?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to prepare nanoemulsions coated with alginate/chitosan for oral insulin delivery. Uncoated nanoemulsions were prepared by homogenization of a water in oil in water (w/o/w) multiple emulsion that was composed of Labrafac® CC, phospholipid, Span™ 80 and Cremorphor® EL. Coating of the nanoemulsions was achieved based on polyelectrolyte cross-linking, with sequential addition of calcium chloride and chitosan to the bulk nanoemulsion dispersion that contained alginate. The particle size of the coated nanoemulsions was about 488 nm and the insulin entrapment ratio was 47.3%. Circular dichroism spectroscopy proved conformational stability of insulin against the preparative stress. In vitro leakage study indicated well-preserved integrity of the nanoemulsions in simulated gastric juices. Hypoglycemic effects were observed in both normal and diabetic rats. The relative pharmacological bioavailability of the coated nanoemulsion with 25 and 50 IU/kg insulin were 8.42% and 5.72% in normal rats and 8.19% and 7.84% in diabetic rats, respectively. Moreover, there were significantly prolonged hypoglycemic effects after oral administration of the coated nanoemulsions compared with subcutaneous (sc) insulin. In conclusion, the nanoemulsion coated with alginate/chitosan was a potential delivery system for oral delivery of polypeptides and proteins.
Introduction
Ever increasing numbers of peptides and proteins are being developed as therapeutic drugs, due to their definite and potent effect.Citation1 These are usually administered parenterally to achieve higher bioavailability and not orally because of their fast degradation in the gastrointestinal tract (GIT) and poor permeability across the intestinal epithelium.Citation2 Approaches taken to improve their oral bioavailability have included protecting against enzymatic degradation and increasing permeability and absorption time.Citation1,Citation3–Citation5
In recent decades, lipid emulsions (such as water in oil [w/o] microemulsionsCitation6 and multiple emulsions) have been widely used in the enhancement of the oral bioavailability of peptides and proteinsCitation7,Citation8 due to their protective ability; improving the fluidity of membrane and transient opening the tight junctions induced by lipid constituents or surfactants.Citation9,Citation10 After oral administration w/o microemulsions are easily transformed into oil in water (o/w) emulsions in the gastric juices. Phase inversion will then release the entrapped drugs and expose them to gastrointestinal degradation by enzymes.Citation11 Therefore, in order to bypass gastric degradation, many studies have administered peptide- or protein-loaded w/o microemulsions by duodenal injection.Citation12 Although water in oil in water (w/o/w) multiple emulsions have shown better stability than w/o microemulsions in gastric juices, water-soluble drugs, including proteins and peptides, will also be released from multiple emulsions very quickly.Citation13 Furthermore, the size of the normal multiple emulsion droplet is relatively large, about 1–100 μm, which limits its uptake by intestinal epithelia or M cells.Citation14 It can be concluded that current nanotechnologies using w/o microemulsion and w/o/w multiple emulsions have had inherent drawbacks when employed to enhance oral bioavailability of proteins and peptides. We assume that these emulsion-based systems will be highly efficient to deliver proteins orally if they can be safely transported through the stomach. The approaches we took in this study were to reduce the w/o/w multiple emulsion size to nanoscale and to coat them with polyelectrolyte polymers.
Alginate (Alg) and chitosan (Chit) are naturally degradable materials that have been used in the oral delivery of peptides and proteins.Citation15,Citation16 Alg and Chit can crosslink together through electrostatic force to form nanoparticles that possess protective abilities as well as the ability to improve permeability and bioadhesion.Citation15,Citation17–Citation20
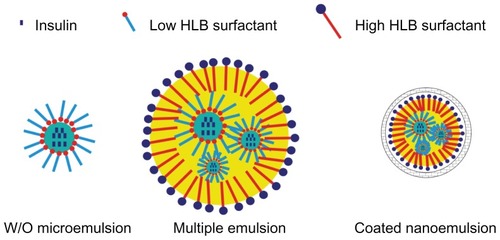
In this study, insulin was chosen as the model protein drug. The improvement of the oral bioavailability of insulin is a tremendous challenge for pharmaceutical scientists, but the success of oral insulin will be of significant value for diabetic patients. This study aimed to decrease the size of multiple emulsion droplets by homogenization and to coat the so-formed nanoemulsion by Alg/Chit through electrostatic cross-linking, to protect the stability of nanoemulsion in GIT (). The primary goal was to evaluate the ability of this Alg/Chit-coated nanoemulsion system to improve the oral bioavailability of insulin. The encapsulation efficiency, size, and in vitro stability in simulated gastric juices were evaluated. The pharmacological bioavailability of Alg/Chit-coated nanoemulsion by oral administration was evaluated in both normal and diabetic rats.
Materials and methods
Material
Porcine insulin (29 IU/mg) was purchased from Xuzhou Wanbang Biochemical Pharmaceutical Co, Ltd (Xuzhou, China). Low-guluronic content, low-viscosity sodium alginate (Alg) (5–20 cP, 1% in water) and low molecular weight chitosan (Chit) (75%–85% deacetylated) were purchased from Sigma- Aldrich (St Louis, MO, USA). Fat-free soybean lecithin was obtained from Lipoid (Lipoid S75; Lipoid GmbH, Ludwigshafen, Germany). Cremophor® EL was provided by BASF (Ludwigshafen, Germany). Labrafac® CC was kindly gifted by Gattefossé Pharma (Saint-Priest, France). High performance liquid chromatography (HPLC)-grade acetonitrile was supplied by Merck (Darmstadt, Germany). All other chemicals were of analytical grade and used as received.
Animals
Male Wistar rats were purchased from Sino-British SIPPR/ BK Lab Animal Co, Ltd (Shanghai, China). Male Goto- Kakizaki (GK) rats were purchased from SLAC Laboratory Animal Co, Ltd (Shanghai, China). All rats were raised in the Animal Centre of Fudan University (Shanghai, China) and were housed in cages under a photoperiod cycled schedule of 12 hours light/12 hours dark, under controlled, air-conditioned temperature and humidity. The rats received standard laboratory feed and were allowed free access to tap water. All of the animal experiments were approved by the Institutional Animal Care and Use Committee of Fudan University.
Preparation of w/o/w multiple emulsion
A two-step process was employed to prepare the w/o/w multiple emulsion.Citation21 Briefly, the w/o primary emulsion was formed by preparing 800 μL insulin solution (150 mg insulin dispersed in 10 mL pH 7.0 Tris-HCl buffer solution [0.1 M]), which was then dissolved with pH 1.0 HCl and added to 8 g of the oily phase (composed of 68.5% Labrafac CC, 25% Span™ 80, and 6.5% phospholipid), with stirring for 15 minutes at 800 rpm. Then this primary emulsion was dropped into 18 g aqueous solution containing 3% Cremophor EL and Alg at different concentrations. The w/o/w multiple emulsion was formed after continuous stirring at 600 rpm for 15 minutes.
Preparation of Alg/Chit-coated nanoemulsion
The w/o/w multiple emulsion was then extruded into nanoscale by high pressure homogenization (AH 100; ATS Engineering Inc, Brampton, ON, Canada) under 60 bar. Then the Alg/Chit-coated nanoemulsion was prepared based on polyelectrolyte cross-linking.Citation19 The process was described as follows: 0.5 mL calcium chloride solution was dropped into 12 mL of the above-mentioned nanoemulsion, with stirring at 600 rpm for 30 minutes. Then, 2 mL Chit solution was added dropwise into it, while stirring at 600 rpm, over 90 minutes. The effects of Alg, Chit, and calcium chloride concentration on the in vitro properties of coated nanoemulsion were evaluated.
Determination of entrapment ratio
The entrapment ratio (ER) was determined indirectly by measuring the leakage ratio, after destroying the coated nanoemulsion. Briefly, 4 mL methanol was added to solubilize 1 mL nanoemulsion. Then, 100 μL HCl (pH 1.0) was added to adjust the final pH to 3.0. Distilled water was added to reach 10 mL and mixed well. Centrifugation was performed at 16000 g for 10 minutes, and the clear supernatant was used to determine insulin by HPLC (as described below). The amount of insulin encapsulated in the nanoemulsion was calculated as the difference between the total amount used to prepare the formulation and the amount of free insulin present in the outer aqueous phase after centrifugation.
Determination of insulin
Porcine insulin was assayed by a reversed-phase HPLC-ultraviolet (UV) method.Citation22 The HPLC system (1100series; Agilent Technologies, Santa Clara, CA, USA) was composed of a quaternary pump, a degasser, an autosampler, a column heater and a tunable ultraviolet detector. A C18 column (Zorbax, 5 μm, 4.6 mm × 150 mm; Agilent) with a C18 precolumn (2 mm × 20 mm; Alltech) was used for detection at 25°C. The mobile phase was composed of acetonitrile and 0.57% phosphoric acid solution (adjusted to pH 2.25 with triethylamine) in a volume ratio of 26/74. A flow rate of 1.0 mL/minute and a detection wavelength of 220 nm were employed.
Characterization of coated and uncoated nanoemulsion
Size and zeta potential
Nanoemulsion size was measured by a photon correlation spectroscopy instrument (NICOMP 380 ZLS Particle Sizing Systems, Port Richey, FL, USA). Samples diluted tenfold with pure water were placed in a disposable cuvette, and photon counts were collected over 5 minutes. The angle was 90°, and the temperature kept at 25°C. The results were calculated with intensity-based Gaussian distribution using the ZPW388-175 software program (Particle Sizing Systems).
The zeta potential measurements were carried out with Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at 25°C. Samples were measured in folded capillary cells integrated with gold electrodes. Three measurements were conducted, and the number of runs in each measurement was automatically determined by the software. The results were expressed as mean ± standard deviation (SD).
Transmission electron microscopy (TEM)
The morphologies of uncoated and Alg/Chit-coated nanoemulsion were observed using transmission electron microscopy (JEM-1230, JEOL). A drop of nanoemulsion was put on 300-mesh, carbon-coated copper grids, and then, extra water was removed using blotting paper. A drop of 1% phosphotungstic acid was added to stain, for 60 seconds, and then dried at ambient conditions. The samples were inspected at an acceleration voltage of 120 kV. The micrographs were recorded at a final magnification of ×12,000.
Conformational stability of insulin
To evaluate the conformational stability of insulin against the preparative stress, circular dichroism (CD) spectroscopy (Jasco J-810; Jasco Corp, Tokyo, Japan) was used. The coated nanoemulsion (1 mL) was destroyed by adding 4 mL methanol and HCl solution (pH 1.0) to 10 mL. After centrifuging at 16,000 g for 10 minutes, insulin was extracted into the supernatant. Insulin was then separated using a solid-phase extraction column (Supelco ENVI-18; Sigma-Aldrich).Citation23 Briefly, the ENVI-18 column was first activated by methanol and balanced by solvent. Then 1 mL supernatant mixture was mounted. Elution was performed with 3 mL aqueous methanol (40%) to wash out impurities and then with 30 mL 60% aqueous methanol to elute the insulin. The insulin concentration was adjusted to 30 μg/mL for the CD test, using HCl solution. Spectra were collected at 20°C, using a 0.5 nm step size, over a wavelength range of 200–300 nm; a band width of 3 nm and a scanning speed of 500 nm/minute, with a 0.25 second response time, were applied. Secondary structure content was estimated employing Jasco w32 secondary structure estimation software (version 1.0).
Leakage of coated nanoemulsion in acidic media
The effect of gastric acid on the integrity of coated nanoemulsion was studied. Nanoemulsion samples (1 mL) were diluted with 10 mL of different acidic media (pH 1.2, 1.6, 2.0, 2.5, and 3.0) and incubated for 30 minutes at 37°C under oscillating shaking at 100 rpm, in order to simulate physiological gastric conditions. The suspension was then centrifuged for 15 minutes at 30,000 g. Then 200 μL clear solution was mixed with 200 μL methanol and centrifuged for 5 minutes at 16,000 g. The ultraclear phase was used to assay the insulin leakage.
The release of insulin from different formulations (coated nanoemulsion, uncoated nanoemulsion, and multiple emulsion) was investigated in pH 2.5 media simulating gastric juice at 37°C, under stirring at 100 rpm. First, 1 mL samples were added in 100 mL release media. Then, 1 mL test samples were withdrawn at determined times (0.25, 0.5, 1, and 2 hours) and centrifuged at 16,000 g. The supernatants were analyzed for insulin by HPLC. The amount of insulin released from the formulation was expressed as a percentage of total insulin, as calculated from the release ratio.
Hypoglycemic effect in normal and diabetic rats
Rats were all fasted overnight for 12 hours before the experiments and allowed a small amount of food after 6 hours during the experiments, to avoid blood glucose dropping due to excessive consumption. Test formulations were administered by gavage (except for instances where this was given subcutaneously, as a reference). Blood samples were collected from the tail vein and the plasma was separated by centrifugation (4000 rpm, 10 minutes) for glucose determination. Glucose oxidase determination assay kits (Shanghai Rongsheng Bio Tech Co, Ltd, Shanghai, China) were used for plasma glucose level determination.
Male Wistar rats (200–250 g) were used as normal rats for the pharmacodynamics study in vivo. The rats were divided into seven groups (six rats per group). The coated nanoemulsions were administered at the insulin doses of 12.5 IU/kg, 25 IU/ kg, and 50 IU/kg, respectively. Free insulin solutions, multiple emulsion, and uncoated nanoemulsion at 50 IU/kg were administered as controls. Meanwhile 1 IU/kg insulin solution was injected subcutaneously as a reference. For the diabetic models, Goto-Kakizaki (GK) rats of 6–7 weeks old were used, with the same dosing and grouping protocol. Plasma glucose levels were plotted against time to evaluate the hypoglycemic effect over time, for up to 24 hours after insulin administration. The area above the curve was calculated using the trapezoidal method. Pharmacological availability (PA) of orally administered insulin was determined according to the following equation, with subcutaneous (sc) insulin as the reference.
Where AACtest is area above the curve of text formulation; AACreference is area above the curve of reference.
Data analysis
The results were expressed as mean ± SD. For group comparison, a one-way analysis of variance (ANOVA) was applied (SPSS 16.0; IBM Corp, Amonk, NY). A difference was considered statistically significant when the P value was less than 0.05.
Results and discussion
Preparation of the coated nanoemulsions
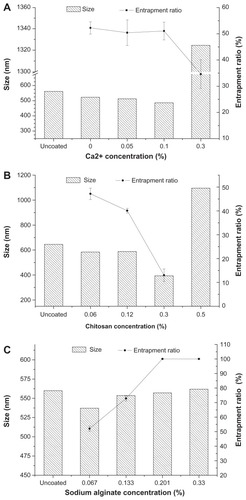
The preparation process of Alg/Chit-coated nanoemulsion was simple and reliable. shows the effect of Ca2+, Alg, and Chit concentration on size and entrapment ratio. According to preliminary experiments, the main factors influencing size and entrapment ratio of coated nanoemulsion were Ca2+, Alg, and Chit concentration, so we designed the factors and levels showed in . At lower Ca2+ concentrations, the nanoemulsion size and entrapment ratio did not change significantly. However, significant variation was observed at Ca2+ concentrations of 0.3%. With increasing concentration of Chit, both the entrapment ratio and size decreased. When the Chit concentration exceeded 0.5%, Alg would form bulk gel with the Chit. The size and entrapment ratio did not seem to change when the Alg concentration was more than 0.067%. The size was smallest when the Alg concentration was 0.067%. The change of zeta potential is shown in . At the beginning, the nanoemulsion appeared negatively charged due to the adsorption of negative Alg. With the addition of positive Ca2+ and Chit, Alg began to complex with them, and the zeta potential decreased. When Chit concentration was over 0.48%, the zeta potential varied towards a drastic positive charge because of excessive Alg-Chit cross-linking.
Table 1 Mean zeta potential values of different formulations to form Alg/Chit-coated nanoemulsion
Figure 2 The effects of Ca2+ (A), Chit (B) and Alg (C) concentration on particle size and entrapment ratio of coated nanoemulsion.
Notes: Mean ± SD, n = 3.
Abbreviations: Chit, Chitosan; Alg, Alginate.
In this study, we employed electrostatic interaction to coat the nanoemulsion. Calcium cations first crosslinked with negatively charged Alg through ionic gelation to form the first shell, and then, Chit interacted with Alg to form a tighter coating. The addition of Ca2+ can increase the acid resistance capacity of the whole system,Citation19 and the outside layer of Chit can further protect the nanoemulsion, exert a mucoadhesive effect, and enhance oral absorption.Citation24 However, an overdose of Ca2+ will lead to formation of bulk gel because Ca2+ initiates ionical cross-linking with coiled alginate structures (through intermolecular interaction).Citation25 It seems that the Ca2+ concentration should be balanced to assure efficient coating and good manufacturing. To a certain extent, Chit would compete with Ca2+ for cross-linking with Alg. With much more Chit in the external water phase, some Alg segments that presented on the surface of nanoemulsion would encircle the Chit to form nanoparticles. Therefore, entrapment ratio decreased with the increase of Chit. In this study, negatively charged Alg segments so much exceeded the positively charged Chit and calcium ions that addition of more Alg would not have had significant influence. However, in a later experiment we found that such nanoemulsions (with too much Alg) had poor stability and exhibited phase separation after just a few days, forming a continuous gel of Alg. In conclusion, the formulation shown in was selected as the optimized formulation to be followed for in vitro and in vivo study.
Table 2 Summary of formulation compositions for in vitro and in vivo study
Morphology of the coated nanoemulsion
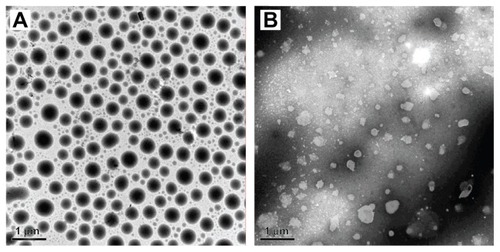
shows the TEM photograph of the uncoated and coated nanoemulsions. The size of these was about 500 nm according to the TEM image, which was similar to sizes determined by dynamic light scattering, 530.2 nm (Polydispersion Index [PI] = 0.407) for the uncoated nanoemulsion and 488.0 nm (PI = 0.396) for the coated nanoemulsion. The droplet shape of the uncoated nanoemulsion was quasicircular and had a smooth profile (). However, the outline of the coated nanoemulsion was rough and irregular (). This phenomenon is similar to reports by others. Citation18,Citation26 Cross-linking between Alg and Chit possibly changed the surface tension, which thus led to surface shrinking and reduction in particle size in the nanoemulsions.
Conformational stability of insulin
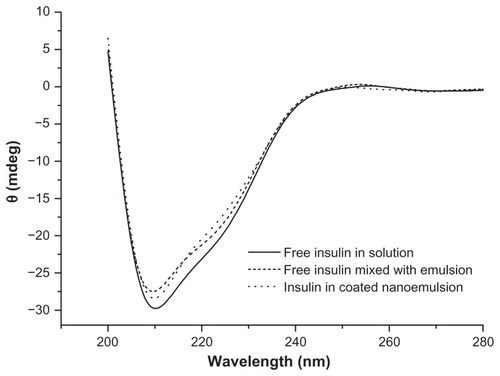
The conformation of proteins or peptides is important for the exertion of optimal therapeutic effect and can be damaged easily under conditions such as high temperature, mechanical manipulation, and exposure to organic solvents. Therefore, conformational stability must be accorded more attention during manipulation of proteins or peptides. Generally, the secondary structures (α-helix and β-fold) can illustrate the efficacy of insulin, and CD is regarded as one of the most effective methods to evaluate the secondary structures of proteins and peptides.Citation27 shows the CD spectra of the insulin secondary structures. The CD spectra of insulin in mixture with emulsion and the coated nanoemulsion were totally identical to the insulin solution, which showed a peak valley at 209 nm and a shoulder at around 225 nm. The secondary structures of insulin in the different samples were very close, with 20%–21% α-helix and 26%–28% β-fold. Such secondary structural analysis confirms that the conformational structure of insulin was stable during the preparative procedures for the Alg/Chit-coated nanoemulsions.
Insulin leakage from coated nanoemulsion in simulated gastric juice
In order to illustrate the protective capability of the coated nanoemulsions in gastric juice, the insulin leakage from the coated nanoemulsions was determined. shows the leakage ratio of insulin, after 30 minutes at 37°C, from the coated nanoemulsion at different pHs. When the media pH was less than 2.0, more than 50% of the insulin leaked from the coated nanoemulsion, and if the pH was more than 2.5, the insulin leakage was very little. In pH 2.5 release media, the insulin release from the uncoated nanoemulsions and the multiple emulsions was rapid, with 70% and 90% of the total insulin released in the first 15 minutes and after 2 hours, respectively. However, about 20% of the insulin was released from the coated nanoemulsion ().
Figure 5 The insulin leakage from coated nanoemulsion in pH 1–3 simulated gastric juice.
Notes: Mean ± SD, n = 3.
Abbreviation: SD, standard deviation.
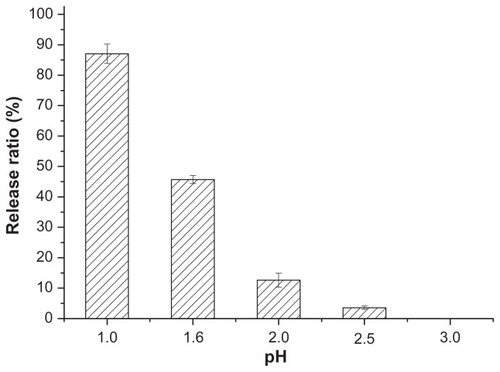
Figure 6 In vitro insulin release profile from Alg/Chit-coated nanoemulsion, uncoated nanoemulsion and multiple emulsion in simulated gastric juice (pH 2.5 media).
Notes: Mean ± SD, n = 3.
Abbreviations: Alg, Alginate; Chit, chitosan.
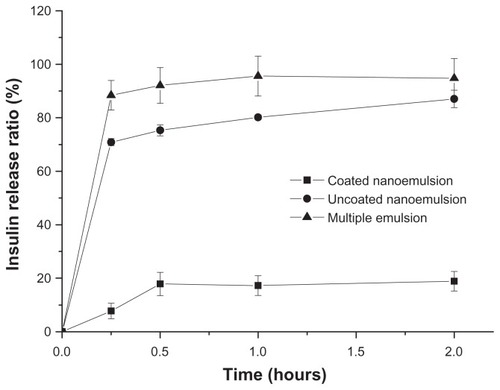
The Alg/Chit shell has certain pH-sensitivity. Too high acidity can change the cross-linking colloid surface and destroy its integrity, causing insulin leakage. As we know, gastric pH varies in different states.Citation26,Citation28 Many reports have suggested that the gastric pH varies from 2.5 to 3.7 in the fasting state due to declined secretion of hydrochloric acid by parietal cells.Citation26,Citation28,Citation29 Since the formulations of this study would be administrated under the fasting state during in vivo studies, the pH 2.5 simulated gastric juice was selected as the release media to illustrate the releases of the coated nanoemulsion, uncoated nanoemulsion, and multiple emulsion. The crosslinking complex prevented the emulsion inversion and then avoided insulin release from the inner phase in simulated gastric environment because of the tight Alg pre-gel network at low pH. The initial about 20% release could be ascribed to free insulin on the surface which escaped from the emulsion in the homogenization process.
Hypoglycemic effect in normal and diabetic rats
The aforementioned in vitro evaluation had indicated the coated nanoemulsion could decrease the leakage of insulin in simulated gastric juice. In order to further prove the in vivo performance, we monitored the hypoglycemic effect after oral administration of various formulations in either normal or diabetic rats. Relative oral bioavailability was calculated on the basis of quantification of blood glucose with subcutaneous (sc) insulin as a reference.
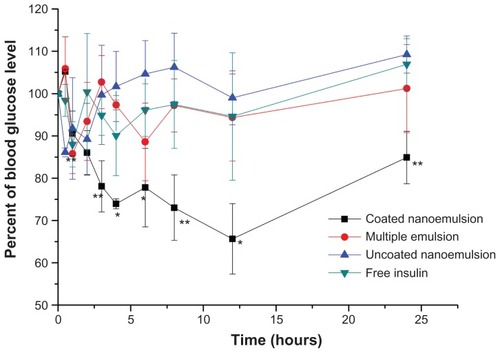
illustrates the glucose level versus time profiles following the administration of the various insulin formulations and coated nanoemulsions with different dosages to normal Wistar rats. Results indicated there was no evident hypoglycemic effect after the oral administration of the insulin solution to normal rats. Similarly, after oral administration of the uncoated nanoemulsion and multiple emulsion as controls, no evident hypoglycemic effects were observed (). However, the blood glucose levels in normal rats decreased remarkably after oral administration of the Alg/Chit-coated nanoemulsion. There may be three factors accounting for the significant hypoglycemic effect of the coated nanoemulsion. Firstly, the nanoscale size increases the chance of uptake by M cells;Citation14 meanwhile, chitosan can reversely open the tight junctions, increasing nanoemulsion paracellular transport.Citation30 Secondly, the Ca2+/Alg/Chit shell protects insulin against proteolytic enzymes.Citation15 Thirdly, Alg and Chit have mucoadhesive properties, which may prolong nanoemulsion retention in the GIT and promote penetration into the mucus layer.Citation31
Figure 7 Plasma glucose level versus time profiles of Wistar rats after oral administration of 50 IU/Kg Alg/Chit-coated nanoemulsion, multiple emulsion, uncoated nanoemulsion, and insulin solution, compared to sc 1 IU/kg insulin.
Notes: Mean ± SD, n = 6; *P < 0.05 and **P < 0.01 compared with control group.
Abbreviations: Alg, Alginate; Chit, chitosan, sc, subcutaneous.
The hypoglycemic effect in normal and GK rats after the administration of different dosages of coated insulin nanoemulsion were shown in and , respectively. Importantly, the blood glucose level achieved the largest decreases (to 65% and 50% of the initial glucose level) after oral administration of the coated nanoemulsion with 50 IU/kg insulin to the normal and diabetic rats, respectively. Compared with sc insulin, the oral coated nanoemulsion showed more sustained and long-term effects. In normal rats, at 12 hours postdose, the glucose level began to increase, recovering to about 90% of the initial level at 24 hours. However, in diabetic rats, the glucose level was continuously maintained at 50%– 60% of the initial level at 6 hours to 24 hours. The prolonged effect also suggests that the GIT retention time was prolonged due to mucoadhesive properties of Chit and Alg. In diabetic rats, the secretion of glucagon was very small and slow due to the poor secretion ability of pancreatic islets. Therefore, the low glucose level could be maintained for a longer time. Meanwhile, the minimum hypoglycemic effect was better than that in the normal rats after administration of the same dose, which was due to a higher initial glucose level in the diabetic rats. The initial glucose level of GK rats is usually 20 to 25 mmol/L compared with 8 to 11 mmol/L of normal Wistar rats. After oral administration of coated nanoemulsion, the glucose level decreased gradually to normal level (about 10 to 12.5 mmol/L) and was maintained for a longer time. There were no sharp decreases in glucose level. The dramatic change of blood glucose concentration may cause glycopenia syndrome, which is harmful to the human body, and this short-term effect cannot satisfy diabetic needs as well.Citation22 Therefore, the administration of a coated nanoemulsion as the insulin delivery system should result in better patient compliance and therapeutic effect.
Figure 8 Plasma glucose level versus time profiles of Wistar rats after oral administration of Alg/Chit-coated nanoemulsion in 50 IU/kg, 25 IU/kg, 12.5 IU/kg and free insulin solution as control.
Notes: Mean ± SD, n = 6; *P < 0.05 and **P < 0.01 by comparing dosage 50 IU/kg with 12.5IU/kg; #P < 0.05 by comparing dosage 25 IU/kg with 12.5 IU/kg; ^P < 0.05 by comparing dosage 50 IU/kg with 25 IU/kg.
Abbreviation: sc, subcutaneous.
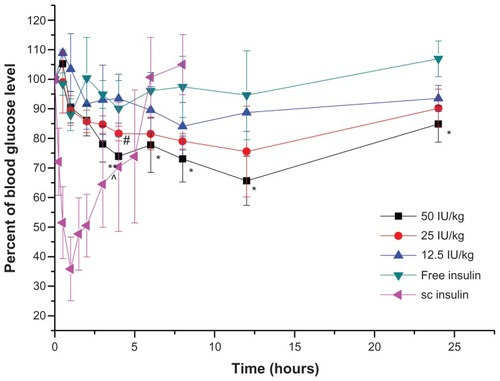
Figure 9 Plasma glucose level versus time profiles of GK rats after oral administration of free insulin solution, Alg/Chit-coated nanoemulsion 50 IU/kg, 25 IU/kg and 12.5 IU/kg, compared with sc 1 IU/kg insulin.
Notes: Mean ± SD, n = 6; *P < 0.05 and **P < 0.01 compared with control group.
Abbreviations: GK, Goto-Kakizaki; sc, subcutaneous.
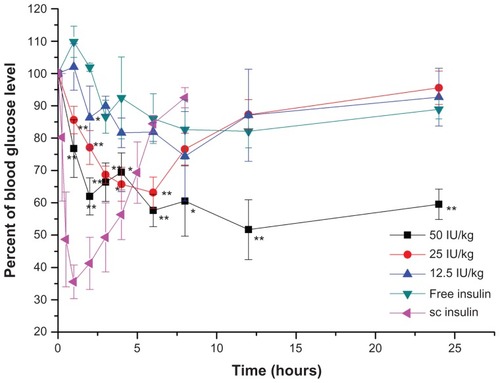
Correspondingly, with the increase in insulin dose, the minimum glucose level was lower and the area above the curve was larger. However, different dosage groups showed different oral pharmacological bioavailability. Compared with sc insulin, the relative pharmacological bioavailability of 12.5, 25, and 50 IU/kg was 8.65% ± 2.19%, 8.42% ± 1.05%, and 5.72% ± 0.55% in normal rats, respectively. Similarly, in diabetic rats, the relative pharmacological bioavailability of 25 and 50 IU/kg was 8.19% ± 0.58% and 7.84% ± 0.29%, respectively. The bioavailability of the high-dose group was lower than that of the low-dose group, which may be understood as follows: high absorption of insulin in the blood may induce an endogenous adjustment by which pancreatic islets will release glucagon to inhibit a glucose decrease.Citation22 Meanwhile, the excessive exogenous insulin may induce a body tolerance, which decreases the proportion of insulin absorbed and the hypoglycemic effects. Although the pancreatic islets of diabetic rats are defective, our model GK rats were genetically modified type 2 diabetic rats whose islets were partly damaged but could still secrete a little insulin and glucagon, albeit not normally.Citation32 Therefore, there were still differences in oral bioavailability with different dosages.
Conclusion
The present study explored the possibility of preparing an oral insulin formulation by combining the advantages of nanoencapsulation and lipid emulsion. The procedure was simple, without any organic reagent addition. Alg/Chit-coated nanoemulsion demonstrated great protection for insulin in simulated gastric media. In the in vivo animal experiments, the coated nanoemulsion lowered glucose levels of GK diabetic rats, at insulin doses of 25 and 50 IU/kg, by up to 60% and 50%, respectively, from their basal glucose level, and the bioavailability of insulin was 8.19% and 7.84%, respectively, which showed the good insulin intestinal absorption. These results suggest that the Alg/Chit-coated nanoemulsion might be developed as a promising approach for the oral delivery of therapeutic proteins.
Acknowledgments
We would like to give thanks for the financial supports received from the Shanghai Municipal Commission of Science and Technology (1052nm03600), the Shanghai Commission of Education (10SG05), the Ministry of Education (NCET-11-0114), and from the National Key Basic Research Program of China (2009CB930300).
Disclosure
The authors report no conflicts of interest in this work.
References
- SoodAPanchagnulaRPeroral route: an opportunity for protein and peptide drug deliveryChem Rev2001101113275330311840987
- LeeHJProtein drug oral delivery: the recent progressArch Pharm Res200225557258412433186
- BilatiUAllémannEDoelkerEStrategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticlesEur J Pharm Biopharm200559337538815760718
- Di MarcoMShamsuddinSRazakKAOverview of the main methods used to combine proteins with nanosystems: absorption, bioconjugation, and encapsulationInt J Nanomed201053749
- RamesanRMSharmaCPChallenges and advances in nanoparticle-based oral insulin deliveryExpert Rev Med Devices20096666567619911877
- ChengMBWangJCLiYHCharacterization of water-in-oil microemulsion for oral delivery of earthworm fibrinolytic enzymeJ Control Release20081291414818474405
- ShimizuMNakaneYEncapsulation of biologically active proteins in a multiple emulsionBiosci Biotechnol Biochem19955934924967537555
- SinghSSinghRVyasSPMultiple emulsion-based systems carrying insulin: development and characterizationJ Microencapsul19951266096158558383
- CilekACelebiNTirnaksizFLecithin-based microemulsion of a peptide for oral administration: preparation, characterization, and physical stability of the formulationDrug Deliv2006131192416401589
- CilekACelebiNTirnaksizFTayAA lecithin-based microemulsion of rh-insulin with aprotinin for oral administration: Investigation of hypoglycemic effects in non-diabetic and STZ-induced diabetic ratsInt J Pharm2005298117618515950411
- TalegaonkarSAzeemAAhmadFJKharRKPathanSAKhanZIMicroemulsions: a novel approach to enhanced drug deliveryRecent Pat Drug Deliv Formul20082323825719075911
- GhoshPKMajithiyaRJUmrethiaMLMurthyRSDesign and development of microemulsion drug delivery system of acyclovir for improvement of oral bioavailabilityAAPS Pharm Sci Tech20067377
- DogruSTCalisSOnerFOral multiple w/o/w emulsion formulation of a peptide salmon calcitonin: in vitro-in vivo evaluationJ Clin Pharm Ther200025643544311123497
- ZaunerWFarrowNAHainesAMIn vitro uptake of polystyrene microspheres: effect of particle size, cell line and cell densityJ Control Release2001711395111245907
- SarmentoBRibeiroAVeigaFSampaioPNeufeldRFerreiraDAlginate/chitosan nanoparticles are effective for oral insulin deliveryPharm Res200724122198220617577641
- NagpalKSinghSKMishraDNChitosan nanoparticles: a promising system in novel drug deliveryChem Pharm Bull (Tokyo)201058111423143021048331
- YinLDingJHeCCuiLTangCYinCDrug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin deliveryBiomaterials200930295691570019615735
- SarmentoBFerreiraDVeigaFRibeiroACharacterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studiesCarbohyd Polym200666117
- SarmentoBRibeiroAJVeigaFFerreiraDCNeufeldRJInsulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexationJ Nanosci Nanotech20077828332841
- ZhangYWeiWLvPWangLMaGPreparation and evaluation of alginate-chitosan microspheres for oral delivery of insulinEur J Pharm Biopharm2011771111920933083
- CournarieFSavelliMPRosilioVInsulin-loaded W/O/W multiple emulsions: comparison of the performances of systems prepared with medium-chain-triglycerides and fish oilEur J Pharm Biopharm200458347748215451521
- NiuMLuYHovgaardLHypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: The effect of cholate type, particle size and administered doseEur J Pharm Biopharm201281226527222369880
- NiuMLuYHovgaardLWuWLiposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradationInt J Nanomedicine201161155116621822379
- GåserødOJolliffeIGHampsonFCDettmarPWSkjåk-BrækGThe enhancement of the bioadhesive properties of calcium alginate gel beads by coating with chitosanInt J Pharm19981752237246
- ZhangNLiJJiangWEffective protection and controlled release of insulin by cationic beta-cyclodextrin polymers from alginate/chitosan nanoparticlesInt J Pharm20103931–2213219
- Yu-HsinLChiung-TongCHsiang-FaLNovel nanoparticles for oral insulin delivery via the paracellular pathwayNanotechnology20071810105102
- PockerYBiswasSBConformational dynamics of insulin in solution. Circular dichroic studiesBiochemistry19801922504350497006683
- LeonSSusannaWPAndrewYPhysiological Factors Related to Drug Absorption5th edNew YorkMcGraw-Hill Medical2004
- ThouzeauCPetersGLe BohecCLe MahoYAdjustments of gastric pH, motility and temperature during long-term preservation of stomach contents in free-ranging incubating king penguinsJ Exp Biol2004207Pt 152715272415201304
- DamgéCReisCPMaincentPNanoparticle strategies for the oral delivery of insulinExpert Opin Drug Deliv200851456818095928
- MaZLimTMLimLYPharmacological activity of peroral chitosan-insulin nanoparticles in diabetic ratsInt J Pharm20052931–227128015778065
- PorthaBLacrazGChaveyAIslet structure and function in the GK ratAdv Exp Med Biol201065447950020217511