?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The purpose of this study was to explore whether magnetic Fe3O4 nanoparticles (Fe3O4-MNP) loaded with cisplatin (Fe3O4-MNP-DDP) can reverse DDP resistance in lung cancer cells and to investigate mechanisms of multidrug resistance in vitro and in vivo. MTT assay showed that DDP inhibited both A549 cells and DDP-resistant A549 cells in a time-dependent and dose-dependent manner, and that this inhibition was enhanced by Fe3O4-MNP. An increased rate of apoptosis was detected in the Fe3O4-MNP-DDP group compared with a control group and the Fe3O4-MNP group by flow cytometry, and typical morphologic features of apoptosis were confirmed by confocal microscopy. Accumulation of intracellular DDP in the Fe3O4-MNP-DDP group was greater than that in the DDP group by inductively coupled plasma mass spectrometry. Further, lower levels of multidrug resistance-associated protein-1, lung resistance-related protein, Akt, and Bad, and higher levels of caspase-3 genes and proteins, were demonstrated by reverse transcriptase polymerase chain reaction and Western blotting in the presence of Fe3O4-MNP-DDP. We also demonstrated that Fe3O4-MNP enhanced the effect of DDP on tumor growth in BALB/c nude mice bearing DDP-resistant human A549 xenografts by decreasing localization of lung resistance-related protein and Ki-67 immunoreactivity in cells. There were no apparent signs of toxicity in the animals. Overall, these findings suggest potential clinical application of Fe3O4-MNP-DDP to increase cytotoxicity in lung tumor xenografts.
Introduction
The incidence of lung cancer is increasing worldwide, and non-small cell lung cancer (NSCLC) poses a serious threat to human life and health.Citation1 Unfortunately, more than half of patients with NSCLC have advanced disease at diagnosis, and have missed the opportunity for surgery, so chemotherapy plays an important role in treatment of the disease. Cisplatin (DDP)-based combination chemotherapies are used as standard treatment for patients with advanced NSCLC and good performance status,Citation2 and have been shown to achieve significant improvement in overall survival and quality of life.Citation3,Citation4 However, DDP-based regimens often have severe side effects, which are a frequent cause of poor tolerability, limited therapeutic efficacy, and drug discontinuation.Citation5 Therefore, novel strategies for treatment of lung cancer are urgently needed.
Recently, one promising strategy to reduce the toxicity of chemotherapy while maintaining its therapeutic effects has been to use drug-coated polymer nanospheres or nanoparticles, which can increase the chemosensitivity of tumor cells.Citation6,Citation7 Previous studies have shown that magnetic Fe3O4 nanoparticles (Fe3O4-MNP) when used as a drug delivery system can enhance the sensitivity of anticancer drugs and reverse drug resistance in DDP-resistant SKOV3 human ovarian cancer cells.Citation8,Citation9 Moreover, an in vivo study has shown that Fe3O4 nanoparticles copolymerized with daunorubicin and 5-bromotetrandrine strongly inhibit growth of xenograft tumors in nude mice.Citation10 However, to date, the signal transduction pathways involved in the beneficial effects of Fe3O4-MNP in the treatment of lung cancer are largely unknown.
Preclinical research on resistance markers is focused on established human cancer cell lines selected for resistance or on xenografts derived from these cell lines. Multidrug resistance, either inherent or acquired, is a serious problem and one of the most important obstacles to successful treatment of lung cancer. Therefore, a better understanding of drug resistance mechanisms and ways to disrupt the relevant signaling might be an efficient strategy to improve survival of patients with lung cancer.Citation11 In the past decade, a number of studies of intrinsic resistance of tumor cells to chemotherapy have focused on transporter proteins, including P-glycoprotein, multidrug resistance-associated protein (MRP), and lung resistance-related protein (LRP),Citation12 which promote resistance by increasing drug efflux, decreasing drug influx, drug inactivation, and alteration of drug targets.Citation13
Patient-derived tumor xenografts may also play a key role in the search for more reliable multidrug resistance markers. There is accumulating evidence that several chemotherapeutic agents involved in the treatment of cancer interfere with the phosphatidylinositol 3-kinase/Akt pathway,Citation14 in which abnormalities of the signal transduction pathway are important in tumorigenesis and tumor progression. Akt is a cytosolic intracellular signal transduction protein that may be a prognostic factor by being closely associated with the development and progression of NSCLC.Citation15 Further, Ki-67 is a nuclear protein of unclear function, but is present in all proliferating cells, including those in normal and tumor tissue.Citation16 It is present during all active phases of the cell cycle (G1, S, G2, and mitosis) and is a biologic tumor marker that follows changes in tumor proliferation between pretreated and treated samples, typically core biopsies and surgical samples.Citation17 Therefore, downregulation of Ki-67 may also be used as a marker of the effect of Fe3O4-MNP loaded with DDP (Fe3O4-MNP-DDP) on NSCLC xenografts.
Motivated by those considerations, the present study investigated a novel therapeutic strategy targeting DDP-resistant lung cancer in vitro and in vivo, and provides theoretical evidence for its clinical application.
Materials and methods
Materials
The following materials were obtained for use in this study: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, St Louis, MO, USA); cisplatin (Qilu Pharmaceutical Co, Ltd, Jinan, People’s Republic of China); RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA); fetal bovine serum (Gibco, Carlsbad, CA, USA); penicillin and streptomycin (Gibco BRL); an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Becton Dickinson, Franklin Lakes, NJ, USA); fluorechrome dye 4, 6-diamidino-2-phenylindole (DAPI, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA); Lipofectamine™ 2000 (Invitrogen Life Technologies, Carlsbad, CA, USA); a reverse transcriptase polymerase chain reaction (RT-PCR) kit (TaKaRa Biotechnology Co, Ltd, Dalian, People’s Republic of China); a bicinchoninic acid protein assay kit (Beijing Com Win Biotech Co, Ltd, Beijing, People’s Republic of China); monoclonal antihuman MRP1, LRP, Bad, Akt1, P-Akt1, caspase-3, β-actin antibody, and horseradish peroxidase-labeled immunoglobulin G (Santa Cruz Biotechnology Inc); monoclonal anti-LRP and Ki-67 antibody for immunocytochemistry (Sigma); and secondary antimouse biotinylated antibody (Gibco BRL).
Preparation of drug-loaded nanoparticles
Following on from previous studies,Citation18 Fe3O4-MNP were synthesized by electrochemical deposition under oxidizing conditions, and their morphology was observed by transmission electron microscopy (JEM-2100, JEOL, Tokyo, Japan). Before use, the Fe3O4-MNP were well dispersed in RPMI-1640 medium containing 10% (v/v) heat-inactivated fetal bovine serum using ultrasound to obtain a colloidal suspension of Fe3O4-MNP. Next, 25 μg/mL Fe3O4-MNP was added under mechanical stirring to a final volume of 200 μL containing a certain concentration of DDP (v/v). The time taken to polymerize Fe3O4-MNP-DDP at 4°C was 24 hours.Citation9
Cell lines
Cisplatin-resistant A549 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 μmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C, 95% relative humidity, and 5% CO2. To maintain their drug resistance, DDP-resistant A549 cells were cultured with 5 μg/mL DDP and then cultured further in DDP-free RPMI-1640 medium for two days before starting the experiment.
Cell proliferation assay
For the cell viability assays, 5 × 104/mL of DDP-resistant A549 cells were incubated with Fe3O4-MNP, DDP, or Fe3O4-MNP-DDP. Meanwhile, A549 cells were incubated with a series of concentrations of DDP, and cells treated with RPMI-1640 medium alone were used as controls. After incubation for 48 hours, 20 μL of MTT solution (5 mg/mL) was added to each well at 37°C in the dark for at least 4 hours. Formazan crystals were dissolved in 200 μL of dimethyl sulfoxide in each well, and reduction of MTT was quantified by absorbance at a measurement wavelength of 540 nm and a reference wavelength of 630 nm using a plate reader (Bio-Rad Laboratories, Tokyo, Japan). The cell inhibition rate was calculated as (1 − A treated cells/A control cells) × 100%. These experiments were repeated at least three times.
Apoptosis assay by flow cytometry
After treatment for 48 hours as described above, the cells were harvested, stained, and evaluated for apoptosis by flow cytometry according to the manufacturer’s protocol. Briefly, 1 × 106 cells were stained with 5 μL of Annexin V-FITC for 20 minutes in the dark, and 10 μL of propidium iodide (5 μg/mL) in 1 × binding buffer was then added to each sample. Next, apoptosis was determined by flow cytometry (FACSCalibur, Becton-Dickinson) using Cell Quest software (BD Biosciences, San Jose, CA, USA).
Immunofluorescence staining and confocal microscopy
Cells were cultured on cover slips, and kept in a 35 mm Petri dish for 16–20 hours before treatment. After being cultured for 48 hours as described earlier, the cells were washed with isotonic phosphate-buffered solution (pH 7.4) and then fixed with 4% paraformaldehyde solution for 20 minutes at 37°C. Next, the cover slips were washed three times with phosphate-buffered solution and stained with 2.5 μg/mL DAPI solution for 10 minutes at room temperature. Finally, the cells were washed twice with phosphate-buffered solution, and morphologic changes in apoptotic tumor cells were observed immediately by confocal microscopy (FluoView® FV1000, Olympus, Tokyo, Japan).
Measurement of intracellular DDP accumulation
Intracellular exchange of DDP was measured by inductively coupled plasma mass spectrometry (ICP-MS, X-7 Series, Thermo Fisher Scientific Inc, Waltham, MA, USA).Citation19 Briefly, 5 × 104/mL cells were incubated with DDP or Fe3O4-MNP-DDP for 48 hours. After incubation, the cells were washed twice with phosphate-buffered solution, and centrifuged at 3000 rpm for 30 minutes. The pellet was then dissolved in 33% nitric acid, and the concentration of DDP was determined by ICP-MS.
Transfection
DDP-resistant A549 human lung cells were cultured as described already.Citation20 Transfection was done with Lipofectamine 2000 under serum-free conditions according to the manufacturer’s recommendations. Briefly, DDP-resistant A549 cells were seeded at a density of 5 × 103 into six-well plates for 24 hours, which grew to 50%–70% confluence by the next day. After washing with phosphate-buffered solution, the DDP-resistant A549 cells were transfected by Lipofectamine 2000 using pEGFP-N1 Vector, as in our recently reported study,Citation21 then incubated at 37°C in a 5% CO2 incubator for 48 hours. The cells were then cultured in medium containing Geneticin 800–1000 μg/mL. The transfected clones were used for transplantation in vivo.Citation22
Tumor model and treatment
BALB/c mice (aged 4 weeks, mean body weight 20 g) were purchased from the Shanghai National Center for Laboratory Animals and kept in a specific pathogen-free environment where temperature was maintained at 22°C and humidity in the range of 40%–50%. Studies involving these mice were performed in adherence with the Guidelines for the Care and Use of Laboratory Animals of the National Institute of Health.
To assess the effect on tumorigenicity, stable transfected DDP-resistant A549 cells were harvested and the BALB/c mice were subcutaneously inoculated on the left abdominal wall with 1 × 107 cells suspended in 0.2 mL of RPMI-1640 medium, for developing the NSCLC xenograft. When tumor sizes in the nude mice had reached an approximate volume of 200 mm3, calculated by the following equation:
where a is the longest diameter and b is the shortest diameter, the tumor-bearing mice were randomly allocated to a control group, a Fe3O4-MNP group, a DDP group, or a Fe3O4-MNP-DDP group (n = 6 per group). Each mouse received an intraperitoneal injection of normal saline (0.2 mL), Fe3O4-MNP (22 mg/kg in 0.2 mL of normal saline), DDP (7.5 mg/kg in 0.2 mL of normal saline), or 22 mg/kg Fe3O4-MNP and 7.5 mg/kg DDP in normal saline. Tumor growth was monitored by multispectral acquisition and analysis (CRi Maestro™ system, Cambridge Research and Instrumentation Inc, Woburn, MA, USA) for four weeks. Thereafter, the mice were sacrificed and their tumor xenografts were harvested and processed for histopathologic analysis to determine protein expression.
RT-PCR assay
After treatment for 48 hours as described above, total cellular RNA was extracted using TRIzol reagent, and RT-PCR was performed according to the manufacturer’s instructions. Briefly, 1 μg samples of total RNA were used to synthesize cDNA at 30°C for 10 minutes, 42°C for 30 minutes, 99°C for 5 minutes, and 5°C for 5 minutes. The newly synthesized cDNA was then amplified by RT-PCR at 94°C for 5 minutes, 94°C for 45 seconds, 55°C for 45 seconds, and 72°C for one minute for 35 cycles. RT-PCR primers were used for MRP1 (526 base pair [bp]) sense: 5′-TCAAATACCTGCTGTTCGG-3′ and antisense: 5′-TGAAGTCCTGTCCTGATGCCA T-3′; LRP (391 bp) sense: 5′-GTGGTGGTAGGAGATGAGTG-3′ and antisense: 5′-CCAGATGTCCACGAGGAGG-3′; Bad (304 bp) sense: 5′-GCTCTTCCTTTGTTCATCTCC-3′ and antisense: 5′-CATCTGGCTCGGGGTTACTGC-3′; Akt1 (159 bp) sense: 5′-TACTCTTTCCAGACCCACGAC-3′ and antisense: 5′-GGACTACCTGCACTCGGAG-3′; caspase-3 (459 bp) sense: 5′-TGTCGATGCAGCAAACCT-3′ and antisense: 5′-CATCCAGTCGCTTGTGC-3′; β-actin (270 bp) sense: 5′-CTACAATGAGCTGCGTGTGGC-3′ and antisense: 5′-CAGGTCCAGACGCAGGATGGC-3′. The PCR products were then separated on 1.5% agarose gel and band intensities were quantified directly on gel photographs by Imager Master VDS image analysis (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Western blot assay
After incubation for 48 hours as described above, the cells were collected and total protein from each sample was separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels using a modified radioimmunoprecipitation assay buffer. The proteins were then transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Western blotting was performed with 1:1500 or 1:1000 dilutions of monoclonal antibodies against either antihuman MRP1, LRP, Bad, Akt1, P-Akt1, caspase-3, or β-actin antibody in 5% nonfat milk, followed by horseradish peroxidase-labeled immunoglobulin G as a secondary antibody (1:2000). The blots were visualized by densitometry scans (ECL system, Amersham Biosciences, Little Chalfont, UK) according to the manufacturer’s instructions.
Immunocytochemistry study
Tissue sections 4 μm thick were cut from representative formalin-fixed and paraffin-embedded blocks, deparaffinized in xylene, and rehydrated routinely before staining. Sections were treated with 7% hydrogen peroxide in methanol for 20 minutes, microwaved for 5 minutes in citrate phosphate buffer (pH 6.0), and incubated with 5% milk in distilled water for 45 minutes, with thorough washing in phosphate-buffered solution between steps.
Individual sections were incubated with monoclonal anti-LRP and Ki-67 antibody (working dilution 1:100) at 4°C overnight. After washing, the sections were reincubated with a secondary antimouse biotinylated antibody (1:1000) in a dark room for one hour. Next, stained slides were examined to identify localization of LRP and Ki-67 immunoreactivity within the cells. Cytoplasmic staining was considered to be positive for LRP, and nuclear staining was considered to be positive for Ki-67.Citation23
Statistical analysis
The data are presented as the mean and standard deviation. All analyses were performed using the Statistical Package for Social Sciences version 13 (SPSS Inc, Chicago, IL, USA). Differences were evaluated using the Student’s t-test or analysis of variance. P < 0.05 was considered to be statistically significant.
Results
Morphology of magnetic Fe3O4 nanoparticles
Transmission electron microscopy indicated that the majority of Fe3O4-MNPs were spherical in shape with particle sizes of about 30 nm ().
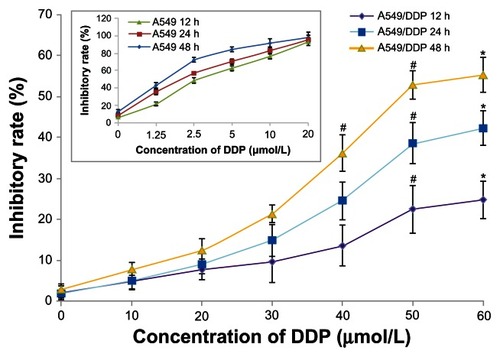
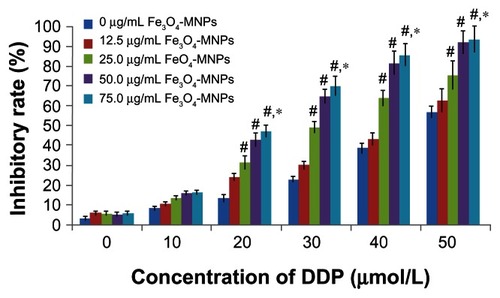
Fe3O4-MNPs enhanced inhibition of DDP in cells
When DDP-sensitive and DDP-resistant A549 cells were treated with various concentrations of DDP for 12, 24, and 48 hours, DDP-sensitive A549 cells were more sensitive to treatment with DDP than were DDP-resistant A549 cells (). MTT assay showed that inhibition of DDP-resistant A549 cells treated with DDP occurred in a time-dependent and dose-dependent manner, and was significantly increased in the presence of Fe3O4-MNP (P < 0.05, ), suggesting that Fe3O4-MNP could reverse DDP resistance and enhance cytotoxicity in DDP-resistant A549 cells. Interestingly, Fe3O4-MNP alone, at concentrations of 12.5 μg/mL to 75 μg/mL, could slightly inhibit proliferation of DDP-resistant A549 cells, and there was no statistically significant difference compared with the control group (P > 0.05, ), suggesting that Fe3O4-MNP did not have marked toxic effects in DDP-resistant A549 cells. On the basis of the findings of our MTT assay and previous research,Citation9 we selected 25 μg/mL as the optimal concentration of Fe3O4-MNP for targeted drug delivery in the present study.
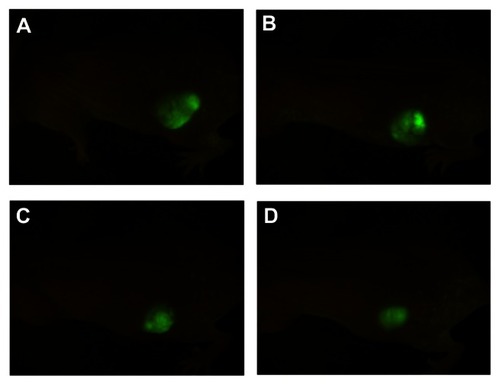
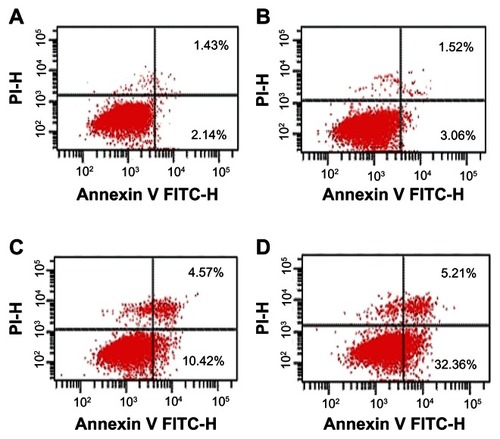
Annexin V-propidium iodide assays for apoptosis
After 48 hours, there was no significant difference in the rate of apoptosis between cells in mice treated with Fe3O4-MNP alone and the control group (P > 0.05). The apoptotic rate was significantly higher in the group treated with Fe3O4-MNP-DDP than in the groups treated with DDP alone or Fe3O4-MNP alone, or the control group (), suggesting that Fe3O4-MNP could enhance DDP-induced apoptosis.
Figure 4 Apoptosis of DDP-resistant A549 cells treated with DDP or Fe3O4-MNP-DDP for 48 hours. (A) Control, (B) 25 μg/mL Fe3O4-MNP, (C) 20 μmol/L DDP, and (D) Fe3O4-MNP-DDP.
Abbreviations: Fe3O4-MNP, magnetic Fe3O4 nanoparticles; DDP, cisplatin; FITC, fluorescein isothiocyanate; PI, propidium iodide.
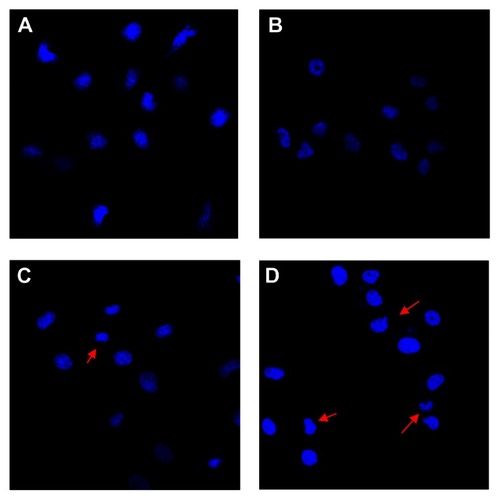
Nuclear morphologic changes in DDP-resistant A549 cells
Nuclear DAPI staining was performed and cells were observed by confocal microscopy to confirm that apoptosis was occurring. DDP-resistant A549 cells in the control and Fe3O4-MNP groups were found to have an intact nuclear structure with consistent low-intensity blue fluorescence (), whereas more apparently apoptotic DDP-resistant A549 cells showing high-intensity blue fluorescence were observed in the DDP and Fe3O4-MNP-DDP groups (). Interestingly, cells in the later stages of apoptosis showing either an irregular cell profile or formation of budding prominences on the cell surface, and apoptotic bodies were subsequently observed more often in the Fe3O4-MNP-DDP group (), indicating that Fe3O4-MNP could induce apoptosis in DDP-resistant A549 cells exposed to DDD.
Figure 5 Changes in nuclear morphology of DDP-resistant A549 cells after treatment for 48 hours (DAPI staining, ×400). (A) Control, (B) 25 μg/mL Fe3O4-MNP, (C) 20 μmol/L DDP, and (D) Fe3O4-MNP-DDP.
Notes: The red arrows indicate the apparent apoptotic cells with incomplete nuclear.
Abbreviations: Fe3O4-MNP, magnetic Fe3O4 nanoparticles; DDP, cisplatin; DAPI, fluorechrome dye 4, 6-diamidino-2-phenylindole.
Accumulation of intracellular DDP
ICP-MS showed that accumulation of intracellular DDP in the Fe3O4-MNP-DDP group was nearly three-fold higher than that in the DDP group (15.28 ± 1.16 μg/L and 5.53 ± 0.09 μg/L, respectively, P < 0.05) when DDP-resistant A549 cells had been treated for 48 hours, suggesting that the presence of Fe3O4-MNP could prevent drug release from resistant cells and allow marked accumulation of DDP inside DDP-resistant A549 cells.
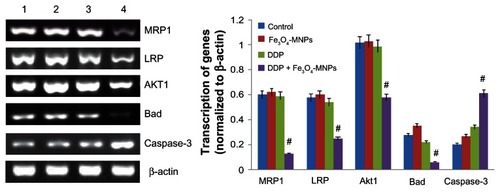
Transcription of genes by RT-PCR
Computer-assisted image analysis indicated that DDP 20 μmol/L had no obvious effect on the transcription of genes in DDP-resistant A549 cells. Of note, less transcription of MRP1, LRP, Akt1, and Bad as well as higher levels of caspase-3 were detected in the Fe3O4-MNP-DDP group than in the control and Fe3O4-MNP groups after incubation for 48 hours (P < 0.05). However, there were no significant differences in genes detected in DDP-resistant A549 cells between the Fe3O4-MNP and control groups (P > 0.05, ).
Figure 6 Transcription of mRNA in DDP-resistant A549 cells using reverse transcriptase polymerase chain reaction after treatment for 48 hours.
Notes: Line 1, control; line 2, 25 μg/mL Fe3O4-MNP; line 3, 20 μmol/L DDP; line 4, Fe3O4-MNP-DDP. #P < 0.05 versus control group.
Abbreviations: Fe3O4-MNP, magnetic Fe3O4 nanoparticles; DDP, cisplatin; MRP, multidrug resistance-associated protein; LRP, lung resistance-related protein.
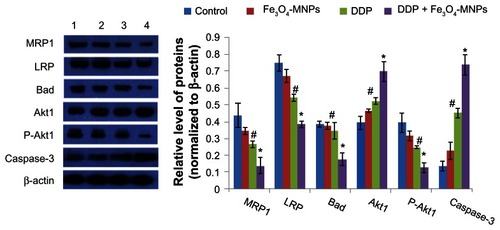
Protein expression detected by Western blotting
After treatment with Fe3O4-MNP-DDP for 48 hours, computer-assisted image analysis showed that levels of MRP1 and LRP proteins were significantly downregulated compared with the control, DDP, and Fe3O4-MNP groups (P < 0.05), and levels of both proteins were markedly decreased in the presence of Fe3O4-MNP (). In contrast, there was no significant difference between the Fe3O4-MNP and control groups (P > 0.05), suggesting that Fe3O4-MNP can enhance downregulation of LRP and MRP1 expression. We also found that levels of caspase-3 and Akt1 proteins in the DDP group were markedly elevated when compared with levels in the control group (P < 0.05), and were enhanced by Fe3O4-MNP (P < 0.05, ). In contrast, Akt1 and Bad protein levels in DDP-resistant A549 cells treated with Fe3O4-MNP-DDP were lower than those in the control group and in the group treated with DDP alone (P < 0.05).
Figure 7 Expression of proteins in DDP-resistant A549 cells after treatment for 48 hours.
Notes: Line 1, control; line 2, 25 μg/mL Fe3O4-MNP; line 3, 20 μmol/L DDP; line 4, Fe3O4-MNP-DDP. #P < 0.05 versus control group; *P > 0.05 versus DDP alone.
Abbreviations: Fe3O4-MNP, magnetic Fe3O4 nanoparticles; DDP, cisplatin; MRP1, multidrug resistance-associated protein-1; LRP, lung resistance-related protein.
Inhibition of tumor growth
A lump with skin-colored wrinkles was observed in the left abdominal wall of each mouse for approximately a week after injection of the transfected DDP-resistant A549 cells, and tumor volumes soon reached a mean of 200 mm3. Thereafter, the BALB/c mice were randomly assigned to the treatment groups and were treated for nearly a month, with tumors growing increasingly larger in the Fe3O4-MNP and control groups, with no obvious difference between these two groups in tumor volume (554 ± 38 mm3 and 417 ± 31 mm3, respectively, P < 0.05, ). Interestingly, tumor volumes in the mice indicated that DDP alone had moderate antitumor efficacy (), whereas subcutaneous tumor growth was suppressed more effectively in mice treated with 22 mg/kg Fe3O4-MNP and 7.5 mg/kg DDP (265 ± 23 mm3 and 149 ± 16 mm3, ), suggesting that the presence of Fe3O4-MNP enhanced the effect of DDP on tumor growth.
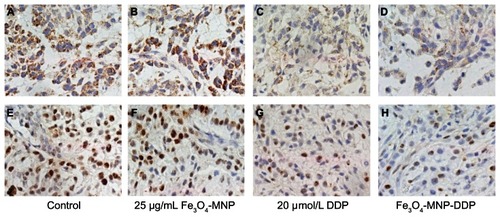
Immunocytochemistry
LRP protein was seen to be overexpressed in the cytoplasm of tumor cells from the control and Fe3O4-MNP groups (), but was markedly decreased after treatment with DDP, with the largest effect seen in the group treated with Fe3O4-MNP-DDP (). We also found the strongest Ki-67 positivity in the control and Fe3O4-MNP groups, with no significant difference in this regard between these two groups (). However, expression of Ki-67 was weaker in tissues treated with Fe3O4-MNP-DDP ().
Toxicity in mice
All of the mice tolerated the study agents satisfactorily, showing no gross signs of cumulative adverse effects, such as weight loss, ruffling of fur, or behavioral and postural changes.
Discussion
Debate continues regarding the value of dose-intense chemotherapy, with analyses suggesting that initially increasing low doses of chemotherapy is of benefit in the treatment of NSCLC, but that little additional benefit is gained using higher doses of chemotherapy.Citation24 Resistance to cisplatin-based chemotherapy is still one of the major obstacles in the treatment of lung cancer, and is generally considered to be related to multidrug resistance genes, apoptosis-related genes, and abnormal function of DNA repair genes,Citation25 although the underlying mechanisms are not yet fully understood. To overcome drug resistance and reduce side effects during chemotherapy, a suitable drug delivery system is required to enhance drug accumulation at the tumor site.
During the last few decades, Fe3O4-MNP have been the most commonly used magnetic nanoparticles, and are a favorable candidate for directing active anticancer agents to tumors in vivo and to protect sensitive tissues from toxicity.Citation26 In light of these characteristics, we have focused on their ability to reverse multidrug resistance in lung cancer cells and the mechanisms involved. The Fe3O4-MNP prepared for use in this study had a spherical shape and a mean particle size of about 30 nm, and are therefore suitable for biologic application in vitro and in vivo. Our cytotoxicity results show that DDP markedly inhibited growth of A549 cells and DDP-resistant A549 cells in a time-dependent and dose-dependent manner, and that this was augmented by Fe3O4-MNP, with no significant effect of Fe3O4-MNP alone on DDP-resistant A549 cells ( and ). ICP-MS showed that intracellular DDP concentrations in cells treated with Fe3O4-MNP-DDP were higher than those in DDP-treated cells, which may be related to the underlying mechanism for nanoparticles entering cells being endocytotic and/or pinocytotic rather than merely a consequence of simple passive permeation,Citation27 thus reducing excretion of DDP. This suggests that the presence of Fe3O4-MNP can effectively prevent drug release from resistant cells and increase intracellular accumulation of DDP in DDP-resistant A549 cells, which may be related to sustained release of DDP into the cytoplasm and reduced excretion. We also found that rates of apoptosis in DDP-resistant A549 cells were higher in the Fe3O4-MNP-DDP group than in the DDP group, which was further demonstrated by the morphologic features of cell nuclei stained with DAPI. All of these observations indicate that Fe3O4-MNP can achieve more cytotoxicity and reverse DDP resistance in lung cancer cells via accumulation of DDP inside cells.
Multiple mechanisms are involved in the cytotoxic effects of DDP, and most believe that the multidrug resistance phenomenon is associated with decreased intracellular accumulation of drugs and overexpression of transmembrane transporters, such as P-glycoprotein and MRP1, both of which are members of the ATP-binding cassette protein transporter superfamily, acting as drugs and/or xenobiotic efflux pumps and modifying drug metabolism via glutathione-S-transferase or cytochrome P450 activity, alterations in DNA repair mechanisms, and modifications in apoptotic signaling.Citation28 MRP, a 190 kDa transmembrane protein, is an ATP-dependent membrane transport protein, and when an anticancer drug enters a tumor cell, MRP uses the hydrolysis energy of ATP to pump the drug back out of the cell, reducing the intracellular drug concentration and increasing drug resistance.Citation29 It is generally accepted that increased MRP expression occurs in tumors with multidrug resistance.Citation30,Citation31 In the present study, we demonstrated that Fe3O4-MNP alone could not decrease MRP1 expression, but could enhance downregulation of MRP1 when combined with DDP, which is consistent with observations in other studies.Citation32 However, some researchers have reported that expression of MRP1 does not occur in response to treatment with cisplatin in lung cancer cell lines or patients,Citation33 and these conflicting observations might be due to different models being used. On the other hand, LRP, an important part of the vault in human cytoplasm and the nuclear membrane, is also suspected of triggering multidrug resistance and being involved in the intracellular distribution of cytotoxic agents.Citation34,Citation35 Accumulating evidence indicates that NSCLC with high expression of LRP is resistant to cisplatin.Citation36,Citation37 In our present study, LRP expression in the Fe3O4-MNP-DDP group was less than that in the DDP group, whereas Fe3O4-MNP alone did not decrease LRP expression in DDP-resistant A549 cells. These results suggest that the regulatory mechanism of both LRP and MRP1 expression is associated with the multidrug resistance phenotype.Citation28
The results of some studies point to the fact that one of the important mechanisms of tumorigenesis and resistance to anticancer drugs is blockade of the pathway triggering apoptosis.Citation38,Citation39 Understanding of the molecular mechanisms of resistance to DDP may lead to improved treatment strategies for DDP-resistant tumors and increased survival in patients with lung cancer. Recent observations indicate that the phosphatidylinositol 3-kinase and mitogen-activated extracellular signal-regulated kinase pathways may contribute to the effect of Akt activation, which has been shown to be a novel mechanism in promoting resistance to DDP in many human malignances, including lung cancer.Citation40,Citation41 To ascertain whether the Akt pathway is activated in DDP-resistant A549 cells, we monitored levels of mRNA and protein for Akt1 and observed less transcription and expression of Bad and Akt1; in contrast, we observed higher protein expression of Akt1 in DDP-resistant A549 cells treated with Fe3O4-MNP-DDP than in those treated with DDP alone. Moreover, phosphorylation of Akt in DDP-resistant A549 cells decreased after treatment with DDP and inhibition of Akt was markedly enhanced by Fe3O4-MNP-DDP. To gain further insight into the mechanism of apoptosis triggered by Fe3O4-MNP-DDP, we used a Western blot assay to determine whether activation of caspase-3 was involved in DDP-induced apoptosis in DDP-resistant A549 cells. Our data show that levels of mRNA and protein for caspase-3 in the Fe3O4-MNP-DDP group were higher than in controls, including both the Fe3O4-MNP and DDP only groups, which further confirms that Fe3O4-MNP enhance the effect of DDP on activation of caspase-3. Bad induces apoptosis by inhibiting members of the antiapoptotic Bcl-2 family, such as Bcl-xL, thereby allowing two other proapoptotic members, Bak and Bax, to form complexes, leading to the release of cytochrome c, activation of caspase-3, and apoptosis.Citation42 In light of the present data and previous findings, the notion that the Akt pathway is involved in cisplatin resistance in lung cancer cell lines is strengthened, and regulation of this pathway may augment apoptosis in DDP-resistant lung cancer cell lines.
It is important to evaluate both the toxicity and potency of any given chemotherapeutic agent in vivo.Citation43 Patient-derived xenograft models could serve as a link between clinical research and in vitro studies in cell lines. The CRi Maestro is an affordable in vivo system for fluorescence-based small animal imaging and offers improved sensitivity, flexibility, and quantitative accuracy for visible and near-infrared labels. In our present study, multispectral acquisition and analysis showed that tumor volumes in BALB/c mice treated with DDP were moderately decreased in size, whereas those treated with Fe3O4-MNP-DDP showed more effective suppression of subcutaneous tumor growth (). Interestingly, either injection of a curative dose of DDP or Fe3O4-MNP had no obvious side effects on the host within a short period of time based on the body weight of mice. Thus, the use of a Fe3O4-MNP formulation is feasible.
On the other hand, preclinical platforms for evaluating novel therapies and prediction markers to monitor standard treatment are required. Immunopositivity for Ki-67 is found in highly proliferative cells, and high Ki-67 immunopositivity is frequently found in poorly differentiated carcinomas.Citation44,Citation45 Our results showed more nuclear Ki-67 immunoreactivity in tumor model tissues, while those treated with Fe3O4-MNP-DDP showed lower nuclear Ki-67 immunoreactivity, indicating that Fe3O4-MNP-DDP can inhibit cell proliferation and tumor growth. Further, other researchers have shown that overexpression of LRP in a subgroup of cisplatin-resistant NSCLC cell lines plays an important role in drug resistance, in which involved the NSCLC cells from toxic impacts of cisplatin by intrinsic mechanism.Citation46 Thus, LRP may be a predictor of the response to treatment in NSCLC. In our present study, high LRP immunoreactivity was observed in the cytoplasm of tumor cells in the control and Fe3O4-MNP groups, whereas that in Fe3O4-MNP-DDP-treated tumor cells was clearly decreased, suggesting that Fe3O4-MNP-DDP can reverse multidrug resistance and kill tumor cells in vivo. On the basis of the antiproliferative effect of Fe3O4-MNP -DDP seen in this study, we postulate that inhibition of growth of solid tumors may also be achievable in vivo.
Conclusion
Overall, the results of this study suggest that Fe3O4-MNP enhance the anticancer activity of DDP in vitro and in vivo without obvious systemic toxicity. Therefore, there is the potential for clinical application of Fe3O4-MNP-DDP to reverse DDP resistance in lung cancer cells.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program 973 of China (2010CB732404), the National Natural Science Funds of the People’s Republic of China (81170492), the Key Medical Disciplines of Jiangsu Province and Nanjing Medical University Science and Technology Development Funds Key Program (2012 NJMU151), and the Science Foundation of Jiangsu Province Cancer Hospital (ZM 200901).
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhouWChristianiDCEast meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and CaucasiansChin J Cancer20113028729121527061
- OheYOhashiYKubotaKRandomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in JapanAnn Oncol20071831732317079694
- TsuboiMOhiraTSajiHThe present status of postoperative adjuvant chemotherapy for completely resected non-small cell lung cancerAnn Thorac Cardiovasc Surg200713737717505412
- PignonJPTribodetHScagliottiGVLung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative GroupJ Clin Oncol2008263552355918506026
- RossiAMaionePGridelliCSafety profile of platinum-based chemotherapy in the treatment of advanced non-small cell lung cancer in elderly patientsExpert Opin Drug Saf200541051106716255664
- FarokhzadOCChengJJTeplyBATargeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivoProc Natl Acad Sci U S A20061036315632016606824
- PerrottaCBizzozeroLFalconeSNitric oxide boosts chemoimmunotherapy via inhibition of acid sphingomyelinase in a mouse model of melanomaCancer Res2007677559756417699758
- XiaGHChenBADingJHEffect of magnetic Fe3O4 nanoparticles with 2-methoxyestradiol on the cell-cycle progression and apoptosis of myelodysplastic syndrome cellsInt J Nanomedicine201161921192721931487
- JiangZChenBAXiaGHThe reversal effect of magnetic Fe3O4 nanoparticles loaded with cisplatin on SKOV3/DDP ovarian carcinoma cellsInt J Nanomedicine20094107114
- ChenBAChengJWuNYReversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5-bromotetrandrine in xenograft nude-miceInt J Nanomedicine20094737819421372
- SinghABoldin-AdamskySThimmulappaRKRNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapyCancer Res20086879757984
- LiJLiZNYuLCAssociation of expression of MRP1, BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapyLung Cancer20106911612219875192
- LongleyDBJohnstonPGMolecular mechanisms of drug resistanceJ Pathol200520527529215641020
- SchatzJHTargeting the PI3K/AKT/mTOR pathway in non-Hodgkin’s lymphoma: results, biology, and development strategiesCurr Oncol Rep201113398406
- OhYHerbstRSBurrisHEnzastaurin, an oral serine/threonine kinase inhibitor, as second- or third-line therapy of non-small-cell lung cancerJ Clin Oncol2008261135114118309949
- UrruticoecheaASmithIEDowsettMProliferation marker Ki-67 in early breast cancerJ Clin Oncol2005237212722016192605
- TorrisiRBagnardiVCardilloAPreoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER-and/or Pg R-positive breast cancer: clinical and biological activityBr J Cancer20089915641571
- ChenBASunQWangXMReversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/AO2 leukemic cellsInt J Nanomedicine2008327728618686787
- BrouwersEEMTibbenMMPluimDInductively coupled plasma mass spectrometric analysis of the total amount of platinum in DNA extracts from peripheral blood mononuclear cells and tissue from patients treated with cisplatinAnal Bioanal Chem200839157758518385987
- MaQSLiPXuMYKu80 is highly expressed in lung adenocarcinoma and promotes cisplatin resistanceExp Clin Cancer Res20123199
- ChenBAMaoPPChengJReversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and MDR-1 shRNA expression vector in leukemia cellsInt J Nanomedicine2010543744420957165
- HellwegCEBaumstark-KhanCHorneckGEnhanced green fluorescent protein as reporter protein for biomonitoring of cytotoxic effects in mammalian cellsAnal Chim Acta2001427191199
- YurdakulAAkyurekNYilmazSPrognostic impact of matrix metalloproteinases (MMP-9 and MMP-2) and vascular endothelial growth factor expression in non-small cell lung cancerTurk J Med Sci201242281288
- StewartDJChiritescuGDahrougeSBanerjeeSTomiakEMChemotherapy dose-response relationships in non-small cell lung cancer and implied resistance mechanismsCancer Treat Rev20073310113717276603
- SevePDumontetCChemoresistance in non-small cell lung cancerCurr Med Chem Anticancer Agents20055738815720263
- ThomasKSayrePResearch strategies for safety evaluation of nanomaterials. Part I: Evaluating the human health implications of exposure to nanoscale materialsToxicol Sci200587316321
- SomaCEDubernetCBentolilaDReversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkyleyanoacrylate nanoparticlesBiomaterials2000211710619673
- BouhamyiaLChantot-BastaraudSZaidiSImmunolocalization and cell expression of lung resistance-related protein (LRP) in normal and tumoral human respiratory cellsJ Histochem Cytochem20075577378217438350
- McGrathTCenterMSAdriamycin resistance in HL60 cells in the absence of detectable P-glycoproteinBiochem Biophys Res Commun1987145117111762886122
- BurgerHFoekensJALookMPRNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic responseClin Cancer Res20039827836
- SteinbachDWittigSCarioGThe multidrug resistance-associated protein 3 (MRP3) is associated with a poor outcome in childhood ALL and may account for the worse prognosis in male patients and T-cell immunophenotypeBlood20031024493449812816874
- BergerWElblingLHauptmannEMickscheMExpression of the multidrug resistance-associated protein (MRP) and chemoresistance of human non-small-cell lung cancer cellsInt J Cancer19977384939334814
- FilipitsMHaddadVSchmidKMultidrug resistance proteins do not predict benefit of adjuvant chemotherapy in patients with completely resected non-small cell lung cancer: International Adjuvant Lung Cancer Trial Biologic ProgramClin Cancer Res2007133892389817606722
- TrillerNKorosecPKernIKosnikMDebeljakAMultidrug resistance in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed diseaseLung Cancer20065423524016934363
- ScheperRJBroxtermanHJSchefferGLOverexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistanceCancer Res199353147514797680954
- van ZonAMossinkMHSchoesterMScheperRJSonneveldPWiemerEACEfflux kinetics and intracellular distribution of daunorubicin are not affected by major vault protein/lung resistance-related protein (vault) expressionCancer Res2004644887489215256459
- BergerWElblingLMickscheMExpression of the major vault protein LRP in human non-small-cell lung cancer cells: Activation by short-term exposure to antineoplastic drugsInt J Cancer20008829330011004683
- GroegerAMEspositoVDe LucaAPrognostic value of immunohistochemical expression of p53, bax, Bcl-2 and Bcl-x (L) in resected non-small-cell lung cancersHistopathology200444546314717670
- TsuruoTNaitoMTomidaAMolecular targeting therapy of cancer: drug resistance, apoptosis and survival signalCancer Sci200394152112708468
- YangXFraserMAbediniMRBaiTTsangBKRegulation of apoptosis-inducing factor-mediated, cisplatin-induced apoptosis by AktBr J Cancer20089880380818283299
- LiuZZhouXDQianGShiXFangJJiangBHAKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6 K1 pathwayCancer Res2007676325633217616691
- WeiMCZongWXChengEHProapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and deathScience200129272773011326099
- CozziPThe discovery of a new potential anticancer drug: a case historyFarmaco20035821322012620417
- RamljakVSucicMVrdoljakDVBorojevicNExpression of Ki-67 and p27 Kip1 in fine-needle aspirates from breast carcinoma and benign breast diseasesDiagn Cytopathol20113933334021488176
- von MinckwitzGSinnHPRaabGClinical response after two cycles compared to HER2, Ki-67, p53, and bcl-2 in independently predicting a pathological complete response after preoperative chemotherapy in patients with operable carcinoma of the breastBreast Cancer Res200810R3018380893
- HaradaTOguraSYamazakiKPredictive value of expression of P53, Bcl2 and lung resistance-related protein for response to chemotherapy in non-small cell lung cancersCancer Sci20039439439912824911