Abstract
Nowadays, refractory diabetic wounds cause a worldwide medical burden. Mesenchymal stem cells derived exosomes (MSC-Exos) show promise as a solid alternative to existing therapeutics in the latest researches, since MSC-Exos share similar biologic activity but less immunogenicity when compared with MSCs. To facilitate further understanding and application, it is essential to summarize the current progress and limitations of MSC-Exos in the treatment of diabetic wounds. In this review, we introduce the effects of different MSC-Exos on diabetic wounds according to their origins and contents and discuss the specific experimental conditions, target wound cells/pathways, and specific mechanisms. In addition, this paper focuses on the combination of MSC-Exos and biomaterials, which improves the efficacy and utilization of MSC-Exos therapy. Together, exosome therapy has high clinical value and application prospects, both in its role and in combination with biomaterials, while novel drugs or molecules loaded into exosomes as carriers targeting wound cells will be development trends.
Introduction
Diabetes is recognized globally as one of the major health events. According to “2021IDF Global Diabetes Map (10th edition)”, the worldwide number of adults with diabetes reached 537 million (10.5%) in 2021, increasing by 16% compared to that of 2019. The harm of diabetes mainly exhibits in its complications, among which unhealed diabetic wounds may lead to lower limb amputation, consuming 20–40% of medical resources per year.Citation1
Since traditional therapy like debridement and wound dressing has little effect, new treatments for diabetic wounds have emerged, such as bioengineered skin substitutes, hyperbaric oxygen therapy, electrical stimulation pulse therapy, stem cell therapy, and so on.Citation2 Among them, stem cells have been widely studied because of their pluripotency, self-renewal, and ability to promote the secretion of regenerative cytokines. Many studies have confirmed that pluripotent stem cells, especially MSCs or some omnipotent progenitor cells, promote diabetic wound healing,Citation3 with exosomes formed and released by stem cells as a critical part of the healing process.Citation4 Exosomes derived from MSCs can transport genetic material and transcription factors to regulate recipient cells and mediate cellular crosstalk among macrophages, endothelial cells (ECs), and fibroblasts.Citation5 More importantly, cell-free therapy has a rare risk of cancer compared to stem cell therapy. Therefore, cell-free therapy with exosomes has become a hot spot regarding diabetic wound healing.Citation6
Due to the heterogeneity of MSCs derived from different tissues, the contents of exosomes may differ from each other, so as do the roles in diabetic wound healing. In addition, methods of extracting MSCs vary from tissue to tissue, and there are different contents in various exosomes, like long non-coding RNA (lncRNA), microRNA (miR), and circular RNA (circRNA). Therefore, it is logical and systematic to classify MSCs by this method. We use the search query combination “(exosomes [Title/Abstract]) AND (diabetes wound) AND (stem cell)” to search literature in 2015–2022 from PubMed, Web of Science, Embase, and so on. After the screening, we selected about 60 articles for the main part of our review, the majority of which are original research articles.
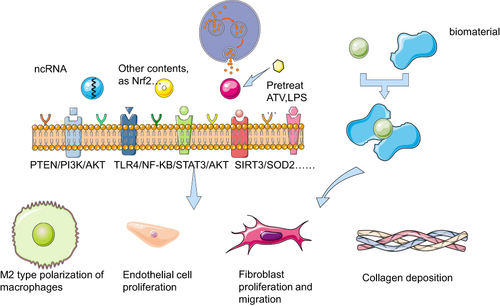
This review will compare the effects of exosomes, which take several kinds of contents, derived from different MSCs on diabetic wound healing, and briefly introduce the related technologies of exosome biomaterials ().
Figure 1 Mechanism of exosomes targeting cells on diabetic wounds with different contents or being combined with biomaterials Exosomes with different contents or pretreatment secreted from MSCs act on repair-related cells in diabetic wounds through some signaling pathways such as PTEN/PI3K/AKT, TLR4/NF-KB/STAT3/AKT, SIRT3/SOD2, to promote wound healing by macrophage M2 polarization, endothelial cell proliferation, fibroblast proliferation and migration, collagen deposition, etc. Exosomes and biomaterials can be used in combination to serve the same purpose in diabetic wounds. The figure was partly generated using Servier Medical Art, provided by Servier (smart.servier.com), licensed under a Creative Commons Attribution 3.0 Unported License.
Normal and Diabetic Wound Healing
Normal Wound Healing
It is known to all that dermal wound healing has four stages, namely hemostasis, inflammation, proliferation, and remodeling, which occur in a linear and overlapping order. After the injury, platelet activation initiates the coagulation cascade to achieve hemostasis. Meanwhile, injured cells release damage-associated factors to activate local macrophages and initiate damage-associated molecular patterns (DAMPs).Citation7 The activation of macrophages, the initiation of DAMPs, and the production of cytokines such as PDGF after platelet attachment together contribute to the recruitment of prolymphocytic neutrophils (PMNs) from circulation.Citation8
The early inflammatory phase is dominated by neutrophils, responsible for the primary defense against pathogens, as well as the release of chemokines to attract monocytes to the wound site. Monocytes differentiate locally into dendritic cells and macrophages. Then, macrophages become the dominant cell type in the inflammatory phase and are responsible for clearing pathogens and other apoptotic cells and secreting proinflammatory cytokines such as IL-1β, IL-6, and TNFα. As the inflammatory phase progresses, the release of some chemokines (eg, CXCL12) promotes the macrophage phenotype conversion from M1 to M2. M2 macrophages, as an anti-inflammatory state, secrete IL-4, IL-10, IL-13, and TGFβ, and trigger tissue repair and remodeling.Citation9,Citation10
During the proliferative phase, keratinocytes are stimulated by cytokines such as PDGF and TGF-β to promote epithelial regeneration. Fibroblasts secrete MMP to degrade the matrix and ECM such as collagen (Col) to repair the wound matrix.Citation11 In the remodeling stage, granulation tissue proliferates; fibroblasts differentiate into myofibroblasts; Col3 is replaced by Col1 in the ECM, and eventually, the mechanical function of the wound is repaired. However, functions such as hair follicle generation are difficult to recover.Citation12
Diabetic Wound Healing
There are many factors contributing to the failure of diabetic wound healing, such as hyperglycemia, chronic inflammation, microcirculation, macrocirculation dysfunction, hypoxia, autonomic, sensory neuropathy, and impaired neuropeptide signal transduction.Citation2 They impair wound healing by affecting the four stages and multiple pathways.
Hyperglycemia results in an accumulation of advanced glycation end products (AGEs). AGEs can reduce the phagocytic capacity of neutrophils and M1 macrophages, and prevent the transformation of M1 into M2.Citation13 In diabetic patients, pro-inflammatory cytokines in both the circulation and local wound are higher than those of normal people, indicating a state of chronic inflammation. Chronic inflammation also delays macrophage transition. Additionally, neutrophils produce massive reactive oxygen species and protease to ruin normal tissues. Fibroblasts and keratinocytes display promoted apoptosis, decreased proliferation, and migration ability,Citation14 with the levels of MMP and matrix degradation being upregulated.
Peripheral vascular disease diminishes the blood supply to the wound, reducing the recruitment of endothelial progenitor cells (EPCs), thereby impeding the formation of microvessels.Citation15 The insufficient blood supply induces local tissue hypoxia, oxidative stress formation, and increased free radicals generation, which causes further tissue damage. Neuropathy in the diabetic foot leads to the loss of protective sensation and a greater burden on the foot, which is another mechanism for impaired wound healing.Citation16 Once the wound forms, normal skin will release neuropeptides. Among them, What is the most well-known products are substance P, neuropeptide Y (NPY), and calcitonin gene-related peptide (CGRP) to improve healing.Citation17,Citation18 However, the secretion is dampened in diabetic scenario.Citation19
The Effect and Mechanism of Exosomes on Diabetic Wound Healing
Overview of Exosomes
Generally, there are two kinds of extracellular vesicles (EVs), ectosomes and exosomes. Ectosomes are vesicles budding from the cell membrane, producing some micro-vesicles or particles. There are also large vesicles with a diameter of 50 nm to 1 μm which ectosomes produce. Exosomes are derived from endosomes, and their diameter is generally 40–160nm (average 100nm).Citation20 There are still difficulties in the isolation, purification, and analysis of exosomes, so the extracted exosomes may not be pure. However, the biological functions that EVs want to achieve are very similar to those of exosomes, so they are also included in the scope of discussion.Citation21
The generation of exosomes is a multi-step process. First, the early endosomes are formed by budding inward of the plasma membrane. Then intraluminal vesicles (ILVs) can be produced similarly from the endosomal membrane. After transporting, several ILVs with their contents become mature multivesicular bodies (MVBs). These MVBs then fuse with the plasma membrane and release ILVs into the extracellular space, which are called exosomes.Citation22 The contents in exosomes are diverse such as proteins, metabolites, DNA, and RNA.Citation23–25 Different exosomes have certain heterogeneity according to the type or quantity of cargo they carry.Citation21
The other is that the origin of exosomes is different.Citation26 Proteins and RNA, DNA, etc. are packaged into exosomes. They can selectively induce specific signals to regulate the development and immune response of recipient cells.
Various MSCs release diverse exosomes. Bone marrow mesenchymal stem cells (BMSCs) are used the most commonly in clinical trials.Citation27 But they are somewhat difficult to obtain and are heterogeneous in the population. lncRNA and miR are one of the main contents of exosomes transported by BMSCs. They do not directly encode proteins but play important roles by regulating cell behavior.Citation28 Another type of MSCs isolated from adipose tissue called adipose-derived stem cells (ADSCs), has several advantages in the isolation process, including the abundance of their source tissue, and an easier and less invasive extraction procedure.Citation29 For example, infrapatellar fat pad-derived MSCs, a kind of ADSCs, are easier to isolate and have the ability to proliferate and differentiate into chondrocytes.Citation30 Therefore, ADSCs are also one of the commonly used stem cells in clinical trials. Although both of them can promote diabetic wound healing, Margherita Pomatto found that the functions of ADSC-Exos and BMSC-Exos are intersected. The molecules carried by ADSC-Exos are mainly related to angiogenesis, which can promote EC migration. BMSC-Exos mainly enhance cell proliferation and viability.Citation31 As a highly vascularized organ, the placenta is not only readily available but also ethically more favorable, with an abundant supply of MSCs. The placental mesenchymal stem cells (PMSCs) are more robust and have stronger proliferation ability than BMSCs, and have stronger long-term growth ability.Citation32,Citation33 Menstrual blood-derived mesenchymal stem cells (MenSCs) have shown higher proliferative capacity than BMSCs. Furthermore, collecting MSCs in menstrual blood is more readily available, non-invasive, and abundant.Citation34
There are also some less-studied sources of stem cells with interesting features. Compared with other MSCs, the hair follicle (HF)-derived mesenchymal stem cells (HFMSCs) have a large number of HFs that do not undergo functional and molecular changes in humans and are easy to collect, independent of gender and age.Citation35 Urine-derived MSCs (USCs) are also promising and can be collected by simple, safe, non-invasive, and low-cost methods. Purified USCs are easy to isolate and do not require enzymatic digestion. USCs have higher telomerase activity. That’s why USCs are capable of producing more cells, without teratoma or tumor.Citation36 Also, USCs are available regardless of sex, age, or health status. Gingival-derived mesenchymal stem cells (GMSCs) are abundant and easily obtained. It has a stronger proliferation ability and more stable morphology. Most importantly, GMSCs showed significant tissue regeneration potential and noteworthy immunomodulatory properties.Citation37 From the above overview, it is important and meaningful to classify and discuss exosomes from the perspective of their origin. All advantages and disadvantages of the MSC-Exos are summarized in .
Table 1 Comparison of Exosomes from Different Sources
In the experiments described in the literature, exosomes are often expressed from corresponding sources of MSCs in vitro. One difference is that some exosomes have predetermined contents before expression, while others undergo certain pre-processing after expression. In vitro, experiments usually employ human umbilical vein endothelial cells (HUVECs), and human dermal fibroblasts (HDF), to observe changes in proliferation, migration, and other cellular functions after exosome treatment. In vivo, experiments involve injecting exosomes into experimental animals such as mice and rats to evaluate their effect on wound healing by assessing macrophage phenotype switching, endothelial proliferation, fibroblast migration, vascular remodeling, collagen deposition, and other wound healing processes.
BMSC-Exos
BMSC is one of the most widely studied types of stem cells, which can differentiate into bone, cartilage, adipose, and other lineages. It is the most commonly used and the most classical MSCs.Citation38 Arsalan Shabbir found that BMSC-Exos could activate important signals like AKT, STAT3, and ERK in target cells. And then target cells will increase the expression of growth factors such as HGF, IL-6, IGF1, and SDF1 to promote epithelial regeneration and vascular reconstruction in diabetic wounds.Citation39
Non-Coding RNAs as Contents
Recent studies have revealed the possible pro-angiogenic function of lncRNAs, specifically HOX transcript antisense RNA (HOTAIR), in extracellular vesicle (EV)-mediated angiogenesis. In vitro, experiments showed that EVs overexpressing HOTAIR can upregulate VEGFA, while in vivo experiments, EVs not only accelerated wound healing but also increased the number of blood vessels in the healing tissue.Citation40,Citation41
Another study found that BMSC-Exos expressing Krüppel-like factor 3 antisense RNA 1 (KLF3-AS1) could reduce the downstream signal miR-383, and the VEGFA decreased, thereby reducing cell proliferation and migration. This study established an exosome KLF3-AS1/miR-383/VEGFA axis in BMSCs concerning diabetic wound angiogenesis.Citation42
Despite being modified by different exogenous factors, exosomes act on wound healing ultimately through similar pathways, among which PTEN/PI3K/AKT is one of the most studied and important. BMSC-exos carrying lncRNA H19 were shown to promote fibroblast migration, and proliferation and decrease apoptosis in the diabetic wound, through the miR-152-3p/PTEN/PI3K/AKT axis.Citation43 A report by the Jianing Ding group demonstrated that BMSC-Exos pretreated with deferoxamine (DFO) exhibits pro-angiogenic properties by miR-126.Citation44 The expression of miR-126 was significantly elevated in DFO-Exos, and miR-126 has been previously recognized to improve wound endothelial proliferation and vascular remodeling via CXCL12 upregulation.Citation45 Moreover, miR-126 can inhibit PTEN on ECs and enhance PI3K/AKT signaling consequently.Citation44
MiR-155 could delay diabetic wound healing by inhibiting FGF-7,Citation46 and worsen wound inflammation through CTLA4 and SOC1 signaling.Citation47 Experiments showed that BMSC-Exos loaded with miR-155 inhibitor lessened the level of miR-155, restore the level of FGF-7, and diminish the expression of pro-inflammatory factors.Citation48 Meanwhile, VEGFA was increased after exosome treatment, which ameliorate vascular reconstruction.
Other Contents
Nrf2
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an antioxidant protein transcription factor, preventing cells from oxidative damage.Citation49 In the study by the Lei Wang group, BMSC-Exos upregulated Nrf2 and downregulated TNF-α and IL-1β in diabetic wounds. Through knockout and activation of Nrf2, it demonstrates that exosomes overexpressing Nrf2 accelerate wound healing by endothelial progenitor cells tube formation.Citation50
PTEN/PI3K/AKT Pathway
The same pathway PTEN/PI3K/AKT acting on different cells (macrophages, ECs) will produce different effects. The normal human body under the same pathway can balance the pro and anti-inflammatory factors. Diabetic patients may be caused by the imbalance of the pathways leading to wound inflammation and prolonged wound healing.
ECs
The pro-angiogenic effect of PI3K/AKT signaling in ECs is inhibited by PTEN via reversing the phosphorylation of PI3K/AKT.Citation51 The downstream molecules of AKT, such as NO produced by eNOS, are closely related to cell proliferation, migration, and angiogenesis.Citation52,Citation53
BMSC-Exos pretreated with atorvastatin (ATV) improves the functions of ECs damaged by high glucose (HG) environment, such as proliferation, migration, tube formation, and VEGF secretion. BMSC-Exos also upregulate the relative expression of angiogenic-related genes (PDGF, bFGF, EGF, and ANG1).Citation54 Through down-regulating PTEN and relieving the inhibition of PI3K/AKT,Citation54 ATV-Exos significantly activate AKT/eNOS pathway; eNOS increased NO production, and protected ECs from apoptosis induced by HG environment.Citation55
Hu et alCitation56 pretreated BMSCs with pioglitazone (PGZ) and isolated PGZ-Exos. The mechanism of PGZ-Exos acts the same as ATV-Exos by PI3K/AKT/eNOS pathway.Citation56
Macrophages
The role of exosomes on macrophage is also partially mediated through PTEN/PI3K/AKT pathway. AKT is a key protein in promoting the M1 polarization of macrophages. Phosphorylation of AKT can promote M1 polarization of macrophages and inhibit M2 polarization, thereby promoting inflammatory response. Therefore, PTEN is required to inhibit the phosphorylation of AKT to inhibit macrophage M1 polarization and suppress inflammatory response.Citation57
In diabetic wounds, BMSC-Exos pretreated with melatonin (MT) significantly inhibited the secretion of IL-1β and TNF-α from macrophages and promoted the expression of IL-10 and Arg-1.Citation58 Mechanism analysis showed PTEN expression was increased, and MT potentiated this effect, thereby inhibiting AKT phosphorylation and promoting macrophage polarization toward M2.Citation58
In summary, BMSC-Exos carrying ncRNA or other contents can regulate inflammation and remodeling of wounds by influencing downstream cytokines such as VEGFA, NO, IL-1, etc through the PTEN/PI3K/AKT or other signaling pathways.
ADSC-Exos
ADSCs are known to secrete paracrine factors and exosomes to promote wound healing, which stimulates fibroblast migration, proliferation, and collagen synthesis.Citation59 ADSC-Exos are also rich in circRNAs, miRs, and lncRNAs. ROS is known to induce the secretion of inflammatory factors and the expression of various adhesion molecules and damage mitochondria by activating the NF-κB signaling pathway.Citation60,Citation61 ADSC-Exos can reduce ROS in cells and improve mitochondrial function under HG. It acts through the SIRT3/SOD2 axis: Increasing the expression of SIRT3 enhanced the activity of downstream superoxide dismutase 2 (SOD2), better-scavenging superoxide, maintaining the redox reaction balance of cells, and reduced the levels of TNF-α, IL-1, IL-6, ICAM-1, VCAM-1 and MCP1.Citation62
Non-Coding RNAs
CircRNA as one of the non-coding RNAs has a 5’-end and 3’ -end connected to form a complete circular structure, which is more stable than linear RNA. CircRNA-mmu_circ_0000250 modified ADSCs-Exos were found to repair EPCs under HG, augment SIRT1 expression through the absorption of miR-128-3p, and promote wound angiogenesis;Citation63 SIRT1 has a great effect on the activation of autophagy and has anti-inflammatory and antioxidant properties.Citation64 By high-throughput sequencing, Rongfeng Shi’s team found that overexpression of circ-Snhg11 in ADSC-Exos reversed EPC dysfunction and promoted M2 polarization of macrophages under HG conditions, by activating miR-144-3p /HIF-1α/VEGF or STAT3 signaling pathways.Citation65 Similarly, another research found that ADSC-Exos overexpressing mmu_circ_0001052 targeted the FGF4 site in DFU through the sponge effect of miR-106a-5p. p-p38 /p38 also increased at the same time. The mmu_circ_0001052+miR-106a-5p/FGF4/p38MAPK signal path was established, which became a potential therapeutic site.Citation66
Individual miRs are susceptible to hydrolysis, so Lv et alCitation67 used electroporation to transfer miR-21-5p into ADSC-Exos. The engineered exosomes were shown to up-regulate MMP-7 expression through the Wnt/β-catenin signaling pathway, thus facilitating the proliferation and migration of keratinocytes, angiogenesis, and collagen remodeling. This proves that exosomes produced by electroporation technology are effective in protecting miRs from hydrolysis, and have certain clinical application value.
lncRNAs have recently been reported to be significant in diabetic complications.Citation68 ADSC-Exos overexpressed lnc00511 can strongly repair EPCs in the HG setting and accelerate the healing of a diabetic wound by inhibiting the ubiquitination and degradation of Twist1.Citation69
Other Contents
Xue Li found that ADSC-Exos could reduce cellular senescence and oxidative stress, and also improve the tube formation ability of EPCs in diabetic patients. The mechanism is to increase the phosphorylation level of SMP30 and VEGFR2 and reduce intracellular ROS and inflammatory factors. When Nrf2 was overexpressed, the above effects were further enhanced and EPCs senescence was finally prevented. Therefore, ADSC-Exos can syndicate with Nrf2 to promote diabetic wound healing.Citation70
Hsu et al found that ADSC-Exos transport TGF-β1 can activate fibroblasts and that the activated fibroblasts produce TGF-β1 in an autocrine manner, leading to more fibroblast proliferation and activation. TGF-β1 is mainly involved in the cell crosstalk mediated by diabetic ADSC-Exos, which is an important early response to initiate wound regeneration.Citation71
There are more ncRNAs carried by ADSC-Exos compared with BMSC-Exos, and the signal pathways are more diverse, such as HIF-1α/VEGF, STAT3, NF-κB, FGF4/p38MAPK and Wnt/β-catenin. Finally, they regulate cytokines and reduce ROS to promote wound healing.
PMSC-Exos
The placenta, as a transient maternal organ, would be disposed of after delivery. Thus, no invasive procedures exist during PMSC extraction. Since the placenta is a highly vascularized organ, it is reasonable to assume that PMSCs have more angiogenic features.Citation72 PMSC includes MSCs derived from different sections of the placenta, such as human amniotic epithelial cells (hAECs), human umbilical cord mesenchymal stem cells (HUCMSCs), and so on.
hAECs
Pei Wei and the group investigated the effects of hAECs-Exos, products of paracrine action from hAECs, on human fibroblasts (HFB) and HUVECs. Experiments in vitro demonstrate hAECs-Exos enhance the proliferation and migration of HFBs and angiogenic activity of HUVECs. When they used high-throughput sequencing, they found that the PI3K-AKT signaling pathway was highly enriched, again focusing on the PI3K-AKT pathway we described previously.Citation73 PI3K-AKT-mTOR pathway plays an important role in promoting angiogenesis and fibroblast function.Citation74,Citation75
HUCMSCs
HUCMSC-Exos have been shown to act on different stages of wound healing: in the inflammatory phase, significantly reduced inflammatory cell infiltration; The proliferative and remodeling phases promote the proliferation of fibroblast cells and vascular ECs,Citation76 and regenerative epithelialization, collagen deposition, and ECM remodeling.Citation77,Citation78
HUCMSC-Exos released after LPS stimulation was found to promote M2 polarization of macrophages. Microarray analysis identified that the treated exosomes carry a specific marker for let-7b, which is speculated to regulate the phenotypic transformation of macrophages by negatively regulating TLR4 expression.Citation79 There are two downstream signals of TLR4: NF-κB can activate the inflammatory response, and STAT3 as a transcriptional repressor can prevent the excessive inflammatory response driven by TLR4.Citation80,Citation81 STAT3 pathway was found to be activated in this experiment. The AKT pathway has been reported to inhibit TLR4/NF-κB activation and subsequent inflammatory responses;Citation82 STAT3 can induce AKT activation to affect immune homeostasis and regulate cell differentiation.Citation83 Ti et alCitation84 also demonstrated that the AKT pathway was highly activated in THP-1 after LPS-pretreated HUCMSC-Exos. Therefore, they suggested that the TLR4/NF-κB/STAT3/AKT is an important way for let-7b on LPS pre-Exos to regulate macrophage polarization and promote wound healing.
The two kinds of PMSC-Exos signal paths have similarities in some links, but they affect wound healing in different stages. hAECs-Exos promote angiogenesis and fibroblast function by PI3K-AKT-mTOR axis, while HUCMSC-Exos regulate macrophage polarization by TLR4/NF-κB/STAT3/AKT axis.
MenSC-Exos
MenSCs have good proliferation and regeneration ability, and it has been shown that MenSCs are pluripotent and can successfully differentiate into mesoderm and ectoderm in vitro.Citation85 A recent study investigated the effects of MenSCs-derived exosomes (MenSC-Exos) on diabetic wounds.Citation86 During the inflammation phase, they found that MenSC-Exos could induce macrophage M1 to M2 polarization. The activity of M1 marker iNOS decreases while the ARG/iNOS ratio increases. The expression of the Rela gene in exosomes is increased, and Rela is a member of the NF-κB family, so it may activate the proliferation and migration of epithelial cells by activating the NF-κB to enhance re-epithelialization. In addition, no corresponding effect was found in MenSC alone, but exosomes alone could cause changes, and the angiogenesis effect was more significant than that of other exosomes.Citation59,Citation87 As for the remodeling stage, in the early, MenSC-Exos promotes the expression of collagen I mRNA and increase the wound closure rate. Late in the repair phase, the expression of collagen III mRNA increases significantly and the Col1:Col3 ratio decreases rapidly, resulting in the reduction of scar.Citation86
HFMSC-Exos
There is a variety of stem cells in the hair follicle, including melanocytes, ECs, and MSCs, corresponding to hair growth, skin repair, and hair follicle repair functions.Citation88,Citation89 Exosomes derived from hair follicle MSCS (HFMSC-Exos) are not quite distinct from classical ADSC-Exos in morphology and protein labeling. Functionally, both Exos increased the proliferation and migration of HDF and angiogenesis of HUVECs. In addition, HFMSC-Exos are also able to protect HDF from apoptosis and oxidative stress under HG conditions, while the mechanism remains unknown.Citation35
USC-Exos
Chun-Yuan Chen and the team studied human urine-derived stem cells (USCs) and exosomes (USC-Exos).Citation36 It was found that USC-Exos could effectively promote the proliferation and migration of fibroblasts, and the angiogenesis of ECs. In particular, USC-Exos expressed a lot of DMBT1. Further analysis indicated that DMBT1 was important in ECs angiogenesis induced by USC-Exos.Citation36 They found that enriched USC-Exos could significantly increase VEGF-A protein level and AKT phosphorylation, suggesting that it may be useful in vascular reconstruction and endothelial repair through PI3K/AKT and VEGFA.Citation90,Citation91
SMSC-Exos
MSCs isolated from synovium, termed synovial MSCs (SMSC), possess tissue specificity: SMSCs have chondrogenic differentiation potential but do not show adipogenic or osteogenic potential.Citation92,Citation93 Shi-Cong Tao used gene overexpression techniques to overexpress miR-126-3p in SMSCs,Citation94 which endows SMSCs with angiogenic functions. After biomaterial loading, the PI3K/AKT and MAPK/ERK pathways were activated to promote the migration of HDF and human dermal microvascular endothelial cells (HMEC-1) and the formation of the capillary network, which played the function of miR-126-3p.Citation95
Combination of Exosome and Biomaterials
There are still certain problems with exosomes for actual clinical use, for example, exosomes may not be stable in the body. Studies have shown that exosomes isolated from B16-BL6 murine melanoma cells have a half-life of only 2 minutes after intravenous injection into mice,Citation96 which may occur to other exosomes. Therefore, combining exosomes with biomaterials may improve this shortcoming. Using biological materials can maintain the biological activity of exosomes and control the release of effective concentrations so that exosomes can act more gently and long-term on the wound.
BMSC
Composite hydrogels, such as chitosan (CS), can be well used for the healing of diabetic wounds. CS and its derivatives have good biocompatibility, biodegradability, and non-toxicity properties and are of extensive use in medicine.Citation97,Citation98 The study by Xinrong Geng reported for the first time the effect of BMSC-Exos loaded carboxyethyl chitosan (CEC) -dialdehyde carboxymethyl cellulose (DCMC) hydrogel on promoting diabetic wound healing in rats.Citation99 The composite hydrogel not only promoted angiogenesis and endothelial proliferation but also induced the generation of M2 macrophages and promoted the anti-inflammatory effect. It not only maintains the required morphological strength and mechanical strength of wound dressings but also shows strong antibacterial and hemostatic effects. It has great application value for the treatment of diabetic wounds.Citation99
ADSC
Biomaterials loaded with ADSC-Exos include some scaffolds, biological dressings, nanomaterials, etc. The research types are more diverse than those of BMSC-Exos. Clinical trials have confirmed that the human acellular amniotic membrane (hAAM) is a good wound dressing, along with a certain effect on wound healing as a stem cell scaffold.Citation100,Citation101 Xiao et al prepared hAAM loaded with ADSC-Exos in vitro and transplanted them into a mouse diabetic model, and observed more macrophages transforming to M2 phenotype, reduced inflammatory cell infiltration, more collagen deposition, and microvessel formation on the wound surface.Citation102
Shiekh et al reported OxOBand, an oxygen-releasing antioxidant wound dressing composed of antioxidant polyurethane (PUAO) and loaded with ADSC-Exos. The Oxoband-bound exosomes can also promote wound healing in diabetic wounds and wounds infected with Staphylococcus aureus and Pseudomonas aeruginosa.Citation103
Shi et al used microfluidic techniques to prepare gel microspheres loaded with ADSCs. It shows good biocompatibility and adaptive biodegradation rates in animal models. Although this method is loaded with ADSCs, studies have shown that it can improve paracrine effects, and ADSCs play their role in wound repair through exosomes.Citation104
Hydrogels are also commonly used to load ADSC-Exos. Wang et al developed a novel FHE hydrogel loaded with exosomes to compose FHE@Exos hydrogel. The hydrogel is composed of Pluronic F127 (F127), oxidized hyaluronic acid (OHA), and EPL, and has various properties such as inject-ability, self-healing, antibacterial activity, and response to exosome release when stimulated. The FHE@Exos hydrogel has a better effect on diabetic wounds than the simple exosome or FHE hydrogel, suggesting that FHE@Exos has a synergistic effect.Citation105 Shilan Shafei used alginate saline gel loaded with exosomes secreted by ADSCs as a bioactive scaffold to promote wound healing. Alginate has high biocompatibility, biodegradability, non-actionability, and high water adsorption performance.Citation106 Tao Jiang developed a smart hydrogel loaded with ADSC-Exos using matrix metalloproteinase-degrading polyethylene glycol (MMP-PEG).Citation107 Although this has certain clinical application value, its long-term therapeutic value needs to be evaluated due to its longevity.
PMSC
The most common biomaterials loaded with HUCMSC are also hydrogels. Jiayi Yang used a unique thermosensitive hydrogel Pluronic F-127 (PF-127). Moreover, it has a porous structure and can prolong the release time of exosomes. PF-127 has been proven to be an ideal exosome-loaded material in terms of biological function and physical properties and has the potential for clinical treatment.Citation108 Yiyao Zhang determined the efficacy of HUCMSCs-Exos surrounded by polyvinyl alcohol/alginate nano hydrogel for the treatment of diabetic wounds.Citation109
GMSC
Shi et al isolated gingival MSC (GMSC) from gingival connective tissue, and loaded exosomes secreted by GMSC into chitosan/silk-based hydrogel. CS has been introduced before. Silk, as a rare biomaterial, can not only promote cell function but also enhance the mechanical strength of hydrogels.Citation110 Quan Shi demonstrated that this hydrogel-loaded GMSC-Exos improved the microenvironment of wound healing and promoted diabetic wound healing by promoting the regeneration, deposition, and remodeling of ECM, microvascular remodeling, and the growth of damaged neurons.Citation37
SMSC
Tao et alCitation95 combined exosomes of SMSC overexpressing miR-126-3p with chitosan materials. They found that using exosomes-CS was able to accelerate regenerating epithelium in vivo, activate angiogenesis, and promote collagen maturation. This underscores the high clinical value of endowing the cellular functions of hard-to-obtain cells to more common cells and exercising them through their exosomes for cell-free therapies. Min Li’s group combined SMSC-Exos overexpressing miR-126-3p with hydroxyapatite/chitosan (HAP/CS) composite hydrogel.Citation111 HAP can induce angiogenesis after implantation;Citation112 Ca2+ ions released from HAP can regulate skin homeostasis and proliferation and differentiation of keratinocytes.Citation111 The exosomes combined with HAP/CS can also promote wound healing through epithelial regeneration, vascular reconstruction, and collagen deposition. All biomaterials are generalized and can be seen in .
Table 2 Types of Biomaterial Combined with Exosome
Conclusion
Abnormal wound healing is one of the serious complications of diabetic patients, which causes a large amount of economic and medical burden worldwide. Wound healing in normal people involves four stages hemostasis, inflammation, proliferation, and remodeling. These four stages influence diabetic wound healing through various factors and mechanisms such as AGEs and neuropathy. Traditional methods have little effect. As a new type of therapy, stem cells can repair wound tissue through exosomes secreted by them. As a kind of extracellular vesicle, exosomes carry proteins, nucleic acids, and other cargo, carry out cellular crosstalk, and exert biological effects. In this review, starting with the stem cell source of exosomes, the exosomes secreted by different stem cells are classified, and their contents and the mechanism of promoting wound healing are described respectively. Common stem cell exosomes are BMSC-Exos, ADSC-Exos, PMSC-Exos, MenSC-Exos, and other cell-Exos. Different exosomes play different roles in wound repair through themselves or changes after being stimulated by some drugs, but all of them intervene in four stages: reducing the inflammatory response, promoting angiogenesis, promoting epithelial growth, and promoting scar formation. In addition, this review also introduces some studies on biomaterials loaded with exosomes for the treatment of diabetic wounds. Biomaterials represented by hydrogels have good compatibility with wounds and synergistic effects on the efficacy of exosomes, embodying certain clinical values. Besides, there are some expecting green biomaterials, like curcumin-containing nano scaffolds which promote osteogenic differentiation of MSCs;Citation113 and silver nanoparticles synthesized by olive leaf extract.Citation114 The entire review presented numerous advances in the in vitro and in vivo experiments of exosomes extracted from different sources of MSCs, giving us hope for the treatment of diabetic wounds. However, only qualitative effects were observed in the in vitro experiments mentioned in the article, and the in vivo experiments were only conducted in experimental animals. Even though biological materials can compensate for the shortcoming of exosomes, their use as foreign substances raises questions about the immunoreaction by the body. Therefore, these studies still have a long way to go before they can be applied clinically.
At present, there is no good method for diabetic wounds. Although stem cell research has been started for a long time, the progress is not smooth considering the histocompatibility. The cell-free therapy of exosomes is an upgraded version of stem cell therapy, with no host rejection and better efficacy. Due to the effective promotion of wound healing from multiple aspects by exosome therapy, which is in contrast to traditional therapy that only protects the wound and relies on the body’s self-healing ability, it can effectively replace traditional therapies. The application of biomaterials has made the action time of exosomes longer and the therapeutic effect better, which is expected to truly implement exosome therapy in clinical practice. Although there are some limitations, exosome therapy remains the most promising approach for diabetic wound healing, especially when combined with biomaterials. Future exosome therapy is expected to achieve controllability in terms of exosome release and action time, by improving the performance of biomaterials. At the same time, some novel drugs or molecules may also be loaded into exosomes as carriers, allowing targeted delivery of drugs to the wound site, and achieving therapeutic effects with the lowest possible drug dosage. With the deepening of research on exosomes, it will have great clinical application value for the treatment of diabetic wounds.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The work is supported by the National Natural Science Foundation of China (82102339) and Hunan Health Commission Research Program (B202304138795) to Hongbo Xu and College Students’ Innovative Entrepreneurial Training Plan Program of the Third Xiangya Hospital of Central South University [grant number 20220035020041].
References
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi:10.1016/s0140-6736(05)67698-2
- Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31(8):817–836. doi:10.1007/s12325-014-0140-x
- Lopes L, Setia O, Aurshina A, et al. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther. 2018;9(1):188. doi:10.1186/s13287-018-0938-6
- Taverna S, Pucci M, Alessandro R. Extracellular vesicles: small bricks for tissue repair/regeneration. Ann Trans Med. 2017;5(4):83. doi:10.21037/atm.2017.01.53
- An Y, Lin S, Tan X, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi:10.1111/cpr.12993
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9). doi:10.3390/ijms18091852
- Rani M, Nicholson SE, Zhang Q, Schwacha MG. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns. 2017;43(2):297–303. doi:10.1016/j.burns.2016.10.001
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi:10.1111/j.1524-475X.2008.00410.x
- Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi:10.1016/j.ejphar.2020.173090
- den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing. Transl Res. 2019;204:39–50. doi:10.1016/j.trsl.2018.10.001
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi:10.2741/1184
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–503. doi:10.1002/path.1427
- Aitcheson SM, Frentiu FD, Hurn SE, Edwards K, Murray RZ. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. 2021;26(16). doi:10.3390/molecules26164917
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. doi:10.1172/jci32169
- Tecilazich F, Dinh TL, Veves A. Emerging drugs for the treatment of diabetic ulcers. Expert Opin Emerg Drugs. 2013;18(2):207–217. doi:10.1517/14728214.2013.802305
- Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med. 2021;13(585). doi:10.1126/scitranslmed.abe4839
- Ekstrand AJ, Cao R, Bjorndahl M, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA. 2003;100(10):6033–6038. doi:10.1073/pnas.1135965100
- Toda M, Suzuki T, Hosono K, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62(6):352–359. doi:10.1016/j.biopha.2008.02.003
- Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. doi:10.1017/s1462399409000945
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–372. doi:10.1016/j.tcb.2015.01.004
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:6478. doi:10.1126/science.aau6977
- Soltani S, Mansouri K, Parvaneh S, Thakor AS, Pociot F, Yarani R. Diabetes complications and extracellular vesicle therapy. Rev Endocr Metab Disord. 2022;23(3):357–385. doi:10.1007/s11154-021-09680-y
- Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688–692. doi:10.1016/j.jmb.2015.09.019
- Pathan M, Fonseka P, Chitti SV, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516–d519. doi:10.1093/nar/gky1029
- Xu YX, Pu SD, Li X, et al. Exosomal ncRNAs: novel therapeutic target and biomarker for diabetic complications. Pharmacol Res. 2022;178:106135. doi:10.1016/j.phrs.2022.106135
- Wen SW, Lima LG, Lobb RJ, et al. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics. 2019;19(8):e1800180. doi:10.1002/pmic.201800180
- Álvarez-Viejo M. Mesenchymal stem cells from different sources and their derived exosomes: a pre-clinical perspective. World J Stem Cells. 2020;12(2):100–109. doi:10.4252/wjsc.v12.i2.100
- Xue M, Zhuo Y, Shan B. MicroRNAs, long noncoding RNAs, and their functions in human disease. Methods Mol Biol. 2017;1617:1–25. doi:10.1007/978-1-4939-7046-9_1
- Bochon B, Kozubska M, Surygała G, et al. Mesenchymal stem cells-potential applications in kidney diseases. Int J Mol Sci. 2019;20(10). doi:10.3390/ijms20102462
- Vahedi P, Moghaddamshahabi R, Webster TJ, et al. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: a review. Int J Mol Sci. 2021;22(17). doi:10.3390/ijms22179215
- Pomatto M, Gai C, Negro F, et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int J Mol Sci. 2021;22(8):8. doi:10.3390/ijms22083851
- Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17(6):1095–1107. doi:10.1089/scd.2007.0154
- Mathew SA, Naik C, Cahill PA, Bhonde RR. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol Life Sci. 2020;77(2):253–265. doi:10.1007/s00018-019-03268-1
- Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6(1):32. doi:10.1186/s13287-015-0013-5
- Las Heras K, Royo F, Garcia-Vallicrosa C, et al. Extracellular vesicles from hair follicle-derived mesenchymal stromal cells: isolation, characterization and therapeutic potential for chronic wound healing. Stem Cell Res Ther. 2022;13(1):147. doi:10.1186/s13287-022-02824-0
- Chen CY, Rao SS, Ren L, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8(6):1607–1623. doi:10.7150/thno.22958
- Shi Q, Qian Z, Liu D, et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904. doi:10.3389/fphys.2017.00904
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi:10.1126/science.276.5309.71
- Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. doi:10.1089/scd.2014.0316
- Born LJ, Chang KH, Shoureshi P, et al. HOTAIR-loaded mesenchymal stem/stromal cell extracellular vesicles enhance angiogenesis and wound healing. Adv Healthc Mater. 2022;11(5):e2002070. doi:10.1002/adhm.202002070
- Lamichhane TN, Leung CA, Douti LY, Jay SM. Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles via regulation of MicroRNAs and long non-coding RNAs. Sci Rep. 2017;7(1):13794. doi:10.1038/s41598-017-14356-2
- Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX, Xu HL. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract. 2022;183:109126. doi:10.1016/j.diabres.2021.109126
- Li B, Luan S, Chen J, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814–826. doi:10.1016/j.omtn.2019.11.034
- Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019;2019:9742765. doi:10.1155/2019/9742765
- Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81. doi:10.1126/scisignal.2000610
- Moura J, Sørensen A, Leal EC, et al. microRNA-155 inhibition restores fibroblast growth factor 7 expression in diabetic skin and decreases wound inflammation. Sci Rep. 2019;9(1):5836. doi:10.1038/s41598-019-42309-4
- Petkovic M, Sørensen AE, Leal EC, Carvalho E, Dalgaard LT. Mechanistic actions of microRNAs in diabetic wound healing. Cells. 2020;9(10). doi:10.3390/cells9102228
- Gondaliya P, Sayyed AA, Bhat P, et al. Mesenchymal stem cell-derived exosomes loaded with miR-155 inhibitor ameliorate diabetic wound healing. Mol Pharm. 2022;19(5):1294–1308. doi:10.1021/acs.molpharmaceut.1c00669
- He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21(13). doi:10.3390/ijms21134777
- Wang L, Cai Y, Zhang Q, Zhang Y. Pharmaceutical activation of Nrf2 accelerates diabetic wound healing by exosomes from bone marrow mesenchymal stem cells. Int J Stem Cells. 2022;15(2):164–172. doi:10.15283/ijsc21067
- Tsugawa K, Jones MK, Sugimachi K, Sarfeh IJ, Tarnawski AS. Biological role of phosphatase PTEN in cancer and tissue injury healing. Front Biosci. 2002;7:e245–51. doi:10.2741/tsugawa
- Zhang W, Bai X, Zhao B, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370(2):333–342. doi:10.1016/j.yexcr.2018.06.035
- Chen YG, Li Z, Wang XF. Where PI3K/Akt meets Smads: the crosstalk determines human embryonic stem cell fate. Cell Stem Cell. 2012;10(3):231–232. doi:10.1016/j.stem.2012.02.008
- Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350. doi:10.1186/s13287-020-01824-2
- Zhang Y, Liu NM, Wang Y, Youn JY, Cai H. Endothelial cell calpain as a critical modulator of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(6):1326–1335. doi:10.1016/j.bbadis.2017.03.021
- Hu Y, Tao R, Chen L, et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021;19(1):150. doi:10.1186/s12951-021-00894-5
- Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198(3):1006–1014. doi:10.4049/jimmunol.1601515
- Liu W, Yu M, Xie D, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):259. doi:10.1186/s13287-020-01756-x
- Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993. doi:10.1038/srep32993
- Deng L, Du C, Song P, et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev. 2021;2021:8852759. doi:10.1155/2021/8852759
- Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10(5):339–352. doi:10.1016/0891-5849(91)90040-a
- Zhang Y, Bai X, Shen K, et al. Exosomes derived from adipose mesenchymal stem cells promote diabetic chronic wound healing through SIRT3/SOD2. Cells. 2022;11(16). doi:10.3390/cells11162568
- Shi R, Jin Y, Hu W, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318(5):C848–c856. doi:10.1152/ajpcell.00041.2020
- Pires Da Silva J, Monceaux K, Guilbert A, et al. SIRT1 protects the heart from ER stress-induced injury by promoting eEF2K/eEF2-dependent autophagy. Cells. 2020;9(2). doi:10.3390/cells9020426
- Shi R, Jin Y, Zhao S, Yuan H, Shi J, Zhao H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed Pharmacother. 2022;153:113463. doi:10.1016/j.biopha.2022.113463
- Liang ZH, Pan NF, Lin SS, et al. Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther. 2022;13(1):336. doi:10.1186/s13287-022-03015-7
- Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 2020;17(5):1723–1733. doi:10.1021/acs.molpharmaceut.0c00177
- Goyal N, Kesharwani D, Datta M. Lnc-ing non-coding RNAs with metabolism and diabetes: roles of lncRNAs. Cell Mol Life Sci. 2018;75(10):1827–1837. doi:10.1007/s00018-018-2760-9
- Qiu J, Shu C, Li X, Ye C, Zhang WC. Exosomes from linc00511-overexpressing ADSCs accelerates angiogenesis in diabetic foot ulcers healing by suppressing PAQR3-induced Twist1 degradation. Diabetes Res Clin Pract. 2021;180:109032. doi:10.1016/j.diabres.2021.109032
- Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14. doi:10.1038/s12276-018-0058-5
- Hsu HH, Wang AYL, Loh CYY, Pai AA, Kao HK. Therapeutic potential of exosomes derived from diabetic adipose stem cells in cutaneous wound healing of db/db mice. Pharmaceutics. 2022;14(6):Jun. doi:10.3390/pharmaceutics14061206
- Chen CY, Liu SH, Chen CY, Chen PC, Chen CP. Human placenta-derived multipotent mesenchymal stromal cells involved in placental angiogenesis via the PDGF-BB and STAT3 pathways. Biol Reprod. 2015;93(4):103. doi:10.1095/biolreprod.115.131250
- Wei P, Zhong C, Yang X, et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8:tkaa020. doi:10.1093/burnst/tkaa020
- Zhang E, Gao B, Yang L, Wu X, Wang Z. Notoginsenoside Ft1 promotes fibroblast proliferation via PI3K/Akt/mTOR signaling pathway and benefits wound healing in genetically diabetic mice. J Pharmacol Exp Ther. 2016;356(2):324–332. doi:10.1124/jpet.115.229369
- Zhang J, Chen C, Hu B, et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 2016;12(12):1472–1487. doi:10.7150/ijbs.15514
- Hade MD, Suire CN, Mossell J, Suo Z. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42(6):2102–2125. doi:10.1002/med.21918
- Teng L, Maqsood M, Zhu M, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate diabetic wound healing via promoting M2 macrophage polarization, angiogenesis, and collagen deposition. Int J Mol Sci. 2022;23(18). doi:10.3390/ijms231810421
- Yan C, Xv Y, Lin Z, et al. Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis. Front Bioeng Biotechnol. 2022;10:829868. doi:10.3389/fbioe.2022.829868
- Bao MH, Feng X, Zhang YW, Lou XY, Cheng Y, Zhou HH. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int J Mol Sci. 2013;14(11):23086–23102. doi:10.3390/ijms141123086
- Gao S, Mao F, Zhang B, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med. 2014;239(3):366–375. doi:10.1177/1535370213518169
- Taetzsch T, Levesque S, McGraw C, et al. Redox regulation of NF-κB p50 and M1 polarization in microglia. Glia. 2015;63(3):423–440. doi:10.1002/glia.22762
- Rao J, Qian X, Li G, et al. ATF3-mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. Am J Transplant. 2015;15(1):76–87. doi:10.1111/ajt.12954
- Ke B, Shen XD, Ji H, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56(2):359–366. doi:10.1016/j.jhep.2011.05.023
- Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi:10.1186/s12967-015-0642-6
- Allickson JG, Sanchez A, Yefimenko N, Borlongan CV, Sanberg PR. Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. Open Stem Cell J. 2011;3(2011):4–10. doi:10.2174/1876893801103010004
- Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–568. doi:10.1002/term.2799
- Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129(11):2182–2189. doi:10.1242/jcs.170373
- Martino PA, Heitman N, Rendl M. The dermal sheath: an emerging component of the hair follicle stem cell niche. Exp Dermatol. 2021;30(4):512–521. doi:10.1111/exd.14204
- Wang B, Liu XM, Liu ZN, et al. Human hair follicle-derived mesenchymal stem cells: isolation, expansion, and differentiation. World J Stem Cells. 2020;12(6):462–470. doi:10.4252/wjsc.v12.i6.462
- Al-Awqati Q. Terminal differentiation in epithelia: the role of integrins in hensin polymerization. Annu Rev Physiol. 2011;73:401–412. doi:10.1146/annurev-physiol-012110-142253
- Renner M, Bergmann G, Krebs I, et al. DMBT1 confers mucosal protection in vivo and a deletion variant is associated with Crohn’s disease. Gastroenterology. 2007;133(5):1499–1509. doi:10.1053/j.gastro.2007.08.007
- Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18(4):301–311. doi:10.1089/ten.TEB.2012.0002
- He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15(12):3809–3821. doi:10.1089/ten.TEA.2009.0188
- Qu K, Wang Z, Lin XL, Zhang K, He XL, Zhang H. MicroRNAs: key regulators of endothelial progenitor cell functions. Clin Chim Acta. 2015;448:65–73. doi:10.1016/j.cca.2015.06.017
- Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2017;6(3):736–747. doi:10.5966/sctm.2016-0275
- Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84. doi:10.1016/j.jbiotec.2013.03.013
- Naseri-Nosar M, Ziora ZM. Wound dressings from naturally-occurring polymers: a review on homopolysaccharide-based composites. Carbohydr Polym. 2018;189:379–398. doi:10.1016/j.carbpol.2018.02.003
- Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9(7):857–879. doi:10.1586/eri.11.59
- Geng X, Qi Y, Liu X, Shi Y, Li H, Zhao L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater Adv. 2022;133:112613. doi:10.1016/j.msec.2021.112613
- Wu Z, Liu X, Yuan D, Zhao J. Human acellular amniotic membrane is adopted to treat venous ulcers. Exp Ther Med. 2018;16(2):1285–1289. doi:10.3892/etm.2018.6331
- Zhou H, Wang L, Zhang C, et al. Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane. Stem Cell Res Ther. 2019;10(1):155. doi:10.1186/s13287-019-1234-9
- Xiao S, Xiao C, Miao Y, et al. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12(1):255. doi:10.1186/s13287-021-02333-6
- Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020. doi:10.1016/j.biomaterials.2020.120020
- Shi M, Gao Y, Lee L, et al. Adaptive gelatin microspheres enhanced stem cell delivery and integration with diabetic wounds to activate skin tissue regeneration. Front Bioeng Biotechnol. 2022;10:813805. doi:10.3389/fbioe.2022.813805
- Wang C, Wang M, Xu T, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi:10.7150/thno.29766
- Shafei S, Khanmohammadi M, Heidari R, et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J Biomed Mater Res A. 2020;108(3):545–556. doi:10.1002/jbm.a.36835
- Jiang T, Liu S, Wu Z, et al. ADSC-exo@MMP-PEG smart hydrogel promotes diabetic wound healing by optimizing cellular functions and relieving oxidative stress. Mater Today Bio. 2022;16:100365. doi:10.1016/j.mtbio.2022.100365
- Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine. 2020;15:5911–5926. doi:10.2147/ijn.S249129
- Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021;120:111671. doi:10.1016/j.msec.2020.111671
- Kapoor S, Kundu SC. Silk protein-based hydrogels: promising advanced materials for biomedical applications. Acta Biomater. 2016;31:17–32. doi:10.1016/j.actbio.2015.11.034
- Li M, Ke QF, Tao SC, Guo SC, Rui BY, Guo YP. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B. 2016;4(42):6830–6841. doi:10.1039/c6tb01560c
- Meagher MJ, Weiss-Bilka HE, Best ME, Boerckel JD, Wagner DR, Roeder RK. Acellular hydroxyapatite-collagen scaffolds support angiogenesis and osteogenic gene expression in an ectopic murine model: effects of hydroxyapatite volume fraction. J Biomed Mater Res A. 2016;104(9):2178–2188. doi:10.1002/jbm.a.35760
- Khezri K, Maleki Dizaj S, Rahbar Saadat Y, et al. Osteogenic differentiation of mesenchymal stem cells via curcumin-containing nanoscaffolds. Stem Cells Int. 2021;2021:1520052. doi:10.1155/2021/1520052
- Ramazanli V, Ahmadov I. Synthesis of silver nanoparticles by using extract of olive leaves; 2022.
