Abstract
Liver cancer remains a global health challenge with a projected incidence of over one million cases by 2025. Hepatocellular carcinoma (HCC) is a common primary liver cancer, accounting for about 90% of all liver cancer cases. The tumor microenvironment (TME) is the internal and external environment for tumor development, which plays an important role in tumorigenesis, immune escape and treatment resistance. Knowing that TME is a unique setting for HCC tumorigenesis, exploration of strategies to modulate TME has attracted increasing attention. Among them, the use of nano-delivery systems to deliver therapeutic agents to regulate TME components has shown great potential. TME-modulating nanoparticles have the advantages of protecting therapeutic agents from degradation, enhancing the ability of targeting HCC and reducing systemic toxicity. In this article, we summarize the TME components associated with HCC, including cancer-associated fibroblasts (CAFs), extracellular matrix (ECM), endothelial cells and immune cells, discuss their impact on the HCC progression, and highlight recent studies on nano-delivery systems that modulate these components. Finally, we also discuss opportunities and challenges in this field.
Introduction
With the rapid increase in the incidence and mortality of liver cancer, it has become a major public health problem worldwide. It is estimated that more than one million people will suffer from liver cancer annually by 2025.Citation1 Liver cancer is reportedly the sixth most common cancer and the fourth leading cause of cancer-related death worldwide in 2018.Citation2 Hepatocellular carcinoma (HCC) is the most common type of primary liver cancers, accounting for about 90% of all primary liver cancers worldwide.Citation3 The HCC prevalence varies geographically and it is the most common cause of death in patients with cirrhosis. HCC occurs in the setting of chronic liver inflammation and it is significantly associated with chronic viral hepatitis infection (hepatitis B or C), alcohol, or toxins. The inflammatory microenvironment provides a favorable growth environment for the HCC development and promotes the abnormal transformation of normal liver cells, such as hepatocytes, stem cells, immune cells and stellate cells.Citation4,Citation5
The tumor microenvironment (TME) is the internal and external environment for tumor development, which plays an important role in tumorigenesis, immune escape and treatment resistance. TME contributes to tumor evasion of immune recognition in three main ways: (1) allowing cancer cells to proliferate massively by suppressing the immune response of the microenvironment; (2) inducing angiogenesis to promote tumor growth under hypoxic conditions; and (3) promoting immune escape of cancer cells.Citation6 The HCC microenvironment exhibits a stronger immunosuppressive effect compared with other tumors, with almost all cellular subpopulations and numerous regulatory mechanisms contributing to the HCC progression. Therefore, the importance of TME in the HCC treatment has received increasing attention in recent years, which drives TME-based researches.
Nano-delivery systems can deliver various chemotherapeutic drugs, therapeutic genes, and photothermal reagents to tumors, thus effectively reducing drug toxicity and resistance, increasing their solubility and improving the targeting ability of therapeutic agents.Citation7,Citation8 Diverse types of engineered nanoparticles have been developed to deliver therapeutic agents to solid tumors. They can protect therapeutic agents from degradation, and the enhanced permeability and retention (EPR) effect facilitates their preferential accumulation at tumors.Citation9 However, passive targeting based on the EPR effect is inefficient and often leads to unpredictable clinical outcomes.Citation9,Citation10 Therefore, researchers have developed the modified nanoparticles to actively target tumor sites. For example, the nanoparticles modified with folic acid could improve their uptake by tumor cells.Citation11 The modified nanoparticles interacted with TME have exhibited encouraging results in the HCC treatment. They kill tumor cells by specifically identifying HCC cells and targeting the HCC microenvironment to release therapeutic agents.
Given the important role that TME plays in the occurrence and progression of HCC, it is of great significance to regulate TME in the HCC treatment. Nano-delivery systems deliver therapeutic agents to regulate TME is a very effective means in that it can affect the HCC progression by regulating signaling pathways, angiogenesis and immune cells. The purpose of this review is to make a comprehensive summary of researches on nano-delivery systems for modulating the HCC microenvironment, in order to gain a better understanding about the relevant TME components in the HCC progression and how nanoparticles modulate these components.
In this review article, we mainly focus on nanoparticles that modulate the HCC microenvironment. We first describe the TME components of HCC and their impact on HCC progression, then discuss the TME- and immune system-related nanoparticles in the HCC treatment, and finally provide an overview on the remaining challenges.
HCC and TME
The HCC microenvironment is composed of cancer cells and stromal cells, including fibroblasts, endothelial cells, macrophages and lymphocytes,Citation12–Citation14 each cell type plays its unique function. The extracellular matrix (ECM) provides a growth environment for these cells. All these intercellular communications ultimately determine how HCC progress. The components of the HCC microenvironment and their roles are shown below ().
Figure 1 The tumor microenvironment components of HCC and their impact on the HCC progression.
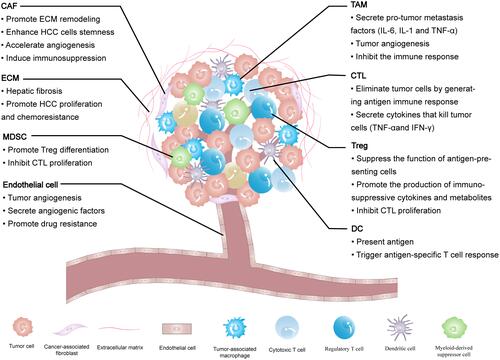
Fibroblasts are found in fibrous matrices, where they participate in wound repair, tissue maturation and inflammatory responses.Citation15 In tumor tissues, normal fibroblasts can activate cancer-associated fibroblasts (CAFs), and they can also arise from endothelial cells, epithelial cells, smooth muscle cells, preadipocytes and bone marrow-derived progenitor cells.Citation16 An increasing number of studies have demonstrated that CAFs are strongly associated with the HCC progression. They not only directly affect HCC cells but interact with other interstitial cells to remold the HCC microenvironment. It was found that CAFs isolated from fresh HCC tumor tissues could induce tumorigenesis and metastasis by promoting the proliferation, self-renewal, migration, invasion and drug resistance of HCC cells.Citation17 ECM regulates the number, morphology, movement and adhesion of cells.Citation18 In physiological conditions, ECM maintains tissue homeostasis, and abnormal ECM is a sign of cancer.Citation19 ECM was observed to increase the hardness of solid tumors in the HCC.Citation20 Endothelial cells interact with pericytes, ECM and basement membrane proteins to maintain their stability and proliferation, playing a vital role in neovascularization.Citation21,Citation22 Studies have shown that tumor-associated endothelial cells have abnormal structures and leaky (porous) cells, which leads to tumor angiogenesis.Citation23,Citation24 Similar to other malignancies, angiogenesis leads to the HCC progression.Citation25
The immune response of TME is an important regulator of progression in many cancers including HCC. Immune cells in the HCC microenvironment include tumor-associated macrophages (TAMs), lymphocytes and dendritic cells (DCs).Citation14,Citation26 TAMs are considered to have two types: classically activated macrophages (M1 macrophages) and alternatively activated macrophages (M2 macrophages).27 M1 macrophages work as a soldier of adaptive immunity to eliminate tumor cells, while M2 macrophages promote tumor proliferation, angiogenesis, and ECM remodeling by suppressing the adaptive immune system.Citation27–Citation29 Lymphocytes affect hepatocarcinogenesis not only by directly acting on the interaction of the adaptive immune system and cytokines but by regulating the innate immune response and angiogenesis.Citation26 DCs are the strongest antigen-presenting cells (APCs) found so far. They control the subsequent differentiation of T cells and regulate their response.Citation30
Given the importance of TME components in the HCC initiation, progression, invasion and metastasis, increased numbers of studies have focused on modulating TME by using nano-delivery systems in the HCC treatment. Therefore, relevant studies on CAF-, ECM-, endothelial cell-, immune system-modulating nanoparticles will be presented below ().
Table 1 The Nanoparticles that Modulate TME and Immune System of HCC
TME-Related Nanoparticles
CAF-Modulating Nanoparticles
Fibroblasts can be activated by a variety of factors during tissue damage, such as growth factors, direct intercellular communication, adhesion molecules in contact with leukocytes, reactive oxygen species,Citation15 and microRNA.Citation31,Citation32 When fibroblasts remain activated after recovery from tissue injury, these activated fibroblasts, also known as the CAFs, may interact with other molecular pathways to promote tumorigenesis. Cytokines may stimulate resident fibroblasts to convert them into CAFs,Citation33 including transforming growth factor-β (TGF-β) and stromal cell-derived factor-1 (SDF-1). In addition, cytokines may convert epithelial or endothelial cells into CAFs, including fibroblast growth factor (FGF), osteocalcin,Citation34 TGF-β, and SDF-1.Citation35,Citation36 CAFs influence the HCC progression through direct or indirect interaction with HCC cells. Furthermore, CAFs promote tumor cells proliferation and the HCC progression by remodeling ECM, inducing angiogenesis, recruiting inflammatory cells, and secreting growth factors and cell inhibitors.Citation37 Current strategies for modulating CAFs include translating CAFs to non-activated state or tumor suppressor phenotype, targeting specific markers of CAFs, and inhibiting the secretion or signaling molecules of CAFs. Due to the limited data about the modulatory effect of nanoparticles on CAFs in the HCC, the following will focus on the current strategies for modulating CAFs in cancer therapy.
Direct elimination of CAFs may disrupt homeostasis and lead to cancer progression. Therefore, putting CAFs into non-activated state or converting them to tumor suppressor phenotype may be a safe and reliable therapeutic approach. It was found in a mouse xenograft model that nanoparticles loaded with plasmids that encode secrete TNF-related apoptosis-inducing ligand (sTRAIL) could cause apoptosis of tumor cells near CAFs.Citation38 Interestingly, it restored the remaining CAFs to a quiescent state, remodeled the TME, and further inhibited tumor growth, which facilitates a second wave of nanotherapeutics.
Another effective therapeutic strategy is to target specific markers on the surface of CAFs, such as α-smooth muscle actin (α-SMA),Citation39 fibroblast activating protein (FAP),Citation40 platelet-derived growth factor receptor α/β (PDGFRα/β)Citation41 and collagen.Citation42 Current researches are mainly focused on the highly expressed FAP in CAFs. FAP is a membrane serine peptidase and a member of the type II serine protease family. Its dipeptidase and collagenase activities are believed to be closely associated with ECM remodeling. FAP is almost absent in healthy tissues but highly expressed in CAFs in various cancers.Citation43 The activity of FAP in the HCC is more than tenfold higher than that in the normal liver.Citation40,Citation44 FAP supports tumor growth by promoting proliferation, migration and invasion of tumor cells in multiple ways. Li et al coupled the FAP-specific single-chain viable fragment (scFV) sequence to the ferritin surface and encapsulated the photosensitizer ZnF16Pc in ferritin nanocages to form scFv-Z@FRT, and then use of photodynamic therapy (PDT) increased tumor uptake of the nanoparticles.Citation45 It was observed that scFV-FAP specific interaction promoted nanoparticles accumulation in tumors and the high ZnF16Pc loading worked in conjugation to cause efficient CAFs eradication. CAP is a cleavable amphiphilic peptide specific to FAP-α. Yu et al developed novel dual-responsive nanoparticles by encapsulating paclitaxel-albumin nanoparticles (HSA-PTX) into CAP-modified thermosensitive liposomes (CAP-TSL) and then added photothermal agent IR-780 to form HSA-PTX@CAP-ITSL.Citation46 HSA-PTX@CAP-ITSL first accumulated at the tumor site where it released HSA-PTX via cleavable CAP responsive to FAP-α. After irradiation by NIR laser, it not only killed tumor cells by thermal effect but promoted HSA-PTX release and facilitated deep penetration into tumor tissue. The anti-tumor effect was verified by both in vivo and in vitro experiments. Mycophenolate mofetil (MMF) is an immunosuppressive agent in liver transplantation. Yang et al constructed MMF prodrugs (MMF-LA) by chemically derivatizing MMF with linoleic acid (LA) and then developed MMF-LA@DSPE-PEG nanoparticles using DSPE-PEG2000 to encapsulate MMF-LA.Citation47 Immunofluorescence showed that MMF-LA@DSPE-PEG accumulated in the positive area of α-SMA, which proves that MMF-LA@DSPE-PEG had a high CAFs targeting ability. By co-seeding LM3 HCC cells and LX2 cells (an activated human hepatic stellate cell line) on the right side at a ratio of 2:1, they constructed a highly fibrotic HCC model to verify the anti-CAFs efficacy of MMF-LA@DSPE-PEG. The results revealed that MMF-LA@DSPE-PEG significantly reduced the CAFs density of HCC and exhibited an even higher anti-tumor activity.
Targeting CAFs-derived cytokines and chemokines is also a promising therapeutic strategy. TGF-β secreted by CAFs promotes angiogenic mimetic formation, tumor progression and invasion,Citation48–Citation50 which is a potential therapeutic target. Morén et al demonstrated that the liver X receptor alpha (LXRα) agonist T0901317 inhibited the HCC growth by eliminating TGF-β-induced CAFs and the fibroblast phenotype of HCC.Citation51 Several TGF-β inhibitors have been developed. For example, the TGF-β receptor inhibitor LY2109761 was shown to effectively inhibit HCC growth and dissemination by blocking the cross-talk between HCC cells and CAFs.Citation52 Gold nanoparticles were also shown to alter cell morphology, migration and molecular markers by disrupting signaling to CAFs and inhibiting their activation by modulating the TGF-β1 expression level.Citation53 In addition, CAFs-derived secretagogues such as chemokines CCL2/5/7, CXCL16, and IL-6 are potential therapeutic targets for CAFs. However, the nanoparticles targeting these cytokines for the HCC treatment need to be further developed.
ECM-Modulating Nanoparticles
ECM is a complex network of multiple macromolecules surrounding cells, with the main components including collagen, elastin, fibronectin, laminin and proteoglycan.Citation54 Structurally, these components constitute the basement membrane and interstitial matrix of the ECM. Studies have shown that the ECM does not act as mere inert support as previously thought, but is an important component of the cellular environment and involved in almost all cellular behaviors.Citation55 ECM provides an important tissue barrier for tumor metastasis. The development, progression, invasion and metastasis of malignant tumors are often accompanied by changes in the expression of the ECM and its cell surface receptors. The growth and metastasis of HCC lead to the destruction and rearrangement of the original liver tissue, while ECM degradation and remodeling are important factors affecting this process. Matrix metalloproteinases (MMPs) are thought to play a key role in ECM degradation and remodeling. The activity of MMPs depends on the balance MMPs and the MMPs inhibitors (TIMPs). The interaction of MMPs and TIMPs maintains the balance of ECM catabolism. In the HCC, this balance is disturbed when MMPs are overexpressed, which disrupts the basement membrane barrier, leading to tumor invasion and metastasis.Citation56 In addition, the stiffness of ECM plays an important role in the HCC development. For instance, lysine oxidase could modulate the stiffness of ECM by promoting covalent cross-linking of collagen fibers and elastin. It is involved in the formation of tumor tissue mesenchyme, which can promote the growth and metastasis of cancer cells.Citation57 It was shown that increased stromal stiffness promoted cancer cells proliferation and chemoresistance, while a soft environment could induce reversible cellular dormancy and stem cell characteristics in the HCC.Citation58
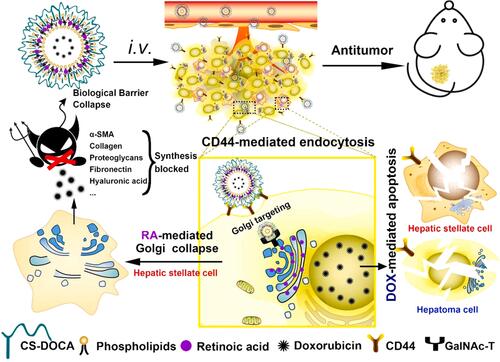
Current researches aim to target various proteins associated with ECM such as collagen, fibronectin and integrin by using novel nano-delivery systems and inhibit HCC proliferation and metastasis by removing these ECM components or altering their interactions with cells. For instance, Ali et al developed Arg-Gly-Asp (RGD) peptide-functionalized gold nanoparticles to target integrins.Citation59 The results showed that tumor cells migration was inhibited by targeting integrins which attenuated local adhesion between the cytoskeleton and ECM via integrin linkage. In addition to direct destruction of the ECM components, some researchers have achieved anti-tumor effects by inhibiting ECM production in the HCC. Hepatic stellate cells (HSCs) are major source of ECM, and the Golgi apparatus of HSCs modifies many ECM components into their final secreted forms, including type I and III collagen, proteoglycans, and fibronectin.Citation60,Citation61 In addition, the Golgi apparatus in cancer cells is required for the secretion of many oncogenic proteins such as MMPs.Citation62 In other words, disruption of the Golgi apparatus in HSCs can reduce ECM formation and inhibit tumor progression. Luo et al designed chondroitin sulfate-modified lipid nanoparticles (LNPs) co-loaded with retinoic acid (RA) and doxorubicin (Dox) (Dox-RA-CSNs), knowing that the two agents can form a complex through electrostatic interaction. This is the first published study reporting the use of RA and Dox for the HCC treatment.Citation63 Dox-RA-CSNs were internalized by HCC cells and targeted the Golgi apparatus of HCC. RA disrupted the Golgi structure and inhibited the production of ECM proteins, while Dox disrupted the function of DNA, leading to apoptosis of cancer cells or HSCs (). It was shown that Dox-RA-CSNs could significantly increase the intertumoral drug concentration and promote the penetration of Dox and RA into HCC cells. In primary liver tumors or mouse HCC (H22) allogeneic tumor models, Dox-RA-CSNs showed significant anti-tumor activity. The dense ECM and elevated interstitial fluid pressure in TME severely impeded the entry of nanoparticles into the tumors due to the large size of the nanoparticles.Citation64 Therefore, avoiding obstruction of the dense ECM is necessary for drug delivery. Small molecule drugs were found to reach the tumor site more easily, thus facilitating deeper drug penetration to the tumors.Citation65 In other words, an ideal delivery system should be able to accumulate around the tumors through active targeting and EPR effects and convert into free small molecules to facilitate tumor penetration. Based on the above idea, Liu et al combined gelatin nanoparticles (GNPs) and the prodrug doxorubicin-lactose (Dox-Lac) to develop ECM-sensitive programmed nano-delivery systems with “size reduction and hydrophilic/hydrophobic transition” (GNPs-Dox-Lac).Citation66 Lac is widely used as a hepatocyte targeting ligands, which can specifically bind to the over-expressed asialoglycoprotein-1 receptor (asgpr1) on hepatocytes and actively internalize through receptor-mediated endocytosis.Citation67 MMP2 in ECM led to degradation of GNPs, which promotes the release of the prodrug Dox-Lac deep into the HCC solid tumor. Finally, pH-responsive dissociation of Dox-Lac in HCC cells led to the release of free Dox, which translocates to the nucleus to induce toxicity. The ECM-sensitive GNPs-Dox-Lac can improve the efficiency of Dox delivery to achieve a tumor suppression rate of 90.8% in vivo.
Figure 2 Schematic representation of how Dox-RA-CSNs target liver cancer cells via CD44-mediated endocytosis, and then target the Golgi apparatus via interaction with GalNAc-T.
Endothelial Cell-Modulating Nanoparticles
HCC is a kind of tumor with a high degree of vascularization. The abnormal formation of tumor new blood vessels is one of the main reasons for the high metastasis rate and poor prognosis of HCC.Citation68 Blood vessels in the HCC are formed in the following ways: instability of microvascular system leads to high permeability of blood vessels and activation of endothelial cells; activated endothelial cells proliferate and migrate to the spinal cord to form new blood vessels, followed by recruitment of activated pericytes to stabilize new blood vessels.Citation69 The expression and release of pro-angiogenic and anti-angiogenic factors remain balanced during angiogenesis, whereas HCC breaks this balance, resulting in the abnormal growth of blood vessels. Aberrant activation of endothelial cells is closely associated with HCC angiogenesis. Activation and migration of vascular endothelial cells are promoted by many angiogenic factors in the HCC microenvironment. Among them, vascular endothelial growth factor (VEGF) is the most important signaling molecule during abnormal vascular growth in the HCC.Citation70 Many studies have demonstrated that the high expression of VEGF is related to the HCC progression.
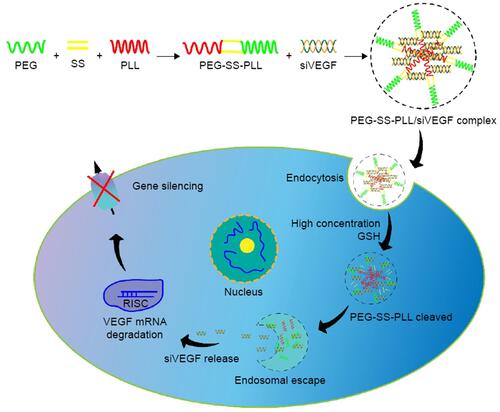
Down-regulating the expression of VEGF mRNA and its protein is a major nano-therapeutic approach against angiogenesis in the HCC. Many studies inhibit VEGF activation by the delivering the small interfering RNA (siRNA) that interferes with VEGF expression to tumor vascular endothelial cells. siRNA can silence the transcriptional translation of specific mRNA and inhibit gene expression,Citation71 but it is easily degraded and engulfed by reticuloendothelial cells during transportation in the body.Citation72 Nano-delivery systems encapsulating siRNA can avoid the above problems. Wang et al developed PEG-polypeptide cationic polymer nanoparticles with peelable PEG shell and disulfide bond-connected PEG-poly(ε-benzyloxycarbonyl-l-lysine) block copolymer (PEG-SS-PLL) to deliver VEGF siRNA (siVEGF) ().Citation73 The experiments demonstrated that PEG-SS-PLL can deliver siRNA to HepG-2 cells and reduce the protein expression of VEGF mRNA. A significant inhibitory effect on tumor growth was observed in the HepG-2 BALB/c mouse model. Xu et al developed CS-SS-9R/BSA-cRGD nanoparticles (CBc-NPs) by grafting nona-arginine (9R) to the chitosan (CS) molecule using a disulfide bond (-SS-) to form CS-SS-9R as a core material. And a cyclic peptide RGD sequence (cRGD) was modified to bovine serum albumin (BSA) as the outer shell.Citation74 It was found that CBc-NPs can specifically bind to the αvβ3 integrin receptors overexpressed in tumor endothelial cells and down-regulate VEGF production of tumor endothelial cells in an autocrine manner. The experiments demonstrated that the expression of VEGF mRNA of EA.hy926 cells was reduced to 11% after siVEGF-loaded CBc-NPs (siVEGF-CBc-NPs) treatment and the inhibition rate of EA.hy926 cells proliferation was as high as 90.25%, probably due to the high uptake by EA.hy926 cells. Anti-angiogenesis experiments, including wound healing, cell migration, cell invasion, capillary formation experiments and CD34 immunohistochemical staining, showed that EA.hy926 cells migration was inhibited after siVEGF-CBc-NPs treatment. Both in vivo and in vitro experiments showed that siVEGF could reduce the distribution density of capillaries. Detection of VEGF mRNA expression in tumor tissues revealed that the inhibitory rate of siVEGF-CBc-NPs on VEGF mRNA expression was as high as 95.54%, while naked siVEGF and non-coding siRNA had no gene silencing effect. Anti-tumor studies in vivo proved that the tumor volume and weight of tumor-bearing mice were reduced and the weight was increased after siVEGF-CBc-NPs treatment, suggesting that siVEGF-CBc-NPs could improve the anti-tumor effect and the life quality of tumor-bearing mice.
Figure 3 Illustration of PEG-SS-PLL catiomer for siVEGF encapsulation and intracellular stimulus-responsive siVEGF release.
Immune System-Related Nanoparticles
The immune system has a dual role in the development and progression of HCC. Some immune cells such as T cells and DCs inhibit tumor growth, while others such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) promote tumor progression.
Due to the unique physical and chemical properties of nanoparticles, nanoparticle-based platforms have been widely studied as strategies for regulating the immune system against cancer. In general, nanoparticles can protect sensitive antigens, proteins and RNAs from degradation or inactivation by enzymes in the physiological environment.Citation75 Additionally, the surface of nanoparticles has a strong modification ability to achieve efficient regulation of immune cells. Addition of long-chain polymers can significantly increase the circulatory half-life, and the targeting ability can be enhanced by attaching specific groups such as antibodies, peptides and ligands to the surface.Citation75,Citation76 Immune system-modulating nano-therapy to improve the efficiency of the HCC treatment and increase the immune response ability is a promising approach. In the following section, we will make a summary of studies using nano-delivery systems to modulate HCC immune cells, including TAMs, lymphocytes, and DCs.
TAM-Modulating Nanoparticles
TAMs are one of the most abundant components in the HCC microenvironment. TAMs can be classified into two types according to their functional characteristics: M1 macrophages and M2 macrophages.Citation27 M1/M2 macrophages have two opposite activities. It is generally believed that M1 macrophages are an inhibitory phenotype, which promotes Th1 response to kill tumor cells and microorganisms, while M2 macrophages are an activated phenotype, which activates Th2 response to promote tissue repair, angiogenesis, immunosuppression, and tumor progression.Citation77 Normally, M1/M2 macrophages co-exist in the microenvironment, and they balance each other under normal physiological conditions. When M2 macrophages prevail, it will contribute to tumorigenesis. The high presence of M2 macrophages has been reported to be closely associated with HCC.Citation78 M2 macrophages release IL-6, IL-1 and TNF-α pro-tumor metastasis factors. In the blood of HCC patients, the level of these three cytokines was found to be abnormally elevated.Citation79,Citation80 Moreover, M2 macrophages are associated with HCC blood vessel growth.Citation81 M2 macrophages increased the tumor volume and promoted intrahepatic metastasis by activating the STAT3 signaling pathway in HCC cell lines,Citation82 suggesting that inhibiting the infiltration of TAMs into tumor tissues and M2 polarization may be an effective treatment approach for HCC.
Sorafenib is a multi-kinase inhibitor that exerts both anti-angiogenic and anti-tumor effects. It is used to treat advanced HCC and significantly prolongs the survival time of patients. However, HCC is resistant to sorafenib, leading to recurrence and metastasis of HCC. CXCR4 is a G protein-coupled receptor whose specific ligand is the chemokine SDF-1. It was found that sorafenib increased the expression of CXCR4/SDF1α in the HCC.Citation83,Citation84 Recent studies have demonstrated that CXCR4 is involved in tumorigenesis and progression by promoting M2 polarization.Citation85 AMD3100, a CXCR4 antagonist, could avoid cancer cells proliferation and M2 polarization by inhibiting the CXCR4/SDF1α axis.Citation84 Gao et al designed CXCR4-targeted lipid-coated poly(lactic-co-glycolic acid) (PLGA) nanoparticles of sorafenib modified with AMD3100 (ADOPSor-NPs) for targeted delivery of sorafenib to HCC.Citation86 The results revealed that ADOPSor-NPs could precisely deliver sorafenib to HCC, block CXCR4/SDF1α and reduce M2 polarization and TAMs infiltration. At the same time, tumor progression was delayed and overall survival was increased in an in situ mouse HCC model. In addition, the team also constructed AMD3100-modified nanoparticles to deliver anti-angiogenic siRNA to HCC (VSAMD-NPs), which together delivered siVEGF and CXCR4 inhibitors to prevent HCC progression and metastasis.Citation87
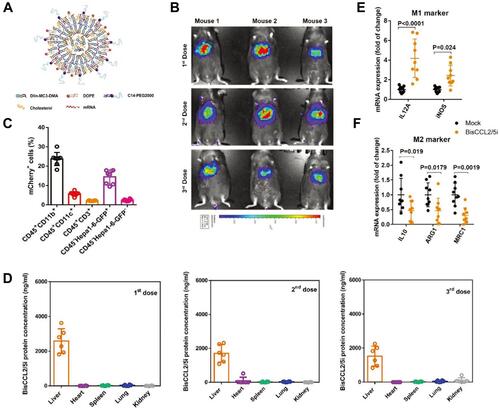
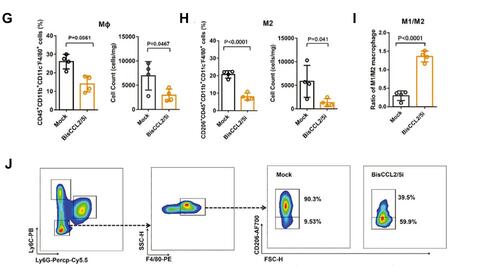
CCL2 and CCL5 are two chemokines that attract TAMs infiltration and induce their polarization to the M2 phenotype. Wang et al evolved a specific CCL2/CCL5 dual inhibitor (BisCCL2/5i) from a single-domain antibody library on the surface of yeast cells.Citation88 They found that BisCCL2/5i could block both CCL2 and CCL5 signaling pathways and effectively promote macrophages polarization toward the M1 phenotype. As it is risky to use of full-length antibodies due to the complexity and long circulating half-life of post-translational modifications, the researchers used Dlin-MC3-DMA LNPs (MC3 LNPs) to load mRNA encoding BisCCL2/5i (BisCCL2/5i-mRNA-LNPs) (). The United States Food and Drug Administration (FDA) has approved the first siRNA drug (ONPATTRO), which relies on MC3 LNPs to deliver siRNA to hepatocytes.Citation89 In other words, MC3 LNPs may possess tumor homing ability to deliver BisCCL2/5i-mRNA to liver malignancies. Experiments have shown that BisCCL2/5i-mRNA-LNPs treatment can not only inhibit TAMs infiltration but induce the polarization of M2 macrophages to M1 subtype. In animal HCC models, BisCCL2/5i-mRNA-LNPs significantly improved the survival of the experimental animals. They also tested the safety of BisCCL2/5i-mRNA-LNPs and found that erythrocytes, white blood cells, body weight, liver function and kidney function were normal in the test group, and no immunotherapy-related adverse events (irAEs) were detected.
Figure 4 Dual blockade of CCL2 and CCL5 via LNP-mediated mRNA delivery of BisCCL2/5i polarizes the macrophage M1 phenotype and reduces the immunosuppression in the TME. (A) Schematic of the mRNA-loaded LNPs. (B) In vivo transfection of luciferase mRNA-LNPs after repeated administration (i.v., every 4 days, in total 3 doses). The luciferase was injected intraperitoneally into the mice 6 h post the administration of luciferase mRNA-LNPs, followed by measuring the luciferase bioluminescence signal using IVIS imaging. n=3 biologically independent samples. (C) The quantification of mCherry-positive cells expressed in murine orthotopic HCC tumor tissue 6 h after injection of mCherry mRNA-LNPs (mCherry mRNA: 0.5 mg kg−1). mRNA was mainly expressed in monocytes (CD45+CD11b+) and tumor cells (Hepa1-6-GFP+) (n=8 biologically independent mice per group). (D) BisCCL2/5i expression in different organs 6 h after each administration of BisCCL2/5i mRNA-LNPs (mRNA: 1 mg kg−1, i.v., 3 days apart). n=6 biologically independent samples. The BisCCL2/5i mRNA was mainly expressed in the liver tissue and the repeated administration resulted in comparable protein level. (E, F) mRNA expression of classic M1 (E) and M2 (F) markers in the HCC tumor tissues 48 h after systemic administration of formulated LNPs as a dose corresponding to 1 mg kg−1 mRNA (Mock, HcRed mRNA). Each data point is an individual sample (n=9); one-way ANOVA and Tukey’s multiple comparisons test. Change of the immunocellular composition in the HCC TME 48 h following Mock mRNA-LNPs and BisCCL2/5i mRNA-LNPs treatments (mRNA: 1 mg kg−1), measured by flow cytometry (n=4 biologically independent samples; unpaired two-tailed Student’s t-test; the experiment was conducted three times independently with similar results). (G, H) The percentage and cell counts of macrophages (G) and their M2 subtype (H) in the total immune cells. (J, I) Representative flow dots of M1- and M2-phenotype macrophages (J) and the ratio of M1/M2 (I). Data are represented as the mean ± SD.
Notes: Reproduced from Wang Y, Tiruthani K, Li Set al mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv Mater. 2021;33(23):e2007603. Copyright © 2021 John Wiley & Sons, Inc. All rights reserved.Citation88
Abbreviations: LNP, lipid nanoparticle; BisCCL2/5i, specific CCL2/CCL5 dual inhibitor; MΦ, macrophages (CD45+CD11b+CD11c−Ly6C−Ly6G−F4/80+).
Hypoxia is common in the HCC and it can induce the formation of inhibitory immune environments such as the accumulation of TAMs, and promote tumor progression.Citation90,Citation91 Dai et al synthesized polydopamine nanoparticles to stabilize oxygen microcapsules (Oxy-Mic-Poly-Nano) via interfacial polymerization, knowing that oxygen microcapsules are good oxygen carriers that can deliver oxygen to the hypoxic sites of HCC.Citation92 They found that the combination of radiotherapy and Oxy-Mic-Poly-Nano enhanced the radiotherapy effect. In a HCC model established by subcutaneous injection of Hep1–6 cells, the proportion of M1 macrophages in R+O (radiotherapy and Oxy-Mic-Poly-Nano) group was 3.1-fold higher than that in the control group, while the proportion of M2 macrophages was 79.9% lower, demonstrating that radiotherapy combined with Oxy-Mic-Poly-Nano could reduce the number of TAMs in the HCC microenvironment and convert M2 macrophages to the M1 phenotype.
Lymphocyte-Modulating Nanoparticles
Lymphocytes are cells with specific immune recognition functions. According to their origins and immune functions, lymphocytes can be divided into three categories:Citation93 (1) T cells, which participate in cellular immunity by directly killing target cells and by assisting or inhibiting antibody production by B cells; (2) B cells, which participate in humoral immunity by producing and secreting immunoglobulins (antibodies); and (3) natural killer cells (NK cells), which spontaneously exert cytotoxic effects independent of antigenic stimulation and have the effect of killing target cells.
T cells are a key component of the adaptive immune system and make a difference in immune defense against pathogens such as viruses, bacteria and tumors. T cells are divided into three types according to their functions: cytotoxic T cell (CTLs), Tregs and helper T cells (Th cells). CTLs, also known as CD8+ T cells due to the presence of their surface protein CD8, are activated by various cytokines to directly recognize and kill tumor cells through intracellular antigens and are responsible for inhibiting tumor cells proliferation.Citation94,Citation95 CTLs directly kill target cells without causing self-damage via two pathways: the perforin/granzyme pathway and the FAS/FAS ligand (FASL) pathway.Citation96 Tregs regulate the body’s immune tolerance and affect the response of immune cells to self and non-self antigens. In immunotherapy, Tregs participate in tumor progression by suppressing immune response. Tregs effectively regulate the immune response through a variety of different mechanisms,Citation97,Citation98 such as the suppression of the function of APCs and the production of immunosuppressive cytokines and metabolites. For example, Tregs make use of membrane-bound cytotoxic T lymphocyte antigen 4 (CTLA-4) to inhibit the function of APCs.Citation99 In various cancers, the presence of high Tregs and low proportion of CD8+ T cells in TME is associated with poor prognosis.Citation100 Th cells coordinate adaptive immune response and contribute to immune protection.Citation101 They help maturation and activation of other lymphocytes, such as B cells, CTLs, and macrophages, and they also recruit various cell populations to sites of infection and inflammation. In one word, T cells play a crucial part in cancer immunotherapy. Knowing that most current anti-HCC immunotherapy studies focus on nanoparticles related to T cells, we will discuss nanoparticles that regulate the vitality of T cells in the following section.
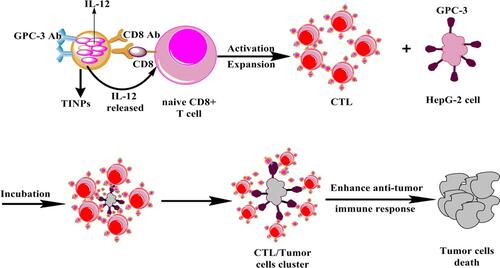
Interleukin 12 (IL-12) is considered to be one of the most effective cytokines to trigger anti-tumor immune response for the reason that it simultaneously activates both innate and adaptive immune response and influences the process of T cells activation.Citation102–Citation105 Li et al designed CD8- and Glypican-3 antibody-modified dual-target PLGA nanoparticles loaded with IL-12.Citation106 The prepared targeted immune nanoparticles (TINPs) could specifically bind to two target cell types, CD8+ T cells and HepG-2 cells, through antibody-antigen interactions. TINPs promoted activation and proliferation of T cells by targeting and delivering IL-12 precisely to T cells (). In addition, they enabled T cells to kill tumor cells effectively by targeting HCC cells to cross-link T cells with tumor cells to form T cell and tumor cell clusters. Experiments showed that TINPs treatment promoted the activation and proliferation of CD8+ T cells. CD107a is a degranulation marker and an indicator of the ability of T cells to degrade target tumor cells.Citation107 The studies found that compared with free IL-12, the expression of CD107a in CD8+ T cells and HepG-2 cells in the presence of TINPs was 5 times higher. Moreover, lactate dehydrogenase assay demonstrated that TINPs improved the lytic capacity of CD8+ T cells against HepG-2. These data indicated that TINPs can significantly enhance the cytotoxic activity of CD8+ T cells.
Figure 5 Scheme showing that TINPs enhance CD8+ T cell functions and cancer immunotherapy.
In addition to direct infusion of IL-12 protein, administration of IL-12 mRNA can achieve efficient delivery of IL-12 to the HCC microenvironment. Lai et al developed LNPs loaded with IL-12 mRNA (IL-12- LNPs).Citation108 After entering the body, IL-12- LNPs released IL-12 mRNA into cells, where they were translated by ribosomes into IL-12 protein, and then activated the immune response and caused the HCC regression. The results showed that CD3+ T cells increased significantly after IL-12-LNPs treatment, and promoted the infiltration of CD3+ Th cells into HCC and surrounding normal liver tissues. CD44 is a marker of T cells activation and can be used to determine the activation status of Th cells. Studies found that the number of recruited CD44+ immune cells increased in normal liver tissues after IL-12- LNPs treatment. It was reported that CD44 expression on CD3+, CD4+ Th cells could enhance Th1 responses by promoting the production of IFN-γ,Citation109,Citation110 indicating that IL-12-LNPs can activate CD44+, CD3+ and CD4+ Th cells and improve the immune response.
Tregs-mediated immunosuppression represents an important obstacle to effective anti-cancer immune response. That is to say, their inactivation or removal contributes to tumor immunotherapy. Interferon-inducible protein-10 (IP-10) is a cytokine of the CXC family of chemokines, which has the functions of chemotaxis and activation of T cells and inducing lymphocytes infiltration in tumor sites.Citation111,Citation112 Lai et al developed folic acid-modified chitosan nanoparticles (IP10-FA-CS-NPs) loaded with IP-10 plasmid.Citation113 Flow cytometric detection of Tregs in the spleen of H22 tumor-bearing mice showed the expression of CD4+ CD25+ FoxP3+ Tregs was 2.70±0.34% after treatment with IP10-FA-CS-NPs, which was significantly lower than that in the other groups. In addition, IP10-FA-CS-NPs also played an anti-tumor role in inhibiting HCC cells proliferation and inducing their apoptosis by promoting IFN-γ and IP-10.
DC-Modulating Nanoparticles
DCs are the most professional APCs in the immune system, and they make a difference in the initiation and regulation of antigen-specific immune response.Citation114,Citation115 Immature DCs have a high endocytosis activity and they can express a variety of intracellular and extracellular pathogen recognition receptors, such as toll-like receptors (TLRs). When DCs are subjected to external stimuli, they are activated and reach a mature antigen presentation state and then migrate to the draining lymphatic organs, where they trigger a strong antigen-specific T cell response by interacting with antigen-specific cells.Citation116,Citation117 Mature DCs can provide three signals to activate naive T cells. Signal 1: Mature DCs down-regulate their endocytic activity, highly express the major histocompatibility complex class I (MHC I) and major histocompatibility complex class II (MHC II) on the surface, and present the processed peptides to the naive CD8+ and CD4+ T cells. Signal 2: Mature DCs massively express accessory molecules and those interact with other receptors in T cells to enhance adhesion and costimulation.Citation118 Signal 3: Mature DCs secrete a large amount of IL-12, which induces T cells activation.Citation119 In the HCC, the decreased ability of DCs to present related antigens may be not only due to the decreased expression of human leukocyte antigen moleculesCitation120 but also due to the weak T cell immune response caused by maturation defects such as endocytosis, allogeneic stimulation and reduced IL-12 secretion.Citation121 Studies have shown that the HCC patients infected with hepatitis B and C viruses have a reduced number of peripheral DCs and impaired function.Citation122,Citation123 Current strategies for the HCC treatment aim to design tumor vaccines promoting DCs maturation.
Development of DC-based anti-tumor vaccines is a promising way of tumor immunotherapy. However, the clinical application is limited due to their low immunogenicity and easy degradation. The use of nano-delivery systems to present antigens has many advantages, such as preventing antigens from being hydrolyzed by enzymes, promoting antigen distribution into APCs, and improving antigen storage shelf life.Citation124 To further improve the activity of tumor vaccines, Yang et al prepared the nanoliposomes encapsulated with H22 hepatoma lysate and conjugated it with both mannose and CpG-ODN (M/CpG-ODN-H22-Lipo).Citation125 It was reported that mannose-modified nanoliposomes can specifically target the mannose receptors that are highly expressed on the surface of DCs to achieve DCs-mediated anti-tumor activity.Citation126 CpG-ODN is an effective immune adjuvant, which can produce strong immune stimulatory effects on a variety of cells and cytokines, and activate the immune response by inducing the expression of costimulatory factors. Cellular uptake and in vivo distribution experiments showed that DC2.4 cells could take up more mannosylated liposomes compared with unmodified liposomes, and the fluorescence intensity of mannose-liposomes and mannose-H22-liposomes was higher at the tumor site, illustrating that mannose modification improved the targeting ability of the liposomes towards the tumor tissue. After 48-h M/CpG-ODN-H22-Lipo treatment, the expression of MHC II and CD80/CD86 molecules on the surface of DC2.4 cells was significantly up-regulated, indicating that M/CpG-ODN-H22-Lipo could activate the maturation of DCs. In addition, M/CpG-ODN-H22-Lipo had good stability. After 10-day incubation in PBS containing 50% serum, M/CpG-ODN-H22-Lipo still retained the ability to activate DCs. Further research on the anti-tumor effect of M/CpG-ODN-H22-Lipo in a BALB/c mouse HCC model demonstrated that mice in the M/CpG-ODN-H22-Lipo group had smaller tumor volume and longer survival time compared with those in the control group. Detection of CD11b+Ly-6G+ MDSCs and CD4+CD25+FoxP3+ Tregs in mice tumor and bone marrow showed that the proportion of MDSCs was reduced after M/CpG-ODN-H22-Lipo treatment. In addition, the percentage of Tregs was decreased, while IFN-γ cells in the spleen and the IgG level in serum were both elevated. These data revealed that M/CpG-ODN-H22-Lipo activated robust humoral and cellular immune response.
Given the poor antigenicity and systemic immunosuppression of traditional anti-tumor vaccines,Citation127 RNA vaccines have been developed to activate the immune response. Compared with traditional tumor vaccines, RNA vaccines have the advantages of avoiding MHC classification restrictions and producing immunogenicity without adjuvants. Zhang et al extracted total RNA from liver cancer cells and constructed RNA-loaded LNPs (RNA-LNPs) targeting DCs.Citation128 It was found that RNA-LNPs could translate and synthesize tumor antigens in DCs, induce high-efficiency anti-HCC specific immune response, and inhibit tumor growth. In vitro studies shown that compared with immature DCs, the expression of CD40, CD80, CD86, CD11c and MHC II molecules on the surface of DCs was increased to a level of mature DCs after RNA-LNPs treatment, indicating that DCs treated with RNA-LNPs can effectively promote the maturation of DCs. T cells proliferation studies found that DCs treated with RNA-LNPs and mature DCs stimulated the growth rate of CD8+ T cells by 51.8% and 51.3% respectively, indicating that the ability of DCs treated with RNA-LNPs to activate immune response is similar to that of mature DCs. In addition, the team also studied the prevention and treatment effects of RNA-LNPs on liver cancer. After mice were intravenously injected with RNA-LNPs, Hepa1-6 was injected subcutaneously to create a model to study the preventive effect of RNA-LNPs on HCC. Compared with the control group, tumor growth in the mice of RNA-LNPs group was significantly delayed. Hepa1-6 was injected subcutaneously and then administered to study the therapeutic effect of RNA-LNPs on HCC. Similar to the preventive effect of RNA-LNPs, its inhibitory effect on tumor growth was stronger than that in the control group. The above data indicated that RNA-LNPs could effectively prevent and inhibit the HCC progression in vivo.
Conclusion and Outlook
In this paper, we review the recent progress in the HCC treatment by using nano-delivery systems to modulate TME. TME is closely related to the HCC progression by promoting the HCC cells invasion and migration through various pathways. TME-related nanoparticles can protect the therapeutic agents from degradation and improve their targeting ability to HCC, thus increasing efficiency in modulating the TME. Current researches mainly focus on the modulation of CAFs, ECM, endothelial cells and the immune system, among which modulation of the immune system in the HCC is believed to have great potential in that it can effectively improve the immune response.
However, limited researches have been reported on the regulation of the HCC microenvironment compared with other cancers. For example, few studies have addressed the regulatory effect of nanoparticles on CAFs, MDSCs and tumor-associated neutrophils, knowing that these cells are also important in the HCC progression. Further studies are necessary. And there are still some problems that need to be solved. Firstly, it is necessary to gain a better understanding about the target components in the HCC microenvironment and how nanoparticles interact with these TME components, as this work will help better design nano-delivery systems to modulate TME. Secondly, more efforts should be devoted to the design of multiple-targeting methods that can simultaneously target multiple TME components in the HCC to achieve better therapeutic effects. Finally, how to overcome the low clinical conversion rate of nanoparticles is also a problem that needs to be explored. In conclusion, the use of nano-delivery systems to regulate TME has shown exciting prospects in the HCC treatment. It is expected that significant progress in the HCC treatment can be achieved in near future.
Acknowledgments
This review was supported by the National Natural Science Foundation of China (No.81772749, 81872220, China); Shanghai Sailing Program (20YF1412100, China); Jiaxing Key Laboratory of Oncological Photodynamic Therapy and Targeted Drug Research as the basic public welfare research project of Zhejiang Province (No.LGF18H160034, China); The New Interdisciplinary Subjects of Pudong New District Health Committee, grant number (PWXx2020-04, China); Shanghai Qingpu District Industry-University-Research Cooperative Development Funding Project (2021-7, China), Program of Shanghai Academic Research Leader (21XD1403400, China), Nature Science Foundation of Shanghai (21ZR1449200, China) and High-level Talents of Fujian University of Chinese Medicine (X2019006-Talents, China).
Disclosure
The authors report no conflicts of interest in this work.
References
- IARC. International Agency for Research on Cancer.GLOBOCAN 2018. Available from: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=11&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nature Reviews Disease Primers. 2021;7:6. doi:10.1038/s41572-020-00240-3
- Nishida N, Kudo M. Oncogenic Signal and Tumor Microenvironment in Hepatocellular Carcinoma. Oncology. 2017;93(Suppl 1):160–164. doi:10.1159/000481246
- Wang K, Sun D. Cancer stem cells of hepatocellular carcinoma. Oncotarget. 2018;9(33):23306–23314. doi:10.18632/oncotarget.24623
- Sevic I, Spinelli FM, Cantero MJ, et al. The role of the tumor microenvironment in the development and progression of hepatocellular carcinoma. J Med. 2019;4:29–45.
- Cho K, Wang X, Nie S, Chen Z, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi:10.1158/1078-0432.Ccr-07-1441
- Zamboni WC, Torchilin V, Patri AK, et al. Best Practices in Cancer Nanotechnology: perspective from NCI Nanotechnology Alliance. Clin Cancer Res. 2012;18(12):3229–3241. doi:10.1158/1078-0432.Ccr-11-2938
- Prabhakar U, Maeda H, Jain RK, et al. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013;73(8):2412–2417. doi:10.1158/0008-5472.Can-12-4561
- Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi:10.1016/j.jconrel.2011.06.001
- Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem. 2006;17(3):603–609. doi:10.1021/bc050335b
- Birgani MT, Carloni V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int J Mol Sci. 2017;18:(2)405. doi:10.3390/ijms18020405
- Leonardi GC, Candido S, Cervello M, et al. The tumor microenvironment in hepatocellular carcinoma. Int J Oncol. 2012;40(6):1733–1747. doi:10.3892/ijo.2012.1408
- Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21(1):35–43. doi:10.1016/j.semcancer.2010.10.007
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi:10.1038/nrc1877
- Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21(1):19–25. doi:10.1016/j.semcdb.2009.10.002
- Yin ZY, Dong CY, Jiang KQ, et al. Heterogeneity of cancer-associated fibroblasts and roles in the progression, prognosis, and therapy of hepatocellular carcinoma. J Hematol Oncol. 2019;12:(1)101. doi:10.1186/s13045-019-0782-x
- Reid LM, Fiorino AS, Sigal SH, Brill S, Holst PA. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology. 1992;15(6):1198–1203. doi:10.1002/hep.1840150635
- Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–1253. doi:10.15252/embr.201439246
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406. doi:10.1083/jcb.201102147
- Dudley AC. Tumor Endothelial Cells. Cold Spring Harbor Perspectives in Medicine. Medicine. 2012;2(3):a006536. doi:10.1101/cshperspect.a006536
- Davis GE, Senger DR. Endothelial extracellular matrix - Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97(11):1093–1107. doi:10.1161/01.RES.0000191547.64391.e3
- Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–1380. doi:10.1016/s0002-9440(10
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15(1):102–111. doi:10.1016/j.gde.2004.12.005
- Finn RS, Zhu AX. Targeting angiogenesis in hepatocellular carcinoma: focus on VEGF and bevacizumab. Expert Rev Anticancer Ther. 2009;9(4):503–509. doi:10.1586/era.09.6
- Santhakumar C, Gane EJ, Liu K, McCaughan GW. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol Int. 2020;14(6):947–957. doi:10.1007/s12072-020-10104-3
- Mills CD. M1 and M2 Macrophages: oracles of Health and Disease. Crit Rev Immunol. 2012;32(6):463–488. doi:10.1615/CritRevImmunol.v32.i6.10
- Amann T, Bataille F, Spruss T, et al. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100(4):646–653. doi:10.1111/j.1349-7006.2009.01087.x
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi:10.1146/annurev.immunol.21.120601.141040
- Tanaka K, Miyata H, Sugimura K, et al. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis. 2015;36(8):894–903. doi:10.1093/carcin/bgv067
- Min A, Zhu C, Peng S, et al. Downregulation of Microrna-148a in Cancer-Associated Fibroblasts from Oral Cancer Promotes Cancer Cell Migration and Invasion by Targeting Wnt10b. J Biochem Mol Toxic. 2016;30(4):186–191. doi:10.1002/jbt.21777
- Kojima Y, Acar A, Eaton EN, et al. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(46):20009–20014. doi:10.1073/pnas.1013805107
- Billottet C, Tuefferd M, Gentien D, et al. Modulation of several waves of gene expression during FGF‐1 induced epithelial‐mesenchymal transition of carcinoma cells. Journal of Cellular Biochemistry. 2008;104(3):826–839. doi:10.1002/jcb.21667
- Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. British Journal of Cancer. 2008;99(9):1375–1379. doi:10.1038/sj.bjc.6604662
- Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Biosci. 2009;19(2):156–172. doi:10.1038/cr.2009.5
- Zhang J, Gu CY, Song QQ, et al. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020;10:(1)127. doi:10.1186/s13578-020-00488-y
- Miao L, Liu Q, Lin CM, et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer Research. 2017;77(3):719–731. doi:10.1158/0008-5472.CAN-16-0866
- Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD. Docetaxel conjugate nanoparticles that target α-smooth muscle actin–expressing stromal cells suppress breast cancer metastasis. J med. 2013;73(15):4862–4871.
- Zou B, Liu X, Zhang B, et al. The Expression of FAP in Hepatocellular Carcinoma Cells is Induced by Hypoxia and Correlates with Poor Clinical Outcomes. J Cancer. 2018;9(18):3278–3286. doi:10.7150/jca.25775
- Beljaars L, Weert B, Geerts A, Meijer DK, Poelstra K. The preferential homing of a platelet derived growth factor receptor-recognizing macromolecule to fibroblast-like cells in fibrotic tissue. Biochemical Pharmacology. 2003;66(7):1307–1317. doi:10.1016/s0006-2952(03)00445-3
- Lakins MA, Ghorani E, Munir H, Martins CP, Shields J. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T Cells to protect tumour cells. Cell Biosci. 2018;9(1):1–9.
- Fitzgerald AA, Weiner LMJC, Reviews M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Reviews. 2020;39(3):783–803. doi:10.1007/s10555-020-09909-3
- Keane FM, Yao T-W, Seelk S, et al. Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. J Med. 2014;4:43–54.
- Li L, Zhou SY, Lv NN, et al. Photosensitizer-Encapsulated Ferritins Mediate Photodynamic Therapy against Cancer-Associated Fibroblasts and Improve Tumor Accumulation of Nanoparticles. Mol Pharmaceut. 2018;15(8):3595–3599. doi:10.1021/acs.molpharmaceut.8b00419
- Yu QW, Qiu Y, Li JP, et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. Article J Control Release. 2020;321:564–575. doi:10.1016/j.jconrel.2020.02.040
- Yang ZT, Zhang L, Zhu H, et al. Nanoparticle formulation of mycophenolate mofetil achieves enhanced efficacy against hepatocellular carcinoma by targeting tumour-associated fibroblast. J Cell Mol Med. 2021;25(7):3511–3523. doi:10.1111/jcmm.16434
- Yang J, Lu Y, Lin -Y-Y, et al. Vascular mimicry formation is promoted by paracrine TGF-β and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Letters. 2016;383(1):18–27. doi:10.1016/j.canlet.2016.09.012
- Giannelli G, Villa E, Lahn M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Research. 2014;74(7):1890–1894. doi:10.1158/0008-5472.CAN-14-0243
- Farazi PA, DePinho R. Hepatocellular carcinoma pathogenesis: from genes to environment. Nature Reviews. Cancer. 2006;6(9):674–687. doi:10.1038/nrc1934
- Morén A, Bellomo C, Tsubakihara Y, et al. LXRα limits TGFβ-dependent hepatocellular carcinoma associated fibroblast differentiation. Oncogenesis. 2019;8(6):36. doi:10.1038/s41389-019-0140-4
- Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S, Giannelli GJH. Down‐regulation of connective tissue growth factor by inhibition of transforming growth factor β blocks the tumor–stroma cross‐talk and tumor progression in hepatocellular carcinoma. Hepatology (Baltimore, Md.). 2010;51(2):523–534. doi:10.1002/hep.23285
- Zhang Y, Elechalawar CK, Hossen MN, et al. Gold nanoparticles inhibit activation of cancer-associated fibroblasts by disrupting communication from tumor and microenvironmental cells. Bioactive Materials. 2021;6(2):326–332. doi:10.1016/j.bioactmat.2020.08.009
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(24):4195–4200. doi:10.1242/jcs.023820
- Hynes RO. The Extracellular Matrix: not Just Pretty Fibrils. Science. 2009;326(5957):1216–1219. doi:10.1126/science.1176009
- Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Gastroenterology. 2013;144(3):512–527. doi:10.1053/j.gastro.2013.01.002
- Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–1017. doi:10.1038/nm.2208
- Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix Stiffness Modulates Proliferation, Chemotherapeutic Response, and Dormancy in Hepatocellular Carcinoma Cells. Hepatology. 2011;53(4):1192–1205. doi:10.1002/hep.24108
- Ali MRK, Wu Y, Tang Y, et al. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. P Natl Acad Sci USA. 2017;114(28):E5655–E5663. doi:10.1073/pnas.1703151114
- Duong HTT, Dong Z, Su L, et al. The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension. Small. 2015;11(19):2291–2304. doi:10.1002/smll.201402870
- Scodeller P, Catalano PN, Salguero N, Duran H, Wolosiuk A, Soler-Illia GJAA. Hyaluronan degrading silica nanoparticles for skin cancer therapy. Nanoscale. 2013;5(20):9690–9698. doi:10.1039/c3nr02787b
- Solis GP, Bilousov O, Koval A, Lüchtenborg AM, Lin C, Katanaev VL. Golgi-Resident Gαo Promotes Protrusive Membrane Dynamics. Cell. Cell. 2017;170(5):939–955.e24. doi:10.1016/j.cell.2017.07.015
- Luo JW, Gong T, Ma LX. Chondroitin-modified lipid nanoparticles target the Golgi to degrade extracellular matrix for liver cancer management. Article Carbohyd Polym. 2020;249:116887. doi:10.1016/j.carbpol.2020.116887
- Suzuki H, Bae YH. Evaluation of drug penetration with cationic micelles and their penetration mechanism using an in vitro tumor model. Biomaterials. 2016;98:120–130. doi:10.1016/j.biomaterials.2016.04.037
- Ling D, Park W, Park S-J, et al. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J Am Chem Soc. 2014;136(15):5647–5655. doi:10.1021/ja4108287
- Liu YY, Li L, Li LJ, et al. Programmed drug delivery system based on optimized ”size decrease and hydrophilicity/hydrophobicity transformation” for enhanced hepatocellular carcinoma therapy of doxorubicin. Nanomed-Nanotechnol. 2018;14(4):1111–1122. doi:10.1016/j.nano.2018.02.006
- Lv F, He X, Wu L, Liu T. Lactose substituted zinc phthalocyanine: a near infrared fluorescence imaging probe for liver cancer targeting. Bioorg Med Chem Lett. 2013;23(6):1878–1882. doi:10.1016/j.bmcl.2012.12.103
- Mukherjee A, Madamsetty VS, Paul MK, Mukherjee S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. Int J Mol Sci. 2020;21:(2)455. doi:10.3390/ijms21020455
- Ho JWY, Pang RWC, Lau C, et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44(4):836–843. doi:10.1002/hep.21353
- Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–137. doi:10.1007/s12072-018-9919-1
- Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi:10.1038/35888
- Wu HP, Feng GS, Liang HM, Zheng CS, Li X. Vascular endothelial growth factor antisense oligodeoxynucleotides with lipiodol in arterial embolization of liver cancer in rats. World J Gastroentero. 2004;10(6):813–818. doi:10.3748/wjg.v10.i6.813
- Wang G, Gao X, Gu G, et al. Polyethylene glycol-poly(epsilon-benzyloxycarbonyl-llysine)-conjugated VEGF siRNA for antiangiogenic gene therapy in hepatocellular carcinoma. Int J Nanomed. 2017;12:3591–3603. doi:10.2147/ijn.S131078
- Xu B, Zhang Y, Yang H, et al. siVEGF-loaded nanoparticle uptake by tumor-associated vascular endothelial cells for hepatocellular carcinoma. Nanomedicine-Uk. 2020;15(13):1297–1314. doi:10.2217/nnm-2020-0082
- Yang ZG, Ma YF, Zhao H, Yuan Y, Kim BYS. Nanotechnology platforms for cancer immunotherapy. Wiley Interdisciplinary Rev. 2020;12:(2)e1590. doi:10.1002/wnan.1590
- Gupta B, Kim JO. Recent progress in cancer immunotherapy approaches based on nanoparticle delivery devices. J Pharmaceutical Investigation. 2021;51(4):399–412. doi:10.1007/s40005-021-00527-x
- Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267(2):204–215. doi:10.1016/j.canlet.2008.03.028
- Dong PP, Ma LJ, Liu LZ, et al. CD86(+)/CD206(+), Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis. Int J Mol Sci. 2016;17:(3)320. doi:10.3390/ijms17030320
- Kong LX, Zhou YJ, Bu H, Lv T, Shi YJ, Yang JY. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J Exp Clin Cancer Res. 2016;35131. doi:10.1186/s13046-016-0412-1
- Ataseven H, Bahcecioglu IH, Kuzu N, et al. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediators Inflamm. 2006:200678380. doi:10.1155/mi/2006/78380
- Peng SH, Deng H, Yang JF, et al. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroentero. 2005;11(41):6521–6524. doi:10.3748/wjg.v11.i41.6521
- Mano Y, Aishima S, Fujita N, et al. Tumor-Associated Macrophage Promotes Tumor Progression via STAT3 Signaling in Hepatocellular Carcinoma. Pathobiology. 2013;80(3):146–154. doi:10.1159/000346196
- Chen Y, Ramjiawan RR, Reiberger T, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61(5):1591–1602. doi:10.1002/hep.27665
- Chen Y, Huang Y, Reiberger T, et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology. 2014;59(4):1435–1447. doi:10.1002/hep.26790
- Cai JJ, Zhang Q, Qian XM, et al. Extracellular ubiquitin promotes hepatoma metastasis by mediating M2 macrophage polarization via the activation of the CXCR4/ERK signaling pathway. Ann Transl Med. 2020;8(15):929. doi:10.21037/atm-20-1054
- Gao DY, Lin TT, Sung YC, et al. CXCR4-targeted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Article Biomaterials. 2015;67:194–203. doi:10.1016/j.biomaterials.2015.07.035
- Liu JY, Chiang T, Liu CH, et al. Delivery of siRNA Using CXCR4-targeted Nanoparticles Modulates Tumor Microenvironment and Achieves a Potent Antitumor Response in Liver Cancer. Mol Ther. 2015;23(11):1772–1782. doi:10.1038/mt.2015.147
- Wang Y, Tiruthani K, Li SR, et al. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv Mater. 2021;33(23):2007603. doi:10.1002/adma.202007603
- Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15(4):313–320. doi:10.1038/s41565-020-0669-6
- Henze A-T, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–3679. doi:10.1172/JCI84427
- Duran SR, Jaquiss RDB. Hepatocellular Carcinoma. N Eng J Med. 2019;381(1):548.
- Dai XM, Ruan J, Guo YX, et al. Enhanced radiotherapy efficacy and induced anti-tumor immunity in HCC by improving hypoxia microenvironment using oxygen microcapsules. Chem Eng J. 2021:422130109. doi:10.1016/j.cej.2021.130109
- Huang Y, Gao X, Chen J. Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy. Acta Pharmaceutica Sinica B. 2018;8(1):4–13. doi:10.1016/j.apsb.2017.12.001
- Xie Q, Ding J, Chen Y. Role of CD8(+) T lymphocyte cells: interplay with stromal cells in tumor microenvironment. Acta Pharmaceutica Sinica B. 2021;11(6):1365–1378. doi:10.1016/j.apsb.2021.03.027
- Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8(+) T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118(4):1390–1397. doi:10.1172/jci34388
- Slaney CY, Kershaw MH, Darcy PK. Trafficking of T Cells into Tumors. Cancer Res. 2014;74(24):7168–7174. doi:10.1158/0008-5472.Can-14-2458
- Jonuleit H, Bopp T, Becker C. Treg cells as potential cellular targets for functionalized nanoparticles in cancer therapy. Nanomedicine-Uk. 2016;11(20):2699–2709. doi:10.1158/0008-5472.CAN-14-2458
- Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–2089. doi:10.1111/cas.14069
- Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3(+) regulatory T cell function. Science. 2008;322(5899):271–275. doi:10.1126/science.1160062
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi:10.1038/nrc3245
- Schorer M, Kuchroo VK, Joller N. Role of Co-stimulatory Molecules in T Helper Cell Differentiation. In: azuma M, Yagita H, eds. Co-Signal Molecules in T Cell Activation: immune Regulation in Health and Disease. Adv Exp Med Biol. 2019;1:153–177.
- Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13(16):4677–4685. doi:10.1158/1078-0432.Ccr-07-0776
- Smyth MJ, Taniguchi M, Street SEA. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165(5):2665–2670. doi:10.4049/jimmunol.165.5.2665
- Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185(5):817–824. doi:10.1084/jem.185.5.817
- Cheever MAM. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi:10.1111/j.1600-065X.2008.00604.x
- Li JY, Lin WS, Chen HJ, Xu ZP, Ye YB, Chen MS. Dual-target IL-12-containing nanoparticles enhance T cell functions for cancer immunotherapy. Cell Immunol. 2020;349104042. doi:10.1016/j.cellimm.2020.104042
- Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254(2):149–154. doi:10.1016/j.cellimm.2008.08.007
- Lai I, Swaminathan S, Baylot V, et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunotherapy Cancer. 2018;4:6125. doi:10.1186/s40425-018-0431-x
- Guan H, Nagarkatti PS, Nagarkatti M. Role of CD44 in the Differentiation of Th1 and Th2 Cells: CD44-Deficiency Enhances the Development of Th2 Effectors in Response to Sheep RBC and Chicken Ovalbumin. J Immunol. 2009;183(1):172–180. doi:10.4049/jimmunol.0802325
- Schumann J, Stanko K, Schliesser U, Appelt C, Sawitzki B. Differences in CD44 Surface Expression Levels and Function Discriminates IL-17 and IFN-gamma Producing Helper T Cells. PLoS One. 2015;10(7):e0132479. doi:10.1371/journal.pone.0132479
- Zhang N, Yang Y, Cheng L, et al. Combination of Caspy2 and IP-10 Gene Therapy Significantly Improves Therapeutic Efficacy Against Murine Malignant Neoplasm Growth and Metastasis. Hum Gene Ther. 2012;23(8):837–846. doi:10.1089/hum.2011.136
- Yates-Binder CC, Rodgers M, Jaynes J, Wells A, Bodnar RJ, Turner T. An IP-10 (CXCL10)-Derived Peptide Inhibits Angiogenesis. PLoS One. 2012;7(7):e40812. doi:10.1371/journal.pone.0040812
- Lai CH, Yu X, Zhuo HQ, et al. Anti-Tumor Immune Response of Folate-Conjugated Chitosan Nanoparticles Containing the IP-10 Gene in Mice with Hepatocellular Carcinoma. J Biomed Nanotechnol. 2014;10(12):3576–3589. doi:10.1166/jbn.2014.2051
- Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269(1):64–73. doi:10.1111/j.1365-2796.2010.02317.x
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi:10.1038/32588
- Geissmann F, Dieu-Nosjean MC, Dezutter C, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196(4):417–430. doi:10.1084/jem.20020018
- Turley SJ, Inaba K, Garrett WS, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288(5465):522–527. doi:10.1126/science.288.5465.522
- Walker WA. Immunology. Curr Opin Gastroenterol. 2007;23(6):644–646. doi:10.1097/MOG.0b013e3282f0769b
- Kaliński P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20(12):561–567. doi:10.1016/s0167-5699(99
- Matsui M, Machida S, Itani-Yohda T, Akatsuka T. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. J Gastroenterol Hepatol. 2002;17(8):897–907. doi:10.1046/j.1440-1746.2002.02837.x
- Ninomiya T, Akbar F, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31(2):323–331. doi:10.1016/s0168-8278(99
- Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188(2):341–350. doi:10.1084/jem.188.2.341
- Kakumu S, Ito S, Ishikawa T, et al. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J Gastroenterol Hepatol. 2000;15(4):431–436. doi:10.1046/j.1440-1746.2000.02161.x
- Reddy ST, van der Vlies AJ, Simeoni E, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–1164. doi:10.1038/nbt1332
- Yang XM, Lai CH, Liu AQ, et al. Anti-Tumor Activity of Mannose-CpG-Oligodeoxynucleotides-Conjugated and Hepatoma Lysate-Loaded Nanoliposomes for Targeting Dendritic Cells In Vivo. J Biomed Nanotechnol. 2019;15(5):1018–1032. doi:10.1166/jbn.2019.2755
- Xi L, Lin ZB, Qiu F, et al. Enhanced uptake and anti-maturation effect of celastrol-loaded mannosylated liposomes on dendritic cells for psoriasis treatment. Article Acta Pharmaceutica Sinica B. 2022;12(1):339–352. doi:10.1016/j.apsb.2021.07.0192211-3835
- Ulmer JB, Mason PW, Geall A, Mandl CW. RNA-based vaccines. Vaccine. 2012;30(30):4414–4418. doi:10.1016/j.vaccine.2012.04.060
- Zhang Y, Xie F, Yin Y, et al. Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles Against Hepatocellular Carcinoma. Int J Nanomed. 2021;16:1553–1564. doi:10.2147/ijn.S291421