 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel particulate delivery matrix based on ionically crosslinked casein (CAS) nanoparticles was developed for controlled release of the poorly soluble anticancer drug flutamide (FLT). Nanoparticles were fabricated via oil-in-water emulsification then stabilized by ionic crosslinking of the positively charged CAS molecules below their isoelectric point, with the polyanionic crosslinker sodium tripolyphosphate. With the optimal preparation conditions, the drug loading and incorporation efficiency achieved were 8.73% and 64.55%, respectively. The nanoparticles exhibited a spherical shape with a size below 100 nm and a positive zeta potential (+7.54 to +17.3 mV). FLT was molecularly dispersed inside the nanoparticle protein matrix, as revealed by thermal analysis. The biodegradability of CAS nanoparticles in trypsin solution could be easily modulated by varying the sodium tripolyphosphate crosslinking density. A sustained release of FLT from CAS nanoparticles for up to 4 days was observed, depending on the crosslinking density. After intravenous administration of FLT-CAS nanoparticles into rats, CAS nanoparticles exhibited a longer circulation time and a markedly delayed blood clearance of FLT, with the half-life of FLT extended from 0.88 hours to 14.64 hours, compared with drug cosolvent. The results offer a promising method for tailoring biodegradable, drug-loaded CAS nanoparticles as controlled, long-circulating drug delivery systems of hydrophobic anticancer drugs in aqueous vehicles.
Introduction
Over the past few decades there has been considerable interest in developing protein nanoparticles as drug delivery vehicles.Citation1,Citation2 The underlying rationale is their exceptional characteristics, namely biodegradability, nonantigenicity, abundant renewable sources, extraordinary binding capacity with various drugs, and the ease of scaling up during manufacture.Citation1,Citation2 Casein (CAS), a generally recognized as safe protein, is the major milk protein that forms an integral part of the daily diet. It possesses a number of interesting properties that make it a good candidate for conventional and novel drug delivery systems.Citation3,Citation4 CAS may be advantageous as an alternative to albumin as a matrix for drug delivery, because it is inexpensive and has better amphiphilicity, good dispersibility, and rapid reconstitution in aqueous systems.Citation3,Citation4
CASs are amphiphilic proteins that can be thought of as block copolymers with high levels of hydrophobic or hydrophilic amino acid residues. Therefore, CASs exhibit a strong tendency to self-assemble into spherical micelles (50–500 nm in diameter, 150 nm average diameter).Citation3,Citation4 Only recently, CAS micelles were harnessed for delivering exogenous hydrophobic bioactives. CAS micelles effectively protected vitamin D2 and docosahexaenoic acid against ultraviolet (UV) light-induced degradation and oxidation, respectively.Citation5,Citation6 In another study, the complexation of curcumin with β-CAS micelles increased the solubility of curcumin at least 2500-fold with enhanced curcumin cytotoxicity to a human leukemia cell line.Citation7 Shapira et alCitation8,Citation9 showed that β-CAS micelles could entrap and deliver hydrophobic chemotherapeutics such as mitoxantrone and paclitaxel, allowing them to be thermodynamically stable in aqueous solutions for oral delivery applications. Recently, Bachar et alCitation10 successfully developed celecoxib-loaded β-CAS micelles with high encapsulation loads for oral delivery.
A desolvation technique has been widely used to prepare protein nanoparticles such as albumin and gelatin, and successfully achieved sustained drug release for prolonged periods of time.Citation2,Citation11 However, this technique has two major drawbacks: the use of organic solvents, and toxic crosslinking chemicals such as glutaraldehyde. Thus, the clinical application of the resulting nanoparticles is hampered by safety concerns. Therefore, the use of nontoxic crosslinking agents (eg, genipin and transglutaminase) emerged as an alternative to glutaraldehyde.Citation2 In our previous study we used a spray-drying technique to generate genipin-crosslinked CAS nanoparticles for prolonged release of alfuzosin hydrochloride.Citation12
Prostate cancer has the highest incidence of all kinds of cancers and is the second most deadly cancer in men, after lung cancer.Citation13 Flutamide (FLT) is an antiandrogenic agent presently used for monotherapy of androgen-dependent prostate cancer.Citation14 It acts by inhibiting the uptake and/or binding of dihydrotestosterone to the target cell receptor, thus interfering with androgen action, which requires stability in blood for a sufficient amount of time.Citation14 However, the low bioavailability of FLT after oral administration could be attributed to its poor wettability, low aqueous solubility, poor permeability, or low concentration at the absorption surface.Citation15 Moreover, FLT undergoes a rapid first-pass hepatic metabolism after oral administration, resulting in a relatively short half-life of 5–6 hours. Reported FLT toxicity includes hepatotoxicity, nausea, and diarrhea. Thus, the pharmacokinetics and dosage characteristics (250 mg three times daily) of FLT make it a suitable candidate for the design of controlled-release delivery systems in order to enhance patients’ compliance and to reduce the incidence of side effects.Citation16
Various attempts have been made to improve FLT bioavailability and control its release behavior, including cyclodextrin inclusion complexes,Citation15–Citation17 colyophilized dispersions,Citation18,Citation19 liquisolid compacts,Citation20 liposomes,Citation21 nanoemulsions,Citation22 and self-nanoemulsifying drug delivery systems.Citation23 In our laboratory,Citation24 oral controlled-release formulas of FLT were developed by combining immediate-release FLT-lyophilized monophase dispersions with prolonged-release FLT-chitosan microspheres. The developed formulas were found effective in providing a prolonged release of FLT for a long period of time after a suitable initial burst release.Citation24
Commonly, nanoparticles composed of biodegradable polymers exhibit controlled release of their drug payload by diffusion or polymer degradation. These systems may provide prolonged exposure of the drug at their site of action once they have accumulated at their target. Nanoparticles have also been used to increase the aqueous solubility of several hydrophobic anticancer drugs via solubilization within the hydrophobic core of the nanoparticles, and to improve their in vivo performance, providing a repository for the drug to be slowly released.Citation25 Therefore, another approach for improving FLT bioavailability and reducing its toxicity could be the formulation of biodegradable FLT-loaded nanoparticles for parenteral administration.
In this study we present the next generation of protein-based nanoparticles prepared via ionic crosslinking, employing CAS as a matrix and sodium tripolyphosphate (TPP) as a polyanionic crosslinker for controlled delivery of FLT. TPP is a nontoxic polyanion that can interact with polycationic polymers via electrostatic forces to form ionic crosslinked networks.Citation24 We suggest this novel system as a potential alternative to the chemically crosslinked protein nanoparticles, so as to avoid their possible toxicity. We attempted to encapsulate FLT into CAS nanoparticles to control its release rate, as well as extending its systemic circulation, comparing its pharmacokinetic parameters with those of drug cosolvent after intravenous administration in rats.
Materials and methods
Materials
CAS from bovine milk, technical grade, and TPP, 90%–95%, practical grade, were purchased from Sigma-Aldrich (St Louis, MO, USA). FLT was kindly donated by Archimica (Origgio, Italy). Polyoxyethylene sorbitan monooleate (Tween 80) was from Riedel-de Häen (Seelze, Germany). Trypsin 2000 U/G from pancreas and sodium azide were obtained from LOBA Chemie Pvt, Ltd (Mumbai, India). Poly(ethylene glycol) 200 (PEG-200) was supplied by Pharaonia Pharmaceuticals (Alexandria, Egypt). Methylene chloride was obtained from ADWIC, El-Nasr Pharmaceutical Chemicals Co (Cairo, Egypt). All other chemicals were of analytical grade and used without further purification.
Preparation of ionically crosslinked FLT-loaded CAS nanoparticles
Aqueous CAS solution (1% w/v) was adjusted to pH 2.0 with 1 N hydrochloric acid. Tween 80 (2% v/v) was added as a surfactant to CAS solution under magnetic stirring (RH basic, Ika Labortechnik, Staufen, Germany). Methylene chloride solution of FLT as the oil phase was mixed with aqueous CAS phase by homogenization (ULTRA-TURAX T25, IKA Labortechnik, Germany) at a speed of 13,000 rpm for 20 minutes to obtain an oil-in-water emulsion. The ratio of oil and aqueous phase was 1:10 v/v. TPP solution (0.5% w/v) was added dropwise to the oil-in-water emulsion under gentle magnetic stirring. After 2 hours of crosslinking, nanoparticles were isolated by centrifugation (Sigma laboratory refrigerated centrifuge, model 3K-30, Germany) at 20,000× g and 10°C for 30 minutes, and subsequently washed several times with Tween 80 solution (0.1% v/v) and water. The particles were lyophilized (CRYODOS-50 Freeze-drier, Telstar Cryodos, Spain) and stored in dry conditions at 25°C. The composition of CAS nanoparticles prepared with different formulation variables is shown in .
Table 1 Composition and characteristics of the formulated ionically crosslinked flutamide (FLT)-loaded casein (CAS) nanoparticles
Characterization of FLT-loaded CAS nanoparticles
Drug loading and incorporation efficiency
The drug loading and incorporation efficiency of FLT-loaded CAS nanoparticles was determined by centrifugation of the colloidal samples at 20,000× g and 4°C for 30 minutes. The nonentrapped FLT in the supernatant obtained after ultracentrifugation of nanoparticles was determined by high-performance liquid chromatography (HPLC) method. The amount of FLT entrapped within nanoparticles was calculated by the difference between the total amount used and the amount presented in the aqueous supernatant phase. All the experiments were carried out in triplicate. The percentage drug loading (%DL) and incorporation efficiency (%IE) for each formulation were calculated using the following equations:
A reverse-phase HPLC method was used for quantifying FLTCitation24 HPLC analysis was carried out with a PerkinElmer series 200 chromatograph (PerkinElmer, Waltham, MA, USA) using a Spheri-5, RP-18, 220 mm × 4.6 mm, 5 μm, column, and a UV detector (PerkinElmer, Waltham, MA, USA). An isocratic solvent system consisting of 75:25 (v/v) methanol/water was used at a flow rate of 1 mL/min and an injection volume of 20 μL, and the peaks were detected at 304 nm. Under these experimental conditions, the total run time was approximately 6 minutes and the retention time was 3.9 minutes. Calibration curves (peak area versus[vs] drug concentration) were linear (R2 > 0.999) over the FLT concentration range of 0.6–60 μg/mL.
Particle size and zeta (ζ) potential
Particle size of FLT-loaded CAS nanoparticles was measured using a dynamic light scattering (DLS) technique with a NanoZS/ZEN3600 Zetasizer (Malvern, Instruments Ltd Malvern, UK). This system is equipped with a 4 mW helium/neon laser at 633 nm wavelength and measures the particle size with the noninvasive backscattering technology at a detection angle of 173° after an at least 100-fold dilution with purified water. All of the DLS measurements were performed at 25.0°C ± 0.1°C at 20-second intervals for three repeat measurements. For the zeta potential measurement, each diluted nanoparticle suspension was put in a universal folded capillary cell equipped with platinum electrodes. The electrophoresis mobility was measured and the zeta potential was calculated by the Dispersion Technology Software provided by Malvern.
Morphological analysis
Transmission electron microscope (TEM) imaging of FLT loaded CAS nanoparticle suspension (F2) was performed on a JEM-100 CX electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV After sample dilution with water (1:1000), a sample drop was placed on a copper grid. The excess was drawn off with a filter paper. Samples were subsequently stained with uranyl acetate solution for 30 seconds and then naturally dried.
Differential scanning calorimetry
To investigate the physical state of FLT inside CAS nanoparticles, thermograms of CAS, FLT, their respective physical mixture, and unloaded and FLT-loaded CAS nanoparticles (F2) were recorded by differential scanning calorimetry model DSC 6 (PerkinElmer). Samples (2–4 mg) were placed in sealed aluminum pans and heated at 10°C/min under nitrogen atmosphere (flow rate 20 mL/min) in the range of 30°C–300°C.
Fourier transform infrared spectra
The Fourier transform infrared (IR) spectra of CAS, FLT, their respective physical mixture, and unloaded and FLT-loaded CAS nanoparticles (F2) were recorded using a Spectrum RXI Fourier transform IR spectrometer (PerkinElmer). Samples were finely ground with IR-grade KBr then pressed into pellets, and IR spectra were taken in transmission over the range of 4000–500 cm−1.
Biodegradability of CAS nanoparticles
To determine the degradation stability of the ionically crosslinked CAS nanoparticles, in vitro degradation was performed by digesting the nanoparticles in pH 7.4 phosphate-buffered saline (PBS) containing trypsin. After centrifugation, the sedimented nanoparticles were redispersed in PBS (pH 7.4) by sonication. Trypsin (2 mg/mL) was added to the nanoparticle suspension and the samples were incubated in a shaking water bath at 37°C ± 0.5°C under mild horizontal shaking at 50 rpm (GFL, type 1083, Gmbh and Co, Burgwedel, Germany). The turbidity of the nanoparticle suspension was monitored spectrophotometrically by recording the percent transmission at 600 nm at specified time intervals using T80 UV/VIS spectrophotometer (PG Instruments Ltd London, UK).
In vitro drug release
In vitro release of FLT from the drug-loaded CAS nanoparticles was assessed using a dialysis bag method and compared with the drug solution in a cosolvent mixture of 0.9% w/v NaCl/ethanol/PEG-200 (2:1:3 v/v/v) (F0). FLT nanoparticles (equivalent to 20 mg drug) were dispersed in PBS (pH 7.4) and placed in a cellulose ester dialysis membrane, cut-off 12–14 kDa, sealed with appropriate universal closures (Spectrum Laboratories, Inc, Rancho Dominguez, CA, USA). The bags were tied to the paddle of the USP XXIV dissolution apparatus II, Type PTWS3 (Pharma Test, Hainburg, Germany), and dialyzed against 900 mL PBS (pH 7.4) containing 0.2% Tween 80 and 0.02% sodium azide as a preservative. The entire system was incubated at 37°C ± 0.5°C under stirring at 100 rpm. At designated time intervals, 5 mL of the release medium was removed and replaced with the same volume of fresh PBS solution. All samples were run in triplicate and filtered through a 0.45 μm membrane filter, and the amount of FLT released was analyzed by HPLC.
In vivo pharmacokinetics
In vivo experiments were performed on male Sprague Dawley rats (200 ± 20 g) housed in stainless steel mesh cages in two groups of eight rats each, under standard conditions of light illumination, relative humidity, and temperature, and they had free access to standard laboratory food and water throughout the study. All procedures were performed according to a protocol approved by the Animal Care and Use Committee of the Faculty of Pharmacy, Alexandria University, Alexandria, Egypt, and in accordance with regulations of the National Research Council’s guide for the care and use of laboratory animals.
For the pharmacokinetic studies, the ionically crosslinked FLT-loaded CAS nanoparticle formulation (F2 was compared with the drug cosolvent (F0).Citation16 Rats were anesthetized with ether inhalation and injected intravenously via the tail vein with a single dose of FLT cosolvent or FLT-loaded CAS nanoparticles (12 mg/kg) per rat. Blood samples (1 mL) were collected from the retro-orbital plexus at designated time intervals (30 minutes, 1, 2, 4, 6, 8, 12, 24, and 48 hours) in ethylenediaminetetraacetic acid-pretreated tubes. Samples were centrifuged immediately at 5000 rpm for 10 minutes. The plasma samples were diluted to 2 mL with methanol, vortexed for 10 minutes, and then centrifuged at 8000 rpm for 20 minutes. A 20 μL amount of the supernatant was injected into the HPLC column to determine the FLT plasma concentration. The different pharmacokinetic parameters were calculated using noncompartmental methods with the software WinNonlin®, Version 3.0 (Pharsight Corporation Ltd, Sunnyvale, CA, USA).
Statistics
All measurements were performed in triplicate, and the results were expressed as arithmetic mean ± standard deviation.
Results and discussion
Formation of CAS nanoparticles
Ionically crosslinked FLT-loaded CAS nanoparticles were successfully prepared by a two-step process. The first step involved the formation of oil droplets (encapsulating FLT) by an oil-in-water emulsion formation. Dichloromethane (CH2Cl2) was chosen due to its ability to diffuse into the aqueous phase at a rapid rate, facilitating particle formation upon evaporation.Citation26 The second step was the solidification of the formed droplets by ionic crosslinking of CAS (pH 2) enveloping the oil droplets with TPP. By decreasing the pH of CAS solution below its isoelectric point (4.6–4.8), the amino groups of CAS become positively charged and can strongly attract the negatively charged phosphate groups of TPP, leading to the formation of ionically crosslinked nanoparticles. shows a photograph of uncrosslinked CAS (1% w/v) and CAS nanoparticles ionically crosslinked with two different concentrations of TPP (1:10 and 1:5 TPP:CAS ratios). It is clear that the visual turbidity of the nanoparticle suspension increases as the TPP concentration increases, indicating a higher crosslinking density.
Figure 1 A photograph illustrating the difference in visual turbidity between: (A) casein (CAS) solution and ionically crosslinked CAS nanoparticles prepared with (B) 1:10 and (C) 1:5 sodium tripolyphosphate (TPP):CAS mass ratios.
Few studies show the formation of protein nanoparticles via ionic interaction as an alternative to chemical crosslinking. The protein nature of CAS enables the formation of nanoparticles via electrostatic complexation with polyanionic polysaccharides, eg, gum Arabic,Citation27 or polycationic polysaccharides, eg, chitosan,Citation28 by adjusting the pH of CAS below or above its pI, respectively. In another study, Rediguieri et alCitation29 investigated the electrostatic complexation between CAS and pectin. At pH > 6, pectin/CAS mixtures phase separate, as both polymers carry negative charges and so repel each other. However, below its pI, CAS molecules become positively charged, and therefore electrostatic attraction between CAS and the anionic pectin occurs, leading to the formation of microparticles.
Characteristics of FLT-loaded CAS nanoparticles
The drug incorporation efficiency obtained from the drug content analysis of nanoparticles ranged from 17.42% to 64.55%, with a drug loading in the range of 2.50%–8.73%, depending upon the formulation variables studied (). The effect of crosslinking density expressed as the TPP:CAS mass ratio on drug encapsulation was studied. shows that FLT incorporation efficiency increased from 27.61% to 64.55% by increasing TPP concentration from 1:20 to 1:3 TPP:CAS mass ratio. A high amount of TPP causes a rise in the crosslinking density of CAS matrix, with a consequential hindrance of the drug escape to the continuous phase during solidification of emulsion droplets, which enhances the drug entrapment while reduced the drug loading. Similar results were obtained by Ajun et alCitation26 where the probucol encapsulating efficiency into chitosan nanoparticles increased by increasing TPP concentration, whereas the drug loading decreased. It was also found that reducing FLT loading led to an enhancement of its incorporation efficiency, with a maximum incorporation (44.09%) attained at an FLT:CAS ratio of 1:10 compared with 17.42% obtained for FLT:CAS ratio of 1:3.
Particle size and zeta potential measurements
DLS results revealed that FLT-loaded CAS nanoparticles exhibited a particle size in the range of 61.9–100.6 nm () with a polydispersity of 0.37–0.59, which could be correlated to the size and composition of CAS micelles (50–500 nm in diameter, 150 nm average diameter). CASs are mixtures of four phosphoproteins, αS1-, αS2-, β-, and κ-CAS, with a molecular weight range of 19–25 kDa, thus producing heterogeneous nanoparticle size distribution.Citation3,Citation4 Shapira et alCitation8 showed that paclitaxel-β-CAS and vinblastine-β-CAS micelles had mean diameters of around 100 nm and 150 nm, respectively, with mono or bimodal particle size distributions for both drugs. In another study conducted by Gaiani et al,Citation30 the hydrodynamic diameter of the rehydrated spray-dried CAS powders was found to be around 210 nm, which may correspond to CAS micelle size, and the suspension was too polydisperse.
reveals a bimodal size distribution of FLT-loaded CAS nanoparticles (F2). The first peak was obtained at 126.5 nm diameter, representing 91.6% of the particles. The second peak was at 10.19 nm diameter, apparently monomeric protein molecules, and represents 8.4% of the particles with a Z-average of 96.85 ± 2.45. Similar bimodal distributions were obtained for different nanoparticle formulations. From , it is also clear that increasing TPP concentration from 1:20 to 1:3 TPP:CAS ratio resulted in a corresponding increase in particle size from 64.83 nm to 100.60 nm, respectively, showing the possibility of modifying particle size. Similar findings were reported by Ajun et al,Citation26 where the size of chitosan nanoparticles increased by increasing TPP concentration.
Figure 2 Particle size (A) and zeta potential (B) distributions of flutamide-loaded casein nanoparticles (F2).
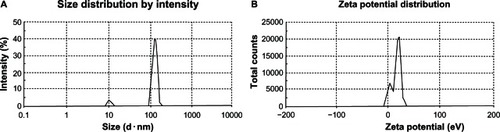
Zeta potential measurements of ionically crosslinked FLT-loaded CAS nanoparticles are presented in . The nanoparticles were positively charged with a zeta potential range of +7.54 mV to +17.30 mV The pure positively charged uncrosslinked CAS exhibited a zeta potential of +20.0 mV (data not shown). This positive charge is a result of the net electrostatic charge on the CAS surface at pH 2.0, ie, below its isoelectric point, where the CAS amino groups become positively charged. Upon CAS crosslinking with the polyanionic TPP, the zeta potential started to decrease to +17.30 mV at TPP:CAS ratio of 1:20 (). This figure revealed a bimodal zeta potential distribution where two populations of particles exist within this sample. About 73% of the particles have a zeta potential of +20.7 mV, whereas 26.1% of the particles have a lower value of +4.45 mV As the TPP concentration increased to 1:3, the zeta potential of the nanoparticles decreased progressively to a value of +7.54 mV
The charge on the nanoparticles is quite low; however, steric stabilization is probably possible through the hydrophilic κ-CAS “hairy” layer, which provides a charged and diffuse surface layer surrounding CAS micelle and stabilizes it through intermicellar electrostatic and steric repulsion, similar to a polyelectrolyte brush.Citation3,Citation4,40 Similar findings were observed by Ye et al,Citation27 where a charge of +15 mV was exhibited by sodium caseinate-gum Arabic nanoparticles. The authors suggested that the presence of hydrophilic gum molecules on the outside of the caseinate aggregate may be enough to sterically stabilize the nanoparticles and consequently prevent their self-aggregation.Citation27
The lyophilized ionically crosslinked CAS nanoparticles were readily dispersible in water, forming a colloidal turbid dispersion with a slight insignificant increase in both particle size and zeta potential after freeze-drying. No additives, eg, mannitol or trehalose, were needed for efficient lyophilization, suggesting the protein itself acts as a cryo-protectant. These findings are in agreement with the work of Bachar et alCitation10 on celecoxib-loaded β-CAS micelles.
Morphological analysis
shows the TEM of ionically crosslinked FLT-loaded CAS nanoparticle formulation (F2). The particles were observed to have a spherical shape with a diameter around 40–50 nm smaller than that obtained from DLS measurements (96.85 nm). This size discrepancy has been previously reported by Wu et alCitation32 where the size of chitosan/TPP nanoparticles loaded with ammonium glycyrrhizinate (20–80 nm), as revealed by TEM, was much smaller than that obtained by DLS (> 120 nm). The DLS method gives the hydrodynamic diameter, and the nanoparticles may shrink during the sample drying and preparation in TEM. The remarkable shrinkage indicates that the nanoparticles have a gel structure and can contain a lot of water, which corroborates with the high water-binding capacity of CAS.Citation31
Figure 3 Transmission electron micrographs of flutamide-loaded casein nanoparticles (F2) at a magnification of 20,000× (A) and 30,000× (B).
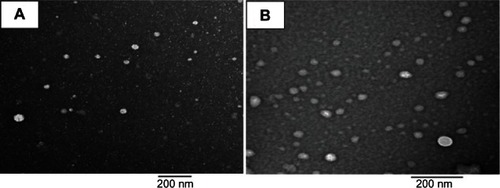
A higher magnification illustrates to a certain extent that the nanoparticles may retain the core shell structure of CAS micelles (). From the micrographs, it is clear that the aggregation of CAS nanoparticles was prevented effectively maybe via the stabilizing effect of hydrophilic shells of κ-CAS.
Solid state characterization
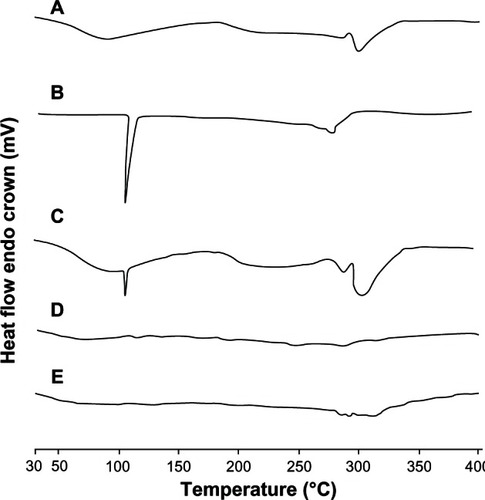
The physical status of FLT formulated in the ionically crosslinked CAS nanoparticles was compared with the free drug using DSC (). A CAS thermogram displayed a broad endothermic peak at 94.8°C due to the evolution of water from the sample, and another one at 205.6°C. FLT in its natural state exists as crystals, which are characterized by the high melting peak around 112.8°C.Citation24 In the CAS/FLT physical mixture, the endothermic peaks of both FLT and CAS were detectable at their original positions. However, when the drug was formulated in CAS nanoparticles, the peak at its original melting point disappeared, and the thermogram was essentially similar to that of unloaded CAS nanoparticles. This may be explained by the total incorporation of the drug into the nanoparticles, suggesting a molecular dispersion of drug inside the protein matrix. Similar results were observed by Puthli and VaviaCitation33 for levonorgestrel-loaded CAS microparticles.
Figure 4 Differential scanning calorimetry thermograms of casein (CAS) (A), flutamide (FLT) (B), CAS-FLT physical mixture (C), unloaded CAS nanoparticles (D), and FLT-loaded CAS nanoparticles (F2) (E).
In order to further characterize possible interactions between the drug and the protein carrier in the solid state, IR spectra of CAS, FLT, TPP, CAS-FLT physical mixture, and ionically crosslinked drug-loaded CAS nanoparticles were recorded (). CAS typically shows absorption bands at 3455.86, 1661.12, 1530.51, and 1235.4 cm−1 that originate from N–H stretching and amide bending vibrations. CAS exhibited another characteristic band at 1415.9 cm−1, attributable to the carboxylate group (O–C–O). FLT has a characteristic peak at 3358.3 cm−1, corresponding to NH stretching vibration of its secondary amino group in addition to the carbonyl stretching peak at 1715.7 cm−1 (C=O amide).Citation17–Citation19
Figure 5 Fourier transform infrared spectroscopy transmission spectra of casein (CAS) (A), flutamide (FLT) (B), CAS-FLT physical mixture (C), sodium tripolyphosphate (D), unloaded CAS nanoparticles (E), and FLT-loaded CAS nanoparticles (F2) (F).
Note: red circles indicate characteristic peaks.
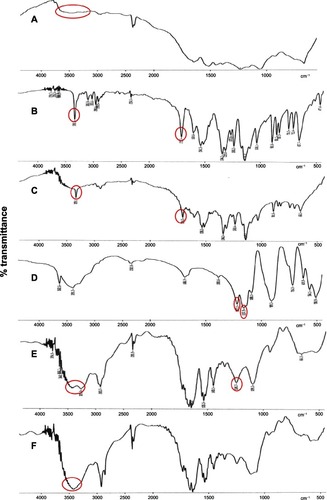
All the characteristic peaks of FLT are present in the physical mixture in their original positions. However, in the spectrum of FLT-loaded CAS nanoparticles, the characteristic peak of FLT at 3358.3 cm−1,corresponding to its amino group, was overlapped with the N–H stretching vibration of CAS at 3455 cm−1. Furthermore, the carbonyl stretching peak of FLT at 1715.7 cm−1 was shifted to 1730.2 cm−1 with much reduced intensity. This result indicated a change in the environment of the carbonyl group of the drug as a consequence of a weak interaction (intermolecular hydrogen bonds) between the drug and protein during the nanoencapsulation process.Citation17–Citation19
From the IR spectrum of TPP, the stretching vibration of the P=O or P–O at 1217.1 cm−1 and 1157.9 cm−1 was observed. On comparing the IR spectrum of TPP-crosslinked CAS nanoparticles with the spectrum of TPP, some peaks disappeared due to interaction among groups of CAS and TPP. The amide bending vibration of CAS at 1235.7 cm−1 and the stretching vibration of the P=O or P–O of TPP at 1217.1 cm−1 disappeared, and a new peak was observed at 1245.7 cm−1 and 1243.4 cm−1 in the spectra of unloaded and FLT-loaded TPP-crosslinked CAS nanoparticles, respectively.
Furthermore, the IR spectrum of the TPP-crosslinked CAS nanoparticles exhibited a new peak at 3270.3 cm−1, indicating that the amino group of CAS may be involved in a bond formation with the phosphate group of TPP, and implying the complex formation via electrostatic interaction between phosphoric groups of TPP and ammonium ions of CAS. At the same time, the 3415.8 cm−1 peak of CAS remains, indicating that not all the amino groups of CAS are involved in the reaction.
Biodegradability of CAS nanoparticles
Several factors, such as particle preparation technique, degradation environments, enzyme activity, surface area, porosity, tortuosity, and size, can affect the degradation of the matrix of protein nanoparticles. At neutral pH, trypsin is expected to attack specific sites on the surface and in the interior of the protein particles. The development of a dense crosslinking matrix for nanoparticles offers resistance against the proteolytic degradation, as it is difficult for the enzymes to penetrate into the particles.Citation34
In our study, proteolysis of ionically crosslinked CAS nanoparticles was measured by a turbidometric method. shows the percentage transmittance values at 600 nm, obtained after digesting CAS nanoparticles with three different crosslinking densities in trypsin. It is clear that the ease with which CAS nanoparticles were degraded by the enzyme trypsin depended on the crosslinking density of the protein. Only 58.47% transmittance was achieved after incubation of CAS nanoparticles with a high crosslinking density (1:3 TPP:CAS mass ratio) in trypsin solution for 96 hours, whereas CAS nanoparticles with a low crosslinking density (1:10 TPP:CAS mass ratio) were completely degraded (98.81% transmittance) after only 72 hours incubation in trypsin solution. As can be seen, the lower the crosslinking density, the faster the rate of degradation of the protein matrix and hence an increase in the percentage transmittance. This suggests that the residence time of nanoparticles in tissue or blood might be controlled by changing the crosslinking density of the CAS matrix.Citation35
Figure 6 Influence of sodium tripolyphosphate (TPP) crosslinking density on the biodegradability of casein (CAS) nanoparticles in trypsin solution measured as % transmittance (T) at 600 nm.
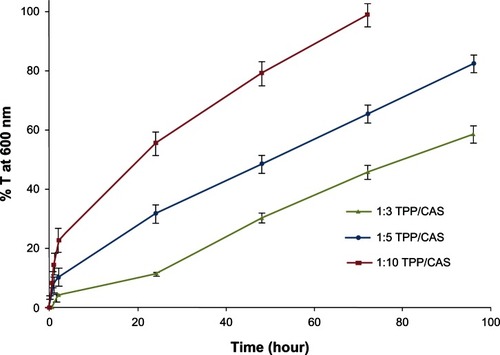
Similar results were obtained by Jayakrishnan et alCitation35 where the protease degradation of CAS microspheres crosslinked with a higher amount of glutaraldehyde was slower than that crosslinked with a lower amount. In another study, Gunasekaran et alCitation34 attributed the resistance of the glutaraldehyde-crosslinked β-lactoglobulin (βLG) nanoparticles against enzymatic attack to their dense crosslinked structure and small portion of basic amino acid composition.
In vitro release of FLT from CAS nanoparticles
A sustained-release pattern is a key issue in the development of colloidal drug delivery systems used in the field of nanomedicine. Ionically crosslinked FLT-loaded CAS nanoparticles prepared under experimental conditions described previously were tested in vitro and released at 37°C in PBS (pH 7.4) containing 0.2% Tween 80 over 96 hours. In general, FLT was released from the CAS nanoparticles very slowly in a continuous way for up to 4 days, showing an almost sustained-release ability of the nanoparticle formulations. On the contrary, it was found that >80% of FLT cosolvent was released within the first 2 hours.
The crosslinking of protein nanoparticles is important for sustained release and targeted drug delivery. From , it can be seen that the higher the crosslinking density, the slower the rate of drug release from the nanoparticles. After 96 hours, 91.54% of FLT was released from nanoparticles crosslinked with a 1:10 TPP:CAS mass ratio, compared with 74.23% from nanoparticles crosslinked with a 1:3 TPP:CAS mass ratio. The nanoparticles prepared with a lower TPP concentration showed a greater overall release. This may be attributed to reduced swelling and drug diffusion from CAS nanoparticles with the increased crosslinking density of the matrices owing to the increasing barrier for drug diffusion by the additional crosslinks formed at higher concentrations of TPP. Thus, it is possible to modulate the release of drugs from the protein matrix by changing the crosslinking density. These data suggest that combining drugs with CAS nanoparticles could prevent release at the injection site and enable the drug to be released slowly in order to sufficiently accumulate at the target site of action.
Figure 7 The influence of sodium tripolyphosphate (TPP) crosslinking density on flutamide (FLT) release from casein (CAS) nanoparticles in phosphate-buffered saline (pH 7.4) at 37°C.
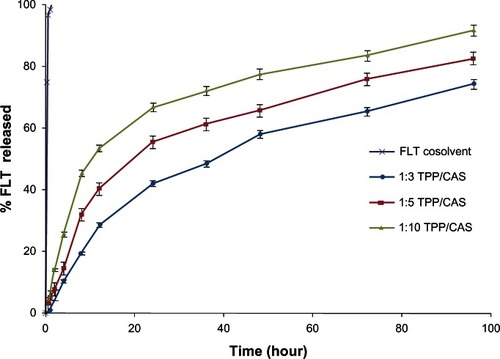
In a previous work, ascorbyl palmitate was more easily released from TPP-crosslinked chitosan nanoparticles at low TPP concentration because of the low-density structure.Citation36 Previous studies showed a sustained-release ability of drugs (eg, mitoxantroneCitation37 and progesteroneCitation38) from glutaraldehyde-crosslinked CAS microparticles, where the rate of drug release was found to decrease with the increase in the glutaraldehyde concentration.
In vivo pharmacokinetics
The mean plasma concentration over time for the FLT cosolvent (F0) and FLT-loaded CAS nanoparticles (F2) after intravenous administration into healthy rats is illustrated in . FLT level in plasma decreased much more rapidly in the case of the FLT cosolvent system (F0) when compared with FLT-CAS nanoparticles. Clearly, FLT-loaded CAS nanoparticles exhibited a longer circulation time and a markedly delayed blood clearance with a drug level of 440.35 ng/mL at 48 hours after administration. On the contrary, drug cosolvent was quickly removed from the circulating system after administration, with a plasma concentration of about 343.45 ng/mL after only 4 hours.
Figure 8 Plasma concentration of flutamide (FLT) following intravenous administration of a single dose of FLT cosolvent (F0) and ionically crosslinked FLT-loaded casein nanoparticles (F2) (12 mg/kg) into healthy rats.
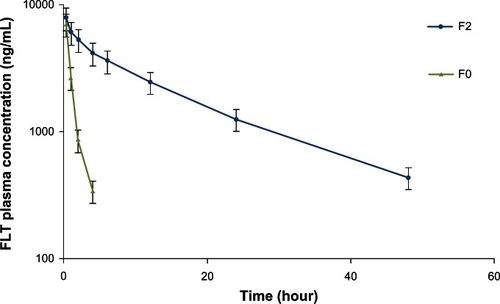
The pharmacokinetic parameters determined through the statistical analysis showed that FLT-loaded CAS nanoparticles (F2) could extend the half-life of FLT from 0.88 hours to 14.64 hours (). Meanwhile, the area under the FLT concentration-time curve (AUCinf) increased by about 12.5-fold for FLT nanoparticles (F2) compared with FLT cosolvent (F0). There was an inverse relationship between the nanocarrier clearance by the reticuloendothelial system (RES) and their prolonged circulation time. The mean clearance value of FLT cosolvent (F0) was 12.6-fold greater than FLT nanoparticles (F2). Therefore, it appears that the longer half-life and pronounced increase in the blood residence time of FLT-loaded CAS nanoparticles was the result of a reduced clearance rate.
Table 2 Pharmacokinetic parameters of flutamide (FLT) after intravenous administration of a single dose of FLT cosolvent (F0) and ionically crosslinked FLT-loaded casein nanoparticles (F2) (12 mg/kg) into healthy rats
It has been found that particles under 200 nm in diameter display a decreased rate of clearance and thus an extended circulation time. In addition, proteins have the possibility of less opsonization by RES through an aqueous steric barrier.Citation2 Therefore, the prolonged circulation of CAS nanoparticles may be due to their ability to reduce the uptake of FLT by the RES, which may reduce side effects associated with the hepatic system. The hydrophobic core of CAS nanoparticles could also retain their drug content in plasma for a longer time.Citation11,Citation25 Based on the findings of the pharmacokinetic study, we postulate that the hydrophilic κ-CAS layer covering the surface of CAS micelles can simulate the role of PEG hydrophilic shell-suppressing opsonization through generating a steric barrier, preventing hydrophobic interactions of plasma opsonins with the particle surface and inhibiting the uptake by RES.Citation39
Conclusions
In this work we investigated a new kind of colloidal system, ionically crosslinked CAS nanoparticles, as a vehicle for effective solubilization and controlled delivery of the poorly soluble antiandrogen FLT. Our results demonstrated that CAS nanoparticles are promising candidates for controlled drug delivery, as they can be easily prepared under mild conditions and they can incorporate bioactive compounds with reasonable entrapment efficiency. Moreover, they presented small particle size (below 100 nm), positive zeta potential, and a good redispersibility after lyophilization. The nanoparticles succeeded in achieving a sustained-release pattern of the drug with the release rate and could be modified via modulating the crosslinking density. In vivo assessment of FLT-loaded CAS nanocarriers demonstrated that they were well tolerated in vivo and caused a significant prolongation of FLT circulation in plasma, compared with drug cosolvent. These properties are extremely useful for intravenous delivery of hydrophobic anticancer drugs that are poorly absorbed.
Acknowledgment
The authors thank Archimica Chemical Company, Italy, for kind donation of the FLT used in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenLRemondettoGESubiradeMFood protein-based materials as nutraceutical delivery systemsTrends Food Sci Technol200617272283
- ElzoghbyAOSamyWMElgindyNAProtein-based nanocarriers as promising drug and gene delivery systemsJ. Control Release2012161384922564368
- LivneyYDMilk proteins as vehicles for bioactivesCurr Opin Colloid Interface Sci2010157383
- ElzoghbyAOAbo El-FotohWSElgindyNACasein-based formulations as promising controlled release drug delivery systemsJ Control Release201115320621621338636
- SemoEKesselmanEDaninoDLivneyYDCasein micelle as a natural nanocapsular vehicle for nutraceuticalsFood Hydrocoll200721936942
- ZimetPRosenbergDLivneyYDRe-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acidsFood Hydrocoll20112512701276
- EsmailiMGhaffariSMMoosavi-MovahediZBeta casein–micelle as a nano vehicle for solubility enhancement of curcumin; Food industry applicationLWT Food Sci Technol20114421662172
- ShapiraAAssarafYGEpsteinDLivneyYDβ-casein nanoparticles as an oral delivery system for chemotherapeutic drugs: Impact of drug structure and properties on co-assemblyPharm Res2010272175218620703895
- ShapiraADavidsonIAvniNAssarafYGLivneyYDβ-Casein nanoparticle-based oral drug delivery system for potential treatment of gastric carcinoma: Stability, target-activated release and cytotoxicityEur J Pharm Biopharm20128029830522085654
- BacharMMandelbaumAPortnayaIPerlsteinDevelopment and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled β-casein micellesJ Control Release201216016417122266050
- ElzoghbyAOSamyWMElgindyNAAlbumin-based nanoparticles as potential controlled release drug delivery systemsJ Control Release201216716818221839127
- ElzoghbyAOSamyWMElgindyNANovel spray-dried genipin-crosslinked casein nanoparticles for prolonged release of alfuzosin hydrochloridePharm Res20133051252223135815
- MartelCLGumerlockPHMeyersFJLaraPNCurrent strategies in the management of hormone refractory prostate cancerCancer Treat Rev20032917118712787712
- GoldspielBRKohlerDRFlutamide: an antiandrogen for advanced prostate cancerDICP1990246166232193461
- ZuoZkwonGStevensonBDiakurJWiebeLIHydroxypropyl-β-cyclodextrin-flutamide inclusion complex. I. Formulation, physical characterization and absorption studies using the Caco-2 in vitro modelJ Pharm Pharmaceut Sci20003220227
- ZuoZTamYKDiakurJWiebeLIHydroxypropyl-β-cyclodextrin flutamide inclusion complex. II. Oral and intravenous pharmacokinetics of flutamide in ratJ Pharm Pharmaceut Sci20025292298
- ElgindyNElkhodairyKMolokhiaAElzoghbyALyophilization monophase solution technique for improvement of the physicochemical properties of an anticancer drug. flutamideEur J Pharm Biopharm20107439740519944160
- ElgindyNElkhodairyKMolokhiaAElzoghbyALyophilization monophase solution technique for preparation of amorphous flutamide dispersionsDrug Dev Ind Pharm20113744645521446829
- ElgindyNElkhodairyKMolokhiaAElzoghbyALyophilized flutamide dispersions with polyols and amino acids: preparation and in vitro evaluationDrug Dev Ind Pharm20113744645521446829
- ElkhodairyKSamyWOptimization and evaluation of micromeritic and release properties of high dose flutamide liquisolid systemsLett Drug Des Discov20129336344
- MurthyRSRUmrethiaMLOptimization of formulation parameters for the preparation of flutamide liposomes by 3(3) factorial 26-term logit modelPharm Dev Technol2004936937715581073
- MadhusudhanBRambhauDApteSSGopinathDOral bioavailability of flutamide from 1-O-alkylglycerol stabilized o/w nanoemulsionsJ Disp Sci Technol20072812541261
- JeevanaJBSreelakshmiKDesign and evaluation of self-nanoemulsifying drug delivery system of flutamideJ Young Pharm201134821607048
- ElgindyNElkhodairyKMolokhiaAElzoghbyABiopolymeric microparticles combined with lyophilized monophase dispersions for controlled flutamide releaseInt J Pharm201141111312021457767
- KumariAYadavSKYadavSCBiodegradable polymeric nanoparticles based drug delivery systemsColloids Surf B Biointerfaces20107511819782542
- AjunWYanSLiGHuiliLPreparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release studyCarbohydr Polym200975566574
- YeAFlanaganJSinghHFormation of stable nanoparticles via electrostatic complexation between sodium caseinate and gum ArabicBiopolym200682121133
- AnalAKTobiassenAFlanaganJSinghHPreparation and characterization of nanoparticles formed by chitosan–caseinate interactionsColloids Surf B Biointerfaces20086410411018294821
- RediguieriCFde FreitasOLettingaMPTuinierRThermodynamic incompatibility and complex formation in pectin/caseinate mixturesBiomacromolecules200783345335417994786
- GaianiCMulletMArab-TehranyEMilk proteins differentiation and competitive adsorption during spray-dryingFood Hydrocoll201125983990
- PanXYuSYaoPShaoZSelf-assembly of β-casein and lysozymeJ Colloid Interf Sci2007316405412
- WuYYangWWangCHuJShoukuanFChitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinateInt J Pharm200529523524515848008
- PuthliSVaviaPGamma irradiated micro system for long-term parenteral contraception: an alternative to synthetic polymersEur J Pharm Sci20083530731718760352
- GunasekaranSKoSXiaoLUse of whey proteins for encapsulation and controlled delivery applicationsJ Food Eng2007833140
- JayakrishnanAKneppWAGoldbergEPCasein microspheres: preparation and evaluation as a carrier for controlled drug deliveryInt J Pharm1994106221228
- YoksanaRJirawutthiwongchaiJArpoKEncapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processesColloids Surf B Biointerfaces20107629229720004558
- KneppWAJayakrishnanAQuiggJMSitrenHSBagnallJJGoldbergEPSynthesis, properties, and intratumoral evaluation of mitoxantrone-loaded casein microspheres in Lewis lung carcinomaJ Pharm Pharmacol1993458878917904628
- LathaMSLalAVKumaryTVSreekumarRJayakrishnanAProgesterone release from glutaraldehyde cross-linked casein microspheres: in vitro studies and in vivo response in rabbitsContraception20006132933410906504
- De KruifCGZhulinaEBκ-Casein as a polyelectrolyte brush on the surface of casein micellesColloids Surf A1996117151159