Abstract
A new organic-inorganic nanohybrid based on zinc-layered hydroxide intercalated with an anti-inflammatory agent was synthesized through direct reaction of salicylic acid at various concentrations with commercially available zinc oxide. The basal spacing of the pure phase nanohybrid was 15.73 Å, with the salicylate anions arranged in a monolayer form and an angle of 57 degrees between the zinc-layered hydroxide interlayers. Fourier transform infrared study further confirmed intercalation of salicylate into the interlayers of zinc-layered hydroxide. The loading of salicylate in the nanohybrid was estimated to be around 29.66%, and the nanohybrid exhibited the properties of a mesoporous-type material, with greatly enhanced thermal stability of the salicylate compared with its free counterpart. In vitro cytotoxicity assay revealed that free salicylic acid, pure zinc oxide, and the nanohybrid have a mild effect on viability of African green monkey kidney (Vero-3) cells.
Introduction
Development of drug delivery systems that can effectively elicit the intended therapeutic effect in patients with minimal adverse reactions has been widely explored in recent years. This research was mainly catalyzed by the challenges in attaining satisfactory pharmacokinetic profiles using conventional drug administration methods.Citation1 The emergence of nanomedicine, a convergence between nanoscience and medicine, has markedly potentiated the revolution in delivery of therapeutic compounds through fabrication of ingenious nanovehicles.Citation2–Citation6
Application of inorganic nanoparticles in drug delivery systems has garnered considerable attention because they possess versatile attributes, including wide availability, good biocompatibility, and rich surface functionality.Citation4 Targeted delivery of a drug molecule to the desired site of action is also made achievable by use of nanoparticles as carriers, which has been found to be exceptionally beneficial in studies involving delivery of chemotherapeutic agents in cancer therapy.Citation7 In addition, nanoparticle-based drug delivery systems can slowly release the carried drugs in order to maintain concentrations at the desired levels for an extended period of time.Citation8 To date, a multitude of inorganic nanoparticles has been extensively employed in drug delivery studies, including iron oxide,Citation9,Citation10 silica nanoparticles,Citation11 calcium phosphate or hydroxyapatite,Citation12,Citation13 gold nanoparticles,Citation14 carbon nanotubes,Citation15 dendrimers,Citation16 layered double hydroxides,Citation17–Citation19 and quantum dots.Citation20,Citation21
Layered double hydroxides (LDHs) are regarded as promising drug delivery carriers because of several notable characteristics. In addition to ease of laboratory preparation and precise tailoring for the required physical and chemical properties,Citation4 LDHs can be versatile carriers because various drugs can be intercalated into the interlayer spacing through ion exchange, and their degradation in an acidic cellular environment liberates the carried drugs in their ionic form, which helps improve their solubility and bioavailability.Citation22 Improved cellular uptake of the drug-LDH nanohybrid has been reported,Citation23 possibly because of its high zeta potential that provides a strong driving force to the surface of a cell.Citation24 LDHs are also excellent candidates for drug delivery systems because they possess good biocompatibility and low cytotoxicity.Citation25–Citation27
LDHs are hydrotalcite-like materials that can be readily synthesized through introduction of base to a suitable mixture of metal salts.Citation28 The structure of the LDH is based on individual layers of metal cations octahedrally arranged with hydroxide groups, in which each hydroxide is surrounded by three metal cations, creating neutral layers. Isomorphic replacement of divalent cations with trivalent cations creates an excess of charge in the layers, which is neutralized by the interlayer anions. It can be represented by the general hydrotalcite formula, [M2+1−x M3+x (OH) 2] x+ (Am−)x/m] · nH2O, where M2+ is divalent cations, M3+ is trivalent cations, and Am− is an exchangeable anion with an m− charge.Citation29 Zinc-layered hydroxide (ZLH) is another type of material made up of brucite-like layers. The layers are modified such that one quarter of the octahedral sites are vacant. Tetrahedrally coordinated zinc cations are located at the up and down side of the vacant octahedra, while the tetrahedra are formed by three hydroxide groups of the brucite-like layers and a water molecule.Citation30,Citation31 The structure can be represented by the general formula M2+ (OH)2−x(Am−)x/m · nH2O, where M2+ is the Zn2+ and Am− is the counter anion with an m− charge.Citation32
Similar to LDH, the use of ZLH as a potential delivery vector is currently being studied extensively. Many successful intercalations have been reported with various therapeutic compounds, including conjugated linoleic acid,Citation33 indole-3-acetic acid,Citation34 gallic acid,Citation35 ascorbic acid, retinoic acid, and α-tocopherol.Citation36
Salicylic acid, also known as 2-hydroxybenzoic acid, is a nonsteroidal anti-inflammatory agent commonly used in the treatment of arthritis. Its use is often associated with adverse effects, including formation of gastric and duodenal ulcers,Citation37 so developing a carrier that might alleviate such effects is desirable. Intercalation of salicylate into different LDH has previously been reported,Citation38–Citation40 but no report on the use of ZLH as the carrier could be found in the literature. In addition, very little information is available on the use of zinc oxide (ZnO) as the starting material for synthesis of a drug-intercalated nanohybrid.Citation24,Citation41 Therefore, to illustrate the use of ZnO for intercalation of organic compounds with therapeutic effects, this study focused on the formation of a nanohybrid compound consisting of an anti-inflammatory active agent, salicylate, and using ZnO as the starting material. The synthesized nanohybrid was later subjected to a cytotoxicity study using in vitro cells.
Materials and methods
Materials
Salicylic acid (98% purity) was purchased from HmbG Chemicals (Hamburg, Germany) and used as received. ZnO of American Chemical Society Standard reagent grade was purchased from Acros Organics (Geel, Belgium) and used without further purification. Deionized water and ethanol were used in all experiments. Dulbecco’s modified Eagle’s medium was purchased from PAA Laboratories (Pasching, Austria), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrasodium bromide (MTT) from Invitrogen (Carlsbad, CA), and used as received. Dimethylsulfoxide of tissue culture grade was purchased from Sigma-Aldrich (St Louis, MO) and used without further purification.
Preparation of salicylate nanohybrids
Salicylate-zinc layered hydroxide nanohybrids (SZNs) were synthesized using ZnO as the starting material by a direct method reported previously.Citation24 Salicylic acid solution (0.1–1.6 mol/L) was prepared by dissolving the appropriate amount of salicylic acid in ethanol, followed by stirring at ambient temperature for 10 minutes. ZnO (0.2 g) was mixed with the solution of salicylic acid and stirred vigorously until the solution became clear. The pH was adjusted to 8.0 using an aqueous solution of NaOH (1.0 mol/L) and vigorously stirred further for 4 hours at ambient temperature. The resulting white precipitate was matured at 70°C in an oil bath shaker for 18 hours. The precipitate was centrifuged and washed thoroughly with deionized water and dried in an oven at 60°C. The powdered material was stored in a sample bottle placed in a container with desiccant for further use and characterization.
Characterization
Powder X-ray diffraction patterns were recorded at 2–60 degrees on a diffractometer (XRD-6000, Shimadzu, Tokyo, Japan) using CuKα radiation at 40 kV and 30 mA. Fourier transform infrared (FTIR) spectra of the materials were recorded over the range of 400–4000 cm−1 on an FTIR spectrophotometer (1725X, Perkin-Elmer, Waltham, MA) using a KBr disc method. Analysis of carbon, hydrogen, nitrogen, and sulfur (CHNS) content in the materials was performed using a Leco model CHNS-932 analyzer (St Joseph, MI), while elemental analysis was performed using inductively-coupled plasma atomic emission spectroscopy (Optima 2000DV, Perkin-Elmer). Thermogravimetric and differential thermogravimetric analysis (TGA-DTG) was carried out using a thermogravimetric analyzer (Mettler Toledo, Greifensee, Switzerland). Surface characterization of the materials was done using a nitrogen gas adsorption-desorption technique at 77 K (ASAP 2000, Micromeritics, Norcross, GA). The surface morphology of the materials was observed using a field emission scanning electron microscope (Nova NanoSEM 230, Hillsboro, OR).
MTT cytotoxicity assay
The cytotoxicity of the synthesized material was determined using the MTT assay. African green monkey kidney (Vero-3) cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum at 37°C with 5% CO2. The cells (5 × 104 cells/mL) were seeded into a 96-well plate and incubated for 24 hours at 37°C with 5% CO2. Test samples were diluted in Dulbecco’s modified Eagle’s medium and 200 μL was added into each well. After 48 hours of incubation, MTT reagent (0.5 mg/mL) in phosphate-buffered saline was added into each well and incubated further for 4 hours at 37°C. The MTT solution was removed, and 100 μL of dimethylsulfoxide was added into each well in order to dissolve formazan crystals. The plate was then analyzed under a microtiter plate reader at 570 nm.
Results and discussion
X-ray diffraction
shows the diffraction patterns of SZN synthesized using various concentrations of salicylic acid (0.1–1.6 mol/L). For ZnO, five intense reflections at 30–60 degrees corresponded to diffractions of 100, 002, 101, 102, and 110 planes, all of which are typical of ZnO.Citation42 The diffraction patterns also revealed that the solid had good crystallinity. Intercalation of anions within the interlayer spacing of ZLH would result in an increment in the basal spacing because of displacement of exchangeable interlayer anions. Inclusion of anions in the ZLH interlayers was also reflected by the presence of peaks at a lower 2θ value, as shown in the diffraction patterns of the SZN nanohybrid. The basal spacing was recorded at 15.78 Å, 15.95 Å, 15.73 Å, and 15.98 Å for 0.2 mol/L, 0.4 mol/L, 0.8 mol/L, and 1.6 mol/L salicylic acid, respectively. Disappearance of the intense ZnO peaks also indicated that the solid was successfully converted into ZLH. No intercalation took place using 0.1 mol/L salicylic acid.
Figure 1 Powder X-ray diffraction patterns for zinc oxide and SZN prepared at various concentrations of salicylic acid.
Note: (·) indicates unknown peak generated by the diffractometer.
Abbreviation: SZN, salicylate-zinc layered hydroxide nanohybrid.
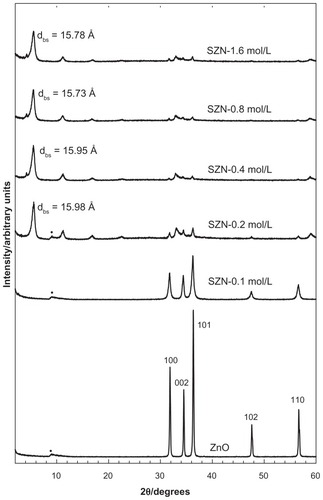
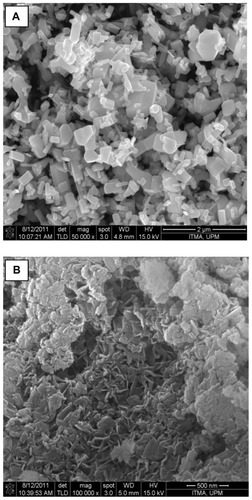
Comparison of the powder X-ray diffraction patterns showed that SZN synthesized using 0.8 mol/L salicylic acid was of relatively purer phase. In contrast, the other three nanohybrids still contained ZnO phase, with SZN synthesized using 0.2 mol/L salicylic acid. exhibiting the highest ZnO intensity. Therefore, the former purer-phase nanohybrid was used for further analysis and labeled as SZN 0.8 mol/L. Formation of a pure-phase nanohybrid could possibly be hampered by use of nonuniform granular-structured ZnO particles (). It is postulated that the larger ZnO particles hindered complete intercalation taking place over a given period of reaction time, thereby resulting in the remainder, ie, unreacted ZnO phase.Citation43
The formation of SZN is believed to have occurred through a dissociation-deposition mechanism. In aqueous conditions, ZnO hydrolyzed into Zn(OH)2 on the surface of the solid particle. The resulting Zn(OH)2 then dissociated at the solution-solid interface to form Zn2+ species, which then reacted with hydroxyls, salicylate anions, and water in solution to generate the layered SZN nanohybrid compound.Citation44
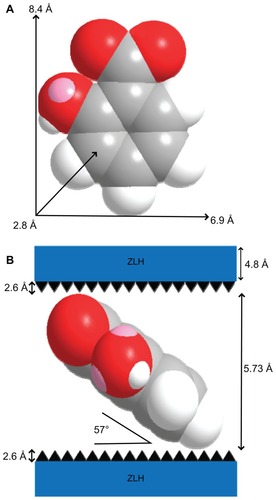
Intercalation of salicylate organic moieties into the interlayer spaces between the metal hydroxide layers resulted in an expansion of the basal spacing, caused by a larger molecular size of salicylate as well as the spatial arrangement of the anions in the intergallery space. shows the three-dimensional size of salicylic acid, as estimated by Chemoffice Ultra 2008 software (Cambridge, MA). The long and short axes and molecular thickness of salicylic acid are 8.4 Å, 6.9 Å, and 2.8 Å, respectively (). Subtracting the thickness of the ZLH inorganic layer (4.8 Å)Citation36,Citation45 and the zinc tetrahedra (2.6 Å each)Citation46 from the d-spacing of SZN obtained from the powder X-ray diffraction spectrum (15.73 Å), the gallery height available for salicylate occupation was 5.73 Å. Because this value was smaller than the dimension of salicylic acid along the y axis and x axis, and bigger than the thickness of two molecules of salicylic acid, the most plausible arrangement is when salicylate anions are arranged as a monolayer with an angle of 57 degrees between the ZLH interlayers, with the carboxylate groups positioned towards the ZLH layers ().
FTIR spectroscopy and elemental analysis
FTIR spectra for ZnO, salicylic acid, and the SZN 0.8 mol/L nanohybrid are shown in . The FTIR spectrum for pure ZnO () displays a characteristic peak at 373 cm−1, attributable to the vibration of the zinc and oxygen sublattices.Citation47 The FTIR spectrum for pure salicylic acid in shows a broad absorption band that spreads from 3229 cm−1 to 2591 cm−1, which can be attributed to the O-H stretching vibration.Citation48 The band at 1656 cm−1 corresponds to the carbonyl group, while those at 1608 cm−1 and 1439 cm−1 refer to the C-C stretching of the aromatic ring.Citation36 The bands caused by C-O and O-H vibration were recorded at 1291 cm−1 and 1236 cm−1, respectively.Citation37
Figure 3 Fourier transform infrared spectra of (A) zinc oxide, (B) salicylic acid, and (C) salicylate-zinc layered hydroxide nanohybrid 0.8 mol/L.
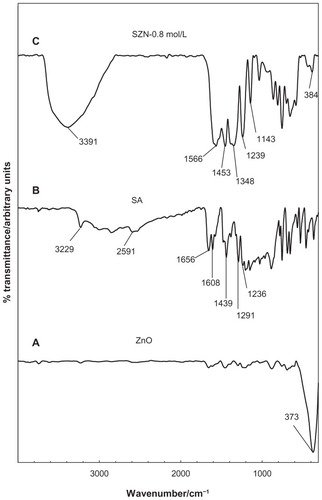
The FTIR spectra for the SZN 0.8 mol/L nanohybrid in illustrates bands characteristic of pure salicylic acid. indicating successful intercalation of the guest anions into the ZLH interlayers. However, the band attributable to C=O vibration of the acid has vanished because salicylic acid is intercalated in the anionic form. In turn, intense bands developed at 1566 cm−1, 1453 cm−1, and 1348 cm−1, which can be attributed to the asymmetric and symmetric carboxylate stretching mode of the salicylate anions.Citation37,Citation49 In addition to that, bands recorded at 1239 cm−1 and 1143 cm−1 are attributed to the C-C stretch in the aromatic ring, while a band corresponding to ZnO absorption is detected at 384 cm−1.Citation24 Successful intercalation of salicylate into ZLH was further supported by CHNS analysis, as shown in . The SZN 0.8 mol/L nanohybrid contained 18.04% carbon (w/w) and 2.09% hydrogen (w/w), and no nitrogen was present in the sample. The loading percentage of salicylate in the nanohybrid was 29.66% (w/w) and analysis using inductively-coupled plasma atomic emission spectroscopy showed that SZN 0.8 mol/L contained 56.65% zinc (w/w).
Table 1 Physicochemical properties of ZnO and SZN 0.8 mol/L
Thermal analysis
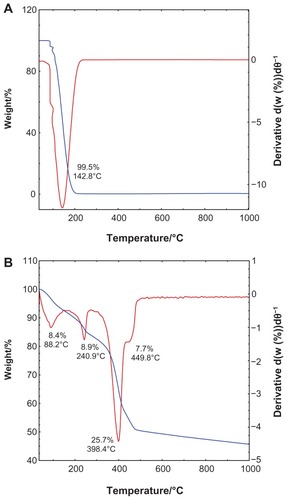
The TGA-DTG analysis obtained for salicylic acid and SZN 0.8 mol/L is shown in . The TGA-DTG thermograms for free salicylic acid () show a single stage of weight loss at a temperature maxima of 142.8°C, which can be attributed to complete combustion of the pure salicylic acid (99.5%). Meanwhile, the thermal behavior of the SZN 0.8 mol/L nanohybrid shows three stages of weight loss between 40.4°C and 491.0°C (). The first stage of weight loss between 40.4°C and 91.5°C is attributed to removal of surface-physisorbed water molecules,Citation41,Citation43 with an 8.4% weight loss. A second band corresponding to intercalated water molecules was not detected during this stage of weight loss, which is further affirmed by the absence of an absorption band at 1600 cm−1 in the FTIR.Citation50 This phenomenon was taken into account during the structural modeling exercise described earlier. The other stages of weight loss are characterized by dehydroxylation of the hydroxide layers and decomposition of the organic moieties, ie, salicylate anions.Citation51 The stages between 179.8°C and 491.0°C accounted for a total weight loss of 42.3%. It is worth noting that decomposition of the intercalated salicylate occurred at a notably higher temperature than that of free salicylic acid, indicating that the thermal stability of salicylate in SZN 0.8 mol/L is enhanced because of the interaction between salicylate anions and the host, ZLH.
Surface properties and surface morphology
shows the adsorption-desorption isotherms for ZnO and SZN 0.8 mol/L. As shown in , the adsorption-desorption isotherm for ZnO is categorized as type IV by the International Union of Pure and Applied Chemistry classification, which is assigned to materials that have a mesoporous-type structure.Citation52 The uptake of adsorbate occurred slowly at low relative pressure in the range of 0.0–0.9, followed by rapid adsorption from 0.9 onwards until an optimum adsorption of approximately 25 cm3/g was achieved. The isotherm of SZN-0.8 mol/L also showed the surface properties of the type IV isotherm.Citation52 Adsorption occurred in a gradually increasing manner from the relative pressure of 0.0 to 0.8. At 0.8, rapid adsorption ensued, until optimum adsorption took place at a higher relative pressure. The optimum uptake was recorded at 290 cm3/g, which was 12-fold higher than that of ZnO. The desorption branch of the hysteresis loop for ZnO was much narrower than that of SZN 0.8 mol/L, indicating that both materials possessed different pore textures. The surface area of both materials as determined by the Brunauer-Emmett-Teller method is shown in . As a result of intercalation, the surface area increased from 4 m2/g for ZnO to 49 m2/g for SZN 0.8 mol/L.
Figure 5 Adsorption-desorption isotherms (A) and Barrett-Joyner-Halenda pore size distribution (B) for ZnO and SZN 0.8 mol/L.
Abbreviations: ZnO, zinc oxide; SZN, salicylate-zinc layered hydroxide nanohybrid.
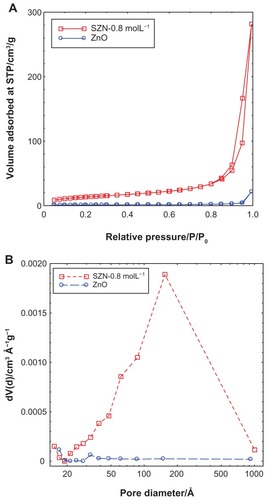
illustrates the Barrett-Joyner-Halenda pore size distribution plots for ZnO and SZN 0.8 mol/L. The pore distribution of ZnO exhibited a peak centered at 32 Å, while SZN 0.8 mol/L showed an intense peak at approximately 154 Å. The pore volume and diameter for ZnO and SZN 0.8 mol/L are tabulated in . The pore volume increased from 0.03 cm3/g to 0.44 cm3/g for ZnO and SZN 0.8 mol/L, respectively, while the pore diameter increased from 17 Å to 153 Å after salicylate was intercalated into the interlayer spacing of ZLH.
The surface morphology of ZnO and the SZN 0.8 mol/L nanohybrid, examined using field emission scanning electron microscopy, is illustrated in . ZnO was revealed as having a nonuniform granular structure, without any specific shape. The material existed in various sizes and shapes (). After intercalation of salicylate, this structure was transformed into an agglomerate, consisting of curled-up sheet like structures, the particle size of which was markedly reduced in comparison with the starting material, ZnO (). This shows that inclusion of salicylate into the interlayer spacing of ZLH resulted in a change in the surface morphology, in which an irregular granular structure was converted into more uniform rolled-up sheets.
MTT cytotoxicity test
The effect of free salicylic acid, ZnO, and the SZN 0.8 mol/L nanohybrid on the viability of Vero-3 cells is shown in . The cytotoxicity test was performed using an MTT assay, in which the ability of metabolically active cells to convert yellow MTT reagent into purple formazan by chemical reduction of the mitochondrial reductase enzyme is measured. As shown in , viability of the Vero-3 cells decreased as the concentration of the test materials increased. All materials were mildly toxic towards Vero-3 cells, resulting in up to 35% cell mortality even at the highest dosage used (25 μg/mL). Interestingly, pure ZnO was found to be the least toxic compared with free salicylic acid and the SZN 0.8 mmol/L nanohybrid, with 92% viability calculated using 25 μg/mL ZnO. On the other hand, both free salicylic acid and the SZN 0.8 mol/L nanohybrid were found to have a similar effect on the viability of Vero-3 cells. However, at higher concentrations (12.5 μg/mL and 25 μg/mL), the latter was more toxic to the Vero-3 cells, with viability at 80% and 73%, respectively.
Figure 7 Cytotoxicity analyses of free SA, ZnO, and SZN 0.8 mol/L at 25–0.195 μg/mL on Vero-3 cells using the MTT assay.
Abbreviations: SA, salicylic acid; SZN, salicylate-zinc layered hydroxide nanohybrid; ZnO, zinc oxide.
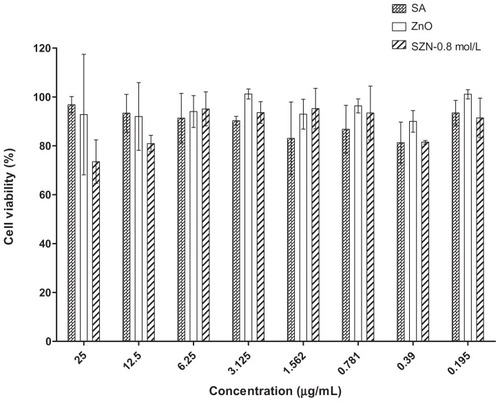
Although all the materials were generally nontoxic, a conclusive effect of concentration on cell viability was regrettably not established in this experiment. The concentrations employed were unable to elicit a more widespread effect on the cells, thereby preventing determination of an IC50 value for each of the test materials. The cells probably needed to be exposed to higher concentrations of the test materials or subjected to a prolonged duration of exposure in order to obtain more comprehensive information on the effect of the test materials on cell viability. Such information is needed before a transfection study entailing investigation of the effects of the nanohybrid at a molecular level can be carried out.
Conclusion
A new nanohybrid compound based on salicylate-intercalated ZLH was synthesized via a simple direct reaction of pure ZnO with salicylic acid solution. The pure-phase nanohybrid was obtained using 0.8 mol/L salicylic acid, and the basal spacing was recorded at 15.73 Å. FTIR analysis showed that the nanohybrid possesses the absorption characteristics of both ZnO and free salicylic acid, while elemental analysis showed that the nanohybrid contained 29.66% (w/w) salicylate. In addition, intercalation of salicylate into the interlayers of ZLH had enhanced thermal stability in comparison with free salicylic acid. It was also found that the Brunauer-Emmett-Teller surface area was increased from 4 m2/g to 49 m2/g after salicylate was intercalated into ZLH. MTT assay revealed that ZnO and SZN 0.8 mol/L had a mild toxic effect on Vero-3 cells, showing a potential for further use in the development of novel inorganic nanoparticle-based drug delivery systems.
Acknowledgments
Funding for this research was provided by the Ministry of Science, Technology, and Innovation of Malaysia under a RUGS grant (05-03-10-1035RU). The Universiti Teknologi Malaysia and the Ministry of Higher Education Malaysia are gratefully acknowledged for providing study leave and a scholarship for MR.
Disclosure
The authors report no conflicts of interest in this work.
References
- TrikeriotisMGhanotakisDFIntercalation of hydrophilic and hydrophobic antibiotics in layered double hydroxidesInt J Pharm200733217618417070662
- FarajiAHWipfPNanoparticles in cellular drug deliveryBioorg Med Chem2009172950296219299149
- ChoKWangXNieSTherapeutic nanoparticles for drug delivery in cancerClin Cancer Res2008141310131618316549
- XuZPZengQHLuGQInorganic nanoparticles as carriers for efficient cellular deliveryChem Eng Sci20066110271040
- KidaneABhattPPRecent advances in small molecule drug deliveryCurr Opin Chem Biol2005934735116006179
- KraljMPavelicKMedicine on a small scaleEMBO Rep200341008101214593436
- GuFXKarnikRWangAZTargeted nanoparticles for cancer therapyNano Today200721421
- SlowingIIVivero-EscotoJLWuCWMesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriersAdv Drug Deliv Rev2008601278128818514969
- WuXTanYMaoHToxic effects of iron oxide nanoparticles on human umbilical vein endothelial cellsInt J Nanomedicine2010538539920957160
- XieJChenKLeeHYUltrasmall c(RGDyK)-coated Fe3O4 nanoparticles and their specific targeting to integrin αvβ3-rich tumor cellsJ Am Chem Soc20081307542754318500805
- LaiCYTrewynBGJeftinijaDMA mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug moleculesJ Am Chem Soc20021254451445912683815
- EppleMKovtunAFunctionalized calcium phosphate nanoparticles for biomedical applicationKey Eng Mater2010441299305
- ChengXKuhnLChemotherapy drug delivery from calcium phosphate nanoparticlesInt J Nanomedicine2007266767418203433
- ShenoyDFuWLiJSurface functionalization of gold nanoparticles using hetero-bifunctional poly(ethylene glycol) spacer for intracellular tracking and deliveryInt J Nanomedicine2006145147217722279
- MadaniSYNaderiNDissanayakeOA new era of cancer treatment: carbon nanotubes as drug delivery toolsInt J Nanomedicine201162963297922162655
- ShaoNSuYHuJComparison of 3 generation polyamidoamine dendrimer and generation 4 polypropylene imine dendrimer on drug loading, complex structure, release behavior, and cytotoxicityInt J Nanomedicine201163361337222267921
- HusseinAlAliSHAl-QubaisiMHusseinMZControlled release and angiotensin-converting enzyme inhibition properties of an antihypertensive drug based on a perindopril erbumine-layered double hydroxide nanocompositesInt J Nanomedicine201272129214122619549
- OhJMParkCBChoyJHIntracellular drug delivery of layered double hydroxide nanoparticlesJ Nanosci Nanotechnol2011111632163521456254
- ChoyJHParkMOhJMBio-nanohybrids based on layered double hydroxideCurr Nanosci20062275281
- WenCJZhangLWAl-SuwayehSATheranostic liposome loaded with quantum dots and apomorphine for brain targeting and bioimagingInt J Nanomedicine201271599161122619515
- OzkanMQuantum dots and other nanoparticles: what can they offer to drug discovery?Drug Discov Today200491065107115582795
- ChoyJHChoiSJOhJMClay minerals and layered double hydroxides for novel biological applicationsAppl Clay Sci200736122132
- ChoyJHKwakSYParkJSCellular uptake behavior of [γ-32P] labeled ATP-LDH nanohybridsJ Mater Chem20011116711674
- HusseinMZAl AliSHZainalZHakimMNDevelopment of antiproliferative nanohybrid compound with controlled release property using ellagic acid as the active agentInt J Nanomedicine201161373138321796241
- MasarudinMJYusoffKAbdul RahimRHusseinMZSuccessful transfer of plasmid DNA into in vitro cells transfected with an inorganic plasmid-Mg/Al-LDH nanobiocomposite material as a vector for gene expressionNanotechnology200920111
- AjatMMohdMYusoffKHusseinMZSynthesis of glutamate- zinc-aluminium-layered double hydroxide nanobiocomposites and cell viability studyCurr Nanosci20084391396
- ChoyJHKwakSYJeongYJParkJSInorganic layered double hydroxides as nonviral vectorsAngew Chem Int Ed Engl2000394041404511093198
- AuerbachSMCarradoKADuttaPKHandbook of Layered MaterialsNew York, NYMarcel Dekker Inc2004
- RivesVLayered Double Hydroxides: Present and FutureNew York, NYNova Science Publishing Inc2001
- MeynMBenekeKLagalyGAnion-exchange reaction of hydroxy double saltsInorg Chem19933212091215
- NewmanSPJonesWComparative study of some layered hydroxide salts containing exchangeable interlayer anionsJ Solid State Chem19991482640
- ArizagaGGCSatyanarayanaKGWypychFLayered hydroxide salts: synthesis, properties and potential applicationsSolid State Ionics200717811431162
- ChoyJHShinJLimSYOhJMOhMHOhSCharacterization and stability analysis of ZnO nanoencapsulated conjugated linoleic acidJ Food Sci201075N636820722942
- YangJHHanYSParkMParkTHwangSJChoyJHNew inorganic-based drug delivery system of indole-3-acetic acid – layered metal hydroxide nanohybrids with controlled release rateChem Mater20071926792685
- HusseinMZGhotbiMYYahayaAHAbd RahmanMZSynthesis and characterization of (zinc-layered-gallate) nanohybrid using structural memory effectMater Chem Phys2009113491496
- HwangSHHanYSChoyJHIntercalation of functional organic molecules with pharmaceutical, cosmeceutical and nutraceutical functions into layered double hydroxides and zinc basic saltsBull Korean Chem Soc20012210191022
- MackowiakPABrief history of antipyretic therapyClin Infect Dis200031Suppl 5S15415611113017
- SilionMHritcuDPopaMIIntercalation of salicylic acid into ZnAl layered double hydroxides by ion-exchange and coprecipitation methodJournal of Optoelectronics and Advanced Materials200911528534
- del ArcoMGutiérrezSMartinCRivesVRochaJSynthesis and characterization of layered double hydroxide (LDH) intercalated with non-steroidal anti-inflammatory drugs (NSAID)J Solid State Chem200417739543962
- TrontoJCrepaldiELPavanPCDe PaulaCCValimJBOrganic anions of pharmaceutical interest intercalated in magnesium aluminum LDHs by two different methodsMol Cryst Liq Cryst2001356227237
- HusseinAlAliSHAl-QubaisiMHusseinMZZainalZHakimMNPreparation of hippurate-zinc layered hydroxide nanohybrid and its synergistic effect with tamoxifen on HepG2 cell linesInt J Nanomedicine201163099311122163163
- FernandesDMSilvaRWinkler HeichenletnerAASynthesis and characterization of ZnO, CuO and a mixed Zn and Cu oxideMater Chem Phys2009115110115
- HusseinMZHashimNYahayaAHZainalZSynthesis and characterization of [4-(2,4-dichlorophenoxybutyrate)-zinc layered hydroxide] nanohybridsSolid State Sciences201012770775
- XingfuZZhaolinHYiqunFSuCWeipingDNanpingXMicrospheric organization of multilayered ZnO nanosheets with hierarchically porous structuresJ Phys Chem C20081121172211728
- MisraCPerrotaAJComposition and properties of synthetic hydrotalcitesClays Clay Miner199240145150
- MarangoniRRamosLPWypychFNew multifunctional materials obtained by the intercalation of anionic dyes into layered zinc hydroxide nitrate followed by dispersion into poly(vinyl alcohol) (PVA)J Colloid Interface Sci200933030330919081109
- Munõz-EspíAChandraAWegnerGCrystal perfection in zinc oxide with occluded carboxyl-functionalized latex particlesCryst Growth Des2007715841589
- TakačMJ-MTopićDVFT-IR and NMR spectroscopic studies of salicylic acid derivatives. II. Comparison of 2-hydroxy- and 2,4- and 2,5-dihydroxy derivativesActa Pharm20045417719115610615
- HusseinMZChanWLSynthesis of organo-mineral nanohybrid material: indole-2-carboxylate in the lamella of Zn-Al-layered double hydroxideMater Chem Phys200485427431
- RoccaECailletCMesbahAFrancoisMSteinmetzJIntercalation in zinc-layered hydroxide: zinc hydroxyheptanoate used as protective material on zincChem Mater20061861866193
- RivesVCharacterization of layered double hydroxides and their decomposition productsMater Chem Phys2002751925
- SingKSWThe use of gas adsorption for the characterization of porous solidsJ Colloid Interface Sci198938113124