Abstract
The emergence of multidrug-resistant bacteria has been deemed a global crisis that affects humans worldwide. Novel anti-infection strategies are desperately needed because of the limitations of conventional antibiotics. However, the increasing gap between clinical demand and antimicrobial treatment innovation, as well as the membrane permeability obstacle especially in gram-negative bacteria fearfully restrict the reformation of antibacterial strategy. Metal-organic frameworks (MOFs) have the advantages of adjustable apertures, high drug-loading rates, tailorable structures, and superior biocompatibilities, enabling their utilization as drug delivery carriers in biotherapy applications. Additionally, the metal elements in MOFs are usually bactericidal. This article provides a review of the state-of-The-art design, the underlying antibacterial mechanisms and antibacterial applications of MOF- and MOF-based drug-loading materials. In addition, the existing problems and future perspectives of MOF- and MOF-based drug-loading materials are also discussed.
Introduction
Infectious diseases have for centuries been a tremendous obstacle to human progress and survival. They continue to rank among the world’s leading causes of death and disability.Citation1 Infectious diseases have placed a tremendous financial and health burden on the entire world along with an ongoing background of unabating infections and the intermittent appearance of both new and old diseases. Due to their broad-spectrum antibacterial activities, antibiotics have been used to treat bacterial infections since Fleming discovered penicillin in 1928.Citation2 However, the increasing overuse and abuse of antibiotics has been found to have deleterious effects, the most prominent effect being the emergence of antibiotic resistance, which has been recognized as a global crisis, leading to more unfavourable prognoses and higher mortalities.Citation3–5 Even now, multidrug-resistant organisms (MDROs) account for up to 50% of the bacterial strains found in health care admission facilities.Citation6 Therefore, to overcome this difficult situation, further development of various antibacterial strategies is highly desired.Citation5
Nanotechnology has been increasingly used in sterilization and infection control during the past ten years as a result of the development of nanomaterials and nanotechnology. Due to their unique antimicrobial mechanisms, antibacterial nanomaterials were found to be less likely to cause bacterial resistance than conventional antibiotics.Citation7 Many nanomaterial agents, including metal nanoparticles (NPs) (eg, Ag-, Cu-, Mn-), metal oxide NPs (eg, CuO, ZnO, Fe3O4, TiO2), and carbon-based nanomaterials (CNMs), have been found to have effective bactericidal functions.Citation7–12 The aforementioned antibacterial nanomaterials have impressive antibacterial activities, but they still have unignorable disadvantages. For example, metal NPs and metal oxide NPs have exhibited antibacterial effects by releasing metal ions, but the short duration of efficacy and the possibility of unexpected damage to normal cells due to the fast release of metal ions have limited their application.Citation13,Citation14
Due to their diverse active sites and exceptional propertiesCitation15 (high surface area, chemical and thermal stability, tuneable pore sizes, and various functionalities on the internal surface), metal-organic frameworks, a class of microporous polymers made of metal nodes and organic linkers, have been extensively studied and tested for use in a variety of fields, such as the fields of environmental pollution,Citation16,Citation17 gas adsorption,Citation18 catalysis,Citation19 and sensing platforms.Citation20 Its distinctive biodegradability, biocompatibility, and low toxicity have facilitated a wider range of biomedical applications.Citation21–24 Compared with other antibacterial agents, MOFs are unique in the following ways: 1) Their components, pore sizes, and structures can be adjusted according to different requirements to achieve various functions. 2) Their high porosity and specific surface area result in a high efficiency of encapsulation/loading of other agents.Citation25 3) Their low toxicity and biodegradability ensure the biosafety of their applications in vivo.Citation13,Citation25 Thus, MOFs are promising both on their own and as drug delivery systems.
Multiple excellent reviews related to MOFs in the biomedical field were published, some of which discussed the applications of MOF-based materials from a broad perspective, ranging from synthesis and functionalization to wide-ranging biomedical applications,Citation25 others discussed the potential antibacterial action of MOFs as drug carriers.Citation26,Citation27 In contrast to these splendid reviews, this review focuses on the up-to-date synthesis methods, discusses potential antibacterial mechanisms from different aspects, and summarizes the current applications of MOF- and MOF-based materials in antimicrobial treatment. In addition, the related future prospects and challenges are discussed. It is hoped that the review will foster a better understanding for researchers of the current situation of MOFs in the antibacterial field.
Synthesis of Metal-Organic Frameworks
The antibacterial effect of nanoparticles is significantly influenced by their particle size and size distribution. Studies have revealed a relationship between the antibacterial action of graphene oxide and its sheet size, which may be because the edge of the graphene oxide sheet causes damage to the bacterial cell membrane through direct physical contact.Citation28 Nanoparticle synthesis techniques have an impact on particle size and size distribution. The steps involved in the formation of nanoparticles according to the LaMer model and the Gibbs-Tompson crystal nucleation theory can be summarized as follows: 1. The monomer concentration increases continuously. 2. The monomer concentration exceeds the nucleation concentration, and nucleation occurs. 3. The monomer concentration decreases, and crystal growth occurs. Generally, nucleation time and crystal growth rate are two factors that can be adjusted to control the nanoparticle size. Microwaves, for example, generate heat quickly, depleting monomers and producing nanoparticles with a smaller size distribution.Citation29
Metal-organic framework materials are a class of organic-inorganic hybrid materials composed of organic ligands and metal ions or clusters, which can be constructed into one-dimensional, two-dimensional, or three-dimensional materials as required.Citation30 They are generally formed by the self-assembly of metal ions and organic ligands through coordination bonds under mild conditions.Citation27 With the development of technology and the increasing application of MOFs, their synthesis methods have become increasingly diversified. The existing synthetic methods include the hydrothermal method,Citation31 solvothermal method,Citation32,Citation33 ionothermal synthesis,Citation34,Citation35 ultrasonicated method,Citation36 spray drying synthesis,Citation37,Citation38 and microwave-assisted hydrothermal synthesis.Citation39,Citation40 Different synthesis techniques have various benefits and drawbacks. The reactions of hydrothermal synthesis and solvent-thermal synthesis occur under high temperature and high pressure, resulting in a high thermal stability of the obtained products. The ultrasonic method allows materials to nucleate evenly and form small crystals, but the purity of the material is inconsistent. Compared with the traditional hydrothermal/solvothermal method, the microwave heating method has a reaction efficiency that is greatly improved, and the prepared materials have high purity. Electrochemical synthesis has a low yield and is prone to the development of by-products. The properties of the produced crystals differ according to the synthetic conditions, which means that the selection of proper synthesis methods in different fields is crucial. For example, Qian et al synthesized MOF-5 by three different methods (hydrothermal synthesis, direct addition method, and adding H2O2 in the direct method) in 2018 and studied the effects of different synthesis methods on the structure, morphology, and other properties. The results showed that crystals with regular cubic structures were synthesized by hydrothermal synthesis, while the crystals synthesized by the direct addition method and the direct method with the addition of H2O2 were larger and had an irregular morphology. provides a summary of recent MOF structures and the synthesis techniques employed by researchers.
Table 1 Synthesis Methods of MOFs in Recent Years
How Do Antibacterial Nanotherapeutics Work?
The antibacterial activity of MOFs is mainly reflected by the possibility of degradation of the structure with the release of the active components in the form of metal ions and organic ligands. In addition, the shape and size of the MOF particles, the existence of metal active centres, and other mechanisms are significant elements that cannot be disregarded.
The antibacterial properties of MOF materials are closely related to the collapse of their structures. At the same time, the release rate of the MOF component depends on its structural stability and subsequently determines its antibacterial effect. For instance, the MOF structure is less stable when soft acids interact with hard bases, and this combination is more likely to cause structural collapse and the release of components, according to Pearson’s HSAB theory. Various materials have different stabilities due to their coordination bonds, which affect how they exert antibacterial effects in vivo. For example, ZIF materials have zeolite-like structures with a tetrahedral topology composed of Zn or Co and imidazole ligands. They have high chemical stability and can maintain their stable frame structures in high-temperature and water environments while being sensitive to pH value and easily decomposing in an acidic environment.Citation71 The pH of the healthy human body ranges from 7.35 to 7.45, which is indicative of an alkaline environment.Citation72 Due to bacterial growth and metabolism, the pH of the microenvironment at the infected location decreases during bacterial infection and becomes acidic. Ge et al constructed an intelligent drug delivery system for the treatment of osteomyelitis by using ZIF-8 as a carrier to load celecoxib with the characteristic of PH response of ZIF materials.Citation73 When they are delivered to specific locations, they disintegrate and release drugs, which avoids the loss of drugs and improves drug utilization. Additionally, the release of antibacterial metal ions caused by the collapse of the structure can also induce antibacterial activity.Citation74–76 The antimicrobial mechanisms of the different components are discussed separately below.
What is the Role of Metal Ions in Antibacterial Nanotherapeutics?
MOFs are regarded as metal ion reservoirs because of their structural features, which enable them to release metal ions steadily and sustainably.Citation77 Metal ions with excellent antibacterial activity should be the first component taken into account when choosing metal particles for MOF materials. Toxicity during transportation and after degradation is another issue to take into account. Therefore, metal ions should have at least the following two characteristics: antibacterial activity and minimal toxicity.
Many metals are indispensable for the biochemistry and metabolism of life. However, when these necessary metals are present in excess, the harm they cause to cells can be fatal.Citation14 The trace elements found in the highest concentrations in the human body are iron, zinc, and copper. They participate in important cellular metabolic activities, such as catalysis and electron transport.Citation14 Due to their potent antibacterial qualities and low toxicity, zinc, copper, and silver are frequently utilized in the field of biomedicine.
Silver Ions
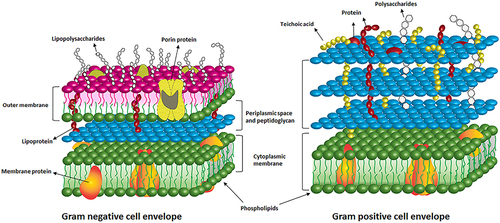
Silver has been demonstrated to have a high antibacterial activity against both gram-positive and gram-negative bacteria. The different inhibitory effects on gram-positive bacteria and gram-negative bacteria are attributed to their different cell wall compositions and material interactions with the cell membrane (as shown in ).Citation78,Citation79
Figure 1 Schematic diagram of the cell membrane of gram-positive and gram-negative bacteria.
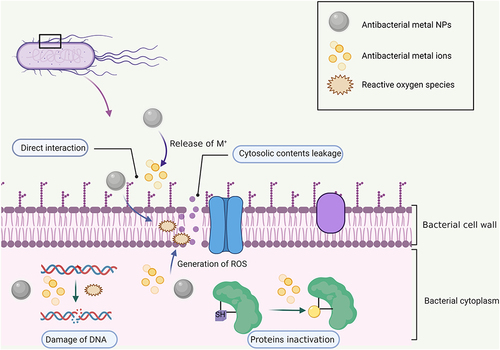
The release of silver ions plays a major role in the antibacterial characteristics of silver, and the mechanisms are basically as follows: 1) Negatively charged bacteria are drawn to positively charged silver ions, which interact with the sulfur-containing proteins in the bacterial cell wall and cause structural damage and cell wall rupture. 2) Silver nanoparticles not only increase membrane permeability and cross the cell membrane but also enter the cell and interact with the contents of the cell, altering their structure and functions. 3) Due to their size and charge, silver nanoparticles can affect metabolic pathways, membranes, and even genetic material by interacting with biological components.Citation81
Copper Ions
Studies have shown that the promising antibacterial and antifungal capabilities of copper nanoparticles are due to both the nanoparticles themselves and the released copper ions, which can interact with proteins that contain sulfur and render them inactive.Citation82,Citation83 Copper has a lower toxicity and antibacterial activity than silver.
The antibacterial activity of copper suggests that the following mechanisms may cause bacterial mortality, similar to that of other metal ions: 1) Through electrostatic contact, copper ions are adsorbed on bacterial cell membranes, influencing the structural integrity and biological activity of the membrane and associated proteins. 2) Some proteins have a strong affinity for copper ions. The normal metabolism of bacteria may be impacted when the concentration of copper ions increases because replacement reactions with elements in proteins or enzymes may result in the inactivation of proteins and enzymes.Citation14
Zinc Ions
Strong bactericidal capabilities against both gram-positive and gram-negative bacteria have been exhibited by zinc nanoparticles.Citation84,Citation85 Additionally, zinc nanoparticles have excellent antifungal properties. Devanand Venkatasubbu et al developed a drug delivery method for treating bone infections using hydroxyapatite and zinc-doped hydroxyapatite, and they demonstrated that the antibacterial property was enhanced with increasing drug concentration and zinc content.Citation84
Due to its role as a crucial cofactor for numerous enzymes, zinc is a trace element that is vital for all living things and has a low harmful effect. Zinc ions at high concentrations have an obvious killing effect on bacteria.Citation86 Despite the scarcity of research, the following three theories dominate the current understanding of zinc’s antibacterial mechanism: 1) Zinc ions released from zinc nanoparticles impair the function of the cell membrane by causing ion concentration differences, which block material transport. 2) Through the positive charge on the bacterial surface, zinc ions directly bind to bacteria on negatively charged cell membranes, where they denature proteins by reacting with functional groups. 3) A large number of free radicals, including OH−, H2O2, and O2−, can be produced by the activation of zinc oxide (ZnO) by UV or visible light. H2O2 can destroy the bacterial cell wall and enter the bacterial body to facilitate sterilization.
Iron Ions
Iron is a trace element that is necessary for life and functions as a cofactor for enzymes in numerous biological pathways, including the transfer of oxygen and carbon dioxide by haemoglobin. However, too much iron can kill bacteria and organisms, and it can also trigger Fenton reactions, which break down proteins, lipids, and DNA.Citation14,Citation87 In TEM images of Fe NP-treated S.li, Lee et al noticed a significant rupture of cell membranes and subsequent leakage of intracellular contents. Because iron has powerful reductive capabilities, the authors hypothesized that iron causes the reductive degradation of functional groups in proteins and outer-membrane lipopolysaccharides. By producing reactive oxygen species, the reaction of Fe2+ with intracellular oxygen or hydrogen peroxide induced by Fe NPs may also cause oxidative stress.Citation88
Other Metal Ions
In addition to the numerous metal ions mentioned above, which have been extensively researched and used in the antibacterial field, there are other relevant metal ions, such as Mn and Co ions. Although the precise antibacterial processes of these metal ions are not fully understood, they have also been proven to have antibacterial effects,Citation89,Citation90 and we hypothesize that these metal ions’ antibacterial mechanisms are comparable to those described above, particularly in the following aspects: 1) Because nanoparticles themselves have positive charges, they can adhere to bacteria by electrostatic adsorption and cause the bacterial surface to disintegrate. 2) Metal NPs release metal ions into bacteria to elicit a toxic effect. 3) The generation of oxidative stress, which results in bacterial death. The antibacterial mechanism of metal ions is shown in .
What is the Role of Organic Ligands in Antibacterial Nanotherapeutics?
The release of organic ligands with antibacterial effects by MOFs can indue antibacterial activity, synergizing with the effect of the released antibacterial metal ions.Citation13 There are several reports of the antibacterial properties of metal ions and chemical ligands. Based on the diversity of MOF components, MOFs can be fabricated from components with antibacterial properties to achieve antibacterial function.Citation14,Citation82,Citation91–94
The antibacterial activity induced by ion release is also influenced by ligand design. Ligand design is one of the important factors affecting the diversity of MOFs, and ligands can affect the topology, stability, and porosity of MOFs.Citation95 MOF-5 ([Zn4O(BDC)3(DMF)9]) and Zn-MOF ([Zn3(BDC)3(H2O)3]4DMF) were developed by Nakhaei et al using the same metal ion and several ligands. This result may be explained by the higher stability of tetranuclear zinc acetate clusters in MOF-5 compared to that of the 2D layered structure in Zn-MOF, which makes it more challenging to release Zn2+ from MOF-5.Citation96
Numerous organic substances, including metallo-organic antimicrobial compounds, organic halogenates, aldehydes, phenols, acyl anilines, heterocyclic acids, and salts, have antibacterial properties. Depending on their actual use, these antibacterial agents can be classified as bactericides, antiseptics, and antimildew agents. Their antibacterial capabilities can be explained in part by the interactions of naturally occurring antibacterial substances with negatively charged bacteria, which changes the permeability of bacterial cell walls and damages their cell membrane. By altering vinyl chloride with trimethylamine, which endows polyvinyl chloride with antibacterial properties, Wu et al fabricated a quaternizated polyvinyl chloride (QPVC) ultrafiltration membrane. When the solution was 50 °C, the QPVC membrane’s antibacterial rate increased to 74.2%.Citation97
However, Kihak Gwon asserts that the ligand is not as important as the central metal and the MOF structure in determining the antibacterial effect, but the antibacterial ligand released after the collapse of the MOF structure still has a certain antibacterial effect.Citation98 An antibacterial Zn-MOF containing hydrazine benzoate linkers was developed by Restrepo et al and was highly effective against S. aureus. It had long-lasting antibacterial properties because of the ligand’s regulated release.Citation86 A new BioMIL-5 made of Zn2+ and azelaic acid (AzA), both of which have intriguing antibacterial effects, was developed by Tamames-Tabar et al. They calculated the MIC/MBC values for AzA and Zn2+, respectively, and reported that this material had additive rather than synergistic antibacterial activity.Citation99
Other Possible Mechanisms
The particle size of a substance is a very important factor in determining its properties. When the particle size of the material is reduced to the nanometre scale, the particle has a significant surface effect due to its small surface area and large surface area, thus allowing better performance.Citation100 Numerous studies have demonstrated that nanomaterials exhibit higher antimicrobial activity than conventional materials and that the antibacterial properties of nanomaterials improve with decreasing particle size.Citation100–102
MOFs can be combined with other substances to elicit corresponding effects in addition to the antibacterial effect they have on their own due to their physical and chemical characteristics. These materials are further discussed in the section on antibacterial applications that follows.
What are the Potential Applications of Metal Nanotherapeutic Platforms in the Antibacterial Field?
According to the explanation of the antibacterial mechanism provided above, the MOF’s constitution determines its outstanding antibacterial characteristics. In addition, outstanding researchers have used MOF materials as drug carriers to continuously develop new nanocomplex materials that exhibit a significant antibacterial effect of “1 + 1> 2”. Below, we discuss examples of MOF antibacterial applications from the following two aspects: pure MOFs as antibacterial agents and MOF-based compounds as antibacterial agents.
Pure Metal Organic Frameworks
Ag-Based MOFs
Ag3(1), composed of 3-phosphonobenzoic acid and Ag+ ions, was developed by Berchel et al. According to the Pearson HSAB hypothesis, 3-phosphonobenzoic acid is a hard base with carboxylic and phosphoric acids. When combined with Ag+ ions, Ag3(1) becomes stable, which is beneficial for the gradual release of Ag+. The minimum bactericidal concentration was determined, and the results showed that Ag3(1) had broad-spectrum antibacterial activity (including multidrug resistant strains) against three Staphylococcus aureus, one Escherichia coli and two Pseudomonas aeruginosa strains.Citation103
Lu et al synthesized two three-dimensional Ag-based MOFs, [Ag2(O-IPA)(H2O)·(H3O)] and [Ag5(PYDC)2(OH)] and tested their antibacterial activity against E. coli and S. aureus by measuring the minimum inhibitory concentration (MIC) and inhibition zone (ZOI). After the experiment, it was concluded that the MICs of [Ag2(O-IPA) (H2O)·(H3O)] and [Ag5(PYDC)2(OH)] were in the range of 5–10 ppm and 10–15 ppm for Escherichia coli and 10–15 ppm and 15–20 ppm for Staphylococcus aureus, which indicated that [Ag2(O-IPA)(H2O)·(H3O)] and [Ag5(PYDC)2(OH)] had better antimicrobial activity than most silver-based antimicrobial materials with MICs of 10–40 ppm. The inhibition zones of [Ag2(O-IPA)(H2O)·(H3O), Ag5(PYDC)2(OH)], commercial Ag-NPs, and pure ligands were measured by the authors. The results showed that the ZOIs of [Ag2(O-IPA)(H2O)·(H3O) and Ag5(PYDC)2(OH)] were larger than those of commercial Ag-NPs, and the diameter of the ZOI of pure ligands was similar to that of filter paper, indicating that they had almost no antibacterial activity. This indicated that the antibacterial activity of Ag-based MOFs is not related to the ligand but depends on the release of the central metal ion. The Ag+ release of [Ag2(O-IPA)(H2O)·(H3O), Ag5(PYDC)2(OH)] and Ag-NPs was also measured by the author. The concentration of Ag+ was 18.76–25.10 ppm in the Ag-MOF solution on the fifth day compared to 6.685–5.942 ppm in the Ag-NP solution, proving that the release of Ag+ was the origin of the antibacterial activity of Ag-based MOFs.Citation104
Cu-Based MOFs
A Cu/H3BTC MOF was developed by Shams et al. Its antibacterial properties against S. aureus and E. coli were tested by ZOI. The ZOI diameters against S. aureus and E. coli were 22 mm and 16 mm, respectively, and the antibacterial activity increased with increasing Cu/H3BTC concentration. By comparing SEM images of the bacteria in the control group, which had a firm and complete cell wall, to those of the bacteria exposed to increasing concentrations of Cu/H3BTC, it was found that the severity of bacterial cell membrane destruction rapidly increased. Therefore, the authors hypothesized that the observed bacteriostatic activity may have been caused by Cu2+ release, which resulted in cell wall and membrane rupture.Citation105
Rauf et al synthesized copper-based coordination polymers [Cu(HBTC)(H2O)3], which were macroscopically nanofibers, by microwave-assisted hydrothermal synthesis. Antibacterial activity was determined by assaying samples for colony count assays against E. coli and S. aureus. The mics of commercial Cu NPs were larger than 250 µg/mL, whereas the mics of nanofibers against E. coli and S. aureus were 200–250 µg/mL and 250–300 µg/mL, respectively. At a concentration of 250 µg/mL, the nanofibers were effective against E. coli and S. aureus. The inhibition rates were 99.9% and 99.1%, compared to 24% for commercial copper NPs at the same concentration. The ligand H3BTC had no obvious inhibitory effect on bacteria, which indicated that the copper-based coordination polymer [Cu(HBTC)(H2O)3] released copper ions to facilitate sterilization.Citation106 Studies have demonstrated that copper-based material treatment can produce ROS, which cause oxidative stress and inhibit bacterial growth.Citation107,Citation108 The ROS produced by commercial Cu NPs and nanofibers were compared by the authors, who discovered that the output of the former was much lower that of the latter.Citation106
Zn-Based MOFs
For the first time, a Zn-MOF was developed by Akbarzadeh et al using the ultrasound-assisted reverse glue method. They then tested the effectiveness of the Zn-antibacterial MOF on six different bacterial strains, including Staphylococcus aureus, Escherichia coli, Salmonella enterica, Klebsiella pneumoniae, Bacillus subtilis, and Acinetobacter baumannii, and the results showed that the Zn-MOF had a good antibacterial effect against the tested bacteria.Citation109
Fe-Based MOFs
Due to its limited antibacterial action under dark conditions, Mil-101 is frequently utilized as a carrier.Citation110,Citation111 Peng et al synthesized MIL-101(Fe) and tuned the unsaturated iron site ratio to increase the potential to produce ROS, making it extremely effective for sterilization in the dark. Experiments showed that when the proportion of unsaturated iron sites was increased, obvious antibacterial activity was observed, and the iron ion leaching experiment confirmed that its antibacterial activity was not determined by the leached iron ions.Citation111
Photodynamic Antibacterial Agents
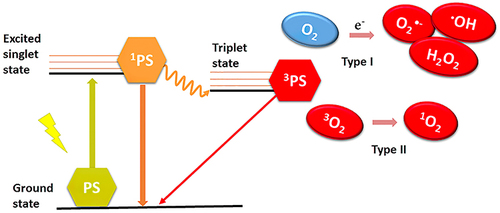
Antibacterial photodynamic therapy (APDT) is a promising alternative to anti-infective therapy that is proposed for photosensitization based on bacteria and photosensitizers (PSs). The mechanism of APDT is as follows: after being exposed to resonant wavelength light, photosensitizers produce reactive oxygen species (ROS), which have a high potential to kill bacteria (as shown in ).Citation80 ROS can act on multiple bacterial targets, thereby providing more opportunities for killing bacteria, and they are less likely to induce drug-resistant bacteria than antibiotics.Citation112,Citation113
Figure 3 Mechanism of APDT to generate ROS.
It has been observed that MOFs, which are self-assembling porous coordination polymers made of metal ions and organic ligands, have photodynamic antibacterial capabilities similar to ZIF-8 and show promise in the treatment of drug-resistant bacterial infections. Due to the designability of MOF components, metal cluster or organic linker tuning can improve the photocatalytic performance of MOFs.Citation114 Combining MOFs and PSs is one strategy that might be used to take full advantage of the benefits of APDT. The surface of MOFs can be modified with photosensitizers, or MOFs can be used as the carrier to load the photosensitizer and transport it to the bacteria to induce antibacterial activity, and the antibacterial metal ions or organic ligands released from MOFs can also have a synergistic antibacterial effect.Citation76,Citation115,Citation116 This point is explained later in this review.
Porphyrins are macromolecular heterocyclic compounds with unique photophysical properties that can produce ROS under irradiation with 660 nm light.Citation117,Citation118 Because of their good biological properties, porphyrins have been widely used as photosensitizers for PDT.Citation119 Sun synthesized a porphyrin-based MOF PCN-224 composed of Zr6 clusters and the TCPP (tetrakis (4-carboxyphenyl)-porphyrin) ligand, which demonstrated great biomedical potential for PDT. A portable band-aid made of chitosan and PCN-224 with the capacity to generate ROS for sterilization when exposed to light irradiation was developed. Moreover, an experiment showed that the combination of PDT and chemotherapy had a strong synergistic effect on killing drug-resistant E. coli.Citation113
Donglin Han introduced Cu2+ into PCN-224 through hydrothermal synthesis to obtain a CuMOF that exhibited a high photodynamic bactericidal efficiency of 99.71% against S. aureus and 97.14% against E. coli under light irradiation at 660 nm within 20 minutes. The doped Cu2+ improved the photocatalytic property of the material by enhancing its capacity to capture photogenerated electrons. The d-d transition of Cu2+ also improved the heat-generation ability of the material.Citation120
Bimetallic PCN-224(Zr/Ti) was developed by Chen et al through cation exchange. The inclusion of Ti dramatically improved photocatalytic performance and prevented ultraviolet light damage to healthy tissues by efficiently extending PCN-224’s light response into the visible region. Studies revealed that PCN-224(Zr/Ti) had a significantly greater antibacterial efficiency than PCN-224. In addition, they created a PLGA-based dressing loaded with PCN-224(Zr/Ti) NPs for use in animal wound models, which showed that the dressing promoted wound healing by killing bacteria and controlling inflammation.Citation121
Yang et al designed an Ag-doped MOF derivative (C-Zn/Ag) with a graphitic-like carbon structure, which was reported to be capable of broad-spectrum optical absorption and efficient photo-to-thermal conversion. The bacteria-killing rate of C-Zn/Ag under near-infrared irradiation was 76%, while the bacteria-killing rate of C-ZIF with only zinc ion release was 43%, showing that the synergy enhanced the antibacterial performance.Citation122
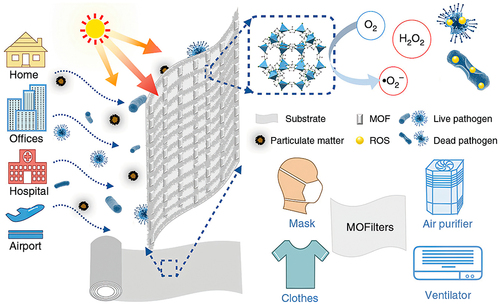
Li et al reported that ZIF-8 exhibited an excellent inactivation efficiency of 99.9999% against E. coli in saline under 2 h of simulated solar irradiation, and they proved that the antibacterial effect depended on the intact ZIF-8 rather than the release of zinc ions in the presence of light irritation because the concentration of zinc ions (2.65 mg L−1) released from ZIF-8 did not reach the minimum inhibitory concentration (MIC, 31.25 mg L−1). They developed a MOFilter mask and proved it was more effective than some commercial masks, providing new ideas for the development and application of porous photocatalytic antibacterial materials. (as shown in ).Citation114
Figure 4 Schematic diagram of MOF-based filter.
Metal Organic Frameworks-Based Compounds
As indicated by the above discussion, we know that pure MOF has a certain bactericidal effect, but its antibacterial effect is relatively limited compared to MOF-based composites. For instance, ZOIs of Zn-MOF Zn2(BDC)2(DABCO) and gentamicin-loaded Zn2(BDC)2(DABCO) against S. aureus and E. coli were studied by Nabipour et al (Zn2(BDC)2(DABCO): ZOI 8 mm for E. coli, 6 mm for S. aureus. Gentamicin loaded Zn2(BDC)2(DABCO): ZOI 9 mm for E. coli, 16 mm for S. aureus).Citation123 Therefore, at present, MOFs are mostly combined with other antibacterial materials to induce a greater bactericidal effect. The large pore size and high specific surface area of MOFs provide a perfect platform for the loading of additional antibacterial substances.
Metal Organic Frameworks Act as Carriers to Load Antibiotics in Antibacterial Therapy
As a class of organic antibacterial agents, antibiotics have been commonly used for the treatment of bacterial infections.Citation124 However, the concentration of antibiotics reaching the infection site is occasionally insufficient to kill germs and cure the infection, as antibiotics are carried in the blood and metabolized by the human body. It has been proposed to use MOF-based materials as carriers to transport antibiotics to the infection site, which can allow the release of drugs through different mechanisms to achieve bactericidal and anti-infection effects. It has been reported that this new antibacterial strategy can induce a synergistic antibacterial effect and lower the dose of antibiotics required to reduce the emergence of bacterial resistance.
An example of a MOF is UiO-66, which is composed of Zr ions and terephthalic acid and may absorb antibiotics into its pores or surface through electrostatic and hydrophobic interactions.Citation125 Nasrabadi et al prepared UiO-66 by the solvothermal method and loaded ciprofloxacin (CIP) into UiO66 to test its drug loading and antibacterial activity.Citation126 The research results revealed that UiO-66 had a very high CIP loading rate of up to 84% and displayed significant antibacterial activity against S. aureus and E. coli. The inhibitory zone of UiO-66-CIP against S. aureus was 24 mm, and the inhibitory zone against E. coli was 22 mm. S. aureus was resistant to CIP, and the inhibitory zone of CIP against E. coli was 14 mm, suggesting that the controlled release system eliminated the drug resistance of S. aureus and had enhanced antibacterial activity against E. coli.Citation126
ZIF-8, composed of zinc ions and dimethylimidazole, was employed to construct a pH-responsive drug delivery system due to its stability in neutral aqueous solution and disintegration in an acidic environment.Citation127 Nabipour et al encapsulated CIP in ZIF-8 through the nanoprecipitation method and compared the drug release rate under different pH values. It was concluded that the amount of CIP encapsulated in ZIF-8 was 21 (w/w) %, and the antibacterial activities were higher than those of ZIF-8 (CIP-ZIF-8: ZOI 46 mm for E. coli, 49 mm for S. aureus. ZIF-8: ZOI 14 mm for E. coli and 12 mm for S. aureus). At pH 5.0, the release rates of CIP were faster than those at pH 7.4, indicating that ZIF-8 is a pH-responsive DDS, and it may be a useful antibiotic delivery system for the treatment of infections.Citation128
Due to its large pore size, noticeable surface area, and capacity for the incorporation of backbone functional groups, the MIL family of MOFs, which are composed of trivalent metal centres and carboxylic acid bridging ligands, show tremendous promise for drug delivery.Citation27 Simon et al used MIL-100 as a carrier of isoniazid to overcome its poor solubility and bioavailability, thereby offering an improvement upon the hepatotoxicity, peripheral neuritis, and emergence of drug-resistant strains that may be caused by long-term isoniazid treatment. They evaluated the physicochemical features of MIL-100 and the adsorption and release of isoniazid. The findings demonstrated that MIL-100 exerted an effective, controlled release of isoniazid and that there were no burst release phenomena during the release process. Moreover, MIL-100 had good biocompatibility, and the above findings suggest that MIL-100 is a promising isoniazid drug delivery system.Citation41
Metal Organic Frameworks-Metal/Metal Oxide Antibacterial Agents
Metal and metal oxides have excellent antibacterial effects on both gram-positive and gram-negative bacteria. Metals, however, have the propensity to agglomerate or oxidize, which reduces their surface area and lessens their antibacterial action.Citation129 Due to their desirable characteristics of large pores, high porosity, and high surface area, MOFs have been extensively studied as templates for immobilizing metal NPs to create MOF-based composites.Citation130,Citation131 For instance, to increase the stability and dispersity of silver nanoparticles, Duan et al immobilized the particles on the surface and in the pores of HKUST-1. The combination of copper ions in HKUST-1 and carboxyl groups in carboxymethylated fibres (CFs) allowed the particles to be uniformly fixed on the fibre surface to form Ag NPs@ HKUST-1@CFs. The antibacterial activities of HKUST-1, Ag NPs@HKUST-1 and Ag NPs@HKUST-1@CFs were determined using the shake flask method, and the results showed that Ag NPs@HKUST-1@CFs had the highest antibacterial activity. The authors asserted that the Ag NPs and the gradual release of copper ions by HKUST-1 were responsible for its antibacterial action.Citation130
Metal Organic Frameworks-Photosensitizer Antibacterial Agents
Bagchi et al developed a dual-stimuli-responsive therapeutic platform against drug-resistant bacteria with hydrophobic PS squaraine (SQ) embedded in a zeolitic imidazolate framework (ZIF-8). The encapsulation of SQ within ZIF-8 limited the aggregation of SQ and improved ROS generation capacity. Moreover, because ZIF-8 is sensitive to pH, hydrolysis in acidic environments enabled SQ to be specifically transported to the site of infection, reducing the likelihood of damaging normal tissues. Furthermore, an experiment demonstrated that ZIF8-SQ had a superior antibacterial effectivity against MRSA even at a very low concentration range under red-light irradiation.Citation115
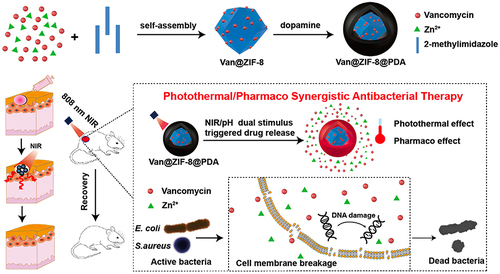
Some researchers developed a NIR/pH dual stimulus-responsive antibacterial formulation by encapsulating antibiotics in photosensitizer-modified MOFs (Van@ZIF-8@PDA). Polydopamine (PDA) had a high antibacterial impact due to its thermal effect when exposed to near-infrared light, which was synergized with the degradation of ZIF-8 to release zinc ions and loaded medicinal drugs (as shown in ).Citation76
Figure 5 Schematic diagram of photothermal synergistic drug antibacterial.
UiO-66 is an ideal drug carrier that can be functionalized with amino groups, nitro groups, etc. In addition, it can electrostatically and hydrophobically attract antibacterial compounds to its pores and surfaces. Lv et al combined UiO-66 with the photosensitizer zinc phthalocyanine to construct an efficient drug-loading system. By electrostatic adsorption and molecular interactions, the chemical antibacterial agent linezolid and lysozyme were successively loaded on the inside and outside of its pores to produce (Li@UiO-66-H4Pc)@lysozyme. Antibacterial experiments were carried out on UiO-66-NH2, UiO-66-H4Pc, Li@UiO-66-H4Pc, and (Li@UiO-66-H4Pc)@lysozyme in the dark and in the light. Li@UiO-66-H4Pc and (Li@UiO-66-H4Pc)@lysozyme both showed various antibacterial activities in the absence of light, whereas UiO-66-NH2 and UiO-66-H4Pc exhibited no apparent antibacterial effects. UiO-66-H4Pc, Li@UiO-66-H4Pc, and (Li@UiO-66-H4Pc)@lysozyme all had apparent dose-dependent antibacterial responses when exposed to laser radiation. These results indicated that antimicrobials and zinc phthalocyanine synergistically kill most bacteria under light. A possible antibacterial mechanism is the destruction of the bacterial cell wall by lysozyme, making it easier for nanomaterials to enter the bacteria to exert a better bactericidal effect. Additionally, the ROS generated under light can kill stubborn bacteria through multiple targets to achieve synergistic photodynamic and chemical antibacterial effects.Citation125
The addition of a photosensitizer endows MOFs with photodynamic antibacterial activity, and MOFs also have a favorable impact on the additional photosensitizer. Li et al, for instance, proposed a photosensitizer-modified ZIF-8 nanocomposite that killed MRSA with high efficiency through the potential synergistic antibacterial effect of the metal ions and photosensitizer. The combination of Ce6 (Chlorin e6) and ZIF-8 made the absorbance intensity of Ce6 more stable and enhanced its ability to generate ROS.Citation116
Other
MOF-Chlorine Disinfectant
In the past two years, there has been a global pandemic of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Citation132 The World Health Organization (WHO) suggested the global use of face masks to prevent COVID-19 infection. SARS-CoV-2 can exist on the surface of various objects, including textile-based materials, such as face masks, for several days, which can produce contamination sources to result in a more widespread infection, so it is urgent to develop personal protective equipment with the ability to kill pathogenic microorganisms.Citation133
Cheung et al designed multifunctional N-chlorine-based textiles coated with UiO-66 and loaded with N-chlorine biocide to develop a material with rapid biocidal activity. Antibacterial experiments showed that the UiO-66-NH-Cl-PET composite demonstrated a rapid bactericidal effect on both gram-positive and gram-negative bacteria, and the sterilization effect reached 107–108 CFU/mL within 5 minutes. Obvious deformation and membrane collapse of treated bacteria were observed under a scanning electron microscope. Due to the presence of a layer of lipopolysaccharide outside the cell wall of gram-negative bacteria, UiO-66-NH-Cl-PET had a lesser antibacterial effect on gram-negative bacteria than on gram-positive bacteria. Additionally, UiO-66-NH-Cl-PET exhibited potent biocidal activity against SARS-CoV-2 and the selective degradation of sulfur mustard and its chemical simulant 2-chloroethyl ethyl sulfide, suggesting that the composite has great potential to be prepared as multifunctional protective wear that can prevent pathogenic microorganisms and sulfur-based chemical warfare agents.Citation133
MOF-Iodine
Teng et al designed a composite coating composed of ZIF-8 and iodine on orthopaedic implants, which not only had a synergistic antibacterial effect but also enhanced the osseointegration of the coated implants. In this study, ZIF-8 functioned as a carrier for povidone-iodine to control its release and induce its antibacterial activity while also using its capacity to produce ROS under infrared radiation to kill bacteria. This suggests the application potential of povidone-iodine a feasible antibacterial agent for orthopaedic applications.Citation134
MOF-Dimethyl Fumarate
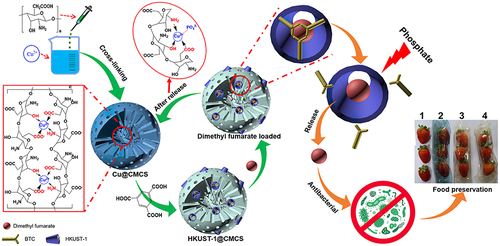
Antibacterial composite materials based on MOFs are also appropriate for industrial uses in food, heating, water, and other fields.Citation135,Citation136 For example, Huang et al combined carboxymethyl chitosan (CMCS) and HKUST-1 to fabricate an ecofriendly, recyclable, long-acting, and intelligent antibacterial agent carrier for loading dimethyl fumarate, which is a nontoxic preservation material with antimicrobial capability. The porosity of the MOFs and the characteristics of polymer processing were fully utilized in the fabrication of the MOF-CMCS composite. Since the active groups such as -COOH, -NH2, and -OH of CMCS may be intimately coupled with the copper ions in HKUST-1, no copper ions are left in the release matrix, avoiding any potential biological safety issues and environmental pollution caused by metal ions. When employed in the food industry, MOF-CMCS can efficiently control the release of dimethyl maleate to induce a long-term antibacterial effect and extend the storage time of food (as shown in ).Citation135
Figure 6 Schematic diagram of the synthesis and antibacterial application of HKUST-1@CMCS.
Among the MOF-based compounds applied as antibacterial agents listed above, MOFs and other materials were combined and complemented each other to achieve a synergistic antibacterial effect of “1+1>2”. The diversity of MOF components makes it possible for them to have synergistic antibacterial effects. In addition to the synergistic effect with other materials, MOFs themselves also exhibit interesting phenomena. For example, some MOFs exhibit antibacterial activity superior to that of their corresponding ligands and exhibit antibacterial activity without ligand release, which may be explained by Tweedy’s chelating theory.Citation137,Citation138 The chelation effect allows the metal ion to share a partial positive charge with the ligand and the possible presence of delocalized electrons during the chelation process, which increases the lipophilicity of the metal and makes it easier for them to cross the lipid-like layer of the bacterial cell membrane to achieve enhanced antibacterial activity.Citation137 For instance, complexes of 2-(5-chloro/nitro-1H-benzimidazol-2-yl)-4-bromo/nitro-phenols (HLx; x =1-4) and zinc (II) nitrate were synthesized, and paper diffusion experiments were performed to assess their antibacterial activity. It was discovered that [Zn(L1)2]·H2O exhibited strong antibacterial activity, whereas the ligand and zinc nitrate alone did not exhibit antibacterial activity.Citation139
In addition to antibacterial applications, MOFs could also be promising in other fields. Tumour therapy is a developing field in which MOFs have great application potential. Substances with pharmacological activity can be chosen as ligands or metal ions for direct assembly to obtain MOFs with therapeutic functions due to the modifiability of the MOF component.Citation140 Surface modification of MOFs can also be performed to avoid their clearance by the immune system, protect the delivered drugs, etc.Citation141 Additionally, MOF-based drug delivery systems have been developed significantly in a number of therapeutic modalities, including chemotherapy, photodynamic therapy, radiation, and immunotherapy, all of which have shown promising therapeutic effects.Citation140,Citation142
Conclusions and Future Prospects
In addition to harming human health, the regional spread of infectious illnesses has a significant negative economic impact because the fear of infection can impede many industries, including consumption, entertainment, and tourism industries.Citation143 In the above discussion, we summarized the various antibacterial mechanisms and applications of various MOFs, which indicated that the application of MOFs and MOF-based materials is a promising therapeutic approach for refractory illnesses caused by resistant bacteria. In terms of infection control, timely and accurate diagnosis of bacterial infections is just as crucial as the subsequent infection therapy.
Monitoring of the corresponding inflammatory indicators, such as C-reactive proteinCitation144 and calcitoninogen,Citation145 is the traditional method used for the clinical diagnosis of bacterial infections, particularly deep infections. However, changes in these inflammatory indicators always occur after the development of the infection. H2S is a metabolite of many microorganisms that can alter the peri-infective microenvironment. Many bacterial strains have been found to be protected by H2S, which lessens the damage that antibiotics may cause to them.Citation146 One study used BI-MOF to monitor H2S in the microenvironment by taking advantage of the strong affinity of Bi for H2S, and the two reacted to produce Bi2S3, which can be triggered by a laser with deep tissue penetration and signalling.Citation147 This can allow the early monitoring of bacterial infections and offers a promising diagnostic tool for the rapid and precise diagnosis of infections. The monitoring function of MOFs was employed in a range of sensors for tracking viruses, microorganisms in drinking water, etc., in addition to assisting in the early identification of bacterial illnesses.Citation148–150
Although MOFs have many advantages compared with other antimicrobial materials, such as the tunability of the composition structure and drug loading capacity, as mentioned above, there are still some factors that limit their practical application. First, MOFs must be synthesized under stringent conditions. Variations in time, temperature, and environment might result in various MOF shapes and sizes, which directly affect the physicochemical characteristics of MOFs. Second, the powder state of MOFs limits its further application, and future research should concentrate on developing MOF-based macrocomposites employing techniques such as electrostatic spinning. Third, the conclusions of studies on MOF toxicity are not clear. The majority of the investigations being conducted right now include short-term cellular or animal tests, and proof of the hazardous consequences of long-term MOF application is insufficient. In addition, MOF toxicity is influenced by their composition, size, shape, and tolerance in living tissues, necessitating a thorough evaluation of the toxicity of various MOFs.
In summary, this paper provides a review of the current research progress of MOFs from the perspective of their applications in the antimicrobial field, including the synthesis, antimicrobial mechanism, and application of MOFs and MOF-based materials. Although significant progress has been achieved, this progress is insufficient, and there are still no studies available on the long-term toxicity of MOFs and real-world therapeutic MOF applications. It is hoped that the antibacterial qualities of MOFs will garner more interest in the fields of water and environmental treatment, biomedical science, and materials science, leading to more research and practical applications of MOFs.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The work was supported by the Shandong Provincial Natural Science Foundation Youth Project (ZR2021QH251) and Clinical Medicine +X Research Project of Affiliated Hospital of Qingdao University (QDFY+X2021055).
References
- Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430(6996):242–249. doi:10.1038/nature02759
- Bentley R. The development of penicillin: genesis of a famous antibiotic. Perspect Biol Med. 2005;48(3):444–452. doi:10.1353/pbm.2005.0068
- Khan J, Tarar SM, Gul I, Nawaz U, Arshad M. Challenges of antibiotic resistance biofilms and potential combating strategies: a review. 3 Biotech. 2021;11(4):169. doi:10.1007/s13205-021-02707-w
- Houghton D. Antimicrobial resistance in the intensive care unit: understanding the problem. AACN Clin Issues. 2002;13(3):410–420. doi:10.1097/00044067-200208000-00007
- Srivastava J, Chandra H, Nautiyal AR, Kalra SJS. Antimicrobial resistance (AMR) and plant-derived antimicrobials (PDAs) as an alternative drug line to control infections. 3 Biotech. 2014;4(5):451–460. doi:10.1007/s13205-013-0180-y
- Lewnard JA, Reingold AL. Emerging challenges and opportunities in infectious disease epidemiology. Am J Epidemiol. 2019;188(5):873–882. doi:10.1093/aje/kwy264
- Xin Q, Shah H, Nawaz A, et al. Antibacterial carbon-based nanomaterials. Adv Mater. 2019;31(45):e1804838. doi:10.1002/adma.201804838
- Goudouri O-M, Kontonasaki E, Lohbauer U, Boccaccini AR. Antibacterial properties of metal and metalloid ions in chronic periodontitis and peri-implantitis therapy. Acta Biomater. 2014;10(8):3795–3810. doi:10.1016/j.actbio.2014.03.028
- Markowska K, Grudniak AM, Wolska KI. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol. 2013;60(4):523–530.
- Hans M, Mathews S, Mücklich F, Solioz M. Physicochemical properties of copper important for its antibacterial activity and development of a unified model. Biointerphases. 2015;11(1):018902. doi:10.1116/1.4935853
- Hu H, Yu L, Qian X, Chen Y, Chen B, Li Y. Chemoreactive nanotherapeutics by metal peroxide based nanomedicine. Adv Sci. 2020;8(1):2000494. doi:10.1002/advs.202000494
- Chen W-J, Tsai P-J, Chen Y-C. Functional Fe3O4/TiO2 core/shell magnetic nanoparticles as photokilling agents for pathogenic bacteria. Small. 2008;4(4):485–491. doi:10.1002/smll.200701164
- Li R, Pan X. Metal-organic-framework-based materials for antimicrobial applications. ACS Nano. 2021;23(15):3808–3848.
- Godoy-Gallardo M, Eckhard U, Delgado LM, et al. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioact Mater. 2021;6(12):4470–4490. doi:10.1016/j.bioactmat.2021.04.033
- Shadpour Mallakpour EN, Hussain CM. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord Chem Rev. 2021;451:245.
- Li L, Zou J, Han Y, et al. Recent advances in Al(iii)/In(iii)-based MOFs for the detection of pollutants. N J Chem. 2022;46(41):19577–19592. doi:10.1039/D2NJ03419K
- Zhong Y, Chen C, Liu S, et al. A new magnetic adsorbent of eggshell-zeolitic imidazolate framework for highly efficient removal of norfloxacin. Dalton Transact. 2021;50(48):18016–18026. doi:10.1039/D1DT03020E
- Qin L, Li Y, Liang FL, et al. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022;341:341. doi:10.1016/j.micromeso.2022.112098
- Bathla A, Lee J, Younis SA, Kim K-H. Recent advances in photocatalytic reduction of CO2 by TiO2– and MOF–based nanocomposites impregnated with metal nanoparticles. Mater Today Chem. 2022;24:100870. doi:10.1016/j.mtchem.2022.100870
- Sohrabi H, Salahshour Sani P, Orooji Y, Majidi MR, Yoon Y, Khataee A. MOF-based sensor platforms for rapid detection of pesticides to maintain food quality and safety. Food Chem Toxicol. 2022;165:113176. doi:10.1016/j.fct.2022.113176
- Yang J, Yang YW. Metal-organic frameworks for biomedical applications. Small. 2020;16(10):e1906846. doi:10.1002/smll.201906846
- Chen J, Cheng F, Luo D, et al. Recent advances in Ti-based MOFs in biomedical applications. Dalton Transact. 2022;51(39):14817–14832. doi:10.1039/D2DT02470E
- Qin L, Liang FL, Li Y, et al. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics. 2022;10(11):202. doi:10.3390/inorganics10110202
- Zhang W, Ye G, Liao D, et al. Recent advances of silver-based coordination polymers on antibacterial applications. Molecules. 2022;27(21):24.
- Yang J, Yang Y-W. Metal-organic frameworks for biomedical applications. Small. 2020;16(10):e1906846.
- Carrillo-Carrión C. Nanoscale metal-organic frameworks as key players in the context of drug delivery: evolution toward theranostic platforms. Anal Bioanal Chem. 2020;412(1):37–54. doi:10.1007/s00216-019-02217-y
- Huxford RC, Della Rocca J, Lin W. Metal-organic frameworks as potential drug carriers. Curr Opin Chem Biol. 2010;14(2):262–268. doi:10.1016/j.cbpa.2009.12.012
- Hatamie S, Ahadian MM, Soufi Zomorod M, et al. Antibacterial properties of nanoporous graphene oxide/cobalt metal organic framework. Mater Sci Engine C. 2019;104:109862. doi:10.1016/j.msec.2019.109862
- Wang S, McGuirk CM, d’Aquino A, Mason JA, Mirkin CA. Metal-Organic Framework Nanoparticles. Adv Mater. 2018;30(37):e1800202. doi:10.1002/adma.201800202
- Liu Y, Zhou L, Dong Y, et al. Recent developments on MOF-based platforms for antibacterial therapy. RSC Med Chem. 2021;12(6):915–928. doi:10.1039/D0MD00416B
- Wu Y, Liu Z, Peng J, Wang X, Zhou X, Li Z. Enhancing selective adsorption in a robust pillared-layer metal-organic framework via channel methylation for the recovery of C2-C3 from natural gas. ACS Appl Mater Interfaces. 2020;12(46):51499–51505. doi:10.1021/acsami.0c15267
- Lee J, Ka D, Jung H, Cho K, Jin Y, Kim M. UiO-66-NH2 and zeolite-templated carbon composites for the degradation and adsorption of nerve agents. Molecules. 2021;26(13):65.
- Kim SN, Park CG, Huh BK, et al. Metal-organic frameworks, NH2-MIL-88(Fe), as carriers for ophthalmic delivery of brimonidine. Acta Biomater. 2018;79:344–353. doi:10.1016/j.actbio.2018.08.023
- Dwibedi D, Ling CD, Araujo RB, et al. Ionothermal synthesis of high-voltage alluaudite Na2+2xFe2-x(SO4)3 sodium insertion compound: structural, electronic, and magnetic insights. ACS Appl Mater Interfaces. 2016;8(11):6982–6991. doi:10.1021/acsami.5b11302
- Ban Y, Li Z, Li Y, et al. Confinement of ionic liquids in nanocages: tailoring the molecular sieving properties of ZIF-8 for membrane-based CO2 capture. Angewandte Chemie. 2015;54(51):15483–15487. doi:10.1002/anie.201505508
- Yuan M, Wang R, Fu W, et al. Ultrathin two-dimensional metal-organic framework nanosheets with the inherent open active sites as electrocatalysts in aprotic Li-O batteries. ACS Appl Mater Interfaces. 2019;11(12):11403–11413. doi:10.1021/acsami.8b21808
- Troyano J, Camur C, Garzon-Tovar L, Carne-Sanchez A, Imaz I, Maspoch D. Spray-drying synthesis of MOFs, COFs, and related composites. Acc Chem Res. 2020;53(6):1206–1217. doi:10.1021/acs.accounts.0c00133
- Carne-Sanchez A, Imaz I, Cano-Sarabia M, Maspoch D. A spray-drying strategy for synthesis of nanoscale metal-organic frameworks and their assembly into hollow superstructures. Nat Chem. 2013;5(3):203–211. doi:10.1038/nchem.1569
- Lucena MA, Oliveira MF, Arouca AM, et al. Application of the metal-organic framework [Eu(BTC)] as a luminescent marker for gunshot residues: a synthesis, characterization, and toxicity study. ACS Appl Mater Interfaces. 2017;9(5):4684–4691. doi:10.1021/acsami.6b13474
- Liu Y, Hori A, Kusaka S, et al. Microwave-assisted hydrothermal synthesis of [Al(OH)(1,4-NDC)] membranes with superior separation performances. Chem Asian J. 2019;14(12):2072–2076. doi:10.1002/asia.201900152
- Simon MA, Anggraeni E, Soetaredjo FE, et al. Hydrothermal synthesize of HF-free MIL-100(Fe) for isoniazid-drug delivery. Sci Rep. 2019;9(1):16907. doi:10.1038/s41598-019-53436-3
- Sun SY, Huang MJ, Wang PC, Lu M. Controllable hydrothermal synthesis of Ni/Co MOF as hybrid advanced electrode materials for supercapacitor. J Electrochem Soc. 2019;166(10):A1799–A1805. doi:10.1149/2.0291910jes
- Tambat SN, Sane PK, Suresh S, Varadan ON, Pandit AB, Sontakke SM. Hydrothermal synthesis of NH2-UiO-66 and its application for adsorptive removal of dye. Adv Powder Technol. 2018;29(11):2626–2632. doi:10.1016/j.apt.2018.07.010
- Butova VV, Budnyk AP, Bulanova EA, Lamberti C, Soldatov AV. Hydrothermal synthesis of high surface area ZIF-8 with minimal use of TEA. Solid State Sci. 2017;69:13–21. doi:10.1016/j.solidstatesciences.2017.05.002
- Bromberg L, Diao Y, Wu HM, Speakman SA, Hatton TA. Chromium(III) terephthalate metal organic framework (MIL-101): HF-free synthesis, structure, polyoxometalate composites, and catalytic properties. Chem Mater. 2012;24(9):1664–1675. doi:10.1021/cm2034382
- Yoon S, Calvo JJ, So MC. Removal of acid orange 7 from aqueous solution by metal-organic frameworks. Crystals. 2019;9(1):54.
- Saeed T, Naeem A, Din IU, et al. Synthesis of chitosan composite of metal-organic framework for the adsorption of dyes; kinetic and thermodynamic approach. J Hazard Mater. 2021;427:127902. doi:10.1016/j.jhazmat.2021.127902
- Guo YY, Dong AR, Huang Q, et al. Hierarchical N-doped CNTs grafted onto MOF-derived porous carbon nanomaterials for efficient oxygen reduction. J Colloid Interface Sci. 2022;606:1833–1841. doi:10.1016/j.jcis.2021.08.180
- Arul P, Huang ST, Gowthaman NSK, Govindasamy M, Jeromiyas N. Surfactant-free solvothermal synthesis of Cu-MOF via protonation-deprotonation approach: a morphological dependent electrocatalytic activity for therapeutic drugs. Microchimica Acta. 2020;187(12). doi:10.1007/s00604-020-04631-x
- Lee CT, Shin MW. Solvothermal growth of Mg-MOF-74 films on carboxylic functionalized silicon substrate using acrylic acid. Surfaces Interfaces. 2021;22:100845. doi:10.1016/j.surfin.2020.100845
- Azad FN, Ghaedi M, Dashtian K, Hajati S, Pezeshkpour V. Ultrasonically assisted hydrothermal synthesis of activated carbon-HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason Sonochem. 2016;31:383–393. doi:10.1016/j.ultsonch.2016.01.024
- Askari H, Ghaedi M, Dashtian K, Azghandi MHA. Rapid and high-capacity ultrasonic assisted adsorption of ternary toxic anionic dyes onto MOF-5-activated carbon: artificial neural networks, partial least squares, desirability function and isotherm and kinetic study. Ultrason Sonochem. 2017;37:71–82. doi:10.1016/j.ultsonch.2016.10.029
- Mirhosseini H, Shamspur T, Mostafavi A, Sargazi G. A novel ultrasonic assisted-reverse micelle procedure to synthesize Eu-MOF nanostructure with high sono/sonophotocatalytic activity: a systematic study for brilliant green dye removal. J Mater Sci. 2021;32(18):22840–22859. doi:10.1007/s10854-021-06762-0
- Azizabadi O, Akbarzadeh F, Danshina S, Chauhan NPS, Sargazi G. An efficient ultrasonic assisted reverse micelle synthesis route for Fe3O4@Cu-MOF/core-shell nanostructures and its antibacterial activities. J Solid State Chem. 2021;294:125.
- Li ZQ, Qiu LG, Xu T, et al. Ultrasonic synthesis of the microporous metal-organic framework Cu-3(BTC)(2) at ambient temperature and pressure: an efficient and environmentally friendly method. Mater Lett. 2009;63(1):78–80. doi:10.1016/j.matlet.2008.09.010
- Al-Attri R, Halladj R, Askari S. Green route of flexible Al-MOF synthesis with superior properties at low energy consumption assisted by ultrasound waves. Solid State Sci. 2022;123:106782. doi:10.1016/j.solidstatesciences.2021.106782
- Amaro-Gahete J, Klee R, Esquivel D, Ruiz JR, Jiménez-Sanchidrián C, Romero-Salguero FJ. Fast ultrasound-assisted synthesis of highly crystalline MIL-88A particles and their application as ethylene adsorbents. Ultrason Sonochem. 2019;50:59–66. doi:10.1016/j.ultsonch.2018.08.027
- Wang YM, Ge SS, Cheng W, et al. Microwave hydrothermally synthesized metal-organic framework-5 derived C-doped ZnO with enhanced photocatalytic degradation of rhodamine B. Langmuir. 2020;36(33):9658–9667. doi:10.1021/acs.langmuir.0c00395
- Sun L, Shao Q, Zhang Y, et al. N self-doped ZnO derived from microwave hydrothermal synthesized zeolitic imidazolate framework-8 toward enhanced photocatalytic degradation of methylene blue. J Colloid Interface Sci. 2020;565:142–155. doi:10.1016/j.jcis.2019.12.107
- Liu Z, Ye J, Rauf A, et al. A flexible fibrous membrane based on copper(II) metal-organic framework/poly(lactic acid) composites with superior antibacterial performance. Biomater Sci. 2021;9(10):3851–3859. doi:10.1039/D1BM00164G
- Chaemchuen S, Zhou K, Mousavi B, et al. Spray drying of zeolitic imidazolate frameworks: investigation of crystal formation and properties. Crystengcomm. 2018;20(25):3601–3608. doi:10.1039/C8CE00392K
- Avci-Camur C, Troyano J, Perez-Carvajal J, et al. Aqueous production of spherical Zr-MOF beads via continuous-flow spray-drying. Green Chem. 2018;20(4):873–878. doi:10.1039/C7GC03132G
- Luz I, Stewart IE, Mortensen NP, Hickey AJ. Designing inhalable metal organic frameworks for pulmonary tuberculosis treatment and theragnostics via spray drying. Chem Commun. 2020;56(87):13339–13342. doi:10.1039/D0CC05471B
- Kubo M, Ishimura M, Shimada M. Improvement of production efficiency of spray-synthesized HKUST-1. Adv Powder Technol. 2021;32(7):2370–2378. doi:10.1016/j.apt.2021.05.024
- Boix G, Troyano J, Garzon-Tovar L, et al. MOF-beads containing inorganic nanoparticles for the simultaneous removal of multiple heavy metals from water. ACS Appl Mater Interfaces. 2020;12(9):10554–10562. doi:10.1021/acsami.9b23206
- Garzon-Tovar L, Cano-Sarabia M, Carne-Sanchez A, Carbonell C, Imaz I, Maspoch D. A spray-drying continuous-flow method for simultaneous synthesis and shaping of microspherical high nuclearity MOF beads. Reaction Chem Engine. 2016;1(5):533–539. doi:10.1039/C6RE00065G
- Zhang X, Li Y, Van Goethem C, et al. Electrochemically assisted interfacial growth of MOF membranes. Matter. 2019;1(5):1285–1292. doi:10.1016/j.matt.2019.06.022
- De Lima Neto OJ, de Oliveira Frós AC, Barros BS, de Farias Monteiro AF, Kulesza J. Rapid and efficient electrochemical synthesis of a zinc-based nano-MOF for Ibuprofen adsorption. N J Chem. 2019;43(14):5518–5524.
- Pirzadeh K, Ghoreyshi AA, Rahimnejad M, Mohammadi MJ. Electrochemical synthesis, characterization and application of a microstructure Cu3 (BTC) 2 metal organic framework for CO2 and CH4 separation. Korean J Chem Engine. 2018;35(4):974–983.
- Wei J-Z, Gong F-X, Sun X-J, et al. Rapid and low-cost electrochemical synthesis of UiO-66-NH2 with enhanced fluorescence detection performance. Inorganic Chem. 2019;58(10):6742–6747. doi:10.1021/acs.inorgchem.9b00157
- Zheng H, Zhang Y, Liu L, et al. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138(3):962–968. doi:10.1021/jacs.5b11720
- Hopkins E, Sanvictores T, Sharma S. Physiology, acid base balance. In: StatPearls. StatPearls Publishing; 2021.
- Ge Y, Wang K, Liu J, et al. A ZIF-8-based multifunctional intelligent drug release system for chronic osteomyelitis. Colloids Surf B Biointerfaces. 2022;212:112354. doi:10.1016/j.colsurfb.2022.112354
- Sava Gallis DF, Butler KS, Agola JO, Pearce CJ, McBride AA. Antibacterial countermeasures via metal–organic framework-supported sustained therapeutic release. ACS Appl Mater Interfaces. 2019;11(8):7782–7791. doi:10.1021/acsami.8b21698
- Au-Duong A-N, Lee C-K. Iodine-loaded metal organic framework as growth-triggered antimicrobial agent. Mater Sci Engine C. 2017;76:477–482. doi:10.1016/j.msec.2017.03.114
- Xiao Y, Xu M, Lv N, et al. Dual stimuli-responsive metal-organic framework-based nanosystem for synergistic photothermal/pharmacological antibacterial therapy. Acta Biomater. 2021;122:291–305. doi:10.1016/j.actbio.2020.12.045
- Uflyand IE, Zhinzhilo VA, Bryantseva J. Synthesis and study of sorption, antioxidant and antibacterial properties of MOF based on cobalt terephthalate and 1, 10-phenanthroline. Eur Radiol. 2021;31(12):4710–4721. doi:10.1007/s00330-020-07477-2
- Anbazhagan S, Azeez S, Morukattu G, Rajan R, Venkatesan K, Thangavelu KP. Synthesis, characterization and biological applications of mycosynthesized silver nanoparticles. 3 Biotech. 2017;7(5):333. doi:10.1007/s13205-017-0961-9
- Arenas-Vivo A, Amariei G, Aguado S, Rosal R, Horcajada P. An Ag-loaded photoactive nano-metal organic framework as a promising biofilm treatment. Acta Biomater. 2019;97:490–500. doi:10.1016/j.actbio.2019.08.011
- Liu Y, Qin R, Zaat SAJ, Breukink E, Heger M. Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. J Clin Transl Res. 2015;1(3):140–167.
- Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. Int J Mol Sci. 2021;22(13):7202. doi:10.3390/ijms22137202
- Perdikaki A, Galeou A, Pilatos G, et al. Ag and Cu monometallic and Ag/Cu bimetallic nanoparticle-graphene composites with enhanced antibacterial performance. ACS Appl Mater Interfaces. 2016;8(41):27498–27510. doi:10.1021/acsami.6b08403
- Wei YH, Chen S, Kowalczyk B, Huda S, Gray TP, Grzybowski BA. Synthesis of stable, low-dispersity copper nanoparticles and nanorods and their antifungal and catalytic properties. J Phys Chem C. 2010;114(37):15612–15616. doi:10.1021/jp1055683
- Devanand Venkatasubbu G, Ramakrishnan V, Kumar J. Nanocrystalline hydroxyapatite and zinc-doped hydroxyapatite as carrier material for controlled delivery of ciprofloxacin. 3 Biotech. 2011;1(3):173–186.
- Wang YW, Cao A, Jiang Y, et al.Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl Mater Interfaces. 2014;6(4):2791–2798.
- Restrepo J, Serroukh Z, Santiago‐Morales J, et al. An antibacterial Zn–MOF with hydrazinebenzoate linkers. Eur J Inorganic Chem. 2017;2017(3):254.
- Gudkov SV, Burmistrov DE, Serov DA, Rebezov MB, Semenova AA, Lisitsyn AB. Do iron oxide nanoparticles have significant antibacterial properties? Antibiotics. 2021;10(7):884. doi:10.3390/antibiotics10070884
- Lee C, Kim JY, Lee WI, Nelson KL, Yoon J, Sedlak DL. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ Sci Technol. 2008;42(13):4927–4933. doi:10.1021/es800408u
- Jayandran M, Haneefa MM, Balasubramanian V. Green synthesis and characterization of Manganese nanoparticles using natural plant extracts and its evaluation of antimicrobial activity. J Appl Pharmac Sci. 2015;5(12):105–110.
- Gupta V, Kant V, Sharma A, et al. Comparative assessment of antibacterial efficacy for cobalt nanoparticles, bulk cobalt and standard antibiotics: a concentration dependant study. Наносистемы. 2020;11(1):78–85.
- Waghmare SR, Mulla MN, Marathe SR, Sonawane KD. Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech. 2015;5(1):33–38. doi:10.1007/s13205-014-0196-y
- Mallick S, Sharma S, Banerjee M, Ghosh SS, Chattopadhyay A, Paul A. Iodine-stabilized Cu nanoparticle chitosan composite for antibacterial applications. ACS Appl Mater Interfaces. 2012;4(3):1313–1323. doi:10.1021/am201586w
- van Hengel IAJ, Putra NE, Tierolf MW, et al. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020;107:325–337. doi:10.1016/j.actbio.2020.02.044
- Sadeghi-Kiakhani M, Hashemi E, Gharanjig K. Inorganic nanoparticles and natural dyes for production of antimicrobial and antioxidant wool fiber. 3 Biotech. 2019;9(12):456. doi:10.1007/s13205-019-1974-3
- Liu J, Wu D, Zhu N, Wu Y, Li G. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci Technol. 2021;109:413–434. doi:10.1016/j.tifs.2021.01.012
- Nakhaei M, Akhbari K, Kalati M, Phuruangrat A. Antibacterial activity of three zinc-terephthalate MOFs and its relation to their structural features. Inorganica Chimica Acta. 2021;522:120353.
- Wu C, Wang Z, Liu S, Xie Z, Chen H, Lu XJ. Simultaneous permeability, selectivity and antibacterial property improvement of PVC ultrafiltration membranes via in-situ quaternization. J Membrane Sci. 2017;548:S0376738817320008.
- Gwon K, Han I, Lee S, Kim Y, Lee DN. Novel metal-organic framework-based photocrosslinked hydrogel system for efficient antibacterial applications. ACS Appl Mater Interfaces. 2020;12(18):20234–20242. doi:10.1021/acsami.0c03187
- Tamames-Tabar C, Imbuluzqueta E, Guillou N, et al. A Zn azelate MOF: combining antibacterial effect. CrystEngComm. 2015;17(2):456–462.
- Al-Heniti S, Umar A. Structural, optical and field emission properties of urchin-shaped ZnO nanostructures. J Nanosci Nanotechnol. 2013;13(1):86–90. doi:10.1166/jnn.2013.7099
- Huang L, Li D-Q, Lin Y-J, Wei M, Evans DG, Duan X. Controllable preparation of Nano-MgO and investigation of its bactericidal properties. J Inorg Biochem. 2005;99(5):986–993. doi:10.1016/j.jinorgbio.2004.12.022
- Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorganic Mater. 2001;3(7):643–646. doi:10.1016/S1466-6049(01)00197-0
- Berchel M, Gall TL, Denis C, et al. A silver-based metal–organic framework material as a ‘reservoir’ of bactericidal metal ions. Aesthet Plastic Surg. 2011;35(5):1000–1003. doi:10.1007/s00266-011-9720-1
- Lu X, Ye J, Zhang D, et al. Silver carboxylate metal–organic frameworks with highly antibacterial activity and biocompatibility. J Inorg Biochem. 2014;138:114–121. doi:10.1016/j.jinorgbio.2014.05.005
- Jo JH, Kim HC, Huh S, Kim Y, Lee DN. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Transactions. 2019;48:8084–8093. doi:10.1039/C9DT00791A
- Rauf A, Ye J, Zhang S, et al. Copper(ii)-based coordination polymer nanofibers as a highly effective antibacterial material with a synergistic mechanism. Dalton Trans. 2019;48(48):17810–17817. doi:10.1039/C9DT03649K
- Sun Y, Jiang X, Liu Y, et al. Recent advances in Cu(II)/Cu(I)-MOFs based nano-platforms for developing new nano-medicines. J Inorg Biochem. 2021;225:111599. doi:10.1016/j.jinorgbio.2021.111599
- Yang Z, Hao X, Chen S, et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J Colloid Interface Sci. 2019;533:13–23. doi:10.1016/j.jcis.2018.08.053
- Akbarzadeh F, Motaghi M, Chauhan NPS, Sargazi G. A novel synthesis of new antibacterial nanostructures based on Zn-MOF compound: design, characterization and a high performance application. Heliyon. 2020;6(1):e03231. doi:10.1016/j.heliyon.2020.e03231
- Hachemaoui M, Mokhtar A, Ismail I, et al. M (M: Cu, Co, Cr or Fe) nanoparticles-loaded metal-organic framework MIL-101(Cr) material by sonication process: catalytic activity and antibacterial properties. Microporous Mesoporous Mater. 2021;323:111244. doi:10.1016/j.micromeso.2021.111244
- Peng S, Li R, Rao Y, et al. Tuning the unsaturated iron sites in MIL-101(Fe) nanoparticles for reactive oxygen species-mediated bacterial inactivation in the dark. Appl Catal B. 2022;316:121693. doi:10.1016/j.apcatb.2022.121693
- Canton M, Sánchez-Rodríguez R, Spera I, et al. Reactive oxygen species in macrophages: sources and targets. Front Immunol. 2021;12:734229. doi:10.3389/fimmu.2021.734229
- Sun Y, Zhao C, Niu J, Ren J, Qu X. Colorimetric band-aids for point-of-care sensing and treating bacterial infection. ACS Central Sci. 2020;6(2):207–212. doi:10.1021/acscentsci.9b01104
- Li P, Li J, Feng X, et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat Commun. 2019;10(1):2177. doi:10.1038/s41467-019-10218-9
- Bagchi D, Bhattacharya A, Dutta T, et al. Nano MOF entrapping hydrophobic photosensitizer for dual-stimuli-responsive unprecedented therapeutic action against drug-resistant bacteria. ACS Appl Bio Mater. 2019;2(4):1772–1780. doi:10.1021/acsabm.9b00223
- Li J, Gopal A, Karaosmanoglu S, et al. Photosensitizer doped zeolitic imidazolate framework-8 nanocomposites for combined antibacterial therapy to overcome methicillin-resistant Staphylococcus aureus (MRSA). Colloids Surf B Biointerfaces. 2020;190:110900. doi:10.1016/j.colsurfb.2020.110900
- Gottfried JM. Surface chemistry of porphyrins and phthalocyanines. Surf Sci Rep. 2015;70(3):259–379.
- Wang J, Zhong Y, Wang X, et al. pH-dependent assembly of porphyrin-silica nanocomposites and their application in targeted photodynamic therapy. Nano Lett. 2017;17(11):6916–6921. doi:10.1021/acs.nanolett.7b03310
- Chen J, Zhu Y, Kaskel S. Porphyrin‐based metal–organic frameworks for biomedical applications. Angewandte Chemie. 2021;60(10):5010–5035. doi:10.1002/anie.201909880
- Han D, Han Y, Li J, et al. Enhanced photocatalytic activity and photothermal effects of cu-doped metal-organic frameworks for rapid treatment of bacteria-infected wounds. Appl Catal B. 2020;261:118248. doi:10.1016/j.apcatb.2019.118248
- Chen M, Long Z, Dong R, et al. Titanium incorporation into Zr‐porphyrinic metal–organic frameworks with enhanced antibacterial activity against multidrug‐resistant pathogens. Small. 2020;16(7):1906240.
- Yang Y, Wu X, He C, et al. Metal-organic framework/Ag-based hybrid nanoagents for rapid and synergistic bacterial eradication. ACS Appl Mater Interfaces. 2020;12(12):13698–13708. doi:10.1021/acsami.0c01666
- Nabipour H, Soltani B, Nasab NA. Gentamicin loaded Zn-2(bdc)(2)(dabco) frameworks as efficient materials for drug delivery and antibacterial activity. J Inorg Organomet Polym Mater. 2018;28(3):1206–1213. doi:10.1007/s10904-018-0781-3
- Lin S, Liu X, Tan L, et al. Porous iron-carboxylate metal–organic framework: a novel bioplatform with sustained antibacterial efficacy and nontoxicity. ACS Appl Mater Interfaces. 2017;9(22):19248–19257. doi:10.1021/acsami.7b04810
- Lv H, Zhang Y, Chen P, Xue J, Jia X, Chen J. Enhanced synergistic antibacterial activity through a smart platform based on UiO-66 combined with photodynamic therapy and chemotherapy. Langmuir. 2020;36(15):4025–4032. doi:10.1021/acs.langmuir.0c00292
- Nasrabadi M, Ghasemzadeh MA, Monfared M. The preparation and characterization of UiO-66 metal–organic frameworks for the delivery of the drug ciprofloxacin and an evaluation of their antibacterial activities. N J Chem. 2019;43(40):16033–16040.
- Lei Z, Tang Q, Ju Y, et al. Block copolymer@ZIF-8 nanocomposites as a pH-responsive multi-steps release system for controlled drug delivery. J Biomater Sci Polym Ed. 2020;31(6):695–711. doi:10.1080/09205063.2020.1713451
- Nabipour H, Sadr MH, Bardajee GR. Synthesis and characterization of nanoscale zeolitic imidazolate frameworks with ciprofloxacin and their applications as antimicrobial agents. N J Chem. 2017;41:7364–7370. doi:10.1039/C7NJ00606C
- Guo C, Cheng F, Liang G, et al. Copper-based polymer-metal–organic framework embedded with Ag nanoparticles: long-acting and intelligent antibacterial activity and accelerated wound healing. Chem Engine J. 2022;435:134915. doi:10.1016/j.cej.2022.134915
- Duan C, Meng J, Wang X, et al. Synthesis of novel cellulose- based antibacterial composites of Ag nanoparticles@ metal-organic frameworks@ carboxymethylated fibers. Carbohydr Polym. 2018;193:82–88. doi:10.1016/j.carbpol.2018.03.089
- Sacourbaravi R, Ansari-Asl Z, Kooti M, Nobakht V, Darabpour E. Fabrication of Ag NPs/Zn-MOF Nanocomposites and their application as antibacterial agents. J Inorg Organomet Polym Mater. 2020;30(11):4615–4621. doi:10.1007/s10904-020-01601-x
- Gil-Manso S, Miguens Blanco I, Motyka B, et al. ABO blood group is involved in the quality of the specific immune response anti-SARS-CoV-2. Virulence. 2022;13(1):30–45. doi:10.1080/21505594.2021.2019959
- Cheung YH, Ma K, van Leeuwen HC, et al. Immobilized regenerable active chlorine within a zirconium-Based MOF textile composite to eliminate biological and chemical threats. J Am Chem Soc. 2021;143(40):16777–16785. doi:10.1021/jacs.1c08576
- Teng W, Zhang Z, Wang Y, et al. Iodine immobilized metal-organic framework for NIR-triggered antibacterial therapy on orthopedic implants. Small. 2021;17(35):e2102315. doi:10.1002/smll.202102315
- Huang G, Li Y, Qin Z, Liang Q, Xu C, Lin B. Hybridization of carboxymethyl chitosan with MOFs to construct recyclable, long-acting and intelligent antibacterial agent carrier. Carbohydr Polym. 2020;233:115848. doi:10.1016/j.carbpol.2020.115848
- Wyszogrodzka G, Marszałek B, Gil B, Dorożyński P. Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov Today. 2016;21(6):1009–1018. doi:10.1016/j.drudis.2016.04.009
- Prathima B, Rao YS, Reddy SA, Reddy YP, Reddy AV. Copper(II) and nickel(II) complexes of benzyloxybenzaldehyde-4-phenyl-3-thiosemicarbazone: synthesis, characterization and biological activity. Spectrochimica Acta Part A. 2010;77(1):248–252. doi:10.1016/j.saa.2010.05.016
- Li R, Chen T, Pan X. Metal-organic-framework-based materials for antimicrobial applications. ACS Nano. 2021;15(3):3808–3848. doi:10.1021/acsnano.0c09617
- Tavman A, Boz I, Birteksoz AS. Spectral characterization and antimicrobial activity of 2-(5-chloro/nitro-1H-benzimidazol-2-yl)-4-bromo/nitro-Phenols and their zinc(II) complexes. Spectrochimica Acta Part A. 2010;77(1):199–206. doi:10.1016/j.saa.2010.05.008
- Yang C, Ke GL, Huan SY, Zhang XB. Advances of metal-organic frameworks in cancer therapy applications. Progress Biochem Biophys. 2020;47(2):105–122.
- Wuttke S, Braig S, Preiss T, et al. MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem Commun (Camb). 2015;51(87):15752–15755. doi:10.1039/C5CC06767G
- Wang L, Qu X, Zhao Y, et al. Exploiting single atom iron centers in a porphyrin-like MOF for efficient cancer phototherapy. ACS Appl Mater Interfaces. 2019;11(38):35228–35237. doi:10.1021/acsami.9b11238
- Smith KM, Machalaba CC, Seifman R, Feferholtz Y, Karesh WB. Infectious disease and economics: the case for considering multi-sectoral impacts. One Health. 2019;7:100080. doi:10.1016/j.onehlt.2018.100080
- Chan Y-L, Liao H-C, Tsay P-K, Chang -S-S, Chen J-C, Liaw S-J. C-reactive protein as an indicator of bacterial infection of adult patients in the emergency department. Chang Gung Med J. 2002;25(7):437–445.
- Andrejaitiene J, Sirvinskas E, Zebrauskiene I. Procalcitonin: a new infection marker. Its use in intensive care. Medicina. 2002;38(5):491–498.
- Toliver-Kinsky T, Cui WH, Toro G, et al. H2S, a bacterial defense mechanism against the host immune response. Infect Immun. 2019;87(1). doi:10.1128/IAI.00272-18
- Yuan K, Huang K, Yang YQ, et al. Multi-roles of nanoscale bismuth metal-organic frameworks: infectious photoacoustic probe and inhibitor of antibiotics tolerant bacteria via targeting endogenous H2S. Nano Today. 2022;47:101683. doi:10.1016/j.nantod.2022.101683
- Li QQ, Wen MJ, Zhang YS, et al. Multiple fluorescence response behaviors towards antibiotics and bacteria based on a highly stable Cd-MOF. J Hazard Mater. 2022;423:127132. doi:10.1016/j.jhazmat.2021.127132
- Guo YF, Han ZS, Min H, et al. A europium-organic framework sensing material for 2-aminoacetophenone, a bacterial biomarker in water. Inorg Chem. 2021;60(12):9192–9198. doi:10.1021/acs.inorgchem.1c01251
- Wang Y, Hu YQ, He QY, et al. Metal-organic frameworks for virus detection. Biosens Bioelectron. 2020;169. doi:10.1016/j.bios.2020.112604