?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In order to improve the oral bioavailability of ibuprofen, ibuprofen-loaded cubic nanoparticles were prepared as a delivery system for aqueous formulations. The cubic inner structure was verified by cryogenic transmission electron microscopy. With an encapsulation efficiency greater than 85%, the ibuprofen-loaded cubic nanoparticles had a narrow size distribution around a mean size of 238 nm. Differential scanning calorimetry and X-ray diffraction determined that ibuprofen was in an amorphous and molecular form within the lipid matrix. The in vitro release of ibuprofen from cubic nanoparticles was greater than 80% at 24 hours, showing sustained characteristics. The pharmacokinetic study in beagle dogs showed improved absorption of ibuprofen from cubic nanoparticles compared to that of pure ibuprofen, with evidence of a longer half-life and a relative oral bioavailability of 222% (P < 0.05). The ibuprofen-loaded cubic nanoparticles provide a promising carrier candidate with an efficient drug delivery for therapeutic treatment.
Introduction
Ibuprofen is a non-steroidal anti-inflammatory drugCitation1,Citation2 inhibiting prostaglandin synthesis but having no effect on the adrenal pituitary axis. In addition, ibuprofen has an analgesic property that is probably related to its anti-inflammatory effect. Moreover, ibuprofen has been proven effective in the treatment of rheumatoid osteoarthritis, ankylosing spondylitis, gout, and Bartter’s syndrome.Citation3,Citation4 However, along with its demonstrated safety and efficacy, ibuprofen plays a limited pharmaceutical role, mainly because of its extremely low aqueous solubility, rapid systemic elimination with a serum half-life of 1.8 hours, and inadequate tissue absorption resulting in poor bioavailability. The conventional ibuprofen tablet has low bioavailability and severe stimulatory effects on the gastrointestinal tract (GIT). Gastric discomfort, nausea, and vomiting are still the most common side effects.
Current trends in ibuprofen research have concentrated on the development of potential delivery systems to increase its aqueous solubility and bioavailability, as well as to achieve controlled delivery of ibuprofen. As a result, the sustained and controlled release preparations of ibuprofen are developed widely, which includes extended-action tablets, modified-release capsules, delayed-release pellets, and sustained-release microspheres.Citation5–Citation9 A modified release-capsule has long been on the market.
Much attention was recently given to bioadhesive delivery systems by increasing the residence time in the GIT, subsequently facilitating the absorption of the drug through adhesion to cellular surfaces. These oral delivery systems certainly represent a promising approach to increasing the bioavailability of ibuprofen.Citation10–Citation12 However, lyotropic liquid crystal nanoparticles, such as reversed bi-continuous cubics, have recently received much more attention for sustained release of the delivered drugs because of their ability to solubilize hydrophilic, hydrophobic, and amphiphilic drug molecules.Citation13 Cubic nanoparticles as oral delivery carriers seem to be advantageous for poorly water-soluble drugs for several reasons. First, being lyotropic, cubic nanoparticles can hold poorly water-soluble drugs in a solubilized state within their lipid bilayers.Citation14 Second, bioadhesive cubic nanoparticles may increase the opportunity for the close contact of drug-loaded nanoparticles with intestinal cell membranes.Citation15 Finally, the cubic phases can enhance the stability of drugs and reduce drug stimulation on the GIT.Citation16
Most studies have focused on glyceryl monooleate (GMO) as a liquid crystal-forming lipid, but glyceride lipids possess ester bonds that are susceptible to degradation by lipase enzymes in the GIT. This phenomenon was previously observed with GMO cubosomes in in vitro digestion models,Citation17 implying a loss of liquid crystal structure in vivo. Therefore, non-digestible lipid phytantriol (PYT) has attracted increasing interest due to its similar phase behavior as GMO’s and improved chemical stability. Phytantriol was found to sustain the absorption of cinnarizine over approximately 48 hours after oral administration, resulting in improved bioavailability. It has been proven that phytantriol can be retained in the stomach for an extended period of time, comparable to GMO, establishing a link between digestibility, gastric retention, and long-term sustained release.Citation18
Cubic liquid crystalline nanostructured particles, like liposomes, are thermodynamically unstable dispersions, requiring energy input often via high-pressure homogenization or ultrasonication to form dispersions in the presence of stabilizers.Citation19 Representative stabilizers include non-ionic block co-polymer pluronics (F127), polyvinyl alcohol (PVA), and polypeptides. These are of interest, as they can provide stability and chemical functionalization for nanoparticles as drug delivery systems.Citation20,Citation21
The abilities of cubic phases to incorporate drugs for controlled release and enhanced drug bioavailability make it an interesting drug delivery system for oral, topical (or mucosal), and intravenous administration, with extensive applications in a multitude of dosage forms.Citation18,Citation22–Citation26 However, few studies have assessed the application of cubic phases in ibuprofen oral drug delivery.
Therefore, the objective of this study was to develop a nanoparticulate delivery system by using PYT and F127, which could solubilize ibuprofen in aqueous media, reaching the clinically relevant concentration and delivering ibuprofen in a controlled manner. Taking advantage of their permeation-enhancing effect, cubic nanoparticles were evaluated in vivo as potential vehicles to improve the oral bioavailability of hydrophobic ibuprofen. A previously reported preparation method was slightly modified to produce particles with suitable size, charge, and stability properties.Citation27 Differential scanning calorimetry (DSC) and powder X-ray diffraction (XRD) were employed to identify the physical state of ibuprofen in the lipid matrix. Finally, the pharmacokinetic profile of orally administered ibuprofen encapsulated in cubic nanoparticles was investigated.
Materials and methods
Materials
Ibuprofen was obtained from Beijing Huafeng United Technology Co, Ltd (Beijing, People’s Republic of China). Phytantriol was purchased from DSM (Basel, Switzerland). Poloxamer 407 (PEO98POP67PEO98) was a gift from BASF (Ludwigshafen, Germany). All chemicals were used as received without further purification. Milli-Q–grade water purified through a Millipore system (ELGA LabWater, Sartorius, UK) was used throughout this study.
Preparation of cubic nanoparticles
Cubic nanoparticles were prepared through the fragmentation of PYT/poloxamer 407 bulk cubic gel.Citation28 Phytantriol (750 mg) and poloxamer 407 (75 mg), at a ratio of 10:1 (w/w), were first melted at 60°C in a hot water bath until they were homogeneous, after which ibuprofen was added to dissolve under continuous stirring. Deionized water (0.25 mL) was then added gradually and the mixture was vortex-mixed to achieve a homogeneous state. After equilibration for 48 hours at room temperature, an optically isotropic cubic phase gel was formed. By adding 10 mL of deionized water, the cubic gel was disrupted by mechanical stirring. Subsequently, the crude dispersion was fragmented for 10 min by intermittent probe sonication (JYD-650, Shanghai, People’s Republic of China) at 200 W energy input using a pulse mode (9-second pulses interrupted by 18-second breaks) under cooling in a 20°C water bath. The resulting milky coarse dispersion was homogenized using a high-pressure homogenizer (Avestin Em-C3, Ottawa, Canada) at certain high pressures and cycles to obtain an opalescent dispersion of the cubic nanoparticles. The final dispersion of cubic nanoparticles was stored at room temperature for further studies.
Particle size and zeta potential measurements
The particle size and size distribution of cubic nanoparticles were determined using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK), based on photon correlation spectroscopy. The dispersion of ibuprofen-loaded cubic nanoparticles was diluted in double-distilled water at 25°C (100 μL sample diluted to 6 mL) for analysis. The mean Z-average diameter and polydispersity index were obtained by cumulate analysis using the MALVERN software. The same suspension was used to measure the zeta potential of nanoparticles. All measurements were performed in triplicate.
Atomic force microscopy
The shape and surface morphology of ibuprofen-loaded cubic nanoparticles were inspected by atomic force microscopy (AFM) (Nanoscope III A, Vecco, Plainview, NY, USA). A drop of an ibuprofen-loaded cubic nanoparticle dispersion was placed on freshly cleaved mica to incubate for 5 minutes, and then the surface of the sample was rinsed with deionized water to remove unbound nanoparticles. Subsequently, the sample was dried in the air and mounted in a microscope scanner for viewing and imaging in the non-contact mode at a frequency of 312 kHz and a scan speed of 2 Hz.
Cryogenic transmission electron microscopy
The inner cubic structure of the nanoparticles was examined by cryogenic transmission electron microscopy (Cryo-TEM) (JOEL JEM-2010, Tokyo, Japan). A thin liquid film of nanoparticles was prepared on a carbon-coated holey film grid. Immediately after blotting, the grid was plunged into precooled liquid ethane for flash freezing. The cryo-grid was then held in a Gatan 626 Cryo-Holder (Gatan Inc, Pleasanton, CA, USA) and the sample was transferred into the cryo-TEM at a constant temperature of −172°C. Samples were viewed under low-dose conditions and all images were recorded digitally by a charge coupled device (CCD) camera (Gatan Inc, 832) at a defocus of 3.000–5.464 μm.
Differential scanning calorimetry
The physical status of ibuprofen encapsulated in cubic nanoparticles was characterized by DSC thermogram analysis (STA 6000 simultaneous thermal analyzer, Perkin Elmer, Waltham, MA, USA). Each 5-mg sample (pure ibuprofen, void cubic nanoparticles, a physical mixture of cubic nanoparticles and ibuprofen, and ibuprofen-loaded cubic nanoparticles) was placed in a standard aluminum pan and then purged with pure dry nitrogen gas at a flow rate of 10 mL/min prior to analysis. The temperature was increased at a rate of 10°C/minute and the heat flow was recorded from 30°C to 140°C.
X-ray diffraction
XRD analysis was carried out to determine the crystallinity of the nanoparticle formulation by using an X-ray powder diffractometer (Bruker AXS, Madison, WI, USA) at 40 kV and 25 mA, with a scanned angle of 3° ≤ 2θ ≤ 40° at a scan rate of 0.9 min−1. XRD patterns were determined for the pure ibuprofen, void cubic nanoparticles, physical mixtures of cubic nanoparticles and ibuprofen, and ibuprofen-loaded cubic nanoparticles.
Encapsulation efficiency
To quantify ibuprofen content encapsulated in cubic nanoparticles after preparation, 0.5 mL of ibuprofen-loaded cubic nanoparticles were added into a centricon (YM-100, Amicon, Millipore, Bedford, Mass, USA) reservoir for centrifuging at 4000 rpm for 15 minutes.Citation29 The filtrate that contained free ibuprofen was removed, and the filtered dispersion was diluted with methanol and analyzed for entrapped ibuprofen content using high-performance liquid chromatography (HPLC).
In vitro drug release
The release tests of ibuprofen-loaded cubic nanoparticles in artificial gastrointestinal fluids at a pH of 1.2 and a pH of 7.4 were conducted using dialysis membrane tubing.Citation30,Citation31 The dispersion of ibuprofen-loaded cubic nanoparticles (equivalent to 4 mg of ibuprofen) was sealed in a dialysis bag (14000 MWCO, Millipore, Boston, MA, USA) and then immersed into pH 1.2- and pH 7.4-release media (250 mL) that were thermostatically maintained at 37°C ± 0.5°C and stirred at a speed of 100 rpm. For comparison, 4 mg of pure ibuprofen were accurately weighed into pH 1.2- and pH 7.4-release media. At time intervals of 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 12.0, and 24.0 h, 2 mL of medium were taken and filtered through a 0.45-μm film, while the same volume of fresh medium was added to the release medium. The concentration of released ibuprofen was then determined by HPLC.
The mechanism of ibuprofen release from the cubic phases was determined by fitting the release rate data into the following equations:
and
where y is the accumulative release percentage; t is the sampling time; k1, k2, and k3 are the release rate constants for EquationEquations (1)(1) , Equation(2)
(2) , and Equation(3)
(3) , respectively; and a1, a2, and a3 are constants for EquationEquations (1)
(1) , Equation(2)
(2) , and Equation(3)
(3) .
In vivo pharmacokinetics study
The main objective of this experiment was to compare the pharmacokinetics of ibuprofen-loaded cubic nanoparticles with those of pure ibuprofen after oral administration. The animal experiment was carried out with the permission of the Ethical Committee of the Sun Yat-sen University (Guangzhou, People’s Republic of China) and performed in accordance with the National Institute of Health and Nutrition Guidelines for the Care and Use of Laboratory Animals. Six healthy female beagle dogs (1.2–2.0 years of age) weighing 12–14 kg were kept in an environmentally controlled breeding room and given standard laboratory chow and water for 1 week, before fasting overnight prior to the experiment. These dogs were randomly divided into two groups (n = 3 for each): dogs in group one were administered pure ibuprofen at a dose of 15 mg/kg dissolved in Milli Q water containing 0.3% (v/v) CMC-Na, while dogs in group two were given ibuprofen-loaded cubic nanoparticles dissolved in distilled water at an equivalent ibuprofen dose of 15 mg/kg by oral gavage. After administration, about 3 mL of blood was collected from the hind leg vein into heparinized tubes at time intervals of 0.17, 0.33, 0.67, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 12.0, and 24.0 hours. The serum was separated and the plasma samples were stored at −20°C until analysis.
Analysis of plasma samples
Calibration samples were prepared by adding proper volumes of standard ibuprofen solution in methanol to drug-free plasma, generating a calibration curve over the drug concentration range of 0.50–75.00 μg/mL (R2 > 0.99). The detection method was validated by evaluating the accuracy, precision, recovery, and limit of quantification and proved to be reliable. For sample analysis, a 200-μL plasma sample aliquot was mixed with 100 μL of a standard ibuprofen solution, then a protein-precipitating methanol agent (500 μL) was added and vortexed for 2 minutes. After 15 minutes of centrifugation at 15,000 rpm, the supernatant was transferred to auto-sampler vials, capped, and placed into the HPLC auto-sampler. Ibuprofen separation was conducted by injecting an aliquot (80 μL) of the sample onto the HPLC column (an Odyssil C18 column, 4.6 × 250 mm, 5 μm) equipped with a guard column (4.6 × 12.5 mm, 5 μm), using a mobile phase consisting of methanol, 0.01 mol/L potassium dihydrogen phosphate, and phosphoric acid (400:100:0.05, v/v) at a flow rate of 1.0 mL/minute. The detection wavelength was set at 263 nm.
Pharmacokinetic and statistical analysis
Pharmacokinetic analysis was carried out by a model independent method using the 3P87 computer program (issued by the State Food and Drug Administration of China for pharmacokinetic study). The Cmax (highest observed concentration during the study period) and Tmax (the time at which Cmax occurred) were directly obtained from the drug concentration time curve. The AUC0−t (the area under the curve to the last measurable concentration) was calculated by the linear trapezoidal rule, and the AUC0−∞ (the area under the curve extrapolated to infinity) was calculated as (AUC0–t + Ct/k), where Ct and k are the last measurable concentration and the elimination constant, respectively. The Mann–Whitney U-test was conducted for statistical analysis. Data were reported as mean values ± SD (standard deviation), and P < 0.05 was assumed to be a statistically significant difference.
Results
Encapsulation efficiency
The encapsulation efficiency of ibuprofen-loaded cubic nanoparticles was evaluated by adding various amounts of ibuprofen (2.7%, 5.5%, 8.3%, and 11.03% in weight) to the dispersed phase during preparation. The ibuprofen encapsulation efficiencies were all over 85% (87.9% ± 2.9%, 88.9% ± 1.5%, 89.6% ± 1.4%, and 86.6% ± 3.1%, respectively), showing no significant influence of original ibuprofen amounts on encapsulation efficiency. This result indicated that the diffusion of ibuprofen into the dispersion medium was negligible due to its high lipophilicity. Therefore, cubic nanoparticles with the highest drug-loading rate of 8.3% were chosen for the following studies.
Particle size and cubic nanoparticle morphology
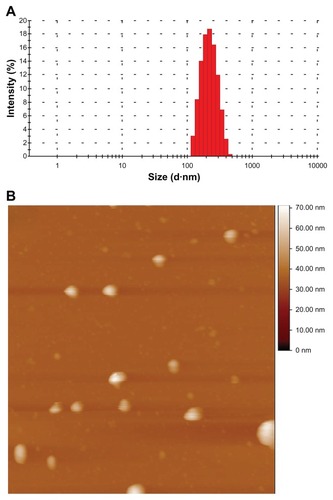
Aqueous-dispersed ibuprofen-loaded cubic nanoparticles were prepared successfully, with a mean particle size of 238.1 ± 2.8 nm and a polydispersity index of 0.096 ± 0.002, as determined by dynamic light scattering ( and ). The size distribution and morphological properties of the ibuprofen-loaded cubic nanoparticles were further investigated by AFM. The AFM images confirmed that the mean particle size was 230 nm (), and that the particles were segregated in a spherical shape with a monodispersed size distribution. Additionally, the average zeta potential of the nanoparticles was −24.8 ± 2.1 mv, which certainly could increase the stability of the nanoparticles in the dispersion ().
Table 1 Formulation and properties of ibuprofen-loaded cubic nanoparticles (n = 3)
Inner structure of cubic nanoparticles
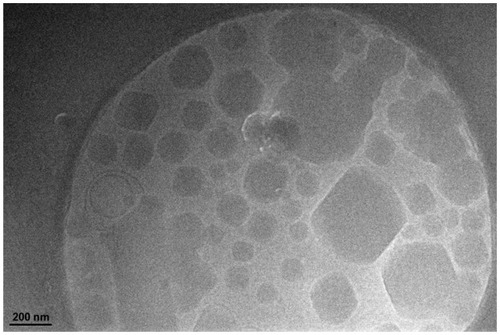
A cryo-TEM image of ibuprofen-loaded cubic nanoparticles () revealed the typical cubic inner structure with a mean particle size of about 230 nm, in line with the above results.
Physicochemical characterizations of drug-loaded cubic nanoparticles
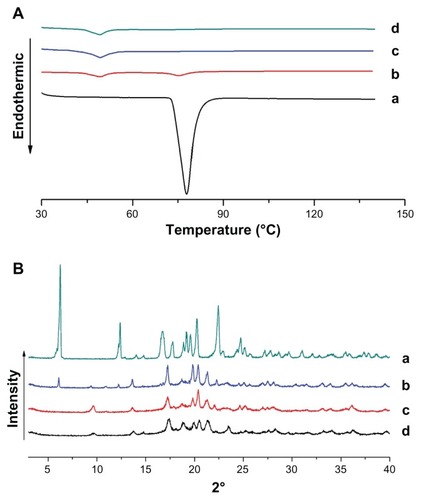
The physical status of ibuprofen encapsulated in the cubic nanoparticles was compared with pure ibuprofen by DSC analysis. The DSC thermograms of pure ibuprofen, void cubic nanoparticles, physical mixtures of cubic nanoparticles and ibuprofen, and ibuprofen-loaded cubic nanoparticles are shown in . Ibuprofen in its natural state exists as crystals, which are characterized by a sharp peak at the melting point (78.1°C). However, when encapsulated in cubic nanoparticles, the peak at this original melting point disappeared. XRD was employed to further investigate the status of ibuprofen in cubic nanoparticle formulation. The characteristic peaks of pure ibuprofen () showed the features of a highly crystalline structure; however, no characteristic ibuprofen peaks were observed when the drug was entrapped in nanoparticles. Both the DSC and XRD results indicated that the drug was molecularly dispersed or in an amorphous state in the cubic nanoparticles, which is favorable to the easy diffusion of drug molecules through the polymeric matrix, resulting in an improved dissolution rate for poorly soluble drugs and sustained release from the nanoparticles.Citation32
In vitro release
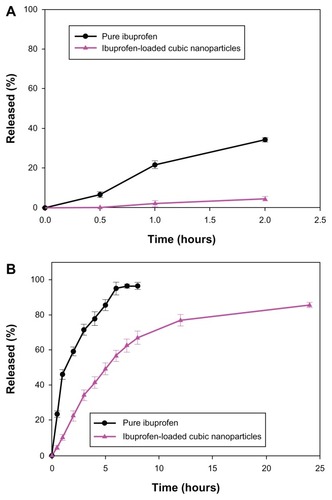
The sustained release of a drug from nanoparticles is an important factor for the successful development of nanoparticle formulations. The release profiles of ibuprofen from pure ibuprofen and cubic nanoparticles () showed obvious slower releases from the latter. At a pH of 1.2, pure ibuprofen released 34.24% within 2 hours, whereas ibuprofen-loaded cubic nanoparticles released only 4.46% (). At a pH of 7.4, pure ibuprofen released 95% within 6 hours, while ibuprofen-loaded cubic nanoparticles released 56.46% (). The release profile of ibuprofen from cubic nanoparticles showed a burst release initially followed by a sustained release over the experimental period. Approximately 85% of the entrapped ibuprofen was released in 24 hours from nanoparticles, in coincidence with the high encapsulation efficiency of ibuprofen in cubic nanoparticles. The observed initial burst release could be due to the dissolving of the adsorbed drug located just at or beneath the surface of the nanoparticles. The ibuprofen entrapped inside the nanoparticles then contributed to the following sustained release, attributed to the unique structure of the cubic nanoparticles.
Figure 4 (A) Drug release from ibuprofen-loaded cubic nanoparticles and pure ibuprofen at pH 1.2 (n = 3). (B) Drug release from ibuprofen-loaded cubic nanoparticles and pure ibuprofen at pH 7.4 (n = 3).
It is known that hydrophobic ibuprofen is mostly distributed in the lipid bilayers, and that the permeation of ibuprofen from the PYT bilayers to the water channels is a rate-limited process. summarizes the results by fitting the release data of ibuprofen-loaded cubic nanoparticles into different release mechanism models. A linear relationship was found between the ibuprofen release rate and the square root of time (R2 > 0.99), indicating the release kinetics can be explained by Higuchi’s equation. In this study, ibuprofen release from cubic nanoparticles was under diffusion control.Citation33
Table 2 Fitting of ibuprofen release data from cubic nanoparticles into various mechanism models (n = 3)
In vivo pharmacokinetics
The oral bioavailability of ibuprofen-loaded cubic nanoparticles was investigated in beagle dogs and compared to that of pure ibuprofen. The key pharmacokinetic parameters are summarized in and the mean ibuprofen concentrations in dog serum at different time intervals are plotted in . After oral administration, ibuprofen cubic nanoparticles were absorbed much slower than pure ibuprofen, with Tmax values being 1.82 ± 0.31 hours and 0.84 ± 0.19 hours (P < 0.05), respectively. It is worth noting that the plasma ibuprofen level of cubic nanoparticles was sustained for over 24 hours, while the drug level of pure ibuprofen dropped below the detection limit at 8 hours. These results implied that an enhanced bioavailability of ibuprofen was achieved through the incorporation of the drug into cubic nanoparticles.
Table 3 Pharmacokinetic parameters (mean ± SD) of ibuprofen in serum after oral administration (n = 3)
Figure 5 Mean plasma ibuprofen concentration after a single oral dose of 15 mg/kg equivalent ibuprofen or ibuprofen-loaded cubic nanoparticles (n = 3).
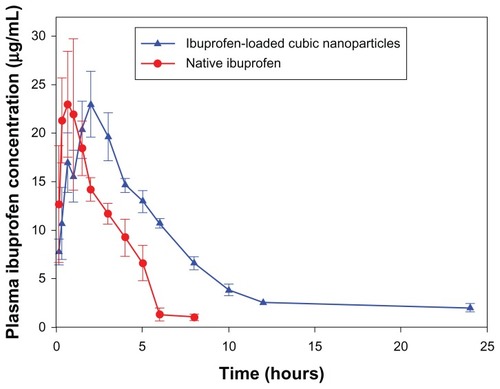
The mean values of AUC0–t and AUC0–∞ were found to be significantly increased for the ibuprofen-loaded cubic nanoparticles (). In addition, the half-life of ibuprofen-loaded cubic nanoparticles was increased by 3.09-fold in comparison with that of pure ibuprofen, indicating that the maximum residence time of ibuprofen in the systemic circulation was extended to 5.39 times for ibuprofen-loaded cubic nanoparticles after oral administration. Indeed, based on the AUC0−∞ values, the calculated relative oral bioavailability of ibuprofen-loaded cubic nanoparticles was about 222% (P < 0.05) when compared to pure ibuprofen.
Discussion
This work aimed to develop an ibuprofen-loaded cubic nanoparticulate system to solve the current problems associated with ibuprofen delivery, which may allow clinical application of this potential drug delivery system as aqueous formulations. First of all, uniform and transparent gels were prepared using PYT/F127 in excess water and analyzed by polarized light microscopy to confirm the formation of a liquid crystalline phase as the diffraction of a dark isotropy was observed. The bulk cubic-phase gels were easily disintegrated into cubic nanoparticles through fragmentation, and the particle size was largely dependent on the homogenization pressure and cycles.Citation19 It is well-known the particle size and size distribution of nanoparticles play important roles in determining the drug release kinetics, cellular uptake, and biodistribution of the drug-loaded nanoparticles, thus influencing the overall bioavailability of the formulation.Citation34–Citation37 In this study, cubic nanoparticles with diameters ranging from 100 to 500 nm in a typical log-normal distribution () were prepared.
Studies conducted by Feng et al reported that nanoparticles below 500 nm in size with a high zeta potential could be efficiently taken up via the lymphatic system (M cells of Peyer’s patches) and cross the membrane of epithelial cells via endocytosis.Citation35 However, Costanzo et al recently reported that nanoparticles with sizes around or below 100 nm showed optimum cellular and nuclear uptake in epithelial and smooth muscle cells.Citation38 In the light of this, we anticipated that nanoparticles with small sizes and high surface charges would be favorable to intestinal uptake and extended circulation half-life, as well as being avoided by the reticuloendothelial system. In actuality, ibuprofen-loaded cubic nanoparticles with a mean particle size of 238 nm and a uniform size distribution were prepared in this study with a homogenization pressure of 1200 bar and nine cycles.
The results of polarized light microscopy and cryo-TEM illustrated the cubic nanostructure in the nanoparticulate formulation; however, it was not clear whether the formulation was a simple mixture of ibuprofen and void cubic nanoparticles or if ibuprofen was indeed encapsulated in cubic nanoparticles. Therefore, evaluation of encapsulation efficiency, DSC, and XRD studies were conducted to confirm the incorporation of drug in cubic nanoparticles. The detected encapsulation efficiency of 89.65% indicated that the majority of the ibuprofen was indeed encapsulated in cubic nanoparticles. Consistently, the DSC and XRD studies verified the disappearance of drug crystallinity in nanoparticles, with no distinctive peak in the DSC profiles and an absence of distinct diffraction peaks in XRD patterns, owing to amorphization or solvation of the drug in the amorphous carrier. This further demonstrated that ibuprofen was encapsulated in the cubic nanoparticles.
The in vitro release experiments indicated that drug release from ibuprofen-loaded cubic nanoparticles in artificial gastrointestinal fluids was much slower than from pure ibuprofen. Consistently, a remarkably sustained release was observed in the in vivo plasma ibuprofen level of ibuprofen-loaded cubic nanoparticles after oral administration. Similar results were also reported in previous literature.Citation17,Citation23
In the current study, the initial burst release of ibuprofen from cubic nanoparticles was probably attributed to either the surface-bound moieties or an aqueous environment allowing increased water penetration. Afterwards, the cubic nanoparticles manifested the sustained release characteristics that appeared to be dependent on the hydrophobicity of ibuprofen incorporated in the cubic nanoparticles. The release behavior fit Higuchi’s equation and could be explained by the diffusion control mechanism. As ibuprofen was dispersed or dissolved in the matrix formed by the cubic phase and stable during the release study, the drug release occurred by diffusing through the aqueous channels of the cubic phase.Citation39
In order to enhance the oral bioavailability of hydrophobic ibuprofen, a muco-adhesive nano-sized carrier was prepared to develop an aqueous nanoparticle formulation. As expected, the pharmacokinetics results certainly indicated an enhanced bioavailability of ibuprofen, as the ibuprofen-loaded cubic nanoparticles were 2.22-fold more bioavailable than pure ibuprofen after oral administration. It is worth mentioning that the drug released from nanoparticles was still detected in plasma 24 hours after oral administration (). The results therefore demonstrated a superior pharmacokinetic disposition of ibuprofen-loaded cubic nanoparticles over pure ibuprofen.
Consistently, the sustained release and enhanced bioavailability of the water-insoluble drug cinnarizine were obtained after oral ingestion of a PYT liquid crystalline matrix. The second absorption phase of the drug from the GIT was proposed to interpret the delayed elimination phenomenon. Sustained absorption of cinnarizine from PHY cubosomes led to significant enhancement in its oral bioavailability when compared to a cinnarizine suspension.Citation18
The ability of cubic nanoparticles to enhance the oral bioavailability of a drug may be attributed to several factors. Cubic nanoparticles can be transported across endothelial cell membranes to enhance the absorption of the incorporated drug. Cubic nanoparticles also possess a lyotropic property that makes it easier for nanoparticles to penetrate the “unstirred water layer” and closely contact cell membranes.Citation15,Citation40,Citation41 Overall, the cubic nanoparticles showed a potential to increase the oral bioavailability of highly lipophilic drugs. The underlying mechanisms of enhancement, however, are still unclear and provoke future research interests.
Conclusion
This study elucidated the idea that a formulation of ibuprofen-loaded cubic nanoparticles prepared through homogenizing bulk cubic phase gels into a cubic dispersion could improve the physicochemical characteristics of the drug. Nanometer-sized cubic nanoparticles with narrow size distributions were obtained under optimal homogenization conditions, and the structure of the dispersed formulation was confirmed as a bicontinuous cubic liquid crystalline phase. The cubic nanoparticles provided a sustained system for the ibuprofen and, more importantly, the sustained release of entrapped ibuprofen resulted in sustained plasma ibuprofen levels and enhanced systemic bioavailability. Thus, the ibuprofen-loaded cubic nanoparticles provide a promising carrier candidate with an efficient delivery of ibuprofen for therapeutic treatment in the near future.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (No 81173002), the National Science and Technology Support Program (No 2012BAI35B02), and the International Science and Technology Cooperation and Exchange Projects (No 2008DFA31080) for their financial support. This work was supported partially by the Ministry of Science and Technology of Zhanjiang (No 2012C3105017) and the College Students Technology Innovation Project of Guangdong (No 1057112043).
Disclosure
The authors report no conflicts of interest.
References
- AbrahmPIndiraniKDesigamaniKNitro-argenine methyl ester, a non-selective inhibitor of nitric oxide synthase reduces ibuprofen-induced gastric mucosal injury in the ratDigest Dis Sci20055091632164016133962
- BradburyFHow important is the role of the physician in the correct use of a drug? An observational cohort study in general practiceInt J Clin Prat2004581442732
- ChavezMLDekorteCJValdecoxib: a reviewCin Ther2003253817851
- WahbiAAHassanEHamdyDSpectrophotometric methods for the determination of ibuprofen in tabletsPak J Pharm Sci2005841616380350
- MurthaJLAndoHYSynthesis of the cholesteryl ester prodrugs cholesteryl ibuprofen and cholesteryl flufenamate and their formulation into phospholipids microemulsionsJ Pharm Sci199483122212287830235
- AbbaspourMRSadeghibFGarekaniHADesign and study of ibuprofen disintegrating sustained-release tablets comprising coated pelletsEur J Pharm Biopharm200868374775917977701
- NagpalMMaheshwariDKRakhaPFormulation development and evaluation of alginate microspheres of ibuprofenJ Young Pharm201241131622523454
- BendasERChristensenJMAyresJWDevelopment and in vitro evaluation of mesalamine delayed release pellets and tableted reservoir-type pelletsDrug Dev Ind Pharm201036439340419740039
- AbdullahGZAbdulkarimMFSalmanIMIn vitro permeation and in vivo anti-inflammatory and analgesic properties of nanoscaled emulsions containing ibuprofen for topical deliveryInt J Nanomedicine2011638739621499428
- ZhangMAkbulutMAdsorption, desorption, and removal of polymeric nanomedicine on and from cellulose surfaces: effect of sizeLangmuir20112720125501255921879763
- PottaSGMinemiSNukalaRKPreparation and characterization of ibuprofen solid lipid nanoparticles with enhanced solubilityJ Microencapsul2011281748121171818
- PergeLRobitzerMGuillemotCNew solid lipid microparticles for controlled ibuprofen release: formulation and characterization studyInt J Pharm20124221–2596722027394
- GuoCYWangJCaoFLLyotropic liquid crystal systems in drug deliveryDrug Discov Today20101523/241032104020934534
- YangDArmitageBMarderSRCubic liquid-crystalline nanoparticlesAngew Chem Int Ed20044344024409
- KorjamoTHeikkinenATMönkkönenJAnalysis of unstirred water layer in vitro permeability experimentsJ Pharm Sci200998124469447919653267
- YaghmurAGlatterOCharacterization and potential applications of nanostructured aqueous dispersionsAdv Colloid Interface Sci2009147–148333342
- BoydBJKhooSMWhittakerDVA lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poor water soluble drug in ratsInt J Pharm20073401/2526017467935
- NguyenTHHanleyTPorterCJBoydBJNanostructured reverse hexagonal liquid crystals sustain plasma concentrations for a poorly water-soluble drug after oral administrationJ Control Release201116429438
- SpicerPTProgress in liquid crystalline dispersions: cubosomesCurr Opin Cpll Interface Sci200510274279
- MuLSeowPNgoAStudy on surfactant coating of polymeric nanoparticles for controlled delivery of anticancer drugColloid Polym Sci200428315865
- YaghmurALaggnerPZhangSModulation of lipidic nanostructures by designer short peptide surfactantsEur J Pharm Sci20083412934
- LaiJLuYYinZPharmacokinetics and enhanced oral bioavailability in beagle dogs of cyclosporine A encapsulated in glyceryl monooleate/poloxamer 407 cubic nanoparticlesInt J Nanomedicine20105132320161984
- YangZWTanYHChenMWDevelopment of amphotericin B-loaded cubosomes through the solemuls technology for enhancing the oral bioavailabilityAAPS Pharm Sci Tech201223112
- PengXSWenXGPanXDesign and in vitro evaluation of capsaicin transdermal controlled release cubic phase gelsAAPS Pharm Sci Tech201011314041410
- HanKWangZPengXTransarterial chemoembolization using docetaxel-loaded phytantriol cubic phase precursor for the treatment of hepatocellular carcinomaJ Pharm Sci201110062240224721491445
- HanKPanXChenMPhytantriol-based inverted type discontinuous cubic phase for vascular embolization and drug sustained releaseEur J Pharm Sci201041569269920883779
- YangZWPengXSTanYHOptimization of the preparation process for an oral phytantriol-based amphotericin B cubosomesJ Nanomater2011201111021808638
- GustafssonJLjusberg-WahrenHAlmgrenMCubic lipid-water phase dispersed into submicron particlesLangmuir1996122046114613
- ChungHKimJUmJSelf-assembled nanocubicle as a carrier for peroral insulin deliveryDiabetologia200245344845111914752
- ZengNGaoXHuQLipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: cellular interaction and in vivo absorptionInt J Nanomedicine201273703371822888230
- PanwarPPandeyBLakheraPPreparation, characterization, and in vitro release study of albendazole-encapsulated nanosize liposomesInt J Nanomedicine2010510110820309396
- JavadzadehYAhadiFDavaranSPreparation and physicochemical characterization of naproxen-PLGA nanoparticlesColloids Surf B Biointerfaces20108149850220719477
- TanakaNImaiNOkimotoKDevelopment of novel sustained-release system, disintegration-controlled matrix tablet (DCMT) with solid dispersion granules of nilvadipineJ Control Release20051082–338639616253377
- FengSSMuLWinKYNanoparticles of biodegradable polymers for clinical administration of paclitaxelCurr Med Chem200411441342414965222
- FengSSMeiLAnithaPPoly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of docetaxeBiomaterials200930193297330619299012
- MittalGSahanaDBhardwajVEstradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivoJ Control Release20071191778517349712
- DesaiMPLabhasetwarVAmidonGLGastrointestinal uptake of biodegradable microparticles: effect of particle sizePharm Res199613183818458987081
- CostanzoPJPattenTESeeryTANanoparticle agglutination: acceleration of aggregation rates and broadening of the analyte concentration range using mixtures of various-sized nanoparticlesLangmuir20062262788279416519483
- ShahJCSadhaleYChilukuriDMCubic phase gels as drug delivery systemsAdv Drug Delive Rev2001472–3229250
- ThomsonABSchoellerCKeelanMLipid absorption: passing through the unstirred layers, brush-border membrane, and beyondCan J Physiol Pharmacol19937185315558306192
- KatneniKCharmanSAPorterCJAn evaluation of the relative roles of the unstirred water layer and receptor sink in limiting the in-vitro intestinal permeability of drug compounds of varying lipophilicityJ Pharm Pharmacol200860101311131918812024