Abstract
The green synthesis of metallic nanoparticles (NPs) has attracted tremendous attention in recent years because these protocols are low cost and more environmentally friendly than standard methods of synthesis. In this article, we report a simple and eco-friendly method for the synthesis of silver NPs using an aqueous solution of Pulicaria glutinosa plant extract as a bioreductant. The as-prepared silver NPs were characterized using ultraviolet–visible spectroscopy, powder X-ray diffraction, transmission electron microscopy, energy-dispersive X-ray spectroscopy, and Fourier-transform infrared spectroscopy. Moreover, the effects of the concentration of the reductant (plant extract) and precursor solution (silver nitrate), the temperature on the morphology, and the kinetics of reaction were investigated. The results indicate that the size of the silver NPs varied as the plant extract concentration increased. The as-synthesized silver NPs were phase pure and well crystalline with a face-centered cubic structure. Further, Fourier-transform infrared spectroscopy analysis confirmed that the plant extract not only acted as a bioreductant but also functionalized the NPs’ surfaces to act as a capping ligand to stabilize them in the solvent. The developed eco-friendly method for the synthesis of NPs could prove a better substitute for the physical and chemical methods currently used to prepare metallic NPs commonly used in cosmetics, foods, and medicines.
Introduction
Metallic nanoparticles (NPs) have attracted the attention of the scientific community and technologists due to their ever-emerging, numerous, and fascinating applications in various fields, including biomedical sciences and engineering.Citation1–Citation3 For instance, the unique optoelectronic and physicochemical properties of metal NPs have already been successfully exploited for the purpose of drug delivery,Citation4 tissue/tumor imaging,Citation5 biosensing,Citation6 catalysis,Citation7 and surface-enhanced Raman scattering-based sensors.Citation8 The commonly employed methods for the synthesis of metallic NPs involve toxic chemicals, hazardous conditions, and costly apparatus. In comparison, the green synthesis of metallic NPs involves biocompatible ingredients under physiological conditions of temperature and pressure. Moreover, the biologically active molecules involved in the green synthesis of NPs act as functionalizing ligands, making these NPs more suitable for biomedical applications.Citation9 Therefore, the development of such protocols to synthesize nontoxic metal NPs is currently of great interest and there is thus a demand for biosynthetic or green methods for this purpose.Citation10
Several methods have been used for the green synthesis of NPs using various biological materials as reducing agents such as microorganisms, marine organisms, micro-fluids, and plant extracts.Citation11–Citation14 Among the most important bioreductants are plant extracts, which are relatively easy to handle, readily available, low cost, and have been well explored for the green synthesis of other nanomaterials.Citation15–Citation19 Various types of metallic NPs have already been synthesized using plant extracts.
Silver (Ag) in the nanoscale form exhibits remarkably unusual physicochemical and biological activities, thus has been widely applied in the health care sector.Citation20,Citation21 In particular, the outstanding antimicrobial properties of Ag NPs have led to the development of a wide variety of nanosilver products, including nanosilver-coated wound dressings, contraceptive devices, surgical instruments, and implants.Citation22,Citation23 Apart from these antimicrobial activities, Ag NPs are also known to possess antifungal, anti-inflammatory, antiviral, anti-angiogenesis, and antiplatelet properties.Citation24–Citation26 Additionally, more recent developments have seen Ag NPs used in room spray, laundry detergent, and wall paint formulations as well as in the textile industry for clothing manufacturing.Citation27–Citation30
The fascinating properties of Ag NPs mostly depend on the size and shape of the NPs. It has also been widely reported that less aggregated, small, and spherical-shaped Ag NPs have proven more effective for most applications than Ag NPs with other morphologies.Citation31–Citation33 Over the years, several studies have been published regarding the green synthesis of Ag NPs with attention paid to controlling the size and shape of the NPs and using plant extracts as reducing agents.Citation34–Citation36 For example, one study examined polydispersed spherical Ag NPs of different sizes ranging from 5 to 100 nm, which were obtained using aqueous coffee and tea leaf extracts, while another study looked at the leaves of different tea species for a similar purpose.Citation37,Citation38 Apart from the leaf, extracts of several other parts of various plants have also been tried as bioreductants for the preparation of Ag NPs, including the tuber of Curcuma longa in powdered form,Citation39 coir of Cocos nucifera,Citation40 root of Morinda citrifolia,Citation41 berry of Solanum xanthocarpum,Citation42 fruit of Terminalia chebula,Citation43 and stem bark of Callicarpa maingayi.Citation44 Additionally, in many other cases, various small whole plants have been used to synthesize Ag NPs.Citation45,Citation46
In the present study, we used the extract of Pulicaria glutinosa, which was collected from local fields in Saudi Arabia as part of our research on Saudi Arabian plants for the development of products with potential economic value.Citation47,Citation48Pulicaria is a relatively large genus of plants belonging to the tribe Inuleae of the daisy family Compositae. It comprises about 100 species distributed from Europe to North Africa and Asia, particularly around the Mediterranean.Citation49 Many plant species of the genus Pulicaria are used in traditional herbal medicines around the world for various purposes such as inflammation, diarrhea, dysentery, back pain, intestinal disorders and menstrual cramps.Citation50 Further, several of the organic compounds present in P. glutinosa plant extract (which were identified via detailed spectroscopic analysis of the plant extract; data to be published elsewhere) contain a large number of phenolic groups, which are known to play a vital role in the reduction of Ag+ ions.Citation51
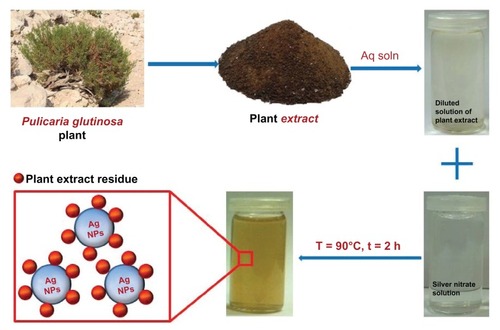
Herein, we report on the green synthesis of Ag NPs via the reduction of Ag ions using an aqueous extract of P. glutinosa plant (). During the study, a detailed analysis was undertaken of the effects of the concentrations of the reactant (silver nitrate [AgNO3]) and reductant (extract of P. glutinosa) as well as the effects of the reaction temperature on the size and morphology of Ag NPs. The as-prepared Ag NPs were characterized using various microscopic and analytical techniques including X-ray powder diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet–visible absorption (UV-Vis) spectroscopy, and transmission electron microscopy (TEM).
Materials and methods
Materials
The whole plant of wild growing P. glutinosa was collected from the hilly area of Al-Hair in central Saudi Arabia during March 2011. The identity of the plant material was confirmed by a plant taxonomist from the Herbarium Division of the College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia. A voucher specimen was deposited in our laboratory as well as in the Herbarium Division of King Saud University with the voucher specimen number KSU-21598. AgNO3 (99.8%) was purchased from Sigma-Aldrich (St Louis, MO, USA).
Preparation of plant extract
The aerial parts of freshly collected P. glutinosa plants were chopped into small pieces. The resultant pieces (245.0 g) were soaked in deionized water (2500 mL) and refluxed for 3 hours. The aqueous solution obtained after reflux was filtered and dried at 50°C under reduced pressure in a rotary evaporator to give a powder of dark brownish color (17.20 g). A small amount of the powdery extract (0.1 g/mL) was used for the synthesis of Ag NPs.
Synthesis of Ag NPs
The reaction mixture was prepared by adding 1 mL of the plant extract to 99 mL of 1 mM AgNO3 (169.87 mg) solution in a 250 mL round-bottom flask, which was mounted with a cooling condenser and magnetic stir bar. The mixture was allowed to stir for 2 hours at 90°C (immediate color change was observed from light yellow to dark brown, and thereafter no further color change was observed even after 2 hours). After 2 hours, the mixture was allowed to cool down before being centrifuged. The centrifugation was performed at room temperature and a speed of 9000 rpm. After washing three times with distilled water, a black powder was obtained that was dried overnight in an oven at 80°C.
To elucidate the effects of the plant extract, various experiments were carried out by mixing 1.0, 2.5, 5.0, 7.5, and 10 mL aqueous solutions of plant extract and 1 mmol of AgNO3 (169.87 mg) in respective amount of Milli-Q water to make a total volume of 100 mL. Similarly, for AgNO3, the quantity of plant extract was kept constant at 7.5 mL, but the amount of AgNO3 was varied in each experiment (1.0, 2.5, and 5.5 mmol). All experiments were carried out at 90°C, except those that were carried out at room temperature (RT) to study the effect of temperature on the synthesis of NPs.
Characterization of NPs
UV-Vis spectroscopy
A Lambda 35 UV-Vis spectrophotometer (PerkinElmer, Waltham, MA, USA) was used to conduct optical measurements. The analysis was performed in quartz cuvettes, using distilled water as a reference solvent. The samples for the ultraviolet (UV) measurements of crude mixture were prepared by diluting 1 mL of mixture (collected at the end of reaction) in 9 mL of water and sonicating for 15 minutes. Pure Ag NP samples for UV-Vis analysis were prepared by diluting 2 mL of pure Ag NP stock solution in 8 mL of water. The stock solution was prepared by dispersing 5 mg of Ag NPs in 5 mL of water and sonicating for 1 hour.
XRD
XRD patterns were obtained on an Ultima IV X-ray powder diffractometer (Rigaku, Tokyo, Japan) using Cu Kα radiation (λ = 1.5418 Å).
TEM and EDX
TEM and EDX was performed on a JEM 1101 transmission electron microscope (JEOL, Tokyo, Japan). The samples for TEM were prepared by placing a drop of primary sample on a copper grid, which was then dried for 6 hours at 80°C in an oven.
FT-IR spectroscopy
FT-IR spectra were obtained on a PerkinElmer 1000 FT-IR spectrometer. To remove any free biomass residue or unbound extract on the surfaces of the NPs, the Ag NPs were repeatedly washed with distilled water; subsequently the product was centrifuged at 9000 rpm for 30 min and dried. The purified Ag NPs were mixed with KBr powder and pressed into a pellet for measurement. Background correction was made using a reference blank KBr pellet.
Results and discussion
P. glutinosa plant extract was used for the synthesis of Ag NPs under facile conditions. It was observed that on the addition of plant extract into the aqueous solution of AgNO3, the color of the solution gradually changed from light yellow to dark brown, indicating the formation of Ag NPs (). Under a similar set of conditions, no change in the color of AgNO3 solution was observed even after 72 hours (). This color change occurred relatively faster at 90°C (in less than 2 hours) than at RT. This observation is in accordance with previously reported study by Mohan Kumar et al, in which the speed of NP formation has been found to increase as the temperature and incubation period increase.Citation46 The formation of the as-prepared Ag NPs was initially monitored by UV-Vis analysis. Typically, Ag NPs exhibit absorption under a visible range of 380–450 nm, depending on the shape and size of the NPs.Citation51,Citation52
Figure 2 Digital photograph of the aqueous solutions of (A) silver nitrate (AgNO3) without addition of the plant extract, (B) AgNO3 with the addition of the plant extract, and photograph of (C) the pure plant extract at a concentration of 0.1 g/mL. Images (A and B) were recorded after 72 hours.

UV-Vis spectroscopic analysis
UV-Vis absorption spectroscopy is an important technique to monitor the formation and stability of metal NPs in aqueous solution. The absorption spectrum of metal NPs is sensitive to several factors, including particle size, shape, and particle–particle interaction (agglomeration) with the medium.Citation52 Therefore, the aqueous bioreduction of Ag+ ions can be effectively monitored by a UV-Vis spectrophotometer. shows the absorption spectra of Ag NPs prepared at different temperatures. The results reveal that the synthesis of Ag NPs using P. glutinosa is significantly faster at elevated temperature (90°C) than at RT.
Figure 3 Ultraviolet–visible (UV-Vis) absorption spectra (A and B) and visual appearance of the silver nanoparticle (Ag NP) solution (C). (A) Comparison between UV-Vis spectra of Ag NPs prepared at room temperature (RT) and at 90°C. (B) Plot of the absorption wavelength of Ag NPs at different temperatures. (C) Color change of the solution at 90°C. The samples shown in (C) were obtained during the reaction and photographs recorded before final workup.
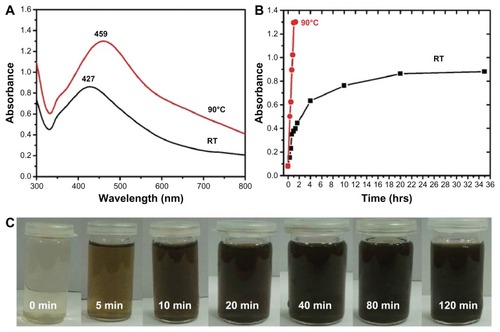
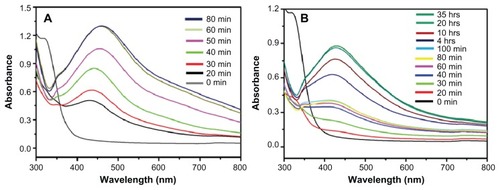
displays the UV-Vis spectra of Ag NPs prepared at RT and 90°C, which were recorded at regular intervals of time. At 90°C, it was observed that the nucleation started very quickly and the formation of Ag NPs was very fast until around 60 minutes had passed, which is clearly reflected by the gradual increase in the intensity of the surface plasmon resonance (SPR) bands (). Thereafter, the process slowed down and, after 80 minutes had passed, no further change in the SPR band was observed, which is an indication of the completion of reaction. However, at RT, the nucleation process was very slow from the beginning and the reaction took almost 20 hours to complete (). At both temperatures, the SPR band shapes remain similar. This trend can be attributed to the gradual and slow nucleation of the NPs, due to the slower rate of reaction at lower temperatures (). Further, the lower intensity of the SPR band of Ag NPs formed at RT in indicates a smaller yield of NPs when compared to the yield of Ag NPs formed at 90°C.
Figure 4 Ultraviolet–visible absorption spectra of silver nanoparticles (Ag NPs) prepared at (A) 90°C using 1 mmol of silver nitrate and 7.5 mL of plant extract and (B) at room temperature using 1 mmol of silver nitrate and 7.5 mL of plant extract.
Note: All spectra were obtained by taking 1 mL of the sample at regular intervals and further diluting it with 9 mL of water then sonicating for 15 minutes.
Additionally, the effects of the plant extract concentration on the synthesis of Ag NPs were also evaluated using UV-Vis spectroscopy. shows the absorption spectra of Ag NPs prepared using different concentrations of plant extract. All experiments were carried out by varying the concentration of P. glutinosa extract, keeping other conditions constant (using 1 mmol AgNO3) at 90°C. It was observed that the concentration of the plant extract exerted a significant effect on the size and size distribution of the NPs (). Generally, a broad peak at a higher wavelength indicates an increase in particle size, whereas a narrow line at a shorter wavelength represents smaller particle size.Citation52 In this case, as the concentration of the plant extract was varied from 1 to 5 mL, it was found that the SPR bands were blue shifted from 455 nm to 422 nm, with a simultaneous increase in the absorption coefficient. In contrast, with further increases in concentration, the bands became sharper and shifted to longer wavelengths – that is, from 422 to 459 nm.
Figure 5 Ultraviolet–visible absorption spectra of silver nanoparticles (Ag NPs) prepared at 90°C for 2 hours using (A) various concentrations of plant extract and keeping the amount of silver nitrate (AgNO3) constant at 1 mmol and (B) various concentrations of AgNO3 and keeping the amount of plant extract constant at 7.5 mL. (C) The diluted solutions of pure Ag NPs (obtained after final workup).
Note: All spectra were measured using same solution concentrations.
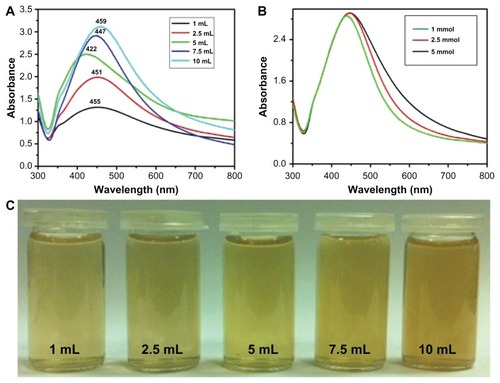
The initial shift toward the shorter wavelength with increasing extract concentration is characteristic of a decrease in particle size, which could be the result of an increase in nucleation due to the enhanced reduction process (like the increased nucleation that occurs in the polyol solution synthesis of metallic NPs). In contrast, further increasing the extract concentration (which also increased the reduction process) resulted in further growth (Ostwald ripening) of the NPs, which gave rise to a red shift of the SPR band (toward a longer wavelength). The sharpness of the absorption band with increasing plant extract concentration was due to the better stabilization of the NPs’ surfaces because the plant extract also acts as surface functionalizing ligand. This is also reflected by the color change of the diluted pure Ag NP solution from light to dark brown as the plant extract concentration increased. A comparative study was also carried out to investigate the effects of AgNO3 concentration (1.0, 2.5, and 5.0 mmol, respectively) on preparation of Ag NPs. shows the UV-Vis spectra of Ag NPs obtained at various aqueous AgNO3 concentrations while the plant extract concentration was kept constant (7.5 mL) at 90°C for 2 hours. It was observed that, as the concentration of AgNO3 increased, the SPR band shifted from 447 nm (1 mmol) to 455 nm (5 mmol), indicating a slight increase in particle size.
is a digital photograph of the diluted solutions of pure Ag NPs prepared with different plant extract concentrations. The darker pure Ag NPs solution prepared with 10 mL of extract indicates the presence of plant extract residue in higher amounts as a capping agent to the Ag NPs. Therefore, at a higher concentration of plant extract, less aggregated but larger NPs were formed. It was also observed that, when the concentration of the plant extract was increased, the intensities of the SPR bands also increased, which indicates that there is a higher yield of Ag NPs at higher concentrations ().Citation40
XRD
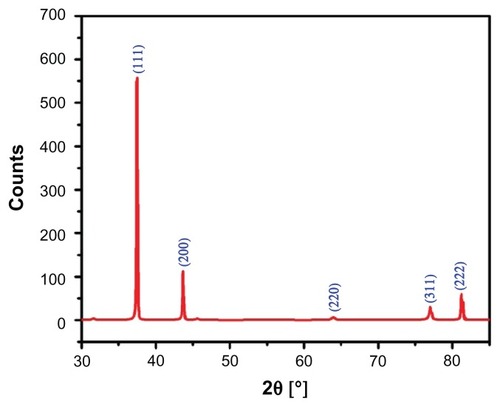
The crystalline nature of the green synthesized Ag NPs using P. glutinosa was confirmed by XRD pattern analysis. The XRD pattern in indicates the face-centered cubic structure of the Ag NPs. There are five distinct reflections in the diffractogram at 37.50° (111), 44.13° (200), 63.91° (220), 76.89° (311), and 81.13° (222). The intense reflection at 111, in comparison to the other four, may indicate the growth direction of the nanocrystals.Citation53 On the basis of the half-width (^) of the 111 reflection in the powder pattern, the average grain size, L – determined by broadening of the 111 reflection by the Scherer formula – is approximately 42 nm. The absence of any additional reflections other than the reflections belonging to the Ag lattice clearly suggests that the green synthesized Ag NP lattice was unaffected by other molecules in the plant extract.
TEM and energy-dispersive X-ray spectroscopy (EDX) analysis
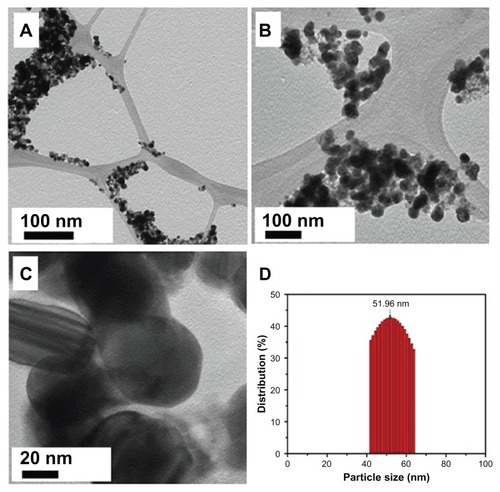
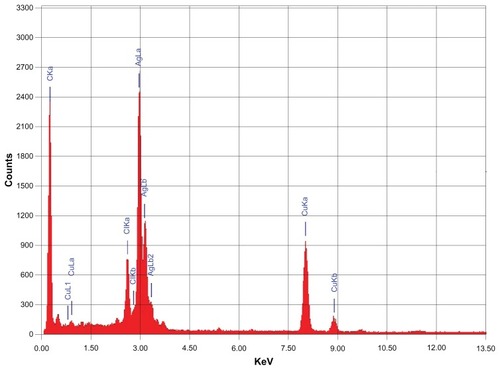
The morphology and size of the green synthesized Ag NPs were studied by TEM. shows the as-synthesized NPs. and C clearly show the spherical morphology of the NPs, with a size range of 40–60 nm (), in which few NPs were agglomerated. The agglomeration of NPs decreased as the plant extract concentration was increased (), which is also evidenced by the UV-Vis spectroscopy results. Further, the elemental composition of the green synthesized sample was also determined by EDX. The EDX spectrum, shown in , reveals the clear elemental composition profile of the green synthesized Ag NPs. The intense signal at 3 keV strongly suggests that Ag was the major element, which has an optical absorption in this range due to the SPR.Citation36 Notably, the other signals in the range of 0.0–0.5 keV – one of which is very intense – represent the typical absorption of carbon and oxygen and thus indicates the presence of the plant extract (as a capping ligand) on the surfaces of the NPs.
FT-IR analysis
The dual role of the plant extract as a bioreductant and capping agent was confirmed by FT-IR analysis of the as-prepared Ag NPs. The sample for the infrared analysis was carefully prepared to exclude any possibility of the presence of any unbound plant extract residue. For this purpose, the as-prepared Ag NPs after final workup were dispersed again in distilled water via 30 minutes of sonication, and subsequently centrifuged at a speed of 9000 rpm for 30 minutes, and this process was repeated twice to isolate the pure Ag NPs and exclude the presence any unbound ligand. shows the FT-IR spectra of P. glutinosa pure plant extract and purified Ag NPs obtained after the bioreduction.
Figure 9 Fourier-transform infrared spectra of pure green synthesized silver nanoparticles (Ag NPs) (line a) and Pulicaria glutinosa plant extract (line b).
Note: The similarities between the spectra strongly suggest the presence of plant extract residue in the Ag NPs as a capping or stabilizing agent.
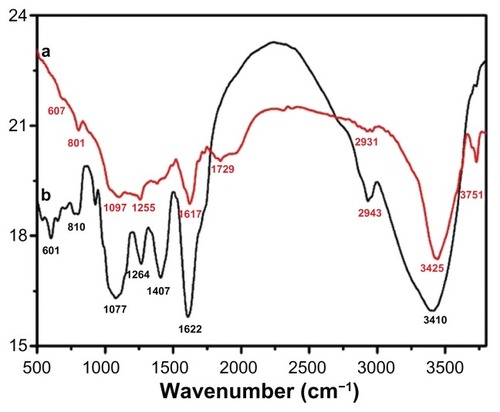
The similarities between the spectra A and B in , with some marginal shifts in peak position, clearly indicate the presence of residual plant extract in the sample as a capping agent to the Ag NPs. Detailed analysis of the plant extract spectra strongly suggested the presence of flavonoids and polyphenols, apart from other phytochemicals, which were mainly responsible for the formation of the Ag NPs by reducing the AgNO3. The spectrum of the plant extract shows the absorption peaks at 3746 and 3410 cm−1 corresponding to the hydrogen-bonded hydroxyl (OH) and the peak at 2943 cm−1 indicates the presence of C–H. The absorption peaks situated around 1753, 1622, and 1407 cm−1 are the characteristic peaks for the C–H, C–C, and C–O stretching, respectively, of the aromatics. The bands at 1264 and 1077 cm−1 indicate the presence of C–O stretching of alcohols, carboxylic acids, and ester and ether groups. Notably, the absorption band at 1974 and 512 cm−1 in the Ag NP spectrum, in addition to peaks present in the plant extract spectrum, represents the Ag NPs’ banding with oxygen from the hydroxyl groups in P. glutinosa compounds.Citation39 Further, the absorption peak centered at 1729 cm−1 points toward the formation of a new C=O group that is either a aldehyde, ketone, or COOH. This strongly suggests that the reduction of Ag was carried out by some hydroxyl groups that get oxidized at the expense of Ag because Ag is reduced.Citation54
Conclusion
Herein, a green approach to the synthesis of Ag NPs using P. glutinosa plant extract has been reported. Applying this method, highly crystalline, spherical-shaped Ag NPs were prepared at ambient conditions without using any harmful reducing or capping agents. The concentration of plant material played a critical role in the size and dispersion of the NPs. Although reduction occurred when the plant extract concentration was low, the NPs prepared using a high concentration of plant extract were less aggregated, indicating that the plant extract acted as a capping ligand, and this was confirmed by FT-IR spectroscopy. Studies on the effect of temperature on the preparation of the NPs showed that the reaction proceeded much faster at an elevated temperature than at RT. Moreover, the size and yield of NPs increased when they were synthesized at 90°C compared with when they were synthesized at RT.
The protocol presented here for the synthesis of Ag NPs can be extended to other metal NPs due to the fact that P. glutinosa plant extract is highly oxidized in nature and is very likely to be able to reduce metals under physiological conditions. Moreover, its abundancy and low cost also make P. glutinosa plant extract potentially attractive for the up-scaling of metallic nanomaterials to explore various catalytic applications.
Acknowledgments
This project was supported by King Saud University, Deanship of Scientific Research, College of Science, Research Center.
Disclosure
The authors declare no conflicts of interest in this work.
References
- ArvizoRRBhattacharyyaSKudgusRAGiriKBhattacharyaRMukherjeePIntrinsic therapeutic applications of noble metal nanoparticles: past, present and futureChem Soc Rev20124172943297022388295
- DykmanLKhlebtsovNGold nanoparticles in biomedical applications: recent advances and perspectivesChem Soc Rev20124162256228222130549
- OtsukaHNagasakiYKataokaKPEGylated nanoparticles for biological and pharmaceutical applicationsAdv Drug Deliv Rev201264246255 Available from: http://dx.doi.org/10.1016/j.addr.2012.09.022
- DoaneTLBurdaCThe unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapyChem Soc Rev20124172885291122286540
- DreadenECEl-SayedMADetecting and destroying cancer cells in more than one way with noble metals and different confinement properties on the nanoscaleAcc Chem Res201245111854186522546051
- BedfordEESpadavecchiaJPradierCMGuFXSurface plasmon resonance biosensors incorporating gold nanoparticlesMacromol Biosci201212672473922416018
- AnKSomorjaiGASize and shape control of metal nanoparticles for reaction selectivity in catalysisChem Cat Chem201241015121524
- BaruahBCraigheadCAbolarinCOne-phase synthesis of surface modified gold nanoparticles and generation of SERS substrate by seed growth methodLangmuir20122843151681517623025402
- LuAHSalabasELSchüthFMagnetic nanoparticles: synthesis, protection, functionalization, and applicationAngew Chem Int Ed Engl20074681222124417278160
- IravaniSGreen synthesis of metal nanoparticles using plantsGreen Chem2011131026382650
- AttardGCasadesúsMMacaskieLEDeplancheKBiosynthesis of platinum nanoparticles by Escherichia coli MC4100: can such nanoparticles exhibit intrinsic surface enantioselectivity?Langmuir201228115267527422329766
- RamanathanRFieldMRO’MullaneAPSmookerPMBhargavaSKBansalVAqueous phase synthesis of copper nanoparticles: a link between heavy metal resistance and nanoparticle synthesis ability in bacterial systemsNanoscale2013562300230623223802
- DhillonGSBrarSKKaurSVermaMGreen approach for nanoparticle biosynthesis by fungi: current trends and applicationsCrit Rev Biotechnol2012321497321696293
- AsmathunishaNKathiresanKA review on biosynthesis of nanoparticles by marine organismsColloids Surf B Biointerfaces201310328328723202242
- GanPPLiSFPotential of plant as a biological factory to synthesize gold and silver nanoparticles and their applicationsRev Environ Sci Biotechnol2012112169206
- GhoshSPatilSAhireMSynthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agentsInt J Nanomedicine2012748349622334779
- SujithaMVKannanSGreen synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterizationSpectrochim Acta A Mol Biomol Spectrosc2013102152323211617
- GeethalakshmiRSaradaDVGold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial propertiesInt J Nanomedicine201275375538423091381
- KouvarisPDelimitisAZaspalisVPapadopoulosDTsipasSAMichailidisNGreen synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extractMater Lett2012761820
- ChenXSchluesenerHJNanosilver: a nanoproduct in medical applicationToxicol Lett2008176111218022772
- WongKKLiuXSilver nanoparticles – the real “silver bullet” in clinical medicine?Med Chem Comm20101125131
- LohseSEMurphyCJApplications of colloidal inorganic nanoparticles: from medicine to energyJ Am Chem Soc201213438156071562022934680
- YouCHanCWangXThe progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicityMol Biol Rep20123999193920122722996
- MonteiroDRSilvaSNegriMSilver nanoparticles: influence of stabilizing agent and diameter on antifungal activity against Candida albicans and Candida glabrata biofilmsLett Appl Microbiol201254538339122313289
- KrishnarajCRamachandranRMohanKKalaichelvanPTOptimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungiSpectrochim Acta A Mol Biomol Spectrosc201293959922465774
- Martínez-GutierrezFThiEPSilvermanJMAntibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticlesNanomedicine20128332833621718674
- ParkMImJShinMHighly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibresNat Nanotechnol201271280380923178335
- AkterTKimWSReversibly stretchable transparent conductive coatings of spray-deposited silver nanowiresACS Appl Mater Interfaces2012441855185922471630
- GottesmanRShuklaSPerkasNSolovyovLANitzanYGedankenASonochemical coating of paper by microbiocidal silver nanoparticlesLangmuir201127272072621155556
- OsórioIIgrejaRFrancoRCortezJIncorporation of silver nanoparticles on textile materials by an aqueous procedureMater Lett201275200203
- MubarakAliDThajuddinNJeganathanKGunasekaranMPlant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogensColloids Surf B Biointerfaces201185236036521466948
- El-NourKMEfthaihaAAl-WarthanAAmmarRASynthesis and applications of silver nanoparticlesArabian Journal of Chemistry201033135140
- GaoMSunLWangZZhaoYControlled synthesis of Ag nanoparticles with different morphologies and their antibacterial propertiesMater Sci Eng C2013331397404
- NjagiECHuangHStaffordLBiosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extractsLangmuir201127126427121133391
- MoultonMCBraydich-StolleLKNadagoudaMNKunzelmanSHussainSMVarmaRSSynthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenolsNanoscale20102576377020648322
- MallikarjunaKSushmaNJNarasimhaGManojLRajuBDPPhytochemical fabrication and characterization of silver nanoparticles by using pepper leaf brothArabian Journal of Chemistry2013 In press
- NadagoudaMNVarmaRSGreen synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extractGreen Chem200810859862
- LooYYChiengBWNishibuchiMRaduSSynthesis of silver nanoparticles by using tea leaf extract from Camellia sinensisInt J Nanomedicine201274263426722904632
- ShameliKAhmadMBZamanianAGreen biosynthesis of silver nanoparticles using Curcuma longa tuber powderInt J Nanomedicine201275603561023341739
- RoopanSMRohitMadhumithaGLow-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activityInd Crops Prod201343631635
- SumanTYRajasreeSRRKanchanaAElizabethSBBiosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extractColloids Surf B Biointerfaces2013106747823434694
- AminMAnwarFJanjuaMRIqbalMARashidUGreen synthesis of silver nanoparticles through reduction with solanum xanthocarpum L Berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pyloriInt J Mol Sci20121389923994122949839
- EdisonTJSethuramanMGInstant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blueProcess Biochem201247913511357
- ShameliKBin AhmadMJaffar Al-MullaEAGreen biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extractionMolecules20121778506851722801364
- ValliJSVaseeharamBBiosynthesis of silver nanoparticles by Cissus quadrangularis extractsMater Lett201282171173 Available from: http://dx.doi.org/10.1016/j.matlet.2012.05.040
- Mohan KumarKSinhaMMandalBKGhoshARSiva KumarKSreedhara ReddyPGreen synthesis of silver nanoparticles using Terminalia chebula extract at room temperature and their antimicrobial studiesSpectrochim Acta A Mol Biomol Spectrosc20129122823322381795
- KhanMMousaAASyamasundarKVAlkhathlanHZDetermination of chemical constituents of leaf and stem essential oils of Artemisia monosperma from central Saudi ArabiaNat Prod Commun201271079108222978234
- Al-OtaibiMSAl-MayoufAMKhanMMousaAAAl-MazroaSAAlkhathlanHZCorrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic mediaArabian Journal of Chemistry2012 In press
- WilliamsCAHarborneJBGreenhamJRGrayerRJKiteGCEaglesJVariations in lipophilic and vacuolar flavonoids among European Pulicaria speciesPhytochemistry200364127528312946426
- StavriMMathewKTGordonAShnyderSDFalconerRAGibbonsSGuaianolide sesquiterpenes from Pulicaria crispa (Forssk.) OlivPhytochemistry20086991915191818448140
- SathishkumarGGobinathCKarpagamKHemamaliniVPremkumarKSivaramakrishnanSPhyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogensColloids Surf B Biointerfaces20129523524022483838
- SmithaSLNissamuddinKMPhilipDGopchandranKGStudies on surface plasmon resonance and photoluminescence of silver nanoparticlesSpectrochim Acta A Mol Biomol Spectrosc200871118619018222106
- PhilipDMangifera indica leaf-assisted biosynthesis of well-dispersed silver nanoparticlesSpectrochim Acta A Mol Biomol Spectrosc201178132733121030295
- KoraAJBeeduSRJayaramanASize-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activityOrg Med Chem Lett201221172722571686