Abstract
Background
A novel dual ligand–modified liposome, folic acid-tethered Pep-1 peptide-conjugated liposomal nanocarrier (FP-Lipo), was designed to overcome the nonselectivity of conventional penetrating peptide-tagged nanoparticulates and to provide the advantage of selective targeting of the folic acid receptor, which is frequently overexpressed on epithelial cancer cells.
Methods
FP-Lipo was prepared by a sequential process of formation of a maleimide-derivatized small unilamellar vesicle, postinsertion of distearoyl phosphatidyl ethanolamine-polyethylene glycol 2000–folate to the vesicle, and Pep-1 peptide conjugation via thiol-maleimide linkage. Conformational and physical characteristics of the FP-Lipo nanocarriers were investigated for the extent of Pep-1 peptide and folic acid on the surface, vesicle size, and zeta potential. In vitro cellular uptake behaviors of the novel carrier containing a fluorescein dextran isothiocyanate probe were examined by spectrophotometry or by confocal laser scanning microscopy.
Results
A novel nanocarrier bearing approximately 750 folate ligands and 100 penetrating peptides per vesicle was successfully prepared. The physical properties were as follows: 140 nm in size; 5 mV in zeta potential; less than 0.3 in polydispersity index. An in vitro cellular uptake study revealed that the FP-Lipo nanocarrier system exhibited more than twofold enhanced translocation into the folic acid receptor–positive HeLa cells compared with the single Pep-1 peptide–modified liposome. Meanwhile, its cellular association and internalization into the folic acid receptor–negative normal HaCaT cells was comparable with that of Pep-1 peptide–modified liposome.
Conclusion
An advanced dual ligand-modified liposome is potentially useful for the treatment of folic acid receptor–positive tumors with high translocation capability of the penetrating peptide–modified liposome.
Introduction
Efficient intracellular delivery of therapeutic agents is one of the major challenges in cancer therapy. Many cytotoxic molecules should be delivered intracellularly to exert their therapeutic action inside the cytoplasm, nuclei, or mitochondria, but cell membranes prevent therapeutic molecules from entering the cells.Citation1 A desirable research direction that addresses this problem is the introduction of cell-penetrating peptides (CPPs) to overcome the permeability barrier.Citation2,Citation3 CPP-modified liposomal nanocarriers have been introduced to improve intracellular delivery of various molecules, from small synthetic compounds to bioactive macromolecules, into tumor cells.Citation4,Citation5 Recently, we reported that liposomes tagged with Pep-1 peptide with a density of several hundreds of peptides could efficiently translocate liposomal drugs into cells.Citation6 In spite of high translocation ability and high efficiency in mediating the translocation of therapeutic molecules into most cells, the clinical application of CPPs appears challenged by a lack of cell specificity.Citation7
Some strategies have been explored to bypass the lack of cell specificity of CPPs and/or CPP-modified nanoparticulate systems, improving delivery selectivity to cancer cells. These include the sequencing of tumor-homing motifs with cell-penetrating peptidesCitation8,Citation9 and the designing of CPPs that are activated in tumor microenvironments, including peritumoral acidic pH, and overexpressed matrix metalloproteinases.Citation10,Citation11 However, even though these systems showed efficient translocation into cells and high selectivity in vitro, their applications may largely depend on either physiological or biological environments.
Folic acid receptor (FR)-α is a glycosylphosphati-dylinositol-anchored membrane protein that is selectively overexpressed in over 90% of ovarian carcinomasCitation12–Citation14 and, to various extents, in other epithelial cancers. FR-β, an isoform of FR-α, is overexpressed in myelogenous leukemias.Citation15,Citation16 A number of FR-targeted therapeutic and imaging agents, including liposomal agents, have been evaluated in pre-clinical studies, with promising results.Citation16–Citation19 Thus, a covalent attachment of folate to the pharmaceutical nanoparticulates by means of spacers permitted the selective binding of the nanocarriers to FR-abundant carcinomas.Citation19 Therefore, as an alternative approach of enhancing the translocation of penetrating peptide–modified liposomes, we hypothesized that the introduction of a folate ligand to Pep-1 peptide–modified liposomes would allow enhanced intracellular uptake to FR-overexpressed tumor cells, with better selectivity.
The aim of this study was to construct dual ligand–modified liposomes (FP-Lipo), novel nanoparticulate carriers, by introducing both folic acid as a targeting moiety and Pep-1 peptide as a CPP. The conformational and physical properties of the nanocarrier were characterized by the determination of the extent of conjugated Pep-1 peptide and folic acid, vesicle size, uniformity, and zeta potential. Further comparisons of FP-Lipo versus Pep-1 peptide–modified liposomes (P-Lipo) or folate-modified liposomes (F-Lipo) were also estimated in terms of cellular uptake and cell specificity, using the cell lines of FR-negative human keratinocyte (HaCaT) and FR-positive cervical cancer (HeLa) cells.
Materials and methods
Materials
Soya phosphatidylcholine (PC), polysorbate 80 (Tween® 80), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), dicyclohexylcarbodiimide (DCC), dithiothreitol, and fluorescein dextran isothiocyanate (FITC-dextran, 4 kDa) were purchased from Sigma-Aldrich (St Louis, MO, USA). Pep-1 peptide (KETWWETWWTEWSQPKKKRKVC, 22 mer) was synthesized by Peptron Co (Daejeon, Korea). Folic acid, dimethylsulfoxide (DMSO) and pyridine were purchased from Duksan Pure Chemical Co, Ltd (Seoul, Korea). Distearoyl phosphatidyl ethanolamine-polyethylene glycol–amine (DSPE-PEG2000-amine), distearoyl phosphatidyl ethanolamine-polyethylene glycol–maleimide (DSPE-PEG2000-mal), and n-[4-(p-maleimidophenyl)butyryl]-phosphatidylethanolamine (PE-PB-mal) were purchased from Avanti® Polar Lipids (Alabaster, AL, USA). A polyethersulfone ultrafiltration membrane was purchased from EMD Millipore (Billerica, MA, USA). Cell culture materials including folate-free RPMI 1640 cell culture medium were obtained from Life Technologies Corp (Carlsbad, CA, USA). 4′, 6-diamidino-2-phenylindole (DAPI) was purchased from F Hoffman-La Roche Ltd (Basel, Switzerland). All other chemicals and reagents purchased from commercial sources were of analytical or cell culture grade.
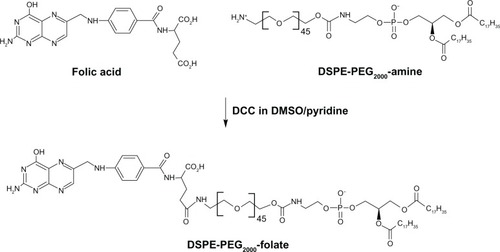
Synthesis of DSPE-PEG2000-folate
DSPE-PEG2000-folate was synthesized as reported previously,Citation20 with slight modifications (). Briefly, excessive folic acid (30.9 mg, 0.07 mM) was dissolved in DMSO (1 mL). DSPE-PEG2000-amine (100 mg, 0.035 mM) and pyridine (0.5 mL, 6.1 mM) were added to the folic acid solution, followed by DCC (32.5 mg, 0.156 mM). The reaction was continued at room temperature for 4 hours, and then, pyridine was removed from the reaction mixture by rotary evaporation. After adding water (12.5 mL) to the mixture, it was centrifuged to remove trace insoluble materials. The supernatant was dialyzed in Spectro/Por® CE Tubing (MWCO 100 kDa; Spectrum Laboratories Inc, Rancho Dominguez, CA, USA) against saline (50 mM, two times with 2000 mL) and water (three times with 2000 mL) to get rid of DMSO and unconjugated reactants. Finally, purified DSPE-PEG2000-folate was obtained in micellized form, with 2 mM concentration.
Figure 1 Schematic of synthesis of DSPE-PEG2000-folate.
Note: The compound was synthesized by DCC chemistry linking the amine group of DSPE-PEG2000-amine and the γ-carboxyl of folic acid.
Abbreviations: DSPE, distearoyl phosphatidyl ethanolamine; PEG2000, polyethylene glycol; DCC, dicyclohexylcarbodiimide.
The formation of DSPE-PEG2000-folate was identified by thin layer chromatography (TLC). A TLC plate (silica gel 60 F254; Merck, Rahway, NJ, USA) was developed with a mobile phase composed of chloroform, methanol, and water at 75:36:6 (v/v). Disappearance of DPSE-PEG2000-amine was confirmed by ninhydrin spray. To determine whether folic acid was linked via an α- or a γ- carboxyl group of folate, enzymatic hydrolysis was employed, as previously reported.Citation20 In the enzymatic hydrolysis method, the linkage of folic acid to DSPE-PEG2000-amine via γ-carboxyl group is selectively cleaved by carboxypeptidase G, and thus, the difference in peak area before and after enzyme treatment represents the amount of γ-carboxyl-linked folate groups.
Preparation of liposomal nanocarriers
Preparation of P-Lipo
The Pep-1 peptide–modified liposomes were prepared by conjugating Pep-1 peptide to a vesicle via a thiol maleimide reaction, as previously reported.Citation6 Briefly, maleimide-derivatized liposomes were prepared by the thin film hydration method.Citation21 PC, Tween 80 and DSPE-PEG2000-mal were dissolved at a molar ratio of 89.5:10:0.5 in chloroform and methanol (1:1), in a round bottom flask. The organic solvent was removed by rotary vacuum evaporation above the lipid transition temperature, and solvent traces were removed under nitrogen gas. The lipid film was then hydrated with aqueous solution containing 10 mg/mL of FITC-dextran, and was sonicated in a bath sonicator (Model 2210; Branson Ultrasonics Co, Danbury, CT, USA) for 20 minutes. Prepared liposomal solution was extruded through a 200 nm polyethersulfone membrane for homogenous size distribution. Pep-1 peptide solution (1 mg/mL), at twofold excess of Pep-1 peptide over maleimide group, was added to vesicles and allowed to react for 12 hours at room temperature in the presence of 0.2 μM concentration of dithiothreitol. Excess cysteine was added to block unreacted maleimide groups on the vesicles. Peptidyl liposomes were isolated from free Pep-1 peptide and cysteine using an ultrafiltration-stirred cell (MWCO 300 kDa; EMD Millipore). Conventional liposomes (C-Lipo) were similarly prepared from PC and Tween 80 (90:10) for use as a reference. The final lipid concentration was 10 mg/mL.
Preparation of F-Lipo
Folate-tethered liposomes were prepared by adding DSPE-PEG2000-folate micelles to liposomal vesicles composed of PC and Tween 80 (89.5:10 in molar ratio), prepared by the film hydration and size-extrusion process as described above. Briefly, unilamellar liposomal solution (1 mL) was incubated with micellized DSPE-PEG2000-folate (40 μL of 2 mM solution) at 40°C for 1 hour. For the purification, the liposomal suspension was ten times diluted with distilled water, to dissociate the folate micelles by lowering DSPE-PEG-folate concentration below critical micellar concentration. Unincoporated folate micelles and FITC-dextran were removed using a stirred ultrafiltration cell (EMD Millipore), in which the removal process was monitored by the disappearance of response in analyses with HPLC (LaChrom Elite®; Hitachi Ltd, Tokyo, Japan) and spectrofluorophotometer (RF-5301PC; Shimadzu Corp, Kyoto, Japan), respectively.
Preparation of FP-Lipo
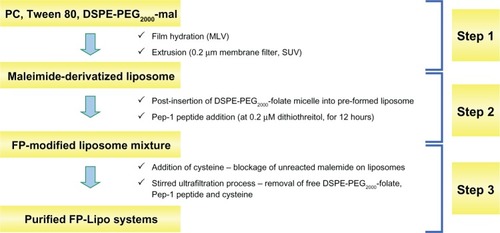
To optimize the dual ligand-conjugated liposomes, four types of dual ligand-conjugated liposomes were prepared by controlling the number of Pep-1 peptides on the vesicle (100 or 350 per vesicle) and by adjusting spacer length with relatively long chain DSPE-PEG2000-mal or short chain PE-PB-mal between the vesicle and Pep-1 peptide (). Dual ligand-conjugated liposomes were constructed by a 3-step process: maleimide-derivatization; postinsertion of DSPE-PEG2000-folate micelle and Pep-1 peptide coupling; and purification of FP-Lipo (). Briefly, PC, Tween 80, and DSPE-PEG2000-mal or PE-PB-mal were dissolved at a molar ratio of 89.5:10:0.15–0.5, in chloroform. Evaporation, hydration, and extrusion processes were performed as described above. Postinsertion of micellized DSPE-PEG2000-folate into the maleimide-derivatized liposomes was carried out using the same method for F-Lipo preparation. Pep-1 peptide was then conjugated to F-Lipo, and prepared FP-Lipo was purified using a stirred ultrafiltration cell.
Table 1 Conformational and physical characteristics of liposomal nanocarriers
Figure 2 Schematic illustration of the three steps used to prepare FP-Lipo: Step 1 maleimide derivatization of liposomes; Step 2 insertion of DSPE-PEG2000-folate to maleimide-derivatized liposomes; and Pep-1 peptide coupling; Step 3 purification of FP-Lipo systems.
Abbreviations: FP-Lipo, dual ligand–modified liposomes; DSPE, distearoyl phosphatidyl ethanolamine; PEG2000, polyethylene glycol; PC, soya phosphatidylcholine; mal, maleimide; MLV, multi-lamellar vesicle; SUV, small unilamellar vesicle.
Characterization of liposomal nanocarriers
Size and zeta potential
Liposomes were diluted with an appropriate volume of water and were examined for size distribution and zeta potential using a dynamic light scattering particle size analyzer (Zetasizer Nano-ZS; Malvern Instruments Ltd Malvern, UK) equipped with a 50 mV laser at a scattering angle of 90°. All measurements were carried out in triplicate under ambient conditions.
Pep-1 peptides on liposomes
The extent of Pep-1 peptide conjugation to liposomes was calculated indirectly by determining the amount of uncoupled peptide by HPLC, as previously reported.Citation6 Briefly a C18 column (Shiseido Co, Ltd Tokyo, Japan) was used with a mobile phase of 0.05% trifluoroacetic acid in water (eluant A) and 0.05% trifluoroacetic acid in acetonitrile (eluant B). The eluant gradient increased from 10% to 60% B in 50 minutes, and the peptide peak was separated with a retention time of 32 minutes. The number of Pep-1 peptide molecules per liposome was determined by the following equation: [Pep-1 peptide]external/[N]vesicles, where [Pep-1 peptide]external and [N]vesicles represent Pep-1 peptides on the vesicle oriented outward and total number of vesicles in the liposomal solution, respectively. [N]vesicle was calculated by the previously reported equation, [N]vesicle = 4πrCitation2 × 2/A, where r and A refer to the radius of the liposomes (65 nm) and the cross-sectional area of the PC head group (0.72 nmCitation2), respectively.Citation22
Folic acids on liposomes
The folate-tethered liposomes (0.2 mL) were disrupted with 2 mL of 10% Triton X-100 to obtain a clear solution. The content of liposomal folate in the solution was determined by HPLC assay of DSPE-PEG2000-folate. A C18 column (Shiseido) was used with a mobile phase of methanol and 10 mM sodium phosphate buffer (pH 7.0, 92:8 v/v). The column eluent was monitored at 285 nm, and the DSPE-PEG2000-folate peak was separated with a retention time of 2.1 minutes. The number of folate molecules per vesicle was calculated using the equation: [Folate]external/[N]vesicle [Folate]external represents the number of folate ligands on the vesicles. Total folate conjugated on the liposome was obtained by HPLC quantification, and we assumed that quantitated folate mostly oriented outward. [N]vesicle was calculated as described above.
Cell culture and in vitro cell uptake study
Cell culture
The human keratinocyte (HaCaT) cell and human cervical cancer (HeLa) cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% and 5% fetal bovine sera, in the presence of antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin). Cells were cultured in a humidified incubator at 37°C in an atmosphere containing 95% air and 5% CO2. For the cellular uptake study, both cell lines were seeded in growth medium at a density of 1 × 10Citation4 per well in a cell culture dish.
In vitro cell uptake study
The liposomal uptake into cultured cells was examined by determining the fluorescence intensity of FITC-dextran, using a fluorescence reader and by visually monitoring the cell association, using a confocal microscope. For the cellular uptake study, HaCaT and HeLa cells were seeded in growth media at a density of 1 × 10Citation5 per well into a 24-well plate on cover slips. After the cells reached 70%–80% confluence, the cells were incubated with liposomal suspensions (500 μM as lipid concentration) containing an equivalent amount of FITC-dextran (0.08 mg/mL) for 4 hours at 37°C. Cells were washed with cold phosphate buffered saline (PBS) (0.01 M, pH 7.4) three times to eliminate the traces of liposomes left in the wells. The cultured cells were lysed in RIPA lysis buffer consisting of 25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate. The cell lysates were then excited at 494 nm, and the emission at 520 nm was determined using a spectrofluorophotometer. Additionally, for microscopic observation, cells were mounted and the fluorescence of probes delivered to cells was visualized using a confocal laser scanning microscope (Zeiss LSM 510 Meta; Carl Zeiss Meditec, Jena, Germany).
In a separate experiment, to study the effect of FR on the liposomal uptake, a competitive binding assay was performed as previously described.Citation23 Briefly, FR-positive HeLa cells were preincubated with 1 mM of folic acid for 1 hour at 37°C. Cells were then washed three times with PBS and treated with each formulation (250 μM as lipid concentration) for 4 hours at 37°C. The following steps were the same as described above.
Cytotoxicity study
HaCaT cells were seeded in growth medium at a density of 1 × 10Citation4 per well into a 96-well plate. After 4 hours, cells were treated with empty liposomal formulations of C-Lipo, P-Lipo, F-Lipo, and FP-Lipo. Cells were then incubated with WST-1 reagent at 37°C, and cell viability was estimated by measuring the absorbance at 450 nm on a microplate reader.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Significance was determined by Student’s t-test, and results were considered to be significant at P < 0.05.
Results
Synthesis and characterization of DSPE-PEG2000-folate
A DSPE-PEG2000-folate conjugate was obtained in micellar solution for postinsertion into the preformed liposomes to prepare the F-Lipo and FP-Lipo. The conjugate was prepared by DCC-mediated coupling of folate to DSPE-PEG2000-amine, in which the γ-carboxyl group of folate was reacted with an amine-terminated DSPE-PEG2000 (). TLC on silica gel 60 F254 showed a new spot (Rf = 0.57), indicating the formation of DSPE-PEG2000-folate. Removal of DSPE-PEG2000-amine (Rf = 0.76) from the reaction mixture was confirmed by ninhydrin spray. The conjugate exhibited a single peak with retention time of 2.1 minutes, and the final product yielded 89.6 mg (79.3%), as determined by HPLC assay (data not shown).
Folate linkage to DSPE-PEG2000-amine via its γ-carboxyl group retains a strong affinity toward its receptor, whereas its α-carboxyl derivatives are not recognized as readily.Citation24,Citation25 Thus, to determine whether folate was effectively linked via the α- or γ-carboxyl group of folate, an enzymatic hydrolysis method was employed. The difference in peak area before and after enzyme treatment ranged from 77% to 83%, indicating that about 80% of the DSPE-PEG2000-folate was γ-carboxyl-linked.
Conformational characteristics of liposomal nanocarriers
In order to construct liposomal formulations bearing defined numbers of targeting ligands, conformational features of liposomal nanocarriers including the number of folic acid and Pep-1 peptide per vesicle were determined (). At first, the amount of folic acid incorporated into liposomes was estimated by HPLC assay of DSPE-PEG2000-folate after vesicle disruption. Insertion efficiencies were found to be between 45% and 50%, indicating that DSPE-PEG2000-folate vesicles were successfully incorporated into the lipid bilayer of preformed liposomes by the postinsertion technique. The average number of folate ligands per vesicle intended for insertion was calculated to be about 750 folate ligands in both the single- and dual-ligand liposomes ().
Conversely, the amount of Pep-1 peptide conjugated to maleimide-derivatized liposomes was estimated indirectly by HPLC assay of free Pep-1 peptide remaining after the coupling reaction. We previously reported that the efficiency of Pep-1 peptide coupling was revealed to be in the range of 78.4% to 80.7% at 2:1 molar ratio of Pep-1 peptide to the maleimide group.Citation6 In this study, coupling efficiencies were the same, at about 80% for both P-Lipo and FP-Lipo, with no differences in penetrating peptide number and type of spacer (PEG2000 and PB), indicating that the peptide conjugated to each vesicle was dominantly influenced by the extent of external maleimide groups on the liposomal surface. The number of Pep-1 peptide molecules attached to liposomes was approximately 350 for P-Lipo and 100–350 for FP-Lipo. FP-Lipo systems were named as FP(100)- or FP(350)-PEG-Lipo and FP(100)- or FP(350)-PB-Lipo, according to the average amount of Pep-1 peptide per vesicle and the type of spacer.
Physical characteristics of liposomal nanocarriers
We investigated the physical characteristics of the liposomal nanocarrier systems in terms of vesicular size, polydispersity index, and zeta potential (). The average size of liposome preparations was found to be about 130–150 nm, by dynamic light scattering. All formulations showed a low polydispersity index, below 0.3, indicating a narrow and homogenous size distribution. C-Lipo and F-Lipo were slightly negative about −7 to −10 mV In contrast, P-Lipo and the four types of FP-Lipo exhibited a positive surface charge due to the attachment of Pep-1 peptide, a positively charged synthetic CPP.
In vitro cell uptake study
Cellular uptake of the novel nanocarriers containing FITC-dextran was investigated quantitatively and microscopically in HaCaT and HeLa cell lines. FITC-dextran was used as a model compound to evaluate the dual-ligand hypothesis because (1) it could be loaded into a liposomal vesicle with ease and it yielded a vesicle that was stable for at least 24 hours, with no significant leakage in all tested formulations; (2) it is easily quantifiable due to its fluorescence properties; and (3) it can hardly transverse the cellular membrane alone.
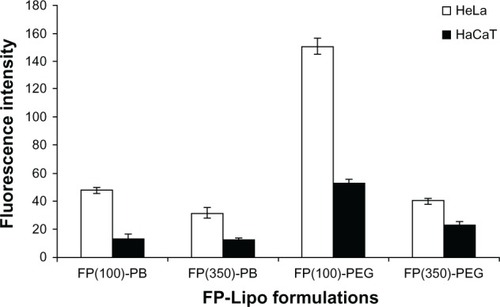
At first, we loaded FITC-dextran into FP-Lipo formulations to compare their cellular uptake behaviors in terms of translocation capability and selectivity. As shown in , all FP-Lipo exhibited the facilitated cellular association and/or internalization of FITC-dextran in the HeLa cells, in which the folic acid receptor was overexpressed compared with HaCaT cells. In particular, FP-PEG-Lipo exhibited higher cellular uptake than that of FP-PB-Lipo, indicating that spacer length might be a critical factor for translocation of FP-Lipo formulations. Moreover, FP(100)-PEG-Lipo exhibited superior cell translocation capability to FP(350)-PEG-Lipo in FR-positive cells, in spite of the lower number of Pep-1 peptide units. Moreover, FP(100)-PEG-Lipo led to about a threefold increase in selective enhancement between target and off-target cells, whereas FP(350)-PEG-Lipo showed less than a twofold enhancement ratio. Hence, FP(100)-PEG-Lipo was considered to be an optimized formulation and was used for further study.
Figure 3 Effect of the number of Pep-1 peptide per vesicle and type of spacer on cell uptake behavior of various FP-Lipo formulations.
Note: Composition of each formulation can be found in .
Abbreviations: FP-Lipo, dual ligand–modified liposomes; FP(100)-PB, FP-Lipo with 100 Pep-1 peptide molecules and PE-PB-mal spacer; FP(350)-PB, FP-Lipo with 350 Pep-1 peptide molecules and PE-PB-mal spacer; FP(100)-PEG, FP-Lipo with 100 Pep-1 peptide molecules and DSPE-PEG2000-mal spacer; FP(350)-PEG, FP-Lipo with 350 Pep-1 peptide molecules and DSPE-PEG2000-mal spacer; PE, phosphatidylethanolamine; PB, phenylbutyryl; mal, maleimide; DSPE, distearoyl phosphatidyl ethanolamine; PEG2000, polyethylene glycol.
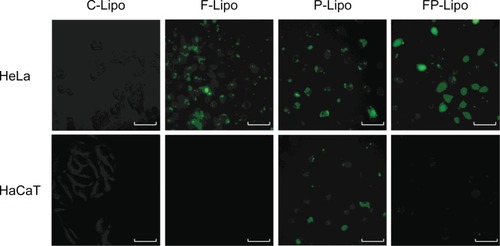
Cellular uptake of FITC-dextran was investigated with various liposomal formulations ( and ). There was an observable difference among the four kinds of liposomal formulations in the two cell lines. The order of the fluorescence intensity in HeLa cells was FP-Lipo > P-Lipo > F-Lipo > C-Lipo. Meanwhile, the uptake efficiency order in HaCaT cells was P-Lipo . FP-Lipo . F-Lipo ≥ C-Lipo (). Compared with C-Lipo, F-Lipo doubled the uptake of the probe in FR-positive HeLa cells, but an insignificant increase in uptake was found in HaCaT cells. CPP-modified liposomes (P-Lipo) facilitated the cellular translocation of the probe remarkably, giving approximately a 4.5-fold greater fluorescence intensity compared with C-Lipo, but there was no difference between HeLa and HaCaT cells. Unlike the nonselective cell-uptake behavior of P-Lipo, FP-Lipo exhibited enhanced selectivity to HeLa cells, revealing a tenfold greater cellular uptake compared with C-Lipo in HeLa cells but only a threefold greater translocation in normal HaCaT cells (). The translocation behaviors of FP-Lipo were further explored by fluorescence microscopy (). As expected from the quantitative analysis results, the cells treated with C-Lipo presented a very weak fluorescence in both cell lines. F-Lipo showed slightly increased fluorescence on the cell surface and cytoplasm of FR-positive cells but little to no fluorescence in the FR-negative cells. On the other hand, numerous green fluorescent spots were found in the cytoplasm with FP-Lipo in the FR-positive cells and to a lesser extent in normal cells. P-Lipo showed increased translocation into cells, with no difference between FR-positive and FR-negative cells ().
Figure 4 The quantitative analysis of FITC-dextran translocated into the cells by various liposomal formulations.
Notes: Values represent mean ± SD (n = 3), and statistical analysis was performed using the Student’s t-test (*P < 0.05 versus paired group).
Abbreviations: FITC-dextran, fluorescein dextran isothiocyanate; FP-Lipo, dual ligand–modified liposomes indicating FP (100)-PEG-Lipo; C-Lipo, conventional liposomes; F-Lipo, folate-modified liposomes; P-Lipo, Pep-1 peptide–modified liposomes.
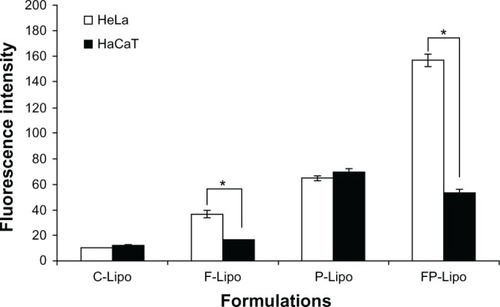
Figure 5 Fluorescence microscopy of HeLa and HaCaT cells incubated with various liposomal formulations containing FITC-dextran at 37°C and pH 7.4 for 4 hours.
Notes: green fluorescence represents cellular translocation of the probe-entrapped liposomal carrier. While peptide conjugation (P-Lipo) increased translocation into both cells with no difference, folate modification (F- and FP-Lipo) manifested the selectivity to FR-positive HeLa cells, resulting in the greatest uptake of FP-Lipo.
Abbreviations: FITC-dextran, fluorescein dextran isothiocyanate; P-Lipo, Pep-1 peptide–modified liposomes; F-Lipo, folate-modified liposomes; FP-Lipo, dual ligand–modified liposomes; C-Lipo, conventional liposomes.
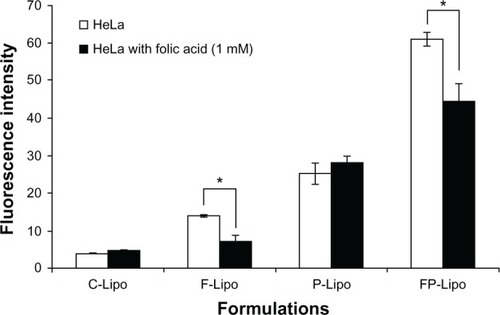
It has been known that folate-tethered liposomal vesicles translocate into cells by receptor-mediated endocytosis.Citation26 To prove the cellular uptake mechanism of this novel dual-ligand system that is partly mediated by the folic acid receptor, excess free folic acid was added to the wells and preincubated with HeLa cells. As expected, in the cases of the C-Lipo and P-Lipo formulations, which lacked folic acid, translocation capabilities were similar in both pretreatment and nontreatment groups (). In contrast, the cellular uptake of F-Lipo and FP-Lipo was significantly suppressed by folic acid pretreatment, with 50% and 25% fluorescence intensity reductions (P < 0.05), respectively, indicating that the folate modification played a major role in the uptake of the liposomal nanocarrier by the HeLa cells.
Figure 6 Effect of FR saturation by free folic acid pretreatment on the cellular uptake of various liposomal formulations.
Notes: 1 mM of free folic acid was added to block Fr on HeLa cells. Values represent mean ± SD (n = 3), and statistical analysis was performed using the Student’s t-test (*P < 0.05 versus paired group).
Abbreviations: FR, folic acid receptor; SD, standard deviation; C-Lipo, conventional liposomes; F-Lipo, folate-modified liposomes; P-Lipo, Pep-1 peptide–modified liposomes; FP-Lipo, dual ligand–modified liposomes.
Cytotoxicity of the FP-Lipo system
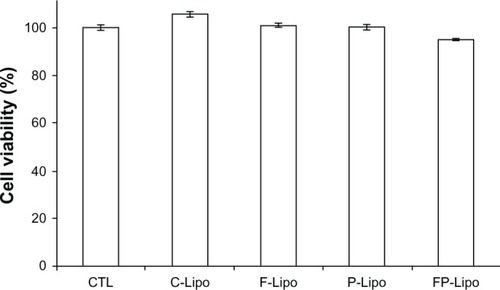
None of the liposome preparations tested caused any significant cytotoxicity to normal mammary epithelial cells at the concentrations used in this experiment (). In comparison with the control group (100%), all the treatment groups were within the range of 95% to 102%, indicating insignificant differences at P < 0.05.
Figure 7 Cytotoxicities of empty liposomal nanocarriers in HaCaT cells by WST-1 assay under the same experimental conditions as in the cell uptake studies.
Notes: Cells were treated with C-Lipo, F-Lipo, P-Lipo, and FP-Lipo for 4 hours, while control cells were treated with double-distilled water (CTL). None of the formulations were cytotoxic to HaCaT cells in the concentrations used for treatment. Data are expressed as mean ± SD (n = 3).
Abbreviations: FITC-dextran, fluorescein dextran isothiocyanate; C-Lipo, conventional liposomes; F-Lipo, folate-modified liposomes; P-Lipo, Pep-1 peptide–modified liposomes; FP-Lipo, dual ligand–modified liposomes; CTL, double-distilled water (control); SD, standard deviation.
Discussion
Recently, CPP-modified nanoparticulate systems have received attention for their excellent translocation capability. Various kinds of CPPs including transactivating transcriptional activator (Tat), arginine-rich peptides (eg, R6, R7, and R9), penetratin, transportan, and Pep-1 peptide were reported as potential ligands for efficient intracellular drug delivery.Citation27–Citation31 The introduction of CPPs to the surfaces of vesicles allows for the efficient translocation of a variety of cargo, including small molecules, nucleic acids, and antibodies, across the plasma membrane.Citation2,Citation3 However, these penetrating peptides could not deliver chemotherapeutic agents with cell specificity. This limitation hampers one of the most promising applications of CPPs; their use in the delivery of drug or imaging agents would have a major relevance if they were able to target specific cells or tissues.
Several strategies have been proposed to enhance the specific cell selectivity, especially by means of either activation of CPPs in a distinctive milieu of the tumor tissue or hybridization of cell-targeting moiety with CPPs. Jiang et alCitation10 constructed a novel carrier composed of anionic neutralizer and cationic CPP, in which both ionic parts were linked via a matrix metalloproteinases (MMP)-cleavable linker. In the MMP-overexpressing cancer tissue, the linker was cleaved and the ionic parts of the chimera could dissociate, allowing the CPP to bind to the surrounding cells. Sawant et alCitation32 developed another strategy to target tumor cells, based on the decrease of pH in tumoral tissue. Until the complex reaches the low pH environment, the Tat peptide is hidden by long-chain PEGs. A decrease of pH in the tumor environment induces the hydrolysis between peptide and PEGs, thereby exposing the CPP. In another study, Myrberg et alCitation8 combined the tumor-homing peptide, PEGA, with the CPP pVEC. The combination of two peptides showed an internalization into tumor cells but not into control tissue, after intravenous injection in tumor-bearing mice.Citation9
As an alternative approach to increasing the translocation of CPP-modified liposomes to cancer cells with specific selectivity, we constructed a dual ligand-modified liposomal carrier, FP-Lipo, by the employment of folic acid and Pep-1 peptide. The FP-Lipo system is 140 nm in size and consists of four different components: PC, Tween 80, DSPE-PEG2000-folate, and DSPE-PEG2000-Pep-1 peptide. PC and Tween 80 are the major components of the liposomal bilayer, carrying the therapeutic agents to the interior compartment. Polyoxyethylene residues in the Tween 80 can cover the liposomal surface and thereby sterically stabilize the system. The DSPE-PEG2000-folate conjugate was incorporated by a postinsertion technique, to render the selectivity of the carrier to FR-overexpressed tumor cells. The number of folic acid molecules in FP-Lipo was set to about 750 molecules per vesicle because maximal cellular uptake of folate-tethered liposomes occurred with 695 to 770 folate ligands per liposome in FR-overexpressed KB cells.Citation33 Pep-1 peptide was attached to the outer surface by a stable maleimide-thiol linkage. The PEG2000, a linking spacer between the vesicle and Pep-1 peptide, helped the peptide to orient outward and ensured the close contact of the two ligands with the cell membrane. The FP-Lipo that used PEG2000 (FP-PEG-Lipo) as a spacer for Pep-1 peptide linkage exhibited significantly higher cell uptake in HeLa cells than the FP-Lipo that used PB as a spacer (FP-PB-Lipo). This might be a result of the steric hindrance effect. Given that the internalization process of peptidyl nanocarriers requires intimate contact with the cell membrane, Pep-1 peptide should be located above the polyoxyethylene residue layer. PEG2000 ensured the direct interaction of penetrating peptides with the cell membrane, whereas the PB group, a relatively short spacer, did not entirely permit the interaction of Pep-1 peptide with the cell membrane. The number of Pep-1 peptide residues was optimized to 100 per vesicle because FP-PEG2000-Lipo with 100 Pep-1 peptides per vesicle exhibited higher cell translocation capability than that with 350 peptides, in spite of the lower number of penetrating peptides. Decrease in the flexibility of the spacer chain in over-tagged liposomes and/or other conformational aspects may explain the disturbed cell uptake of nanocarriers with 350 Pep-1 peptides, but further investigations are required to verify this assumption.
In vitro cellular uptake study revealed that the optimized FP(100)-PEG-Lipo nanocarrier system exhibited significantly enhanced translocation of FITC-dextran into the FR-positive cancer cells than FR-negative normal cells, with excellent uptake-enhancing capability ( and ). Unlike the nonselective cell uptake behavior of P-Lipo, FP-Lipo led to a threefold enhancement in selectivity between target and offtarget cells. Although the F-Lipo system was also capable of achieving similar selectivity to the dual-ligand formulation, its translocation efficiency, expressed as fluorescence intensity, was only about one-fourth of translocation efficiency of the dual-ligand formulation, in the FR-positive cancer cells. From these results, we presumed that after folic acid is modified on the surface of the P-Lipo, the FP-Lipo possess high affinity to the folic acid receptor, which allows the nanoparticulates to be intimately bound and taken up by the FP-positive tumor cells. Moreover, the binding of FP-Lipo to FR in the cell membrane may provide closer contact of Pep-1 peptide with the cell membrane and sequentially, translocate the nanocarrier across the cellular membrane.
In looking further into the details of fluorescence intensity values for different formulations, we noticed formulation effects on the cell uptake behaviors of FP-Lipo, for two variables: folate ligand and Pep-1 peptide. In HeLa cells (FR-overexpressed cells), the fluorescence intensity values of C-Lipo, F-Lipo, P-Lipo, and FP-Lipo (FP(100)-PEG-Lipo) were 10, 36, 64, and 157, respectively (). The value of FP-Lipo was greater than the sum of F- or P-Lipo, indicating that both folic acid and Pep-1 peptide synergistically facilitated the intracellular delivery of FP-Lipo in FR-positive cells. Folic acid modification on the surface of the liposomes allows the nanocarriers to be easily bound and taken up by the tumor cells, where the folic acid receptor is highly expressed.Citation34 Moreover, although the mechanism for translocation of Pep-1 peptide into the cells is not clearly understood, an earlier study suggests that the internalization routes of Pep-1 peptides or Pep-1 peptide/proteinaceous drug complexes are energy-independent and are preferentially mediated via direct interaction of Pep-1 peptide with cell membranes.Citation35 Pep-1 peptide strongly interacts with the lipid bilayer, causing local perturbation and subsequent membrane penetration.Citation36 Meanwhile, the fluorescence intensities of C-Lipo, F-Lipo, P-Lipo, and FP-Lipo in HaCaT cells were 12, 16, 70, and 53, respectively. In normal cells, due to the absence of FR, only Pep-1 peptide triggered the intracellular delivery of FP-Lipo, thus providing similar fluorescence intensity with P-Lipo. This observation correlates very well with the observations of declined cellular uptake of FP-Lipo by the folic acid pretreatment, suggesting that FR-oriented cellular binding and association is partially involved in the cellular uptake of FP-Lipo (). In summary, we found selective enhancement of the FP-Lipo system in FR- overexpressed tumor cells, when compared with P-Lipo. Therefore, we conclude that this novel dual-ligand liposomal system, FP(100)-PEG-Lipo, could be a new platform for selective delivery of chemotherapeutic agents to cancer cells.
Conclusion
A major obstacle for developing a penetrating peptide-modified nanoparticle is the delivery of the pharmacologically active agent to its site of action with selectivity. In this study, as an advanced CPP-modified nanoparticulate system, we constructed the folate-tethered Pep-1 peptide–modified liposomal system and successfully demonstrated the potential capability for selective delivery of chemotherapeutic agents to FR-positive cancer cells.
Acknowledgment
This research was supported by the Basic Science Research Program through the National Research Fund (NRF) awarded by Ministry of Education, Science, and Technology (2011-0009876). We are particularly grateful for the assistance with the cell uptake study given by Department of Biomedical Science and Research Institute for Bioscience and Biotechnology, Hallym University.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorchilinVPRecent approaches to intracellular delivery of drugs and DNA and organelle targetingAnnu Rev Biomed Eng2006834337516834560
- GoncalvesEKitasESeeligJBinding of oligoarginine to membrane lipids and heparan sulfate: structural and thermodynamic characterization of a cell-penetrating peptideBiochemistry20054472692270215709783
- HerbigMEWellerKMMerkleHPReviewing biophysical and cell biological methodologies in cell-penetrating peptide (CPP) researchCrit Rev Ther Drug Carrier Syst200724320325517956214
- TsengYLLiuJJHongRLTranslocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: a kinetic and efficacy studyMol Pharmacol200262486487212237333
- TorchilinVPRammohanRWeissigVLevchenkoTSTAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitorsProc Natl Acad Sci USA200198158786879111438707
- KangMJKimBGEumJYDesign of a Pep-1 peptide-modified liposomal nanocarrier system for intracellular drug delivery: Conformational characterization and cellular uptake evaluationJ Drug Target201119749750520738150
- VivèsESchmidtJPèlegrinACell-penetrating and cell-targeting peptides in drug deliveryBiochim Biophys Acta20081786212613818440319
- MyrbergHZhangLMäeMLangelUDesign of a tumor-homing cell-penetrating peptideBioconjug Chem2008191707518001077
- MäeMMyrbergHEl-AndaloussiSLangelUDesign of a tumor homing cell-penetrating peptide for drug deliveryInt J Pept Res Ther20091511115
- JiangTOlsonESNguyenQTRoyMJenningsPATsienRYTumor imaging by means of proteolytic activation of cell-penetrating peptidesProc Natl Acad Sci USA200410151178677177215601762
- SethuramanVABaeYHTAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumorsJ Control Release2007118221622417239466
- WuMGunningWRatnamMExpression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervixCancer Epidemiol Biomarkers Prev199989770782
- ElnakatHRantnamMDistribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapyAdv Drug Deliv Rev20045681067108415094207
- WeitmanSDLarkRHConeyLRDistribution of the folate receptor GP38 in normal and malignant cell lines and tissueCancer Res19925212339634011596899
- RossJFWangHBehmFGFolate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemiaCancer199985234835710023702
- PanXQZhengXShiGWangHRatnamMLeeRJStrategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acidBlood2002100259460212091353
- SudimackJLeeRJTargeted drug delivery via the folate receptorAdv Drug Deliv Rev200041214716210699311
- ZhaoXBLeeRJTumor-selective targeted delivery of genes and antisense oligodeoxyribonucleotides via the folate receptorAdv Drug Deliv Rev20045681193120415094215
- LeeRJLowPSFolate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitroBiochim Biophys Acta1995123321341447865538
- GabizonAHorowitzATGorenDTargeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes: in vitro studiesBioconjug Chem199910228929810077479
- NagarsenkerMSLondheVYNadkarniGDPreparation and evaluation of liposomal formulations of tropicamide for ocular deliveryInt J Pharm19991901637110528098
- CornellBAMiddlehurstJSeparovicFThe molecular packing and stability within highly curved phospholipid bilayersBiochim Biophys Acta198059824054107378411
- XiangGWuJLuYLiuZLeeRJSynthesis and evaluation of a novel ligand for folate-mediated targeting liposomesInt J Pharm20083561–2293618258394
- LeamonCPLowPSDelivery of macromolecules into living cells: a method that exploits folate receptor endocytosisProc Natl Acad Sci USA19918813557255762062838
- WangSLuoJLantripDADesign and synthesis of [111In] DTPA-folate for use as a tumor-targeted radiopharmaceuticalBioconjug Chem1997856736799327130
- LeeRJLowPSDelivery of liposomes into cultured KB cells via folate receptor-mediated endocytosisJ Biol Chem19942695319832048106354
- GreenMLoewensteinPMAutonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator proteinCell1988556117911882849509
- PoogaMHällbrinkMZorkoMLangelUCell penetration by transportanFASEB J199812167779438412
- MaioloJRFerrerMOttingerEAEffects of cargo molecules on the cellular uptake of arginine-rich cell-penetrating peptidesBiochim Biophys Acta20051712216117215935328
- JoliotAPernelleCDeagostini-BazinHProchiantzAAntennapedia homeobox peptide regulates neural morphogenesisProc Natl Acad Sci USA1991885186418681672046
- MorrisMCDepollierJMeryJHeitzFDivitaGA peptide carrier for the delivery of biologically active proteins into mammalian cellsNat Biotechnol200119121173117611731788
- SawantRMHurleyJPSalmasoS“SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriersBioconjug Chem200617494394916848401
- ElmquistALindgrenMBartfaiTLangelUVE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functionsExp Cell Res2001269223724411570816
- StellaBMarsaudVArpiccoSBiological characterization of folic acid-conjugated poly(H2NPEGCA-co-HDCA) nanoparticles in cellular modelsJ Drug Target200715214615317365286
- DeshayesSHeitzAMorrisMCCharnetPDivitaGHeitzFInsight into the mechanism of internalization of the cell-penetrating carrier peptide Pep-1 through conformational analysisBiochemistry20044361449145714769021
- HenriquesSTCastanhoMATranslocation or membrane disintegration? Implication of peptide-membrane interactions in pep-1 activityJ Pept Sci200814448248718181239