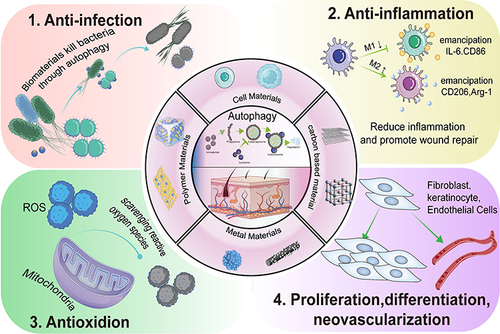
Abstract
Autophagy, a self-renewal mechanism, can help to maintain the stability of the intracellular environment of organisms. Autophagy can also regulate several cellular functions and is strongly related to the onset and progression of several diseases. Wound healing is a biological process that is coregulated by different types of cells. However, it is troublesome owing to prolonged treatment duration and poor recovery. In recent years, biomaterials have been reported to influence the skin wound healing process by finely regulating autophagy. Biomaterials that regulate autophagy in various cells involved in skin wound healing to regulate the differentiation, proliferation and migration of cells, inflammatory responses, oxidative stress and formation of the extracellular matrix (ECM) have emerged as a key method for improving the tissue regeneration ability of biomaterials. During the inflammatory phase, autophagy enhances the clearance of pathogens from the wound site and leads to macrophage polarization from the M1 to the M2 phenotype, thus preventing enhanced inflammation that can lead to further tissue damage. Autophagy plays important roles in facilitating the formation of extracellular matrix (ECM) during the proliferative phase, removing excess intracellular ROS, and promoting the proliferation and differentiation of endothelial cells, fibroblasts, and keratinocytes. This review summarizes the close association between autophagy and skin wound healing and discusses the role of biomaterial-based autophagy in tissue regeneration. The applications of recent biomaterials designed to target autophagy are highlighted, including polymeric materials, cellular materials, metal nanomaterials, and carbon-based materials. A better understanding of biomaterial-regulated autophagy and skin regeneration and the underlying molecular mechanisms may open new possibilities for promoting skin regeneration. Moreover, this can lay the foundation for the development of more effective therapeutic approaches and novel biomaterials for clinical applications.
Introduction
Cellular autophagy is a lysosome-mediated degradative process by which cells digest their own components (eg, animal cells) for the maintenance of normal intracellular physiological activities and homeostasis, thereby playing a crucial role in maintaining the homeostasis of the cellular microenvironment. Cellular autophagy is often regarded as a survival response mechanism of organisms to protect the internal and external cellular environment from damage when external nutrients are scarce or the cell is injured by external sources. Cellular autophagy is a highly conserved eukaryotic cellular pathway that is relevant to human health. Autophagy is essential for normal homeostasis and healthy function of the skin. Moreover, it can promote wound healing and allow cell cycle regulation to undergo self-renewal.Citation1
Skin, the body’s largest barrier composed of multiple cells, maintains the dynamic balance of organisms through metabolic and immune functionsCitation2 and protects organisms from pathogens. However, skin wound healing is plagued by multiple problems.Citation3 Wound healing is an extremely complex biological process that involves joint involvement and the synergistic effect of numerous cell types.Citation4 Health problems such as inflammation, oxidative stress, and bacterial infections can slow down the wound-healing process and cause severe pain to the patient. In addition, prolonged treatment leads to the consumption of huge amounts of medical resources and creates a burden on the healthcare system.Citation5
Skin damage leads to an inflammatory response caused by necrotic tissue and microorganisms invading the sites of tissue injury, where a large number of immune cells, such as neutrophils, monocytes, and macrophages, remove cellular debris and microorganisms and reduce the accumulation of intracellular reactive oxygen species (ROS) and the secretion levels of inflammatory factors. Autophagy plays a critical role in skin repair by promoting the role of functional and immune cells in accelerating the repair of wounded skin. A study by Birmingham’s group revealed a positive correlation between autophagy activation and resistance to pathogenic infections. Moreover, Salmonella typhimurium can activate autophagy, thereby inhibiting its multiplication in cells and protecting them from bacterial damage.Citation6 A study by Dasa et al highlighted that vitamin D enhanced macrophage autophagy, which led to enhanced expression of anti-inflammatory factors and a reduction in the inflammatory response, thus promoting skin healing.Citation7
Skin is a complex organ composed of organic substances (such as collagen, etc.) with a multilevel structure ranging from microscopic nano- to macroscopic tissues.Citation8 Therefore, biomaterials offer great promise in wound repair. In recent years, research on biomaterials for skin wound repair and tissue regeneration has attracted much interest.Citation9,Citation10 In the clinical applications, biomaterials allowed more precise targeting, regulated cellular and in vivo microenvironment-dependent homeostasis, and accelerated wound healing.Citation11,Citation12 In addition, the mechanisms underlying the regulation of cellular homeostasis by biomaterials have been extensively studied. Biomaterial-mediated autophagy has been shown to play an important role in cell phagocytosis and clearance, maintenance of cellular functions, cell differentiation, and in vitro and in vivo immune stress.Citation13 The extremely complex homeostatic system of organisms can prevent the accumulation of abnormal materials (eg, protein aggregates) by activating degradation pathways. Therefore, biomaterials entering the body are easily recognized as foreign substances that activate a series of cellular defense mechanisms (eg, autophagy).Citation14 Biomaterials play an important role in regulating autophagy in different diseases, thus affecting disease progression.Citation15 Biomaterials have been shown to regulate bacterial infection, the inflammatory response, oxidative stress, cell proliferation, and differentiation by regulating autophagy during skin wound repair, thereby promoting wound healing.Citation16 Certain investigations have mainly focused on the relationship between autophagy and skin wound healing,Citation1 and this review is the first to discuss the role of biomaterial-based autophagy in skin repair and regeneration. We summarized the relationship between autophagy and skin regeneration based on a literature review and recent advances in the field of skin repair and regeneration by biomaterial-induced autophagy. In addition, we outlined the association between autophagy, biomaterials, and wound repair, particularly the mechanism underlying the regulation of wound repair by biomaterial-targeted autophagy (). It is possible that new strategies for promoting skin wound healing might be developed by comprehending the relationship between skin wound healing and autophagy-modulating biomaterials as well as their underlying molecular mechanisms.
Molecular Mechanism of Autophagy
Definition and Classification of Autophagy
Two common degradation pathways exist for compounds within normal cells, namely, the ubiquitin‒proteasome pathway, which primarily degrades short-lived proteins within the cell, and the autophagic pathway, which predominantly degrades long-lived proteins and some damaged organelles within the cytoplasm.Citation17 Autophagy, the term proposed by Nobel laureate Christian de Duve in 1963, is mainly activated through a series of stimuli that can influence cellular microenvironments, such as starvation, stress, and hypoxia.Citation18 Lysosomes, major organelles involved in autophagy, play a key role in regulating the cellular microenvironment. During autophagy, cytoplasmic macromolecules, aggregated proteins, damaged organelles, and pathogens are delivered to lysosomes and digested to generate amino acids, nucleotides, sugars, and other nutrients to adapt to the changing cellular microenvironment.Citation19–22
Autophagy is mainly classified into macroautophagy, molecular chaperone-mediated autophagy (CMA), and microautophagy.Citation20 The term autophagy generally refers to macroautophagy. The autophagosomal membrane formed by the nonribosomal regions of the rough endoplasmic reticulum and the Golgi apparatus encapsulate proteins and further produce autophagic vesicles. These vesicles are degraded upon binding to lysosomes. Microautophagy involves the direct wrapping of proteins by lysosome membranes for protein degradation. The intracytoplasmic protein-bound molecular chaperones in CMA are translocated into the lysosomal compartment and digested.Citation23–25 A majority of studies on autophagy have focused on macroautophagy and its associated pathways. Therefore, this review also primarily discusses macroautophagy and its underlying mechanisms.
Autophagic Process
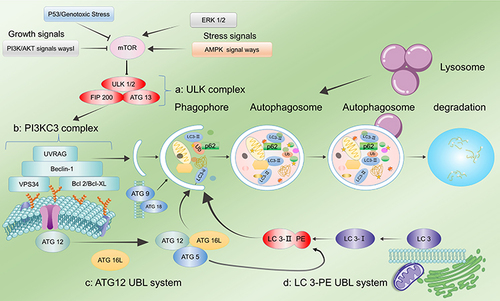
The autophagic process consists of four stages, namely, induction of autophagy, membrane extension and formation of autophagosomes, fusion of autophagosomes with lysosomes, and degradation of products within autophagic lysosomes. The entire autophagic process is a dynamic equilibrium process (autophagic flux).Citation26 The process and regulation of autophagy are shown in . The first stage of the autophagic process is the induction phase for autophagosome formation. Several factors are responsible for the activation of autophagy in organisms, such as nutritional deficiency, oxidative stress, and pathogen attack. These autophagy-inducing signals lead to the formation of continuously expanding “liposome-like” membrane structures in the cytoplasm. This structure is not spherical but flattened, similar to a bowl-shaped structure composed of lipid bilayers. It can be observed under electron microscopy (phagophore), which is one of the gold standards in autophagy detection.Citation27 Mammalian target of rapamycin (mTOR) is a key regulator of autophagy. Under optimal growth conditions, activated mTOR can phosphorylate Unc-51-like-kinase 1 (ULK1) and autophagy-related 13 (ATG13) to inhibit autophagy. However, when the body is affected by nutrient deficiency and under oxidative stress, mTOR activity is inhibited, leading to dephosphorylation of ULK1 and ATG13, thereby activating autophagy.Citation28 Adenosine monophosphate-activated protein kinase (AMPK), another key protein in autophagy regulation, is a master regulator of cellular metabolism that is involved in the restoration of energy homeostasis. When AMPK is activated under changed-energy conditions, mTOR is inhibited, thereby inducing autophagy. The activation of AMPK promotes autophagy by directly activating the ULK kinase core complex through phosphorylation.Citation18 The second stage involves the extension of the isolation membrane and autophagosome formation, in which the phagophore is continuously elongated to enclose all the cytoplasmic components, including organelles, in a bowl-like structure and then closes to become a closed spherical autophagosome. The detection of autophagosomes under an electron microscope is additional evidence of autophagy.Citation29 Autophagosomes are characterized by two features, namely, a bilayer membrane structure and the inclusion of cytoplasmic components such as mitochondria and endoplasmic reticulum fragments. The extension of the membrane in autophagic vesicles leads to the formation of unique double-membrane structures of autophagosomes.Citation19,Citation22 The stimulation of the ULK kinase core complex leads to the activation of Beclin-1, which binds to VPS15 and VPS34 (PI3KC3 complex). This complex recruits lipophilic proteins to form autophagic vesicle membrane precursors and binds to Atg14 or UVRAG to complete autophagosome membrane formation, leading to autophagosome maturation and transport.Citation30 The two ubiquitination modifications, namely, ATG5-ATG12-ATG16 and microtubule associated proteins 1A and 1B (LC3)-phosphatidylethanolamine, are critical processes that regulate autophagosome formation and maturation. During the autophagy induction phase, there is hydrolytic cleavage of LC3 by ATG4 protein to generate LC3-I. LC3-I is then activated by ATG7, ATG3, and ATG12-ATG5-ATG16 complexes and coupled to phosphatidylethanolamine (PE) in the membrane to generate LC3-II. In contrast to ATG5-ATG12-ATG16, LC3-II is present on both the inner and outer surfaces of autophagosomes. Although the ATG5-ATG12-ATG16 complex detaches from the vesicle after the closure of the autophagosome membrane, a portion of LC3-II remains covalently bound to the membrane. Therefore, LC3-II can be used as a marker of autophagic activity within cellsCitation20,Citation29,Citation31,Citation32 and is an essential indicator for the detection of autophagy.Citation33 In the third stage, autophagosomes (fully enclosed phagophores) fuse with the cytosol. When the autophagosome is completely formed, ATG4 cleaves LC3-II attached to the outer membrane from the PE lipid and is released back into the cytoplasm. The fusion of autophagosomes with lysosomes requires the joint activity of the lysosomal membrane protein LMAP-1 and the small GTPase Rab7.Citation17,Citation21,Citation34,Citation35 In the fourth stage, autophagosomes fuse with lysosomes to form autolysosomes. The inner membrane of the autophagosome is degraded by lysosomal enzymes, and the content of the autophagosome merges with that of the lysosome. In addition, the “cargo” of the autophagosome is degraded, and the resulting degradation products (eg, amino acids, fatty acids, etc.) are transported back to the cytosol for reuse by the cell, while the residues are either excreted or retained in the cell.Citation25,Citation36–38 Moreover, before the degradation of autolysosomes, a new tubular structure is formed and shed from its upper part to form a new lysosome, thus facilitating the normal physiological activities of the cell.
Figure 2 Regulation of autophagy. (a) The ULK1 complex undergoes dephosphorylation in response to external nutrient deprivation or external cell injury to induce autophagy. (b) The PI3KC3 complex, ATG 9, and ATG 18 mediate the formation of autophagosome membranes and the recruitment of ATG. (c) ATG 12 UBL system. (d) The LC3-PE UBL system is involved in autophagosome maturation and transport. Autophagy is strongly related to several factors, among which mTOR plays a key role. AMPK and ERK 1/2 upregulate autophagy by inhibiting mTOR phosphorylation, while the P53 and PI3K/AKT pathways lead to the inhibition of autophagy.
Common Methods for Assessing Autophagy
The assessment of autophagosome formation by transmission electron microscopy has become the gold standard in autophagy detection due to the subcellular structure of autophagosomes.Citation39 In addition, variations in the LC3-II/I ratio, Beclin-1, and P62 (SQSTM1) can also be detected using Western blotting to evaluate the autophagic process. These three proteins are most important in the autophagy detection process and can provide sufficient evidence of autophagy.Citation40 Upon induction of autophagy, cytoplasmic-type LC3 (ie, LC3-I) enzymatically cleaves off a small segment of the polypeptide to form LC3-II. Therefore, the LC3-II/I ratio is proportional to the level of autophagy. Beclin-1, which is also proportional to the level of autophagy, is strongly associated with the extension and formation of autophagic vesicle membranes and is essential for the detection of autophagy in several experiments.Citation41 However, the level of P62 (SQSTM1) is inversely proportional to the level of autophagy. As p62 bound to substrates is degraded by protein hydrolases during lysosomal degradation, the degradation of p62 is generally considered a marker of activated autophagy. In addition to Western blotting, flow cytometry is also a commonly used method for the detection of autophagy.Citation42 Moreover, the mRFP-GFP-LC3 dual fluorescence indicator system has been widely used in autophagy research.Citation43 However, in the analysis of clinical tissues, the adenovirus transfection method is still less applicable. All these methods have been widely used in experiments on autophagy, and their combined use and cross-validation can yield more accurate detection results.
Role of Autophagy in Skin Wound Repair
Skin, the largest organ of the body made up of three layers of tissue, the epidermis, dermis, and subcutaneous tissue, is the natural barrier protecting the internal organs of the body and plays a crucial role in maintaining homeostasis.Citation44 The skin is the first line of defense of the body’s immune defense mechanism and protects the body against stimuli caused by mechanical, chemical, and pathogenic factors (microorganisms).Citation45 However, the skin is susceptible to damage due to its constant exposure to external environmental factors, such as external injuries, burns, ulcers, and other skin problems. These events can lead to the disruption of the overall skin structure and basic functions of the skin, resulting in abnormal metabolism of the body and disturbance of the immune system.Citation11 Normal wound healing is divided into four phases, namely, hemostasis, inflammation, proliferation, and remodelling. These four phases involve many types of cells and require synchronous immune responses, the absence of which can lead to a failure of wound healing.Citation46
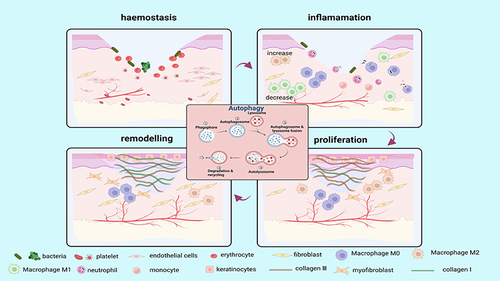
Autophagy has been recognized as an important factor influencing wound repair (). However, the impact of autophagy can vary depending on the stage of trauma and external disturbing factors.Citation47 Several factors can induce autophagy during the wound-healing process, such as infection, inflammation, oxidative stress, and nutritional deficiencies, which can delay wound repair.Citation48 Autophagy protects cells from damage and enhances cell proliferation, anti-inflammation, and antioxidant capacity during the initial stages of wound repair. For example, stem cells from human exfoliated deciduous teeth-derived exosomes promoted wound healing with less itching in the LPS-induced wound model by stimulating macrophage autophagy.Citation49 Additionally, in diabetic trauma models, melatonin protects endothelial progenitor cells (EPCs) against advanced glycation end product (AGE)-induced damage via autophagy activation to accelerate wound healing in vivo.Citation50 However, enhanced autophagy can also negatively impact wound healing. For example, endothelial cell-derived small extracellular vesicles can decrease collagen synthesis and delay cutaneous wound healing by reducing the expression of ERK1/ERK2 and triggering the autophagy of fibroblasts.Citation51 In addition, in diabetic trauma models, AGEs can induce autophagy and modulate M1 macrophage polarization, thereby impeding skin wound healing.Citation52 Thus, the effect of autophagy on wound repair is strongly related to various factors, such as the type of cells and the time to repair.
Figure 3 The role of autophagy in skin wound healing. Autophagy is involved in four phases of wound healing (hemostasis, inflammation, proliferation, and remodelling). Autophagy facilitates the survival, proliferation and migration of neutrophils, macrophages, endothelial cells, keratinocytes and fibroblasts, thereby improving their biological function and enhancing wound healing. (Image created with BioRender.com).
Mechanisms Underlying Autophagy-Regulated Skin Wound Repair
Autophagy-Regulated Antibacterial Effects
Severe infections are the most common and inevitable impediment to wound healing due to the susceptibility of open skin wounds.Citation53 The organism activates immune cells, and these cells induce inflammation to destroy invading microorganisms upon detection of microbial infection. Once the invading microorganism is recognized by the host immune cells, autophagy is activated. This mechanism can protect the organism by activating the host immune system while destroying the pathogen (). In general, there are two types of autophagy-induced phagocytosis of microorganisms, namely, direct phagocytosis of intracellular microorganisms by autophagosomes and LC3-associated phagocytosis, where LC3 recruitment to phagosomes can promote phagosome maturation and bacterial degradation. Both pathways can function simultaneously as host defense mechanisms against infections.
Figure 4 Autophagy in bacterial infection. (a) Removal of bacteria from the host cell by autophagy. After rapamycin or IFNγ treatment, bacteria such as M. tuberculosis and S. Typhimurium were digested by phagocytosis. Reproduced from Cemma M, Brumell JH. Interactions of pathogenic bacteria with autophagy systems. Curr Biol. 2012;22(13):R540–R545.Copyright © 2012, Elsevier Inc.Citation62 (b) After rapamycin treatment and Streptococcus pneumoniae infection, LC3 levels in neutrophils significantly increased, promoting autophagy. (c) Quantitative analysis of the LC3 levels in neutrophils. (t-tests) (d) Quantitative analysis of NET expression in neutrophils. (t-tests) (e) Expression of NETs was significantly reduced after inhibition of autophagy in neutrophils using the autophagy inhibitors 3-MA, Atg5, and siRNA, indicating that autophagy is essential for NET formation in neutrophils in humans infected with pneumococci. Reproduced from Ullah I, Ritchie ND, Evans TJ. The interrelationship between phagocytosis, autophagy and formation of neutrophil extracellular traps following infection of human neutrophils by Streptococcus pneumoniae. Innate Immun. 2017;23(5). © The Author(s) 2017. Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/).Citation55 (f) Significant increase in the expression levels of LC3 in macrophages of wild-type and autophagy-deficient mice after reduced Mycobacterium tuberculosis following ambroxol treatment. Reproduced from Choi SW, Gu Y, Peters RS, et al. Ambroxol induces autophagy and potentiates rifampin antimycobacterial activity. Antimicrob Agents Chemother. 2018;62(9):e01019–18. Copyright © 2018, American Society for Microbiology. Citation61*P<0.05, **P<0.01, relative to the Basal group.
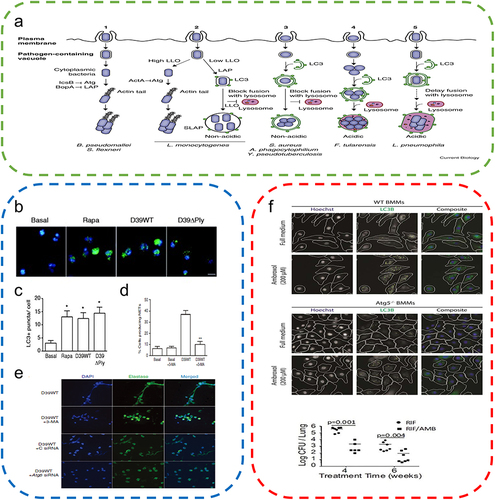
A large number of cells, predominantly neutrophils and macrophages, are recruited around the wound during the wound-healing process to remove pathogens, foreign bodies, and dead cells. Both of these types of cells are also strongly related to autophagy. Neutrophils are the most abundant cells in the human immune system and are first recruited at the wound site.Citation54 According to a recent study, following infection with Streptococcus pneumoniae, neutrophils induce autophagy and enhance phagocytic activity for the rapid clearance of intracellular bacteria. However, the administration of autophagy inhibitors significantly reduces the phagocytic activity of neutrophils.Citation55 Furthermore, autophagy not only phagocytizes bacteria but also initiates the preventive process. ATG5 can activate autophagy upon Mycobacterium tuberculosis infection, aiding in the enhanced recruitment of neutrophils to the sites of infection to counteract pathogens to prevent lung infection by Mycobacterium tuberculosis.Citation56 Neutrophils not only phagocytose pathogens but also release antimicrobial proteins and extracellular chromatin structures with specific granular proteins to fight infection.Citation57 In a recent study involving a mouse model with a bone marrow-specific autophagy-deficient phenotype, neutrophils inhibited neutrophil degranulation and affected the release of neutrophil extracellular traps (NETs) due to unactivated autophagy, resulting in a significant reduction in the antimicrobial capacity of neutrophils in vivo.Citation58
Macrophages are responsible for the phagocytosis of cellular debris, toxins, and pathogens in the body. Therefore, macrophages also play a crucial role in fighting bacterial infections. When an organism is infected, a large number of macrophages accumulate at the sites of infection, phagocytize, and digest pathogens. A recent study revealed that during Mycobacterium tuberculosis infection, adenosine triphosphate (ATP) activated autophagy, leading to the significant killing of intracellular mycobacteria, thereby countering bacterial infection.Citation59 In an ATG5-knockout mouse model, suppression of autophagy expression led to a significant increase in susceptibility to infection by Listeria monocytogenes and Toxoplasma gondii.Citation60 Although most bacteria are lysed by autophagy,Citation61 some bacteria can escape autophagy and even use it for multiplication in large numbers. Shigella castellani can escape from the phagosome into the host cell cytoplasm. In addition, it can use the cell surface virulence protein lcsB to interfere with autophagy pathway-related recognition to evade cellular clearance.Citation62 Furthermore, Francisella tularensis can disrupt the phagosomal membrane and enter the host cell cytoplasm to multiply in large numbers, leading to infection. Then, it re-enters the body through the autophagic pathway and spreads its cells through cellular autophagy.Citation63 Thus, autophagy activation is important for the phagocytosis of bacteria by neutrophils and macrophages. The inhibition of bacterial infection by autophagy plays a key role in wound healing. However, certain bacteria may escape autophagy, which needs to be further verified and investigated.
Autophagy-Regulated Inflammatory Response
Cytokine-stimulated monocytes and lymphocytes migrate into the wound after skin damage and differentiate into macrophages for the removal of necrotic tissue and pathogens, thereby activating the autoimmune system and leading to inflammation. Macrophages, which are critical in the initiation and maintenance of inflammation, are effector cells of the body’s innate immune system with functions such as the identification and removal of pathogens, killing target cells, antigen presentation, and immunomodulation.Citation64,Citation65 Macrophages can be polarized to assume different phenotypes and exhibit different functions in different microenvironments. They can differentiate into M1 macrophages in response to lipopolysaccharide (LPS), ROS, and other factors and perform the functions of eliminating pathogens and releasing proinflammatory factors during the early stages of inflammation. Anti-inflammatory cytokines such as IL-4 and IL-10 induce the differentiation of macrophages into M2 macrophages, which can suppress the immune response, promote tissue repair and reconstruction, and regulate angiogenesis during the later stages of inflammation.Citation66 Thus, macrophages play a crucial role in skin repair. Macrophages are one of the bridges connecting autophagy and inflammation.Citation67,Citation68 When autophagy disturbs the polarization of macrophages, autophagy in macrophages is dysfunctional and promotes polarization toward the M1 phenotype. However, activation of autophagy promotes M2 macrophage polarization to attenuate the inflammatory response ().Citation69,Citation70 When autophagy is induced in macrophages with autophagy activators such as thymosin β4 and punicalagin, they can trigger M2 macrophage polarization, which attenuates the inflammatory response and accelerates wound healing.Citation71,Citation72 In contrast, the administration of autophagy inhibitors leads to M1 macrophage polarization, resulting in more severe tissue damage.Citation73
Figure 5 Autophagy attenuates the inflammatory response. (a) Regulation of macrophage functions by the autophagic pathway. Reproduced from Wu MY, Lu JH. Autophagy and macrophage functions: inflammatory response and phagocytosis. Cells. 2019;9(1):E70. Copyright © 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).Citation76 (b) RvD2 promotes NLRP3 degradation through autophagy. (One-way ANOVA) Reproduced from Cao L, Wang Y, Wang Y, Lv F, Liu L, Li Z. Resolvin D2 suppresses NLRP3 inflammasome by promoting autophagy in macrophages. Exp Ther Med. 2021;22(5):1222. © Cao et al. This is an open access article distributed under the terms of Creative Commons Attribution.Citation70 (c) Vitamin D augments autophagic flux in macrophages of UV exposed mouse skin. Reproduced from Das LM, Binko AM, Traylor ZP, Peng H, Lu KQ. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15(5):813–826. Copyright © 2019, The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group. Creative Commons Attribution-NonCommercial-NoDerivatives License.Citation7 *P<0.05.
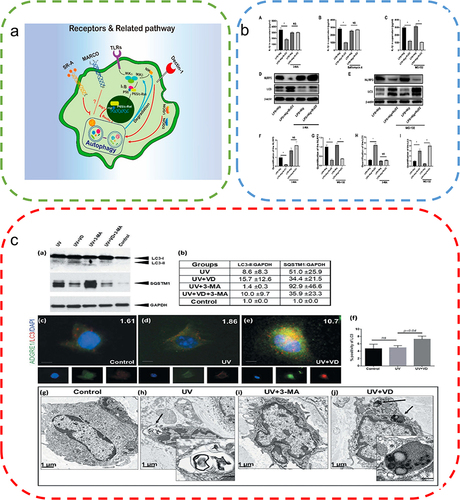
Secretion of cytokines is one of the major pathways by which macrophages influence the inflammatory microenvironment.Citation74 The stimulation of autophagy with rapamycin blocked interleukin-1 beta (IL-1β) secretion and promoted its degradation. Autophagy inhibition led to increased secretion of IL-1β.Citation75 However, autophagy inhibition by reduced expression of ATG7 led to the secretion of large amounts of IL-1β, while the common proinflammatory factors interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) were unaffected.Citation76 Thus, autophagy plays an important role in the secretion and degradation of cytokines produced by macrophages, which is not simply the result of the immune system’s response to inflammatory stimuli but involves several mechanisms that need further elucidation. The nuclear factor kappa-B (NF-κB) pathway is also a major pathway through which macrophages regulate inflammation. Autophagy has a strong relationship with NF-κB and is regulated by the NF-κB pathway.Citation77 In several cellular models, NF-κB activation inhibited TNF-α-induced autophagy.Citation77 In a mouse model, p65, a member of the NF-κB family, upregulated beclin 1 expression and induced autophagy.Citation78 Although autophagy can regulate inflammatory responses and macrophage polarization, it is still challenging to determine the key regulatory points linking autophagy to macrophage polarization, which need to be studied in detail.
Autophagy-Regulated Oxidative Stress
Oxidative stress is believed to play a crucial role in the wound-healing process.Citation79 Oxidative stress is primarily triggered by excess ROS in cells. ROS, the reduction products of oxygen with only a single electron, include superoxide anions, hydrogen peroxide, free radicals, and many other peroxides with the potential to oxidize proteins, lipids, and other substances.Citation80,Citation81 Several cells produce moderate amounts of ROS under normal physiological conditions, which are not harmful to organisms. Low levels of ROS can play important roles in rapid wound healing, angiogenesis, and pathogen clearance after skin injury.Citation12 However, high levels of ROS can lead to severe damage to the cells surrounding the wound.Citation82
ROS play a critical role in the initiation of autophagyCitation83 (). On the one hand, stimuli such as starvation, pathogens, or death receptors activate ROS and induce autophagy.Citation84 On the other hand, oxidatively damaged organelles are also delivered to lysosomes for degradation and recycling by autophagy.Citation85 ROS can initiate autophagy, and autophagy activation can then perform antioxidant functions to remove excessive ROS from the body.Citation86,Citation87 Thus, autophagy is strongly associated with ROS. After the treatment of human dermal fibroblasts with H2O2 in an oxidative stress model, the administration of remifentanil (an opioid receptor agonist) significantly enhanced autophagy, attenuated oxidative stress responses, and promoted fibroblast proliferation to maintain cellular activity.Citation88 Transcription factor EB (TFEB), identified as a major regulator of autophagy, is a key mediator of the cellular response to oxidative stress. Large intracellular production of ROS can induce rapid nuclear translocation of TFEB to promote autophagy activation and lysosomal degradation.Citation89 There is a complex interplay between ROS and autophagy. ROS-induced autophagy can reduce oxidative stress and lead to cell survival, whereas the absence of autophagy leads to increased oxidative stress and ROS accumulation.
Figure 6 Autophagy alleviates ROS accumulation around the wound. (a) High levels of ROS stimulate TFEB dephosphorylation and activate autophagy, thereby removing excess ROS. (b) Sulforaphane induces low levels of ROS to activate the Nrf2 pathway and promotes TFEB-dependent autophagy to eliminate ROS. Reproduced from Li D, Ding Z, Du K, Ye X, Cheng S. Reactive oxygen species as a link between antioxidant pathways and autophagy. Oxid Med Cell Longev.2021;2021:5583215. Copyright © 2021 Dan Li et al. Creative Commons Attribution License.83 (c) Examination of the changes in LC3 and P62 protein expression by Western blotting to evaluate the effects of AGEs indicated the promotion of autophagy by AGEs. (One-way ANOVA) (d) Apoptosis was detected by the annexin V-FITC/PI kit, which indicated that autophagy inhibition exacerbated AGEs/ROS -induced apoptosis. Reproduced from Xu L, Fan Q, Wang X, Zhao X, Wang L. Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis. 2016;7(11):e2445. Published by Nature Publishing Group under Creative Commons Attribution 4.0 International License.Citation87 **P<0.01.
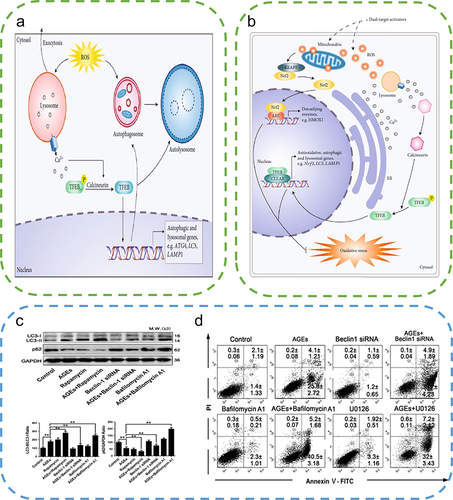
However, antioxidants or autophagy activators as single agents may not be effective against complex diseases. Dual-target therapeutics targeting both autophagy and oxidative stress could be the major direction for future research. Several natural compounds have been demonstrated to reduce oxidative stress and induce autophagy. Curcumin, a multifunctional drug, has anti-inflammatory, antioxidant, and proangiogenic effects and has been used in several disease models.Citation90 Curcumin can significantly activate the transformation of LC3 I to LC3 II and P62 degradation by inducing autophagy, thereby attenuating the effects of oxidative stress in atherosclerosis.Citation91 Resveratrol, a common ROS scavenger, was demonstrated to enhance the activity of antioxidant enzymes, namely, superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX), and reduce the levels of malondialdehyde (MDA).Citation92 In addition, resveratrol can induce autophagy through the AMPK pathway.Citation93 These dual-targeted drugs that target both antioxidants and autophagy overcome the inability of single therapy using antioxidants or autophagy activators in treating diseases associated with autophagy dysfunction and oxidative stress, indicating better therapeutic efficacy.
ROS are also strongly associated with macrophages, as they play a critical role in the induction and maintenance of M1 macrophage polarization.Citation94 The massive accumulation of ROS in mitochondria affects M1 macrophage polarization. In contrast, the decrease in ROS promotes M2 macrophage polarization. In NOX-deficient mice, ROS production is suppressed, thereby promoting an M2 macrophage phenotype.Citation95 However, in a recent study, ROS production was suppressed in NOX-deficient mice, whereas M2 macrophages expressed significantly less CD163 and interleukin 10 (IL-10).Citation96 The underlying molecular mechanisms of macrophage polarization are still obscure, particularly the effect of ROS on macrophage differentiation. Therefore, the mechanism of action of ROS on M1/M2 macrophage polarization still needs further investigation.
Autophagy, ROS, and macrophage polarization are interconnected. Autophagy activation promotes macrophage polarization toward the M2 phenotype (a large M2 polarization effect), the removal of ROS and the attenuation of oxidative stress in the body, thereby improving wound regeneration. This multifaceted therapy has also been applied on a large scale in other diseases. Zhao et al meticulously discussed the autophagy-activating, antioxidant, and anti-inflammatory capabilities of bicyclol against acute liver injury. Bicyclol effectively promoted the transformation of LC3 II, activated nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), and showed a better protective effect in liver injury.Citation29 In macrophages, metformin decreased the inflammatory response by autophagy activation, thereby increasing the expression of LC3 and decreasing the expression of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory vesicles. Overall, metformin attenuates the inflammatory response and protects against ischemic myocardial injury through the autophagy-ROS-NLRP3 axis.Citation97 Conversely, loss of autophagic function significantly promotes macrophage inflammation. Impaired mitochondrial function leads to excessive ROS production and autophagy inhibition to activate macrophage polarization toward the M1 phenotype.Citation98 Thus, the dual-target roles of ROS and inflammation in the treatment of skin wounds have been investigated.Citation99 However, therapeutic strategies targeting the autophagy-ROS-macrophage polarization axis are limited and may be a major direction for future research.
Autophagy Promotes Angiogenesis and Skin Cell Growth
Wound healing is a complex process involving the hyperblastosis of granulation tissue and scar tissue formation. The proliferation and differentiation of endothelial cells, fibroblasts, and keratinocytes affect the wound healing rate and scar formation. Angiogenesis is critical to skin wound repair. The newly formed blood vessels provide oxygen and nutrients to the tissues and remove waste products from the tissues. Autophagy activators induce autophagy in endothelial cells, which significantly improves pyroptosis and reduces inflammation and apoptosis.Citation100 In addition, autophagy protects endothelial cells from ROS-induced oxidative damage. Curcumin induces autophagy in human umbilical vein endothelial cells coincubated with hydrogen peroxide, thus improving their viability.Citation101 According to a recent study, autophagy activation via the AMPK-mTOR and FOXO3a pathways significantly restored endothelial function in human umbilical vein endothelial cells and endothelial progenitor cells and accelerated neoangiogenesis.Citation102,Citation103 However, the administration of autophagy inhibitors such as bafilomycin A1 significantly inhibited angiogenesis, thereby affecting the recovery of damaged cells.Citation104
Fibroblast proliferation and collagen deposition by fibroblasts for reconstitution of the fibrin matrix are the key factors affecting wound healing. Fibroblasts can migrate into the wound site due to their contractible properties, synthesize and release ECM, and repair the tissue to restore skin integrity.Citation105 Autophagy had a considerable influence on fibroblast survival. The stimulation of fibroblasts by TGF-β activated autophagy via TFEB transcription factors, which significantly decreased apoptosis to maintain fibroblast activity. However, the administration of autophagy inhibitors significantly increased the expression of apoptosis-related proteins and significantly reduced type I collagen secretion.Citation106 In addition, urolithin A (UroA) promotes autophagy through Nrf2 activation and attenuates ROS accumulation, thus protecting dermal fibroblasts from ultraviolet A (UVA)-induced aging.Citation107 Fibrosis and scar formation have become major therapeutic challenges in wound healing.Citation47 Autophagy can stimulate fibroblast apoptosis and inhibit fibrosis formation. In addition, p75 neurotrophic factor receptor (p75NTR) can inhibit the PI3K/Akt/mTOR pathway and activate autophagy to inhibit hypertrophic scar fibroblast (HSF) proliferation, migration, and ECM deposition.Citation108 However, hypertrophic scar tissues show higher levels of autophagy than normal tissues. Autophagy inhibition induces excess fibroblasts in vivo to undergo apoptosis, thereby inhibiting fibrosis.Citation109 Autophagy not only promotes fibroblast activation but also induces fibroblast apoptosis, thereby inhibiting fibrosis. Different drug dosages and administration schedules to induce autophagy are the primary reasons for the differences in efficacy. Briefly, autophagy of fibroblasts is expected to become a new target for skin repair and the treatment of skin fibrosis in the future.
Keratinocytes, which form the basis of skin structure, are involved in wound epithelialization and can differentiate into stratified epidermis and epithelium, thus playing multiple roles essential for wound repair.Citation110 In ATG5/ATG7 autophagy-deficient mice, autophagy inhibition significantly attenuates the migration, proliferation, and differentiation of keratinocytes.Citation111 In chronic trauma-induced hyperglycemic and hypoxic environments, autophagy has been intricately linked with keratinocyte proliferation. In the hyperglycemic environment, the p38/MAPK pathway is downregulated and accompanied by the inactivation of autophagy and impaired migration of keratinocytes.Citation112 The BNIP3 pathway is upregulated in hypoxic keratinocytes, which effectively stimulates autophagy activation and migration of keratinocytes. In addition, the accumulation of excessive ROS leads to the activation of the P38 and JNK-MAPK pathways, which in turn induces autophagy. Thus, multiple pathways simultaneously promote the migration of keratinocytes.Citation113 Overall, autophagy plays an important role in the migration of keratinocytes during wound repair and is a novel promising direction for future research.
Applications of Autophagy-Based Biomaterials in Skin Repair
Skin wound healing is a complex, multicellular process that requires cooperation among cells and precise regulation of cell proliferation and differentiation for ECM remodelling.Citation114 Biomaterials have proven to be an attractive strategy that can be applied to skin wound healing due to their controlled drug delivery and excellent drug release rates.Citation44 The design and fabrication of biomaterials require interdisciplinary approaches involving experts from biochemistry, biophysics, and materials science to impart excellent physical and chemical properties, with biocompatibility being a key functional requirement.Citation115 The previous definition of biocompatibility, which referred to the absence of toxicity or harmful effects on biological systems, has recently been replaced by a more complex concept; that is, biomaterials can promote biological recognition, which facilitates cellular proliferation, migration, and differentiation.Citation116 One of the strategies used to achieve this goal is to regulate cellular oxidative stress and inflammation through autophagy. Autophagy plays key roles in the wound healing process, such as promoting macrophage polarization, alleviating tissue damage by M1-type macrophages, enhancing tissue remodelling by M2-type macrophagesCitation7 to promote tissue repair,Citation117 and enhancing anti-inflammatory and anti-infective activities. In addition, autophagy promotes the proliferation, migration, and differentiation of cells associated with wound repair.Citation118 Recent literature has reported that biomaterials accelerate the skin healing process through autophagy ().Citation119 This section focuses on the effects of different types of biomaterials on autophagy and briefly describes their biological and medical applications ().
Table 1 Regulation of Biomaterial-Based Autophagy in Skin Repair
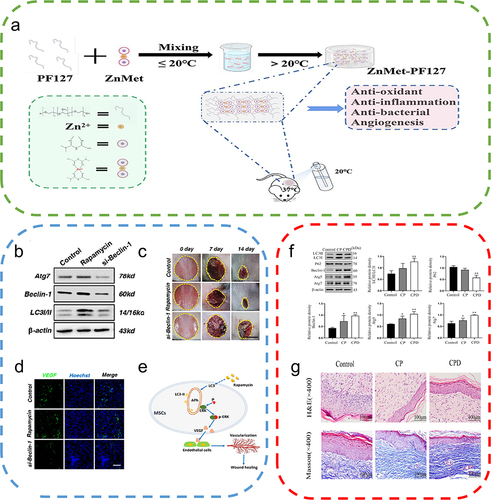
Figure 7 Autophagy-based biomaterials promote wound repair. (a) Schematic diagram of the preparation process and application of ZnMet-Pluronic F127. Reproduced from Liu Z, Tang W, Liu J, et al. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact Mater. 2023;20:610–626. © 2022 The Authors. CC BY-NC-ND license. Citation122 (b) Changes in ATG7, Beclin-1, and LC3 protein levels after treatment of mesenchymal stem cells (MSCs) with the autophagy activator rapamycin and the autophagy inhibitor si-Beclin-1. (c) Cutaneous wound healing in different groups of mice, with rapamycin-treated MSCs exhibiting the highest therapeutic effect on wound healing. (d) Immunofluorescence staining of VEGF in wound area tissue after 2 weeks, where rapamycin-treated MSCs promoted the expression of VEGF on the wound surface. (e) Autophagy regulates ERK phosphorylation to increase VEGF release from MSCs, which in turn promotes endothelial cell vascularization. Reproduced from An Y, Liu WJ, Xue P, et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018;9(2):58. Published by Nature Publishing Group under Creative Commons Attribution 4.0 International License.Citation129 (f) Effect of the nanocomposite membrane loaded with chitosan and polyvinylpyrrolidone (CP) and the nanocomposite membrane loaded with chitosan, polyvinylpyrrolidone and dihydroquercetin (CPD) on the expression of LC3II/I, P62, Beclin-1, ATG5, and ATG7, with CPD significantly promoting autophagy-related protein expression. (One-way ANOVA) (g) Images of H&E-stained and Masson-stained skin wounds treated with nanocomposite membrane CP and CPD on day 16. Reproduced from Zhang J, Chen K, Ding C, et al. Fabrication of chitosan/PVP/dihydroquercetin nanocomposite film for in vitro and in vivo evaluation of wound healing. Int J Biol Macromol. 2022;206:591–604. Copyright © 2022, Elsevier Inc.Citation121 *P<0.05, **P<0.01, relative to the control group.
Polymer Materials
Polymeric materials are widely used in medical diagnosis and disease treatment because of their diverse biological functions, high biocompatibility, and chemical stability.Citation120 Different types of polymeric nanomaterials have been extensively developed, such as fibrous membranes, hydrogels, and microspheres. Recently, nanofibrous membranes have attracted much attention as a result of their simple preparation process and biocompatibility. Zhang et al prepared novel chitosan/PVP/DHQ composite nanofibrous films using electrospinning technology, which led to significant inhibition of the PI3K/AKT/mTOR pathway and a decrease in the expression levels of p-PI3K, p-Akt, and p-mTOR, thus promoting the conversion of LC3-I to LC3-II. In addition, they significantly increased the expression levels of Beclin-1, ATG5, and ATG7, promoted p62-dependent autophagic degradation, and increased the expression levels of pankeratin, vascular endothelial growth factors (VEGF), and platelet endothelial cell adhesion molecule-1 (CD31) to a certain extent, thus demonstrating excellent antibacterial and antioxidant properties. Therefore, polymeric materials with multifunctional and multitargeted mechanisms can promote skin wound healing.Citation121 The composites of different designs have been receiving increased attention, and novel composites are continuously being developed. Hydrogels have multiple active ingredients and excellent structures with high stability and safety. Zinc and metformin (ZnMet complex) could inhibit reactive oxygen species production through activation of autophagy, thereby protecting cells from oxidative stress-induced damage. Thermosensitive hydrogels in combination with the ZnMet complex can form a novel skin wound healing material. This hydrogel promotes the healing of traumatic skin defects and burn injuries by promoting cell proliferation, angiogenesis, and collagen formation.Citation122 Hydrogels are also highly effective for other skin diseases. Nonpolluting amphoteric sulfated polysulfo betaine methacrylate (SBMA) hydrogels have been shown to activate autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway, reducing inflammation for rapid remodelling of ECM, and improving pressure ulcer (PU) healing.Citation123 Hydrogel microspheres, which are microporous-based assembled scaffolds that can mimic tissue diversity, can protect drugs from untimely exposure and lead to effective drug delivery and the timely release of drugs. Therefore, they have great research potential. Polylactic acid-glycolic acid (PLGA) microspheres can lead to sustained release of platelet-derived growth factor (PDGF). In addition, PDGF-PLGA hydrogels have excellent biocompatibility and support the growth and migration of HUVECs and 3T3 cells. However, PDGF-PLGA hydrogels promote wound healing by inhibiting autophagy.Citation124 The administration of the autophagy activator rapamycin significantly reduced skin wound healing in animals. These two contrasting effects of autophagy on wound healing may be the result of differences in the class of macromolecules or traumatic events. In addition, the activation degree of autophagy at different time points after injury may also be different. Therefore, there is still a need to further investigate the roles of autophagy-based biomaterials in skin injury to suggest future directions for the applications of biomaterials in skin repair.
Cellular Materials
Cell therapy is an emerging medical treatment that plays an important role in the repair and regeneration of functional tissues and organs and has shown great promise in the treatment of wound repair.Citation125 Stem and progenitor cells have high regenerative potential and can promote angiogenesis in tissue regeneration, especially during wound healing.Citation126 Encapsulation of adipose-derived stem cells (ASCs) in three-dimensional polyethylene glycol-fibrin (FPEG) gels leads to enhanced expression of anti-smooth muscle α-actin (α-SMA) and platelet-derived growth factor receptor-β (PDGFR-β) to increase collagen deposition and wound remodelling by modulating the vascularization of the wound surface.Citation127 Despite their excellent efficacy, their direct application at the trauma site has some concerns, such as the risk of host immune rejection and the low survival rate of transplanted stem cells.Citation128 These concerns can be well addressed by autophagy activation. Autophagy determines the therapeutic effect of MSCs in wound healing by regulating the survival of loaded cells on biomaterials. Furthermore, AURKA regulates autophagy through FOXO3a to inhibit the apoptosis of adipose stem cells (ADSCs) induced by a high glucose environment, thereby maintaining the activity of ADSCs and promoting the healing of diabetic wounds.Citation42 Stem cells can not only participate in repair through differentiation but also promote repair based on paracrine signaling. Transplanted MSCs promote ERK phosphorylation upon activation of autophagy and promote VEGF paracrine secretion to accelerate angiogenesis in endothelial cells.Citation129 MSCs have the same effect on refractory wounds. MSCs can increase autophagy in tissues surrounding diabetic wounds and improve diabetic wound healing by removing advanced glycosylation end products.Citation130 Stimulation of electrospun membrane-loaded EPCs with adenosine can effectively promote diabetic wound healing. In addition, adenosine promotes EPC autophagy and rapid differentiation, thereby decreasing their energy requirements to ensure their activity in the diabetic trauma environment, ultimately increasing microangiogenesis.Citation131 Briefly, cellular materials play an important role in skin repair via autophagy. Cellular biomaterials have become a hot research field with enormous clinical significance in wound repair due to their high biocompatibility.
Metallic Nanomaterials
Metallic nanomaterials, one of the most comprehensively studied biomaterials exhibiting autophagy induction, have received much attention in recent times for their applications in tissue regeneration because of their excellent mechanical properties, chemical stability, and biocompatibility. Silver nanoparticles (AgNPs) have been extremely useful in clinical medicine due to their proven antibacterial activity. AgNPs have strong chemical stability and characteristic antiviral and antifungal activities and are often doped in medical devices. According to a previous study, AgNP–porcine dermal extracellular matrix (PADM) hydrogels were prepared by embedding AgNPs into a PADM hydrogel. The AgNPs were uniformly distributed in the PADM hydrogel and slowly released at body temperature while maintaining the particle size. The AgNP–PADM hydrogel exhibited significant antioxidant properties and was effective in killing both gram-positive and gram-negative bacteria.Citation132 Gold nanoparticles (AuNPs) are biomaterials that have been widely used in healthcare applications due to their superior stability and biocompatibility. AuNPs can promote wound repair through a photothermal effect. In a recent study, hydroxyapatite (HAp) coatings doped with AuNPs and polydopamine (PDA) stimulated tissue repair, promoted granulation tissue formation, and improved wound healing under 808 nm light.Citation133 It has been established that autophagy acts as a bridge between metallic nanomaterials and wound repair. AgNPs reduced the accumulation of ROS and HO-1 in NIH-3T3 cells by activating autophagy via upregulation of LC3, thereby attenuating apoptosis.Citation134 Iron oxide nanomaterials (IONPs), one of the most commonly used metallic nanomaterials, are widely used in diagnostic imaging and magnetic resonance imaging.Citation135 Superparamagnetic iron oxide nanoparticles (SPIONs) significantly alleviate LPS-induced sepsis by activating autophagy, preventing microbial infection, and increasing IL-10 expression in RAW264.7 cells.Citation136 IONPs have been shown to reduce inflammatory responses in human monocytes and can be taken up by human monocytes to increase cell viability and attenuate inflammation.Citation137 Metal-organic frameworks (MOFs) have unique physical and chemical properties and exhibit excellent photothermal characteristics, high surface area, and superior stability. MOF induced autophagy in mouse fibroblasts via the inhibition of the mTOR pathway as well as the enhancement of Beclin1 and Atg5, thereby preventing excess ROS in cells and maintaining cellular activity.Citation138
Briefly, metallic nanomaterials induce autophagy via multiple signaling pathways and exhibit different effects on wound repair and regeneration. Therefore, investigating metallic nanomaterial-induced autophagy not only further elucidates the molecular mechanism of autophagy but also provides new insight into skin wound healing.
Carbon-Based Nanomaterials
Carbon is one of the most common and abundant elements in the Earth’s crust. Carbon atoms can form bonds with other carbon atoms in various ways to form different carbon isomers, resulting in several superior carbon-based nanomaterials, such as 0D fullerenes, nanodiamonds (NDs), and 2D graphene and their derivatives.Citation139 These carbon-based nanomaterials are widely used in biomedical applications and have been successfully applied for tissue regeneration and drug delivery.Citation140 Fullerenes exhibit potent anti-inflammatory and antibacterial properties that can promote wound healing.Citation141 According to Gao’s group, hydrophilic fullerene tris-C60 significantly reduced the release of proinflammatory factors and enhanced apoptosis and proliferation of human skin keratinocytes.Citation142 In addition, almost all types of carbon-based nanomaterials have been shown to possess antimicrobial effects, and the major mechanism of action involves bacterial outer membrane damage. Carbon-based nanomaterials have great benefits in wound healing due to the vulnerability of skin wounds to bacterial infections.Citation140 Cheng et al designed a detachable arginine-loaded cerium dioxide-graphene nanocomposite (ACG-NC), which can effectively produce ROS under sunlight to kill microbes and accelerate wound healing.Citation143 Carbon-based nanomaterials can also play a regulatory role by modulating autophagy. Low doses of NDs and SiO2-NPs enhanced wound healing ability by slowing aging during serial passaging. Furthermore, NP treatment induced the activation of Nrf2- and FOXO3-mediated cellular stress responses to improve the maintenance, repair, and cellular functions of human facial skin fibroblast 1 (FSF1).Citation144 Thus, the anti-inflammatory and antibacterial effects of carbon-based nanomaterials establish their close association with all stages of wound healing. Furthermore, the effects of biomaterials are directly dependent on their doses, which is a key factor that should be considered during the development of such biomaterials.
Summary and Prospects
Autophagy regulation has resulted in promising wound healing results in animals. Biomaterials can modulate autophagy, prevent intra- and extracellular stresses, and regulate the physiological mechanism of traumatized cells, all of which lead to a positive effect on promoting wound healing. Surface modifications and structural alterations of biomaterials, especially multicomponent nanomaterials, can increase or decrease the autophagic flux in tissue cells. Some biomaterials can induce autophagy, promote the regeneration of damaged skin tissues, and exert antimicrobial, anti-inflammatory, antioxidant, and pro-cellular proliferation and differentiation effects. Appropriate levels of autophagy enhance cellular metabolism and are essential for cellular energy cycling to maintain homeostasis. Although excessive autophagy may lead to fibrosis, normal levels of autophagy at the right stage can stabilize cellular activity and exert a housekeeping role in physiological processes. Exploring the molecular mechanisms underlying biomaterial-induced autophagy can help to develop biomaterials with rational design. Understanding the autophagy mechanism will lead to finer-tuned regulation of autophagy in cells and tissues, resulting in a potential new therapeutic strategy.
Several challenges still exist for the applications of autophagy-based biomaterials in promoting wound repair. On the one hand, polymers and cells can effectively encapsulate drugs and increase their stability, which can prolong drug release and increase bioactivity, thereby improving therapeutic efficacy. However, it cannot be overlooked that the two materials have several shortcomings, such as weak antibiotic capacity. Most metallic and carbon-based nanomaterials can utilize a nonantibiotic treatment modality for highly effective antibacterial activity. However, they can activate apoptosis-related genes and further induce cell apoptosis mechanisms due to their poor biocompatibility, which delays wound healing. On the other hand, studies on nanomaterial-induced autophagy are still inadequate. Therefore, the relationships between the specific pathways of autophagy and physicochemical properties of biomaterials, such as composition, structure, and properties, are still unclear, and there is a need for more systematic and in-depth studies. In addition, biomaterial-based autophagy-targeted delivery to specific cells is still not well understood. The major cells associated with wound healing are macrophages, endothelial cells, fibroblasts, and keratinocytes. Specific cells can perform various regulatory functions via autophagy to promote wound repair. Finally, it can be stated that most of the current studies are preclinical studies involving cell cultures or animals and that sufficient clinical validation is lacking. Nevertheless, there are many opportunities that remain untapped. Currently, several biomaterial-based approaches are potentially effective in wound healing. The selection of high-quality biomaterials, optimization of size, shape, charge, and surface modifications of the biomaterials, and improvement in their clinical safety and performance will be the key to future studies. Biomaterial-modulated autophagy for wound healing has become a promising path for further research and development and an effective treatment option for wounds. This novel approach has future research and development prospects in the field of regenerative medicine and will certainly open a new chapter in tissue engineering in the future.
Abbreviations
AMPK, adenosine monophosphate-activated protein kinase; AGE, advanced glycation end product; ATP, adenosine triphosphate; ATG, autophagy related; ASC, adipose-derived stem cells; ADSCs, adipose stem cells; AgNPs, silver nanoparticles; AuNPs, gold nanoparticles; ACG-NC, arginine-loaded cerium dioxide-graphene; nanocomposite; CMA, chaperone-mediated autophagy; CP, chitosan and polyvinylpyrrolidone; CPD, chitosan polyvinylpyrrolidone and dihydroquercetin; CD31, platelet endothelial cell adhesion molecule-1; ECM, extracellular matrix; EPCs, endothelial progenitor cells; FSF1, facial skin fibroblast 1; GSH-PX, glutathione peroxidase; Hap, hydroxyapatite; HSF, hypertrophic scar fibroblast; LPS, lipopolysaccharide; IL-1β, interleukin-1 beta; IL-6, interleukin 6; IONPs, iron oxide nanomaterials; LC3, microtubule-associated proteins 1A and 1B; MOF, metal-organic frameworks; mTOR, mammalian target of rapamycin; MDA, malondialdehyde; MSCs, mesenchymal stem cells; NETs, neutrophil extracellular traps; Nrf2, nuclear factor-erythroid 2 p45-related factor 2; NDs, nanodiamonds; PU, pressure ulcer; PLGA, polylactic acid-glycolic acid; PADM, porcine dermal extracellular matrix; PDGF, platelet-derived growth factor; p75NTR, p75 neurotrophic factor receptor; PDA, polydopamine; PNA, poly(N-isopropylacrylamide-acrylic acid; PLLA, poly (L-lactic acid); ROS, reactive oxygen species; SOD, superoxide dismutase; SBMA, sulfated polysulfo betaine methacrylate; SPIONs, superparamagnetic iron oxide nanoparticles; TNF-α, tumor necrosis factor-α; TFEB, transcription factor EB; ULK1, unc-51-like-kinase 1; UroA, urolithin a; UVA, ultraviolet a; VEGF, vascular endothelial growth Factors; ZnMet complex, complexes of zinc and metformin.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the Central Finance Supports Local Colleges and Universities Talent Development Funding from Heilongjiang Provincial Department of Finance (2020GSP09), the Natural Science Foundation of Heilongjiang Province (LH2020H076), the National Natural Science Foundation of China (82072051, 81903233 and 81874250) and the Basic Scientific Research Project of University belonging to Heilongjiang (2019-KYYWF-0987).
References
- Ren H, Zhao F, Zhang Q, Huang X, Wang Z. Autophagy and skin wound healing. Burns Trauma. 2022;10:tkac003. doi:10.1093/burnst/tkac003
- Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev. 2014;69–70:81–102. doi:10.1016/j.addr.2013.12.006
- Pirot F, Kalia YN, Stinchcomb AL, Keating G, Bunge A, Guy RH. Characterization of the permeability barrier of human skin in vivo. Proc Natl Acad Sci U S A. 1997;94(4):1562–1567. doi:10.1073/pnas.94.4.1562
- Lin JY, Lo KY, Sun YS. Effects of substrate-coating materials on the wound-healing process. Materials. 2019;12(17):2775. doi:10.3390/ma12172775
- Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–239. doi:10.1016/j.addr.2018.12.014
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281(16):11374–11383. doi:10.1074/jbc.M509157200
- Das LM, Binko AM, Traylor ZP, Peng H, Lu KQ. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15(5):813–826. doi:10.1080/15548627.2019.1569298
- Naomi R, Bahari H, Ridzuan PM, Othman F. Natural-based biomaterial for skin wound healing (Gelatin vs. collagen): expert review. Polymers. 2021;13(14):2319. doi:10.3390/polym13142319
- Farazin A, Torkpour Z, Dehghani S, et al. A review on polymeric wound dress for the treatment of burns and diabetic wounds. Int J Basic Sci Med. 2021;6(2):44–50. doi:10.34172/ijbsm.2021.08
- Ahmady AR, Razmjooee K, Saber-Samandari S, Toghraie D. Fabrication of chitosan-gelatin films incorporated with thymol-loaded alginate microparticles for controlled drug delivery, antibacterial activity and wound healing: in-vitro and in-vivo studies. Int J Biol Macromol. 2022;223(Pt A):567–582. doi:10.1016/j.ijbiomac.2022.10.249
- Li R, Liu K, Huang X, et al. Bioactive materials promote wound healing through modulation of cell behaviors. Adv Sci. 2022;9(10):e2105152. doi:10.1002/advs.202105152
- Zhang S, Li Y, Qiu X, et al. Incorporating redox-sensitive nanogels into bioabsorbable nanofibrous membrane to acquire ROS-balance capacity for skin regeneration. Bioact Mater. 2021;6(10):3461–3472. doi:10.1016/j.bioactmat.2021.03.009
- Feng X, Zhang Y, Zhang C, et al. Nanomaterial-mediated autophagy: coexisting hazard and health benefits in biomedicine. Part Fibre Toxicol. 2020;17(1):53. doi:10.1186/s12989-020-00372-0
- Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. doi:10.1186/1743-8977-9-20
- Guo L, He N, Zhao Y, Liu T, Deng Y. Autophagy modulated by inorganic nanomaterials. Theranostics. 2020;10(7):3206–3222. doi:10.7150/thno.40414
- Chaudhari AA, Vig K, Baganizi DR, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17(12):E1974. doi:10.3390/ijms17121974
- De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28(1):435–492. doi:10.1146/annurev.ph.28.030166.002251
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–1109. doi:10.1038/ncb1007-1102
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995. doi:10.1126/science.1099993
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8(11):931–937. doi:10.1038/nrm2245
- Levine B. Cell biology: autophagy and cancer. Nature. 2007;446(7137):745–747. doi:10.1038/446745a
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi:10.1016/j.cell.2007.12.018
- Feng D, Liu L, Zhu Y, Chen Q. Lysosome biology in autophagy. Exp Cell Res. 2013;319(12):1697–1705. doi:10.1016/j.yexcr.2013.03.034
- Yang X, Yu DD, Yan F, et al. The role of autophagy induced by tumor microenvironment in different cells and stages of cancer. Cell Biosci. 2015;5(1):14. doi:10.1186/s13578-015-0005-2
- Li WW, Li J, Bao JK. Microautophagy: lesser-known self-eating. Cell Mol Life Sci. 2012;69(7):1125–1136. doi:10.1007/s00018-011-0865-5
- Martens S, Fracchiolla D. Activation and targeting of ATG8 protein lipidation. Cell Discov. 2020;6(1):23. doi:10.1038/s41421-020-0155-1
- Papinski D, Kraft C. Atg1 kinase organizes autophagosome formation by phosphorylating Atg9. Autophagy. 2014;10(7):1338–1340. doi:10.4161/auto.28971
- Yang Z, Huang J, Geng J, Nair U, Klionsky DJ, Brodsky J. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol Biol Cell. 2006;17(12):5094–5104. doi:10.1091/mbc.e06-06-0479
- Zhao TM, Wang Y, Deng Y, et al. Bicyclol attenuates acute liver injury by activating autophagy, anti-oxidative and anti-inflammatory capabilities in mice. Front Pharmacol. 2020;11:463. doi:10.3389/fphar.2020.00463
- Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177(7):1682–1699. doi:10.1016/j.cell.2019.05.026
- Li YF, Ouyang SH, Tu LF, et al. Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics. 2018;8(20):5713–5730. doi:10.7150/thno.28778
- Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2012;13(1):7–12. doi:10.1038/nrm3249
- Zhang Q, Xiao L, Xiao Y. Porous nanomaterials targeting autophagy in bone regeneration. Pharmaceutics. 2021;13(10):1572. doi:10.3390/pharmaceutics13101572
- Nishimura T, Tooze SA. Emerging roles of ATG proteins and membrane lipids in autophagosome formation. Cell Discov. 2020;6(1):32. doi:10.1038/s41421-020-0161-3
- Liu K, Zhao E, Ilyas G, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11(2):14. doi:10.1080/15548627.2015.1009787
- Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–676. doi:10.1038/45257
- Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. doi:10.1038/nature04723
- Yim WWY, Mizushima N. Lysosome biology in autophagy. Cell Discov. 2020;6(1):6. doi:10.1146/annurev.ph.28.030166.002251
- Wei Y, Liu M, Li X, Liu J, Li H. Origin of the autophagosome membrane in mammals. BioMed Res Int. 2018;2018:1–9. doi:10.1155/2018/1012789
- He C, Baba M, Cao Y, Klionsky DJ, Brodsky JL. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Brodsky JL, ed. Mol Biol Cell. 2008;19(12):5506–5516. doi:10.1091/mbc.e08-05-0544
- Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi:10.1038/nature04724
- Yin Y, Chen F, Li J, Yang J, Li Q, Jin P. AURKA enhances autophagy of adipose derived stem cells to promote diabetic wound repair via targeting FOXO3a. J Invest Dermatol. 2020;140(8):1639–1649.e4. doi:10.1016/j.jid.2019.12.032
- Yu P, Zhang C, Gao CY, et al. Anti-proliferation of triple-negative breast cancer cells with physagulide P: ROS/JNK signaling pathway induces apoptosis and autophagic cell death. Oncotarget. 2017;8(38):64032–64049. doi:10.18632/oncotarget.19299
- Kamalathevan P, Ooi PS, Loo YL. Silk-based biomaterials in cutaneous wound healing: a systematic review. Adv Skin Wound Care. 2018;31(12):565–573. doi:10.1097/01.ASW.0000546233.35130.a9
- Murray RZ, West ZE, Cowin AJ, Farrugia BL. Development and use of biomaterials as wound healing therapies. Burns Trauma. 2019;7:2. doi:10.1186/s41038-018-0139-7
- Das S, Baker AB. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol. 2016;4:82. doi:10.3389/fbioe.2016.00082
- Migneault F, Hébert MJ. Autophagy, tissue repair, and fibrosis: a delicate balance. Matrix Biol J Int Soc Matrix Biol. 2021;100–101:182–196. doi:10.1016/j.matbio.2021.01.003
- Ceccariglia S, Cargnoni A, Silini AR, Parolini O. Autophagy: a potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy. 2020;16(1):28–37. doi:10.1080/15548627.2019.1630223
- Xie Y, Yu L, Cheng Z, et al. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J Nanobiotechnology. 2022;20(1):239. doi:10.1186/s12951-022-01446-1
- Jin H, Zhang Z, Wang C, et al. Melatonin protects endothelial progenitor cells against AGE-induced apoptosis via autophagy flux stimulation and promotes wound healing in diabetic mice. Exp Mol Med. 2018;50(11):1–15. doi:10.1038/s12276-018-0177-z
- Zeng T, Wang X, Wang W, et al. Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clin Sci. 2019;133(9):CS20190008. doi:10.1042/CS20190008
- Guo Y, Lin C, Xu P, et al. AGEs induced autophagy impairs cutaneous wound healing via stimulating macrophage polarization to M1 in diabetes. Sci Rep. 2016;6:36416. doi:10.1038/srep36416
- Breitkreutz D, Koxholt I, Thiemann K, Nischt R. Skin basement membrane: the foundation of epidermal integrity--BM functions and diverse roles of bridging molecules nidogen and perlecan. BioMed Res Int. 2013;2013:179784. doi:10.1155/2013/179784
- Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi:10.1146/annurev-pathol-020712-164023
- Ullah I, Ritchie ND, Evans TJ. The interrelationship between phagocytosis, autophagy and formation of neutrophil extracellular traps following infection of human neutrophils by Streptococcus pneumoniae. Innate Immun. 2017;23(5). doi:10.1177/1753425917704299
- Kimmey JM, Huynh JP, Weiss LA, et al. Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature. 2015;528(7583):565–569. doi:10.1038/nature16451
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi:10.1126/science.1092385
- Bhattacharya A, Wei Q, Shin JN, et al. Autophagy is required for neutrophil-mediated inflammation. Cell Rep. 2015;12(11):1731–1739. doi:10.1016/j.celrep.2015.08.019
- Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M, Lammas DA. ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunol. 2008;9:35. doi:10.1186/1471-2172-9-35
- Zhao Z, Fux B, Goodwin M, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4(5):458–469. doi:10.1016/j.chom.2008.10.003
- Choi SW, Gu Y, Peters RS, et al. Ambroxol induces autophagy and potentiates rifampin antimycobacterial activity. Antimicrob Agents Chemother. 2018;62(9):e01019–18. doi:10.1128/AAC.01019-18
- Cemma M, Brumell JH. Interactions of pathogenic bacteria with autophagy systems. Curr Biol. 2012;22(13):R540–R545. doi:10.1016/j.cub.2012.06.001
- Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103(39):14578–14583. doi:10.1073/pnas.0601838103
- Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunol Rev. 2014;262(1):56–73. doi:10.1111/imr.12224
- Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 2019;97(3):258–267. doi:10.1111/imcb.12236
- Qiu P, Liu Y, Zhang J. Review: the role and mechanisms of macrophage autophagy in sepsis. Inflammation. 2019;42(1):6–19. doi:10.1007/s10753-018-0890-8
- Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16(10):1014–1024. doi:10.1038/ni.3273
- Clarke A, Simon A. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2018;19:170–183. doi:10.1038/s41577-018-0095-2
- Saitoh T, Akira S. Regulation of inflammasomes by autophagy. J Allergy Clin Immunol. 2016;138(1):28–36. doi:10.1016/j.jaci.2016.05.009
- Cao L, Wang Y, Wang Y, Lv F, Liu L, Li Z. Resolvin D2 suppresses NLRP3 inflammasome by promoting autophagy in macrophages. Exp Ther Med. 2021;22(5):1222. doi:10.3892/etm.2021.10656
- Cao Y, Chen J, Ren G, Zhang Y, Tan X, Yang L. Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients. 2019;11(11):2794. doi:10.3390/nu11112794
- Renga G, Oikonomou V, Stincardini C, et al. Thymosin β4 limits inflammation through autophagy. Expert Opin Biol Ther. 2018;18(sup1):171–175. doi:10.1080/14712598.2018.1473854
- Takahama M, Akira S, Saitoh T. Autophagy limits activation of the inflammasomes. Immunol Rev. 2018;281(1):62–73. doi:10.1111/imr.12613
- Unanue ER, Beller DI, Calderon J, Kiely JM, Stadecker MJ. Regulation of immunity and inflammation by mediators from macrophages. Am J Pathol. 1976;85(2):465–478.
- Harris J, Hartman M, Roche C, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011;286(11):9587–9597. doi:10.1074/jbc.M110.202911
- Wu MY, Lu JH. Autophagy and macrophage functions: inflammatory response and phagocytosis. Cells. 2019;9(1):E70. doi:10.3390/cells9010070
- Qing G, Yan P, Xiao G. Hsp90 inhibition results in autophagy-mediated proteasome-independent degradation of IkappaB kinase (IKK). Cell Res. 2006;16(11):895–901. doi:10.1038/sj.cr.7310109
- Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009;29(10):2594–2608. doi:10.1128/MCB.01396-08
- Dunnill C, Patton T, Brennan J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14(1):89–96. doi:10.1111/iwj.12557
- Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114(3):524–537. doi:10.1161/CIRCRESAHA.114.300559
- D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi:10.1038/nrm2256
- Sahu A, Jeon J, Lee MS, Yang HS, Tae G. Antioxidant and anti-inflammatory activities of Prussian blue nanozyme promotes full-thickness skin wound healing. Mater Sci Eng C. 2021;119:111596. doi:10.1016/j.msec.2020.111596
- Li D, Ding Z, Du K, Ye X, Cheng S. Reactive oxygen species as a link between antioxidant pathways and autophagy. Oxid Med Cell Longev. 2021;2021:5583215. doi:10.1155/2021/5583215
- Zhang SW, Feng JN, Cao Y, Meng LP, Wang SL. Autophagy prevents autophagic cell death in tetrahymena in response to oxidative stress. Dong Wu Xue Yan Jiu Zool Res. 2015;36(3):167–173.
- Baechler BL, Bloemberg D, Quadrilatero J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy. 2019;15(9):1606–1619. doi:10.1080/15548627.2019.1591672
- Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17(9):422–427. doi:10.1016/j.tcb.2007.07.009
- Xu L, Fan Q, Wang X, Zhao X, Wang L. Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis. 2016;7(11):e2445. doi:10.1038/cddis.2016.322
- Yoon JY, Park CG, Park BS, Kim EJ, Byeon GJ, Yoon JU. Effects of remifentanil preconditioning attenuating oxidative stress in human dermal fibroblast. Tissue Eng Regen Med. 2017;14(2):133–141. doi:10.1007/s13770-017-0030-9
- Raben N, Puertollano R. TFEB and TFE3: linking lysosomes to cellular adaptation to stress. Annu Rev Cell Dev Biol. 2016;32:255–278. doi:10.1146/annurev-cellbio-111315-125407
- Lee YJ, Kim NY, Suh YA, Lee C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol. 2011;15(1):1–7. doi:10.4196/kjpp.2011.15.1.1
- Momtazi-Borojeni AA, Abdollahi E, Nikfar B, Chaichian S, Ekhlasi-Hundrieser M. Curcumin as a potential modulator of M1 and M2 macrophages: new insights in atherosclerosis therapy. Heart Fail Rev. 2019;24(3):399–409. doi:10.1007/s10741-018-09764-z
- Cheng L, Jin Z, Zhao R, Ren K, Deng C, Yu S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int J Clin Exp Med. 2015;8(7):10420–10428.
- Wang XH, Zhu L, Hong X, et al. Resveratrol attenuated TNF-α–induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp Biol Med. 2016;241(8):848–853. doi:10.1177/1535370216637940
- Kohchi C, Inagawa H, Nishizawa T, Soma GI. ROS and innate immunity. Anticancer Res. 2009;29(3):817–821.
- Padgett LE, Burg AR, Lei W, Tse HM. Loss of NADPH oxidase-derived superoxide skews macrophage phenotypes to delay type 1 diabetes. Diabetes. 2015;64(3):937–946. doi:10.2337/db14-0929
- Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23(7):898–914. doi:10.1038/cr.2013.75
- Fei Q, Ma H, Zou J, et al. Metformin protects against ischaemic myocardial injury by alleviating autophagy-ROS-NLRP3-mediated inflammatory response in macrophages. J Mol Cell Cardiol. 2020;145:1–13. doi:10.1016/j.yjmcc.2020.05.016
- Yuan Y, Chen Y, Peng T, et al. Mitochondrial ROS-induced lysosomal dysfunction impairs autophagic flux and contributes to M1 macrophage polarization in a diabetic condition. Clin Sci. 2019;133(15):1759–1777. doi:10.1042/CS20190672
- Dai J, Zhang X, Wang Y, Chen H, Chai Y. ROS-activated NLRP3 inflammasome initiates inflammation in delayed wound healing in diabetic rats. Int J Clin Exp Pathol. 2017;10(9):9902–9909.
- Meng Q, Li Y, Ji T, et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor α-mediated autophagy. J Adv Res. 2021;28:149–164. doi:10.1016/j.jare.2020.08.010
- Han J, Pan XY, Xu Y, et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8(5):812–825. doi:10.4161/auto.19471
- Guo H, Ding H, Yan Y, et al. Intermittent hypoxia-induced autophagy via AMPK/mTOR signaling pathway attenuates endothelial apoptosis and dysfunction in vitro. Sleep Breath Schlaf Atm. 2021;25(4):1859–1865. doi:10.1007/s11325-021-02297-0
- Zha S, Li Z, Chen S, Liu F, Wang F. MeCP2 inhibits cell functionality through FoxO3a and autophagy in endothelial progenitor cells. Aging. 2019;11(17):6714–6733. doi:10.18632/aging.102183
- Liang P, Jiang B, Li Y, et al. Autophagy promotes angiogenesis via AMPK/Akt/mTOR signaling during the recovery of heat-denatured endothelial cells. Cell Death Dis. 2018;9(12):1152. doi:10.1038/s41419-018-1194-5
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi:10.1038/nrm809
- Zhou L, Liu Z, Chen S, et al. Transcription factor EB-mediated autophagy promotes dermal fibroblast differentiation and collagen production by regulating endoplasmic reticulum stress and autophagy-dependent secretion. Int J Mol Med. 2021;47(2):547–560. doi:10.3892/ijmm.2020.4814
- Liu W, Yan F, Xu Z, et al. Urolithin A protects human dermal fibroblasts from UVA-induced photoaging through NRF2 activation and mitophagy. J Photochem Photobiol B. 2022;232:112462. doi:10.1016/j.jphotobiol.2022.112462
- Shi W, Wu Y, Bian D. p75NTR silencing inhibits proliferation, migration, and extracellular matrix deposition of hypertrophic scar fibroblasts by activating autophagy through inhibiting the PI3K/Akt/mTOR pathway. Can J Physiol Pharmacol. 2021;99(4):349–359. doi:10.1139/cjpp-2020-0219
- Cao C, Wang W, Lu L, et al. Inactivation of Beclin-1-dependent autophagy promotes ursolic acid-induced apoptosis in hypertrophic scar fibroblasts. Exp Dermatol. 2018;27(1):58–63. doi:10.1111/exd.13410
- Talagas M, Lebonvallet N, Berthod F, Misery L. Cutaneous nociception: role of keratinocytes. Exp Dermatol. 2019;28(12):1466–1469. doi:10.1111/exd.13975
- Qiang L, Yang S, Cui YH, He YY. Keratinocyte autophagy enables the activation of keratinocytes and fibroblasts and facilitates wound healing. Autophagy. 2021;17(9):2128–2143. doi:10.1080/15548627.2020.1816342
- Li L, Zhang J, Zhang Q, et al. High glucose suppresses keratinocyte migration through the inhibition of p38 MAPK/autophagy pathway. Front Physiol. 2019;10:24. doi:10.3389/fphys.2019.00024
- Zhang J, Zhang C, Jiang X, et al. Involvement of autophagy in hypoxia-BNIP3 signaling to promote epidermal keratinocyte migration. Cell Death Dis. 2019;10(3):234. doi:10.1038/s41419-019-1473-9
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
- Travan A, Donati I, Marsich E, et al. Surface modification and polysaccharide deposition on BisGMA/TEGDMA thermoset. Biomacromolecules. 2010;11(3):583–592. doi:10.1021/bm9011419
- Rapino M, Di Valerio V, Zara S, et al. Chitlac-coated thermosets enhance osteogenesis and angiogenesis in a co-culture of dental pulp stem cells and endothelial cells. Nanomaterial. 2019;9(7):E928. doi:10.3390/nano9070928
- Gomes LC, Dikic I. Autophagy in antimicrobial immunity. Mol Cell. 2014;54(2):224–233. doi:10.1016/j.molcel.2014.03.009
- Chandel S, Manikandan A, Mehta N, et al. The protein tyrosine phosphatase PTP-PEST mediates hypoxia-induced endothelial autophagy and angiogenesis via AMPK activation. J Cell Sci. 2021;134(1):jcs250274. doi:10.1242/jcs.250274
- Han YF, Sun TJ, Han YQ, Xu G, Liu J, Tao R. Clinical perspectives on mesenchymal stem cells promoting wound healing in diabetes mellitus patients by inducing autophagy. Eur Rev Med Pharmacol Sci. 2015;19(14):2666–2670.
- Mozumder MS, Mairpady A, Mourad AHI. Polymeric nanobiocomposites for biomedical applications. J Biomed Mater Res B Appl Biomater. 2017;105(5):1241–1259. doi:10.1002/jbm.b.33633
- Zhang J, Chen K, Ding C, et al. Fabrication of chitosan/PVP/dihydroquercetin nanocomposite film for in vitro and in vivo evaluation of wound healing. Int J Biol Macromol. 2022;206:591–604. doi:10.1016/j.ijbiomac.2022.02.110
- Liu Z, Tang W, Liu J, et al. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact Mater. 2023;20:610–626. doi:10.1016/j.bioactmat.2022.06.008
- Li Y, Jiang S, Song L, et al. Zwitterionic hydrogel activates autophagy to promote extracellular matrix remodeling for improved pressure ulcer healing. Front Bioeng Biotechnol. 2021;9:740863. doi:10.3389/fbioe.2021.740863
- Xu K, An N, Zhang H, et al. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J Drug Deliv Sci Technol. 2020;55:101405. doi:10.1016/j.jddst.2019.101405
- Wang X, Rivera-Bolanos N, Jiang B, Ameer GA. Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv Funct Mater. 2019;29(23):1809009. doi:10.1002/adfm.201809009
- Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107(8):3317–3322. doi:10.1073/pnas.0905432106
- Zamora DO, Natesan S, Becerra S, et al. Enhanced wound vascularization using a dsASCs seeded FPEG scaffold. Angiogenesis. 2013;16(4):745–757. doi:10.1007/s10456-013-9352-y
- Ni X, Ou C, Guo J, et al. Lentiviral vector-mediated co-overexpression of VEGF and Bcl-2 improves mesenchymal stem cell survival and enhances paracrine effects in vitro. Int J Mol Med. 2017;40(2):418–426. doi:10.3892/ijmm.2017.3019
- An Y, Liu WJ, Xue P, et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018;9(2):58. doi:10.1038/s41419-017-0082-8
- Han Y, Sun T, Tao R, Han Y, Liu J. Clinical application prospect of umbilical cord-derived mesenchymal stem cells on clearance of advanced glycation end products through autophagy on diabetic wound. Eur J Med Res. 2017;22(1):11. doi:10.1186/s40001-017-0253-1
- Chen W, Wu Y, Li L, et al. Adenosine accelerates the healing of diabetic ischemic ulcers by improving autophagy of endothelial progenitor cells grown on a biomaterial. Sci Rep. 2015;5(1):11594. doi:10.1038/srep11594
- Dong Q, Zu D, Kong L, et al. Construction of antibacterial nano-silver embedded bioactive hydrogel to repair infectious skin defects. Biomater Res. 2022;26(1):36. doi:10.1186/s40824-022-00281-7
- Xu X, Liu X, Tan L, et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018;77:352–364. doi:10.1016/j.actbio.2018.07.030
- Lee YH, Cheng FY, Chiu HW, et al. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials. 2014;35(16):4706–4715. doi:10.1016/j.biomaterials.2014.02.021
- Jin Z, Dun Y, Xie L, et al. Preparation of doxorubicin-loaded porous iron Oxide@ polydopamine nanocomposites for MR imaging and synergistic photothermal-chemotherapy of cancer. Colloids Surf B Biointerfaces. 2021;208:112107. doi:10.1016/j.colsurfb.2021.112107
- Xu Y, Li Y, Liu X, et al. SPIONs enhances IL-10-producing macrophages to relieve sepsis via Cav1-Notch1/HES1-mediated autophagy. Int J Nanomedicine. 2019;14:6779–6797. doi:10.2147/IJN.S215055
- Wu Q, Jin R, Feng T, et al. Iron oxide nanoparticles and induced autophagy in human monocytes. Int J Nanomedicine. 2017;12:3993–4005. doi:10.2147/IJN.S135189
- Shen S, Li L, Li S, Bai Y, Liu H. Metal–organic frameworks induce autophagy in mouse embryonic fibroblast cells. Nanoscale. 2018;10(38):18161–18168. doi:10.1039/C8NR04459G
- Dalla Colletta A, Pelin M, Sosa S, Fusco L, Prato M, Tubaro A. CARBON-BASED nanomaterials and SKIN: an overview. Carbon. 2022;196:683–698. doi:10.1016/j.carbon.2022.05.036
- Rahman MA, Barkat HA, Harwansh RK, Deshmukh R. Carbon-based nanomaterials: carbon nanotubes, graphene, and fullerenes for the control of burn infections and wound healing. Curr Pharm Biotechnol. 2022;23(12):1483–1496. doi:10.2174/1389201023666220309152340
- Zhou Z. Liposome formulation of fullerene-based molecular diagnostic and therapeutic agents. Pharmaceutics. 2013;5(4):525–541. doi:10.3390/pharmaceutics5040525
- Gao J, Hsing-Lin W, Rashi I. Suppression of proinflammatory cytokines in functionalized fullerene-exposed dermal keratinocytes. J Nanomater. 2010;2010:1–9. doi:10.1155/2010/416408
- Cheng Y, Chang Y, Feng Y, et al. Hierarchical acceleration of wound healing through intelligent nanosystem to promote multiple stages. ACS Appl Mater Interfaces. 2019;11(37):33725–33733. doi:10.1021/acsami.9b13267
- Mytych J, Wnuk M, Rattan SIS. Low doses of nanodiamonds and silica nanoparticles have beneficial hormetic effects in normal human skin fibroblasts in culture. Chemosphere. 2016;148:307–315. doi:10.1016/j.chemosphere.2016.01.045