Abstract
Many types of nanoparticles (NPs) are tested for use in medical products, particularly in imaging and gene and drug delivery. For these applications, cellular uptake is usually a prerequisite and is governed in addition to size by surface characteristics such as hydrophobicity and charge. Although positive charge appears to improve the efficacy of imaging, gene transfer, and drug delivery, a higher cytotoxicity of such constructs has been reported. This review summarizes findings on the role of surface charge on cytotoxicity in general, action on specific cellular targets, modes of toxic action, cellular uptake, and intracellular localization of NPs. Effects of serum and intercell type differences are addressed. Cationic NPs cause more pronounced disruption of plasma-membrane integrity, stronger mitochondrial and lysosomal damage, and a higher number of autophagosomes than anionic NPs. In general, nonphagocytic cells ingest cationic NPs to a higher extent, but charge density and hydrophobicity are equally important; phagocytic cells preferentially take up anionic NPs. Cells do not use different uptake routes for cationic and anionic NPs, but high uptake rates are usually linked to greater biological effects. The different uptake preferences of phagocytic and nonphagocytic cells for cationic and anionic NPs may influence the efficacy and selectivity of NPs for drug delivery and imaging.
Introduction
Nanoparticles (NPs) can be applied in the medical sector as sensors, in cell and organ imaging, drug delivery, implants, and implant coatings. Surface charge is the most important factor affecting NPs in terms of their function in imaging and drug delivery. In these applications, inorganic carbon, metal, metal oxides, and sulfides as well as a variety of organic and biodegradable NPs were used (). Many NPs are tested in preclinical studies, but only polymer-based, lipid-based, protein-based NPs and nanocrystals are approved for drug delivery, while iron oxide NPs are in clinical use for magnetic resonance imaging and drug delivery. Most approved NP formulations are formulations of conventional compounds for improved drug delivery, particularly in oncology.
Table 1 Overview of nanoparticle (NP) formulations in development for imaging and drug delivery with examples for approved drugs, with indication of the most important fields of applicationCitation172
Reasons for the relatively low number of approved particles are, among others, problems in reproducibility and long-term stability of NP formulations and lack of guidelines for relevant biological testing. The attachment of functional groups and coatings to prevent uptake by the reticuloendothelial system increases the variety of NP preparations. As each parameter can be varied, a great number of NPs could be designed. For a faster development of efficient particles, it would be very useful to identify correlations of specific surface properties to cellular effects. Studies on polystyrene particles, where size and charge can be changed in a controlled way, have been widely used as models.
Positively charged constructs are used in nonviral gene transfection, and studies on gene-delivery systems with cationic liposomes and cationic polymers help to understand the role of positive surface charge. Cationic lipid/DNA complexes (lipoplexes) enter cells by endocytosis or direct penetration through the cell membrane after interaction of the cationic lipopolyamines with proteoglycans of the cell membrane. For subsequent delivery of DNA to the nucleus, degradation in the lysosomes is prevented by different mechanisms. Lipoplexes have protonable amine groups that slow down the acidification of endosomes, and thereby slow down endosome–lysosome transition.Citation1 Xu and SzokaCitation2 proposed the following mode of action: anionic lipids from the cytoplasmic facing monolayer of the endosome flip-flop in the membrane and diffuse laterally to form charge neutral ion pairs with the lipoplexes. Thereby, the DNA is released from the lipoplex and from the endosome. The mechanism of gene delivery by cationic polymers (polyplexes) is slightly different. Cationic polymers form complexes with the negatively charged DNA, and still possessing a net positive surface charge, bind to the negatively charged plasma membrane of the target cells to a higher degree than negatively charged or neutral molecules.Citation3 For release of the genetic material, these complexes are transported via the endosomal–lysosomal system into the endosomes where these complexes are cleaved by enzymes into polyamines and DNA. The polyamines buffer H+ and cause lysosomal Cl accumulation with subsequent osmotic swelling and lysis of the endosomes, thereby preventing degradation of the DNA by lysosomal nucleases. This mechanism is termed the “proton sponge” effect. The released DNA passes to the nucleus and integrates into the nuclear DNA.
The use of cationic NPs is limited by their cytotoxicity. For poly(propylene imine) dendrimers, other candidates for nonviral gene transfer, the relation of primary amine groups and toxicity has been clearly shown.Citation4 Shielding of the amine groups by functionalization decreased the toxicity of these constructs.Citation5 This review aims to clarify if cationic NPs interact with other cellular targets, act by other cytotoxic mechanisms and use other uptake routes than anionic and neutral NPs.
Cytotoxicity
The cytotoxicity of NPs depends on particle parameters like morphology, such as aspect ratio/shape and size. Hydrophobicity, surface area in terms of roughness and porosity, and surface charge influence the capacity to produce reactive oxygen species (ROS), determine binding sites for receptors, and influence dispersion and aggregation of the particles. Cytotoxicity is also often due to contamination, solubility and release of toxic components, and adsorption of compounds. On the other hand, biological parameters such as cell type used for the study or the culture and exposure conditions (eg, cell density, particle concentration, medium composition, temperature), also influence cytotoxicity.
Main influencing factors for cytotoxicity are material, size, shape, composition, surface charge, and surface hydrophobicity. The correlation of cytotoxic effect and size has been studied in many papers. For nonphagocytic cells, small size correlates with increased cytotoxicity. In vitro experiments showed higher cytotoxicity of well-dispersed mesoporous silica and amorphous silica, dolomite, ZnO, Ni, Ag, and polystyrene NPs compared to the respective microparticles.Citation6–Citation15 When particles smaller than 100 nm are compared, still-smaller particles act more toxically than larger ones (quantum dots,Citation16 TiO2Citation17). In contrast to these studies, no differences have been reported for 10–100 nm silica particles compared to 45 μm ones,Citation18 and for nickel ferrite NPs.Citation19 Okuda-Shimazaki et al demonstrated the importance of the aggregation state and showed that larger aggregates of TiO2 NPs acted more cytotoxically than smaller ones.Citation20
Phagocytes such as macrophages and monocytes react more strongly to microparticles than to NPs. One study reported higher cell damage for silica microparticles than for NPs,Citation21 and another study noticed absence of cell damage in THP-1 cells for 30–70 nm silica NPs, while 1000 nm particles acted cytotoxically.Citation22
Compared to nonphagocytic cells, THP-1 cells are also much more resistant to 20–200 nm silver and to 21 nm TiO2 NPs.Citation23 Size-dependent toxicity studies in vivo are less conclusive: systemic toxicity upon intraperitoneal application of 10 nm and 50 nm iron oxide particles was higher than that after dosing with 1000 nm particles.Citation24 When applied intraocularly however, 4000 nm magnetic iron oxide particles caused more toxicity than 50 nm particles.Citation25
It is also generally accepted that fiber-shaped NPs of a given material are more reactive and toxic compared with spherical particles: carbon nanotubes, for instance, are generally more toxic than fullerenes.Citation26,Citation27 Hydrophobicity is often linked to surface charge, but at the same surface charge, NPs with hydrophobic surfaces, eg, oleic acid-coated nickel ferrite and stearic acid-coated TiO2 particles reacted more cytotoxically than the respective noncoated particles.Citation19,Citation28 In the following sections, the influence of charge on cytotoxicity and cellular uptake will be described in more detail.
Charge-dependent cytotoxicity
For cytotoxic action, both charge density and charge polarity play a role. Charged NPs, eg, gold particles, are more cytotoxic than neutral forms,Citation29 and positively charged ZnO, silica, silica-titania hollow, and gold nanoparticles act more cytotoxically than negative variants of similar size in nonphagocytic cells.Citation30–Citation34 Cytotoxic action of poly(amidoamine) (PAMAM) dendrimers is correlated with the number of primary amino groups,Citation35 and cytotoxicity of PAMAM dendrimers decreased when amine groups were neutralized with acetyl groups.Citation36 Also, in in vivo experiments, high numbers of primary amine groups increased the toxicity of dendrimers.Citation37 This rule, however, does not apply to all NPs. For some NPs, eg, poly(lactic-co-glycolic acid) (PLGA) particles, charge appears to play no role,Citation38 or other parameters, eg, porosity for mesoporous SiO2 particles, are more important than surface charge.Citation39 The lack of negative effects of positively charged PLGA particles could be due to the use of chitosan, a polysaccharide with excellent biological properties, as coating material.Citation38 Shielding of cationic groups by functionalization and polethylene glycol (PEG)ylation decreased both cytotoxicity and efficacy in NPs where efficacy and cytotoxicity were linked to cationic charge.Citation40
In contrast to nonphagocytic cells, phagocytic cells preferentially interact with negatively charged particles, presumably due to the ingestion of bacteria, which also displays a net negative charge.Citation41 The stronger interaction of phagocytes with negatively charged particles may be the reason for the higher cytotoxicity of anionic cyanoacrylic NPs compared to cationic ones.Citation42 In line with the low importance of cationic charge for macrophage uptake and cytotoxicity, shielding of the positive surface charge by PEGylation displayed only a small effect on cellular uptake and cytotoxicity in these cells, whereas marked decrease in membrane damage, lipid peroxidation, and oxidative stress were seen in nonphagocytic neuroblastoma cells.Citation43 It would, however, be oversimplistic to explain these effects only by neutralization of the surface charge, because both functionalization and coating also markedly increase particle size, another key parameter for NP cytotoxicity. Conclusions on surface-charge effects, therefore, are only valid when comparing functionalized or nonfunctionalized particles of similar sizes. When comparing functionalized PLGA NPs with different coatings for tumor targeting, the cationic NPs were slightly more effective than anionic ones, and both accumulated to a higher extent in tumor tissue than bare Pluronic-coated ones.Citation44
In general, NPs may interact with a variety of cellular targets to cause adverse effects ().
Figure 1 Targets for cytotoxicity of nanoparticles (NPs).
Notes: NPs may act through extracellular generation of reactive oxygen species (ROS) (1), they may physically damage the plasma membrane by causing holes (2) or bind to membrane proteins like nicotinamide adenine dinucleotide phosphate-oxidase (3), Ca2+ channels (4), and membrane receptors (5), thereby inducing oxidative signaling, increasing intracellular Ca2+ levels and activating second-messenger cascades. Inside the cells, NPs may interfere with mitochondrial metabolism (6), causing generation of radicals and induction of apoptosis. Intracellular ROS generation by NPs or by metals from lysosomal degradation (7) as well as lysosomal disruption (8) and direct binding to components of the cytoskeleton (9) and the induction of structural alterations of proteins (10) are additional modes of toxic actions. In the nucleus, interference with the transcription machinery and oxidative damage of the DNA (11) may occur.
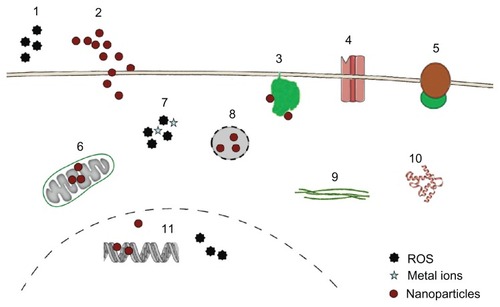
Plasma membrane
NPs may cause focal dissolution of the plasma membrane and hole formation and perturbation of the internal membrane structure. Focal dissolution by carbon particles and loss of membrane folds induced by brookite NPs in exposed cells were observed by electron microscopy.Citation45,Citation46 Plasma-membrane folds in Madin-Darby canine kidney cells (MDCK) disappeared upon exposure to brookite NPs.Citation46 The authors speculated that generation of ROS induced lipid peroxide formation in the membranes, thereby decreasing their flexibility. Wang et al,Citation47 by contrast, showed passive penetration of quantum dots by increasing membrane fluidity. These effects, however, do not inevitably lead to cell death. To repair plasma-membrane damage, either by pore formation through endogenous factors (complement, perforin) or by exogenous factors (bacterial toxins), cells possess several repair mechanisms. Disruption of membrane integrity leads to influx of Ca2+ and can be repaired by exocytosis of internal membranes, endocytosis of the permeabilized site, and shedding of the injured membrane through microparticle formation.Citation48 Repair of the membrane occurs within seconds, and the remodeling of the cortical actin takes a few minutes.Citation49
Electrophysiological measurements and studies with unilamellar lipid vesicles indicated the transient disruption of plasma-membrane integrity upon the passive entry of silica NPs into cells.Citation50,Citation51 Pores < 1 μm in diameter can be closed by sealing the plasma membrane around the hole, and it is likely that plasma-membrane damage by NPs that led to decreased viability exceeded the repair capacity of the cells.
To get insight into the molecular mechanism of plasmamembrane damage, several groups used supported lipid bilayers.Citation52,Citation53 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) lipid bilayers supported on solid substrates do not exactly represent the composition of the plasma membrane in vivo, because they usually consist of only one type of lipid and lack the asymmetric distribution of the lipids and the presence of proteins in real plasma membranes, but they can mimic mechanical processes and metabolism of the plasma membrane quite well.
Using bilayer models and computer models of DMPC, the influence of size and surface charge on the interaction with lipids and hole formation was studied and several mechanisms identified.Citation54 Large cationic G7 PAMAM dendrimers were able to cause holes in intact bilayers, whereas the smaller cationic G5 dendrimers increased the size of preexisting holes but did not generate new holes. Neutral dendrimers adsorbed to the edges of preexisting holes,Citation55 and removed lipids from the edge of the hole, and formed dendrimer–lipid aggregates.Citation56 According to Lin et al, cationic gold particles (2.2 nm) can disrupt 20 × 20 nm lipid bilayers but not 28 × 28 nm lipid bilayers.Citation53 The capacity for hole formation was influenced by the density of the particle’s cationic charge, the negativity of the lipid bilayer, surface tension, temperature, and salt concentration. Simulation using coarse-grained representations suggests that the degree of gold particle–cell interaction can be tuned by variation of the surface charge. Strongly cationic particles create defective areas across the entire surface of the outer leaflet of the bilayer, and a hydrophilic pore with highly disordered lipids at the edge is formed.Citation52 In these models, cationic NPs could penetrate better through plasma membranes than anionic particles.
NPs can also cause effects at the plasma membrane by interaction with membrane-bound proteins. Binding to nicotinamide adenine dinucleotide phosphate-oxidase leads to generation of ROS,Citation57 activation of voltage-gated Ca2+ channels to intracellular Ca2+ changes,Citation58 and the activation of membrane receptors to activation of the second-messenger pathways.Citation59,Citation60 Binding to membrane receptors is intended for therapeutic interventions, eg, the binding of human epidermal growth-factor receptor 2 (HER2)-coated NPs in diagnosis and treatment of HER2 high-expressing breast carcinoma cells.Citation61 Also, uncoated, nontargeted NPs bind to epithelial growth factor receptor and β1 integrin receptors and activate the respective signaling pathways.Citation62
Intracellular targets of NPs are mitochondria, lysosomes, nucleus, and intracellular proteins.
Mitochondria
Swelling of mitochondria occurred after cellular exposure to quantum dotsCitation63 and decrease of the mitochondrial membrane potential has been reported for silver, TiO2 and alumina NPs.Citation64–Citation66 The increase in mitochondrial membrane permeability was induced either by disruption of the respiratory chain or by changes in Bax and Bcl-2 expression, which lead to disruption of mitochondrial metabolism, increased ROS production, adenosine diphosphate-induced depolarization, release of cytochrome C, and induction of apoptosis.Citation67,Citation68 Whereas no obvious morphological damage of lysosomes and mitochondria was reported for carboxyl polystyrene particles of different sizes,Citation10 amine-functionalized polystyrene particles damaged mitochondria and lysosomes in astrocytoma cells.Citation69
Lysosomes
Lysosomes are likely targets for ROS-producing NPs because they are very sensitive to oxidative stress.Citation70 Healthy lysosomes may increase the cytotoxicity of NPs by the release of leachable metal ions (eg, from iron oxide NPsCitation71), which then generate cellular oxidative stress. Lysosomes as targets for cytotoxicity have been revealed for quantum dots and silicon NPs. Costaining with lysosome markers revealed swollen lysosomes upon exposure to quantum dots.Citation72 Other groups reported morphological alterations upon exposure to cationic polystyrene particlesCitation69 and cytotoxicity of silicon NPs caused by permeabilization of lysosomes.Citation73 Especially for cationic NPs and polymers, swelling and disruption of lysosomes due to buffering of H+ is a major mode of cytotoxic action.Citation74 When lysosomal membranes are damaged, a high amount of hydrolytic enzymes is released, leading to degradation of intracellular macromolecules. Independent from the release of hydrolytic enzymes, a correlation of cytotoxicity and lysosomal localization has been described for CeO2 NPs.Citation75 Anionic CeO2 NPs were taken up into lysosomes and caused cell death, whereas cationic NPs were localized in the cytoplasm of viable tumor cells. The extent of cellular uptake was not correlated with this cytotoxicity, and it was not clear from this study how lysosomal localization was linked to cytotoxicity.
Autophagy, the intracellular disposal mechanism to remove and degrade undesirable substances, can be activated by cellular stress. The cellular amount of autophagosomes upon exposure to gold NPs, iron oxide NPs, fullerenes, carbon nanotubes, and quantum dots was increased due to oxidative stress, disruption of cytoskeleton, and mitochondrial damage.Citation76–Citation79 In the absence of metals, either as an integrative part of the particles or as contamination, accumulation of autophagosomes has only been reported in cells exposed to NPs with positive surface charge, cationic polymeric NPs, polyplexes, and cationic dendrimers, and not for anionic NPs.Citation80–Citation82
Nucleus
NPs may inhibit cell division and arrest cytokinesis, an action often seen in combination with other effects on DNA. Many NPs (<50 nm) can get into the nucleus,Citation67,Citation83 but localization in the nucleus is not a prerequisite for action on the DNA because intracellular NPs can gain access to the genetic material during mitosis when the nuclear membrane breaks down. In earlier descriptions of the nuclear pores, passage of particles as large as 25 nm has been reported.Citation84 Later studies report the nuclear pore as an hourglass-like channel with a diameter of 45–70 nm.Citation85 In both studies, the dynamic size of the pore was mentioned, which also allows the entry of larger (90 nm) nuclear-targeted NPs into the nucleus.Citation86 The access to the nucleus, in addition to size, depends on surface charge: noncharged silica NPs can enter the nucleus, whereas the same particles are retained in the cytoplasma when they are functionalized with amine or carboxyl groups.Citation87
Studies on isolated DNA revealed thermal stabilization by cationic but not by anionic poly(l-lysine) NPs.Citation88 This interaction may present a mechanical obstacle to polymerase motion along the DNA chain, leading to inhibition of transcription.Citation89 Also, aberrant clusters of topoisomerase I induced by SiO2 NPs can cause alterations in DNA transcription.Citation90 Genotoxic effects by NPs occur either directly or by oxidative damage of DNA. The consequences of ROS in the nucleus are point mutations in the DNA and double-strand breaks, which have been accused of causing alterations of DNA structure, mitosis, and transcription. High surface activity in the form of ROS generation or through Ti–O or Ti–N bonds could cause DNA alterations induced by Ag and TiO2 NPs.Citation67,Citation91 Neither cationic nor anionic polystyrene particles interacted with chromosome reorganization.Citation92 Extranuclear inhibition of translation can occur through interference of NPs with mRNA-stabilizing proteins.
Intracellular proteins
NPs have a high affinity to macromolecules, particularly to proteins. This binding may increase protein stability, decrease it and interfere with protein function, or have no effect on the protein.Citation93 Intracellular TiO2 NPs induced conformational changes in tubulin and inhibited tubulin polymerization,Citation94 and thereby could impair cell division, cellular transport, and cell migration. NPs such as CeO2, quantum dots, copolymer particles, and carbon nanotubes may also lead to protein aggregation and fibrillation.Citation95 The formation of protein aggregates may promote the development of several neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and prion diseases. Fullerenes, polymeric NPs, and quantum dots have also been reported to prevent the formation of protein aggregates in diseases like Alzheimer’s, and potentially could be useful for the prevention of these diseases.Citation96–Citation98
Dendrimers, carbon nanotubes, alumina NPs, and chitosan NPs modulate the architecture of intercellular tight junctions by disruption and thereby decrease the transepithelial electrical resistance of cell monolayers.Citation64,Citation99–Citation101 Lipid NPs do not affect tight junction proteins, and silver NPs increase the barrier function of endothelial monolayers.Citation102,Citation103 The role of surface charge on these effects is largely unknown.
The different sensitivity of plasma membrane and intracellular organelles to NPs’ surface charge may lead to charge-dependent modes of cytotoxicity. On this topic, however, few studies are available.
Charge-dependent differences in the mode of cytotoxic action
It appears that positively charged NPs either directly or by detachment of adsorbed polymers (eg, polyethylenimine) cause membrane damage, whereas anionic particles cause intracellular damage. Although the mechanism of damage by anionic particles is not clear, a correlation of lysosomal localization and cytotoxicity has been identified for nanoceria particles.Citation75 In one study, where variations of size (30 nm, 150 nm, 500 nm) and surface charge (cationic, anionic, neutral) were evaluated, the relation of surface charge to cytotoxicity was more complex. In the 30 nm and 500 nm zeolite particles, surface charge had only a small effect on cytotoxicity, but marked differences between positively and negatively charged 150 nm zeolite particles were seen in epithelial (human embryonic kidney cells).Citation104 This may be due to the fact that 150 nm particles possessed the highest charge densities. Amine-functionalized NPs acted more by disruption of membrane integrity, whereas carboxyl-functionalized ones induced apoptosis to a greater extent. In macrophages (RAW cells); however, the 150 nm carboxyl-functionalized particles showed more membrane disruption and more apoptosis than the ones with amine and thiol surface functionalization, corroborating the specific role of anionic charge for macrophages.
Serum effects
Coating with bovine serum albumin (BSA) or the presence of serum in the incubation medium reduced cytotoxicity for many NPs. Polystyrene particles, PLGA particles, polysaccharide NPs, and iron oxide NPs acted less cytotoxically on nonphagocytic cells in the presence of serum.Citation3,Citation9,Citation105,Citation106 Particularly, serum reduces the effects on membrane integrity. Potential causes for the mitigating effect of protein include instability of the suspension in the presence of proteins and masking of the reactive surface of the NPs, avoiding the interaction of the NPs with the plasma membrane and the generation of ROS. The decreased cytotoxicity in the presence of serum was usually correlated with a lower cellular uptake.Citation105 In phagocytic cells, where increased cytotoxicity in the presence of serum was reported,Citation107 serum coating is known to increase the cellular uptake of particles.Citation108
Cellular uptake
Similar to cytotoxicity, cellular uptake is influenced by size, shape, material, surface charge, and surface hydrophobicity. Nonphagocytic cells take up spherical NPs between 20 and 50 nm at the highest rates.Citation61,Citation109–Citation112 Enterocytes are an exception to this rule, because they preferentially ingest particles in the range between 100 and 200 nm.Citation113 Phagocytic cells, by contrast, preferentially ingest particles between 2 and 3 μm,Citation114 and phagocytose NPs to a lower extent. Phagocytes contain a higher amount of small supermagnetic iron oxide particles than of ultrasmall supermagnetic iron oxide particles,Citation115 and they phagocytose particles < 300 nm less well than 5 μm particles.Citation116 Well-dispersed 20–200 nm silver particles are taken up by phagocytic (THP-1) cells to a lower degree than by nonphagocytic (A549 and HepG2) cells.Citation23 Aggregates of silver NPs, however, are taken up by phagocytes to a higher extent.Citation117
For iron oxide particles, size appears to be a stronger determinant for uptake than surface charge.Citation118
Charge-dependent cellular uptake and intracellular localization
Studies on the effect of charge density and of the kind of charge (positive, negative) in nonphagocytic cells showed that charged polystyrene and iron oxide particles are taken up better than their uncharged counterparts.Citation119–Citation121 When charged groups on the surface were present, positively charged particles were generally better taken up than negatively charged ones. Cells ingest positively charged gold and silver particles, superparamagnetic iron oxide particles, hydroxylapatite, silicon dioxide, lipid particles, poly(lactic acid), chitosan, polymeric particles, and polystyrene particles to a higher extent than the respective anionic ones.Citation122–Citation131
Lunov et al studied the preferential uptake of anionic particles by phagocytic cells in more detail.Citation132 They compared the uptake of polystyrene particles in differentiated macrophages to that of monocytes and observed a preferential uptake of the carboxylated particles by macrophages and a higher uptake of amino-functionalized particles in monocytes. Macrophages have a higher phagocytic activity towards many bacteria than monocytes,Citation133 and if the preference for anionic particles is linked to phagocytic activity, are expected to display a greater uptake than the less phagocytic monocytes.
The role of surface charge of polystyrene particles and quantum dots on cellular uptake is controversial. Carboxylated 1 μm and 50 nm polystyrene particles were ingested to a higher degree by alveolar type I cells,Citation134 whereas Fazlollahi et alCitation135 showed preferential uptake of cationic polystyrene particles in MDCK cells. For quantum dots, some groups reported preferential uptake of anionic quantum dots,Citation136,Citation137 and others that of positively charged quantum dots.Citation138 Ryman- Rasmussen et alCitation139 did not find any differences between the uptakes of positively and negatively charged quantum dots. Different degrees of hydrophobicity of the functionalized particles may be one reason for the disparate results. Bu et al also assessed the surface hydrophobicity of the quantum dots they used and speculated that the increased uptake of anionic particles may be caused by a higher hydrophobicity of these particles compared to the corresponding neutral and positive ones.Citation140 When studying the uptake of polystyrene particles in alveolar macrophages, Makino et al suggested that the preference of cells to ingest charged particles in their study could also be due to the greater softness of amine and carboxyl-functionalized particles compared to plain ones.Citation141
Serum effects
Both positively and negatively charged NPs bind serum and albumin, but coverage differs between the particles. The change-dependent coverage of carboxylated polystyrene particles with serum was higher than that of positively charged ones,Citation142 whereas positively charged CeO particles bound BSA better than negatively charged ones.Citation143 Similarly, reports on the effect of BSA and serum on cellular uptake showed controversial findings: cells ingested BSA precoated NPs to a lower degree than uncoated ones, as reported by Baier et al,Citation105 but absorbed serum-coated cationic CeO and mesoporous silicon particles to a higher extent, according to data from other groups.Citation142,Citation144
Mechanisms of cellular entry
Under physiological conditions, NPs may enter the cells via passive and active transport. Passive transport of NPs into cells is relatively rare (eg, gold particlesCitation52,Citation145), and most NPs enter cells by endocytosis. The mechanisms for passive uptake have only partly been identified. Arviso et al suggested perturbation of the membrane potential by positively charged gold particles with flipping of membrane areas as the mode of uptake.Citation146 An orderly arrangement of hydrophilic and hydrophobic ligands at the particle surface facilitates passive entry for gold NPs and lipid particles. When the ligands were arranged as stripes, the particles were able to translocate easily across the membrane, while in the random arrangement endocytosis occurred.Citation147–Citation149
Endocytosis serves to absorb molecules from the extracellular space by invagination of the plasma membrane and formation of intracellular vesicles. The first type of endocytosis discovered was clathrin-mediated endocytosis, but in the meantime several additional types of endocytosis have been identified. For the study of NPs, in general, a simplified classification into the four routes of clathrin- mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis, and clathrin-independent and caveolae-independent pathways is usedCitation150–Citation153 (, adapted from Perez-Martinez et alCitation154).
Figure 2 Simplified representation of active uptake mechanisms in nonphagocytic cells.
Notes: Nanoparticle (●) uptake has been evaluated mainly according to macropinocytosis, represented here as only one route, through macropinosome (MP), clathrin-mediated uptake by clathrin-coated pits (CC), and caveolae-dependent uptake by caveosomes (Cav). Uptake by clathrin-independent caveolae-independent endocytosis, which includes flotillin-, Arf6-, Cdc42-, and RhoA-dependent uptake, is also presented as only one route. Fluid-phase endocytosis, which mainly uses the clathrin-coated pits, is not depicted as a separate route. All pathways deliver their content to endosomes (E), late endosomes (LE) and lysosomes (L); the content of caveolosomes may also be delivered to the endoplasmic reticulum (ER) and the Golgi apparatus. Vesicular transport through the cell occurs through transcytotic vesicles (TV). © 2012, Elsevier. Reproduced with permission from Fröhlich E, Roblegg E. Models for oral uptake of nanoparticles in consumer products. Toxicology. 2012;291(1–3):8.Citation179
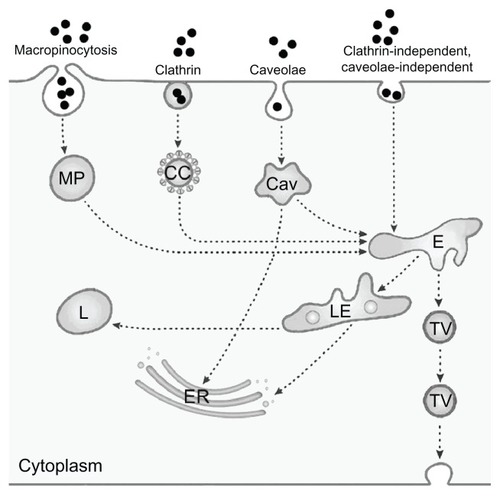
The endocytic routes described so far are receptor-mediated and transport with high efficiency. Bulk flow of substances from the extracellular space, by contrast, occurs through fluid-phase endocytosis, formerly termed pinocytosis. Citation155 This transport is nonsaturable and has a low capacity. Most endocytic pathways include lysosomes, where a variety of macromolecules can be degraded. If substances, however, enter by caveolae-mediated endocytosis, they may also be delivered to the endoplasmatic reticulum and to the Golgi apparatus, thereby avoiding degradation in lysosomes.Citation156
In addition to transcellular transport, NPs can use the paracellular route to pass epithelial monolayers. Opening of the tight junctions by NPs may present an option for drug delivery across the blood–brain barrier but surface charge appears not to play the most important role. Although cationic albumin particles were able to cross this barrier, tight junctions remained intact.Citation157 Studies using dendrimers of different sizes with cationic and anionic charge also suggest that (large) size is more relevant for opening of tight junctions than surface charge.Citation158
presents an overview of studies on surface-dependent particle uptake and shows that no general rules have been identified so far. When positively and negatively charged chitosan and poly(lactic acid) particles were compared in the same study, both types of particles used the same (clathrin-mediated) uptake mechanism.Citation159,Citation160 Quantum dots can be ingested by clathrin and clathrin-independent caveolae-independent endocytosis.Citation173,Citation174 Controverse findings were also reported for cationic polystyrene NPs. Clathrin-mediatedCitation175, macropinocytototicCitation177 and caveolae-dependentCitation178 routes were described. Plain polystyrene NPs used clathrin-independent endocytosis, whereas positively charged NPs are taken up via clathrin-coated vesicles.Citation175 The authors also showed that upon inhibition of the clathrin-mediated uptake, plain NPs were ingested by macropinocytosis as an alternative route.Citation119 The influence of size is obvious in the uptake of 24 nm and 43 nm anionic polystyrene particles: the smaller particles were taken up by the clathrin-independent caveolae-independent route, whereas the larger ones were ingested by clathrin-mediated endocytosis.Citation176 Cell-specific differences also play a role: cationic polystyrene particles were taken up by LAMP-1- positive endosomes in the macrophage cell line RAW 264.7 and by caveolae in BEAS-2 cells.Citation162 Similar differences were also reported for dendrimers, which were taken up by clathrin-mediated endocytosis in Caco-2 cellsCitation163 and by macropinocytosis in A549 cells.Citation164 Foster et al reported very different rates of particle uptake in the respiratory cell lines A459 and Calu-3.Citation165 For the interpretation of these data, problems related to working with uptake inhibitors have to be taken into account. This includes inhibition of more than one route due to low specificity of the inhibitors, uptake by compensatory mechanisms when one route is blocked, alterations of plasma-membrane proteins, disruption of the cortical actin cytoskeleton, and inhibition of vesicle trafficking, etc.Citation166
Table 2 Routes of endocytic uptake in nonphagocytic cells
The cell-specific expression of endocytic routes may explain the observed differences in the endocytic routes used, in the amount of particle uptake, and in the velocity of this uptake. It is, for instance, known that smooth-muscle cells, fibroblasts, adipocytes, and endothelial cells have an incredible amount of caveolae,Citation167 and therefore preferentially use this route. This leads not only to a different intracellular localization but also to different velocity of uptake. The clathrin- mediated pathway is faster than the clathrin-independent caveolin-independent uptake,Citation160 and therefore particles using this route accumulate faster in cells. Asati et al propose to exploit differences in the uptake routes between normal and tumor cells to develop cytostatic NP-based drugs.Citation75
Not only cell entry by different uptake routes but also the escape of cationic particles from the endosomal–lysosomal system could explain the charge-dependent differences in the intracellular localization of anionic PLGA and mesoporous and chitosan particles.Citation129,Citation130,Citation168
The predictive value of the aforementioned surface charge-dependent cellular studies is currently not clear. For chemicals, a large multicentre evaluation study identified a rather good correlation (R2 = 0.77) between IC50 values in cytotoxicity screening assays and human acute poisoning with various chemicals.Citation169 For NPs, few comparative data are available, which suggests a low predictive value for inhalation exposureCitation170 and a good correlation for parenteral exposure.Citation171
Conclusions
Cationic surface charge for most NPs correlates with higher cellular uptake and greater cytotoxicity in nonphagocytic cells. Cationic NPs appear to cause plasma-membrane disruption to a greater extent and anionic NPs apoptosis. Anionic NPs are better ingested and act more cytotoxically in phagocytic cells. The presence of serum appears to reduce NP uptake in nonphagocytic cells, but increases it in phagocytic cells. The differences between phagocytic and nonphagocytic cells have to be taken into account in the design of medical NPs.
Acknowledgments
Financial support by the Austrian Research Science Grant P22576-B18 is gratefully acknowledged. The author thanks Gabriella Salas for English-language editing.
Disclosure
The author reports no conflicts of interest in this work.
References
- El OuahabiAThiryMPectorVFuksRRuysschaertJMVandenbrandenMThe role of endosome destabilizing activity in the gene transfer process mediated by cationic lipidsFEBS Lett199741421871929315683
- XuYSzokaFCJrMechanism of DNA release from cationic liposome/DNA complexes used in cell transfectionBiochemistry19963518561656238639519
- NafeeNSchneiderMSchaeferUFLehrCMRelevance of the colloidal stability of chitosan/PLGA nanoparticles on their cytotoxicity profileInt J Pharm2009381213013919450671
- AgasheHBDuttaTGargMJainNKInvestigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimerJ Pharm Pharmacol200658111491149817132212
- DuttaTGargMJainNKPoly(propyleneimine) dendrimer and dendrosome mediated genetic immunization against hepatitis BVaccine20082627–283389339418511160
- Al-RawiMDiabateSWeissCUptake and intracellular localization of submicron and nano-sized SiO(2) particles in HeLa cellsArch Toxicol201185781382621240478
- AlbersCEHofstetterWSiebenrockKALandmannRKlenkeFMIn vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrationsNanotoxicology Epub October 21, 2011
- FröhlichEKueznikTSambergerCRobleggEWrightonCPieberTRSize-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymesToxicol Appl Pharmacol2010242332633219909766
- FröhlichEMeindlCRobleggEGriesbacherAPieberTRCytotoxicity of nanoparticles is influenced by size, proliferation and embryonic origin of the cells used for testingNanotoxicology20126442442321627401
- FröhlichESambergerCKueznikTCytotoxicity of nanoparticles independent from oxidative stressJ Toxicol Sci200934436337519652459
- HeQZhangZGaoYShiJLiYIntracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticlesSmall20095232722272919780070
- LinWHuangYWZhouXDMaYIn vitro toxicity of silica nanoparticles in human lung cancer cellsToxicol Appl Pharmacol2006217325225917112558
- NairSSasidharanADivya RaniVVMenonDManzoorKRainaSRole of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cellsJ Mater Sci Mater Med200920Suppl 1S235S24118716714
- PatilGKhanMIPatelDKSultanaSPrasadRAhmadIEvaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic responses of micro- and nano-particles of dolomite on human lung epithelial cells A(549)Environ Toxicol Pharmacol201234243644522785077
- PietruskaJRLiuXSmithABioavailability, intracellular mobilization of nickel, and HIF-1alpha activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticlesToxicol Sci2011124113814821828359
- LovricJBazziHSCuieYFortinGRWinnikFMMaysingerDDifferences in subcellular distribution and toxicity of green and red emitting CdTe quantum dotsJ Mol Med200583537738515688234
- ZhangYYuWJiangXLvKSunSZhangFAnalysis of the cytotoxicity of differentially sized titanium dioxide nanoparticles in murine MC3T3-E1 preosteoblastsJ Mater Sci201122819331945
- ChaKEMyungHCytotoxic effects of nanoparticles assessed in vitro and in vivoJ Microbiol Biotechnol20071791573157818062241
- YinHTooHPChowGMThe effects of particle size and surface coating on the cytotoxicity of nickel ferriteBiomaterials200526295818582615949547
- Okuda-ShimazakiJTakakuSKanehiraKSonezakiSTaniguchiAEffects of titanium dioxide nanoparticle aggregate size on gene expressionInt J Mol Sci20101162383239220640159
- ChongCSCaoMWongWWEnhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine deliveryJ Control Release20051021859915653136
- MorishigeTYoshiokaYInakuraHCytotoxicity of amorphous silica particles against macrophage-like THP-1 cells depends on particle-size and surface propertiesPharmazie201065859659920824960
- LankoffASandbergWJWegierek-CiukAThe effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cellsToxicol Lett2012208319721322108609
- KatsnelsonBADegtyarevaTDMinigalievaIISubchronic systemic toxicity and bioaccumulation of Fe3O4 nano- and microparticles following repeated intraperitoneal administration to ratsInt J Toxicol2011301596821398218
- RajuHBHuYVedulaADubovySRGoldbergJLEvaluation of magnetic micro- and nanoparticle toxicity to ocular tissuesPloS One201165e1745221637340
- PolandCADuffinRKinlochICarbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot studyNat Nanotechnol20083742342818654567
- TakagiAHiroseANishimuraTInduction of mesothelioma in p53+/– mouse by intraperitoneal application of multi-wall carbon nanotubeJ Toxicol Sci200833110511618303189
- OnumaKSatoYOgawaraSNano-scaled particles of titanium dioxide convert benign mouse fibrosarcoma cells into aggressive tumor cellsAm J Pathol200917552171218319815711
- SchaeublinNMBraydich-StolleLKSchrandAMSurface charge of gold nanoparticles mediates mechanism of toxicityNanoscale20113241042021229159
- BaekMKimISYuJChungHEChoyJHChoiSJEffect of different forms of anionic nanoclays on cytotoxicityJ Nanosci Nanotechnol20111121803180621456296
- BhattacharjeeSde HaanLHEversNMRole of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cellsPart Fibre Toxicol201072520831820
- GoodmanCMMcCuskerCDYilmazTRotelloVMToxicity of gold nanoparticles functionalized with cationic and anionic side chainsBioconjug Chem200415489790015264879
- OhWKKimSChoiMCellular uptake, cytotoxicity, and innate immune response of silica-titania hollow nanoparticles based on size and surface functionalityACS Nano2010495301531320698555
- RuizendaalLBhattacharjeeSPournazariKSynthesis and cytotoxicity of silicon nanoparticles with covalently attached organic monolayersNanotoxicology200934339347
- NahaPCDavorenMLyngFMByrneHJReactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cellsToxicol Appl Pharmacol20102461–2919920420846
- McNernyDQLeroueilPRBakerJRUnderstanding specific and nonspecific toxicities: a requirement for the development of dendrimer-based pharmaceuticalsNanomed Nanobiotechnol201023249259
- ChauhanASDiwanPVJainNKTomaliaDAUnexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimersBiomacromolecules20091051195120219348417
- MuraSHillaireauHNicolasJInfluence of surface charge on the potential toxicity of PLGA nanoparticles towards Calu-3 cellsInt J Nanomedicine201162591260522114491
- YuTMaluginAGhandehariHImpact of silica nanoparticle design on cellular toxicity and hemolytic activityACS Nano2011575717572821630682
- LuoXFengMPanSWenYZhangWWuCCharge shielding effects on gene delivery of polyethylenimine/DNA complexes: PEGylation and phospholipid coatingJ Mater Sci Mater Med20122371685169522481628
- CorpeWAttachment of Marine Bacteria to Solid SurfacesNew YorkAcademic Press19701
- TomitaYRikimaru-KanekoAHashiguchiKShirotakeSEffect of anionic and cationic n-butylcyanoacrylate nanoparticles on NO and cytokine production in Raw264.7 cellsImmunopharmacol Immunotoxicol201133473073721457109
- HoskinsCWangLChengWPCuschieriADilemmas in the reliable estimation of the in-vitro cell viability in magnetic nanoparticle engineering: which tests and what protocols?Nanoscale Res Lett201277722247975
- ChungYIKimJCKimYHThe effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targetingJ Control Release2010143337438220109508
- Panessa-WarrenBWarrenJWongSMisewichJBiological cellular response to carbon nanoparticle toxicityJ Phys Condens Matter20061833S2185
- RyabchikovaEMazurkovaNShikinaNIsmagilovZThe crystalline forms of titanium dioxide nanoparticles affect their interactions with individual cellsJ Med Chem Biol Radiol Def20108
- WangTBaiJJiangXNienhausGUCellular uptake of nanoparticles by membrane penetration: a study combining confocal microscopy with FTIR spectroelectrochemistryACS Nano2012621251125922250809
- DraegerAMonastyrskayaKBabiychukEBPlasma membrane repair and cellular damage control: the annexin survival kitBiochem Pharmacol201181670371221219882
- MellgrenRLA new twist on plasma membrane repairCommun Integr Biol20114219820021655439
- de PlanqueMRAghdaeiSRooseTMorganHElectrophysiological characterization of membrane disruption by nanoparticlesACS Nano2011553599360621517083
- MuQHondowNSKrzemiskiLBrownAPJeukenLJRoutledgeMNMechanism of cellular uptake of genotoxic silica nanoparticlesPart Fibre Toxicol2012912922823932
- LinJZhangHChenZZhengYPenetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationshipACS Nano2010495421542920799717
- LinJQZhengYGZhangHWChenZA simulation study on nanoscale holes generated by gold nanoparticles on negative lipid bilayersLangmuir201127138323833221634406
- HongSHesslerJBanaszak HollMLeroueilPMeckeAOrrBPhysical interactions of nanoparticles with biological membranes: the observation of nanoscale hole formationChem Health Saf20061331620
- MeckeAMajorosIJPatriAKBakerJRJrHollMMOrrBGLipid bilayer disruption by polycationic polymers: the roles of size and chemical functional groupLangmuir20052123103481035416262291
- MeckeAUppuluriSSassanellaTMDirect observation of lipid bilayer disruption by poly(amidoamine) dendrimersChem Phys Lipids2004132131415530443
- KlotzLSiesHCellular Generation of Oxidants: Relation to Oxidative StressWeinheimWiley2009
- BrownDMHutchisonLDonaldsonKStoneVThe effects of PM10 particles and oxidative stress on macrophages and lung epithelial cells: modulating effects of calcium-signaling antagonistsAm J Physiol20072926L1444L1451
- PeuschelHSydlikUHaendelerJc-Src-mediated activation of Erk1/2 is a reaction of epithelial cells to carbon nanoparticle treatment and may be a target for a molecular preventive strategyBiol Chem2010391111327133220868224
- SydlikUBierhalsKSoufiMAbelJSchinsRPUnfriedKUltrafine carbon particles induce apoptosis and proliferation in rat lung epithelial cells via specific signaling pathways both using EGF-RAm J Physiol Lung Cell Mol Physiol20062914L725L73316751223
- JiangWKimBYRutkaJTChanWCNanoparticle-mediated cellular response is size-dependentNat Nanotechnol20083314515018654486
- UnfriedKSydlikUBierhalsKWeissenbergAAbelJCarbon nanoparticle-induced lung epithelial cell proliferation is mediated by receptor-dependent Akt activationAm J Physiol Lung Cell Mol Physiol20082942L358L36718083769
- LovricJChoSJWinnikFMMaysingerDUnmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell deathChem Biol200512111227123416298302
- ChenLYokelRAHennigBToborekMManufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculatureJ Neuroimmune Pharmacol20083428629518830698
- Freyre-FonsecaVDelgado-BuenrostroNLGutierrez-CirlosEBTitanium dioxide nanoparticles impair lung mitochondrial functionToxicol Lett2011202211111921315139
- TeodoroJSSimoesAMDuarteFVAssessment of the toxicity of silver nanoparticles in vitro: a mitochondrial perspectiveToxicol in Vitro201125366467021232593
- AshaRaniPVLowKahMunGHandeMPValiyaveettilSCytotoxicity and genotoxicity of silver nanoparticles in human cellsACS Nano20093227929019236062
- PiaoMJKangKALeeIKSilver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosisToxicol Lett201120119210021182908
- BexigaMGVarelaJAWangFCationic nanoparticles induce caspase 3-, 7- and 9-mediated cytotoxicity in a human astrocytoma cell lineNanotoxicology20115455756721142842
- BairdSKKurzTBrunkUTMetallothionein protects against oxidative stress-induced lysosomal destabilizationBiochem J2006394Pt 127528316236025
- SoenenSRivera-GilPMontenegroJParakWDe SmedtSBraeckmansKCellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluationNanotoday201165446465
- FunnellWRMaysingerDThree-dimensional reconstruction of cell nuclei, internalized quantum dots and sites of lipid peroxidationJ Nanobiotechnol200641010
- ThibodeauMSGiardinaCKnechtDAHelbleJHubbardAKSilica-induced apoptosis in mouse alveolar macrophages is initiated by lysosomal enzyme activityToxicol Sci2004801344815056807
- NelAEMadlerLVelegolDUnderstanding biophysico-chemical interactions at the nano-bio interfaceNat Mater20098754355719525947
- AsatiASantraSKaittanisCPerezJMSurface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticlesACS Nano2010495321533120690607
- Johnson-LylesDNPeifleyKLockettSFullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunctionToxicol Appl Pharmacol2010248324925820713077
- LiJJHartonoDOngCNBayBHYungLYAutophagy and oxidative stress associated with gold nanoparticlesBiomaterials201031235996600320466420
- KhanMIMohammadAPatilGNaqviSAChauhanLKAhmadIInduction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticlesBiomaterials20123351477148822098780
- SternSTZolnikBSMcLelandCBClogstonJZhengJMcNeilSEInduction of autophagy in porcine kidney cells by quantum dots: a common cellular response to nanomaterials?Toxicol Sci2008106114015218632727
- EidiHJoubertONemosCDrug delivery by polymeric nanoparticles induces autophagy in macrophagesInt J Pharm20124221–249550322119964
- VercauterenDDeschoutHRemautKDynamic colocalization microscopy to characterize intracellular trafficking of nanomedicinesACS Nano20115107874788421923168
- LiCLiuHSunYPAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathwayJ Mol Cell Biol200911374519516051
- BoyogluCBoyoglu-BarnumSSoniSThe intracellular co-localizations of different size of gold nanoparticles NSTINanotechnology 2011: Bio Sensors, Instruments, Medical, Environment and EnergyCRC Press20123489492
- AdamSAThe nuclear pore complexGenome Biol200129REVIEWS000711574060
- PetersRTranslocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionalityTraffic20056542142715813752
- NitinNLaConteLRheeWJBaoGTat peptide is capable of importing large nanoparticles across nuclear membrane in digitonin permeabilized cellsAnn Biomed Eng200937102018202719657743
- NabeshiHYoshikawaTArimoriAEffect of surface properties of silica nanoparticles on their cytotoxicity and cellular distribution in murine macrophagesNanoscale Res Lett2011619321711578
- KamataHZinchenkoAMurataSEffects of cationic and anionic nanoparticles on the stability of the secondary structure of DNAColloid Polymer Sci20112891213291335
- ZinchenkoAALuckelFYoshikawaKTranscription of giant DNA complexed with cationic nanoparticles as a simple model of chromatinBiophys J20079241318132517142281
- ChenMvon MikeczAFormation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticlesExp Cell Res20053051516215777787
- LiNMaLWangJInteraction between nano-anatase TiO2 and liver DNA from mice in vivoNanoscale Res Lett20095110811520652136
- LiuYLiWLaoFIntracellular dynamics of cationic and anionic polystyrene nanoparticles without direct interaction with mitotic spindle and chromosomesBiomaterials201132328291830321810539
- LynchIDawsonKAProtein-nanoparticle interactionsNanotoday200831–24047
- GheshlaghiZNRiaziGHAhmadianSGhafariMMahinpourRToxicity and interaction of titanium dioxide nanoparticles with microtubule proteinActa Biochim Biophys Sin200840977778218776989
- LinseSCabaleiro-LagoCXueWFNucleation of protein fibrillation by nanoparticlesProc Natl Acad Sci U S A2007104218691869617485668
- MakarovaEGGordonRYPodolskiIYFullerene C60 prevents neurotoxicity induced by intrahippocampal microinjection of amyloid-beta peptideJ Nanosci Nanotechnol201212111912622523954
- Cabaleiro-LagoCQuinlan-PluckFLynchIInhibition of amyloid beta protein fibrillation by polymeric nanoparticlesJ Am Chem Soc200813046154371544318954050
- YooSIYangMBrenderJRInhibition of amyloid peptide fibrillation by inorganic nanoparticles: functional similarities with proteinsAngew Chem Int Ed Engl201150225110511521495130
- GoldbergDSGhandehariHSwaanPWCellular entry of G3.5 poly (amido amine) dendrimers by clathrin- and dynamin-dependent endocytosis promotes tight junctional opening in intestinal epitheliaPharm Res20102781547155720411406
- CoyucoJCLiuYTanBJChiuGNFunctionalized carbon nanomaterials: exploring the interactions with Caco-2 cells for potential oral drug deliveryInt J Nanomedicine201162253226322125408
- VllasaliuDExposito-HarrisRHerasATight junction modulation by chitosan nanoparticles: comparison with chitosan solutionInt J Pharm20104001–218319320727955
- RogerELagarceFGarcionEBenoitJPLipid nanocarriers improve paclitaxel transport throughout human intestinal epithelial cells by using vesicle-mediated transcytosisJ Control Release2009140217418119699246
- SheikpranbabuSKalishwaralalKLeeKJVaidyanathanREomSHGurunathanSThe inhibition of advanced glycation end-products- induced retinal vascular permeability by silver nanoparticlesBiomaterials20103182260227119963272
- PetushkovAIntraJGrahamJBLarsenSCSalemAKEffect of crystal size and surface functionalization on the cytotoxicity of silicalite-1 nanoparticlesChem Res Toxicol20092271359136819580308
- BaierGCostaCZellerABSA adsorption on differently charged polystyrene nanoparticles using isothermal titration calorimetry and the influence on cellular uptakeMacromol Biosci201111562863821384550
- Petri-FinkASteitzBFinkaASalaklangJHofmannHEffect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): colloidal stability, cytotoxicity, and cellular uptake studiesEur J Pharm Biopharm200868112913717881203
- CliftMJBhattacharjeeSBrownDMStoneVThe effects of serum on the toxicity of manufactured nanoparticlesToxicol Lett2010198335836520705123
- RogersWJBasuPFactors regulating macrophage endocytosis of nanoparticles: implications for targeted magnetic resonance plaque imagingAtherosclerosis20051781677315585202
- ChithraniBDGhazaniAAChanWCDetermining the size and shape dependence of gold nanoparticle uptake into mammalian cellsNano Lett20066466266816608261
- GaoHShiWFreundLBMechanics of receptor-mediated endocytosisProc Natl Acad Sci U S A2005102279469947415972807
- LuFWuSHHungYMouCYSize effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticlesSmall20095121408141319296554
- WangSHLeeCWChiouAWeiPKSize-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering imagesJ Nanobiotechnol2010833
- WinKYFengSSEffects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugsBiomaterials200526152713272215585275
- ChampionJAWalkerAMitragotriSRole of particle size in phagocytosis of polymeric microspheresPharm Res20082581815182118373181
- RaynalIPrigentPPeyramaureSNajidARebuzziCCorotCMacrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10Invest Radiol2004391566314701989
- GonzalezOSmithRLGoodmanSBEffect of size, concentration, surface area, and volume of polymethylmethacrylate particles on human macrophages in vitroJ Biomed Mater Res19963044634738847354
- WangHWuLReinhardBMScavenger receptor mediated endocytosis of silver nanoparticles into J774A1 macrophages is heterogeneousACS Nano2012687122713222799499
- YuSSLauCMThomasSNSize- and charge-dependent nonspecific uptake of PEGylated nanoparticles by macrophagesInt J Nanomedicine2012779981322359457
- JiangXMusyanovychARockerCLandfesterKMailanderVNienhausGUSpecific effects of surface carboxyl groups on anionic polystyrene particles in their interactions with mesenchymal stem cellsNanoscale2011352028203521409242
- ThorekDLTsourkasASize, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cellsBiomaterials200829263583359018533252
- VillanuevaACaneteMRocaAGThe influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cellsNanotechnology2009201111510319420433
- BrandenbergerCRothen-RutishauserBMuhlfeldCEffects and uptake of gold nanoparticles deposited at the air-liquid interface of a human epithelial airway modelToxicol Appl Pharmacol20102421566519796648
- ChenLMcCrateJMLeeJCLiHThe role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cellsNanotechnology2011221010570821289408
- ChoJCarusoFInvestigation of the interactions between ligand-stabilized gold nanoparticles and polyelectrolyte multilayer filmsChem Mater2005171745474553
- MarquisBJLiuZBraunKLHaynesCLInvestigation of noble metal nanoparticle ζ-potential effects on single-cell exocytosis function in vitro with carbon-fiber microelectrode amperometryAnalyst2011136173478348621170444
- LorenzMRHolzapfelVMusyanovychAUptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cellsBiomaterials200627142820282816430958
- MillerCRBondurantBMcLeanSDMcGovernKAO’BrienDFLiposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomesBiochemistry1998373712875128839737866
- GeYZhangYXiaJEffect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake in vitroColloids Surf B Biointerfaces200973229430119564099
- Harush-FrenkelORozenturEBenitaSAltschulerYSurface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cellsBiomacromolecules20089243544318189360
- YueZGWeiWLvPPSurface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticlesBiomacromolecules20111272440244621657799
- MailanderVLandfesterKInteraction of nanoparticles with cellsBiomacromolecules20091092379240019637907
- LunovOSyrovetsTLoosCDifferential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell lineACS Nano2011531657166921344890
- DrutzDJIntracellular fate of Neisseria gonorrhoeaeBrooksGGotschlichEHolmesKSawyerWYoungFImmunobiology of Neisseria gonorrhoeaeWashingtonAmerican Society for Microbiology19781232235
- KempSJThorleyAJGorelikJImmortalization of human alveolar epithelial cells to investigate nanoparticle uptakeAm J Respir Cell Mol Biol200839559159718539954
- FazlollahiFAngelowSYacobiNRPolystyrene nanoparticle trafficking across MDCK-IINanomedicine20117558859421310266
- JaiswalJKMattoussiHMauroJMSimonSMLong-term multiple color imaging of live cells using quantum dot bioconjugatesNat Biotechnol2003211475112459736
- SakaiNMatsuiYNakayamaATsudaAYonedaMFunctional-dependent and size-dependent uptake of nanoparticles in PC12J Phys Conf Ser201130412049
- DuanHNieSCell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatingsJ Am Chem Soc2007129113333333817319667
- Ryman-RasmussenJPRiviereJEMonteiro-RiviereNASurface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytesJ Invest Dermatol2007127114315316902417
- BuQYanGDengPNMR-based metabonomic study of the sub-acute toxicity of titanium dioxide nanoparticles in rats after oral administrationNanotechnology2010211212510520203358
- MakinoKYamamotoNHiguchiKHaradaNOhshimaHTeradaHPhagocytic uptake of polystyrene microspheres by alveolar macrophages: effects of the size and surface properties of the microspheresColloids Surf B Biointerfaces20032713339
- EhrenbergMSFriedmanAEFinkelsteinJNOberdorsterGMcGrathJLThe influence of protein adsorption on nanoparticle association with cultured endothelial cellsBiomaterials200930460361019012960
- PatilSSandbergAHeckertESelfWSealSProtein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potentialBiomaterials200728314600460717675227
- FerratiSMackAChiappiniCIntracellular trafficking of silicon particles and logic-embedded vectorsNanoscale2010281512152020820744
- TaylorUKleinSPetersenSKuesWBarcikowskiSRathDNonendosomal cellular uptake of ligand-free, positively charged gold nanoparticlesCytometry A201077543944620104575
- ArvizoRRMirandaORThompsonMAEffect of nanoparticle surface charge at the plasma membrane and beyondNano Lett20101072543254820533851
- CarneyRPCarneyTMMuellerMStellacciFDynamic cellular uptake of mixed-monolayer protected nanoparticlesBiointerphases201271–41722589060
- LiYLiXLiZGaoHSurface-structure-regulated penetration of nanoparticles across a cell membraneNanoscale20124123768377522609866
- VermaAUzunOHuYSurface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticlesNat Mater20087758859518500347
- DohertyGJMcMahonHTMechanisms of endocytosisAnnu Rev Biochem20097885790219317650
- HowesMTMayorSPartonRGMolecules, mechanisms, and cellular roles of clathrin-independent endocytosisCurr Opin Cell Biol201022451952720439156
- SahayGAlakhovaDYKabanovAVEndocytosis of nanomedicinesJ Control Release2010145318219520226220
- SandvigKPustSSkotlandTvan DeursBClathrin-independent endocytosis: mechanisms and functionCurr Opin Cell Biol201123441342021466956
- Perez-MartinezFCGuerraJPosadasICenaVBarriers to non-viral vector-mediated gene delivery in the nervous systemPharm Res20112881843185821225319
- KhalilIAKogureKAkitaHHarashimaHUptake pathways and subsequent intracellular trafficking in nonviral gene deliveryPharmacol Rev2006581324516507881
- PartonRGCaveolae meet endosomes: a stable relationship?Dev Cell20047445846015469832
- LuWTanYZHuKLJiangXGCationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrierInt J Pharm20052951–224726015848009
- KitchensKMKolhatkarRBSwaanPWEddingtonNDGhandehariHTransport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: influence of size, charge and fluorescent labelingPharm Res200623122818282617094034
- ZakiNMNastiATirelliNNanocarriers for cytoplasmic delivery: cellular uptake and intracellular fate of chitosan and hyaluronic acid-coated chitosan nanoparticles in a phagocytic cell modelMacromol Biosci201111121747176021954171
- Harush-FrenkelODebottonNBenitaSAltschulerYTargeting of nanoparticles to the clathrin-mediated endocytic pathwayBiochem Biophys Res Commun20073531263217184736
- IversenTFrerkerNSandvigKEndocytosis and Intracellular Trafficking of Quantum Dot–Ligand BioconjugatesHobokenJohn Wiley & Sons2010
- MiglioreLCoppedeFEnvironmental-induced oxidative stress in neurodegenerative disorders and agingMutat Res20096741–2738418952194
- KitchensKMForakerABKolhatkarRBSwaanPWGhandehariHEndocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cellsPharm Res200724112138214517701324
- PerumalOPInapagollaRKannanSKannanRMThe effect of surface functionality on cellular trafficking of dendrimersBiomaterials20082924–253469347618501424
- FosterKAYazdanianMAudusKLMicroparticulate uptake mechanisms of in-vitro cell culture models of the respiratory epitheliumJ Pharm Pharmacol2001531576611206193
- IvanovAPharmacological Inhibition of Endocytotic Pathways: is it Specific Enough to be useful?New YorkHumana Press200840
- PartonRGSimonsKThe multiple faces of caveolaeNat Rev Mol Cell Biol20078318519417318224
- ChenYChenHZhangSStructure-property relationships in manganese oxide – mesoporous silica nanoparticles used for T1-weighted MRI and simultaneous anti-cancer drug deliveryBiomaterials20123372388239822177841
- ClemedsonCDierickxPJSjostromMThe prediction of human acute systemic toxicity by the EDIT/MEIC in vitro test battery: the importance of protein binding and of partitioning into lipidsAltern Lab Anim200331324525615612867
- KrollAPillukatMHHahnDSchnekenburgerJCurrent in vitro methods in nanoparticle risk assessment: limitations and challengesEur J Pharm Biopharm200972237037718775492
- LiYLiuJZhongYBiocompatibility of Fe(3)O(4)@Au composite magnetic nanoparticles in vitro and in vivoInt J Nanomedicine201162805281922131827
- LedetGMandalTNanomedicine: emerging therapeutics for the 21st centuryUS Pharm2012373711
- XiaoYForrySPGaoXHolbrookRDTelfordWGTonaADynamics and mechanisms of quantum dot nanoparticle cellular uptakeJ Nanobiotechnol2010813
- ZhangLWMonteiro-RiviereNAMechanisms of quantum dot nanoparticle cellular uptakeToxicol Sci2009110113815519414515
- JiangXDausendJHafnerMSpecific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cellsBiomacromolecules201011374875320166675
- LaiSKHidaKManSTPrivileged delivery of polymer nanoparticles to the perinuclear region of live cells via a nonclathrin, non-degradative pathwayBiomaterials200728182876288417363053
- DausendJMusyanovychADassMUptake mechanism of oppositely charged fluorescent nanoparticles in HeLa cellsMacromol Biosci20088121135114318698581
- XiaTKovochichMLiongMZinkJINelAECationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathwaysACS Nano200821859619206551
- FröhlichERobleggEModels for oral uptake of nanoparticles in consumer productsToxicology20122911–38