Abstract
Introduction
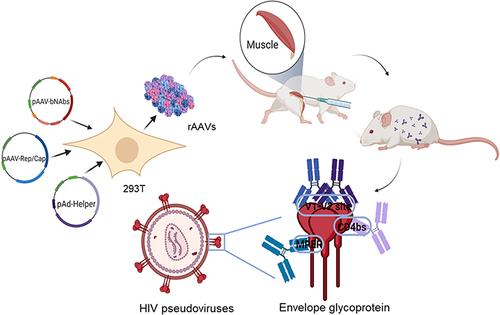
Broadly neutralizing antibodies (bNAbs) have the ability to neutralize a considerable breadth of genetically diverse human immunodeficiency virus (HIV) strains. Passive immunization can potentially provide protection against HIV infection in animal models. However, the direct antibody infusion effect is limited due to the short half-life and deficient immunogenicity of the antibody. As an alternative strategy, we propose the use of nano viral vectors, specifically the adeno-associated virus (AAV), to continuously and systematically produce bNAbs against HIV.
Methods
Plasmids expressing bNAbs PG9, PG16, 10E8, and NIH45-46 antibodies were constructed, targeting three different epitopes of HIV. Additionally, the bNAbs gene mediated by rAAV8 was administered to generate long-term expression with a single injection. We established both single and combined immunization groups. The neutralizing activity of antibodies expressed in mice sera was subsequently evaluated.
Results
The expression of bNAbs in BALB/c mice can last for >24 weeks after a single intramuscular injection of rAAV8. Further studies show that neutralization of the HIV pseudovirus by sera from co-immunized mice with rAAV8 expressing 10E8 and PG16 was enhanced compared with mice immunized with 10E8 or PG16 alone.
Conclusion
The prolonged expression of neutralizing antibodies can be maintained over long periods in BALB/c mice. This combined immunization is a promising candidate strategy for HIV treatment.
Introduction
The envelope glycoprotein (Env) of the human immunodeficiency virus (HIV) is the only virus-encoded protein present on the virion surface. The precursor of HIV Env is gp160, which is cleaved by furin into a heterodimer consisting of surface glycoprotein gp120 and transmembrane glycoprotein gp41.Citation1 Functional Env manifests as a trimer of gp120-gp41 heterodimers on the surface of infectious virions.Citation2 gp120 binds to cellular proteins CD4 and CCR5/CXCR4, whereas gp41 mediates the fusion between the virus and the host cell membrane.Citation3
Broadly neutralizing antibodies (bNAbs) are capable of recognizing a myriad of HIV-1 strains, including those less susceptible to surface alterations, and effectively decrease HIV-1 levels in the human body by inhibiting their replication.Citation4 These antibodies play a crucial role in preventing the virus from invading target cells, finalizing intracellular assembly, and releasing new viral particles. Furthermore, they increase viral clearance via phagocytosisCitation5 and eradicate infected cells through an FcγR-dependent mechanism.Citation6 Neutralizing antibodies also inhibit HIV-1 cell-to-cell spread.Citation7 The analysis of these antibodies revealed four major vulnerable sites of the Env virus, namely, the CD4 binding site (CD4bs),Citation8 gp120 interface trimerization glycan-dependent epitopes on variable loop 1 and variable loop 2 (V1/V2) on body apexCitation9 or variable loop 3 (V3),Citation10 and the membrane proximal outer region (MPER) of gp41.Citation11
Long-term treatment of HIV-1 infection necessitates repetitive injections due to the antibodies’ short half-life within the body.Citation12 Adeno-associated virus (AAV) vectors exhibit potential as gene vectors for treating HIV-1 infection owing to their desirable gene delivery properties, nonpathogenic nature, and lack of immunotoxicity.Citation13,Citation14 Moreover, utilizing the self-complementary double-stranded DNA form of the vector genome facilitates faster expression dynamics.Citation15 AAV vectors can be further optimized by engineering and fine-tuning viral capsids to enhance their transmission properties.Citation16 To date, at least 18 preclinical and clinical rAAV studies have been conducted related to HIV-1 research.Citation17 Nevertheless, the limited breadth and potency of bNAbs, along with viral drug resistance, impede their clinical application.Citation8 To overcome these limitations, combined immunization employing multiple antibodies targeting diverse areas could yield superior results.Citation18 In our previous study, we used rAAV8 to carry two anti-HIV bNAbs for the passive co-immunization of mice, leading to an elevated neutralization rate of HIV-1 strains compared to the injection of rAAV8 antibodies expressing 10E8 and NIH45-46.Citation19
In this study, we employ four types of rAAV8 to transfer bNAbs targeting distinct regions of HIV Env, including 10E8 against gp41 MPER, NIH45-46 against gp120 CD4, and PG9 and PG16 against gp120 V1/V2. After evaluating HIV-1 antibodies in 293T cells, we measure the biological functions of antibodies in mice. Furthermore, we examine the effects of joint targeting of Env through combined immunization to achieve superior neutralization.
Material and Methods
Construction of Recombinant Adeno-Associated Virus 8 Nano-Vectors
The rAAV8 vector was synthesized by Vigene Biosciences Inc. (Shandong, China). Employed as an expression vector, the rAAV8 vector contains KpnI and BglII restriction sites flanked by inverted terminal repeats (ITRs) ( and ). The full antibody genes were successfully assembled into a single open reading frame. The CAG promoter was deployed to stimulate and enhance antibody expression. The sequences encoding human bNAbs PG9 (3U4E), PG16 (3MUG), 10E8 (4G6F), and NIH45-46 (3U7W), retrieved from the Protein Data Bank, were inserted into the vector (Table S1).
Figure 1 Antibody expression cassettes. (A) Schematic representation of bNAb constructs. Kpn I and Bgl II restriction sites are labeled atop the schematic. The expression vector components are labeled as follows: coding regions are located between the ITRs; CAG, promoters of the encoding protein; ss, human IL2 signal sequence leading to antibody secretion; HC, heavy chain antibody gene; LC, light chain antibody gene; Furin, furin cleavage site; 2A, FMDV 2A sequence. (B) Schematic representation of AAV expression plasmid.
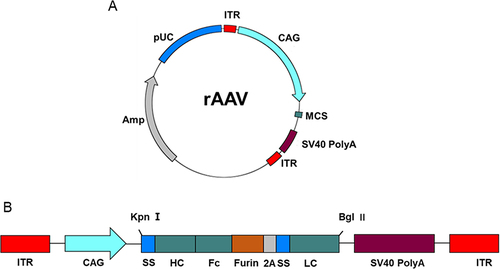
The human IL2 signal sequence (MYRMQLLSCIALSLALVTNS) was added to the N-terminus of the heavy and light chains to facilitate protein secretion. The furin cleavage site (RAKR), linked to the foot-and-mouth disease virus (FMDV) 2A self-processing sequence (QLLNFDLLKLAGDVESNPGP), was employed between the heavy and light chains.Citation20 This furin cleavage site sequence, situated between the heavy chain of the mAb and 2A sequence, serves to counteract potential adverse effects of residual 2A residues on the heavy chain of the mAb in vivo.Citation21 rAAV8 encoding the red fluorescent protein (RFP), referred to as AAV-RFP, was used as a control. The corresponding antibody constructs were termed as PG9-rAAV8, PG16-rAAV8, 10E8-rAAV8, and NIH45-46-rAAV8.
Packaging and Purification of Recombinant Adeno-Associated Virus 8 for Broadly Neutralizing Antibodies
rAAV8 production was realized according to a previously described method.Citation22 Three plasmids were co-transfected into HEK293T cells (ATCC, MD, USA): rAAV8 vectors with sequences flanked by the ITRs encoding the antibody, a packaging plasmid containing rAAV8 rep and cap genes, and a helper plasmid encoding adenovirus helper functions. After 72 hours of cultivation, the transfected cells were harvested and extracted using trichloromethane. The samples underwent centrifugation after the addition of 1 M sodium chloride, followed by the addition of 10% (w/v) PEG8000 to the collected supernatant. The mixture was then left on ice for 1 hour. Subsequently, trichloromethane was added, and the resulting precipitate was collected after centrifugation at 12,000 rpm and 4 °C for 10 minutes. The precipitate was resuspended in PBS, and the purified rAAV8 supernatant was stored at −80 °C.
Expression of Broadly Neutralizing Antibodies in-vitro
To evaluate antibody expression in cultured cells, HEK293T cells were seeded in a 12-well plate and infected with 1.0 × 10¹⁰ viral genomes (vg) of rAAV8. Cells treated with AAV8-RFP served as controls for fluorescence observation following transfection.
The expression of bNAbs PG9, PG16, 10E8, and NIH45-46 in HEK293T cells was verified through Western blot analysis. The cell culture supernatant was collected and examined via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and native-PAGE. Post-SDS-PAGE or native-PAGE, the protein was transferred to nitrocellulose membranes, which were subsequently blocked with 5% nonfat dry milk for 1 hour. The membranes were then incubated with horseradish peroxidase (HRP) goat anti-human antibody IgG (Beijing Dingguo Inc., Beijing, China) for 45 minutes. The membranes were incubated with the ECL Detection Kit (Beyotime Biotech, Shanghai, China) and then exposed using Tanon 5200 Chemiluminescence Imaging System (Tanon, Shanghai, China).
Evaluation of Purified Recombinant Adeno-Associated Virus 8
rAAV8 purity was evaluated through SDS-PAGE. rAAV8 particles were loaded onto 10% acrylamide gels, and the resultant bands were stained with Coomassie blue. Morphological analysis was conducted using a transmission electron microscope (TEM; JEOL Ltd., Tokyo, Japan). Samples were prepared by placing them on carbon-formvar copper grids and staining negatively with phosphotungstic acid, as previously described.Citation23
Determination of Recombinant Adeno-Associated Virus 8 Nano-Vector Titer
The rAAV8 vector titer was quantified through quantitative polymerase chain reaction (qPCR), as previously described.Citation24 Each sample, complemented with TransStart Top Green qPCR SuperMix (TransGen, Beijing, China), was loaded into the CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). The vector titer was calculated based on a standard curve, which was established using a purified DNA plasmid diluted in a series of 10-fold increments.
Animal Procedures and Sample Collection
Female BALB/c mice, aged 4–6 weeks and weighing 18.0–24.0 g, were procured from Liaoning Changsheng Biotechnology Co. Ltd. (Liaoning, China). All animal trials were performed in accordance with the Administration of Affairs Concerning Experimental Animals, as approved by the State Council of the People’s Republic of China (11-14-1988), and with the Institutional Animal Care and Use Committee of Jilin University’s approval (permit number: 2021SY0718). Mice were grouped into groups of five and received a single intramuscular injection of rAAV8 into the gastrocnemius muscle. Eight groups were established, with the first four groups individually immunized with 1.0 × 10¹¹ vg of rAAV8 expressing the PG9, PG16, 10E8, or NIH45-46 antibody. The fifth and sixth groups received combined immunizations, termed Comb1 and Comb2, respectively. The seventh group was immunized with rAAV8 RFP, whereas the eighth group received an equal volume of PBS. Images of AAV8-RFP-treated mice were acquired weekly for the first 4 weeks, then every 4 weeks thereafter, using the Living Image System. For the enzyme-linked immunosorbent assay (ELISA) and neutralization analyses, serum samples were collected from the caudal vein at four-week intervals. Mice were euthanized 24 weeks postintramuscular injection.
Evaluation of Broadly Neutralizing Antibodies Binding Activities
96 well ELISA plates were incubated overnight with BG505 or gp41 (Immune Technology, NY, USA) at 4 °C. The plates were blocked using PBS containing 3% bovine serum albumin. Serum samples were serially diluted 10-fold, ranging from 1: 100 to 1: 100,000. HRP-conjugated goat anti-human IgG was added as a marker, followed by the addition of the substrate tetramethyl benzidine. The reaction was terminated using 2 M H2SO4. The absorbance at 450 nm was measured using an iMarkTM Microplate Reader (Bio-Rad, Hercules, CA, USA).
HIV-1 Neutralization Assay
Both HIV-1 tier 1 (SF162 and MW965) and tier 2 (SC422661.8, HIV-16936-2.21, TRO11, X2278, 246F3, CNE8, CH119, Du156, PVO.04, RHPA.7, and X1632) pseudoviruses were prepared to facilitate neutralization detection, as previously described.Citation25 In brief, HEK293 T cells were transfected with an Env-deficient plasmid (pSG3ΔEnv) and an HIV-1 Env-expressing plasmid at a mass ratio of 2: 1 using polyethylenimine. After a 72-hour co-transfection period, the HIV-1 Env pseudovirus was harvested by sterile filtering of the cell culture supernatant at 0.22 μm. Serial dilutions of the supernatant from HEK293T cells and serum samples were prepared at 1: 30, 1: 90, 1: 270, and 1: 810. The pseudovirus (2000 tissue culture infective dose (2000 TCID50)) was incubated with the samples for 1 h, followed by seeding of TZM-bl cells (ATCC; MD, USA) with diethylaminoethyl-dextran (DEAE-dextran) (Sigma, St. Louis, MO, USA). After a 48-hour incubation period, the cell culture medium was discarded, and luciferase was added in darkness, the luminescence of which was then measured using a 2030 Multilabel Reader (Promega, Madison, WI, USA). The 50% inhibitory dose (ID50) was determined as the serum sample dilution value capable of neutralizing 50% of HIV-1 pseudoviruses.
Statistical Analysis
All graphs represent mean values ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). Multiple t-tests were employed for comparisons between groups. P values of <0.05 were deemed statistically significant.
Results
Assessment of Recombinant Adeno-Associated Virus 8 Purity
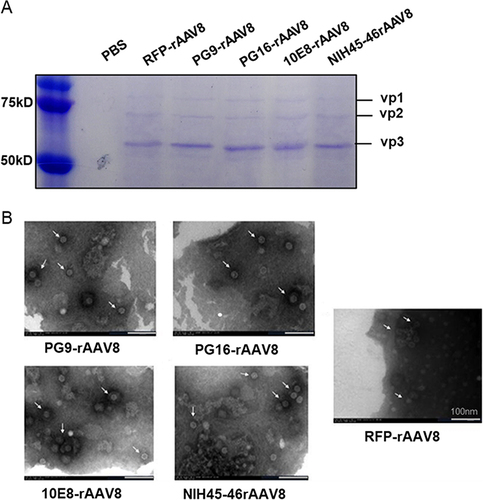
rAAV8 purity was determined using SDS-PAGE and TEM analyses. The three visible bands identified on SDS-PAGE correspond to the VP1, VP2, and VP3 proteins (), which constitute the AAV capsids at an approximate 1: 1: 10 ratio.Citation26 TEM results confirm the successful encapsulation of rAAV8 nanoparticles, exhibiting a uniform icosahedral morphology consistent with their natural form ().
Recombinant Adeno-Associated Virus 8 Mediated Antibody Expression
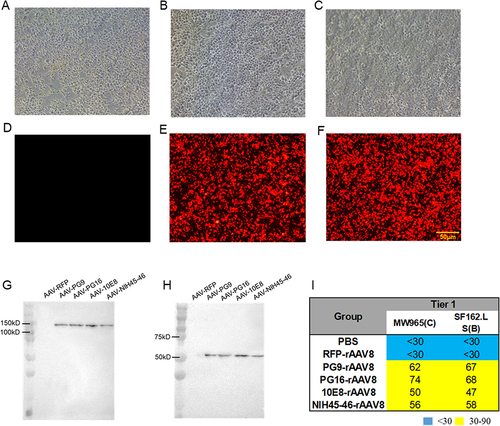
To evaluate transduction efficiency, HEK293T cells were infected separately with PG9-rAAV8, PG16-rAAV8, 10E8-rAAV8, and NIH45-46-rAAV8. The fluorescence intensity of AAV-RFP in HEK293T cells augmented over time (0, 48, 72 h) (). Western blotting was utilized to quantify the expression of PG9, PG16, 10E8, and NIH45-46 in the supernatants of infected HEK293T cells. Dimerized antibodies, visible at approximately 150 kD under nonreducing conditions, were mediated by PG9-rAAV8, PG16-rAAV8, 10E8-rAAV8, and NIH45-46-rAAV8 (). Under reducing conditions, the antibody heavy chain could be observed at approximately 50 kD (). Notably, no antibody bands were detected in RFP-rAAV8 infected cells under either condition. In vitro experimental results demonstrate that antibodies expressed from HEK293T cells neutralize two tier 1 strains of HIV-1 pseudoviruses (SF162.LS and MW965) (), whereas no visible neutralizing activity against the HIV-1 pseudovirus was detected in RFP-rAAV8 infected cells (ID50 < 30). The SDS-PAGE shows the same results (Figure S1).
Figure 3 AAV-mediated antibody expression in HEK293T cells. RFP-rAAV8 was used as a negative control. The infection of 293T cells with RFP-rAAV8 was observed under a fluorescence microscope at 0h (A and D), 48h (B and E), and 72h (C and F). Scale, 50 μm. (G) Under non-reducing conditions, full-length dimerized antibodies and (H) under reducing conditions, the heavy chain of antibodies mediated by PG9-rAAV8, PG16-rAAV8, 10E8-rAAV8, and NIH45-46-rAAV8. (I) ID50 of expressed antibodies in HEK293T cell culture medium against tier 1 pseudoviruses.
High Transduction Efficiency of Recombinant Adeno-Associated Virus 8 and Persistent Broadly Neutralizing Antibodies Expression in vivo
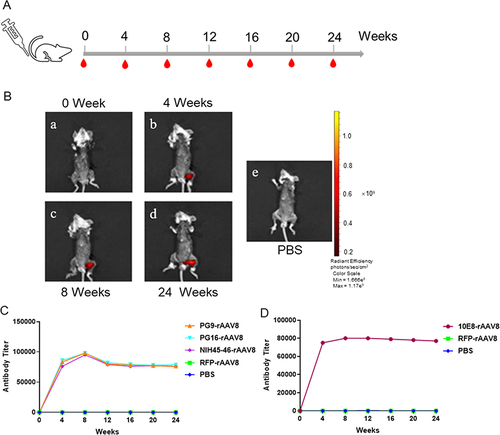
The immunization schedule is depicted in . To monitor rAAV8s expression in Balb/c mice, the biophotonic imaging system reveals visibly expressed RFP in the leg muscle tissue of mice at 0, 4, 8, and 24 weeks postimmunization (). The radiant efficiency persisted at similar levels for at least 24 weeks, indicating that AAV-mediated foreign genes were effectively expressed in Balb/c mice. The antibody titers against BG505/gp41 protein were detected by ELISA, with preimmune sera serving as negative controls. Notably, 4 weeks following a single muscle injection, PG9-rAAV8, PG16-rAAV8, 10E8-rAAV8, and NIH45-46-rAAV8 were detected at relatively high concentrations, and the antibody titer peaked at 8 weeks, maintaining relative stability until 24 weeks ( and ).
Figure 4 Gene expression mediated by rAAV8s in BALB/c mice. (A) Immunization schedule with a single injection of rAAV8 in BALB/c mice. (B) Biophotonic imaging of rAAV8 expressing RFP in BALB/c mouse muscle at 0, 4, 8, and 24 weeks. PBS is the corresponding control group. (C) The BG505 peptide was used to detect the concentration of PG9, PG16, and NIH45-46 antibodies. (D) Concentrations of the 10E8 antibody were assessed using gp41 protein.
Sustained Anti-HIV Neutralization of Individual Broadly Neutralizing Antibodies
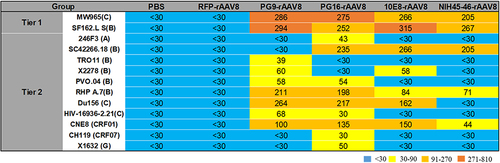
Tier 1 (MW965.26 and SF162.LS) and tier 2 (SC422661.8, HIV-16936-2.21, TRO11, X2278, 246F3, CNE8, CH119, DU156, PVO.04, rhPA.7, X1632) HIV-1 envelope pseudoviruses were used to assess the neutralizing activities of sera from BALB/c mice 24 weeks post-immunization (). The serum samples from the PG9-rAAV8, PG16-rAAV8, 10E8-rAAV8, and NIH45-46-rAAV8 groups were capable of potently neutralizing SF162.LS, MW965, CNE8, and RHPA.7 pseudoviruses. Furthermore, the aforementioned serum samples could neutralize 7, 9, 5, and 3 subtypes of the 11 tier 2 pseudoviruses, respectively (ID50 > 30).
Combined in vivo Immunization Enhanced Neutralization Breadth and Potency
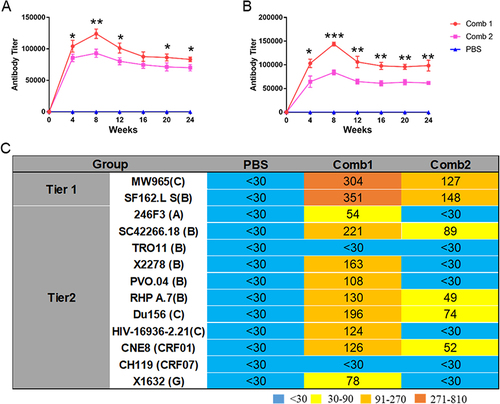
Given the results of individual immunizations, PG16 and 10E8 antibodies were selected for combined immunization, along with the co-immunization of all four antibodies. Four weeks following a single muscle injection, relatively high concentrations of the mixed antibodies were detected, as shown in and . The antibody concentrations in the Comb1 group were higher than those in the Comb2 group. An expanded neutralization breadth and enhanced neutralization potency were observed in the Comb1 combined immunization group, compared to the single injection group, whereas the Comb2 group displayed decreased neutralization breadth and potency ().
Figure 6 Antibody expression and neutralizing activity of antibodies expressed by combined rAAV8s. (A) The BG505 protein was used to detect the titer of co-expressed antibody. (B) The titer of the co-expressed antibody was assessed using the gp41 peptide. (C) ID50 of expressed antibodies in BALB/c mice sera against tier 1 and tier 2 HIV-1 isolates. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The high mutation and replication rates of HIV pose significant challenges to vaccine development.Citation27,Citation28 However, bNAbs have the potential to combat the virus by participating in the body’s innate response.Citation29 The administration of potent anti-HIV-1 monoclonal antibodies is emerging as a promising strategy for HIV-1 prevention and treatment.Citation30,Citation31 AAV vectors show promise as a delivery method owing to their long-term stability and low immunogenicity,Citation32,Citation33 with AAV8 proving to be particularly advantageous owing to its affinity for muscle transduction, marked increase in transgene expression in skeletal muscle,Citation34 and lower prevalence in seropositive individuals.Citation35 Previous research confirmed the feasibility of utilizing rAAV8-mediated bispecific bNAbs (BibNAbs) gene delivery to sustain HIV-1 neutralizing activity. Notably, BibNAbs expression and neutralizing activity in mouse serum persisted for 24 weeks following a single administration of rAAV8.Citation36
In this study, 4 rAAV8 vectors were employed for in vivo intramuscular injections to facilitate the expression of antibodies against the HIV-1 envelope protein. Tier 1 HIV-1 pseudoviruses were chosen to assess the neutralization activity of antibodies expressed in the supernatant of HEK293T cells, whereas multiple tier 1 and tier 2 HIV-1 pseudoviruses were selected to determine antibody-mediated neutralization in sera from immunized BALB/c mice. The results indicate that the antibody titer in mice peaked at 8 weeks postimmunization and then stabilized for a certain duration, with serum antibody titers detectable above 5×104 mIU/mL for over 24 weeks. The neutralizing activity of the mouse serum demonstrated that antibodies expressed in the PG9-rAAV8 and PG16-rAAV8 groups had superior neutralizing capabilities against HIV pseudoviruses compared to those expressed in the 10E8-rAAV8 and NIH45-46-rAAV8 groups.
In a manner similar to existing combination antiretroviral therapy (cART) protocols for HIV-1 control, the use of a combination of antibodies to suppress HIV-1 has been proposed for quite some time.Citation37,Citation38 Studies indicate that the in vitro neutralization activity of dual, triple, and quadruple bNAbs combinations targeting four different epitopes considerably enhance the neutralization breadth compared to their single bNAb counterparts.Citation39 Furthermore, repeated injections of a mix of three or five bNAbs in NOD Rag1−/− IL2RγNULL mice were capable of entirely suppressing the virus.Citation40 These studies suggest that bNAb combinations are beneficial in preventing and treating HIV-1. However, these studies either tested the neutralizing activity of antibodies in vitro or required continuous injections of antibodies into animals. In our study, we designed two combined immune groups: Comb1 (PG16-rAAV8 and 10E8-rAAV8) and Comb2 (PG9-rAAV8, PG16-rAAV8, 10E8-rAAV8, and NIH45-46-rAAV8). Our results show that the neutralizing activity of sera from mice in the Comb1 group surpassed that of the sera from mice immunized individually. Nevertheless, the neutralizing activity of sera from mice in the Comb2 group was not enhanced. This can be attributed to the combination of the broad-spectrum bNAb 10E8 and the high-potency bNAb PG16 being more conducive to HIV neutralization.Citation8 It was previously hypothesized that certain antibodies, like PG9 and PG16, which target largely overlapping epitopes, exhibit mutual inhibition.Citation9 Furthermore, conformational changes in PG9 and PG16 induced by NIH45-46 binding to CD4 sites could result in the loss of antibody targeting epitopes.Citation11 This underscores the importance of prudent antibody selection in combined immunization. In future studies, we intend to conduct a more detailed evaluation of combined passive immunization with multiple antibody combinations in animal models.
Conclusion
The combined immunization approach was found to enhance HIV-1 neutralization effects. This form of passive combined immunization potentially improves the efficacy of HIV immunotherapy and offers a novel method of passive immunization for further research. Our study of the combined immunization of HIV bNAbs provides a reference for future investigations in this field.
Abbreviations
AIDS, Acquired Immunodeficiency Syndrome; AAV, Adeno-Associated Virus; bNAbs, Broadly Neutralizing Antibodies; BibNAb, Bispecific Broadly Neutralizing Antibody; CD4bs, CD4-Binding Site; DEAE-dextran, Diethylaminoethyl-Dextran; ECL, Enhanced Chemiluminescence; ELISA, Enzyme-Linked Immunosorbent Assay; Env, Envelope Glycoprotein; FMDV, Foot-and-Mouth Disease Virus; HIV, Human Immunodeficiency Virus; HRP, Horseradish Peroxidase; ID50, 50% Inhibitory Dose; ITRs, Inverted Terminal Repeats; MPER, Membrane-Proximal External Region; PEI, Polyethylenimine; PBS, Phosphate Buffer Saline; qPCR, Quantitative PCR; rAAV8, Recombinant Adeno-Associated Virus 8; RFP, Red Fluorescent Protein; SDS-PAGE, Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis; TCID50, 50% Tissue Culture Infective Dose; TEM, Transmission Electron Microscopy; V1/V2, Variable Loops 1 and 2; V3, Variable Loop 3; vg, Viral Genomes.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Changchun Science and Technology Bureau (Grant number: 21ZY15), and the Jilin Scientific and Technological Development Program (Grant numbers: 20220204008YY and 20210204197YY).
References
- Ward AB, Wilson IA. Insights into the trimeric HIV-1 envelope glycoprotein structure. Trends Biochem Sci. 2015;40(2):101–107. doi:10.1016/j.tibs.2014.12.006
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280(5371):1884–1888. doi:10.1126/science.280.5371.1884
- Chen Y, Jin H, Tang X, et al. Cell membrane-anchored anti-HIV single-chain antibodies and bifunctional inhibitors targeting the gp41 fusion protein: new strategies for HIV gene therapy. Emerg Microbes Infect. 2022;11(1):30–49. doi:10.1080/22221751.2021.2011616
- Wibmer CK, Moore PL, Morris L. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS. 2015;10(3):135–143. doi:10.1097/COH.0000000000000153
- Cardozo-Ojeda EF, Perelson AS. Modeling HIV-1 within-host dynamics after passive infusion of the broadly neutralizing antibody VRC01. Front Immunol. 2021;12:710012. doi:10.3389/fimmu.2021.710012
- Lu CL, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1–infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352(6288):1001–1004. doi:10.1126/science.aaf1279
- Malbec M, Porrot F, Rua R, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013;210(13):2813–2821. doi:10.1084/jem.20131244
- Wu X, Yang ZY, Li YX, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi:10.1126/science.1187659
- Walker LM, Phogat SK, Chan-Hui P-Y, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi:10.1126/science.1178746
- Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi:10.1038/nature10373
- Huang JH, Ofek G, Laub L, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–412. doi:10.1038/nature11544
- Hsu DC, Schuetz A, Imerbsin R, et al. TLR7 agonist, N6-LS and PGT121 delayed viral rebound in SHIV-infected macaques after antiretroviral therapy interruption. PLoS Pathog. 2021;17:e1009339.
- Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15(7):445–451. doi:10.1038/nrg3742
- Berns KI, Linden RM. The cryptic life style of adeno associated virus. BioEssays. 1995;17(3):237–245. doi:10.1002/bies.950170310
- McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi:10.1038/sj.gt.3301514
- Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 2012;19(6):694–700. doi:10.1038/gt.2012.20
- Gardner MR. Promise and progress of an HIV-1 cure by adeno-associated virus vector delivery of anti-HIV-1 biologics. Front Cell Infect Microbiol. 2020;10:176. doi:10.3389/fcimb.2020.00176
- Wagh K, Bhattacharya T, Williamson C, et al. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog. 2016;12(3):e1005520. doi:10.1371/journal.ppat.1005520
- Yu YJ, Fu L, Jiang XY, et al. Expression of HIV-1 broadly neutralizing antibodies mediated by recombinant adeno-associated virus 8 in vitro and in vivo. Mol Immunol. 2016;80:68–77. doi:10.1016/j.molimm.2016.10.011
- Balazs AB, Chen J, Hong CM, Rao DS, Yang LL, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–84. doi:10.1038/nature10660
- Fang J, Qian JJ, Yi SL, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23(5):584–590. doi:10.1038/nbt1087
- Wu XB, Dong XY, Wu ZJ, et al. A novel method for purification of recombinant adeno-associated virus vectors on a large scale. Chin Sci Bull. 2001;46(6):485–489. doi:10.1007/BF03187263
- Fu L, Li Y, Hu Y, et al. Norovirus P particle: an excellent vaccine platform for antibody production against Alzheimer’s disease. Immunol Lett. 2015;168(1):22–30. doi:10.1016/j.imlet.2015.09.002
- Rohr UP, Wulf MA, Stahn S, Steidl U, Haas R, Kronenwett R. Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. J Virol Methods. 2002;106(1):81–88. doi:10.1016/S0166-0934(02)00138-6
- Bi JP, Li FS, Zhang M, et al. An HIV-1 vaccine based on bacterium-like particles elicits Env-specific mucosal immune responses, Immunol. Lett. 2020;222:29–39.
- Daniel B, Juan JA, Hassibullah A, Tomas C, Joseph P, Daniel G. Generation of infectious recombinant Adeno-associated virus in Saccharomyces cerevisiae. PLoS One. 2017;12(3):e0173010.
- Gao F, Weaver EA, Lu ZJ, et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J Virol. 2005;79(2):1154–1163. doi:10.1128/JVI.79.2.1154-1163.2005
- Gaschen B, Taylor J, Yusim K, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296(5577):2354–2360. doi:10.1126/science.1070441
- Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236(1):265–275. doi:10.1111/j.1600-065X.2010.00910.x
- Ng CT, Jaworski JP, Jayaraman P, et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 2010;16(10):1117–1119. doi:10.1038/nm.2233
- Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med. 2019;25(4):547–553. doi:10.1038/s41591-019-0412-8
- Mays LE, Wang L, Lin J, et al. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol Ther. 2014;22(1):28–41. doi:10.1038/mt.2013.134
- Mueller C, Gernoux G, Gruntman AM, et al. 5 year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol Ther. 2017;25(6):1387–1394. doi:10.1016/j.ymthe.2017.03.029
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99(18):11854–11859. doi:10.1073/pnas.182412299
- Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–712. doi:10.1089/hum.2009.182
- Li S, Qiao YB, Jiang S, Wang B, Kong W, Shan YM. Broad and potent bispecific neutralizing antibody gene delivery using adeno-associated viral vectors for passive immunization against HIV-1. J Control Release. 2021;338:633–643. doi:10.1016/j.jconrel.2021.09.006
- Mehandru S, Vcelar B, Wrin T, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81(20):11016–11031. doi:10.1128/JVI.01340-07
- Kong R, Louder MK, Wagh K, et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol. 2015;89(5):2659–2671. doi:10.1128/JVI.03136-14
- Florian K, Stromberg AH, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–122. doi:10.1038/nature11604
- Pejchala R, Walkerb LM, Stanfielda RL, et al. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci USA. 2010;107(25):11483–11488. doi:10.1073/pnas.1004600107