Abstract
Multiresistance among microorganisms to common antimicrobials has become one of the most significant concerns in modern medicine. Nanomaterials are a new alternative to successfully treat the multiresistant microorganisms. Nanostructured materials are used in many fields, including biological sciences and medicine. Recently, it was demonstrated that the bactericidal activity of zero-valent bismuth colloidal nanoparticles inhibited the growth of Streptococcus mutans; however the antimycotic potential of bismuth nanostructured derivatives has not yet been studied. The main objective of this investigation was to analyze the fungicidal activity of bismuth oxide nanoparticles against Candida albicans, and their antibiofilm capabilities. Our results showed that aqueous colloidal bismuth oxide nanoparticles displayed antimicrobial activity against C. albicans growth (reducing colony size by 85%) and a complete inhibition of biofilm formation. These results are better than those obtained with chlorhexidine, nystatin, and terbinafine, the most effective oral antiseptic and commercial antifungal agents. In this work, we also compared the antimycotic activities of bulk bismuth oxide and bismuth nitrate, the precursor metallic salt. These results suggest that bismuth oxide colloidal nanoparticles could be a very interesting candidate as a fungicidal agent to be incorporated into an oral antiseptic. Additionally, we determined the minimum inhibitory concentration for the synthesized aqueous colloidal Bi2O3 nanoparticles.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Keywords:
Introduction
The occurrence of microorganisms living in cooperative, aggregated communities is called a biofilm. Biofilms can form on many kinds of surfaces and interfaces, including the human body.Citation1 The most common biofilms associated with human disease episodes are the fungi of the genus Candida, most notably Candida albicans, which causes both superficial and systemic disease. C. albicans biofilm is commonly found in children and elderly persons with a deficient immune system, and it is the etiological agent of oral moniliasis; the mortality of patients with invasive candidiasis can be as high as 40%.Citation2,Citation3 Fungal infections are difficult to eradicate, requiring high quantities and longtime exposure to an antimycotic agent to efficiently eliminate the etiological pathogen.Citation4
Multiresistance among pathogen microorganisms has become one of the most important problems in current medicine.Citation5 Since early reports in 1980, new evidence has suggested the emergence of multiresistant strains of Candida, suggesting that multiresistance is not an exclusive phenomenon of bacteria.Citation6,Citation7 The absence of new alternatives to efficiently treat these multiresistant microorganisms is a real problem, and it is urgent to synthesize new broad spectrum drugs to fight antimicrobial resistance.
Typically, bismuth is found as bismuthinite (bismuth sulfide), bismite (bismuth oxide [Bi2O3]), and bismuthite (bismuth carbonate).Citation8 In medicine, bismuth, in the form of bismuth subsalicylate, has been employed as an antidiarrheal to treat nauseas, vomiting, and stomach pain.Citation9
The chemistry of the binary oxides of bismuth is dominated by the +3 oxidation state. Most of the work on bismuth, concentrates on Bi2O3. Bi2O3 shows a distinctive polymorphism, including the following solid state phases: α-Bi2O3, β-Bi2O3, γ-Bi2 δ-Bi2O3, and the recently characterized ɛ-Bi2O3Citation10 The α-Bi2O3 is the most thermodynamically stable phase at room temperature and pressure. So, under standard reaction conditions in aqueous solutions, the α-Bi2O3 is formed a poorly water-soluble specie that carries surface hydroxyl groups.Citation11 α-Bi2O3 is a basic oxide and its Bi-O bonds are predominantly ionic;Citation12 it is a p-type semiconductor material.Citation10
Bi2O3 is a derivative of great technological importance, and it is used in the manufacture of glass and ceramic products, and also, as catalyst in the oxidation of hydrocarbons. It is widely used in applications, such as microelectronics, and sensor and optical technologyCitation13,Citation14
Nanoparticles (NPs) have large surface areas, and therefore, they have increased interactions with biological targets. We recently demonstrated the antibacterial effectiveness of zero-valent bismuth NPs at inhibiting the growth of Streptococcus mutans;Citation15 however; at the present time the antimycotic potential of bismuth nanostructured derivatives are not known. In this report, we present evidence of the antimycotic properties of Bi2O3 NPs against the growth of C. albicans and its capability to eliminate the fungal biofilm – the biocidal activity of Bi2O3 NPs was very similar to that obtained with chlorhexidine, a commonly used oral antiseptic and commercial antifungals as nystatin and terbinafine. Additionally, the C. albicans antifungal capacities of bulk Bi2O3 material and bismuth nitrate, the precursor salt used in the Bi2O3 colloidal NPs synthesis, are compared.
Materials and methods
Synthesis of Bi2O3 NP
For the synthesis of Bi2O3 nanoclusters (Bi2O3 NCs), the following chemical reagents were used: bismuth nitrate pentahydrate (Bi(NO3)3 5H2O), 98% (Sigma-Aldrich, St Louis, MO, USA); Bi2O3, 99.999% trace metals basis (Sigma-Aldrich); potassium hydroxide (KOH), 85% (Meyer, Tlahuac, Mexico City Mexico); argon (Ar), 99.998% (Praxair Inc, Danbury, CT, USA); and deionized water, 18.2 Ω (Milli Q®; Thermo Fisher Scientific, Waltham, MA, USA). These chemical substances were used as they were received.
We used a typical preparation method for the production of the Bi2O3 NP colloids, as follows: First, all water to be used in the synthesis was boiled and after cooling to room temperature, was bubbled with argon gas for 15 minutes in order to prevent carbonation. Subsequently, 0.0274 g of Bi(NO3)3 5H2O were dissolved in 24.5 mL of the prepared water. This solution was heated under vigorous magnetic stirring, at 80°C. Then, 0.5 mL of an aqueous solution of KOH 0.032 M were added to the Bi(NO3)3 5H2O solution. The final concentrations of bismuth and hydroxide ions were 2 × 10−3 M and 6 × 10−3 M, respectively. It is worth noting that during the entire process of dissolution and mixing of the reagents, a weak stream of argon was blowing over the surface of the reaction mixture. The newly synthesized yellowish Bi2O3 powder NPs were isolated by centrifugation at 14,000 rpm, for 30 minutes. Afterwards, they were washed with previously decarbonated water, three times. Finally, the powdered Bi2O3 NPs were once again resuspended in water and stored at room temperature for approximately 30 minutes, until their use. In order to prove the reproducibility of this synthesis method these colloids were prepared in triplicate.
Characterization of Bi2O3 NPs
The needle-shaped morphology of Bi2O3 NPs was determined by transmission electron (TEM) micrographs obtained using a JEOL 1200-EXII (JEOL Ltd Tokyo, Japan) of 40–120 KV. Single drops of the ethanol nanoparticle suspension were deposited onto 200 mesh copper grids coated with a carbon/collodion layer.
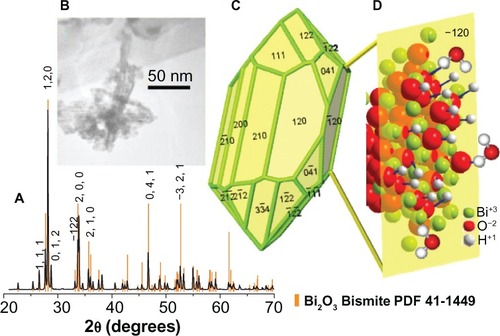
The Bi2O3 monoclinic phase identification was obtained from the X-ray diffraction pattern (PDF 41-1449 card), recorded on a Bruker D2-Phaser diffractometer (Bruker Corp, Billerica, MA, USA), using Cu Kα radiation, 1.5406 Å (30 kV, 10 mA). The crystallite size, 77 nm, was determined using the Scherrer equation. From the X-ray diffraction pattern and the TEM images, we observed needle-shaped crystallites, which indicated a textured material (in the 120 direction) ().
Figure 1 Structural and morphological characterization of Bi2O3 NPs, the crystalline habit, and the idealization of the hydroxyl ions and water molecules interactions with the −120 surface plane.
Notes: (A) Bi2O3 NPs X-ray diffraction pattern. This sample was synthesized in water, as described in the Materials and methods section. In this case, the observed peaks match with the α-phase (monoclinic) of Bi2O3. (B) TEM image of the Bi2O3 NPs clearly shows the needle shape exhibited by these nanoclusters. (C) This model shows the crystal structure of a representative particle, with the respective exposed planes. (D) The images shows a (−120) plane sketch, in particular, the enhanced interaction of bismuth atoms with hydroxyl ions and water molecules.
Abbreviations: Bi2O3, bismuth oxide; NP, nanoparticle; TEM, transmission electron microscope.
Antimycotic activity of Bi2O3 NPs against C. albicans growth
C. albicans (No 90029) was obtained from the American Type Culture Collection, (Manassas, VA, USA). The antifungal effect of Bi2O3 NPs on C. albicans growth was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Biotium Inc, Hayward CA, USA),Citation16,Citation17 following the instructions of the manufacturer. C. albicans was grown in Trypticase™ Soy Broth (TSB) (BD Biosciences, Franklin Lakes, NJ, USA) at 37°C, overnight in aerobic conditions. The fungal cells were counted, using a Neubauer chamber, and 1 × 10Citation4 cells were inoculated in 100 μL of TSB medium, in a 96-well polystyrene plate. Three wells with only TSB medium were used as controls for growth of C. albicans. Chlorhexidine 2% (Ultradent Products Inc, South Jordan, UT, USA) was used as a positive antimicrobial control; additionally, terbinafine 1% (Novartis Pharmaceuticals Corp, Basel, Switzerland) and commercial Bi2O3 were used as antifungal positive controls.
Hereinafter, the concentrations given refer to the initial concentration of the respective bismuth precursor. We used 50 μL of a 2 mM of Bi2O3 NP dispersion, in a total volume of 150 μL, to interfere with bacterial growth. As additional controls, 2 mM of Bi2O3 (bulk material) aqueous suspension and 2 mM of Bi(NO3)3 5H2O aqueous solution (the precursor bismuth salt), were added separately to cells, to compare the antifungal activities of the metallic salt, bulk material, and nanostructured samples. In this research, we avoided the use of organic solvents in order to prevent possible interference with the antifungal and cytotoxic properties of the Bi2O3 NPs.
The 96-well plate was incubated at 37°C overnight. Next, 10 μL of MTT was added to each well, and the plate was protected against light and incubated at 37°C for 2 hours. Next, 200 μL of dimethyl sulfoxide was added to dissolve the reduced MTT species. The amount of live cells was determined using a microplate absorbance reader (iMark™; Bio-Rad Laboratories, Philadelphia, PA, USA), at 595 nm. The experiments were repeated three times, and the measured optical density was analyzed by descriptive statistics.
Determination of minimal inhibitory concentration (MIC) of Bi2O3 NPs
The MIC was determined as described in a previous work.Citation18 Briefly, a 5 tube in the McFarland scale, with 1 × 109 CFU, was obtained (McFarland scale are fungal solutions. There are 1–9 ranges in this scale with a specific number of microbes in each one): C. albicans was grown in TSB agar and incubated at 37°C for 24 hours. One colony was inoculated in 5 mL of TSB medium and incubated at 37°C for 24 hours. The fungal count was determined with a Neubauer chamber. Tubes with a final concentration of 1 × 106 CFU were obtained by dilution of the 5 tube in the McFarland scale. The Bi2O3 NP suspension was diluted to final concentrations of 0.25, 0.5, 1, and 1.5, from the 2 mM stock. Then, 1 mL of each Bi2O3 NP dispersion was mixed with a fungal culture medium and then, incubated at 37°C for 18 hours. The MIC was determined by the presence or absence of turbidity in the different tubes containing the NPs.
Biofilm inhibitory activity of Bi2O3 NPs
The antibiofilm activity of the Bi2O3 NPs was determined by fluorescence microscopy, following the methodology described above. To observe the biofilm, SYTO® 9 green fluorescent nucleic acid stain (Life Technologies, Carlsbad CA, USA) was added to a final concentration of 20 μM.Citation19,Citation20 The 96-well plate was incubated for 30 minutes at room temperature and protected against light. The C. albicans biofilm was visualized with a Carl Zeiss Z1 Axio Inverter microscope (Carl Zeiss Meditec, Jena, Germany), at 485 nm.
Cytotoxicity of Bi2O3 NPs on culture cells by fluorescence microscopy
It is important to emphasize that we did not find any report concerning cytotoxicity of common bismuth nanostructured derivatives. We found one related publication describing the stability, cytotoxicity, and potential use of KBi(H2O)2[Fe(CN)6] 3H2O NPs – of course, this cyano species is extremely toxic.Citation21
In this research we used monkey kidney (Vero) cells to analyze the cytotoxic effect of Bi2O3 NPs. A confluent monolayer of Vero cells (CCL-81 ; ATTCC) was grown in minimal essential media (MEM) (Life Technologies) supplemented with 10% of Fetal Bovine Serum (FBS) (Life Technologies), at 37°C with 5% of CO2, in a Nunc® Lab-Tek® Chamber Slide™ System (16 wells; Thermo Fisher Scientific). This was exposed to 2 mM of Bi2O3 NPs for 24 hours, and the possible cytotoxic effect was detected by fluorescence microscopy. For the negative control, cells without NPs were employed. The Vero cells were washed three times with phosphate-buffered saline (PBS), and 4′,6-diamidino-2-phenylindole (DAPI) (Abcam Inc, Cambridge, UK) was added to stain the nucleus of cells.Citation22 Cytotoxicity was interpreted as the presence of a degraded or amorphous nucleus, observing at 365 nm with an inverter Carl Zeiss microscope.
Results
Synthesis and characterization of Bi2O3 NPs
The colloidal dispersions of Bi2O3 NPs prepared from 2 × 10−3 M Bi(NO3)3 5HO and 6 × 10−3 M of KOH in deionized water yielded a fine beige powder with an approximate crystallite size of 77 nm. The X-ray diffraction pattern of the Bi2O3 NP powder showed the presence of α-phase nanocrystallites and the absence of other possible compounds (like carbonated elements) generated during their synthesis (). The synthesized Bi2O3 NPs remained stable at room temperature for 15 days, and no visible trace of carbonation was observed since the suspended particles in water kept their yellowish color. This established that the antimycotic and antibiofilm properties were associated only with the presence of Bi2O3 NPs.
Antimycotic activity of Bi2O3 NPs against C. albicans growth
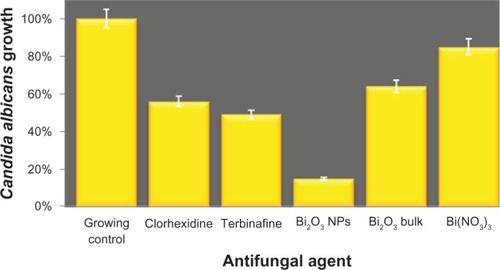
To explore the possible antifungal activity of Bi2O3 NPs, their effect on C. albicans growth was determined. The results showed that Bi2O3 NPs reduced the number of fungi by 85%, in comparison with the control fungi grown in medium. At the same time, the treatment with 2% chlorhexidine and 1% terbinafine (inhibition controls) showed a 44% and 51% of reduction in the number of fungi, respectively, when compared with nontreated cells. The antifungal effect of Bi2O3 NPs was two times better when compared with the bulk Bi2O3 material (), suggesting that nanostructured Bi2O3 is more effective as an antifungal agent.
Figure 2 Antifungal activity of bismuth oxide nanoparticles against Candida albicans growth.
Notes: The y axis shows the optical density units of C. albicans growth. C. albicans culture without inhibitor was used as the growth control, whereas chlorhexidine 2%, terbinafine 1%, Bi2O3 (bulk material), and Bi(NO3)3 · 5H2O were used as positive inhibition controls. For these experiments, we used a concentration of 2 mM of the respective bismuth species.
Abbreviations: Bi2O3, bismuth oxide; Bi(NO3)3 · 5H2O, bismuth nitrate pentahy-drate; NP, nanoparticle.
Determination of minimal inhibitory concentration of Bi2O3 NPs
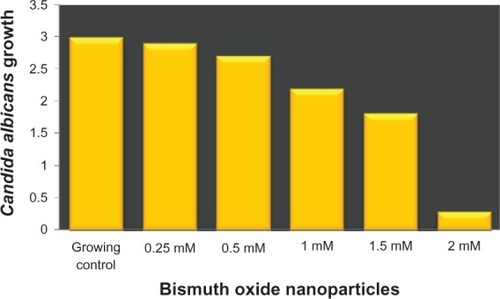
In order to characterize the antifungal activity of Bi2O3 NPs, we determined their MIC. The result obtained was 1.5 mM (). This result is important to know as it represents the minimal quantity of Bi2O3 NPs that is required to effectively interfere with C. albicans growth.
Figure 3 MIC of bismuth oxide nanoparticles against Candida albicans growth.
Notes: The y axis shows the optical density units of C. albicans growth; the x axis shows the different concentrations of Bi2O NPs analyzed. C. albicans culture without inhibitor was used as a growth control. Experiments were carried out three times and each time by triplicate. Average results were obtained from the three independent experiments.
Abbreviations: Bi2O3, bismuth oxide; MIC, minimal inhibitory concentration; NP, nanoparticle.
Biofilm inhibitory activity of Bi2O3 NPs
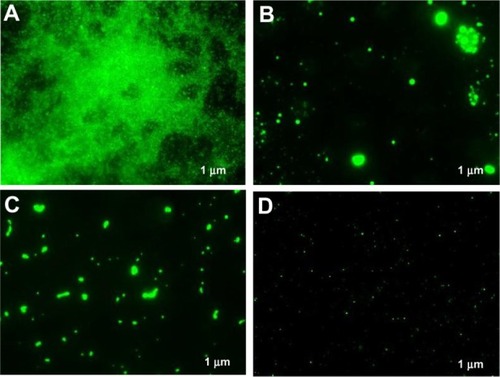
In the previous experiment, we measured the antimycotic activity of Bi2O3 NPs. In order to analyze the possible biofilm inhibition of C. albicans by bismuth nanoclusters, the antibiofilm activity of Bi2O3 NPs was determined by fluorescence microscopy. The results showed a complete inhibition of biofilm formation by chlorhexidine (), terbinafine (), and Bi2O3 NPs (), compared with the control (). The results did not change when Bi2O3 NPs were added at different postinoculation times – we tested the biofilm inhibitory activity at 6 and 18 hours postinoculation, obtaining similar results (data not shown). These data indicate that Bi2O3 NPs have an antibiofilm activity that is as effective as chlorhexidine and terbinafine.
Figure 4 Inhibition of Candida albicans biofilm detected by fluorescence microscopy, after 24 hours. As a growth control, C. albicans was added to culture media; chlorhexidine 2% and terbinafine 1% were employed as positive inhibition controls. In these experiments, we used a concentration of 2 mM Bi2O3 NPs. (A) growth control; (B) chlorhexidine; (C) terbinafine; and (D) Bi2O3 NPs.
Note: The bar indicates 1 μm.
Abbreviations: Bi2O3, bismuth oxide; NP, nanoparticle.
Cytotoxicity of Bi2O3 NPs in culture cells, by fluorescence microscopy
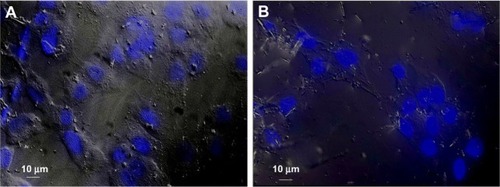
The cytotoxic effect of Bi2O3 NPs was evaluated in monkey kidney (Vero) cells by fluorescence microscopy. The results showed that Bi2O3 NPs did not promote cytotoxic effects in the Vero cells at 24 hours of exposure compared with the cells without NP. The nuclei and, indeed, all the cells looked very similar in the presence or absence of Bi2O3 NPs (). These results suggest the absence of cytotoxicity by Bi2O3 NPs, under our experimental conditions.
Figure 5 Cytotoxicity of bismuth oxide nanoparticles detected by fluorescence microscopy. The possible cytotoxic effect of bismuth oxide nanoparticles was evaluated in monkey kidney cells by staining the nuclei with DAPI and visualizing cell morphology with DIC. As positive control, Vero cells were used. (A) Positive control and (B) Vero cells, after interacting with bismuth oxide nanoparticles, for 24 hours.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; DIC, differential interference contrast microscopy.
Discussion
In this study, we presented evidence of the antimycotic activity of Bi2O3 NPs. Their efficacy in inhibiting C. albicans growth was significantly better than that of chlorhexidine, nystatin, and terbinafine. These results indicate that Bi2O3 NPs are better antifungal agents than the most commonly used oral antiseptic and commercial antifungal agents. To be assured that the antifungal effect was due to the nanostructured Bi2O3, this was compared with the antifungal effect of bulk Bi2O3. The Bi2O3 NPs were two times more effective than the polycrystalline Bi2O3 in inhibiting fungal growth. This result was very important because it demonstrated the effectiveness of the nanostructured material versus the same bulk compound. Bismuth salicylate, a typical molecular bismuth derivative, has been employed to treat fungal infections but was not shown to inhibit the fungal growth,Citation23 suggesting that it is the nanostructured particle of Bi2O3 that is conferred with the antimicrobial properties described earlier.Citation15 These results suggest that Bi2O3 NPs could be very interesting antifungal agents. Previously, it was reported that zinc, titanium, and silver NPs have very good antifungal activity;Citation24–Citation26 however, it is not possible to establish any quantitative antibiofilm activity comparison, due to the type of published results.
Inorganic nanostructures have many applications in fields like the biological sciences and medicine. NPs have been applied as coating materials and in treatments and diagnosis.Citation27 NPs of titanium dioxide, silver, diamonds, iron oxides, carbon nanotubes, and biodegradable polymers have all been studied for their use in diagnosis and treatments. NPs of silver, copper oxides, and selenium have been reported to have antimicrobial activity.Citation28–Citation30 The advantages of inorganic NPs are their high surface-to-volume ratios, different shapes, many structural defects, (the presence of irregularities in the crystal lattice of the nanostructured bismuth oxide) and of course, their nanoscale size, which allows more active sites to interact with biological systems such as bacteria, fungi, and viruses. This is the most important difference between NPs and the typical organic molecular antimicrobial agents, and could minimize the risk of developing antimicrobial resistance.
The mechanism of the antimicrobial activity of inorganic NPs is not completely understood, and their precise mechanism of action against bacteria and fungi remains to be fully elucidated. It has been shown that positive charges on the metal ion are critical for the antimicrobial activity, allowing for the electrostatic attraction between a negatively charged cell membrane and the positively charged NPs.Citation31 It has also been reported that silver NPs can damage DNA, alter gene expression, and affect the membrane-bound respiratory enzymes.Citation32–Citation34
As previously mentioned, Bi2O3 has a marked basic character, and when this compound is suspended in water, it tends to be associated with hydroxyl ions and water molecules on its surface, similar to titanium dioxide, zinc oxide, tin dioxide, and other metal oxides.Citation35–Citation39 When Bi2O3 is nanostructured, its ionic character is enhanced compared with the same bulk material, ie, it becomes a “harder” species with respect to its electrons (according to Pearson’s theory of hard and soft acids and bases).Citation40 As a consequence, when Bi2O3 is nanostructured, its dissociating character increases, as does the overall surface of the material. Therefore, the amount of OH−1 ions and water molecules bound to the surfaces of the nanocrystallites is higher than in the same bulk material, as is sketched in (). Thus, Bi2O3 NPs dispersed in water are a chemical species with a highly negative surface electric potential; subsequently, these nanostructured species are strongly basic aggregation points, conferring them with a lethal character for the immersed C. albicans fungi. It has been reported that several microorganisms are very sensitive to alkaline media, among them, C. albicans.Citation41 We suggest that this is the basis for the fungicidal character of Bi2O3 nanoclusters. Additionally, we cannot discard antifungal activity mechanisms involving free radicals, due to the presence of CO2 in the culture mediaCitation42 and the inherent α-Bi2O3 NPs photocatalytic properties.Citation43 However, more experimental evidences are needed to support these hypotheses.
In parallel to their antifungal activity, the Bi2O3 NPs had the potential to interfere with biofilm formation of C. albicans. This antibiofilm activity of the Bi2O3 NPs was also studied. Surprisingly, the antibiofilm formation effect was complete in the media with the Bi2O3 NPs. This effect was unexpected since Bi2O3 NPs only reduced cell growth and did not completely inhibit it. To explain this observation, we suggest that 85% of cells were inactivated during inoculation with Bi2O3 NPs and that surviving cells were insufficient to build a biofilm. It may be that they went into planktonic and stress states due the presence of the Bi2O3 NPs, and it is possible that they were lost during the wash of dye excess. In the presence of chlorhexidine and Bi2O3 NPs, we only observed (via fluorescence microscopy) cellular debris on a dark background – mainly DNA of dead fungus with accumulates of dye. Morphologically, these dye accumulates clearly differed from fungal biofilm.
It is noteworthy that there are published reports of fungal growth inhibition, including inhibition of C. albicans, where inorganic NPs, such as diamond-, silver-, gold-, platinum-, and palladium-based NPs, were used as antifungal agents.Citation44 Particularly, silver (Ag) NPs have also been shown to inhibit yeast growth,Citation31 and their antifungal activity against certain species of CandidaCitation45 is well documented. These studies show evidence for the molecular mechanism of Ag NPs activity, whereby Ag NPs act on and inhibit a number of oxidative enzymes, such as yeast alcohol dehydrogenase, through the generation of reactive oxygen species.Citation31 Ag NPs have been demonstrated to exhibit a desirable and promising antifungal effect in several studies, with no serious side effects to the host.Citation46 However, the results and the experimental conditions used in the aforementioned studies are not comparable with those of this research.
The possible cytotoxic effect of Bi2O3 NPs was explored in monkey kidney (Vero) cells by fluorescence microscopy. No change was detected in the morphology of the nuclei or, indeed, of all cells in the presence or absence of Bi2O3 NPs, suggesting the lack of cytotoxic effect after 24 hours of exposure. Earlier, the toxicity of metal oxide NPs, mainly Ag oxide (Ag2O), zinc oxide (ZnO), and iron oxide (Fe2O3),Citation47 was reported. The possible toxicity of Bi2O3 NPs was analyzed using the same concentration of NPs as was employed in those antifungal assays. The preliminary results of this study reveal no cytotoxicity of colloidal Bi2O3 NPs, at least under the experimental conditions previously described. This lack of cytotoxicity can be attributed to the very small amount of inorganic nanomaterial that interacted with the Vero cells. The total Bi2O3 nanocluster mass and doses of 0.15 μg/mL introduced were not enough to cause cell damage.
For particle toxicity, three factors are crucial: size, shape, and chemical composition. A reduction in the size of a nanosized particle results in an increase in the specific surface area of the nanostructured powder. Therefore more chemical species may attach to its surface, which enhances its reactivity and results in an increase in its toxic effects.Citation48,Citation49
In this case, the colloidal Bi2O3 NPs had a relatively small size; however, the number of nanoclusters introduced into the Vero cell culture medium, was significantly low compared with the total loads of the other two derivatives of bismuth (Bi2O3 in bulk form and bismuth nitrate).
In this work we focused on the effectiveness of Bi2O3 NPs in inhibiting the growth of C. albicans. All together, the experimental data suggest that Bi2O3 NPs could be an interesting alternative to combat the fungal infections at the origin of biofilms. The property of Bi2O3 NPs could be used in oral health, supporting the antifungal activity of oral antiseptics. Further experiments will be necessary to determine the possible toxicity of Bi2O3 NPs and analyze the genotoxicity (apoptosis, DNA fragmentation, DNA damage) in human fibroblasts cultures and analyze their potential use in humans.
In conclusion, Bi2O3 NPs of 77 nm average size have an antimycotic activity inhibiting the growth of C. albicans, as well as an antibiofilm activity. This behavior is different from the one of the corresponding bulk material; so, the observed activity is a size-dependent property, an explanation that was proposed first time. Additionally, our results suggest that the Bi2O3 NPs, under the experimental tested conditions and concentrations, do not exhibit cytotoxicity.
Acknowledgments
The authors wish to thank Vilma Suarez-Martinez and Myriam De la Garza-Ramos, from FO UANL, for support in growing the C. albicans, and Ernesto Torres, from FM UANL, for providing the Vero cell line. Donaji Velasco-Arias and Rene Hernandez-Delgadillo thank CONACyT for the Ph D scholarships. Claudio Cabral-Romero wishes to thank the Research Department-UANL for financial support (Paicyt CN-775-11) and CONACyT for financing the project (No 183825). Also, David Diaz wants to express his gratitude to DGAPA-UNAM and CONACyT, for their financial support (projects PAPIIT IN 101009 and SEP-CB-132094, respectively). Finally, Inti Zumeta-Dubé is grateful for the postdoctoral fellowship award from UE-CONACyT, BisNano project.
Disclosure
The authors report no conflicts of interest in this work.
References
- CostertonJWOverview of microbial biofilmsJ Ind Microbiol19951531371408519468
- ChandraJKuhnDMMukherjeePKHoyerLLMcCormickTGhannoumMABiofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistanceJ Bacteriol2001183185385539411514524
- ReyesAReyesACStudies on Candida albicans infection in Filipino children. II. Prevalence of oral moniliasisActa Med Philipp1956–1957131–415115513508064
- FortúnJAntifungal therapy update: new drugs and medical usesEnferm Infecc Microbiol Clin201129Suppl 53844 Spanish22305668
- FalagasMEFragoulisKNKarydisIA comparative study on the cost of new antibiotics and drugs of other therapeutic categoriesPloS One20061e1117183637
- MarchaimDLemanekLBheemreddySKayeKSSobelJDFluconazole-resistant Candida albicans vulvovaginitisObstet Gynecol201212061407141423168767
- SasseCDunkelNSchäferTThe stepwise acquisition of fluconazole resistance mutations causes a gradual loss of fitness in Candida albicansMol Microbiol201286353955622924823
- KirkREOthmerDFEncyclopedia of Chemical Technology5th edHobokenJohn Wiley and Sons Inc2004
- Figueroa-QuintanillaDSalazar-LindoESackRBA controlled trial of bismuth subsalicylate in infants with acute watery diarrheal diseaseNew Engl J Med199332823165316588487823
- MehringMFrom molecules to bismuth oxide-based materials: Potential homo- and heterometallic precursors and model compoundsCoord Chem Rev20072517–89741006
- CoxPAThe Elements: Their Origin, Abundance, and DistributionOxfordOxford University Press1989
- EarnshawAGreenwoodNChemistry of the Elements1st edPergmon Press LtdOxford England1984
- AdamianZNAbovianHVAroutiounianVMSmoke sensor on the base of Bi2O3 sesquioxideSens Actuators B Chem1996351–3241243
- LeontieLCaramanMAlexeMHarnageaCStructural and optical characteristics of bismuth oxide thin filmsSurface Science2002507510480485
- Hernandez-DelgadilloRVelasco-AriasDDiazDZerovalent bismuth nanoparticles inhibit Streptococcus mutans growth and formation of biofilmInt J Nanomedicine201272109211322619547
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- LiuYPetersonDAKimuraHSchubertDMechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reductionJ Neurochem19976925815939231715
- AndrewsJMDetermination of minimum inhibitory concentrationsJ Antimicrob Chemother200148Suppl 151611420333
- YueHEastmanPSWangBBAn evaluation of the performance of cDNA microarrays for detecting changes in global mRNA expressionNucleic Acids Res2001298E41E4111292855
- FreyTNucleic acid dyes for detection of apoptosis in live cellsCytometry19952132652748582249
- PereraVSHaoJGaoMNanoparticles of the novel coordination polymer KBi(H2O)2[Fe(CN)6] H2O as a potential contrast agent for computed tomographyInorg Chem201150177910791221797245
- KubistaMAkermanBNordénBCharacterization of interaction between DNA and 4′,6-diamidino-2-phenylindole by optical spectroscopyBiochemistry19872614454545533663606
- AlharbiSAMashatBHAl-HarbiNAWainwrightMAloufiASAlnaimatSBismuth-inhibitory effects on bacteria and stimulation of fungal growth in vitroSaudi J Biol Sci2012192147150
- LipovskyANitzanYGedankenALubartRAntifungal activity of ZnO nanoparticles – the role of ROS mediated cell injuryNanotechnology2011221010510121289395
- TatlıdilİSökmenMBreenCCleggFBurukCKBacaksızEDegradation of Candida albicans on TiO2 and Ag-TiO2 thin films prepared by sol–gel and nanosuspensionsJournal of Sol-Gel Science Technology20116012332
- HwangISLeeJHwangJHKimKJLeeDGSilver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicalsFEBS J201227971327133822324978
- ColvinVLThe potential environmental impact of engineered nanomaterialsNat Biotechnol200321101166117014520401
- SondiISalopek-SondiBSilver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteriaJ Colloid Interface Sci2004275117718215158396
- RenGHuDChengEWVargas-ReusMAReipPAllakerRPCharacterisation of copper oxide nanoparticles for antimicrobial applicationsInt J Antimicrob Agents200933658759019195845
- TranPAWebsterTJSelenium nanoparticles inhibit Staphylococcus aureus growthInt J Nanomedicine201161553155821845045
- KimJSKukEYuKNAntimicrobial effects of silver nanoparticlesNanomedicine2007319510117379174
- FengQLWuJChenGQCuiFZKimTNKimJOA mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureusJ Biomed Mater Res200052466266811033548
- YamanakaMHaraKKudoJBactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysisAppl Environ Microbiol200571117589759316269810
- BraggPDRainnieDJThe effect of silver ions on the respiratory chain of Escherichia coliCan J Microbiol19742068838894151872
- XuJJChenMDFuDGPreparation of bismuth oxide/titania composite particles and their photocatalytic activity to degradation of 4-chlorophenolTransactions of Nonferrous Metals Society of China2011212340345
- LindanPJDWater chemistry at the SnO2(110) surface: the role of inter-molecular interactions and surface geometryChem Phys Lett20003284–6325329
- BatzillMBergermayerWTanakaIDieboldUTuning the chemical functionality of a gas sensitive material: Water adsorption on SnO2(101)Surf Sci20066004L29L32
- DuncanDAAllegrettiFWoodruffDPWater does partially dissociate on the perfect TiO2 (110) surface: A quantitative structure determinationPhys Rev B2012864045411
- NoeiHQiuHWangYLöfflerEWöllCMuhlerMThe identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopyPhys Chem Chem Phys200810477092709719039343
- PearsonRGChemical hardness and density functional theoryJ Chem Sci20051175369377
- Guerreiro-TanomaruJMCornélioALAndolfattoCSallesLPTanomaru-FilhoMpH and antimicrobial activity of Portland cement associated with different radiopacifying agentsISRN Dent2012201246901923119173
- MedinasDBCerchiaroGTrindadeDFAugustoOThe carbonate radical and related oxidants derived from bicarbonate bufferIUBMB Life2007594–525526217505962
- MuruganandhamMAmuthaRLeeGJHsiehSHWuJJSillanpääMFacile fabrication of tunable Bi2O3 self-assembly and its visible light photocatalytic activityJ Phys Chem C2012116231290612915
- ChwalibogASawoszEHotowyAVisualization of interaction between inorganic nanoparticles and bacteria or fungiInt J Nanomedicine201051085109421270959
- KimKJSungWSMoonSKChoiJSKimJGLeeDGAntifungal effect of silver nanoparticles on dermatophytesJ Microbiol Biotechnol20081881482148418756112
- SardiJCScorzoniLBernardiTFusco-AlmeidaAMMendes GianniniMJCandida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic optionsJ Med Microbiol201362Pt 1102423180477
- KumarVKumariAGuleriaPYadavSKEvaluating the toxicity of selected types of nanochemicalsRev Environ Contam Toxicol20122153912122057930
- LinkovISatterstromFKCoreyLMNanotoxicology and nanomedicine: making hard decisionsNanomedicine20084216717118329962
- SuhWHSuslickKSStuckyGDSuhYHNanotechnology, nanotoxicology, and neuroscienceProg Neurobiol200987313317018926873