Abstract
Docetaxel (DTX) is one of the most important anticancer drugs; however, the severity of its adverse effects detracts from its practical use in the clinic. Magnetic nanoparticles of Fe3O4 (MgNPs-Fe3O4) can enhance the delivery and efficacy of anticancer drugs. We investigated the effects of MgNPs-Fe3O4 or DTX alone, and in combination with prostate cancer cell growth in vitro, as well as with the mechanism underlying the cytotoxic effects. MgNPs-Fe3O4 caused dose-dependent increases in reactive oxygen species levels in DU145, PC-3, and LNCaP cells; 8-hydroxydeoxyguanosine levels were also elevated. MgNPs-Fe3O4 alone reduced the viability of LNCaP and PC-3 cells; however, MgNPs-Fe3O4 enhanced the cytotoxic effect of a low dose of DTX in all three cell lines. MgNPs-Fe3O4 also augmented the percentage of DU145 cells undergoing apoptosis following treatment with low dose DTX. Expression of nuclear transcription factor κB in DU145 was not affected by MgNPs-Fe3O4 or DTX alone; however, combined treatment suppressed nuclear transcription factor κB expression. These findings offer the possibility that MgNPs-Fe3O4–low dose DTX combination therapy may be effective in treating prostate cancer with limited adverse effects.
Introduction
Prostate cancer is the most common cancer affecting men, and the second leading cause of cancer death in the United States.Citation1 The incidence and mortality rates of prostate cancer vary greatly among different geographic areas and ethnic groups. Although the incidence of prostate cancer in Japan remains low compared with that in the United States, it has been increasing in recent years. However, by 2020, prostate cancer is projected to surpass stomach cancer as the most frequently diagnosed cancer in Japanese men.Citation2
Several management options are available when prostate cancer is diagnosed at an early stage, including watchful waiting, surgery, cryosurgery, radiation therapy, and hormonal therapy. For advanced prostate cancers, surgical or medical ablation of androgens is regarded the optimal first-line treatment. In most patients treated by androgen deprivation, disease progression will continue until reaching a stage referred to as castration-resistant prostate cancer (CRPC). Progression to a hormonal refractory state is a complex process, involving both selection and outgrowth of preexisting clones of androgen-independent cells as well as adaptive upregulation of genes that help cancer cells survive and grow after androgen ablation.Citation3
Although the effects of several anticancer drugs for prostate cancer have been evaluated in vitro and in animal experiments in vivo, most have little or no impact on the survival of patients with CRPC or metastatic prostate cancer.Citation4,Citation5 Docetaxel (DTX), a semisynthetic toxoid produced from the needles of the European yew tree, is the first chemotherapy agent to improve survival in CRPC, and the US Food and Drug Administration has recommended a 3-week DTX-prednisone regimen as a first-line treatment option for CRPC patients.Citation6–Citation8 Although DTX-based chemotherapy may provide some benefits, most CRPC patients do not realize them, and the average survival remains relatively brief. Moreover, the current regimen requires the administration of high doses of DTX, which causes toxic reactions and thereby precludes the use of DTX as a monotherapy.Citation9 To reduce toxicity and to improve the survival and quality of life of CRPC patients, novel therapeutic strategies targeting the molecular basis of androgen- and chemo-resistance of prostate cancer using a reduced but equieffective dose of DTX should be developed.
Cancer nanotechnology offers great potential for cancer diagnosis, targeted treatment, and monitoring.Citation10 Researchers are exploring the use of nanoparticles (NPs) ranging in length from 1 nm to 100 nm in two or three dimensions to detect, image, monitor, and treat cancers. Among the rapidly evolving types of NPs, magnetic NPs (MgNPs) – biocompatible and superparamagnetic nanomaterials with chemical stability and low toxicity – are especially promising.Citation11 The combination of MgNPs with anticancer agents has been applied to leukemia, lung, and pancreatic cancer cells in vitro and to xenograft-injected nude mice.Citation12–Citation15 MgNPs composed of Fe3O4 (MgNPs-Fe3O4) are being widely investigated for use as targeted drug carriers. The aim of this study was to evaluate the effect of treatment with MgNPs-Fe3O4 or MgNPs-Fe3O4 combined with DTX on prostate cancer cell growth in vitro. We also explored the mechanism underlying MgNPs-Fe3O4-induced cell death, focusing on the effect of MgNPs-Fe3O4 treatment on the production of reactive oxygen species (ROS).
Materials and methods
Physical characterization of MgNPs-Fe3O4
MgNPs-Fe3O4 were obtained from the Toda Kogyo Corporation (Otake, Hiroshima, Japan) and had the following characteristics: spherical shape; an average particle size of 10 nm in powder and 8–10 nm as measured by transmission electron microscopy (TEM); a size of 60–100 nm as measured by dynamic light scattering (DLS); a zeta potential of −30 to −40 mV at a pH of 10; and a surface area in powder of 100–120 m2/g.
Preparation of MgNPs-Fe3O4
After ultraviolet sterilization of the particles, MgNPs-Fe3O4 stocks were prepared by suspending particles in Roswell Park Memorial Institute (RPMI)-1640 with supplements to yield final concentrations of 1 μg/mL, 10 μg/mL, or 100 μg/mL, followed by sonication at 30 W for 10 minutes with an Ultrasonic Homogenizer VP-050 (TAITEC, Koshigaya, Saitama, Japan).
Docetaxel
DTX was purchased from Sigma-Aldrich (St Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO; stock solution). Stock solutions were aliquoted and stored at −20°C to avoid repetitive freeze-thaw cycles. Stock solutions were serially diluted using culture medium to prepare working solutions.
Cell lines
LNCaP, DU 145, and PC-3 human prostate cancer cell lines were purchased from American Tissue Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 100 U/mL penicillin-streptomycin in 5% CO2 at 37°C. The human normal prostate stromal cell (PrSC) line was obtained from BioWhittaker® (Lonza Walkersville, Inc, Walkersville, MD, USA) and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 100 U/mL of penicillin G, 100 μg/mL of streptomycin, ITH (5 μg/mL insulin, 5 μg/mL transferrin, and 1.4 μmol/L hydrocortisone), and 5 ng/mL of bFGF in 5% CO2 at 37°C.
Characterization of MgNPs-Fe3O4 suspension
MgNP-Fe3O4 suspensions and their cellular localization were characterized using the following methods.
Dynamic light scattering (DLS)
The average hydrodynamic size and size distribution of MgNPs-Fe3O4 in media were determined by DLS using a Fiber-Optics Particle Analyzer FPAR-1000 (Otsuka Electronics Co, Ltd Hirakata, Osaka, Japan). DU145 cells were incubated with MgNPs-Fe3O4 (1 μg/mL, 10 μg/mL, or 100 μg/mL).
Transmission electron microscopy (TEM)
DU145 cells were incubated with MgNPs-Fe3O4 (10 μg/mL). After incubation for 24 hours, cells were collected washed three times with phosphate buffered saline (PBS), and fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) at 4°C for 4 hours. The resulting samples were postfixed with 2% osmium tetraoxide at 4°C for 2 hours, dehydrated and embedded in epoxy resin. Ultrathin sections (80 nm) were then stained with uranyl acetate and lead citrate, and observed by TEM.
Measurement of intracellular reactive oxygen species
ROS were measured using the CM-H2DCFDA assay (Life Technologies, Carlsbad CA, USA), according to the manufacturer’s instructions. DU145 cells (1.0 × 105 cells/well) were incubated with MgNPs-Fe3O4 (1 μg/mL, 10 μg/mL, or 100 μg/mL) for 24 hours in the absence or presence of N-acetylcysteine (NAC; 10 mM) (Sigma-Aldrich Co); NAC was added 3 hours before treatment with MgNPs-Fe3O4. A stock solution of CM-H2DCFDA (5 mM) was freshly prepared in DMSO and diluted to a final concentration of 1 μM in PBS. Cells were washed with PBS followed by incubation with 50 μL of working solution of fluorochrome marker CM-H2 DCFDA for 30 minutes. Fluorescent imaging was recorded using an IX2 N-FL-1 microscope (Olympus Corporation, Tokyo, Japan), and analyzed using imaging software (Adobe Photoshop Elements 8; Adobe Systems Incorporated San Jose, CA, USA). As a positive control, cells were treated with H2O2 (100 μM) for 24 hours.
Analysis of 8-hydroxydeoxyguanosine in DNA
The MgNPs-Fe3O4 (1 μg/mL, 10 μg/mL, or 100 μg/mL) were added to wells containing DU145, PC-3, or LNCap cells (5.0 × 106 cells), and incubated for 72 hours at 37°C (5% CO2). Nuclear deoxyribonucleic acid (DNA) of the cells was isolated by the sodium iodide method. Analysis of 8-hydroxydeoxyguanosine (8-OH-dG) was performed as previously described.Citation16 The 8-OH-dG levels were measured by high performance liquid chromatography electrochemical detection. The amount of 8-OH-dG in the DNA was determined through comparisons with the authentic standards, and expressed as the number of 8-OH-dG per 106 deoxyguanosine (dG).
AlamarBlue® assay
Cell viability was determined using the AlamarBlue® assay (Alamar Biosciences, Inc, Sacramento, CA, USA), according to the manufacturer’s instructions. Briefly, cells were seeded in 24-well plates (1.0 × 104 cells/well); cells were treated with DTX (0.1 μM, 1 μM, 10 μM, or 100 μM) or DTX (1 nM) plus MgNPs-Fe3O4 (1 μg/mL, 10 μg/mL, or 100 μg/mL) for 48 hours at 37°C (5% CO2). AlamarBlue® was added to each well at 10% volume and was incubated for 200 minutes. Metabolically active cells reduced the dye into a fluorescent form; fluorescence intensity was measured using a plate reader (excitation/emission: 570 nm/600 nm; Viento XS, DS Pharma Biomedical Co, Ltd Suita, Osaka, Japan). Fluorescence intensity was used to estimate cell viability by linear interpolation between the emission from cells treated with 0.1% saponin (0% viability) and that from untreated cells (100% viability).
Flow cytometry (FCM) analysis for cell apoptosis
The apoptotic peak (sub-G1) of cells was measured using FCM. DU145 cells (1.0 × 106 cells) were seeded in 100 mm culture dishes; cells were either untreated (control), or treated with DTX (1 nM) or MgNPs-Fe3O4 (10 μg/mL or 100 μg/mL) in the absence or presence of DTX (1 nM). Aspirated medium was collected to determine the amount of floating cells and cell debris as indicators of cell death. Cells were collected and fixed in ice-cold 70% ethanol and stored at −20°C before use. In preparation for use, cells were washed with PBS and resuspended in PBS before incubation with ribonuclease (0.5 mg/mL) at room temperature for 30 minutes. After the addition of 1 mg/mL of propidium iodide (PI; Sigma-Aldrich), the cells were passed through a 40 mm nylon mesh for analysis using an LSRII flow cytometer (BD Bioscience Franklin Lakes, NJ, USA). The fluorescence intensities of PI were measured by FCM, and the number of cells in the sub-G1 peak was determined. Quantification of the fraction was performed with ModFit LT for Mac 3.0 (Verity Software House, Topsham, ME, USA).
Annexin-V assay was used to detect the early phases of apoptosis. Apoptosis was assessed by monitoring the expression of phosphatidylserine on the outer leaflet - an early marker of apoptotic cell death. Phosphatidylserine was stained with fluorescein isothiocyanate (FITC)-labeled Annexin V Loss of membrane integrity as a consequence of necrosis was detected using PI staining of DNA. Briefly, DU145 cells (1.0 × 106) were either untreated (control) or treated with DTX (1 nM), or with MgNPs-Fe3O4 (10 μg/mL or 100 μg/mL) for 24 hours in the absence or presence of DTX (1 nM). After incubation, cells were harvested gently washed twice in ice-cold PBS, collected by centrifugation, and then stained using an Annexin V-FITC Kit (Beckman Coulter, Inc, Fullerton, CA, USA) according to the manufacturer’s instructions. Cells were then stained with Annexin V and PI for analysis by FCM within 1 hour of staining using the FL1 (FITC) and FL3 (PI) lines.
Western blot analysis
Cells were lysed in Radioimmunoprecipitation assay buffer (Sigma-Aldrich) containing protease inhibitors (Sigma-Aldrich). Total protein concentration was determined by Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of lysates were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Merck Millipore, Billerica, MA, USA). Membranes were blocked with a blocking reagent (NOF Corporation, Tokyo, Japan) for 1 hour at room temperature, and incubated overnight at 4°C with the respective primary antibodies in Tris-buffered saline and Tween 20 (TBST). The membranes were washed with TBST three times and incubated with diluted horseradish peroxidase-conjugated secondary antibodies (1:3000 for nuclear factor κB [NFκB]; 1:10,000 for β-actin) for 1 hour at room temperature. After three additional washes, membranes were detected using an enhanced chemiluminescence kit (GE Healthcare UK Ltd, Little Chalfont, UK). Antibodies against NFκB and β-actin were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA) and Sigma-Aldrich, respectively; antirabbit and antimouse horseradish peroxidase-conjugated secondary antibodies were purchased from GE Healthcare (GE Healthcare UK Ltd).
Statistical analysis
All experiments were repeated at least three times. Data are represented as the mean ± standard deviation. Data were analyzed using an unpaired Student’s t-test with or without Welch’s correction and ANOVA. Differences were considered statistically significant at P < 0.05.
Results
MgNPs-Fe3O4 characterization in cell culture medium
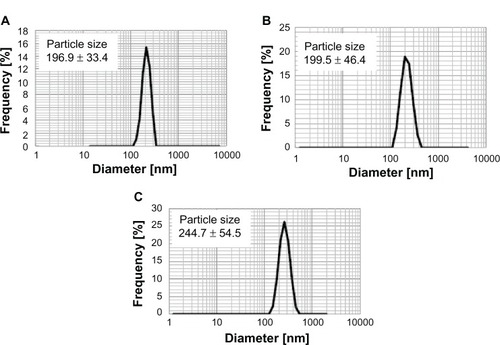
shows the mean hydrodynamic diameter of MgNPs-Fe3O4 in medium with supplements as measured by DLS. The mean hydrodynamic diameter of MgNPs-Fe3O4 increased with increasing concentration, suggesting that aggregation is enhanced at higher concentrations.
Cellular uptake
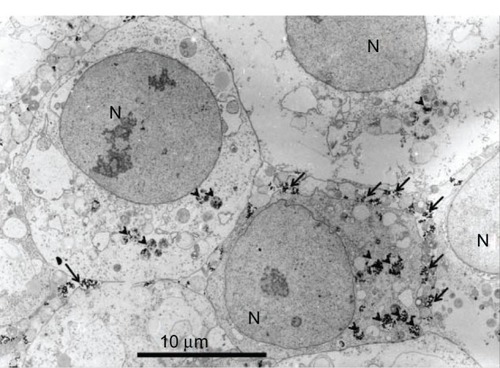
Cellular uptake of MgNPs-Fe3O4 was evident from TEM microphotographs (). MgNPs-Fe3O4 were localized within intracellular vesicles.
ROS production
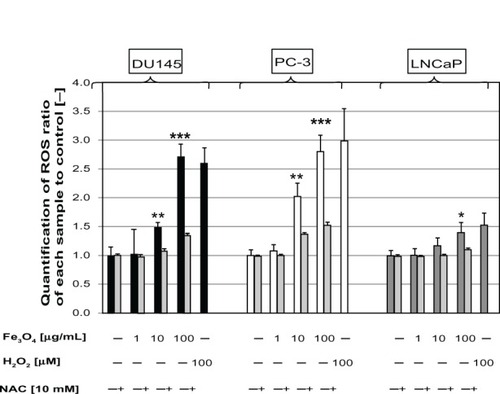
MgNPs-Fe3O4 caused dose-dependent increases of ROS production in DU145 and PC-3 cells; a significant increase in LNCaP cells was evident only at the highest dose. Treatment with 100 μg/mL of MgNPs-Fe3O4 elicited a response comparable to that evoked by H2O2 (). Among the three cell lines, ROS levels in the DU145 and PC-3 lines were higher than that in the LNCaP line. Pretreatment with NAC attenuated the MgNPs-Fe3O4-induced rise in ROS in all three prostate cancer cell lines ().
Figure 3 Production of intracellular ROS in DU145, PC-3, and LNCaP cells after treatment with MgNPs-Fe3O4 for 24 hours in the absence or presence of NAC.
Notes: Data are presented as the mean ± SD of three independent experiments. *Significantly different from the untreated control at P < 0.05; **significantly different from the control at P < 0.01; ***significantly different from the untreated control at P < 0.001.
Abbreviations: ROS, reactive oxygen species; MgNPs-Fe3O4, Fe3O4 magnetic nanoparticles; NAC, N-acetylcysteine; SD, standard deviation.
8-OH-dG levels in DNA
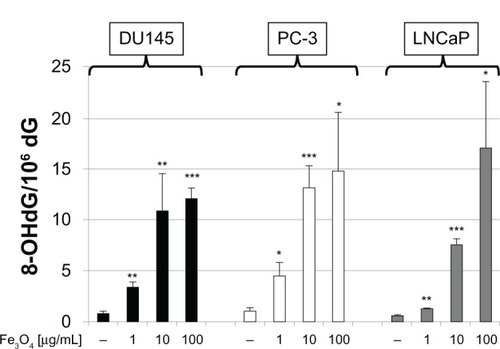
The 8-OH-dG levels in the DNA in all three prostate cancer cell lines increased in a dose-dependent manner (). The 8-OH-dG levels of DU145 and PC-3 cells exposed to 10 μg/mL of MgNPs-Fe3O4 were 13-fold to 14-fold greater than that of the untreated control cells.
Figure 4 8-OH-dG levels in DU145, PC-3, and LNCaP cells after 72 hours of treatment with MgNPs-Fe3O4.
Notes: Data are presented as the mean ± SD of three independent experiments. *Significantly different from the untreated control at P < 0.05; **significantly different from the control at P < 0.01; ***significantly different from the untreated control at P < 0.001.
Abbreviations: 8-OH-dG, 8-hydroxydeoxyguanosine; MgNPs-Fe3O4, Fe3O4 magnetic nanoparticles; SD, standard deviation.
Effect of MgNPs-Fe3O4, DTX, and MgNPs-Fe3O4–DTX combinations on cell viability
MgNPs-Fe3O4 alone reduced the viability of LNCaP and PC-3 cells, but had little or no effect on the viability of DU145 and PrSC cells (). These results suggest that the cytotoxicity of MgNPs-Fe3O4 may be dependent on the cell type of the prostate cancer cell line. DTX alone decreased cell viability in a dose-dependent manner in all three cancer cell lines (). Combined treatment with MgNPs-Fe3O4 and DTX enhanced the inhibitory effect of DTX; in PC-3 cells, 100 μg/mL of MgNPs-Fe3O4 plus 1 nM of DTX reduced cell viability so it was similar to that caused by 10 nM DTX alone. These data suggest that MgNPs-Fe3O4 may be beneficial in reducing the DTX dose it may and thereby overcome the safety limitations of DTX.
Figure 5 Effect of MgNPs-Fe3O4 exposure on cell line viability. Effect of MgNPs-Fe3O4 exposure on the viability of (A) DU145, (B) PC-3, (C) LNCaP, and (D) PrSC cell lines.
Notes: Data are presented as the mean ± SD of three independent experiments. *Significantly different from the control at P < 0.05.
Abbreviations: MgNPs-Fe3O4, Fe3O4 magnetic nanoparticles; SD, standard deviation.
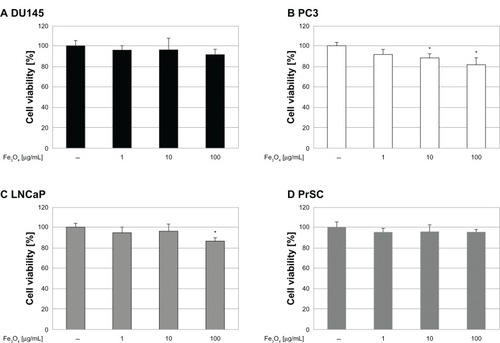
Figure 6 Effect of DTX alone or in combination with MgNPs-Fe3O4 on cell viability. Effect of DTX alone or in combination with MgNPs-Fe3O4 on the viability of (A) DU145, (B) PC-3, and (C) LNCaP cell lines.
Notes: Data are presented as the mean ± SD of three independent experiments. *Significantly different from the control at P < 0.05; **significantly different from the control at P < 0.01.
Abbreviations: DTX, docetaxel; MgNPs-Fe3O4, Fe3O4 magnetic nanoparticles; SD, standard deviation.
Effect of MgNPs-Fe3O4, DTX, and MgNPs-Fe3O4–DTX combinations on cell death
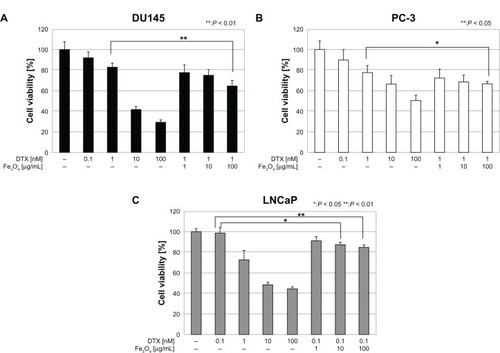
An apoptotic fraction of cells containing subdiploid amounts of DNA was detected as a sub-G1 peak (). MgNPs-Fe3O4 caused a dose-dependent increase in the percentage of DU145 cells in the sub-G1 fraction; similarly, DTX alone elicited a rise in the percentage of cells in the sub-G1 fraction. Combined treatment with MgNPs-Fe3O4 plus DTX augmented the effect compared to either treatment alone; this enhancement was dose-dependent.
Figure 7 Flow cytometry analysis of apoptosis of DU145 cells. Panels represent the following treatments: (A) untreated (control); (B) MgNPs-Fe3O4 (10 μg/mL); (C) MgNPs-Fe3O4 (100 μg/mL); (D) DTX (1 nM); (E) DTX (1 nM) + MgNPs-Fe3O4 (10 μg/mL); and (F) DTX (1 nM) + MgNPs-Fe3O4 (100 μg/mL).
Notes: Cells were incubated with each condition for 24 hours. The percentage of cells in the sub-G1 phase was quantified for each plot.
Abbreviations: MgNPs-Fe3O4, Fe3O4 magnetic nanoparticles; DTX, docetaxel.
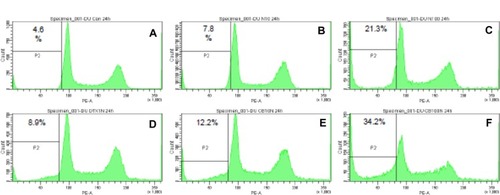
Neither MgNPs-Fe3O4 nor DTX alone increased Annexin V/PI staining (). Conversely, a significant increase in the percentage of apoptotic cells was observed during the combined treatment with NPs-Fe3O4 and DTX compared to the untreatment, the treatment with MgNPs-Fe3O4 alone, or DTX alone (P < 0.05).
Figure 8 Effects of MgNPs-Fe3O4 and docetaxel (DTX) alone or in combination on apoptosis in DU145 cells. (A) representative FCM using Annexin V/PI staining of one set of triplicate experiments. N10: MgNPs-Fe3O4 (10 μg/mL); N100: MgNPs-Fe3O4 (100 μg/mL); DTX: DTX (1nM); CB10: DTX (1nM) + MgNPs-Fe3O4 (10 μg/mL); and CB100: DTX (1 nM) + MgNPs-Fe3O4 (100 μg/mL). (B) Percentages of apoptotic cells from FCM analysis.
Notes: Data are presented as the mean ± SD of three independent experiments. Results show that the combination of 10 μg/mL or 100 μg/mL of MgNPs-Fe3O4 with 1 nM of DTX induced significant apoptosis in DU145 cells compared to untreated cells, cells treated with 10 μg/mL or 100 μg/mL of MgNPs-Fe3O4 alone, or 1nM of DTX alone (*P < 0.05).
Abbreviations: MgNPs-Fe3O4, Fe3O4 magnetic nanoparticles; DTX, docetaxel; PI, propidium iodide; DMSO, dimethyl sulfoxide; FCM, flow cytometry analysis; SD, standard deviation.
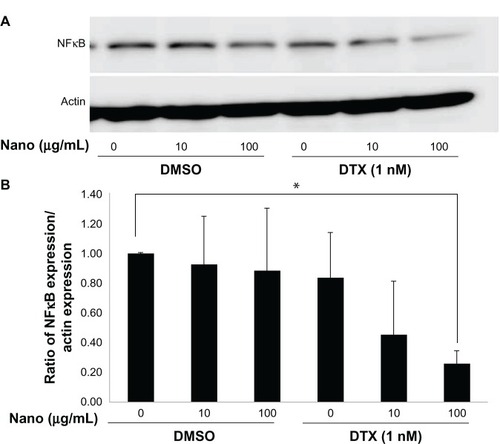
Effect of MgNPs-Fe3O4 and MgNPs-Fe3O4–DTX combinations on NFκB expression in DU145 cells
Treatment with MgNPs-Fe3O4 alone did not lower NFκB expression in DU145 cells; conversely, treatment with MgNPs-Fe3O4–DTX combinations inhibited NFκB expression in a dose-dependent manner ().
Figure 9 Effects of MgNPs-Fe3O4 in the absence or presence of DTX on NFκB expression in DU145 cells. (A) Western blot analysis. (B) Densitometric analysis of NFkB/actin expression ratio.
Notes: Cells were treated for 48 hours. The ratio of NFκB expression/actin expression represents the mean ± SD of three independent experiments. Results show that NFkB expression decreased in DU145 cells treated with 100 μg/mL of MgNPs-Fe3O4 with 1 nM of DTX compared to untreated cells (*P < 0.05).
Abbreviations: MgNPs-Fe3O4, Fe3O4 magnetic nanoparticles; DMSO, dimethyl sulfoxide; DTX, docetaxel; NFκB, nuclear factor κB; SD, standard deviation.
Discussion
DTX remains the cornerstone of chemotherapy for treating prostate cancer when castration resistance is documented and secondary hormone therapy is ineffective. However, to be effective, DTX must be administered at such high doses that can induce significant toxicity.Citation9,Citation17 To overcome this drawback, combination therapies have been developed; DTX combined with tyrosine kinase or bcl-2 inhibitors are currently in Phase II studies for treating CRPC.Citation17 Drug-delivery assemblies consisting of a nanocarrier, a targeting agent, and DTX have also been developed. For example, NC-6301 – a polymeric micelle with DTX – shows less toxicity than native DTX in vivo; NC-6301 is a nanoscale drug delivery system approximately 100 nm in a diameter.Citation18 In the present study, we found that DTX alone has a strong anticancer effect, and the cytotoxic effect of a low concentration (1 nM) is augmented by MgNPs-Fe3O4.
Many studies have focused on the use of NPs, especially MgNPs, in theranostics.Citation10,Citation11 Due to their biocompatibility and stability, iron oxide MgNPs, particularly magnetic Fe3O4 and its oxidized and more stable form, maghemite γ-Fe2O3, are superior for biomedical applications compared to other metal oxide NPs. Moreover, iron oxide NPs may have additional utility as a contrast agent in magnetic resonance imaging or as a carrier for drug delivery.Citation11–Citation15 In the present study, we focused on MgNPs-Fe3O4 because of their potential to treat CRPC. This stems from the intrinsic properties of the magnetic core combined with the drug loading capability and the biomedical properties of MgNPs conferred by different surface coatings. Iron oxide MgNPs have also effectively been used in combination with chemotherapy and hyperthermia to overcome drug resistance in a leukemia xenograft model,Citation19 and with doxorubicin under a static magnetic field to enhance the doxorubicin-mediated cytotoxicity of MCF-7 cells.Citation20
Results from several studies suggest that the cytotoxic effects of MgNPs are dependent on the metal and target cell type.Citation21,Citation22 CuO, ZnO, and CuZnFe2O4, but not Fe2O3 or Fe3O4, were highly toxic to the human lung epithelial cell line A549; CuO NPs were especially effective in inducing a significant increase in ROS production.Citation21 In BRL 3A liver cells, only silver NPs were highly toxic; Fe3O4, tungsten, aluminum, and MnO2 exhibited little or no toxicity.Citation23 Conversely, iron oxide MgNPs caused hepatic and renal damage when administered to mice,Citation22 and the reduced viability of J774 macrophages in vitro.Citation24
ROS act as a second messenger in cell signaling and are involved in various biological processes, such as growth and survival in normal cells. Oxidative stress reflects a redox imbalance within the cells and usually results from the net accumulation of intracellular ROS, which are not detoxified by cellular antioxidative agents.Citation25 In cancer cells, the production of ROS is typically increased; since they play important roles in initiation, progression, and metastasis, ROS are considered oncogenic. However, ROS are also implicated in triggering cell death, including that of cancer cells; thus, their production is desirable in chemotherapy, radiotherapy, and photodynamic therapy. This dual role of ROS has led to the development of two paradoxical ROS-manipulation strategies in cancer treatment.Citation25 One strategy is to treat tumor cells with antioxidants, such as through the dietary administration of red wine and green tea polyphenols to prevent cancer. The other strategy is to provide pro-oxidant therapy, which consists of generating ROS directly and inhibiting antioxidative enzyme systems in tumor cells. In the present study, MgNPs-Fe3O4 exhibited mild cytotoxicity toward PC-3 and LNCaP, but not toward DU145 and PrSC cells. The LNCaP and PC-3 cell lines have previously been reported to have unique redox state properties, including the production of different levels of oxidative damage products and antioxidant proteins; these differences may provide new insights into the possible uses and dangers of using pro-oxidants or antioxidants as cancer therapeutic agents.Citation26–Citation28 We found that the MgNPs-Fe3O4-induced increase in ROS was most robust in the DU145 and PC-3 cell lines; however, the levels of 8-OH-dG, an index of oxidative DNA damage, were comparably elevated in all three cell lines.
The transcription of antiapoptotic genes is activated by the NFκB signaling pathway, resulting in cell survival. The NFκB signaling pathway also plays a critical role in cancer development and progression, and in the development of tumor resistance to chemotherapy and radiation therapy,Citation29 particularly in the transition toward CRPC.Citation30 Previous studies demonstrating a relationship between elevated NFκB and a worse prognosis support this notion.Citation31,Citation32 Thus, the NFκB pathway has become an important target in the development of novel anticancer treatments. The combination of magnetic NPs with either adriamycin or daunorubicin has been reported to increase p53 levels and decrease NFκB protein levels, leading to increased apoptosis in Raji lymphoma cells.Citation33 In the present study, treatment with MgNPs-Fe3O4 or DTX alone had no effect on the expression of NFκB in DU145 cells; however, treatment with MgNPs-Fe3O4–DTX combinations decreased expression in a dose-dependent manner. This result is unique because many NPs have been reported to activate the NFκB pathway via activation of mitogen-activated protein kinase cascades by an oxidative stress response.Citation34 Thus, our results suggest that the decrease in NFκB expression resulting from treatment with MgNPs-Fe3O4–DTX combinations may be uncoupled from ROS generation. Although the chemical components involved in NFκB inhibition and ROS production have been identified, the contribution of MgNPs-Fe3O4 exposure to the mechanisms of induction and action remains unclear. Further studies such as those measuring NFκB DNA-binding activity are needed.
Conclusion
We found that MgNPs-Fe3O4 significantly increased ROS production in prostate cancer cell lines and induced oxidative DNA damage; the cytotoxic effects of MgNPs-Fe3O4 alone were mild. Treatment with a combination of MgNPs-Fe3O4 and a low dose of DTX enhanced the inhibitory effect of DTX alone on prostate cancer cell growth in vitro, and also suppressed NFκB expression. These findings offer the possibility that MgNPs-Fe3O4 may allow the dose of DTX to be reduced without decreasing its antitumor activity.
Acknowledgments
We thank Dr T Yabana for TEM analysis, and Dr M Yoneda for his pathological preparation. This research was supported in part by a Grant-in-Aid for the Global COE Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant-in-Aid for Research on Risk of Chemical Substances from the Ministry of Health, Labor and Welfare of Japan, and a Research Grant-in-Aid from Magnetic Health Science Foundation.
Disclosure
The authors report no conflicts of interest in this work. The authors have no financial interests in or financial conflict with the subject matter discussed in this manuscript.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- TabataNOhnoYMatsuiRPartial cancer prevalence in Japan up to 2020: estimates based on incidence and survival data from population-based cancer registriesJpn J Clin Oncol200838214615718245517
- SoAGleaveMHurtado-ColANelsonCMechanisms of the development of androgen independence in prostate cancerWorld J Urol20052311915770516
- DiazMPattersonSGManagement of androgen-independent prostate cancerCancer Control200411636437315625524
- LassiKDawsonNAUpdate on castrate-resistant prostate cancer: 2010Curr Opin Oncol201022326326720177381
- TannockIFde WitRBerryWRTAX 327 InvestigatorsDocetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancerN Engl J Med2004351151502151215470213
- PetrylakDPTangenCMHussainMHDocetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancerN Engl J Med2004351151513152015470214
- NiraulaSTannockIFBroadening horizons in medical management of prostate cancerActa Oncol201150Suppl 114114721604955
- LeeSYChoJSYukDYObovatol enhances docetaxel-induced prostate and colon cancer cell death through inactivation of nuclear transcription factor-kappaBJ Pharmacol Sci2009111212413619834284
- MisraRAcharyaSSahooSKCancer nanotechnology: application of nanotechnology in cancer therapyDrug Discov Today20101519–2084285020727417
- ShubayevVIPisanicTR2ndJinSMagnetic nanoparticles for theragnosticsAdv Drug Deliv Rev200961646747719389434
- ChengJWuWChenBAEffect of magnetic nanoparticles of Fe3O4 and 5-bromotetrandrine on reversal of multidrug resistance in K562/A02 leukemic cellsInt J Nanomedicine2009420921619918367
- WangCZhangHChenBYinHWangWStudy of the enhanced anticancer efficacy of gambogic acid on Capan-1 pancreatic cancer cells when mediated via magnetic Fe3O4 nanoparticlesInt J Nanomedicine201161929193521931488
- ZhangGDingLRenegarRHydroxycamptothecin-loaded Fe3O4 nanoparticles induce human lung cancer cell apoptosis through caspase-8 pathway activation and disrupt tight junctionsCancer Sci201110261216122221435100
- SatoATamuraYSatoNMelanoma-targeted chemo-thermo-immuno (CTI)-therapy using N-propionyl-4-S-cysteaminylphenol-magnetite nanoparticles elicits CTL response via heat shock protein-peptide complex releaseCancer Sci201010191939194620594194
- KawaiKLiYSKasaiHAccurate measurement of 8-OH-dG and 8-OH-Gua in mouse DNA, urine and serum: effects of X-ray irradiationGenes and Environment2007293107114
- GalskyMDVogelzangNJDocetaxel-based combination therapy for castration-resistant prostate cancerAnn Oncol201021112135214420351071
- HaradaMIwataCSaitoHNC-6301, a polymeric micelle rationally optimized for effective release of docetaxel, is potent but is less toxic than native docetaxel in vivoInt J Nanomedicine201272713272722745540
- RenYZhangHChenBMultifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistanceInt J Nanomedicine201272261226922619560
- AljarrahKMhaidatNMAl-AkhrasMAMagnetic nanoparticles sensitize MCF-7 breast cancer cells to doxorubicin-induced apoptosisWorld J Surg Oncol2012106222533492
- KarlssonHLCronholmPGustafssonJMöllerLCopper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubesChem Res Toxicol20082191726173218710264
- MaPLuoQChenJIntraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in miceInt J Nanomedicine201274809481822973100
- HussainSMHessKLGearhartJMGeissKTSchlagerJJIn vitro toxicity of nanoparticles in BRL 3A rat liver cellsToxicol In Vitro200519797598316125895
- NaqviSSamimMAbdinMConcentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stressInt J Nanomedicine2010598398921187917
- WangJYiJCancer cell killing via ROS: to increase or decrease, that is the questionCancer Biol Ther20087121875188418981733
- ChenYWangJFraigMMDefects of DNA mismatch repair in human prostate cancerCancer Res200161104112412111358834
- TrzeciakARNyagaSGJarugaPLohaniADizdarogluMEvansMKCellular repair of oxidatively induced DNA base lesions is defective in prostate cancer cell lines, PC-3 and DU-145Carcinogenesis20042581359137015044326
- ChaiswingLBourdeau-HellerJMZhongWOberleyTDCharacterization of redox state of two human prostate carcinoma cell lines with different degrees of aggressivenessFree Radic Biol Med200743220221517603930
- GasparianAVGuryanovaOAChebotaevDVShishkinAAYemelyanovAYBudunovaIVTargeting transcription factor NFkappaB: comparative analysis of proteasome and IKK inhibitorsCell Cycle20098101559156619372735
- JainGCronauerMVSchraderMMöllerPMarienfeldRBNF-κB signaling in prostate cancer: a promising therapeutic target?World J Urol201230330331022085980
- KoumakpayiIHLe PageCMes-MassonAMSaadFHierarchical clustering of immunohistochemical analysis of the activated ErbB/PI3K/Akt/NF-kappaB signalling pathway and prognostic significance in prostate cancerBr J Cancer201010271163117320216540
- MacKenzieLMcCallPHatziieremiaSNuclear factor κB predicts poor outcome in patients with hormone-naive prostate cancer with high nuclear androgen receptorHum Pathol20124391491150022406367
- JingHWangJYangPKeXXiaGChenBMagnetic Fe3O4 nanoparticles and chemotherapy agents interact synergistically to induce apoptosis in lymphoma cellsInt J Nanomedicine20105999100421187919
- MaranoFHussainSRodrigues-LimaFBaeza-SquibanABolandSNanoparticles: molecular targets and cell signallingArch Toxicol201185773374120502881