (0.05% diaminobenzidine and 0.005% hydrogen peroxide in distilled water).
Evaluation of the secondary structure
To verify the integrity of the secondary structure of the BSA in this NEDDS, circular dichroic spectra of the BSA aqueous solution and three batches of the BSA NEDDS placed at room temperature for 30, 90, and 180 days were acquired at room temperature by using a Chirascan spectropolarimeter (Jasco 810, Easton, MD) equipped with ultraviolet light (190–250 nm) with a 0.01−cm path length and a step size of 1 nm. The lamp housing was purged with nitrogen, and an average of three scans was obtained. BSA was extracted from the NEDDS by using the previously mentioned protocol.
Evaluation of the tertiary structure
To verify the integrity of the tertiary structure of BSA in this NEDDS, we used Fourier transform infrared (FTIR) spectroscopy. FTIR spectra of the BSA aqueous solution and three batches of the BSA NEDDS stored at room temperature for 30, 90, and 180 days were obtained using a Spectrum One spectrophotometer (Lambda 950; PerkinElmer, Waltham, MA) separately 500, 550, 600, and 200 times diluting with water. Four samples were prepared using the potassium bromide disk method and examined using the transmission mode. A spectral range of 400–4000 cm−1 with a resolution of 2 cm−1 (accumulation of 64 scans) was used in order to obtain good-quality spectra. By monitoring the peak frequencies and locations of certain bands, all possible chemical changes and interactions were determined.
Statistical analysis
Values are expressed as means ± standard deviation. Statistical significance was determined using one-way analysis of variance by the least-significant-difference test. P < 0.05 was considered significantly different.
Results
Construction of pseudoternary phase diagrams
Pseudoternary phase diagrams were separately constructed for each Smix ratio (). A Smix with a ratio (Km) of 2:1 in a mixture of Cremophor EL-35 (surfactant), propylene glycol (cosurfactant), isopropyl myristate (oil), and normal saline (aqueous medium) showed a large nanoemulsion area (). shows that the 3:1 Smix formula generated the smallest nanoemulsion area. The area of nanoemulsion increased with Smix proportions of 1:1 and 2:1 (). On the basis of these pseudoternary phase diagrams, we chose the Smix of Cremophor EL-35/propylene glycol at a ratio of 2:1, which showed the largest nanoemulsion area, as the main formulation for the BSA NEDDS.
Figure 1 Pseudoternary phase diagrams of the BSA NEDDS, composed of the following constituents: oil, isopropyl myristate; surfactant, Cremophor EL-35; and cosurfactant, propylene glycol; drug-loading value was 45 mg/mL. (A) Surfactant and cosurfactant mixture ratio (Smix), 1:1; (B) Smix, 2:1; (C) Smix, 3:1.
Note: The red area represents the nanoemulsion delivery system region.
Abbreviations: BSA, bovine serum albumin; NEDDS, nanoemulsion drug-delivery system; IPM, isopropyl myristate.
Preparation of the BSA NEDDS formulation
It is important to determine the surfactant concentration accurately and to use the optimum concentration in the formulation. According to the results of the different concentrations and dosages of BSA, the BSA aqueous solution was added to the mixture of Cremophor EL-35, propylene glycol, and isopropyl myristate. BSA (45 mg/mL) was dissolved in the optimized mixture of 18% Cremophor EL-35, 9% propylene glycol, 3% isopropyl myristate (all w/w) and slowly added to normal saline under gentle stress to produce a transparent nanoemulsion-delivery system using the phase-inversion method.
Characterization of the BSA NEDDS
The droplets in the novel BSA NEDDS appeared dark and the surroundings were bright, such that a “positive” image was observed using transmission electron microscopy (). We randomly selected 500 droplets on the electron micrograph, and calculated the average particle size according to the scale and droplet-diameter measurements and statistical data. The results showed that the droplet particle sizes were mostly within the range of 1–100 nm, with an average size of approximately 21.0 nm. Examination of the atomic-force microscopy images revealed that most of the droplets exhibited spherical morphology, with a mean diameter of approximately 20 nm (). The droplets were solid, with surfaces mostly rugged with sags and crests. No droplets aggregated, especially in the BSA stabilized with the nanoemulsion, suggesting its stability, although proteins in the neighboring droplets might have been in contact with each other. The differences observed among these morphological evaluations were essentially due to the fact in the Nano ZS90, the hydrodynamic radius of the particle was measured. On the contrary, transmission electron microscopy and atomic-force microscopy were performed on nanoemulsions of surface ions and solvent and nonsolvent molecules. Consequently, the actual mean diameter (obtained by transmission electron microscopy) was generally far smaller than the hydrodynamic diameter. shows that the average particle size in the novel BSA NEDDS was 21.8 nm, 75% of the particles were <50 nm and 90% <60 nm in size, and the range of distribution was very narrow. We determined the polydispersity index (PDI) value, which measures the spread of the particle-size distribution, in which a small value indicates a narrow particle-size range.Citation22 In general, the novel BSA NEDDS exhibited a relatively limited size distribution, with a PDI value of 0.170, indicating a close size distribution and good stability of the nanoemulsion due to the reduced Ostwald ripening. shows that the average particle size in the novel BSA NEDDS was −24.21 mV and remained in the −40-mV to 40-mV range. The viscosity, refractive index, and dispersant refractive index were 0.8872 cP, 1.59 nDCitation20, and 1.330, respectively.
Drug-loading and encapsulation efficiency
The bicinchoninic acid method is a basic, classical technique used to detect BSA content. The standard curve equation is y = 0.0008x + 0.1636, r2 = 0.9948, and the linear range is 25–1000 μg/mL. The drug-loading content and encapsulation efficiency of the BSA NEDDS can be measured in comparison to a standard curve. In this study, we obtained high encapsulation efficiency (>90%) and enough drug loading (45 mg/mL).
Stability study of the BSA NEDDS
Thermodynamic stability
After six cycles, the BSA NEDDS did not show any phase separation, turbidity, precipitation, drug separation, demulsification, or creaming. These observations suggest that this system has good thermodynamic stability at temperatures between 4°C and 25°C. Thermostability also differentiates nanoemulsion from emulsions, which are kinetically stable and eventually show phase separation. Therefore, the formulations obtained in our study were tested for thermodynamic stability by using centrifugation and heating–cooling and freeze–thaw cycles. Thermodynamic stability analysis showed no turbidity, phase separation, precipitation, drug separation, demulsification, or creaming before or after centrifugation (13,000 × g, 30 minutes) within the temperature range of 4°C–25°C.
Effects of different temperatures and pH values
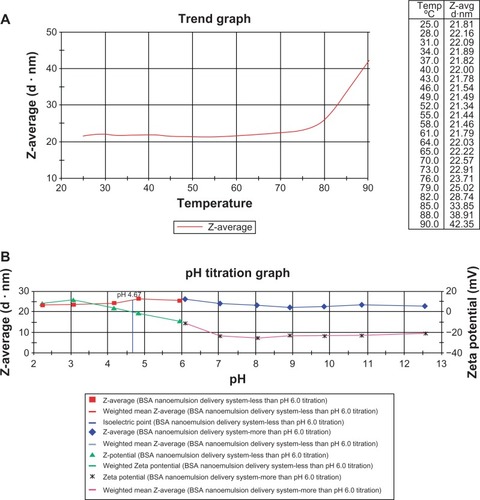
Effects of the increasing temperatures or pH values on the particle size and zeta potential were determined using the Nano ZS90, as shown in . shows the effect of increasing temperature on particle size of the BSA NEDDS. The particle size did not change significantly (it remained in the 20–30-nm range) at temperatures up to 80°C and increased rapidly at higher temperatures (>80°C), but remained under 100 nm. shows that no obvious change in zeta potential was observed within the pH range of 1–13, and the value remained between −40 mV and 20 mV. The average particle size of the novel BSA NEDDS remained at 20–30 nm at different pH values (). These results showed that the average particle size did not obviously change on using high temperature up to 80°C () and that the zeta potential remained in the −40-mV to 40-mV range at different pH values (). The average particle size of the novel BSA NEDDS remained within the range of 20–30 nm at different pH values ().
Figure 3 (A and B) Effect of the different conditions on the BSA NEDDS. (A) Effect of particle size on the increasing temperature of the BSA NEDDS; (B) Effect particle size and zeta potential on the increasing pH values of the BSA NEDDS.
Abbreviations: BSA, bovine serum albumin; NEDDS, nanoemulsion drug-delivery system.
Short- and long-term stability
The stability test showed that the novel BSA NEDDS did not change in visible appearance (ie, flocculation, stratification, precipitation, or creaming and emulsification) either in the natural state or after high-speed centrifugation (13,000 × g, 30 minutes). The main physical characteristics results – particle size, polydispersity index, condition, zeta potential, electrophoresis mobility and melting point – measured using the Nano ZS90 at 20°C are shown in . The BSA NEDDS, which was stored at room temperature for 0, 30, 60, 90, and 180 days, was very stable, and the particle size of this system was within the range of 20–30 nm (). Fluctuations in particle size were very small, and there was no change observed even after storage at room temperature for 180 days. The zeta potential remained between −20 mV and −30 mV (). Melting point and mobility and condition () results showed no obvious changes either. There were also no visible changes in the main physical characteristics of the BSA NEDDS.
Figure 4 (A–F) Long-term stability results for the BSA NEDDS. (A) Particle size; (B) polydispersity index; (C) conductivity; (D) zeta potential; (E) electrophoresis mobility; (F) melting point.
Abbreviations: BSA, bovine serum albumin; NEDDS, nanoemulsion drug-delivery system.
shows that the sodium dodecyl sulfate polyacrylamide gel electrophoresis gels of the BSA aqueous solution and this system stored at room temperature for 30, 60, 90, and 180 days generated a clear band in lanes 1–5. At the same time points, BSA aqueous solution showed no obvious bands in lanes 6–9. These results imply that no changes occurred in the BSA NEDDS when it was stored at room temperature for 180 days.
Figure 5 (A and B) Long-term stability results for BSA in the NEDDS. (A) Primary structural integrity changes in BSA in the NEDDS when stored at room temperature for 180 days. Lane 1, BSA aqueous solution; lane 2, BSA NEDDS stored at room temperature for 30 days; lane 3, BSA NEDDS stored at room temperature for 60 days; lane 4, BSA NEDDS stored at room temperature for 90 days; lane 5, BSA NEDDS stored at room temperature for 180 days; lane 6, BSA aqueous solution stored at room temperature for 30 days; lane 7, BSA aqueous solution stored at room temperature for 60 days; lane 8, BSA aqueous solution placed at room temperature for 90 days; lane 9, BSA aqueous solution stored at room temperature for 180 days; lane 10, prestained protein ladder. (B) Relative content and bioactivity of the BSA in the nanoemulsion delivery system stored at room temperature for 180 days.
Note: No obvious changes in relative content and bioactivity were observed between the fresh samples and BSA NEDDS stored at room temperature for 30, 90, and 180 days. Abbreviations: BSA, bovine serum albumin; NEDDS, nanoemulsion drug-delivery system.
Stability study of the BSA in the BSA NEDDS
Relative content and bioactivity analysis
showed no differences in the relative content among the fresh sample and the samples stored at room temperature for 30, 60, 90, and 180 days. The relative content and bioactivity of these samples did not show any decrease after storage at room temperature for 180 days. The results showed that BSA in the BSA NEDDS has very high stability. BSA retained high bioactivity when the BSA NEDDS was stored at room temperature for 180 days.
Primary structural integrity and structural specificity
The results of the evaluation of structural integrity are shown in , which shows that the aqueous BSA solution (lane 10), the supernatant nanoemulsion (lane 6), and the BSA emulsion (lane 8) each had one bright, clear band, each with a molecular weight of 66.4 kDa. The supernatant and precipitate of the blank nanoemulsion or emulsion had no obvious bands (lanes 1–4). The precipitate of this nanoemulsion-drug delivery system (lane 7) and the emulsion (lane 9) of BSA had one very narrow and dark band at 66.4 kDa. The results for lanes 7 and 9 show that the surfactant and cosurfactant partly precipitate the protein, but the supernatants of the nanoemulsion and emulsion gave more intense bands, so most of BSA in the nanoemulsion and emulsion was present in the supernatant. Meanwhile, the results showing that the supernatant and precipitate of the blank nanoemulsion or emulsion had no band showed that sodium dodecyl sulfate polyacrylamide gel electrophoresis can be adapted to the evaluation of structural integrity. Thus, the structural integrity of the novel BSA NEDDS was well maintained and showed no obvious degradation. In the structural specificity evaluation by Western blotting (), the protein lanes are similar to those observed in , and these bands are close to the 66-kDa markers. Once again, one clear band was evident in the BSA aqueous solution (lane 10), the nanoemulsion supernatant (lane 6), and the BSA emulsion (lane 8), while the supernatant and precipitate from the blank nanoemulsion and emulsion had no obvious bands (lanes 1–4). The precipitates of the novel BSA NEDDS (lane 7) and emulsion (lane 9) lacked clear bands. Meanwhile, the results showing that the supernatant and precipitate of the blank nanoemulsion or emulsion (lanes 1–4) had no bands suggested that Western blotting methods can also be adapted to the evaluation of structural specificity. Overall, good structural integrity and specificity of the BSA NEDDS are demonstrated in . For example, no obvious degradation was detected in the BSA NEDDS, but the supernatant and precipitate of the blank nanoemulsion and emulsion lacked obvious bands (). The supernatant of the nanoemulsion and emulsion showed obvious, intact bands (). These results showed good immunogenicity of the novel BSA NEDDS without obvious degradation of BSA.
Figure 6 (A and B) Primary structural integrity and specificity results for the BSA in the NEDDS. (A) Sodium dodecyl sulfate polyacrylamide gel electrophoresis of BSA; (B) Western bolt analysis of BSA. Lane 1, supernatant of blank nanoemulsion; lane 2, precipitate of blank nanoemulsion; lane 3, supernatant of blank emulsion; lane 4, precipitate of blank emulsion; lane 5, prestained protein ladder; lane 6, supernatant of the BSA nanoemulsion; lane 7, precipitate BSA nanoemulsion; lane 8, supernatant of BSA emulsion; lane 9, precipitate of BSA emulsion; lane 10, BSA aqueous solution.
Abbreviations: BSA, bovine serum albumin; NEDDS, nanoemulsion drug-delivery system.
Integrity of the secondary structure
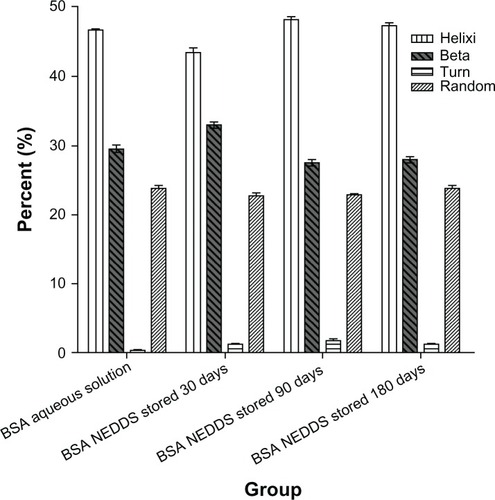
To correlate the small changes in the aromatic environment observed with a possible change in the secondary structure of BSA, we assessed circular dichroic spectra in the ultraviolet region, which corresponds to the protein backbone. shows the circular dichroic spectrum of the BSA aqueous solution in comparison with the circular dichroic spectrum of the BSA extracted from nanoemulsion stored at room temperature for 30, 90, and 180 days. Both spectra showed clear minima of a similar magnitude at 209 nm and 222 nm, which are indicative of the known α-helical conformation of BSA. The reduced maximum at 210 nm indicated a relative loss of the helical structure of BSA following encapsulation and extraction from this NEDDS. In addition, the ratio of α-helix, β-sheets, turns and random is not changed compared with that of the native aqueous BSA solution in , in the secondary structure. These results suggest that BSA underwent adsorption at the interface within the emulsion system, which is commonly associated with some degree of unfolding. Importantly, the similarity characterize peak was about 210 nm and also its main secondary structure ratio of α-helix and β-sheets did not change between the native aqueous BSA solution and its NEDDS when stored at room temperature for 180 days in the circular spectra. Turn and random of BSA showed part change compared the BSA NEDDS with native aqueous BSA solution.
Figure 7 (A–D) Secondary structural spectra of the BSA in the NEDDS. (A) BSA aqueous solution; (B) BSA NEDDS stored at room temperature for 30 days; (C) BSA NEDDS stored at room temperature for 90 days; (D) BSA NEDDS stored at room temperature for 180 days.
Note: Arrows represent BSA absorption peaks.
Abbreviations: BSA, bovine serum albumin; NEDDS, nanoemulsion drug-delivery system.
Integrity of the tertiary structure
The BSA stability of the tertiary structure was measured by FTIR spectroscopy. In and , FTIR spectra of BSA aqueous solutions showed peaks at 3445.54 cm−1, 2073.25 cm−1, 1651.09 cm−1, 1543.11 cm−1, 1455.79 cm−1, 1403.38 cm−1, 1308.93 cm−1, 1245.23 cm−1, 1162.07 cm−1, 1114.44 cm−1, 1019.18 cm−1, 409.45 cm−1, and 401.15 cm−1. The BSA NEDDS stored at room temperature for 30 days showed peaks at 3445.98 cm−1, 2960.90 cm−1, 2928.27 cm−1,1643.72 cm−1, 1547.08 cm−1, 1513.11 cm−1, 1455.28 cm−1, 1388.11 cm−1,1351.77 cm−1, 1293.05 cm−1,1249.53 cm−1, 1181.92 cm−1, 1103.03 cm−1, 943.77 cm−1, and 445.17 cm−1. The BSA NEDDS stored at room temperature for 90 days showed peaks at 3423.61 cm−1, 2975.64 cm−1, 2924.37 cm−1, 2888.64 cm−1, 1924,15 cm−1, 1651.09 cm−1, 1512.45 cm−1, 1454.77 cm−1, 1420.07 cm−1, 1383.24 cm−1, 1332.75 cm−1, 1269.24 cm−1, 1185.99 cm−1, 1088.44 cm−1, 1047.94 cm−1, 947.74 cm−1, and 880.00 cm−1. The BSA NEDDS stored at room temperature for 180 days showed peaks at 3444.22 cm−1, 2975. 21 cm −1, 2539. 36 cm −1, 2412. 35 cm −1, 2133. 42 cm −1, 1923. 02 cm −1, 1651. 08 cm −1, 1519.29 cm−1, 1455.12 cm−1, 1416.10 cm−1, 1383.80 cm−1, 1328.78 cm−1, 1274.41 cm−1, 1185.89 cm−1, 1088.11 cm−1, 1048.26 cm−1, 943.77 cm−1, and 880.10 cm−1. From these data, the BSA aqueous solution and the three samples stored at room temperature for 30, 90, and 180 days were mainly characterized by a band at around 1653 cm−1, which was assigned to the α-helix conformation (1658–1650 cm−1) and can also be observed in free BSA (1653.43 cm−1). The peaks at 3435 cm−1, 1640 cm−1, 1415 cm−1, and 1109 cm−1were due to the stretching of O−H, COO (asymmetric), COO (symmetric), and C−O−C, respectively. The characteristic band of the BSA proteins at 1650 cm−1 and 1530 cm−1 are due to the C=O stretching band of the carboxyl functional group on the tryptophan moiety as well as the carboxylate group. The FTIR spectroscopy results showed that the tertiary structure of BSA did not change in comparison with that of the BSA in the aqueous solution and the three samples stored at room temperature for 30, 90, and 180 days.
Table 1 Fourier transform infrared spectra results of the bovine serum albumin (BSA) nanoemulsion drug-delivery system (NEDDS)
Discussion
In this study, we successfully developed the BSA NEDDS with a high encapsulation efficiency (>90%) and drug-loading (45 mg/mL) using the phase-inversion method and the pseudoternary phase diagram. The entrapment efficiency and drug loading of this delivery system was measured by a microbicinchoninic acid assay, which is a traditional method for measuring the protein content of a drug.Citation27 The BSA loaded into the nanoemulsion was separated by centrifugation, and the supernatant was analyzed by a microbicinchoninic acid assay. Centrifugation is normally used to determine the stability of a delivery system after emulsion/nanoemulsion formation. Importantly, it is a standard for distinguishing between nanoemulsion and traditional emulsions. It is also the easiest and most widely used and effective method for separating molecules. We converted the nanoemulsion to an emulsion with absolute ethanol and separated BSA by high-speed centrifugation (13,000 × g, 30 minutes). For comparison, soy protein isolate emulsions and gels were centrifuged in a microcentrifuge tube at 10,000 × g for 20 minutes at room temperature.Citation28 These results also confirmed that this delivery system is thermodynamically stable in these particular concentrations of oil, surfactant, and water, with no phase separation, creaming, or cracking.Citation29
These results show that the stability of a protein drug can be improved greatly in our research, which we attribute to the following characteristics of this system. First, we used a novel nanoemulsion-drug delivery system, which is one of the most promising techniques for the delivery of drugs. Its advantages include higher storage stability and thermodynamic stability.Citation30–Citation32 We chose the nanoemulsion-drug delivery system formula to apply to a protein drug because it has a gel phase that forms through the preparation process. Second, we obtained the largest area of this nanoemulsion-drug delivery system by using pseudoternary diagrams to determine the best formula. Phase diagrams that provided information about the boundaries between different phases as a function of composition and other variables, such as temperature, were constructed. From this information, structural organization can be inferred.Citation33 Therefore, the choice of oil and surfactant and the mixing ratio of oil to surfactant/cosurfactant are key issues in producing the nanoemulsion. The use of the phase-inversion method, a spontaneous low-emulsification-energy method also called the titration method, to prepare the BSA NEDDS is also important. Such systems can be prepared simply by mixing oil, water, surfactant, and cosurfactant in the appropriate proportions, followed by mild agitation. The order of component mixing is generally not critical, because the nanoemulsion-drug delivery system is formed spontaneously. Nevertheless, the driving forces are small, and it can take a long time for the system to reach equilibrium.Citation34 Aqueous titration or spontaneous emulsification has been successfully investigated for preparing nanoemulsions of many lipophilic drugs in previous articles.Citation34 Some studies on the nanoemulsion-delivery system have frequently used high-energy methods, especially homogenization.Citation22 Homogenization pressure significantly influences the properties of emulsions because the shear forces and turbulence produced during homogenization, both of which are pressure-dependent, affect the particle size and size distribution.Citation35 It has also been shown that disruption of any of these interactions will shift this delicate balance and destabilize the protein. The chemical and physical stability of proteins can also be compromised by environmental factors, such as pH, ionic strength, temperature, high pressure, nonaqueous solvents, metal ions, detergents, adsorption, and agitation and shearing.Citation36 We chose the phase-inversion method, which is a low-energy emulsification method, to avoid destabilizing the protein drug. Finally, the BSA NEDDS formula does not include any physical reagents such as inorganic solvents (such as methylene chloride or acetone) that might destroy the protein drug, in contrast to other delivery systems. We used simpler preparation techniques compared to those used to generate microparticles, nanospheres, and nanoparticles, which may also contribute to the enhanced stability of the protein drug.
It is known that the stability of protein drugs remains one of the key hurdles to their successful entry into the market. Therefore, many factors must be considered for these drugs: structural integrity and specificity of the primary, secondary, and tertiary protein structures, relative content, and biological activity. Many traditional methods are very sensitive and useful for evaluating the structural integrity and specificity of proteins, including centrifugation, micro-BCA method, sodium dodecyl sulfate polyacrylamide gel electrophoresis, Western blotting, enzyme-linked immunosorbent assay, circular dichroic spectrometry, and FTIR spectrometry. In this study, the blank nanoemulsion or emulsion did not interfere with the identification of BSA in the nanoemulsion. The essential characteristics and thermodynamic stability of the novel BSA NEDDS were also evaluated. BSA has been widely used with many drug-delivery systems such as nanoparticles, microparticles, emulsions, gels, and liposomes because it is a common and model protein. In this study, a novel BSA NEDDS was designed and successfully prepared by the phase-inversion method and using pseudoternary phase diagrams. The results of stability tests, including assessments of particle size and zeta potential under different temperatures or pH values and the integrity of the primary, secondary, and tertiary structures of BSA, have shown that the BSA NEDDS is very stable and greatly enhances the stability of the protein drug BSA. These data will enhance the reliability of the nanoemulsion-drug delivery system for use as an oral protein drug-delivery system and change the route of drug delivery, ie, injection, and the requirement of freezing drugs. In future, we intend to study some more advanced methods involving high-performance liquid chromatography, capillary electrophoresis, and protein drugs such as human serum albumin. Also, the stability of the BSA nanoemulsion-delivery system stored at different temperatures of 4°C, 25°C, 40°C, and 45°C and 65°C should be researched further.
Conclusion
In the present study, a NEDDS for oral administration of the standard-model protein drug BSA was successfully developed. The optimum nanoemulsion formulation was a mixture of Cremophor EL-35, propylene glycol, and isopropyl myristate at a ratio of 18:9:3 (w/w/w). The BSA NEDDS showed a narrow particle-size distribution, with a PDI value of 0.170, a spherical average particle size of 21.8 nm, and zeta potential of −24.8 mV. This nanoemulsion-drug delivery system is very stable with high encapsulation efficiency and 45 mg/mL drug loading. It also ensured good integrity of the primary, secondary, and tertiary structures; the system showed good specificity and bioactivity when stored at room temperature for 180 days. These results show that the NEDDS can greatly enhance the stability of a protein drug while retaining good integrity, specificity, and bioactivity. Thus, this delivery system can greatly enhance stability and be employed for oral administration of protein drugs.
Acknowledgements
Thanks to China National Key Hi-Tech Innovation Project for the R&D of Novel Drugs (No 2008ZX09101-033) and National Natural Science Foundation of China (No 81172892). We are grateful to the Shanghai Laboratory of Malvern Instruments, UK, for the zeta-potential and particle-size measurements. We are also grateful to Xueheng Yang of Chongqing University for detecting the BSA NEDDS by atomic-force microscopy.
Disclosure
The authors report no conflicts of interest in this work.
References
- CapelleMAGurnyRArvinteTHigh throughput screening of protein formulation stability: practical considerationsEur J Pharm Biopharm200765213114817107777
- SakulkuUNuchuchuaOUawongyartNPuttipipatkhachornSSoottitantawatARuktanonchaiUCharacterization and mosquito repellent activity of citronella oil nanoemulsionInt J Pharm20093721–210511119162149
- AlmeidaAJSoutoESolid lipid nanoparticles as a drug delivery system for peptides and proteinsAdv Drug Deliv Rev200759647849017543416
- LeeKYYukSHPolymeric protein delivery systemsProg Polym Sci2007327669697
- BilatiUAllémannEDoelkerEStrategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticlesEur J Pharm Biopharm200559337538815760718
- RathoreNRajanRSCurrent perspectives on stability of protein drug products during formulation fill and finish operationsBiotechnol Adv2008243504514
- Carrillo-NavaEDohnalVCostasMInfinite dilution activity coefficients for toluene in aqueous solutions of the protein stabilizers glycerol, ethylene glycol, glucose, sucrose and trehaloseJ Chem Thermodyn2002344443456
- SunHWLiuWZouQMHelicobacter pylori vaccine delivery systems: research progressJ Int Pharm Res2009366431434 Chinese
- YangLCuiFCunDTaoAShiKLinWPreparation, characterization and biodistribution of the lactone form of 10-hydroxycamptothecin (HCPT)-loaded bovine serum albumin (BSA) nanoparticlesInt J Pharm200734016317217482779
- RodriguesMMASimioniARPrimoFLSiqueira-MourabMPMoraisPCTedescoACPreparation, characterization and in vitro cytotoxicity of BSA-based nanospheres containing nanosized magnetic particles and/or photosensitizerJ Magn Magn Mater2009321016001603
- WangYWangXLuoGDaiYAdsorption of bovin serum albumin (BSA) onto the magnetic chitosan nanoparticles prepared by a microemulsion systemBioresour Technol20089993881388417892932
- ZhangCChengYQuGPreparation and characterization of galactosylated chitosan coated BSA microspheres containing 5-fluorouracilCarbohydr Polym2008723390397
- KimBSOhJMKimKSBSA-FITC-loaded microcapsules for in vivo deliveryBiomaterials2009321090290919027943
- SharmaNBansalMVishtSSharmaPKKulkarniGTNano-emulsion: A new concept of delivery systemChronicles of Young Scientists20101226
- ChenHKhemtongCYangXChangXGaoJNanonization strategies for poorly water-soluble drugsDrug Discov Today2011167–835436020206289
- GaoFZhangZBuHNanoemulsion improves the oral absorption of candesartan cilexetil in rats: performance and mechanismJ Control Release2011149216817420951749
- ShafiqSShakeelFKharRKEnhanced stability of ramipril in nanoemulsion containing cremophor-EL: a technical noteAAPS Pharm Sci Tech20089410971101
- ShakeelFBabootaSAhujaAAliJFaisalMSShafiqSStability evaluation of celecoxib nanoemulsion containing Tween 80Thai J Pharm Sci20083249
- KongMParkHJStability investigation of hyaluronic acid based nanoemulsion and its potential as transdermal carrierCarbohydr Polym201183313031310
- KongMChenXParkHJDesign and investigation of nanoemulsified carrier based on amphiphile-modified hyaluronic acidCarbohydr Polym2011832462469
- WangLLiXZhangGDongJEastoeJOil-in-water nanoemulsions for pesticide formulationsJ Colloid Interface Sci2007314123023517612555
- YuanYGaoYZhaoJMaoLCharacterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditionsFood Res Int20084116168
- SilvaHDCerqueiraMASouzaBWSNanoemulsions of β-carotene using a high-energy emulsification–evaporation techniqueJ Food Eng2011102130135
- KlangVMatskoNZimmermannAMVojnikovicEValentaCEnhancement of stability and skin permeation by sucrose stearate and cyclodextrins in progesterone nanoemulsionInt J Pharm20103931–215216020434531
- BakerJRJrBielinskaALandersJJanczakKCaoPEnhanced systemic and mucosal immune responses in mice immunized with recombinant Bacillus anthracis protective antigen (rPA) using a novel nanoemulsion adjuvantJ Allergy Clin Immunol20041132S292
- ShafiqSSushmaTAhmadFJDesign and development of oral in water rampipril nanoemulsion formulation: in vitro and in vivo evaluationJ Biomed Nanotechnol200732844
- GutierroIHernándezRMIgartuaMGascónARPedrazJLSize dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheresVaccine2002211–2677712443664
- XinGuLydiaJEustonSREffects of different oils on the properties of soy protein isolate emulsions and gelsFood Res Int2009428925932
- ShafiqSShakeelFEffect of Labrasol on self-nanoemulsification efficiency of ramipril nanoemulsionPharmazie2009641281281720095139
- ShakeelFRamadanWAhmedMAInvestigation of true nanoemulsions for transdermal potential of indomethacin: characterization, rheological characteristics, and ex vivo skin permeation studiesJ Drug Target200917643544119527114
- BaliVAliMAliJNovel nanoemulsion for minimizing variations in bioavailability of ezetimibeJ Drug Target200118750651920067438
- PatelARVaviaPRPreparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrateAAPS Pharm Sci Tech200793E344E352
- Shafiq-un-NabiSShakeelFTalegaonkarSFormulation development and optimization using nanoemulsion technique: a technical noteAAPS Pharm Sci Tech200782E12E17
- Shafiq-un-NabiSShakeelFTalegaonkarSFormulation development and optimization using nanoemulsion technique: a technical noteAAPS Pharm Sci Tech2007282832
- FlouryJDesrumauxALardièresJEffect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsionsInnov Food Sci Emerg Technol200012127134
- WangWInstability, stabilization, and formulation of liquid protein pharmaceuticalsInt J Pharm1999185212918810460913