Abstract
Purpose
The aim of this study was to investigate the biomechanical stability provided by a novel, polylactic acid/nano-sized, β-tricalcium phosphate, bioabsorbable, self-retaining cervical fusion cage (BCFC).
Methods
Quasistatic nonconstraining torques (maximum 1.5 NM) induced flexion, extension, lateral bending (±1.5 NM), and axial rotation (±1.5 NM) on 32 sheep cervical spines (C2–C5). The motion segment C3–C4 was first tested intact; the following groups were tested after complete discectomy: autologous tricortical iliac crest bone graft, Medtronic–Wego polyetheretherketone (PEEK) cage, Solis PEEK cage, and BCFC. The autologous bone graft group was tested with an anterior plate. The mean range of motion (ROM) was calculated from the load-displacement curves.
Results
BCFC significantly decreased ROM in lateral bending and axial rotation compared to other implants, and no significant difference in ROM between two types of PEEK cages and BCFC could be observed in flexion and extension. Anterior cervical plate (ACP) significantly decreased ROM in flexion and extension, but no significant difference in ROM between BCFC and bone graft plus ACP could be determined in lateral bending and axial rotation.
Conclusion
The BCFC device showed better stability to autologous tricortical iliac crest bone graft and PEEK cages in single-level anterior cervical discectomy and fusion models and thus may be a potential alternative to the current PEEK cages.
Introduction
Anterior cervical discectomy and fusion (ACDF) is widely applied in the treatment of cervical disc herniation and cervical spondylosis.Citation1,Citation2 Tricortical iliac crest bone graft has been the gold standard to fill the residual space after surgical disc removal.Citation3 Although autologous grafts obtained from the anterior iliac crest achieve high fusion rates, it brings significant disadvantages, including bone absorption, graft extrusion, and pseudoarthrosis. These deficiencies have led to a rapid increase in the use of a cervical spine interbody fusion cage (CSIFC). A metallic cage was first used in ACDF. The shortcomings of the metallic cage, including cage migration, subsidence, stress shielding, and obscured postoperative radiologic assessment, have already been reported.Citation4,Citation5 Carbon fiber and polyetheretherketone (PEEK) cages are radiolucent and less stiff, which allows radiologic evaluation and reduces stress shielding.Citation6,Citation7 However, carbon fiber and PEEK are still nonabsorbable materials and therefore cannot lead to completely biological interbody fusion. Despite satisfactory initial results, long-term problems, such as subsidence and breakage of the cage, have been noticed.Citation8,Citation9
The development of bioabsorbable materialsCitation10,Citation11 and inherent limitations of current nonabsorbable CSIFC devices have promoted the study of bioabsorbable cages. Bioabsorbable cages can be designed with similar stiffness to human bone to reduce stress shielding of the inside graft, and adequate postoperative assessment of interbody fusion would be possible. Polylactic acid (PLA) and its copolymer have a long history of safe clinical use. Although it can also be designed as an interbody fusion cage, PLA acidic degradation products can cause asepsis inflammation, which will damage the microenvironment of bone formation. Kandziora et alCitation12 and Frost et alCitation13 have reported osteolysis around lumber and cervical fusion cages made of PLA. Previous studies have indicated that the incorporation of β-tricalcium phosphate (β-TCP) into PLA materials can both enhance its osteoconductivity and buffer acid products.Citation14,Citation15 Additionally, nano-sized β-TCP shows improved mechanical properties and tunable degradability compared to micro-sized powders.Citation16,Citation17 Therefore, it is a potentially promising approach to design composite fusion devices combining the advantages of the two biodegradable materials and overcoming the disadvantage of each.
Anterior plates and screws are often employed for additional support to promote interbody fusion, reduce the rate of pseudoarthrosis, and maintain the spinal curvature and intervertebral height. Internal fixation devices may cause complications, such as plate migration, screw breakage and pullout, stress shielding, and even spinal cord or nerve injuries, that may require further treatment.Citation18 Under the premise of ensuring high fusion rates, how can these side effects be reduced or minimized? Further research into these aspects is necessary.
Recently, we developed a novel bioabsorbable cervical fusion device (BCFC) fabricated by the composite of PLA and nano-sized β-TCP; its anchoring clips, which can be implanted into the endplates of vertebrae, are designed to provide the primary biomechanical stabilization. The purpose of this study was to compare the biomechanical stability of BCFC with tricortical iliac crest bone graft and PEEK cages and with a tricortical iliac crest bone graft plus anterior cervical plate (ACP).
Materials and methods
Specimen preparation
In this study, 32 cervical spines (C2–C5) of 2-year-old adult female sheep (average weight 66.3 ± 5.1 kg) were prepared for biomechanical tests. Each specimen was radiographically screened to rule out pathologic abnormalities. The motion segment C3–C4 was isolated, and the musculature and fascia were carefully removed, preserving ligaments, discs, and joint capsules intact. En bloc specimens were obtained fresh frozen and thawed at room temperature.
After intact spinal analysis, a complete discectomy of C3–C4 with resection of the anterior longitudinal ligament was performed to simulate essential clinical features. The intervertebral disc height of C3–C4 was adjusted to 5 mm or 6 mm after the endplates were shaved with a high-speed diamond burr. All implants were inserted from 1 mm to 2 mm excess of preoperative disc height.
Cervical spine interbody fusion cages
The implants used for the biomechanical test were tricortical iliac crest bone graft (), Medtronic cage (Medtronic, Minneapolis, MN), which has a cylindrical hollow center and a flat superior surface (); and Solis cage (Stryker, Kalamazoo, Michigan), which has a hollow center, a convex superior surface, and 1 mm titanium spikes bilaterally on both inferior and superior surfaces (). Cervical cages were implanted according to the manufacturer’s instructions. To allow comparison among the different implants, bone graft and cages of similar height, width, and depth were used. The volume of the cages and their hollow centers were determined by the water displacement technique, according to Archimedes’ principle.
Figure 1 The different implants tested in the study. (A) Autologous bone graft was obtained from the anterior iliac crest. (B) Medtronic cage has a cylindrical hollow center and a flat superior surface. Inset: lateral view of a Medtronic cage. (C) Solis cage has 1 mm titanium spikes bilaterally on both inferior and superior surface. Inset: lateral view of a Solis cage. (D) BCFC device, composite of an interbody fusion cage and two anchoring clips. Inset: lateral and dorsal views and of assembled BCFC device.
Abbreviation: BCFC, bioabsorbable, self-retaining cervical fusion cage.

BCFC cervical fusion device
The BCFC device is a PLA/nano-sized β-TCP composite cage (containing 30 wt% β-TCP) with two anchoring clips (containing 10 wt% β-TCP) () that can be implanted, with custom-made instrumentation, in the superior and inferior endplates of vertebrae to aid in expulsion resistance.
Study protocol
Specimens (n = 32) were randomly assigned to four groups after intact testing: A, autologous tricortical iliac crest bone; B, Medtronic cage; C, Solis cage; and D, BCFC device (). Eight spines were tested in each group. Specimens in Group A were also tested with ACP (Medtronic, Minneapolis, MN) ().
Figure 2 The different instrumentation techniques tested in the study. Depicted are lateral radiographs of sheep cervical spine (C2–C5) with the different instrumentations from left to right: (A) native sheep cervical spine; (B) bone graft; (C) Medtronic cage; (D) Solis cage; (E) BCFC device; (F) bone graft plus ACP.
Abbreviations: BCFC, bioabsorbable, self-retaining cervical fusion cage; ACP, anterior cervical plate.
Biomechanical testing apparatus and measurement system
All cervical spines were kept moist by spraying saline throughout the biomechanical test. For testing, C2 and C5 were mounted in pots using polymethylmethacrylate. After the lower pot was rigidly fixed to the material testing apparatus, nondestructive biomechanical testing was performed by using a mechanical testing system machine (MTS system; Zwick–Roell, Ulm, Germany). A pure moment was applied to the C3 vertebra through servomotors. Each cervical spine was subjected to an unconstrained pure moment of ±1.5 NM at a motor rate of 1°/s for a total of load/unload cycles; relaxing for 60 seconds was allowed to minimize viscoelastic response before the data of the third cycle were used for analysis. Resultant three-dimensional range of motion (ROM) of each segment was tracked by the motional analysis system.
Data management and analysis
The ROM for each surgical implant was compared with other implants and with the intact specimen. For multiple comparison procedures, an analysis of variance (ANOVA) was used followed by Student–Newman–Keuls test for post hoc analysis. The values are given as mean ± standard deviation. Statistical evaluation was supported by SPSS 15.0 (IBM Corporation, Armonk, NY).
Results
Data of the cages
Height, width, and depth of the implants along with results of volume of the cages and their hollow centers are provided in . The Solis cage had the greatest hollow volume for graft filling among these cages. The BCFC cage had a larger hollow volume than the Medtronic cage.
Table 1 Height, width, depth, and volume of the cervical spine interbody fusion cages and bone graft
Comparison between BCFC and stand-alone interbody implants
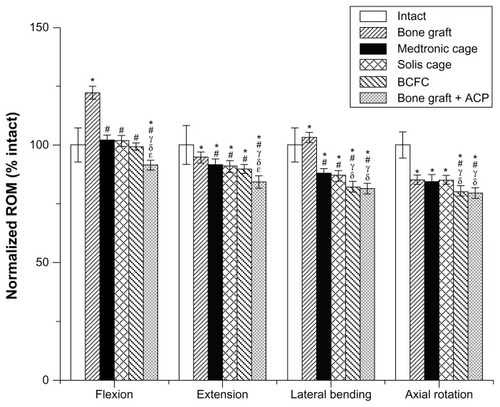
summarizes the ROM results for all groups. In comparison to the intact motion segment, the tricortical iliac crest bone showed a significantly higher ROM in flexion, extension, and axial rotation (P < 0.05). No significant difference in the ROM among the Medtronic cage, Solis cage, and BCFC device could be determined in flexion or extension. The BCFC device demonstrated a significantly lower ROM in lateral bending and axial rotation (P < 0.05).
Figure 3 Graph showing the variation in the biomechanical ROM in all loading models for all groups.
Notes: *, #, γ, δ, ɛ significance with respect to intact bone graft, Medtronic cage, Solis cage, BCFC, and bone graft plus ACP, respectively (P < 0.05).
Abbreviations: ROM, range of motion; BCFC, bioabsorbable, self-retaining cervical fusion cage; ACP, anterior cervical plate.
Comparison among stand-alone interbody implants, BCFC, and bone graft plus ACP
Compared to the intact motion segment, the Medtronic cage, Solis cage, and BCFC device, additional ACP significantly (P < 0.05) decreased ROM of the tricortical iliac crest bone in flexion and extension. In lateral bending and axial rotation, bone graft plus ACP showed slightly better stability than BCFC but with no statistical significance.
Discussion
The present study was performed to evaluate the primary biomechanical stability of a BCFC, a novel cervical fusion device. The cage and anchoring clips were fabricated with different contents of nano-sized β-TCP, according to unpublished data from our previous study (unpublished data). In that study, PLA/nano-sized composite rods were obtained from an axial compression molding process, and the in vitro degradation, mechanical properties, and cytocompatibility of the composites were investigated to screen suitable material for fabricating the cage and anchoring clips. To the best of our knowledge, we are the first to use a PLA and nano-sized β-TCP composite to fabricate a bioabsorbable cervical interbody fusion device.
Although ACDF is the proverbial “gold standard” to treat cervical disc herniation and cervical spondylosis, controversy remains about the choice of the best instrumentation for ACDF. The ideal CSIFC for ACDF is influenced by several factors, including materials, maximum graft filling of the intervertebral space, optimal surface contact area of graft and vertebral body, and biomechanical stability. Competition exists between cage volume and graft volume. One important biologic factor for CSIFC is having the smallest possible cage volume, which allows the maximum graft filling of the intervertebral space.Citation19 For this purpose, the hollow center of the BCFC was designed as a double cylinder, and the convex superior surface of the BCFC leads to a large contact area of graft and vertebral body. The present study showed that both the Solis cage and BCFC with the convex superior surfaces were more stable than the Medtronic cage with a flat surface.
Various materials have been used for manufacturing CSIFC for ACDF. PEEK is a nonabsorbable biopolymer and lacks osteoconductivity. The PEEK cage is now widely applied because its elasticity modulus is close to that of cortical bone.Citation18 Despite satisfactory initial results, the long-term effects of using the PEEK cage alone in ACDF are still unknown. The novel BCFC device composite of PLA and nano-sized β-TCP can be absorbed completely in vivo. Absorption and bone healing are simultaneous (creeping substitution), leading to complete fusion and new bone formation. Consequently, there are no long-term effects, such as toxic or allergic reactions or cage migration and breakage.
A sufficient compressive stiffness is crucial for the application of a bioabsorbable cage, otherwise destruction of the cage might lead to loss of disc space height and migration of breakdown products to the spinal canal.Citation20 The compressive stiffness of BCFC has not been evaluated. However, our unpublished data suggested that PLA/nano-sized composite material containing 30 wt% β-TCP might have sufficient stiffness for interbody stabilization. In contrast, composite material containing 10 wt% β-TCP, which was more flexible and thus more resistant to deformation, might be suitable for fabricating the anchoring clips. Although the compressive stiffness of BCFC is still lower than that of the PEEK cage, BCFC has already demonstrated the ability to withstand loads and limited ROM in a sheep model. Although ACDF without any additional anterior support is a proven and widely accepted surgical technique, especially at the single level, anterior plating offers more immediate stability and increases fusion rate of the operated segment; however, side effects make it a controversial procedure. The Solis cage design in terms of its shape and the presence of titanium spikes minimizes the need for an anterior plate device and its associated complications. However, a recent study has reported a prevalence of 25.5% subsidence and 14.9% intervertebral nonunion after using a stand-alone Solis cage in ACDF.Citation8 To overcome these problems, anchoring clips of the BCFC are designed to preserve the natural anatomic profile. In the comparison between the BCFC and stand-alone implants, the present study demonstrated biomechanical equivalence in flexion and extension. In lateral bending and axial rotation, the BCFC showed significantly better stability than other implants. The self-retaining anchor provides immediate solid fixation between the cage and the adjacent vertebral bodies without additional anterior plating systems, simulating the effect of anterior plates and screws. Furthermore, bone graft ACP showed slightly better stability than BCFC in lateral bending and axial rotation; however, with no statistical significance.
The limitations of this study should be mentioned. Although anatomical, biomechanical, and bone mineral density evaluation of sheep and goat cervical spine have shown good comparability with human spine, the biomechanical performance of tested implants in the human in vivo may differ significantly from the results obtained in the sheep in vitro study due to the complex loading conditions of human cervical spine in vivo. In vivo animal study is required for further validation of using the BCFC device in the ACDF model.
Conclusion
The biomechanical study showed better stability of the novel BCFC device than traditional cervical interbody devices in a single-level ACDF model. The results suggest that it may be a viable alternative to current PEEK cages and anterior plate devices.
Acknowledgment
This work was supported by the National Basic Research Program of China (973 program) (2009CB930002), Shanghai International Science and Technology Partnership Program (11540702700), Natural Science Foundation of China (31170925), and the National Natural Science Foundation of China (30970718).
Disclosure
The authors report no conflicts of interest in this work.
References
- LiedBRoenningPASundsethJAnterior cervical discectomy with fusion in patients with cervical disc degeneration: a prospective outcome study of 258 patients (181 fused with autologous bone graft and 77 fused with a PEEK cage)BMC Surg2010101020302673
- NiuCCLiaoJCChenWJOutcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cagesJ Spinal Disord Tech201023531031620124907
- MillerLEBlockJESafety and effectiveness of bone allografts in anterior cervical discectomy and fusion surgerySpine (Phila Pa 1976)201136242045205021304437
- KeplerCKRawlinsBAMesh cage reconstruction with autologous cancellous graft in anterior cervical discectomy and fusionJ Spinal Disord Tech201023532833220087220
- LiaoJCNiuCCChenWJPolyetheretherketone (PEEK) cage filled with cancellous allograft in anterior cervical discectomy and fusionInt Orthop200832564364817639386
- LiuHPloumisALiCPolyetheretherketone cages alone with allograft for three-level anterior cervical fusionISRN Neurol2012Article ID 452703
- HellbuschLCSpanglerWJBowderARadiographic PEEK double-lucency finding after anterior cervical discectomy and fusion with local autograft and PEEK spacer: a preliminary studyJ Neurosurg Spine201216324825022195610
- YangJJYuCHChangBSSubsidence and nonunion after anterior cervical interbody fusion using a stand-alone polyetheretherketone (PEEK) cageClin Orthop Surg201131162321369474
- MarottaNLandiATarantinoRFive-year outcome of stand-alone fusion using carbon cages in cervical disc arthrosisEur Spine J201120Suppl 1S8S1221404034
- HuangDZuoYZouQReinforced nanohydroxyapatite/polyamide66 scaffolds by chitosan coating for bone tissue engineeringJ Biomed Mater Res B Appl Biomater20121001515721953937
- CrouzierTSailhanFBecquartPThe performance of BMP-2 loaded TCP/HAP porous ceramics with a polyelectrolyte multilayer film coatingBiomaterials201132307543755421783243
- KandzioraFPflugmacherRScholzMBioabsorbable interbody cages in a sheep cervical spine fusion modelSpine (Phila Pa 1976)2004291718451855185615534403
- FrostABagouriEBrownMOsteolysis following resorbable poly-L-lactide-co-D, L-lactide PLIF cage use: a review of casesEur Spine J201221344945421881864
- HuttunenMAshammakhiNTormalaPFibre reinforced bioresorbable composites for spinal surgeryActa Biomater20062557558716807156
- AunobleSClementDFrayssinetPBiological performance of a new beta-TCP/PLLA composite material for applications in spine surgery: in vitro and in vivo studiesJ Biomed Mater Res A200678241642216721799
- PoliniAPisignanoDParodiMOsteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factorsPLoS One2011610e2621122022571
- LinKChangJShenRThe effect of powder properties on sintering, microstructure, mechanical strength and degradability of beta-tricalcium phosphate/calcium silicate composite bioceramicsBiomed Mater20094665009
- ZhouJXiaQDongJComparison of stand-alone polyetheretherketone cages and iliac crest autografts for the treatment of cervical degenerative disc diseasesActa Neurochir (Wien)2011153111512220924769
- KandzioraFPflugmacherRSchaferJBiomechanical comparison of cervical spine interbody fusion cagesSpine (Phila Pa 1976)200126171850185711568693
- KandzioraFPflugmacherRKleemannRBiomechanical analysis of biodegradable interbody fusion cages augmented With poly(propylene glycol-co-fumaric acid)Spine (Phila Pa 1976)200227151644165112163726