Abstract
Chitosan, a natural biodegradable polymer, is of great interest in biomedical research due to its excellent properties including bioavailability, nontoxicity, high charge density, and mucoadhesivity, which creates immense potential for various pharmaceutical applications. It has gelling properties when it interacts with counterions such as sulfates or polyphosphates and when it crosslinks with glutaraldehyde. This characteristic facilitates its usefulness in the coating or entrapment of biochemicals, drugs, antigenic molecules as a vaccine candidate, and microorganisms. Therefore, chitosan together with the advance of nanotechnology can be effectively applied as a carrier system for vaccine delivery. In fact, chitosan microspheres have been studied as a promising carrier system for mucosal vaccination, especially via the oral and nasal route to induce enhanced immune responses. Moreover, the thiolated form of chitosan is of considerable interest due to its improved mucoadhesivity, permeability, stability, and controlled/extended release profile. This review describes the various methods used to design and synthesize chitosan microspheres and recent updates on their potential applications for oral and nasal delivery of vaccines. The potential use of thiolated chitosan microspheres as next-generation mucosal vaccine carriers is also discussed.
Introduction
Vaccination is cost-effective, and probably the best preventable strategy against most diseases.Citation1 Traditionally, vaccines are administered parenterally via an intramuscular or subcutaneous route.Citation2,Citation3 This process of vaccine delivery incurs difficulties such as needle phobia, low patient compliance, short half-life, potential contamination while using needles, and a necessity for highly trained personnel. As a result, oral and nasal vaccination has been paid considerable attention as a way to overcome such potential drawbacks and eliminate the problems associated with parenteral administration of vaccines.Citation4 Better yet, parenteral vaccination mostly stimulates systemic immunity, whereas mucosal vaccination tends to confer both systemic and mucosal immune responses.Citation5 In regard to mucosal administration of protein drugs or vaccines, microspheres are well known for their controlled delivery formulation,Citation6–Citation8 which would provide a long-lasting boosting effect and enhance the effectiveness of the immune response against infectious diseases.Citation8
Chitosan has well-defined properties including bioavailability, biocompatibility, low cost, and an ability to open the intracellular tight junction; therefore, it has been suggested as a suitable polymeric material for mucosal delivery.Citation9 Desirable properties of chitosan can be determined from its molecular weight (MW) and degree of deacetylation (DD). It has been reported that high MW chitosan enhances the absorption of various compounds across the mucosal barrier.Citation9,Citation10 Due to its cationic property, positively charged chitosan would have an electrostatic interaction with the negatively charged mucosal surface.Citation11 Moreover, chitosan possesses mucoadhesivity, beneficial for prolonging the retention time at the mucosal area for a controlled and sustained therapeutic effect.Citation4 Nontoxicity is another prerequisite property of chitosan, which can be effectively applied for mucosal delivery of vaccines as a form of the microparticulate system. In an aqueous environment, chitosan swells and forms a gel-like layer, favorable for the interaction of polymers with glycoprotein in mucous. In the case of nasal delivery, chitosan possesses good bioadhesive properties and can reduce the rapid clearance of vaccine from the nasal cavity where it could be delivered to nasal-associated lymphoid tissue – the induction and effector sites for vaccine-induced immune responses.Citation11
General aspects of chitin and chitosan
Chitin is an abundant source of chitosan, a unique cationic polysaccharide superior to any man-made cationic derivatives.Citation12 In general, it comprises the skeletal materials in invertebrates. It is also found in egg shells of nematodes and rotifer as well as in the cuticles of arthropods, exoskeletons, peritrophic membranes, and cocoons of insects. In the fungal walls, chitin varies in crystallinity, degree of covalent bonding to other wall components, and DD.Citation12 It was reported as the principal component of protective cuticles of crustaceans such as crabs, shrimps, prawns, and lobsters.Citation11
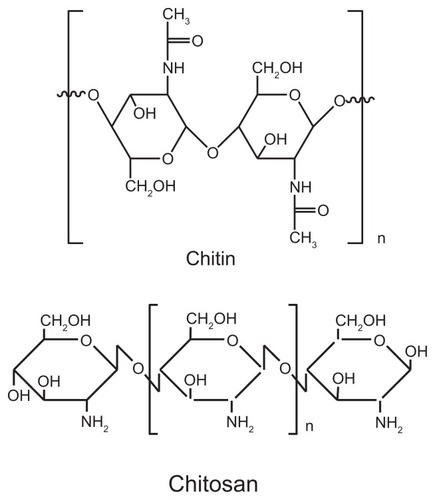
Chitosan, a natural linear polyaminosaccharide obtained by alkaline deacetylation of chitin, is the second most abundant polysaccharide next to cellulose.Citation12 It is made up of copolymers of glucosamine and N-acetyl-glucosamine, while chitin is a straight homopolymer composed of β-(1, 4)-linked N-acetyl-glucosamine units.Citation13–Citation15 Chitosan has one primary amino and two free hydroxyl groups for each C6 building unit (). Due to the presence of abundant amino groups, chitosan carries a positive charge and thus reacts with negatively charged polymers as well as with mucosal surfaces, making it a useful polymer for mucosal delivery.Citation11 Many studies have reported the use of chitosan in the formation of gels, nanoparticles, and microspheres for drug delivery application.Citation12
Chitosan microspheres (CMs)
Extensive research has been carried out to exploit the use of chitosan as a drug or vaccine carrier.Citation11 Indeed, chitosan has been used for prolonged and targeted delivery of drug and macromolecules. CMs can be a better option due to their ability for sustained release and improved bioavailability of target molecules. CMs also enhance the uptake of hydrophilic substances across epithelial cells.Citation11 It has been reported that a strong interaction between cationic CMs and anionic glycosaminoglycan receptors can retain the microspheres at the target site of the capillary region.Citation16 CMs have been applied in the oral,Citation9 parenteral,Citation17 and nasal deliveryCitation10,Citation18,Citation19 of encapsulated vaccine, DNA, or small interfering ribonucleic acid transfection studies.Citation20–Citation23
Biodegradability, biocompatibility, and safety of CMs
Biodegradability and biocompatibility play important roles in the metabolic process of chitosan in the body. It has been suggested that for systemic absorption a suitable MW (30–40 kDa) is essential for renal clearance dependent on the type of the polymer.Citation24 When the size of the polymer is larger than this range, then degradation is necessary for the polymer to be eliminated from the body. Degradation of chitosan is known to occur in vertebrates by lysosomes and several bacterial enzymes.Citation24 The biodegradability of chitosan in living organisms is dependent on its DD of chitin wherein the degradation rate decreases with an increase in DD.Citation25,Citation26 Primarily, chitosan is degraded sufficiently and eliminated properly in most cases when given adequate conditions.Citation27 Chitosan, as any other drug delivery materials, should be preferentially degraded after the efficient delivery of vaccine to the target site. The digestion of chitosan was found to be species-dependent and also dependent on the availability of the amine group in the composition of chitosan.Citation27
The safety of chitosan has been extensively studied and it was found that it is a biologically compatible polymer with a minimal toxicity.Citation28,Citation29 Many countries including Japan, Italy, and Finland have approved the use of chitosan for dietary application.Citation30 It has also been approved by the Food and Drug Administration for wound dressing application in the USA.Citation31 As chitosan is considered a nontoxic and nonirritant material, it is widely applied as a potential excipient in pharmaceutical formulations as well as in cosmetic industries. It is biocompatible for both healthy and infected skin.Citation32 It has been described that the median lethal dose for an oral administration of chitosan in rodents was >16 g/kg,Citation31 suggesting that it is safe and the risk of side effects after oral administration is negligible. On the other hand, Dash et al found that the toxicity of chitosan was dependent on its DD and MW.Citation24 As MW and concentration increased, the toxicity of chitosan also increased. It was noted that the toxicity of high DD chitosan was greatly increased by changes in MW and concentration when compared to that of low DD. Interestingly, chitosan and its derivatives were toxic to several bacteria, fungi, and parasites.Citation33–Citation35 This could be beneficial to controlling infectious diseases; however, the precise mechanism behind this inhibitory effect is yet to be further examined. It has been reported that no significant pyrogenic and toxic effects of chitosan were found in mice, rabbits, and guinea pigs.Citation36 In a fat chelation study, 4.5 g/day chitosan in humans was reported to be nontoxic.Citation37 It was noted, however, that in both of these studies the MW and DD were not specified.Citation36,Citation37 It has been reported that chitosan nanoparticles with 80 kDa MW and 80% DD showed no toxicity in mice when orally delivered at 100 mg/kg.Citation38 Moreover, chitosan solution exposed to nasal mucosa showed no significant changes in mucosal cell morphology compared to the control.Citation10 Collectively, chitosan exhibits minimal toxicity and side effects, which opens the possibility for its application and adoption in vaccine delivery as a safe and biocompatible material.
Bioavailability of CMs
Most vaccines are administered by parenteral injection because the bioavailability of mucosally delivered vaccines via the oral or nasal route is generally low.Citation39 These vaccines are sometimes impermeable to the mucosal barrier owing to their large MW and hydrophilic characteristics. Moreover, they can be easily degraded by the proteolytic enzymes present at the mucosal site. On the other hand, parenteral injections require a relatively high dose because the in vivo half-life of the vaccine is generally no more than a few hours which is considered one of the major problems of parenteral administration.Citation39 Thus, an improved system that can provide a sustained and controlled delivery of vaccine with maximum bioavailability is a priority.
Chitosan is not only nontoxic and biodegradable but it also exhibits excellent mucoadhesive properties and permeation-enhancing effect of the delivery materials across the cell surface, especially the mucosal area.Citation39 CMs also have potential applications for enhancing the adsorption of mucosally administered biomacromolecules through the paracellular route.Citation40,Citation41 They have the potential to loosen up the tight junction between epithelial cells and to reduce transepithelial electrical resistance.Citation42 It is worthwhile mentioning that mucoadhesivity is another potential benefit to using CMs for improved drug adsorption because cationic chitosan interacts with the anionic mucosal layer, which has sialic acid moieties. This adhesivity offers various advantages for an enhanced uptake of the therapeutic vaccines at the site of the induction phase: (1) mucoadhesive CMs could strongly reduce degradation of the vaccine by proteases at the absorption membrane by providing an intimate interaction with intestinal mucosa; (2) the adhesion of vaccine-loaded CMs to the mucosal layer provides an excessive driving force by a high concentration gradient towards the absorption membrane, leading to enhanced paracellular uptake; and (3) the mucoadhesive properties of chitosan provides a prolonged residual time of CMs on mucosal tissue, leading to drug absorption for an extended period of time and thus improving its bioavailability.Citation40,Citation41 Patil et al found a strong interaction between mucin in the nasal mucus layer and CMs, which resulted in rapid absorption and high bioavailability. Moreover, CMs were cleared slowly from the nasal cavity, also improving bioavailability.Citation43 In another study, Wang et al emphasized the enhancement of drug bioavailability using both the mucoadhesivity and permeation-enhancing effect of CMs,Citation39 suggesting that CMs could not only protect vaccines from degradation but also improve permeation, uptake, and bioavailability of the drug. They further defined the parameters, such as size and distribution, of CMs that are important for improving drug bioavailability, reproducibility, and repeatability as well as steady release behavior.Citation39 Producing equal sized CMs is very difficult, and the size distribution would be too broad if the microspheres are prepared by mechanical stirring or ultrasonication technique, which are common methods for CM preparation.Citation44 These could limit their vaccine delivery application. Firstly, the poor reproducibility of equal sized CMs may result in poor repeatability on release behavior and efficacy among the different batches. Secondly, the therapeutic efficacy can hardly be achieved with irregular sized CMs and a broad size distribution. Thirdly, a broad size distribution of CMs would result in poor bioavailability of the vaccine. Fourth, the side effects of vaccine therapy would likely be increased.Citation44 Therefore, particle size is an important factor that should be taken into account in the application and pharmacodynamic effect of vaccine-loaded CMs. Thus, it is important to prepare CMs of uniform size with a narrow size distribution and controlled release profile for their effective application in mucosal vaccine delivery.
Low off-target immunogenicity of CMs
One of the major concerns of a vaccine carrier system is the unwanted immunogenicity and pathogenicity caused by off-target reactions between the carrier itself and the body’s immune system.Citation45 This is the major disadvantage of using bioengineered viruses or bacteria as delivery vehicles for vaccines.Citation45 Therefore, polymeric carriers have been investigated as a useful alternative for the efficient delivery of vaccines without unwanted immunological outcomes. In this regard, chitosan can be considered a powerful polymer candidate because it has enormous potential for use as a vaccine carrier system that possesses low off-target immunogenicity,Citation46 suggesting that it will limit unwanted off-target immune reactions with the body’s normal immune function and not interfere with the actual vaccine-mediated immune response which is to be loaded. Several reports have also suggested that chitosan and its derivatives could be useful for drug delivery application without any significant off-target immunogenicity.Citation47,Citation48 Therefore, CMs (without vaccine loaded) are expected to neither alter normal immunological activity and biological function in the body nor interfere with the vaccine efficacy by showing unwanted off-target immunogenicity.
Preparation of CMs
Different methods have been studied and applied to prepare CMs for the delivery of drugs and vaccines. Several methods are discussed here in detail and are summarized in .
Table 1 Advantages and disadvantages of chitosan microspheres prepared by various methods
Interaction with anions
Ionotropic gelation
The counterions that are used in the ionotropic gelation method can be divided into two main categories: low MW counterions (eg, pyrophosphate, tripolyphosphate, tetrapolyphosphate, octapolyphosphate, hexametaphosphate, octyl sulfate, lauryl sulfate, hexadecyl sulfate, and cetyl stearyl sulfate) and high MW counterions (eg, alginate, κ-carrageenan, and polyaldehydrocarbonic acid). Briefly, chitosan solution is added dropwise into magnetically stirred aqueous counterions. The beads are removed from the solution by filtration, washed with distilled water, and dried.Citation11 CMs encapsulated with an atrophic rhinitis vaccine prepared by ionotropic gelation were nasally administered, which enhanced cytokine (tumor necrosis factor-α [TNF-α]) and nitric oxide production as an indication of immune stimulating activity.Citation49
Emulsification and ionotropic gelation
In the emulsification and ionotropic gelation method, an aqueous solution of chitosan is added to a nonaqueous continuous phase (isooctane and emulsifier) to form a water-in-oil emulsion. Sodium hydroxide solution is then added at different intervals, leading to ionotropic gelation. The microspheres, thus formed, are removed by filtration, washed, and then dried.Citation50 It has been suggested that the conventional emulsification and ionotropic gelation method for preparation of CMs provides irregular microparticles, whereas spherical microparticles with a diameter of about 10 μm can be obtained when employing a modified process. In one modified process, gelatin is used, which allows the ionic crosslinking of chitosan/gelatin (water-in-oil emulsion) to take place under coagulation conditions at a low temperature.Citation51 Several other crosslinking agents have been used for surface modification of chitosan/gelatin microspheres: the surface was very smooth in sodium sulfate or sodium citrate crosslinked chitosan/gelatin microspheres; however, large gaps were observed in chitosan/tripolyphosphate microspheres.Citation51 It has been reported that the increase of stirring speed leads to a decrease in diameter and a narrower size distribution.Citation51
Complex coacervation
Sodium alginate, sodium carboxymethyl cellulose, κ-carrageenan, and sodium polyacrylic acid can be used for complex coacervation with chitosan to form microspheres after the interionic interaction between oppositely charged polymers. For example, potassium chloride and calcium chloride were used to formulate the coacervate capsules of chitosan–alginate and chitosan–κ-carrageenan, respectively, and the obtained capsules were hardened in the counterion solution before washing and drying.Citation52–Citation54
Crosslinking methods
Emulsion crosslinking method
Water insoluble reagents can be simply dispersed in chitosan solution and entrapped by the emulsion crosslinking process. Glutaraldehyde, formaldehyde, and genipin have been widely used as crosslinking agents for the preparation of CMs. In the emulsion crosslinking method, chitosan solution is first prepared by dissolving chitosan with acetic acid. This solution is then added to liquid paraffin containing a surfactant, forming a water-in-oil emulsion before the addition of a crosslinking agent. The formed microspheres are filtered, washed with suitable solvent, and dried.Citation17,Citation55,Citation56
Multiple emulsion method
The multiple emulsion method is probably the best way to increase the entrapment efficiency of the target molecule in CMs. In this method, a primary emulsion (oil-in-water) is first formed (nonaqueous solution containing the target molecule in chitosan solution). This primary emulsion is then added to an external oil phase to form multiple emulsions (oil-in-water-in-oil) followed by either the addition of glutaraldehyde (as a crosslinking agent) or the evaporation of an organic solvent.Citation12 CMs, loaded with hydrophobic reagents, were found to have better morphological characteristics and yield when prepared by the multiple emulsion method.Citation12
Thermal crosslinking method
In the thermal crosslinking technique, CMs are prepared with different thermal conditions in various steps. Orienti et al reported CM preparation by the thermal crosslinking method using citric acid, which served as crosslinking agent.Citation57 Citric acid was added to chitosan solution in acetic acid (2.5% weight/volume) and then cooled to 0°C before adding to corn oil. After stirring for 2 minutes, the emulsion was then added dropwise to corn oil by maintaining the temperature at 120°C. Then, the crosslinking was performed under vigorous stirring (1000 rpm) for 40 minutes and the microspheres obtained were filtered, washed, dried, and sieved.Citation57
Crosslinking with a naturally occurring agent
Genipin, a naturally occurring crosslinking agent, has also been used to prepare CMs by the spray drying method, which provides small particle size, low crystallinity, and good sphericity.Citation58 It was reported that genipin crosslinked CMs had better biocompatibility and slower degradation rate than glutaraldehyde crosslinked CMs.Citation59,Citation60 The microspheres used as an injectable chitosan-based drug delivery system revealed low toxicity.
Emulsion droplet coalescence method
Tokumitsu et al developed the emulsion droplet coalescence method for CM preparation, which implements the principle of both emulsion crosslinking and precipitation.Citation61 In this method, precipitation is usually induced by coalescence of chitosan droplets with sodium hydroxide. Briefly, a drug containing stable emulsion solution of chitosan is prepared in liquid paraffin oil. This emulsion is mixed with another stable emulsion containing a chitosan aqueous solution of sodium hydroxide with high-speed stirring, which allows the droplets of each emulsion to collide randomly and coalescently. This results in the precipitation of chitosan droplets with small particle size. CMs loaded with gadopentetic acid were prepared using this method for gadolinium neutron capture therapy.Citation61 Gadopentetic acid interacts electrostatically with amino groups of chitosan since it is a bivalent anionic compound. A range of nanosized particles and a high loading of gadopentetic acid were obtained through this emulsion droplet coalescence method compared to the conventional emulsion crosslinking method.Citation61
Precipitation or coacervation method
Chitosan precipitates when it interacts with an alkaline solution since it is not soluble in an alkaline pH medium. In this method, chitosan particles are prepared by dropping chitosan solution into an alkaline solution (eg, sodium hydroxide, sodium hydroxide–ethanediamine, or sodium hydroxide–methanol) through a compressed air nozzle, which produces coacervate droplets. Particles are collected by precipitation or centrifugation before excessive washing with hot and cold water, respectively.Citation62 The particle sizes can be controlled by varying the diameter of the compressed air nozzle together with the pressure. A crosslinking agent can also be used to harden the particles,Citation62 which would be beneficial because of its slow release. Sodium sulfate was also used to prepare CMs using this precipitation technique. Recombinant human interleukin-2-loaded CMs were prepared by a dropwise addition of sodium sulfate-containing recombinant human interleukin-2 solution in acidic chitosan solution. As a result, chitosan was precipitated and recombinant human interleukin-2 was incorporated when CMs were formed.Citation63 Of note, this method is devoid of any crosslinking agent.
Reversed micellar method
Reverse micellar is the stable liquid mixture of oil, water, and surfactants dissolved in organic solvents. To this mixture, an aqueous solution of chitosan and the target molecule are added before the addition of a crosslinking agent such as glutaraldehyde.Citation62 Mitra et al described the preparation of doxorubicin–dextran conjugate-encapsulated chitosan nanoparticles.Citation64
Sieving method
Agnihotri and Aminabhavi developed a method to prepare clozapine-loaded CMs.Citation65 In this method, a thick jelly mass of chitosan was prepared in 4% acetic acid and crosslinked with glutaraldehyde. The crosslinked nonsticky jelly mass was passed through a sieve to get microparticles of a suitable size, which were then washed with 0.1 N sodium hydroxide to remove unreacted glutaraldehyde and dried overnight at 40°C. As a result, a high loading efficiency of clozapine (98.9%) was achieved. However, the particles were irregular in shape with an average size of 543–698 μm. The irregular shape and size of the particles is one of the major disadvantages of this method, which could affect the bioavailability of CMs in vivo. However, an in vitro and in vivo study demonstrated a controlled and sustained release of the drug.Citation65
Solvent evaporation method
The solvent evaporation method involves the formation of emulsion between a polymer solution and an immiscible continuous phase – either aqueous (oil-in-water) or nonaqueous (water-in-oil). This can be done by using liquid paraffin/acetone. The target molecule dissolved in acetone is dispersed in chitosan solution and the mixture is emulsified in liquid paraffin while stirring. The microsphere suspension is filtered, washed, and dried. Magnesium stearate can be added as an agglomeration preventing agent. It appears that the average particle size decreases when the amount of magnesium stearate used in the preparation is increased.Citation66
Spray drying method
Spray drying is one of the most widely investigated methods of preparing CMs in which chitosan solution is sprayed and then air-dried followed by the addition of a crosslinking agent. He et al prepared CMs by spray drying multiple emulsions (oil-in-water-in-oil or water-in-oil-in-water) to entrap cimetidine and famotidine into microspheres. The drug was released in a sustained and controlled fashion compared to the other microspheres prepared by traditional spray drying or the oil-in-water emulsion method.Citation67
Vaccine delivery through CMs
CMs have been examined for the mucosal delivery of vaccines. A variety of chitosan-based carrier systems with their functional properties for oral and nasal delivery is shown in . Here, the utility of CMs for oral and nasal vaccination in vitro and in vivo are discussed.
Table 2 Chitosan-based carrier systems with functional properties for the delivery of (model) vaccines through oral and nasal routes
Oral delivery
Oral delivery of vaccines has numerous advantages over conventional needle injection and is a well accepted route of vaccination. However, most vaccines are still administered by injection due to the lack of a proper delivery system to reach the induction site and to enhance the effector responses. Although oral delivery is probably the preferred administrative route of vaccines, especially for children, it causes degradation of the antigens in the gastrointestinal track and also shows inefficient targeting to the site of action when delivered in a naked form.Citation68 Therefore, developing an effective delivery system has been considered the primary task in the oral vaccination field. To gain adequate immune responses after oral delivery, the vaccine should reach the M-cells of Peyer’s patches in the gut avoiding the acidic pH condition of the stomach and enzymatic degradation. Even if the vaccine nearly reaches Peyer’s patches, the immune response is not always induced due to the inability of antigens to gain access to Peyer’s patches and because of inefficient uptake at the induction site. Several studies have shown that the uptake by M-cells was significantly enhanced and degradation of protein and peptide vaccines in the gastrointestinal track was prevented after the incorporation of vaccine with CMs.Citation59,Citation68–Citation71 Due to its nontoxicity and potent antigen binding properties, chitosan has been considered a promising tool for oral vaccination.Citation68
Extensive research on CMs for mucosal vaccine delivery, in particular, the uptake of CMs in murine Peyer’s patches in vitro and in vivo, was carried out by van der Lubben et al.Citation59,Citation68,Citation70,Citation71 They prepared a human intestinal M-cell model by coculturing Caco-2 and Raji-cells and investigated the uptake of CMs.Citation59 No morphological changes in the monolayer were observed and this model was used to examine the in vitro uptake of CMs for oral vaccine delivery.Citation59 They found that CMs can be taken up by the epithelium of Peyer’s patches. It has been reported that the size of microparticles should be <10 μm for efficient uptake by M-cells and to reach the dome of Peyer’s patches.Citation3 Indeed, CMs used in the study were much smaller than 10 μm and therefore suitable for M-cell uptake.Citation68 Since chitosan is biodegradable, van der Lubben et al further claimed that antigen was freed from CMs after uptake by M-cells.Citation68
Therapeutic use of CMs for oral and nasal delivery has been examined. A diphtheria toxoid (DT) was used to examine the enhancement of both systemic and local immune responses.Citation70 Unloaded CMs, DT-loaded CMs, and DT in phosphate-buffered saline (PBS) were delivered into mice by oral and nasal administration. DT associated with alum was subcutaneously immunized in mice as a positive control. A strong systemic and local immune response was found against DT in mice administered orally with different doses of DT-loaded CMs when compared to the mice fed with DT in PBS. Furthermore, a dose-dependent anti-DT immunoglobulin G (IgG) response in sera was found after oral administration of DT-loaded CMs. On the other hand, the systemic immune response (IgG) induced by DT-associated CMs were ten times higher than that induced with DT in PBS after nasal delivery.Citation70
CMs were also examined after oral delivery of tetanus toxoid (TT) to induce systemic and local immune responses.Citation69 TT-loaded CMs were prepared by the ionic crosslinking method using sodium tripolyphosphate. Unloaded CMs, TT-loaded CMs, and naked TT in PBS were orally administered in mice, and TT absorbed on aluminum phosphate was administered intramuscularly as a positive control. TT-loaded CMs enhanced a strong systemic and local immune response in a dose-dependent manner at 3 weeks after the oral delivery of vaccine compared to TT in PBS. They observed that a four-fold higher dose was needed for TT-loaded CMs to get a similar IgG response to the positive control. The study was also carried out at different time points to understand the kinetics of the immune response based on the level of IgG. It was found that the IgG response could be observed at day 14 and was increased after boosting at day 22. At day 29, the IgG level was lower than at day 22; however, it still maintained a higher concentration than TT in PBS at all the time points investigated. On the other hand, IgA levels were not significantly different at day four; however, the levels were significantly (P < 0.01) higher in TT-loaded CMs than in TT in PBS at days eight, 14, and 22.Citation69 These results suggest that the encapsulated vaccine in CMs enhanced the systemic as well as local immune responses compared to the nonencapsulated vaccine, rendering a safe and effective form of oral vaccination. Further studies on cellular immune responses including memory effect of B-cells and T-cells will ensure the solid effectiveness of CMs for vaccine delivery.
At first glance, chitosan would not be considered suitable for oral vaccination since it is a pH-sensitive polymer. It is soluble at acidic pH and becomes insoluble at about pH 6.5. It has been suggested that an enteric coating can protect chitosan from the acidic stomach.Citation12 When this reaches the intestine, the enteric layer dissolves at high pH and the antigen-encapsulated chitosan core is exposed to enzymes. In this state, chitosan can protect the encapsulated antigen from enzymatic degradation and most importantly can lead the antigen to reach the induction site of Peyer’s patches for immune stimulation. For this, Hori et al developed Eudragit®-coated CMs and evaluated ovalbumin as an oral immune delivery system.Citation72 The ovalbumin-loaded CMs prepared by the emulsification-solvent evaporation method showed high ovalbumin content and an appropriate size for the efficient uptake by Peyer’s patches. A comparable systemic IgG response was found after the oral administration of ovalbumin-loaded CMs in mice. Moreover, a higher intestinal mucosal IgA response was achieved using ovalbumin-loaded CMs by delivery of the microspheres toward Peyer’s patches, where they were subsequently uptaken by the M-cells and the entrapped ovalbumin was released in a controlled fashion.Citation72 In another study, Cho et al reported a mucoadhesive and pH-sensitive thiolated Eudragit-coated CM, designed to enhance mucoadhesivity and bioavailability of the carrier at the target site. They found strong mucoadhesive properties in vitro and in vivo,Citation4 suggesting that Eudragit-coated CMs were a potential carrier for the oral delivery of vaccines.
Recently, hepatitis B surface antigen-loaded CMs were formulated, characterized, and optimized in vitro and in vivo for effective oral delivery of hepatitis B surface antigen against chronic hepatitis B.Citation73 An emulsion solvent evaporation technique was applied to prepare CMs, with the addition of protease inhibitors and permeation enhancers to overcome the limitation of the enzymatic and permeation barrier. In vitro drug release, in vivo efficacy, and importantly the effect of different storage conditions were studied to test the practicality of the system. An enhanced stability of the antigen was found when using the microspheres for a period of 4 months at room temperature, suggesting a possible way to overcome the tedious and expensive requirement of cold chain storage in the vaccine industry. Importantly, the study signifies a potential strategy for effective oral administration of hepatitis B surface antigen using the biodegradable CM system.Citation73
Recently, Uddin et al developed an albumin–chitosan mixed matrix microsphere (ACM)-filled capsule formulation for oral administration of Typhoid Vi® antigen (TVA) to demonstrate antigen-specific systemic and mucosal immune responses.Citation74 TVA-loaded ACMs were filled into hard gelatin capsules with enteric coating. The physicochemical characterization such as particle size, zeta potential, swelling, and disintegration rates of the microspheres were favorable for oral delivery of the microencapsulated vaccine. In vivo studies showed that the oral delivery of TVA-loaded ACMs had similar IgG and IgA responses with those of the parenteral vaccination group, suggesting that TVA-loaded ACMs had the potential to induce antigen-specific immune responses when delivered via oral administration.Citation74
Nasal delivery
Nasal administration of vaccines has been reported to enhance bioavailability and improve efficacy.Citation12 An effective humoral and cell-mediated immune response can be achieved through nasal delivery of vaccines when the appropriate delivery system is used as a carrier for particulate antigens.Citation75,Citation76 Nasal-associated lymphoid tissue, present at the nasal epithelium and containing immunocompetent cells, would be an ideal target site for the nasal delivery of vaccines to induce an immune response.Citation75,Citation76 It has been suggested that nasal-associated lymphoid tissue epithelium has similar types of immune cells that are present in the M-cells of Peyer’s patches in gut-associated lymphoid tissue and is located just below the epithelial surface, which contains macrophages, dendritic cells, lymphoid follicles (mostly B-cells), and intrafollicular areas (mostly T-cells) in a network. At these sites, particulate antigens are mainly taken up and/or transported across the cells by transcytosis without any extensive degradation. It has been well described that increased epithelial permeability influences the particulate antigen uptake across the epithelial mucosa.Citation77–Citation84 Importantly, chitosan has the ability to increase membrane permeability when used as a delivery system for nasal vaccination.Citation85
However, antigen delivery through nasal administration sometimes results in poor immune responses. Several factors including limited diffusion of particulate antigens across the mucosal barrier, rapid clearance of particulate drug or vaccine formulation from the mucosal surface, and enzymatic degradation because of instability of the particulate carrier are associated with this.Citation86,Citation87 In order to overcome these problems, chitosan might be one of the best options for nasal administration of vaccines due to its ability to increase the retention time when it binds to the mucosal membrane.Citation12 Several reports also demonstrated that chitosan enhanced mucosal absorption of vaccines with adjuvant activity to improve mucosal immunity after nasal administration.Citation10,Citation88,Citation89
Cho and colleagues conducted extensive research on CMs for intranasal delivery of vaccines to induce the immune response in vitro and in vivo.Citation49,Citation90–Citation94 They used Bordetella bronchiseptica dermonecrotoxin (BBD), a causative agent and a major virulence factor for atrophic rhinitis – a disease that causes huge economic damage in the swine industry. BBD-loaded CMs were prepared by the ionic gelation process using tripolyphosphate.Citation49 The morphology of vaccine-loaded microspheres was observed as aggregated shapes, whereas unloaded microspheres were quite spherical. The average particle size of BBD-loaded CMs was 4.39 μm, which provides a condition for effective delivery of the vaccine to nasal-associated lymphoid tissue for immune induction. The size of unloaded CMs was about 1.94 μm, indicating that CMs became enlarged after vaccine loading. The release studies further demonstrated that when the MW of chitosan decreased, more BBD was released. It was also found that encapsulated BBD had greater release at higher pH than lower pH. The secretion of TNF-α and nitric oxide from the murine macrophages treated with BBD-loaded CMs indicated that the cells stimulated with BBD-loaded CMs produced TNF-α and nitric oxide in a time-dependent manner at a similar level to cells stimulated with BBD alone or lipopolysaccharide. It is important to mention that BBD-loaded CMs induced a steadily increasing immune stimulating effect in the macrophages, whereas it began to decrease at 80 hours poststimulation with lipopolysaccharide.Citation49
An in vivo study was carried out in mice that measured IgG and IgA in sera, nasal wash, and saliva after intranasal administration of BBD-loaded CMs.Citation90 The IgA levels in nasal wash increased in a time- and dose-dependent manner after intranasal administration of BBD-loaded CMs. However, such immune response was not detected in saliva, suggesting that CMs successfully delivered the vaccine to nasal-associated lymphoid tissue after intranasal administration and induced a higher systemic and local immune response. Although in vitro and in vivo results showed CMs as a potential carrier for nasal delivery, BBD-loaded CMs were found in aggregated shapes because of physical and storage instabilities.Citation90
To overcome this instability problem, chitosan was modified by covalent conjugation with polyethylene glycol to form pegylated chitosan.Citation92 The pegylated CMs (PCMs) were prepared through a similar ionic gelation process. The average particle size of BBD-loaded PCMs was <10 μm, their shape was spherical, and they were physically more stable compared to BBD-loaded CMs. Due to better stability, the vaccine was released from BBD-loaded PCMs in a more steady fashion than in BBD-loaded CMs. The study further showed that macrophages secreted TNF-α and nitric oxide in a time-dependent manner after exposure to BBD, BBD-loaded CMs, BBD-loaded PCMs, and lipopolysaccharide. However, a significantly higher TNF-α secretion was found in the cells treated with BBD-loaded PCMs than cells exposed to BBD-loaded CMs and BBD alone. Moreover, TNF-α secretion increased in a sustained fashion in the cells exposed to BBD-loaded PCMs, whereas it began to decline at 48 hours poststimulation with lipopolysaccharide.Citation92
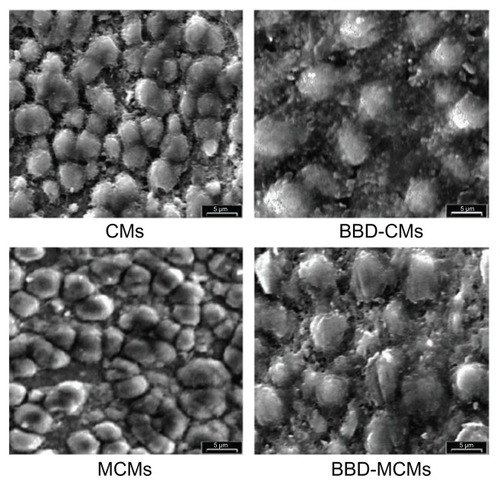
To increase the target specificity, another study was carried out with mannosylated CMs (MCMs) with encapsulated BBD to target macrophage mannose receptors and increase immune stimulating activity.Citation91 Colocalization of BBD-loaded MCMs and the macrophage receptors was confirmed by confocal laser scanning microscope. The results showed that macrophages exposed to BBD-loaded MCMs secreted higher TNF-α and interleukin-6 than that of BBD-loaded CMs and BBD alone. Furthermore, BBD-specific IgA response was found to be significantly higher in saliva and serum after intranasal immunization with BBD-loaded MCMs in mice compared with BBD-loaded CMs,Citation91 suggesting that the MCMs extensively assisted in stimulating macrophages for induction and enhancement of immune activity. The representative scanning electron microscope photographs of CMs and MCMs (BBD loaded and unloaded) are shown in .Citation91
Figure 2 Scanning electron microscope photographs of CMs, BBD-loaded CMs, MCMs, and BBD-loaded MCMs (5000×).
Notes: Bar represents 5 μm. Reprinted from Biomaterials, 29(12). Jiang HL, Kang ML, Quan JS, et al. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization, 1931–1939. Copyright 2008 with permission from Elsevier.Citation91
Abbreviations: BBD, Bordetella bronchiseptica dermonecrotoxin; CM, chitosan microsphere; MCM, mannosylated chitosan microsphere.
Soane et al performed extensive research using different types of chitosan and concluded that chitosan could be used as a nasal vaccine delivery carrier without any harmful effects.Citation95,Citation96 To investigate this, the cilia beat frequency was studied in guinea pigs after nasal administration of chitosan solution for 28 days and found that none of the chitosan induced the changes of cilia beat frequency, indicating a safety profile of chitosan for nasal delivery.Citation95 They further investigated the bioadhesive properties of CMs via nasal administration using three different formulations: chitosan solution, CMs, and starch microspheres, which was followed by the examination of clearance properties in human subjects. The clearance rate was 21 minutes for the control, 41 minutes for the chitosan solution, 68 minutes for the starch microspheres, and 84 minutes for the CMs. This result indicates that CMs have better bioadhesive properties and are able to significantly reduce the drug clearance rate and prolong the residence time of the delivered vaccine in nasal mucosa, resulting in enhanced bioavailability and efficacy.Citation96
Several reports demonstrated the concomitant use of CMs as a mucosal adjuvant and as a vaccine delivery system. A vaccine formulation with CMs and a nontoxic LTK63 mutant of heat-labile toxin induced significantly higher IgG titers in sera and IgA in nasal washes after intranasal delivery in mice.Citation88 A modified N-trimethyl chitosan microparticulate system also showed higher antigen-specific antibody responses in sera, nasal, and vaginal wash.Citation97 Chitosan–DNA nanospheres with intranasal delivery exhibited significant responses of cytotoxic T-cell response and interferon-γ as well as antigen specific-IgG and IgA, rendering a strong humoral and cell-mediated immune response.Citation98
CMs were prepared with Pluronic® F127 as an immunomodulating and stabilizing agent to enhance the stability for controlled drug release and adjuvanticity.Citation94 Pluronic, a triblock copolymer of polyethylene oxide and polypropylene oxide (polyethylene oxide-b-polypropylene oxide-b-polyethylene oxide) commonly known as poloxamer, has a variety of pharmaceutical applications and has become one of the most extensively investigated temperature-sensitive materials.Citation99 F127 is water soluble and has a good drug release profile, which makes it a potent drug delivery carrier for a variety of therapeutic and bioactive agents.Citation100–Citation104 When Westerink et al intranasally immunized antigen-loaded F127/CMs into mice, it significantly increased systemic and mucosal immune responses compared to those of control groups,Citation105 suggesting that the stabilization of protein antigens by F127 enhances the immune response of F127/CMs compared to chitosan alone. This study demonstrated a nasal vaccine delivery strategy for enhancement of the immune response via a synergistic effect of chitosan and F127. In another study, intraperitoneally and subcutaneously injected F127/cytosine–phosphate–guanosine and F127/CM formulations significantly enhanced antigen-specific systemic antibody responses compared to the antigens delivered with cytosine–phosphate–guanosine or CMs alone,Citation106 suggesting that F127 might have an adjuvant effect when used in combination with chitosan. Therefore, application of a delivery system that combines adjuvants with various modes of action is beneficial to maximizing immune response.
Limitations of CMs
Besides the enormous advantages of CMs such as biodegradability, nontoxicity, permeation enhancing effects, and an ability to open the tight junction between epithelial cells as described earlier, there are some limitations as well. Cho and colleagues performed several studies on CMs for vaccine delivery.Citation49,Citation90–Citation94 They found that the vaccine-loaded CMs self-aggregated at 2 weeks after preparation, although it was effective in inducing immune responses including cytokine expression in vitro and antigen-specific IgG and IgA responses in vivo after nasal delivery.Citation49,Citation90 To make stable and nonaggregated CMs, they used F127 to prepare F127/CMs which showed spherical morphology with no aggregation at an extended period of time after preparation. This was due to the hydrophilic polyethylene oxide chains of F127 that hindered the self-aggregation of CMs.Citation94 F127/CMs showed much improved immune activity in vitro and in vivo and also exhibited potential protection against infection compared to CMs alone.Citation94
Several other studies described the instability of CMs in acidic media, especially when prepared by the precipitation method. CMs prepared by sodium sulfate precipitation were found to have poor acidic stability.Citation107 This acidic instability was initiated by the addition of sodium sulfate to chitosan acetic acid solution which led to an ionic neutralization of the positively charged amine groups of chitosan, providing poorly soluble chitosan derivatives.Citation107 After the addition of acid (increasing proton concentration), the equilibrium shifted to the solubilizing range for chitosan, thus dissolving the CMs.Citation30 In another study, sulfadiazine-loaded chitosan beads were prepared using tripolyphosphate; however, it was found that the beads had poor mechanical strength.Citation108 Collectively, there are some limitations of CMs that can be overcome by modifying the CMs. For example, F127 is a good strategy to improve the stability and mechanical strength of CMs. Additionally, structural modifications of chitosan (eg, thiolated chitosan) might improve the stability and functionality of CMs.
Thiolated CMs as a modified and improved form of a chitosan-based mucosal vaccine carrier
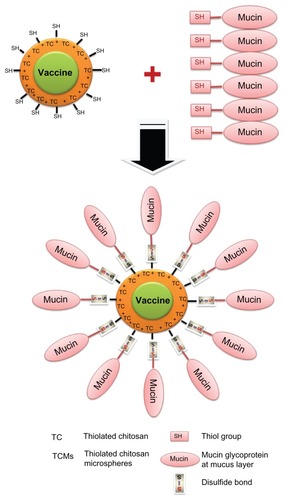
Thiolated polymers (ie, thiomers) have gained considerable attention – especially for vaccine delivery – because they are one of the most promising polymers with multifunctional properties including strong mucoadhesivity, enhanced permeation effects, protection ability, stability, and enhanced bioavailability of drugs.Citation109–Citation114 Among various thiomer-based carriers, thiolated CMs (TCMs) are highly popular because of their strong mucoadhesiveness and ability to control and extend drug release profiles with improved permeation ability.Citation115–Citation119 TCMs can be prepared by immobilizing the thiol-bearing chain on the polymeric backbone of chitosan (). The strong mucoadhesivity of TCMs is obtained through the formation of disulfide bonds between the thiol groups of TCMs and cysteine-rich subdomains of mucin glycoproteins at the mucosal surface ().Citation120 The permeability through the mucosal surface can be enhanced by using TCMs instead of unmodified CMs. Increased permeability is achieved by opening the tight junction after the inhibition of protein tyrosine phosphatase, a key enzyme involved in the closing process of tight junction.Citation110 Due to the formation of inter- and intramolecular disulfide bonds through TCMs, a compact three-dimensional network is generated which allows controlled drug release and leads to high cohesivity. Moreover, TCMs exhibit a reversible opening of the tight junction, which leads to better permeation effects than unmodified CMs.Citation115,Citation117,Citation120 In the case of first-generation thiomers, thiolated chitosan derivatives are prepared by conjugating thiol-bearing aliphatic ligands to the amino groups of chitosan. For example, N-acetyl-cysteine, 6-mecaptonicotinic acid, thioglycolic acid, glutathione, and 2-iminothiolane are the aliphatic thiol-bearing ligands with functional carboxyl groups which form amide bonds with the amino groups of chitosan by carbodiimide to synthesize the thiomers of chitosan.Citation118,Citation121–Citation125 CMs prepared by these thiomers exhibit strong mucoadhesivity, biocompatibility, and enhanced permeability and absorption after oral and nasal administration.
Figure 3 Representative structure of thiolated chitosan: (A) general structure of thiolated chitosan modified by an –SH group (X: linker) and (B) chitosan-N-acetyl-cysteine (modification of chitosan at the D-glucosamine unit by N-acetyl-cysteine).
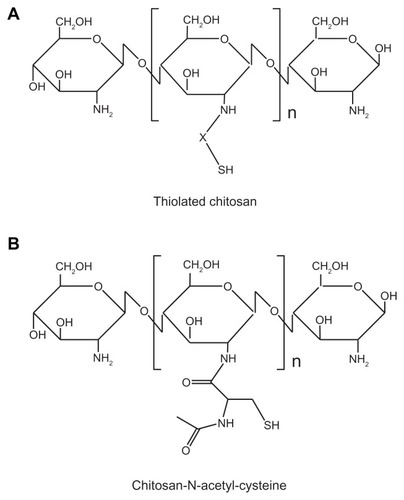
Figure 4 Schematic representation of functional interaction between TCMs and mucin in mucosal vaccine delivery.
Abbreviation: TCM, thiolated chitosan microsphere.
It is important to note that thiomers bearing free thiol groups are relatively unstable in solution because they are prone to oxidize at pH ≥ 5, leading to a self-crosslinking of the polymer. Different approaches have been attempted to delay oxidation and inhibit the self-crosslinking reaction. As an example of a next-generation thiomer, the aromatic thiol-bearing ligands are extraordinary candidates for delaying the oxidation process and protecting the thiol groups of the thiolated polymers.Citation126 Recently, Bernkop-Schnurch et al performed several studies using aromatic thiol-bearing ligands for the synthesis of S-protected thiolated chitosan and evaluated their efficacy as mucosal drug delivery carriers.Citation109,Citation127,Citation128 To prepare the S-protected thiolated chitosan, the thiol-bearing ligand was covalently attached to chitosan as the first step of modification. In the second step, the thiol group of thiolated chitosan was protected by the formation of disulfide bonds with aromatic thiol-bearing ligands. The S-protected thiolated chitosan exhibited improved mucoadhesivity, enhanced permeation effect, inhibited efflux pump, bioavailability, and controlled release profile compared to the corresponding thiolated and unmodified polymers,Citation109,Citation127,Citation128 demonstrating that TCMs prepared using S-protected thiolated chitosan are a promising chitosan-based mucoadhesive polymer for the development of various mucosal vaccine delivery systems.
Conclusion and future perspectives
Among various investigated vaccine carriers, CMs hold enormous promise as a delivery vehicle for both oral and nasal administration. This review has discussed and evaluated various methods for preparation of CMs which could help to design more and better functionalized chitosan-based carrier systems. This study demonstrated that vaccine-loaded CMs could be prepared with suitable and appropriate particle sizes, which is a very important factor in the delivery of the vaccine to the induction site of mucosa-associated lymphoid tissue for proper immune stimulation. Furthermore, both systemic and local immune responses can be induced in a dose- and time-dependent manner through vaccine-loaded CMs. The nontoxic, highly bioavailable, mucoadhesive, and biodegradable nature of chitosan and its particulate form is the main reason that it could become a successful vaccine carrier in the near future. Furthermore, the much improved properties of modified CMs (eg, TCMs), such as increased mucoadhesivity, membrane permeability, stability, and controlled/extended release of the encapsulated vaccine, show that they are a promising candidate for a potent vaccine carrier system. Further research and the ability to modify chitosan may improve structural and physicochemical properties, increasing the potential of CM systems. New possibilities in the field of targeted vaccine delivery may be unlocked once various specific ligands (targeting moieties such as mannose and folate) that can be conjugated with chitosan derivatives have been designed and examined for specific interactions with preferred cell types. However, there are many challenges including low physical and mechanical stability, irregular particle size and distribution, and low target specificity that have hindered the efficacy, practical use, and commercialization of CMs. Thus, considering these factors, carefully designed and better functionalized CMs could be prepared for fruitful future application.
Acknowledgments
This research was supported by the Cooperative Research Program for Agriculture, Science, and Technology Development (PJ 007611) and the Next-Generation BioGreen 21 Program (PJ81272011), Rural Development Administration, Republic of Korea. This work was also partially supported by grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2010-0027222, 2010-0003291) and by the Agriculture Research Center Program of the Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea. MA Islam and J Firdous were supported by the Brain Korea 21 Program.
Disclosure
The authors report no conflicts of interest in this work.
References
- PackDWTiming is everythingNat Mater20043313313414991008
- GiudiceELCampbellJDNeedle-free vaccine deliveryAdv Drug Deliv Rev2006581688916564111
- MitragotriSImmunization without needlesNat Rev Immunol200551290591616239901
- QuanJSJiangHLKimEMpH-sensitive and mucoadhesive thiolated Eudragit-coated chitosan microspheresInt J Pharm20083591–220521018490120
- McgheeJRMesteckyJDertzbaughMTEldridgeJHHirasawaMKiyonoHThe mucosal immune system: from fundamental concepts to vaccine developmentVaccine199210275881539467
- EylesJESharpGJWilliamsonEDSpiersIDAlparHOIntra nasal administration of poly-lactic acid microsphere co-encapsulated Yersinia pestis subunits confers protection from pneumonic plague in the mouseVaccine19981676987079562689
- JanesKACalvoPAlonsoMJPolysaccharide colloidal particles as delivery systems for macromoleculesAdv Drug Deliv Rev2001471839711251247
- MiFLShyuSSChenCTSchoungJYPorous chitosan microsphere for controlling the antigen release of Newcastle disease vaccine: preparation of antigen-adsorbed microsphere and in vitro releaseBiomaterials199920171603161210482415
- ArturssonPLindmarkTDavisSSIllumLEffect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2)Pharm Res1994119135813617816770
- IllumLFarrajNFDavisSSChitosan as a novel nasal delivery system for peptide drugsPharm Res1994118118611897971722
- SinhaVRSinglaAKWadhawanSChitosan microspheres as a potential carrier for drugsInt J Pharm20042741–213315072779
- KumarMNMuzzarelliRAMuzzarelliCSashiwaHDombAJChitosan chemistry and pharmaceutical perspectivesChem Rev2004104126017608415584695
- KasHSChitosan: properties, preparations and application to microparticulate systemsJ Microencapsul19971466897119394251
- KatoYOnishiHMachidaYApplication of chitin and chitosan derivatives in the pharmaceutical fieldCurr Pharm Biotechnol20034530330914529420
- SinglaAKChawlaMChitosan: some pharmaceutical and biological aspects – an updateJ Pharm Pharmacol20015381047106711518015
- GalloJMHassanEEReceptor-mediated magnetic carriers: basis for targetingPharm Res1988553003043244640
- JameelaSRKumaryTVLalAVJayakrishnanAProgesterone-loaded chitosan microspheres: a long acting biodegradable controlled delivery systemJ Control Release1998521–217249685932
- Jabbal-GillIFisherANRappuoliRDavisSSIllumLStimulation of mucosal and systemic antibody responses against Bordetella pertussis filamentous haemagglutinin and recombinant pertussis toxin after nasal administration with chitosan in miceVaccine19981620203920469796062
- WitschiCMrsnyRJIn vitro evaluation of microparticles and polymer gels for use as nasal platforms for protein deliveryPharm Res199916338239010213368
- AlamehMDe JesusDJeanMLow molecular weight chitosan nanoparticulate system at low N:P ratio for nontoxic polynucleotide deliveryInt J Nanomedicine201271399141422457597
- JeanMAlamehMDe JesusDChitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetesEur J Pharm Sci2012451–213814922085632
- LuoYZhaiXMaCAn inhalable β2-adrenoceptor ligand-directed guanidinylated chitosan carrier for targeted delivery of siRNA to lungJ Control Release20121621283622698944
- YangYLiuXZhangDChitosan/VEGF-sIRNA nanoparticle for gene silencingJ Control Release2011152Suppl 1e160e16122195822
- DashMChielliniFOttenbriteRMChielliniEChitosan – a versatile semi-synthetic polymer in biomedical applicationsProg Polym Sci20113689811014
- XuJMcCarthySPGrossRAKaplanDLChitosan film acylation and effects on biodegradabilityMacromolecules1996291034363440
- YangYMHuWWangXDGuXSThe controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivoJ Mater Sci Mater Med200718112117212117619982
- KeanTThanouMBiodegradation, biodistribution and toxicity of chitosanAdv Drug Deliv Rev201062131119800377
- AraiKKineemakiTFujitaTToxicity of chitosanBull Tokai Reg Fish Res Lab1968568994
- HiranoSSeinoHAkiyamaYNonakaIBiocompatibility of chitosan by oral and intravenous administrationsPolym Mater Sci Eng198859897901
- IllumLChitosan and its use as a pharmaceutical excipientPharm Res1998159132613319755881
- WedmoreIMcManusJGPusateriAEHolcombJA special report on the chitosan-based hemostatic dressing: experience in current combat operationsJ Trauma200660365565816531872
- ShajiJJainVLodhaSChitosan: a novel pharmaceutical excipientInt J Pharm Appl Sci2010111128
- JumaaMFurkertFHMullerBWA new lipid emulsion formulation with high antimicrobial efficacy using chitosanEur J Pharm Biopharm200253111512311777759
- GuoZChenRXingRNovel derivatives of chitosan and their antifungal activities in vitroCarbohydr Res2006341335135416343460
- PujalsGSune-NegreJMPerezPIn vitro evaluation of the effectiveness and cytotoxicity of meglumine antimoniate microspheres produced by spray drying against Leishmania infantumParasitol Res200810261243124718278586
- RaoSBSharmaCPUse of chitosan as a biomaterial: studies on its safety and hemostatic potentialJ Biomed Mater Res199734121288978649
- GadesMDSternJSChitosan supplementation and fecal fat excretion in menObes Res200311568368812740459
- SonajeKLinYHJuangJHWeySPChenCTSungHWIn vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin deliveryBiomaterials200930122329233919176244
- WangLYGuYHSuZGMaGHPreparation and improvement of release behavior of chitosan microspheres containing insulinInt J Pharm20063111–218719516436319
- ThanouMVerhoefJCJungingerHEOral drug absorption enhancement by chitosan and its derivativesAdv Drug Deliv Rev200152211712611718935
- ThanouMVerhoefJCJungingerHEChitosan and its derivatives as intestinal absorption enhancersAdv Drug Deliv Rev200150Suppl 1S9110111576697
- BorchardGLuessenHLDe BoerAGVerhoefJCLehrCMJungingerHEThe potential of mucoadhesive polymers in enhancing intestinal peptidedrug absorption. III: effects of chitosanglutamate and carbomer on epithelial tight junctions in vitroJ Control Release199639131138
- PatilSBabbarAMathurRMishraASawantKMucoadhesive chitosan microspheres of carvedilol for nasal administrationJ Drug Target201018432133120199172
- JoscelyneSMTragardhGMembrane emulsification – a literature reviewJ Memb Sci20001691107117
- YooJWIrvineDJDischerDEMitragotriSBio-inspired, bioengineered and biomimetic drug delivery carriersNat Rev Drug Discov201110752153521720407
- JayakumarRChennazhiKPMuzzarelliRATamuraHNairSVSelvamuruganNChitosan conjugated DNA nanoparticles in gene therapyCarbohydr Polym201079118
- JreyssatyCShiQWangHEfficient nonviral gene therapy using folate-targeted chitosan–DNA nanoparticles in vitroISRN Pharm2012201236927022474605
- TongHShiQFernandesJCLiLDaiKZhangXProgress and prospects of chitosan and its derivatives as non-viral gene vectors in gene therapyCurr Gene Ther20099649550219807649
- JiangHLParkIKShinNRIn vitro study of the immune stimulating activity of an atrophic [correction of athrophic] rhinitis vaccine associated to chitosan microspheresEur J Pharm Biopharm200458347147615451520
- LimLYWanLSCThaiPYChitosan microspheres prepared by emulsification and ionotropic gelationDrug Dev Ind Pharm19972310981985
- ShuXZZhuKJChitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelationJ Microencapsul200118223724511253940
- DalyMMKnorrDChitosan–alginate complex coacervate capsules: effects of calcium chloride, plasticizers, and polyelectrolytes on mechanical stabilityBiotechnol Prog1988427681
- NishiokaYKyotaniSOkamuraMRelease characteristics of cisplatin chitosan microspheres and effect of containing chitinChem Pharm Bull (Tokyo)19903810287128732076575
- OhyaYTakeiTKobayashiHOuchiTRelease behaviour of 5-fluorouracil from chitosan-gel microspheres immobilizing 5-fluorouracil derivative coated with polysaccharides and their cell specific recognitionJ Microencapsul1993101198383199
- PavanettoFPeruginiPContiBModenaTGentaIEvaluation of process parameters involved in chitosan microsphere preparation by the o/w/o multiple emulsion methodJ Microencapsul19961366796888933353
- ThanooBCSunnyMCJayakrishnanACross-linked chitosan microspheres: preparation and evaluation as a matrix for the controlled release of pharmaceuticalsJ Pharm Pharmacol19924442832861355537
- OrientiIAiedehKGianasiEPontiCZecchiVChitosan-indomethacin conjugates. Effect of different substituents on the polysaccharide molecule on drug releaseArch Pharm Pharm Med Chem19963295245250
- MiFLTanYCLiangHFSungHWIn vivo biocompatibility and degradability of a novel injectable-chitosan-based implantBiomaterials200223118119111762837
- van der LubbenIMvan OpdorpFAHengeveldMRTransport of chitosan microparticles for mucosal vaccine delivery in a human intestinal M-cell modelJ Drug Target200210644945612575734
- YooJSKimYJKimSHChoiSHStudy on genipin: a new alternative natural crosslinking agent for fixing heterogaft tissueKorean J Thorac Cardiovasc Surg201144319720722263152
- TokumitsuHIchikawaHFukumoriYChitosan–gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion-droplet coalescence technique and characterizationPharm Res199916121830183510644070
- MitraADeyBChitosan microspheres in novel drug delivery systemsIndian J Pharm Sci201173435536622707817
- Ozbas-TuranSAkbugaJAralCControlled release of interleukin-2 from chitosan microspheresJ Pharm Sci20029151245125111977100
- MitraSGaurUGhoshPCMaitraANTumor targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrierJ Control Release2001741–331732311489513
- AgnihotriSAAminabhaviTMControlled release of clozapine through chitosan microparticles prepared by a novel methodJ Control Release200496224525915081216
- BogatajMMrharAGrabnarIThe influence of magnesium stearate on the characteristics of mucoadhesive microspheresJ Microencapsul200017449950810898089
- HePDavisSSIllumLSustained release chitosan microspheres prepared by novel spray drying methodsJ Microencapsul199916334335510340219
- van der LubbenIMVerhoefJCvan AelstACBorchardGJungingerHEChitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patchesBiomaterials200122768769411246962
- AhireVJSawantKKDoshiJBRavetkarSDChitosan microparticles as oral delivery system for tetanus toxoidDrug Dev Ind Pharm200733101112112417852363
- van der LubbenIMKerstenGFretzMMBeuveryCVerhoefJCJungingerHEChitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in miceVaccine20032113–141400140812615436
- van der LubbenIMKoningsFABorchardGVerhoefJCJungingerHEIn vivo uptake of chitosan microparticles by murine Peyer’s patches: visualization studies using confocal laser scanning microscopy and immunohistochemistryJ Drug Target200191394711378522
- HoriMOnishiHMachidaYEvaluation of Eudragit-coated chitosan microparticles as an oral immune delivery systemInt J Pharm20052971–222323415885938
- PremalethaKLicyCDJoseSSaraladeviAShirwaikarAShirwaikarAFormulation, characterization and optimization of hepatitis B surface antigen (HBsAg)-loaded chitosan microspheres for oral deliveryPharm Dev Technol201217225125821108582
- UddinANBejugamNKGayakwadSGAktherPD’SouzaMJOral delivery of gastro-resistant microencapsulated typhoid vaccineJ Drug Target200917755356019563303
- ChiouCJTsengLPDengMCMucoadhesive liposomes for intranasal immunization with an avian influenza virus vaccine in chickensBiomaterials200930295862586819608270
- PineSBarackmanJOttGO’HaganDIntranasal immunization with influenza vaccine and a detoxified mutant of heat labile enterotoxin from Escherichia coli (LTK63)J Control Release2002851–326327012480330
- CarrRMLolachiCMAlbaranRGRidleyDMMontgomeryPCO’SullivanNLNasal-associated lymphoid tissue is an inductive site for rat tear IgA antibody responsesImmunol Invest1996255–63873968915676
- ClearyPPZhangYParkHSNasal associated lymphoid tissue and M cells, a window to persistent streptococcal infectionsIndian J Med Res2004119Suppl576015232163
- DebertinASTschernigTTonjesHKleemannWJTrogerHDPabstRNasal-associated lymphoid tissue (NALT): frequency and localization in young childrenClin Exp Immunol2003134350350714632758
- GillRFPirockinaiteGO’SullivanNLMontgomeryPCNasal-associated lymphoid tissue is not an absolute requirement for the induction of rat tear IgA antibody responsesCurr Eye Res20103511820021248
- HopkinsSFisherGKraehenbuhlJPVelinDNasal-associated lymphoid tissue – a site for vaccination and pathogen entrySTP Pharm Sci1998814751
- LiangBHylandLHouSNasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of miceJ Virol200175115416542011333927
- OwenSJBatzloffMChehrehasaFNasal-associated lymphoid tissue and olfactory epithelium as portals of entry for Burkholderia pseudomallei in murine melioidosisJ Infect Dis2009199121761177019456230
- ZuercherAWCoffinSEThurnheerMCFundovaPCebraJJNasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responsesJ Immunol200216841796180311823512
- McNeelaEAO’ConnorDJabbal-GillIA mucosal vaccine against diphtheria: formulation of cross reacting material (CRM197) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal deliveryVaccine2000199–101188119811137256
- DonovanMDFlynnGLAmidonGLAbsorption of polyethylene glycols 600 through 2000: the molecular weight dependence of gastrointestinal and nasal absorptionPharm Res1990788638682235883
- SarkarMADrug metabolism in the nasal mucosaPharm Res199291191589391
- BaudnerBCGiulianiMMVerhoefJCRappuoliRJungingerHEGiudiceGEThe concomitant use of the LTK63 mucosal adjuvant and of chitosan-based delivery system enhances the immunogenicity and efficacy of intranasally administered vaccinesVaccine20032125–263837384412922117
- IllumLJabbal-GillIHinchcliffeMFisherANDavisSSChitosan as a novel nasal delivery system for vaccinesAdv Drug Deliv Rev2001511–3819611516781
- KangMLKangSGJiangHLIn vivo induction of mucosal immune responses by intranasal administration of chitosan microspheres containing Bordetella bronchiseptica DNTEur J Pharm Biopharm200663221522016531027
- JiangHLKangMLQuanJSThe potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunizationBiomaterials200829121931193918221992
- JiangHLParkIKKangMLImmune stimulating activity of an atrophic rhinitis vaccine associated to pegylated chitosan microspheres in vitroPolym Adv Technol2007183220225
- JiangHLParkIKShinNRYooHSAkaikeTChoCSControlled release of Bordetella bronchiseptica dermonecrotoxin (BBD) vaccine from BBD-loaded chitosan microspheres in vitroArch Pharm Res200427334635015089042
- KangMLJiangHLKangSGPluronic F127 enhances the effect as an adjuvant of chitosan microspheres in the intranasal delivery of Bordetella bronchiseptica antigens containing dermonecrotoxinVaccine200725234602461017485148
- SoaneRJFrierMPerkinsACJonesNSDavisSSIllumLEvaluation of the clearance characteristics of bioadhesive systems in humansInt J Pharm19991781556510205625
- SoaneRJHinchcliffeMDavisSSIllumLClearance characteristics of chitosan based formulations in the sheep nasal cavityInt J Pharm20012171–218319111292554
- BaudnerBCVerhoefJCGiulianiMMProtective immune responses to meningococcal C conjugate vaccine after intranasal immunization of mice with the LTK63 mutant plus chitosan or trimethyl chitosan chloride as novel delivery platformJ Drug Target2005138–948949816332574
- KumarMBeheraAKLockeyRFIntranasal gene transfer by chitosan–DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infectionHum Gene Ther200213121415142512215263
- WangPLJohnstonTPEnhanced stability of two model proteins in an agitated solution environment using poloxamer 407J Parenter Sci Technol19934741831898410567
- MorikawaKOkadaFHosokawaMKobayashiKEnhancement of therapeutic effects of recombinant interleukin-2 on a transplantable rat fibrosarcoma by the use of a sustained release vehicle, pluronic gelCancer Res198747137413491675
- KangMLChoCSYooHSApplication of chitosan microspheres for nasal delivery of vaccinesBiotechnol Adv200927685786519583998
- MiyazakiSTobiyamaTTakadaMAttwoodDPercutaneous absorption of indomethacin from pluronic F127 gels in ratsJ Pharm Pharmacol19954764554577674126
- VeyriesMLCouarrazeGGeigerSControlled release of vancomycin from poloxamer 407 gelsInt J Pharm1999192218319310567749
- ZhangLParsonsDLNavarreCKompellaUBDevelopment and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofurJ Control Release2002851–3738112480313
- WesterinkMASmithsonSLSrivastavaNBlonderJCoeshottCRosenthalGJProJuvant (Pluronic F127/chitosan) enhances the immune response to intranasally administered tetanus toxoidVaccine2001205–671172311738734
- CoeshottCMSmithsonSLVerderberEPluronic F127-based systemic vaccine delivery systemsVaccine200422192396240515193401
- BertholdACremerKKreuterJPreparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for antiinflammatory drugsJ Control Release19963911725
- BodmeierROhKHPramarYPreparation and evaluation of drug-containing chitosan beadsDrug Dev Ind Pharm198915914751494
- DunnhauptSBarthelmesJThurnerCCWaldnerCSakloetsakunDBernkop-SchnurchAS-protected thiolated chitosan: synthesis and in vitro characterizationCarbohydr Polym201290276577222839999
- HauptsteinSBernkop-SchnurchAThiomers and thiomer-based nanoparticles in protein and DNA drug deliveryExpert Opin Drug Deliv2012991069108122703388
- IslamMABajracharyaPKangSKMucoadhesive alginate/poly (L-lysine)/thiolated alginate microcapsules for oral delivery of Lactobacillus salivarius 29J Nanosci Nanotechnol20111187091709522103131
- IslamMAJiangHLQuanJSMucoadhesive and pH-sensitive thiolated Eudragit microspheres for oral delivery of Pasteurella multocida antigens containing dermonecrotoxinJ Nanosci Nanotechnol20111154174418121780423
- LeeWJChaSShinMIslamMAChoCSYooHSInduction of Th1 polarized immune responses by thiolated Eudragit-coated F4 and F18 fimbriae of enterotoxigenic Escherichia coliEur J Pharm Biopharm201179222623121571066
- LeeWJChaSShinMEfficacy of thiolated Eudragit microspheres as an oral vaccine delivery system to induce mucosal immunity against enterotoxigenic Escherichia coli in miceEur J Pharm Biopharm2012811434822306699
- IqbalJShahnazGPereraGHintzenFSartiFBernkop-SchnurchAThiolated chitosan: development and in vivo evaluation of an oral delivery system for leuprolideEur J Pharm Biopharm20128019510221964316
- MillottiGSambergerCFrohlichESakloetsakunDBernkop-SchnurchAChitosan-4-mercaptobenzoic acid: synthesis and characterization of a novel thiolated chitosanJ Mater Chem2010201224322440
- ShahnazGVetterABarthelmesJThiolated chitosan nanoparticles for the nasal administration of leuprolide: bioavailability and pharmacokinetic characterizationInt J Pharm20124281–216417022421322
- TalaeiFAziziEDinarvandRAtyabiFThiolated chitosan nanoparticles as a delivery system for antisense therapy: evaluation against EGFR in T47D breast cancer cellsInt J Nanomedicine201161963197521976973
- WerleMBernkop-SchnurchAThiolated chitosans: useful excipients for oral drug deliveryJ Pharm Pharmacol200860327328118284806
- Bernkop-SchnurchAKastCEGuggiDPermeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systemsJ Control Release20039329510314636716
- MillottiGPereraGViglCPicklKSinnerFMBernkop-SchnurchAThe use of chitosan-6-mercaptonicotinic acid nanoparticles for oral peptide drug deliveryDrug Deliv201118319019721039318
- MillottiGSambergerCFrohlichEBernkop-SchnurchAChitosan-graft-6-mercaptonicotinic acid: synthesis, characterization, and biocompatibilityBiomacromolecules200910113023302719821557
- SaboktakinMRTabatabaieRMMaharramovARamazanovMADevelopment and in vitro evaluation of thiolated chitosan–poly(methacrylic acid) nanoparticles as a local mucoadhesive delivery systemInt J Biol Macromol201148340340721215774
- SaremiSAtyabiFAkhlaghiSPOstadSNDinarvandRThiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: preparation, in vitro and ex vivo evaluationInt J Nanomedicine2011611912821289989
- DunnhauptSBarthelmesJHombachJSakloetsakunDArkhipovaVBernkop-SchnurchADistribution of thiolated mucoadhesive nanoparticles on intestinal mucosaInt J Pharm20114081–219119921295123
- BurnerUJantschkoWObingerCKinetics of oxidation of aliphatic and aromatic thiols by myeloperoxidase compounds I and IIFEBS Lett1999443329029610025950
- DunnhauptSBarthelmesJIqbalJIn vivo evaluation of an oral drug delivery system for peptides based on S-protected thiolated chitosanJ Control Release2012160347748522542699
- DunnhauptSBarthelmesJRahmatDS-protected thiolated chitosan for oral delivery of hydrophilic macromolecules: evaluation of permeation enhancing and efflux pump inhibitory propertiesMol Pharm2012951331134122489677