?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Zoledronic acid, an inhibitor of osteoclast-mediated bone resorption, has been shown to have both direct and indirect antitumor activity. However, its use in extraskeletal malignancy is limited due to rapid uptake and accumulation within bone. Polyinosinic acid-polycytidylic acid [poly (I:C)] is a synthetic double-stranded RNA with direct antitumor cytotoxicity if it can be delivered to tumor cells intracellularly.
Methods
Cationic lipid-coated calcium phosphate nanoparticles (LCP) were developed to enable intracellular codelivery of zoledronic acid and poly (I:C). LCP codelivering zoledronic acid and poly (I:C) were prepared using an ethanol injection method. Briefly, the ethanol solution of lipids was rapidly injected into newly formed calcium phosphate crystals containing poly (I:C) and zoledronic acid, and the mixture was then sonicated briefly to form LCP. The LCP were fully characterized for mean diameter size and zeta potential, efficiency in loading zoledronic acid, cytotoxic effect in a B16BL6 melanoma cell line in vitro, and antitumor effect in B16BL6 melanoma-bearing mice.
Results
LCP with a mean diameter around 200 nm and a narrow size distribution (polydispersity index 0.17) and high zoledronic acid encapsulation efficiency (94%) were achieved. LCP loaded with zoledronic acid and poly (I:C) had significantly greater antitumor activity than the free drugs in the B16BL6 melanoma cell line (P < 0.05). Furthermore, codelivery of zoledronic acid and poly (I:C) by LCP had higher cytotoxicity than delivering poly (I:C) alone by LCP (P < 0.05), indicating a synergism between zoledronic acid and poly (I:C). Finally, the antitumor study in melanoma-bearing mice also demonstrated synergism between zoledronic acid and poly (I:C) codelivered by LCP.
Conclusion
Cationic lipid-coated calcium phosphate nanoparticles constructed for codelivery of zoledronic acid and double-stranded RNA poly (I:C) had better antitumor activity both in vitro and in vivo. Future preclinical development of LCP encapsulating zoledronic acid and poly (I:C) for the treatment of human cancer is under way.
Introduction
Zoledronic acid and other nitrogen-containing bisphosphonates are potent inhibitors of osteoclast proliferation, and are used to treat osteoporosis and cancer-related bone metastases.Citation1 Moreover, preclinical studies have demonstrated the antitumor effects of zoledronic acid in various nonskeletal tumor models, including breast cancer, myeloma, and others, suggesting that zoledronic acid has direct tumor inhibitory activity.Citation2 Zoledronic acid can directly induce apoptosis in tumor cells, and inhibit tumor cell growth and angiogenesis.Citation3–Citation6 For example, zoledronic acid directly suppresses cell proliferation and induces apoptosis in highly tumorigenic prostate and breast cancers.Citation7 The anticancer activity of zoledronic acid has also been seen in the induction of γδ T-cell activation needed to initiate both the innate and the adaptive immune responses.Citation8,Citation9
However, zoledronic acid is rapidly eliminated after intravenous injection due to preferential uptake and accumulation within bone, which results in ineffective concentrations of zoledronic acid in nonskeletal cancer tissues.Citation10 In addition, accumulation of zoledronic acid in bone would pose a serious health risk.Citation11 These problems could be solved by a nanoparticle drug delivery system shielding the zoledronic acid from directly binding to bone whilst in the circulation.Citation12–Citation16 The enhanced permeation and retention effect of the nanoparticle drug delivery system can also improve accumulation of zoledronic acid in solid tumors.Citation17,Citation18
Synthetic double-stranded RNA polyinosinic-polycytidylic acid [poly (I:C)], a ligand for endosomal receptor TLR3,Citation19 can induce expression of inflammatory cytokines and type I interferon via the NF-κB, mitogen-activated protein kinase, and interferon regulatory factor 3 pathways, so enhancing the antitumor immune response.Citation11,Citation20,Citation21 Furthermore, poly (I:C) can be a ligand for cytoplasmic melanoma differentiation-associated gene 5.Citation22 In human melanoma, transfection of poly (I:C) into the cytoplasm can induce autophagy-mediated apoptosis via the melanoma differentiation-associated gene 5-mediated signaling pathway.Citation21,Citation23,Citation24 Thus, delivering poly (I:C) to solid tumor tissue can induce tumor apoptosis directly if delivery to the cytoplasm can be achieved.
Complexes containing nucleic acid and calcium phosphate precipitates have been used in gene transfection studies for many years.Citation25 They can be taken up easily by cells through endocytosis and subsequently dissolved in endosomes.Citation26 Also, calcium phosphate has low toxicity and good biocompatibility because it is the inorganic component of hard biological tissues, ie, bones and teeth.Citation27 Therefore, it has the potential to be a good carrier system for drug delivery, especially for nucleic acid-based therapeutics. However, large aggregates are easily formed as a result of rapid crystal growth, so use of these complexes is limited to in vitro gene transfection.Citation28 The key issue when fabricating calcium phosphate nanoparticles is to halt continuous growth of crystals. Synthesis of calcium phosphate nanoparticles can be done by various methods, including wet precipitation,Citation29 solid state reaction,Citation30 flame spray pyrolysis,Citation31 and hydrothermal,Citation32 spray-drying,Citation33 micelle-mediated,Citation34 reverse micelle-mediated,Citation35,Citation36 and double emulsion-mediated synthesis.Citation37 These calcium phosphate nanoparticle fabrication methods usually involve multiple steps, which are not easily scaled up in the pharmaceutical manufacturing process.
In this study, we devised a simple method of wet precipitation combined with cationic lipid surface coating for fabrication of calcium phosphate nanoparticles and codelivery of zoledronic acid and poly (I:C). Being a polyanion, poly (I:C) is not only a therapeutic agent, but has a unique role in the process of fabricating calcium phosphate nanoparticles. In this study, binding poly (I:C) prevented continuous growth of calcium phosphate crystals and imparted a negative charge to the surface of calcium phosphate crystals for subsequent cationic lipid coating. We also tested whether codelivery of zoledronic acid and poly (I:C) has a synergistic inhibitory effect on growth of the mouse melanoma both in vitro and in vivo.
Materials and methods
Materials
DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) was purchased from Avanti Polar Lipids Inc (Alabaster, AL). Poly (I:C) was sourced from EMD Chemicals (San Diego, CA). Zoledronic acid was obtained from Alexis Corporation (San Diego, CA). Cholesterol and tetrabutylammonium hydrogen sulfate were purchased from JT Baker (Phillipsburg, NJ). Calcium nitrate, phosphate salt, and all other reagents were purchased from VWR International (West Chester, PA). Fetal bovine serum, Dulbecco’s Modified Eagle’s Medium, and other reagents needed for cell culture were purchased from Mediatech (Manassas, VA). A mouse melanoma cell line (B16BL6) was obtained from the National Cancer Institute (Frederick, MD).
Preparation of lipid-coated calcium phosphate nanoparticles
Calcium phosphate nanoparticles were prepared by a wet precipitation method ().Citation28,Citation38 Briefly 500 μL of aqueous solution of calcium chloride (6.25 mM) was mixed with 500 μL of aqueous solution of diammonium hydrogen phosphate (3.74 mM). The pH of both solutions was preadjusted to 9.0 with 0.1 M NaOH.Citation39 Mixing was accomplished by rapidly injecting both solutions using a 1 mL insulin syringe into a 1.5 mL tube into which 15 μL of poly (I:C) aqueous solution (5 μg/μL or higher concentrations in some experiments) was added before injection. The suspension was then mixed well by vortex for 10 seconds. Finally, 7.5 μL of zoledronic acid 10 mg/mL was added to the calcium phosphate-poly (I:C) complex to form a calcium phosphate-poly (I:C)-zoledronic acid core.
Figure 1 Fabrication of lipid-coated calcium phosphate nanoparticles.
Abbreviations: CaP, calcium phosphate; LCP, lipid-coated calcium phosphate nanoparticles; poly (I:C), polyinosinic acid-polycytidylic acid.
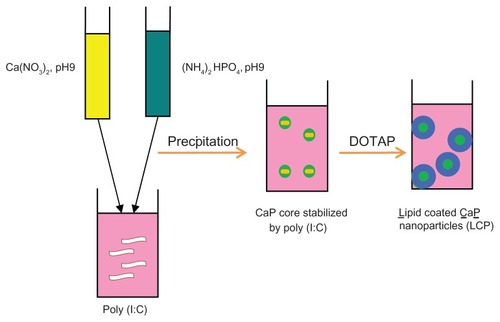
The lipid mixture, composed of DOTAP-cholesterol (1:1 mol/mol), was dissolved in absolute ethanol and coated onto the surface of the calcium phosphate-poly (I:C)- zoledronic acid complex. Briefly, 80 μL of the lipid mixture in 25 mg/mL ethanol solution was rapidly injected into the calcium phosphate-poly (I:C)-zoledronic acid core, and the mixture was then sonicated for 2 minutes in a bath sonicator to form lipid-coated nanoparticles consisting of a lipid shell and calcium phosphate-poly (I:C)-zoledronic acid core. The nanoparticles formed were extensively dialyzed against pH 8.0 Tris-HCl buffer to remove residual ethanol.
Preparation of cationic liposomes
DOTAP cationic liposomes were prepared using the ethanol injection method.Citation40 Briefly, 80 μL of lipid ethanol solution (DOTAP to cholesterol, 1:1, 25 mg/mL) was rapidly injected into a 1.5 mL tube containing 1.0 mL of filtered sterilized water, and the suspension was then sonicated in a bath sonicator for 2 minutes to form liposomes. The cationic liposomes formed were extensively dialyzed against water to remove the residual ethanol.
Preparation of nanoparticles
Because poly (I:C) has a unique role in stabilizing LCP, LCP containing various amounts of poly (I:C) (25, 75, 150, 300 μg/mL, respectively) was prepared to investigate the effect of amount of poly (I:C) on nanoparticle size. The mean diameters of the calcium phosphate nanoparticles, cationic liposomes, and LCP were compared between the three formulations. LCP containing various amounts of zoledronic acid (25, 50, and 75 μg/mL) and a fixed amount of poly (I:C) (75 μg/mL) were prepared using the method described above and evaluated for particle size and cytotoxicity in a B16BL6 melanoma cell line.
Particle size and zeta potential of calcium phosphate nanoparticles, cationic liposomes, and LCP
Mean diameter and polydispersity index were used to characterize the size distribution of the nanoparticles. The particle size and zeta potential of the different formulations were determined at room temperature using a particle sizer (Nicomp Model 380/ZLS, Particle Sizing Systems, Santa Barbara, CA). Each sample was diluted 20-fold with 0.22 μL of filtered water before measurement. All values were calculated as the mean of the three separate batches.
Quantitative analysis and calculation of zoledronic acid encapsulation efficiency in LCP
Quantitative analysis of zoledronic acid was performed by reverse-phase high-performance liquid chromatography on a Luna C18(2) column (3 μm, 150*4.6 mm) using a mobile phase composed of 5:95 methanol to phosphate buffer (8 mM, pH 7.4), 3 mM tetrabutylammonium hydrogen sulfate, and 2.3 μg/mL ethylenediamine tetra-acetic acid at a flow rate of 0.6 mL per minute at room temperature, with ultraviolet detection at 215 nm.Citation41 Briefly, 1 mL of liposomes or nanoparticles was centrifuged through a centrifugal device with a molecular cutoff of 100,000 (Nanosep, Pall Life Sciences, Menlo Park, CA) at 12,000 rpm for 20 minutes. The supernatants containing free zoledronic acid were then carefully collected, and the zoledronic acid concentration was determined by high-performance liquid chromatography.Citation42 The zoledronic acid encapsulation efficiency was calculated by the following equation:
Stability study
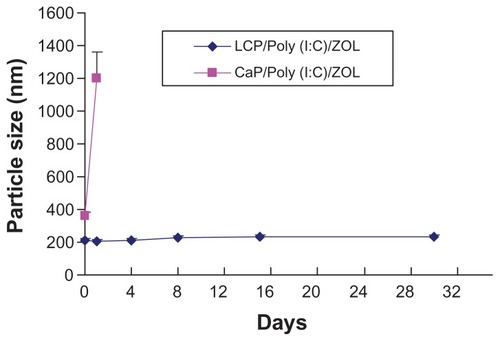
To determine the stability of LCP under storage conditions, LCP was freshly prepared and then stored at 4°C in 10 mM pH 8.0 Tris-HCl buffer, and the particle size and zoledronic acid encapsulation efficiency were then monitored during 30 days of storage.
Cell viability assay
In vitro cytotoxicity was determined by MTT assay, as described previously.Citation43 The B16BL6 cells were grown in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The B16BL6 cells were seeded in 96-well plates at 8 × 103 cells/well in 100 μL of medium. After 16 hours, the cells were treated with the different formulations diluted in Dulbecco’s Modified Eagle’s medium to provide the same mass concentration (μg/mL) of poly (I:C), zoledronic acid, or their combination, and incubated for 48 hours prior to MTT assay. Briefly, MTT reagent was added to the culture medium at a final concentration of 0.5 mg/mL and the cells were incubated at 37°C for an additional 4 hours. Finally, the medium was removed, the cells with dye compounds were dissolved in dimethylsulfoxide, and adsorption was measured at 544 nm using a microplate reader (Fluostar, BMG LabTech GmbH, Ortenberg, Germany). Viability was expressed as a percentage of the untreated control cells (100% viability).
Animal study
Female 8-week-old Charles River C57BL/6 mice were used to evaluate the in vivo efficacy of the different formulations. The animal experiments complied with the rules set down in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee. B16BL6 mouse melanoma cells were used as the tumor development model. B16BL6 cells (5 × 105) were inoculated subcutaneously into the mice to induce tumor formation and growth. After 5 days, when the tumor volume had reached approximately 50 mm3, mice were randomly divided into four groups (n = 6) to receive phosphate-buffered solution (control), free zoledronic acid, LCP-poly (I:C), or LCP-poly (I:C)-zoledronic acid, and received four peritumoral injections on days 0, 2, 4, and 6 after tumor formation at a dose of 4.5 μg per mouse. The doses of 4.5 μg per mouse were calculated as free zoledronic acid or poly I:C or their 1:1 combination in the three treatment groups mentioned above. Following treatment, the animals were monitored regularly for body weight, tumor growth, and survival. Tumor volumes were assessed by measuring two perpendicular diameters with digital calipers and using the formula (L*W2)/2, where L is the longest diameter and W is perpendicular to L.Citation42 Results are expressed as the mean tumor volume ± standard deviation for six mice.
Statistical analysis
The Student’s t-test was used to identify significant differences between groups. P < 0.05 was considered to be statistically significant.
Results and discussion
Nanoparticle preparation and characterization
As shown in , the diameter of LCP without poly (I:C) prepared by the newly formed calcium phosphate crystals and DOTAP was about 8.3 μm, suggesting no affinity between calcium phosphate crystals and DOTAP. Therefore, the surface of the calcium phosphate crystal needed to be functionalized by negative charges for further coating with cationic liposomes. Poly (I:C), a negatively charged synthetic nucleic acid, was chosen to functionalize the surface of calcium phosphate because of its high affinity for calcium phosphate and therapeutic effects. We then investigated the effect of poly (I:C) on the particle size of LCP. The particle size was still large with the addition of poly (I:C) 25 μg/mL (). However, the diameter of LCP was dramatically decreased to the size range of nanoparticles (around 200 nm) by addition of poly (I:C) 75 μg/mL to the preparation. There was no significant difference in particle size between the three poly (I:C) concentrations used (75, 150, 300 μg/mL), indicating that 75 μg/mL of poly (I:C) was enough to prevent continuous growth of calcium phosphate crystals. Meanwhile, under such conditions, the charge (+/−) to N/P ratio (for DOTAP and poly (I:C), respectively), was 4:1, and this ratio was appropriate for DOTAP coating to achieve colloidal stability of LCP. It was confirmed that all the poly (I:C) was associated with LCP, with an N/P ratio of 4:1 by agarose gel retardation assay (data not shown). The agarose gel retardation assay also indicated that increasing poly (I:C) to >150 μg/mL in the preparation resulted in gradual accumulation of free poly (I:C) in the formulation (data not shown), so an N/P ratio of 4:1 was used for subsequent experiments.
Figure 2 (A) Effect of poly (I:C) on particle size of LCP without zoledronic acid. (B) Effect of ratio of poly (I:C) to zoledronic acid on particle size of LCP.
Abbreviations: LCP, lipid-coated calcium phosphate nanoparticles; poly (I:C), polyinosinic acid-polycytidylic acid; zol, zoledronic acid.
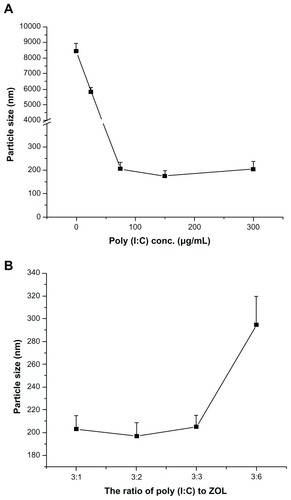
In order to codeliver poly (I:C) and zoledronic acid, different weight ratios of poly (I:C) to zoledronic acid with a fixed 75 μg/mL amount of poly (I:C) were used in the fabrication of LCP, and their sizes were compared (). There were no obvious increases in LCP particle size when the weight ratios were varied from 3:1 to 3:3 [poly (I:C) to zoledronic acid], but the size increased to around 300 nm at a ratio of 3:6. Because zoledronic acid has high affinity for calcium phosphate,Citation12 it will compete with poly (I:C) binding to calcium phosphate, leading to compromise of the stabilization effects of poly (I:C).
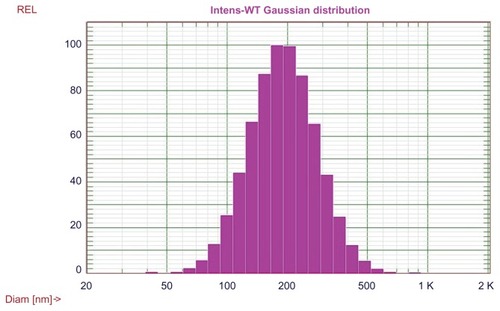
Four different delivery systems were prepared and characterized, and the results were summarized in , which shows that LCP is capable of delivery of poly (I:C) alone. The size of LCP-poly (I:C) is around 180 nm. The lipoplex formed by mixing of cationic liposomes, poly (I:C), and zoledronic acid, had the smallest particle size of 138 nm but the broadest size distribution (polydispersity index 0.33) of all the formulations tested. It appeared that only 42% of the total amount of zoledronic acid was associated with the lipoplex. This is probably due to the rapid diffusion of zoledronic acid from the lipoplex because the molecular weight of zoledronic acid is less than 300 Da. The LCP-poly (I:C)- zoledronic acid, formed by a calcium phosphate core and DOTAP coating, had higher zoledronic acid encapsulation efficiency (94%) and a relatively small size (205 nm). Being one of the bisphosphonates, zoledronic acid anions are suitable for coordinating calcium cations in the calcium phosphate phase through bidentate chelation.Citation44 The high affinity between calcium phosphate and zoledronic acid decreased the diffusion of zoledronic acid from LCP. The lipid coating added another diffusion barrier to zoledronic acid, leading to overall high encapsulation efficiency. Interestingly, without a lipid coating, the freshly prepared intermediate core formed by calcium phosphate, poly (I:C), and zoledronic acid had a negative charge (−10 mV) and a high loading efficiency for zoledronic acid (90%), suggesting that the major contribution to the high encapsulation efficiency of zoledronic acid in LCP was the high affinity between zoledronic acid and calcium phosphate. The size distribution of LCP-poly (I:C)-zoledronic acid is shown in .
Table 1 Diameter, polydispersity index, zeta potential, and encapsulation efficiency of different nanoparticles
Figure 3 Size distribution of LCP-poly (I:C)-zoledronic acid.
Abbreviations: LCP, lipid-coated calcium phosphate nanoparticles; poly (I:C), polyinosinic acid-polycytidylic acid.
We then studied the stability of LCP in pH 8.0 Tris- HCl buffer stored at 4°C for 30 days. Although the calcium phosphate-poly (I:C)-zoledronic acid complex had a relatively small size of 360 nm when freshly prepared, its size gradually increased to more than 1 μm after 24 hours (). However, LCP-poly (I:C)-zoledronic acid demonstrated good colloidal stability. shows that the particle size of LCP-poly (I:C)-zoledronic acid remained unchanged over 30 days. Therefore, the cationic DOTAP coating on the LCP nanoparticle contributed to its good colloidal stability. The cationic liposome drug delivery system has been widely studied for gene transfection, vaccine delivery, and antitumor targeting. LCP may have some characteristics similar to those of a cationic liposome, such as good colloidal stability. However, more experiments are required for a better understanding of the difference between these two drug delivery systems.
Codelivery of poly (I:C) and zoledronic acid had superior cytotoxicity in melanoma cells
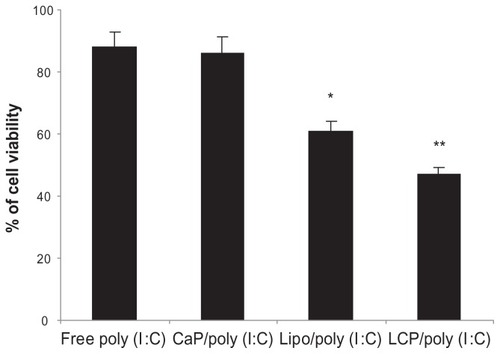
It has been reported that poly (I:C) induces direct apoptotic cytotoxicity in cancer cells, including melanoma,Citation45 lung cancer,Citation46 and breast cancer,Citation45,Citation47 if poly (I:C) can be delivered into the cytoplasm.Citation48 Therefore, we formulated poly (I:C) using calcium phosphate nanoparticles, a DOTAP lipoplex, and LCP, and compared their cytotoxicity in B16BL6 mouse melanoma cells. As shown in , at the same poly (I:C) concentration of 0.5 μg/mL, LCP-poly (I:C) showed the highest cytotoxicity to B16BL6 cells, followed by the poly (I:C)-lipoplex, and finally poly (I:C)-calcium phosphate nanoparticles. Cell viability was 47.1%, 61.2%, and 86.3%, respectively. Blank calcium phosphate nanoparticles, DOTAP liposomes, or their combination had no cytotoxic effect on B16BL6 cells (data not shown). The enhanced cytotoxic effect of LCP could be explained by delivery of more poly (I:C) into the cytoplasm by LCP. It has been reported that the calcium phosphate core in LCP is unstable in an acidic endosomal environment, so its decomposition to free calcium and phosphate ions in the endosome will increase osmotic pressure and cause swelling and rupture of the endosomes, and release of their contents into the cytosol.Citation26,Citation49,Citation50 However, without DOTAP, the negatively charged calcium phosphate-poly (I:C) nanoparticles are not easily taken up by melanoma cells, leading to a compromised cytotoxic effect.Citation48
Figure 5 In vitro cytotoxicity of free poly (I:C), CaP-poly (I:C), Lipoplex-poly (I:C), and LCP-poly (I:C) in a B16BL6 cell line, with a poly (I:C) concentration of 0.5 μg/mL.
Notes: *P < 0.05, compared with free poly (I:C); **P < 0.05, compared with lipoplex-poly (I:C).
Abbreviations: CaP, calcium phosphate; LCP, lipid-coated calcium phosphate nanoparticles; poly (I:C), polyinosinic acid-polycytidylic acid.
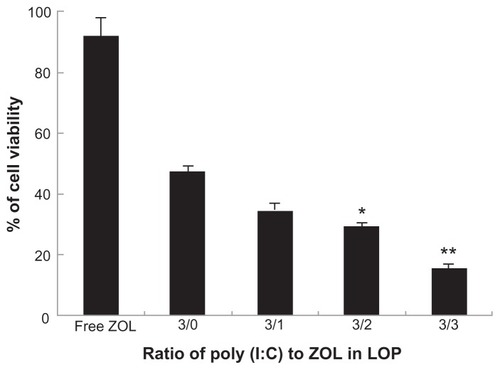
Next, the cytotoxicity of LCP codelivering poly (I:C) and zoledronic acid was tested in B16BL6 cells. Like free poly (I:C), free zoledronic acid at a concentration of 0.5 μg/mL had no cytotoxic effect in B16BL6 cells. We then prepared LCP codelivering poly (I:C) and zoledronic acid in different ratios, but the combined concentration of both drugs (poly (I:C) + zoledronic acid) in LCP was kept constant (0.5 μg/mL) in the subsequent MTT assay. The results demonstrated that the drug combination was more potent in its ability to generate cytotoxicity in melanoma cells than were the individual drugs used alone (). As shown in , the cell viabilities after treatment with free zoledronic acid and LCP-poly (I:C) were 91.8% and 49.0%, respectively. Codelivery of poly (I:C) and zoledronic acid (1:1) by LCP showed enhanced cell cytotoxicity, with a cell viability of 14.4%. Interestingly, the greater the amount of zoledronic acid in the LCP-poly (I:C)-zoledronic acid complex, the greater the potency of the cytotoxicity to B16BL6 cells, although the combined concentration of the two drugs was the same. However, from previous formulation studies, zoledronic acid could not be increased to more than 50%, because more zoledronic acid led to instability of LCP during the fabrication process.
Figure 6 In vitro cytotoxicity of LCP-poly (I:C)-zoledronic acid in B16BL6 cell line with different weight ratios of poly (I:C) to zoledronic acid.
Notes: The concentration of total drugs (poly (I:C) + zoledronic acid) was 0.5 μg/mL. *P < 0.05, compared with the ratio of 3/0; **P < 0.01, compared with the ratio of 3:0.
Abbreviations: LCP, lipid-coated calcium phosphate nanoparticles; poly (I:C), polyinosinic acid-polycytidylic acid; ZOL, zoledronic acid.
Tumor inhibition by codelivering poly (I:C) and zoledronic acid in melanoma-bearing mice
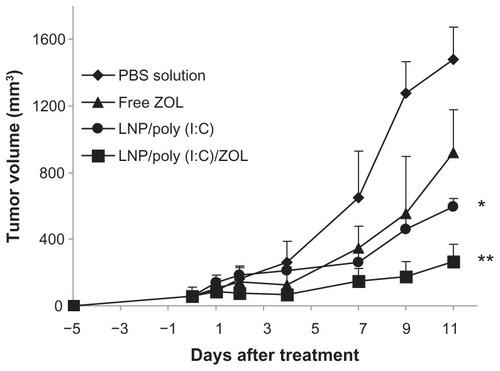
Based on the superior cytotoxicity of LCP codelivering poly (I:C) and zoledronic acid in the B16BL6 melanoma cell line, we investigated its antitumor activity in B16BL6 tumor-bearing mice. The tumors were allowed to grow subcutaneously for 5 days, and the tumor-bearing mice were then treated with phosphate-buffered solution (control), free zoledronic acid, LCP-poly (I:C), or LCP-poly (I:C)-zoledronic acid by peritumoral injection on days 0, 2, 4, and 6. As shown in , the control mice had the largest tumors at day 11. Mice treated with LCP-poly (I:C)-zoledronic acid showed significant tumor growth inhibition compared with the other groups (P < 0.05). Mice treated with LCP-poly (I:C) also showed slight tumor growth inhibition.
Figure 7 Tumor growth inhibition by poly (I:C) and zoledronic acid codelivered by LCP in melanoma-bearing mice.
Notes: *P < 0.05 compared with PBS; **P < 0.01 compared with PBS.
Abbreviations: LCP, lipid-coated calcium phosphate nanoparticles; poly (I:C), polyinosinic acid-polycytidylic acid; ZOL, zoledronic acid; PBS, phosphate-buffered solution.
Poly (I:C) has multiple actions in the inhibition of tumor growth, including direct apoptosis effects on tumor cells and modulation of the immune system.Citation51–Citation55 One of the side effects of poly (I:C) is induction of toxic cytokines, so its use in cancer is limited.Citation56–Citation58 In our study, by encapsulating zoledronic acid and poly (I:C) in LCP, the effective doses of both poly (I:C) and zoledronic acid could be decreased, so this formulation may have less toxic effects. The current peritumoral injection of LCP-poly (I:C)-zoledronic acid in melanoma-bearing mice confirmed the in vivo efficacy of codelivery of poly (I:C) and zoledronic acid by LCP. However, further development of LCP by surface PEGylation and targeting ligands is required for intravenous injection, and is suitable for treatment of other type of tumors.
Conclusion
LCP were developed for simultaneous delivery of zoledronic acid and poly (I:C). Lipid-coated nanoparticles including a calcium phosphate core and lipid shell had a narrow particle size distribution and high loading efficiency for poly (I:C) and zoledronic acid, with good stability. In addition, codelivery of zoledronic acid and poly (I:C) offered superior antitumor activity in both in vitro and in vivo studies. These results suggest a potential for future preclinical development of zoledronic acid and poly (I:C)-encapsulating LCP for the treatment of human cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- AaproMSColemanREBone health management in patients with breast cancer: current standards and emerging strategiesBreast201221181921958673
- BenzaidIMonkkonenHStresingVHigh phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivoCancer Res201171134562457221646473
- LeeMVFongEMSingerFRGuenetteRSBisphosphonate treatment inhibits the growth of prostate cancer cellsCancer Res20016162602260811289137
- SenaratneSGPirianovGMansiJLArnettTRColstonKWBisphosphonates induce apoptosis in human breast cancer cell linesBr J Cancer20008281459146810780527
- ClezardinPBisphosphonates’ antitumor activity: an unravelled side of a multifaceted drug classBone2011481717920655399
- GiraduoEHanahanDZoledronic acid inhibits angiogenesis and impairs tumorigenesis in a mouse model of cervical carcinogenesisHaematological Reports200633941
- AlmubarakHJonesAChaisuparatRZhangMMeillerTFScheperMAZoledronic acid directly suppresses cell proliferation and induces apoptosis in highly tumorigenic prostate and breast cancersJ Carcinog201110221297922
- KunzmannVWilhelmMAdjuvant zoledronic acid for breast cancer: mechanism of action?Lancet Oncol2011121199199221968221
- CiminiEPiacentiniPSacchiAZoledronic acid enhances Vdelta2 T-lymphocyte antitumor response to human glioma cell linesInt J Immunopathol Pharmacol201124113914821496396
- CaragliaMMarraMNaviglioSBottiGAddeoRAbbruzzeseAZoledronic acid: an unending tale for an antiresorptive agentExpert Opin Pharmacother201011114115420001436
- SalzanoGMarraMPorruMSelf-assembly nanoparticles for the delivery of bisphosphonates into tumorsInt J Pharm20114031–229229721055454
- ScheperMChaisuparatRCullenKMeillerTA novel soft-tissue in vitro model for bisphosphonate-associated osteonecrosisFibrogenesis Tissue Repair20103620359336
- FarokhzadOCLangerRImpact of nanotechnology on drug deliveryACS Nano200931162019206243
- AgratiCMarianecciCSennatoSMulticompartment vectors as novel drug delivery systems: selective activation of Tγδ lymphocytes after zoledronic acid deliveryNanomedicine2011715316121034859
- van RooijenNvan Kesteren-HendrikxEClodronate liposomes: perspectives in research and therapeuticsJ Liposome Res200212819412604042
- DanenbergHDGolombGGroothuisALiposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbitsCirculation2003108222798280414610008
- MarraMSalzanoGLeonettiCNanotechnologies to use bisphosphonates as potent anticancer agents: the effects of zoledronic acid encapsulated into liposomesNanomedicine20117695596421453789
- GuoSHuangLNanoparticles escaping RES and endosome: challenges for siRNA delivery for cancer therapyJ Nanomater20112011
- HafnerACorthesyBTextorMMerkleHTuning the immune response of dendritic cells to surface-assembled poly(I:C) on microspheres through synergistic interactions between phagocytic and TLR3 signalingBiomaterials201132102651266121216459
- ZhengYAnHYaoMScaffolding adaptor protein Gab1 is required for TLR3/4- and RIG-I-mediated production of proinflammatory cytokines and type I IFN in macrophagesJ Immunol2010184116447645620435932
- BeschRPoeckHHohenauerTProapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cellsJ Clin Invest200911982399241119620789
- Alonso-CurbeloDSoengasMSSelf-killing of melanoma cells by cytosolic delivery of dsRNA: wiring innate immunity for a coordinated mobilization of endosomes, autophagosomes and the apoptotic machinery in tumor cellsAutophagy20106114815019923903
- ChengYSXuFAnticancer function of polyinosinic-polycytidylic acidCanc Biol Ther2011101212191223
- TormoDChecinskaAAlonso-CurbeloDTargeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cellsCancer Cell200916210311419647221
- JordanMSchallhornAWurmFMTransfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formationNucleic Acid Res19962445966018604299
- LiJChenYCTsengYCMozumdarSHuangLBiodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA deliveryJ Control Release2010142341642119919845
- ZhangMKataokaKNano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agentsNanotoday200946508517
- BishtSBhaktaGMitraSMaitraApDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene deliveryInt J Pharm2005288115716815607268
- SokolovaVVRadtkeIHeumannREppleMEffective transfection of cells with multi-shell calcium phosphate-DNA nanoparticlesBiomaterials200627163147315316469375
- SilvaCGracaMSombraAValenteMStructural and electrical study of calcium phosphate obtained by a microwave radiation assisted procedurePhysica B Condens Matter20094048–1115031508
- ChoJSKoYNKooHYKangYCSynthesis of nano-sized biphasic calcium phosphate ceramics with spherical shape by flame spray pyrolysisJ Mater Sci Mater Med20102141143114920052521
- LiKTjongSCHydrothermal synthesis and biocompatibility of hydroxyapatite nanorodsJ Nanosci Nanotechnol20111112104441044822408923
- XuHHMoreauJLSunLChowLCNanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibitionDent Mater201127876276921514655
- ThachepanSLiMMannSMesoscale crystallization of calcium phosphate nanostructures in protein (casein) micellesNanoscale20102112400240521080570
- DasguptaSBoseSReverse micelle-mediated synthesis and characterization of tricalcium phosphate nanopowder for bone graft applicationsJ Am Ceram Soc2009921125282536
- HanJTanTLooJUtilizing inverse micelles to synthesize calcium phosphate nanoparticles as nano-carriersJ Nanopart Res201113834413454
- ShumHBandyopadhyayABoseSWeitzDDouble emulsion droplets as microreactors for synthesis of mesoporous hydroxyapatiteChem Mater2009212255485555
- ErgunCEvisZWebsterTSahinFCSynthesis and microstructural characterization of nano-size calcium phosphates with different stoichiometryCeram Int201137971977
- SokolovaVKnuschkeTKovtunABuerJEppleMWestendorfAThe use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activationBiomaterials201031215627563320417963
- ZhouCYuBYangXLipid-coated nano-calcium-phosphate (LNCP) for gene deliveryInt J Pharm20103921–220120820214964
- ShmeedaHAmitayYGorinJTzemachDDelivery of zoledronic acid encapsulated in folate-targeted liposome results in potent in vitro cytotoxic activity on tumor cellsJ Control Release20101461768320462513
- MarraMSalzanoGLeonettiCNew self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative studyBiotechnol Adv201230130230921741464
- SudimackJJGuoWTjarksWLeeRJA novel pH-sensitive liposome formulation containing oleyl alcoholBiochim Biophys Acta200215641313712100993
- KimCWYunYPLeeHJHwangYSKwonIKLeeSCIn situ fabrication of alendronate-loaded calcium phosphate microspheres: controlled release for inhibition of osteoclastogenesisJ Control Release20101471455320600398
- ChengYSXuFAnticancer function of polyinosinic-polycytidylic acidCancer Biol Ther2010101219122320930504
- ForteGRegaAMorelloSPolyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancerJ Immunol2012188115357536422516955
- SalaunBCosteIRissoanMCLebecqueSJRennoTTLR3 can directly trigger apoptosis in human cancer cellsJ Immunol200617684894490116585585
- HirabayashiKYanoJInoueTInhibition of cancer cell growth by polyinosinic-polycytidylic acid/cationic liposome complex: a new biological activityCancer Res199959174325433310485480
- SokolovaVEppleMInorganic nanoparticles as carriers of nucleic acids into cellsAngew Chem Int Ed Engl20084781382139518098258
- GrahamFLvan der EbAJA new technique for the assay of infectivity of human adenovirus 5 DNAVirology19735224564674705382
- ShirAOgrisMRoedlWWagnerELevitzkiAEGFR-homing dsRNA activates cancer-targeted immune response and eliminates disseminated EGFR-overexpressing tumors in miceClin Cancer Res20111751033104321196415
- KublerKtho PeschCGehrkeNImmunogenic cell death of human ovarian cancer cells induced by cytosolic poly(I:C) leads to myeloid cell maturation and activates NK cellsEur J Immunol201141103028303921728171
- WuCYYangHYMonieAIntraperitoneal administration of poly(I:C) with polyethylenimine leads to significant antitumor immunity against murine ovarian tumorsCancer Immunol Immunother20116081085109621526359
- InaoTHarashimaNMonmaHAntitumor effects of cytoplasmic delivery of an innate adjuvant receptor ligand, poly(I:C), on human breast cancerBreast Cancer Res Treat201213418910022203435
- HarashimaNInaoTImamuraROkanoSSudaTHaradaMRoles of the PI3K/Akt pathway and autophagy in TLR3 signaling-induced apoptosis and growth arrest of human prostate cancer cellsCancer Immunol Immunother201261566767622038398
- SteelmanAJLiJpoly(I:C) promotes TNFalpha/TNFR1-dependent oligodendrocyte death in mixed glial culturesJ Neuroinflammation201188921812954
- NgoiSMSt RoseMCMenoretAMPresensitizing with a Toll-like receptor 3 ligand impairs CD8 T-cell effector differentiation and IL-33 responsivenessProc Natl Acad Sci U S A201210926104861049122689946
- MorahanPSMunsonAERegelsonWCommerfordSLHamiltonLDAntiviral activity and side effects of polyriboinosinic-cytidylic acid complexes as affected by molecular sizeProc Natl Acad Sci U S A19726948428464337241