Abstract
Recently nanoparticles have been extensively studied and have proven to be a promising candidate for cancer treatment and diagnosis. In the current study, we examined the chemo-sensitizing activity of a mixture of nanodiamond (ND) and nanoplatinum (NP) solution known as DPV576, against multidrug-resistant (MDR) human myeloid leukemia (HL60/AR) and MDR-sensitive cells (HL60). Cancer cells were cultured with different concentrations of daunorubicin (DNR) (1 × 10 −9−1 × 10 −6 M) in the presence of selected concentrations of DPV576 (2.5%–10% v/v). Cancer cell survival was determined by MTT assay, drug accumulation by flow cytometry and confocal laser scanning microscopy (CLSM), and holes and structural changes by atomic force microscopy (AFM). Co-treatment of HL60/AR cells with DNR plus DPV576 resulted in the reduction of the IC50 to 1/4th. This was associated with increased incidences of holes inside the cells as compared with control untreated cells. On the other hand, HL60 cells did not show changes in their drug accumulation post-treatment with DPV576 and DNR. We conclude that DPV576 is an effective chemo-sensitizer as indicated by the reversal of HL60/AR cells to DNR and may represent a potential novel adjuvant for the treatment of chemo-resistant human myeloid leukemia.
Introduction
Nanoparticles have shown promising results for cancer therapies. Several studies have shown the success of drug delivery and cell targeting using nanoparticles.Citation1 One example is the conjugation of trastuzumab (Herceptin®) to doxorubicin-carrying nanoparticles which allows transport of the chemotherapeutic agent specifically to tumor cells.Citation2 In addition, antibody-conjugated nanoparticles have the potential to be used in active targeted drug delivery.Citation3 Other nanoparticles that have been studied for their use in cancer therapies include: iron oxide,Citation4 poly(D,L-lactide-co-glycolide)/montmorillonite,Citation5 poly(D,L-lactic acid),Citation6 nickel,Citation7 and human serum albumin.Citation2,Citation8,Citation9
The development of multidrug resistance (MDR) to anticancer agents by tumor cells is a major obstacle in the chemotherapeutic cure of cancer.Citation10 Therefore, larger dosages of these anticancer drugs must be applied, leading to increased toxicity; side effects include myelosuppression, carcinogenic effects, alopecia, myalgia, thrombocytopenia, congestive heart failure, and immune suppression.Citation11–Citation16 There are many drugs that have been shown to reverse MDR in MDR-associated protein (MRP), including: probenecid, an inhibitor of organic anion transport;Citation17 genistein, an inhibitor of tyrosine kinase;Citation18 buthionine sulfoximine, an inhibitor of glutathione synthesis;Citation19 ethacrynic acid to reverse glutathione S-transferase-mediated resistance; and calcium channel blockers and calmodulin inhibitors to reverse MDR.Citation20 However, these agents have been shown to be toxic in humans when used at very high doses. These considerations prompted us to investigate a new modulator of MDR with lower toxicity. Earlier studies showed that a mixture of two nanoparticles - nanodiamond (ND) and nanoplatinum (NP) - in liquid form (DPV576) and in fabric form (DPV576-C), show immune modulatory effects.Citation21,Citation22 In the current study we extend our research with DPV576 to examine its chemo-sensitizing activity against DNR-resistant human myeloid leukemia (HL60/AR) in vitro.
Materials and methods
Tumor cell lines and culture conditions
Human multidrug-resistant (MDR) myeloid leukemia (HL60/AR) cells and MDR sensitive (HL60) cells were used in the present study. HL60/AR cells were kindly provided by Dr Gollapudi at the University of California (Irvine, CA, USA). HL60 cells were purchased from the American Tissue and Culture Collection (ATCC) (Manassas, VA, USA). Tumor cells were maintained in our laboratory in a complete medium (CM) that consisted of RPMI-1640, supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and 100 μg/mL streptomycin, and penicillin.
Drugs and chemicals
DNR and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO, USA). DPV576, a liquid mixture of nanodiamond (ND) and nanoplatinum (NP) solution was used. DPV576 was supplied by Venex Company (Atsugi, Kanagawa, Japan). The particle diameters of NP and ND were 10–20 nm and 100–200 nm, respectively and their final concentrations were 50 ng/mL NP and 5 μg/mL ND.
Drug sensitivity assay
Drug sensitivity was determined using a colorimetric MTT assay. This assay is based on the reduction of tetrazolium salt MTT by a mitochondrial dehydrogenase from a colorless to a blue-colored formazan product in viable cells that can be measured spectrometrically The amount of formazan produced is proportional to the number of living cells. Cells (1 × 104/well) were seeded in 96-well plates and cultured in triplicate in the presence or absence of various concentrations of DPV576 (2.5%–10% v/v) and with or without selected concentrations of DNR (1 × 10 −9−1 × 10−6 M). The final volume of medium in each well after addition of DPV576 and/or DNR was 200 mL. The cultures were incubated at 37°C for 3 days, after which 50 μg of MTT was added to each well, and the cultures incubated for an additional 4 hours. The plates were centrifuged the medium carefully removed the formazan crystals solubilized with acid alcohol, and the plates read at 590 nm using an ELISA plate reader (Molecular Devices, Menlo Park, CA, USA). The 50% inhibitory concentration (IC50) was determined as the drug concentration, which resulted in a 50% reduction in cell viability. The IC50 was determined by plotting the logarithm of the drug concentration versus the survival rate of the treated cells.
Daunorubicin (DNR) accumulation
The accumulation into cells of DNR a fluorescent compound was studied by flow cytometry, as has been previously described.Citation17 Briefly, cells were incubated in the presence or absence of DPV576 (2.5%–10% v/v) at 37°C for 15 minutes. DNR (2 μM) was then added to the cells, gently mixed and incubated at 37°C for 45 minutes. Accumulation of DNR was measured by flow cytometry using a FACScan (Becton Dickinson, Franklin Lakes, NJ, USA), the fluorescence intensity was recorded from histograms, and the data was expressed as mean fluorescence channel numbers (MFC).
AFM imaging
HL60/AR cells (0.5 × 106) were cultured with DPV576 (10% v/v) for 24 hours then exposed to DNR (2 μM) for 45 minutes. Results were compared to those of cells treated with DNR alone and DPV576 alone. Cytospin preparations (Shandon Southern Institute, Sewickley, PA, USA) of cells under different treatment conditions were air dried fixed in 100% MeOH for 5 minutes, and prepared for AFM studies. Dimension 5000 AFM (Veeco, Plainview, NY, USA) under contact mode was used to image the HL60/AR cells with OTESP silicon probes (Veeco). Topographic height images were recorded at 512 × 512 pixels at a scan rate of 0.8 Hz. Image processing was performed using SPIP Software (Image Metrology, Hørsholm, Denmark). Usually an MLCT-AFM tip (with a ‘k’ value of 0.03 N/m) contributes to the broadening effect because of its specific geometryCitation23
Confocal imaging
Confocal laser scanning microscopy (CLSM) was used to detect DNR accumulation for HL60/AR cells treated with DNR alone and DNR plus DPV576. Cytospin preparations, as mentioned above, were prepared for confocal imaging. The CLSM was operated in reflectance mode using a 515 nm laser line.Citation24
Hole/vacuole analysis using light microscopy of Giemsa-stained preparations
Morphological assessment of hole induction in HL60/AR cells by DPV576 treatment was conducted in Giemsa-stained cytospin preparations. HL60/AR cells (0.5 × 106) were cultured with DPV576 (10% v/v) for 24 hours then exposed to DNR (2 μM) for 45 minutes. The cells (0.3 × 105) were then treated with either DPV576, DNR, DPV576 plus DNR, or saline. Afterwards, the samples were centrifuged on slides at 200 g for 5 minutes using a cytospin cytocentrifuge. Slides were air dried fixed in 100% MeOH for 5 minutes, and then stained with 4% Giemsa solution for 15 minutes as has been previously described.Citation25
Statistical analysis
Statistical significance was determined by Student’s t-test. Differences were considered significant at the P < 0.05 level.
Results
The effects of DPV576 on the susceptibility of HL60/AR cancer cells to DNR were examined at the levels of both cell survival and drug accumulation.
HL60/AR cell survival
HL60/AR cells were cultured with DNR at different concentrations (1 × 10 −9−1 × 10−6 M) in the presence or absence of DPV576 for 3 days. Cell survival and IC50 values were then determined by MTT assay. shows that DNR, as expected, inhibited the survival of cancer cells in a dose-dependent manner and that the IC50 of DNR alone was 3.1 μM. However, when the cells were co-cultured with DPV576 plus DNR, we noticed a decrease in cell survival that was also dose dependent of DPV576 and maximized at 10% v/v. Subsequently, the IC50 was significantly reduced, reaching 0.8 μM at 10% v/v.
Figure 1 Effect of DPV576 on the reversal of DNR resistance in HL60/AR cells. Cancer cells (1 × 104 well −1) were seeded in 96-well plates with DNR (1 × 10−9 to 1 × 10−6 M) and cultured in the presence or absence of various concentrations of DPV576 (2.5, 5, and 10% v/v) for 3 days. Cell survival was determined by MTT assay. Data represents the mean ± SD from three individual experiments, each in triplicate. The IC50 of DNR with DPV576 required 1/4th the amount of DNR as compared to the IC50 of DNR alone.
Abbreviations: DNR, daunorubicin; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; IC50, 50% inhibitory concentration.
![Figure 1 Effect of DPV576 on the reversal of DNR resistance in HL60/AR cells. Cancer cells (1 × 104 well −1) were seeded in 96-well plates with DNR (1 × 10−9 to 1 × 10−6 M) and cultured in the presence or absence of various concentrations of DPV576 (2.5, 5, and 10% v/v) for 3 days. Cell survival was determined by MTT assay. Data represents the mean ± SD from three individual experiments, each in triplicate. The IC50 of DNR with DPV576 required 1/4th the amount of DNR as compared to the IC50 of DNR alone.Abbreviations: DNR, daunorubicin; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; IC50, 50% inhibitory concentration.](/cms/asset/5bc5d8e3-a1ff-482d-96f4-9ceba64d48f8/dijn_a_43417_f0001_c.jpg)
Drug accumulation
Flow cytometry
To determine if the observed DPV576-enhanced accumulation of DNR cytotoxicity in HL60/AR cells is related to an alteration in drug transport, we studied accumulation of DNR by flow cytometry. The results show that DPV576 at concentrations of 2.5%, 5%, and 10% v/v significantly enhances the accumulation of DNR in HL60/AR cells (). On the other hand, HL60 cells did not show changes in drug accumulation post-treatment with DPV576 and DNR (), suggesting that DPV576 had no significant effect on the accumulation of DNR in HL60 cells.
Figure 2 Effect of DPV576 on DNR accumulation in HL60/AR and HL60 cells using flow cytometry. Cancer cells (1 × 104) were incubated with DNR (2 μm) with or without DPV576 (2.5, 5, and 10% v/v) and drug accumulation was assessed using flow cytometry, and is expressed as MFC number. (A) HL60/AR cells; (B) HL60 cells; (C) dot plot and histogram overlays of HL60/AR cells with and without DNR and DPV576. Forward and side scatter was used to exclude debris and dead cells.
Notes: Data represent the mean + SD of three experiments; *P < 0.05; **P < 0.001. Ten thousand cells were acquired for analysis by flow cytometry.
Abbreviations: DNR, daunorubicin; MFC, mean fluorescence channel.
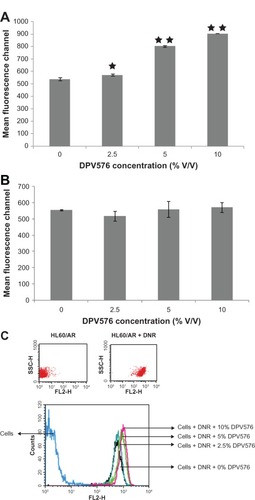
Confocal studies
HL60/AR cells treated with DNR in the presence and absence of DPV576 were examined by CLSM. Cells exposed to low concentrations of DNR alone showed very faint brightness (); similarly, cells treated with low and high doses of DNR also showed a faint brightness (). On the other hand, cells exposed to high concentrations of DNR with DPV576 (–) showed the greatest degree of brightness. Note the presence of multiple holes in these cells. Note also the apoptotic HL60/AR cells as characterized by an increased nuclear to cytoplasmic ratio in the early stages of apoptosis (–) and membrane blebbing in the later stages of apoptosis ().
Figure 3 Effect of DPV576 on DNR accumulation in HL60/AR cells using CLSM. Cells displayed little to no brightness as shown in (A) without DNR and without DPV576, (B) cells with only DPV576, and (C) DNR alone. Cells exposed to both DNR and DPV576 show the highest degree of brightness (D–H).Note the increased degree of brightness in all of these co-treated cells. Also note the normal nuclear to cytoplasmic ratio in (D), increased ratio in (E), and further increased ratio in (F). also shows apoptotic HL60/AR cells. Note the cells with prominent membrane blebbing (G and H).
Abbreviations: CLSM, confocal laser scanning microscopy; DNR, daunorubicin.
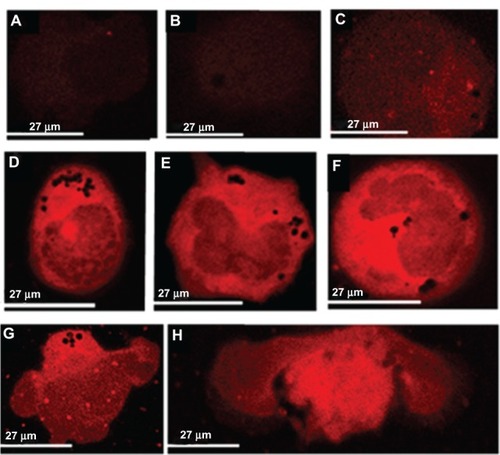
AFM studies
AFM studies were carried out to examine the hole formation in HL60/AR cells treated with DNR in the presence or absence of DPV576. Results show that hole formation is detected in the control () and in DNR only treated cells (). However, hole formation is increased post-treatment with DPV576 (). On the other hand, for HL60/AR cells treated with DNR plus DPV576, AFM detected marked increases in the size and number of holes (–). These holes ranged from 40–500 nm in depth, and 0.1–2.5 μm in diameter, and were situated in the cytoplasm and the nucleus. Contrast images of correspond to and regions in dark orange indicate the depth of the holes. shows an apoptotic HL60/AR cell with membrane blebbing.
Figure 4 HL60/AR cells were cultured with DPV576 (10% V/V) for 24 hours then exposed to DNR (2 μM) for 45 minutes. Cytospin preparations of cells under different treatment conditions were air dried, fixed in MeOH, and prepared for AFM studies. Preparation showed no significant changes in hole formation in control cells untreated with DNR and DPV576 (A) and DNR only treated (B). DPV576 only treated (C) showed an increase in the number of holes. Preparation of DPV576 plus DNR treated cells (D–F) showed more frequent and larger-sized holes (white arrows). Contrast images of (D and E) correspond to (G and H), respectively, and regions in dark orange indicate the depth of the holes (black arrows). Finally, (I) shows an apoptotic cancer cell with signs of membrane blebbing.
Abbreviations: AFM, atomic force microscopy; DNR, daunorubicin.
Discussion
Several studies have shown promising results for the role of nanoparticles in the reversal of chemo/radio-sensitization in cancer cells. Nanoparticles loaded with chemotherapeutic drugs have been used to successfully deliver drugs to the cytoplasm, nucleus, and other specific organelles.Citation26–Citation28 For example, DNR-loaded magnetic nanoparticles of Fe3O4 can overcome MDR.Citation29 In another study, nickel nanoparticles have shown increased cancer cell membrane permeability.Citation7 However, the use of nanoparticles as DNR sensitizers has been of major concern due to their known toxicity. Exposure by inhalation to fine-grained nickel nanoparticles can cause cancer formation,Citation30 illness, and in some cases, death.Citation31 Cobalt nanoparticles have also been shown to display similar levels of toxicityCitation32 and tumor formation.Citation33
Unlike other nanoparticles, DPV576 exhibits low levels of toxicity and yet reverses MDR in HL60/AR cells. Our preliminary results showed that DPV576 is less toxic as demonstrated by its ability to enhance human T cell and B cell proliferation in vitro (data not shown). Further studies by Schrand et al reported that NDs are non-toxic to a variety of cell types and did not produce significant reactive oxygen species.Citation34 In addition, the intratracheally instilled NDs showed no significant adverse effects in the lungs of mice as evaluated through histopathological and ultrastructural examinations.Citation35 Further studies suggest that NDs may have several biomedical applications due to their unique properties such as large specific surface areas, stable photoluminescence, and ability to be functionalized with biomolecules.Citation36,Citation37 Moreover, DPV576 has shown immune modulatory effects such as activation of human monocyte-derived dendritic cellsCitation21 and modulation of murine T lymphocytes by a mixture of ND/NP coated onto fabrics (DPV576-C) in mice.Citation22 In addition, results of the current study show that DPV576 reverses MDR in HL60/AR by a mechanism that involves hole induction.
The mechanisms of MDR in cancer cells have been the focus of research. These include a decreased uptake of hydrophilic drugs which require transporters to enter cells and an increased energy-dependent efflux of hydrophobic drugs that can enter cancer cells through the plasma membrane by diffusion. Other factors to be considered in reversing MDR include the decreased sensitivity to drug-induced apoptosis and induction of drug-detoxifying mechanisms.Citation38,Citation39 In the last four decades, several attempts have been made to reverse MDR in MRP, and several chemosensitizers have been developed. These include: probenecid,Citation17 genistein,Citation18 buthionine sulfoximine,Citation19 ethacrynic acid, and calcium channel blockers.Citation20 In addition, hole induction by several nanoparticles represents an additional mechanism for reversing MDR.Citation7
Our results indicate that DPV576 is an effective agent in the reversal of MDR as exemplified by the reduction of the IC50 to 1/4th upon exposure to DPV576. We identified via flow cytometry an increased accumulation of DNR in HL60/AR cells that was associated with the presence of several holes. The ability of different nanoparticles to induce holes has been reported in several types of cells. These include: transient holes caused by exposure of the cell membrane to monolayered protected nanoparticles;Citation40 cationic nanoparticles and macromolecules that generate transient holes in several biological membranes;Citation41 and an amine-modified nanoparticle treatment which resulted in hole induction in transformed human alveolar epithelial type 1-like cells.Citation42 The idea that such nanoparticles have the ability to alter the permeability of the respective cell membrane and thus facilitate the relevant drug uptake is very significant.
The observation of the hole-inducing capabilities of DPV576 may represent a possible mechanism by which this agent reverses MDR. Cancer cells have been shown to phagocytize microorganisms and other cells, and subsequently, vacuoles can be seen in these cancer cells. Many tumor cells exhibit phagocytic activity, including: phagocytosis of titanium particles by sarcoma L929 cells;Citation43 elastic fibers by dermatofibroma cells;Citation44 erythrocytes and bacteria by adenocarcinomas;Citation45,Citation46 Candida albicans by lymphatic tumor cells;Citation47 and Saccharomyces cerevisiae by human breast, oral, and colon cancer cells.Citation48,Citation49 Cancer cells can also phagocytize other cells such as lymphocytesCitation50,Citation51 and neutrophils.Citation52 In addition, phagocytosis of one tumor cell by another tumor cell has been reportedCitation53 and is referred to as cannibalism. In the current study, we detected the presence of holes in the control untreated HL60/AR cells () via AFM, which may be attributed to their cannibalistic activity. Treatment with DPV576 increased hole formation in cells () and showed a three-fold increase in the percentage of cells with multiple holes (≥4) as compared to control untreated cells () and DNR only treated cells (). However, HL60/AR cells co-treated with DPV576 plus DNR showed holes more frequent in number and which appeared larger in size, ranging from 40–500 nm in depth and 0.1–2.5 μm in diameter, and were situated in the cytoplasm and the nucleus (–). AFM studies were further confirmed using CLSM imaging.
Another mechanism by which DPV576 might kill HL60/AR cells is through interference with MRP and drug transport via modulation of the transport function of MRP. MRP is a member of the ATP-binding cassette superfamily of membrane transport proteins and has been suggested to play a role in the reduction of drug accumulation.Citation19 Normally, MDR cells overexpress P-glycoproteins (P-gp); using a nanoformulation of drugs is one way of bypassing the P-gp pumps. Since HL60/AR is a cell line that is known to overexpress MRP,Citation17 the data suggests that DPV576 might induce HL60/AR cell apoptosis through interference with drug transport in addition to inducing holes in the cancer cell membrane.
Several studies suggest that DPV576 serves a dual purpose in the fight against cancer – in addition to its ability to induce holes in cancer cells, it has been shown to have an immune modulatory effect.Citation21,Citation22 Such properties of DPV576 make this agent advantageous over other nanoparticles.
Conclusion
This study strongly suggests that DPV576 reverses resistance in HL60/AR cells by increasing cellular DNR accumulation via a mechanism that may involve induction of holes in the cellular membrane. It should be investigated as a unique candidate for drug delivery and MDR therapy with less toxic effects.
Acknowledgments
The authors are greatly indebted to Dr Sastry Gollapudi, Professor of Immunology, Division of Basic and Clinical Immunology, University of California, Irvine, CA, USA, for assistance in revision of the manuscript. The authors would also like to thank Venex Company, Atsugi, Kanagawa, Japan, for providing DPV576. Confocal laser scanning microscope work was performed at the California Nanosystems Institute Advanced Light Microscopy/Spectroscopy Shared Resource Facility at the University of California, Los Angeles, supported with funding from the National Institutes of Health – National Center for Research Resources shared resources grant (CJX1-443835-WS-29646) and the National Science Foundation Major Research Instrumentation grant (CHE-0722519). Atomic force microscopy imaging was performed at Nano and Pico Characterization Lab at the California Nanosystems Institute.
Disclosure
The authors report no conflicts of interest in this work.
References
- LuJLiongMShermanSMesoporous silica nanoparticles for cancer therapy: energy-dependent cellular uptake and delivery of paclitaxel to cancer cellsNanobiotechnology200732899519936038
- AnhornMGWagnerSKreuterJLangerKvon BriesenHSpecific targeting of HER2 overexpressing breast cancer cells with doxorubicin loaded trastuzumab-modified human serum albumin nanoparticlesBioconjug Chem200819122321233118937508
- YousefpourPAtyabiFVasheghani-FarahaniEMovahediAADinarvandRTargeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumabInt J Nanomedicine201161977199021976974
- ChenTChengTChenCTargeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRIJ Biol Inorg Chem200914225326018975017
- SunBRanganathanBFengSSMultifunctional poly (D,L-lactidecoglycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancerBiomaterials200829447548617953985
- Cirstoiu-HapcaABossy-NobsLBucheggerFGurnyRDelieFDifferential tumor cell targeting of anti-HER2 (Herceptin®) and anti-CD20 (Mabthera®) coupled nanoparticlesInt J Pharm2007331219019617196347
- GuoDWuCLiJSynergistic effect of functionalized nickel nanoparticles and Quercetin on inhibition of the SMMC-7721 cells proliferationNanoscale Res Lett20094121395140220651919
- SteinhauserISpänkuchBStrebhardtKLangerKTrastuzumab modified nanoparticles: optimisation of preparation and uptake in cancer cellsBiomaterials200627284975498316757022
- SteinhauserIMLangerKStrebhardtKMSpänkuchBEffect of trastuzumab-modified antisense oligonucleotide-loaded human serum albumin nanoparticles prepared by heat denaturationBiomaterials200829294022402818653231
- SharmaSSantiskulvongCBentolilaLARaoJDorigoOGimzewskiJKCorrelative nanomechanical profiling with super-resolution F-actin imaging reveals novel insights into mechanisms of cisplatin resistance in ovarian cancer cellsNanomedicine20128575776622024198
- SingalPKIliskovicNDoxorubicin-induced cardiomyopathyN Engl J Med1999339139009059744975
- FrancisPAKrisMGRigasJRGrantSCMillerVAPaclitaxel (Taxol) and docetaxel (Taxotere): active chemotherapeutic agents in lung cancerLung Cancer19951S163S1727551925
- FossellaFVLeeJSBerilleJHongWKSummary of phase II data of docetaxel (Taxotere), an active agent in the first- and second-line treatment of advanced non-small cell lung cancerSemin Oncol199522222297740327
- StraussGMLynchTJEliasADA phase I study of ifosfamide/carboplatin/etoposide/paclitaxel in advanced lung cancerSemin Oncol199522470747544029
- SandersonBJFergusonLRDennyWAMutagenic and carcinogenic properties of platinum-based anticancer drugsMutat Res19963551–259708781577
- SantinADHermonatPLRavaggiAEffects of concurrent cisplatinum administration during radiotherapy vs radiotherapy alone on the immune function of patients with cancer of the uterine cervixInt J Radiat Oncol Biol Phys2000484997100611072156
- GollapudiSKimCHTranBNSanghaSGuptaSProbenecid reverses multidrug resistance in multidrug resistance-associated protein-overexpressing HL60/AR and H69/AR cells but not in P-glycoprotein-overexpressing HL60/Tax and P388/ADR cellsCancer Chemother Pharmacol19974021501589182837
- VersantvoortCHSchuurhuisGJPinedoHMGenistein modulates the decreased drug accumulation in non-P-glycoprotein mediated multidrug resistant tumour cellsBr J Cancer19936859399468105867
- ChumanYChenZSSetoKReversal of MRP-mediated vincristine resistance in KB cells by buthionine sulfoximine in combination with PAK-104PCancer Lett1998129169769714337
- PhillipsPCAntineoplastic drug resistance in brain tumorsNeurol Clin1991923834041682794
- GhoneumMGhoneumAGimzewskiJNanodiamond and nanoplatinum liquid, DPV576, activates human monocyte-derived dendritic cells in vitroAnticancer Res201030104075407921036722
- GhoneumMGhoneumATolentinoLGimzewskiJModulation of aged murine T lymphocytes in vivo by DPV576-C, a nanodiamond- and nanoplatinum-coated materialIn Vivo201024214114620363985
- SharmaSRasoolHIPalanisamyVStructural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopyACS Nano2010441921192620218655
- PetrouIHeuRStranickMA breakthrough therapy for dentin hypersensitivity: how dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teethJ Clin Dent2009201233119489189
- KumagaiKItohKSuzukiRHinumaSSaitohFStudies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicitiesJ Immunol198212913883946979572
- AllenTMCullisPRDrug delivery systems: Entering the mainstreamScience20043031818182215031496
- CholerisELittleSRMongJAPuramSVLangerRPfaffDWMicroparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female miceProc Natl Acad Sci USA20071044670467517360582
- MisraRSahooSKCoformulation of doxorubicin and curcumin in poly(D,L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cellsMol Pharm20118385286621480667
- ChenBALaiBBChengJDaunorubicin-loaded magnetic nanoparticles of Fe3O4 overcome multidrug resistance and induce apoptosis of K562-n/VCR cells in vivoInt J Nanomedicine2009420120819918366
- SanoNShibataMIzumiKOtsukaHHistopathological and immunohistochemical studies on nickel sulfide-induced tumors in F344 ratsJpn J Cancer Res1988792122212835348
- PhillipsJIGreenFYDaviesJCMurrayJPulmonary and systemic toxicity following exposure to nickel nanoparticleAm J Ind Med20105376376720623660
- LisonDDe BoeckMVerougstraeteVKirsch-VoldersMUpdate on the genotoxicity and carcinogenicity of cobalt compoundsOccup Environ Med20015861966211555681
- HansenTClermontGAlvesABiological tolerance of different materials in bulk and nanoparticulate form in a rat model: sarcoma development by nanoparticlesJ R Soc Interface200631176777517015296
- SchrandAMHuangHCarlsonCAre diamond nanoparticles cytotoxic?J Phys Chem B200711112717201422
- YuanYWangXJiaGPulmonary toxicity and translocation of nanodiamond in miceDiam Relat Mater201019291299
- XingYDaiLNanodiamonds for nanomedicineNanomedicine20094220721819193186
- MochalinVNShenderovaOHoDGogotsiYThe properties and applications of nanodiamondsNat Nanotechnol201171112322179567
- SzakácsGPatersonJKLudwigJABooth-GentheCGottesmanMMTargeting multidrug resistance in cancerNat Rev Drug Discov20065321923416518375
- GottesmanMMMechanisms of cancer drug resistanceAnnu Rev Med20025361562711818492
- VermaAUzunOHuYSurface structure-regulated cell membrane penetration by monolayer protected nanoparticlesNat Mater20087758859518500347
- LeroueilPRHongSYMeckeABakerJROrrBGHollMMNanoparticle interaction with biological membranes: Does nanotechnology present a janus face?Accounts Chem Res200740335342
- RuenraroengsakPNovakPBerhanuDRespiratory epithelial cytotoxicity and membrane damage (holes) caused by amine-modified nanoparticlesNanotoxicology2012619410821352086
- OsanoEKishiJTakahashiYPhagocytosis of titanium particles and necrosis in TNF-alpha resistant mouse sarcoma L929 cellsToxicol In Vitro2003171414712537961
- KiyoharaTKumakiriMKobayashiHOhkawaraALaoLMAtrophic dermatofibroma. Elastophagocytosis by the tumor cellsJ Cutan Pathol200027631231510885409
- Marin-PadillaMErythrophagocytosis by epithelial cells of a breast carcinomaCancer197739310851089199342
- VandenbergheJVerheyenALauwersSGeboesKSpontaneous adenocarcinoma of the ascending colon in Wistar rats: the intracytoplasmic presence of a Campylobacter-like bacteriumJ Comp Pathol198595145553973110
- GhoneumMGrewalIBrownJOsborneRElembabiHGillGPhagocytosis of candida albicans by lymphatic tumour cells in vitroActa Histochem2003105212713312831164
- GhoneumMGollapudiSPhagocytosis of Candida albicans by metastatic and non metastatic human breast cancer cell lines in vitroCancer Detect Prev2004281172615041073
- GhoneumMHamiltonJBrownJGollapudiSHuman squamous cell carcinoma of the tongue and colon undergoes apoptosis upon phagocytosis of Saccharomyces cerevisiae, the baker’s yeast, in vitroAnticancer Res2005252A98198915868937
- GhoneumMSalemFShumSSPerryLGillGIn situ lymphophagocytosis by nonlymphoreticular neoplasmsNat Immun Cell Growth Regul19876277873600677
- GhoneumMSalemFAllenHGillGPhagocytosis of autologous lymphocytes by cervical preneoplastic and neoplastic cellsNat Immun Cell Growth Regul1988742392483237234
- SinghalNHandaUBansalCMohanHNeutrophil phagocytosis by tumor cells – a cytological studyDiagn Cytopathol201139855355520949458
- OverholtzerMMailleuxAAMouneimneGA nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasionCell2007131596697918045538