Abstract
Magnetic nanoparticles heated by an alternating magnetic field could be used to treat cancers, either alone or in combination with radiotherapy or chemotherapy. However, direct intratumoral injections suffer from tumor incongruence and invasiveness, typically leaving undertreated regions, which lead to cancer regrowth. Intravenous injection more faithfully loads tumors, but, so far, it has been difficult achieving the necessary concentration in tumors before systemic toxicity occurs. Here, we describe use of a magnetic nanoparticle that, with a well-tolerated intravenous dose, achieved a tumor concentration of 1.9 mg Fe/g tumor in a subcutaneous squamous cell carcinoma mouse model, with a tumor to non-tumor ratio > 16. With an applied field of 38 kA/m at 980 kHz, tumors could be heated to 60°C in 2 minutes, durably ablating them with millimeter (mm) precision, leaving surrounding tissue intact.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Ferromagnetic material is composed of microscopic interacting domains. Once these domains are aligned by a field, they remain oriented and the material is magnetized. For magnetite, Fe3O4, the domain size is 15–80 nm.Citation1 Subdomain nanoparticles align and respond to a magnetic field, but when the field is removed, the thermal motion is high enough to randomly reorient them, leaving no residual magnetization. These magnetic materials are termed “superparamagnetic.” The first superparamagnetic ferrofluids were formed by finely grinding magnetic material. For intravenous (IV) use, superparamagnetic iron oxide particles (or just “magnetic nanoparticles” [MNPs]) do not aggregate, thus avoiding emboli. MNPs heat up in an alternating magnetic field (AMF), either by physical rotation (the Brownian effect) or moving the magnetic moment without particle movement (the Néel effect).Citation2
In 1957, Gilchrist et al first used magnetic particles to heat tissues with a 1.2 MHz magnetic field.Citation3 Application to hyperthermia treatments and cancer followed.Citation4–Citation6 Since then, many studies have ensued to harness this technology for potential clinical use (reviewsCitation7–Citation12). In addition to direct tissue heating, MNPs can be incorporated into drug delivery systems that involve heat releasing the drug.Citation13–Citation17 For example, MNPs have been trapped either in the core or in between the lipid bilayer of thermosensitive liposomes and, on AMF heating, shown to release encapsulated drugs.Citation13,Citation18–Citation20 A chain of three 20 nm MNPs were attached to loaded liposomes and shown to release doxorubicin and exhibit mouse tumor control over 17 days using an unusually low 10 kHz field applied for 3 hours at a time.Citation21 When positively charged cisplatin ionically bound to phosphate-starch coated MNPs was heated, it was shown to release the drug and kill cells.Citation22 In another study, a thermosensitive polymer was layered onto MNPs covalently coupled to doxorubicin with an acid-labile hydrazine bond that showed release on heating with AMF and a pH of 5.3 (the pH of endosomes).Citation23 Hydrophobic and hydrophilic drugs have also been encapsulated, via emulsification, with MNPs in a polyvinyl alcohol polymer that demonstrated drug release when heated with an AMF and mouse tumor control over 30 days.Citation24 Oleic acid/Pluronic®-coated MNPs were associatively loaded with daunorubicin and 5-bromotetrandrine and effectively treated tumors for 12 days after AMF heating – these were shown to decrease P-glycoprotein and Bcl-2 expression while increasing Bax and caspase-3 expression. which may assist in combating multidrug resistance.Citation25 Gels incorporating MNPs implanted into tumors have also been developed.Citation26 Much progress has also been made in developing better quality magnetic nanoparticles that: are constructed using high temperature crystallization;Citation27 heat better;Citation28,Citation29 have different coatings, such as dextran,Citation30,Citation31 polyethylene glycol (PEG),Citation32 dopamine,Citation33 silanes,Citation34 and gold;Citation35,Citation36 have low Curie temperatures for heat control;Citation37 and for liposomal encapsulation.Citation17,Citation38–Citation40
Direct intratumoral injections of MNPs followed by induction heating has shown some benefit in controlling tumor growth.Citation38,Citation41–Citation49 Direct intratumoral injection was used in the first MNP hyperthermia clinical trial treating a prostate cancer using a 100 kHz machine designed for human patients,Citation50 and later in human glioma trialsCitation51,Citation52 which demonstrated safety and some benefit. Heating was obtained, but due to inhomogeneous MNP distribution, complete tumor eradication was not possible. Although direct intratumoral injections have the advantages of achieving high concentrations of MNPs and limiting systemic toxicity, they have the severe disadvantages of not generally covering tumors adequately,Citation41,Citation52 being invasive, and not being amenable to small metastatic tumor growths. In contrast, IV administration, although also not uniform, covers irregular tumor shapes more precisely, even small tumors (as has been shown with similar-sized gold nanoparticlesCitation53,Citation54) and is minimally invasive. Although IV administration does not result in a homogeneous tumor loading, the distribution is more global and thorough rather than the punctate distribution from direct injections.Citation55,Citation56 Complete uniformity is not required, since heating will fill in by conduction or surround low concentration regions. More complete tumor treatment appears better attainable with IV distributions. Previous attempts to implement IV MNPs followed by AMF heating showed some efficacy but were not able to fully ablate tumors, as the required concentration was not reached in the tumors.Citation57–Citation59 From calculations, test tube experiments, and in vitro cell hyperthermia, it appears that ~0.1%–0.4% iron by weight is required for adequate heating in a tumor.Citation60,Citation61 A barrier to this approach has been the toxicity of the MNPs at a level that achieves the required tumor loading after IV injection. Here, we present results attaining 0.19% iron in subcutaneous tumors after a nontoxic IV injection, enabling durable tumor ablation after AMF hyperthermia.
Materials and methods
MNPs
A commercially available “biocompatible” type of magnetic nanoparticles was evaluated in these studies (catalog number 9900, Nanoprobes, Yaphank, NY, USA). Specific loss power (SLP) was measured by published methods.Citation62 Briefly, 1.2 mL of a 2.1 mg Fe/mL MNP solution was placed in an Eppendorf tube insulated with Styrofoam in the AMF (980 kHz, 38 kA/m). A fiber-optic thermocouple was inserted to measure the temperature over time. Using the initial slope of heating, the SLP was calculated using the formula: SLP = (C × V)/m × dT/dt, where C is the volume-specific heat capacity of the sample (Cwater = 4185 J kg−1 K−1), V is the sample volume, and m is the mass of ironCitation58 (not Fe3O4 or compound molecular weight). Typically, in 5.3 seconds, the temperature of the sample rose by 4.2°C, whereas that of water alone rose by 0.2°C. The heating rate of water alone was subtracted from the MNP sample heating rate. A small volume of water (1.2 mL) was used since the heating coil was only one turn. Larger volumes would lead to averaging from regions having lower applied field.
Electron microscopy
Low-magnification transmission electron microscope images were taken with an FEI BioTwinG transmission electron microscope (Hillsboro, OR, USA). High-resolution lattice images and diffraction patterns were taken with a JEOL ARM200CF double-corrected S/TEM operating at 200 keV (Tokyo, Japan). One microliter of 70 mg Fe/mL purified iron particles in water was dispersed into 1 mL acetone. The solution (50 μL) was applied to an ultrathin carbon film on holey carbon support film (400 copper mesh; Ted Pella, Redding, CA, USA) and air dried.
Dynamic light scattering
One microliter of 70 mg Fe/mL purified iron particles in water was dispersed into 1 mL water, 0.2-micron filtered and measured with a 90Plus Particle Size Analyzer (Brookhaven Instruments, Holtsville, NY, USA). Results are reported here for lognormal intensity analysis and error as standard error of the mean.
Tissue culture
Murine squamous cell carcinoma SCCVII cells (American Type Culture Collection, Manassas, VA, USA) were grown in Gibco® Dulbecco’s Modified Eagle Medium (Life Technologies, Carlsbad, CA, USA) supplemented with Gibco 10% calf serum (Life Technologies) and Gibco Antibiotic-Antimycotic (Life Technologies). Cells were incubated at 37°C and 10% CO2.
Subcutaneous tumors
SCCVII squamous cell carcinoma tumors were initiated by injecting 200,000 cells in a total volume of 50 μL containing 50% Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA) subcutaneously in the thighs of 8–10-week-old NCr nude mice (Taconic, Hudson, NY, USA). Tumors were treated with hyperthermia 10–11 days after implantation when they were ~150 mm3. Mice were euthanized when tumors reached 1000 mm3. All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council of the National Academies. The protocol was approved by the Institutional Animal Care and Use Committee of the State University of New York at Stony Brook.
Iron injections
Iron nanoparticles were concentrated to 130 mg Fe/mL in 80% phosphate-buffered saline (PBS) – 10 mM phosphate buffer, pH 7.4, 140 mM NaCl – and injected intravenously via a tail vein at 1.7 g Fe/kg body weight (bw).
Maximum tolerated dose (MTD50)
Three mice in each group were intravenously injected with 0.8, 1.7, 2.6, 3.4, 4.2, and 5.1 g Fe/kg MNPs. Body weights were monitored once per day over 2 weeks and once per week for 1 month. “MTD50” is here defined as the dose at which 50% of animals lost > 15% of their original body weight any time within 1 month.
Pharmacokinetics
Female NCr nude mice were subcutaneously implanted with SCCVII cells as described above. The animals were intravenously injected with MNPs (1.7 g Fe/kg) once the tumors reached ~0.15 cc and three mice per time point were killed at various time points thereafter. Tissues were harvested weighed and analyzed for iron content. After subtraction of iron from control mice (without MNP injection), the means and standard error of the means were plotted. Six time points were assayed: 5 minutes, 1 hour, and 4, 8, 24, and 96 hours. Blood half-life was analyzed as a two-component decay with exponential fitting using a two-phase half-life model with Prism 5 software (GraphPad La Jolla, CA, USA).
Iron measurement
To release iron, the tissues were first digested with a strong acid mixture of 1 M H2SO4 and 1 M HNO3 and heated to 60°C. After tissues were mostly dissolved (~30–40 minutes), HCl was added at 3:1 HCl:HNO3 ratio. Triton X-100 (final 10%) was also added to solubilize cell membranes.
Tissue iron content was measured by a colorimetric method adapted from Ceriotti and Ceriotti.Citation64 Briefly, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) was added to the digested tissue samples to 0.1 M and the pH adjusted to 3 with 10 N KOH. Ascorbic acid (final 10%) was added to reduce the ferric ions to ferrous ions. Finally, FerroZine™ reagent (3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate; Sigma-Aldrich, St Louis, MO, USA) was added to the solution to form a purple-colored complex. The absorption was measured at 562 nm and compared with a standard curve. This method was further calibrated by the Nano-technology Characterization Laboratory at the National Institutes of Health using inductively coupled plasma mass spectroscopy using a National Institute of Standards and Technology iron standard. The tissue iron concentration of mice without MNPs was subtracted from the MNP-injected mice tissue concentrations.
Induction equipment
A 10 kW induction heater with a single turn, 2.5 cm diameter coil operating at 980 kHz and 38 kA/m (model SI-10KWHF, Superior Induction, Pasadena, CA, USA) was used for treatment, or alternatively model IMH5.0 (MSI Automation, Inc., Wichita, KS, USA). Field strength was measured with a two-dimensional magnetic field probe (model 0015, 100 kHz to 1 MHz, AMF Life Systems, Rochester, MI, USA).
Hyperthermia treatment
Mice were anesthetized intraperitoneally with ketamine (100 mg/kg)/xylazine (8 mg/kg) and positioned in a Plexiglas holder such that one leg extended downward through a 1 cm hole and this was anchored to a lower plate via dental floss loosely tied around the ankle. The holder was attached to a computer-controlled stepping motor (T-LS80-I; Zaber, Vancouver, BC, Canada) that oscillated the mouse leg vertically through the center of the coil with a stroke of 25 mm, encompassing the ~6 mm tumors and surrounding tissue. The oscillation speed was 4 mm/second with a period of 6 seconds. The surface temperature of the tumor and surrounding skin was monitored using a FLIR SC300 series thermal camera (FLIR Systems, Wilsonville, OR, USA). Internal tumor temperatures were monitored with fiber-optic thermocouples (Reflex-4, Neoptix Canada, La Malbaie, QC, Canada) on some mice to determine the correlation between internal versus external temperatures. The mice legs were scanned in the AMF 24 hours after IV injection of MNPs. To monitor and limit normal tissue damage from heat conduction from the heated tumor into surrounding tissue, the field was applied until the skin 0.8 cm from the tumor edge reached 50°C, which typically took ~2 minutes.
Results
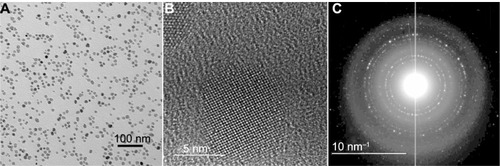
We evaluated the properties and in vivo use of a newly available type of biocompatible magnetic particles with a core of Fe3O4 (magnetite) and a 2000 MW PEG coating. Electron microscopy showed the iron oxide core to be 11.3 ± 2.3 nm in size (). High-resolution imaging and the diffraction pattern were consistent with Fe3O4 cores ().Citation64 Dynamic light scattering indicated that the MNPs had a hydrodynamic diameter of 23.8 ± 0.1 nm and a polydispersity of 0.087. Their efficiency of heating in an AMF (38 kA/m, 980 kHz), characterized by SLP, was 754 W/g(Fe). “SLP,” also termed “specific absorption rate,” is the rate of energy absorbed from the applied AMF per unit mass. A control sample of water showed no measurable heating.
Figure 1 Transmission electron microscopy images of the magnetic nanoparticles. (A) Particle cores measured to be 11.3 ± 2.3 nm (scale bar = 100 nm). (B) High-resolution lattice image of a 9.9 nm particle showing its crystalline core (scale bar = 5 nm). (C) Electron diffraction pattern, identifying cores as Fe3O4 (scale bar = 10 nm−1).
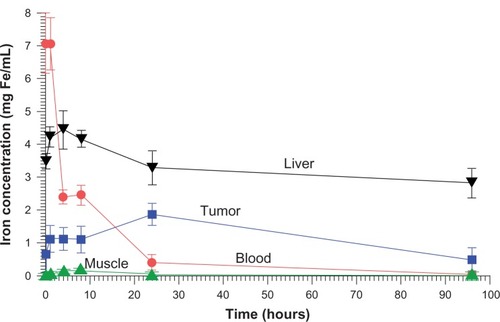
Pharmacokinetics was measured after injection of the dose used for therapy (IV 1.7 g Fe/kg). The concentration of iron in various tissues after subtraction of normal body iron is shown in . For the measurement times assayed the tumor concentration peaked at 1.9 ± 0.3 mg Fe/cc at 24 hours. The highest muscle concentration occurred at 8 hours, 0.12 ± 0.02 mg Fe/cc, giving a peak tumor to peak non-tumor (surrounding muscle) ratio of 15.8. At 24 hours, the muscle content could not be distinguished from normal muscle iron content (0.068 mg Fe/ccCitation66), which would give a tumor to non-tumor ratio of >16.0 at 24 hours. Blood clearance exhibited a rapid early half-life of 2.0 hours followed by a slow component half-life of 14.0 hours.
Figure 2 Biodistribution of iron (after subtraction of normal tissue iron) over time.
Notes: The maximum iron concentration in the tumor from the points measured was at 24 hours post-injection, reaching 1.9 mg Fe/mL. Time points were: 5 minutes, 1 hour, and 4, 8, 24, and 96 hours.
An initial toxicity study determined the MTD50 (defined as the dose at which 50% of animals lost > 15% of original body weight any time within 1 month) to be 4.7 g Fe/kg. Mice IV injected at 3.4 g Fe/kg have now survived >12 months without showing any clinical signs of toxicity.
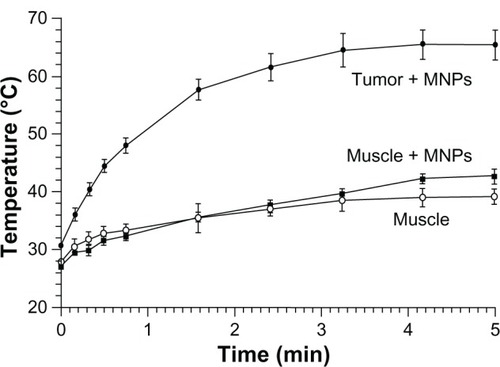
Nude mice with subcutaneous squamous cell carcinomas (SCCVII) implanted in their legs were heated by placing the legs in an AMF (). A stepping motor was used to scan the leg through the field so that it would be heated uniformly. A fiber-optic thermocouple was placed in the tumor center in test animals to determine the difference between the surface (measured with an infrared camera) and center of the tumor. This difference was less than ±2°C (which has also been observed by othersCitation67), so the external temperature was used so as not to invasively disturb tissues. The tumor heating rate is shown in . These experiments showed that tumors could be rapidly heated to ablative temperatures (60°C in 2 minutes) after a well-tolerated IV injection of MNPs. Due to the 16:1 MNP ratio of tumor to non-tumor surrounding tissue, normal tissue (with the same IV MNP injection and field) was found to have a temperature of 36°C after 2 minutes. Lowering the MNP injection by one-half (0.85 g Fe/kg) or lowering the field by one-half also resulted in ineffective treatment levels of a temperature < 42°C after 2 minutes. AMF alone without MNPs resulted in a leg temperature of 36°C after 2 minutes (). We also noted that tumors after 2.6 g Fe/kg IV administration were heated to 82°C in 2 minutes.
Figure 3 Thermal image of subcutaneous tumor being heated by an alternating magnetic field.
Notes: The leg was scanned up and down vertically to make the field uniform over the leg. The tumor can be observed to have specifically heated (red region).
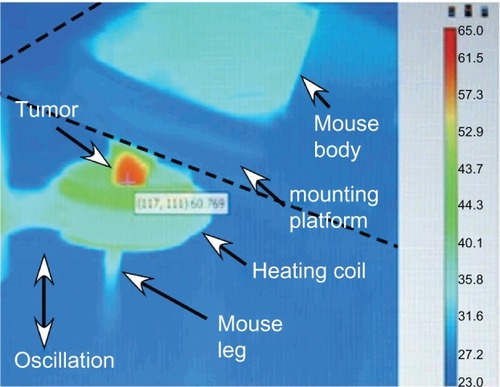
Figure 4 Heating of tissues in the magnetic field: 24 hours after an intravenous injection of magnetic nanoparticles (MNPs) (1.7 g Fe/kg) – tumor (filled circles) and leg muscle (no tumor, filled squares) tissues.
Notes: Also shown is heating of leg muscle tissue with no injection of MNPs (open circles). The alternating magnetic field applied was 38 kA/m at 980 kHz. Tumors (average size of 206 mm3, three averaged) equilibrated at 66°C after 5 minutes, muscle with MNPs reached 42°C, and muscle without MNPs reached 39°C. Three mice were used per group.
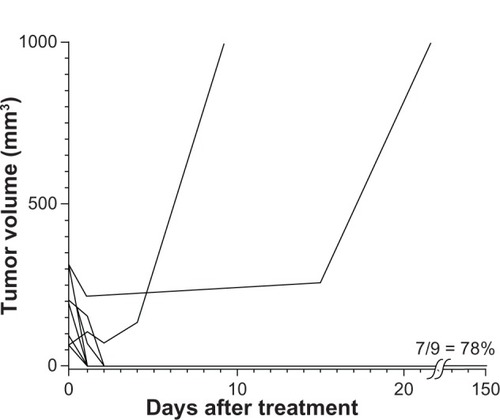
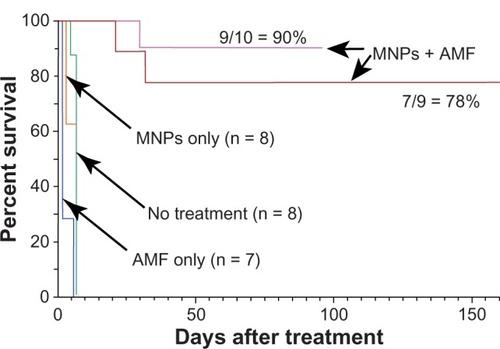
For therapy, mice with subcutaneous squamous cell carcinomas implanted on their legs were treated by IV injection of 1.7 g Fe/kg then their legs were heated 24 hours later using the magnetic field. Results are shown in . The amount of IV-administered MNPs delivered to the tumors was enough in combination with the field strength to effectively ablate nearly all tumors (78%–90%, results of two independent experiments). Control treatments (ie, no treatment, magnetic field treatment only, or MNP treatment only) had no measurable effect on tumor growth or survival. Successfully treated tumors were rapidly liquefied and resorbed in 1–2 days (). After complete remission (at 160 days), mice had virtually the same leg diameter at the place of the tumor (5.47 mm average) as at their untreated contralateral leg (5.53 mm average) with no leg dysfunction, indicating that the treatment was well confined with less than ~1 mm of normal tissue damage.
Figure 5 Magnetic nanoparticle (MNP) hyperthermia treatment.
Notes: Intravenous injection of 1.7 g Fe/kg and followed 24 hours later by exposure to an alternating magnetic field (38 kA/m, 980 kHz, 2 minutes) resulted in durable ablation of tumors (7/9 = 78%, n = 9, absence of palpable tumor). A repeated experiment showed 90% (n = 10) thermoablation. Controls – no treatment, magnetic field only, and only magnetic nanoparticles – had no measurable effect on survival.
Abbreviations: AMF, alternating magnetic field; n, number of animals per group.
Discussion
The extraordinary efficacy attained in our study for an extremely aggressive tumorCitation68 can be attributed to a combination of six factors: (1) IV delivery that adequately loads carcinomas, (2) low systemic toxicity (MTD50 4.8 g Fe/kg) that enabled sufficiently high tumor loading (0.19% Fe) for effective heating, (3) good tumor to non-tumor ratio (>16:1), (4) MNPs that heated efficiently (SLP 754 W/g), (5) use of a high magnetic field (38 kA/m), and (6) use of a high frequency (980 kHz). Previous studies have indicated better heating with increased concentration, SLP,Citation60 field strength, and frequency,Citation69 thus stressing the importance of maximizing each parameter. The SLP also depends on the size and polydispersity of the MNPs, with larger and more uniform MNPs performing better.Citation27 However, for the fields and size of MNPs used in this study, increased polydispersity may actually be preferable.Citation1 The SLP of the particles used here (754 W/g) compares favorably to conventional particles, ~100–300 W/g (9 nm size, 500 kHz, 37.3 kA/m29) but is lower than constructs containing zinc, cobalt, and manganese, which reach 4000 W/g.Citation29 However, other constructs may affect toxicity and delivery. PEG of MW 2000 is appropriate, since tumor uptake is not significantly different from higher MW PEG coatings and PEG of lower MW results in shorter blood half-life and higher macrophage uptake.Citation70 PEG of higher MW is more viscous and leads to potential problems with high-concentration injections.Citation71 While there were a number of contributory factors to achieving durable remissions, the main advance in our study was the use of IV delivery. Although the resulting distribution of nanoparticles is not uniform throughout the tumor after IV administration, it leads to thorough tumor encasement,Citation53,Citation54 which can cut off blood supply (oxygen and nutrients) to central hypoxic regions, compared with direct injections that are punctate and can leave tumor regions untreated.Citation41,Citation52,Citation72 Arterial administration of magnetic microparticles causing emboli in liver tumors followed by AMF heating was found to be vastly superior to direct injection of the same amount and AMF heating to the same temperature.Citation67 A heating strategy does not require perfect homogeneity, because the heat will either extend to adjacent cells that have fewer MNPs or starve entrapped regions. Ideally, the heating should cover the tumor’s growing edge to be consistently effective, precisely where the leakage of IV nanoparticles is greatest.Citation53,Citation73–Citation75 In comparison with direct intratumoral injection, IV injection has the additional advantage of precisely loading many tumors simultaneously, which could then be treated in one application – a much needed strategy for metastases.
The present study focused on obtaining long-lasting cancer abatement in vivo to address the substantial obstacles encountered in transitioning from cell studies to animals with tumors. What appears exciting in vitro may easily fail in vivo. The translation from mice to humans is also fraught with uncertainty and new reasons for potential failure. Personnel at MagForce (Berlin, Germany) are to be commended for their construction of an appropriate AMF machine for humans and application to human prostateCitation50 in 2005 and more recently to human gliomas.Citation51,Citation52,Citation76 Direct intratumoral injection of MNPs into recurrent gliomas and AMF heating combined with radiotherapy resulted in a survival time (from primary diagnosis, “OS-1”) of 23.2 months compared with 14.6 months (taken from another published study) with radiotherapy only.Citation76,Citation77 Another objective of the present study was to follow tumors for at least 3 months, since frequently a treatment that appears promising after 10–30 days has really only killed some of the tumor cells and tumors reappear after a month or more.Citation78 In and , our results are compared with some other in vivo MNP treatments reported thus far. shows studies using direct intratumoral injections of the MNPs and shows those attempting intravenous administration. Our study (to 160 days) was the longest; many others report tumor response to only 14–35 days. For direct intratumoral injection (), several studies reported tumor-free survival. For example, Ito et alCitation42 impressively controlled 1.5 cm tumors for at least 120 days by multiply retreating with intratumoral MNP injections and AMF heating treatments. It appears that direct intratumoral injections can be effective but are restricted by invasiveness and ability to produce adequate tumor MNP coverage. Fewer studies have been reported using IV injections (); those that have, all reported some tumor growth inhibition but all animals died of tumor overgrowth. Our study is the only one showing long term survival after IV administration. For other IV treatments, the maximum iron injected was 100 mg Fe/kg, presumably limited by toxicity. As has been noted previously, it is difficult to achieve the required tumor concentration by IV administration.Citation79 In our study, 1.7 g Fe/kg was used and delivered a sufficient amount to the tumor. Another striking difference between our study and others is that only a single heat treatment of 2 minutes duration was used, while all other studies utilized a treatment time of at least 20 minutes and some performed multiple treatments.
Table 1 Direct intratumoral injection of magnetic nanoparticles
Table 2 Intravenous injection of magnetic nanoparticles
The amount of iron used here is considerably larger than that used in other iron imaging or therapy applications. It was used for proof of principle to demonstrate that highly effective selective tumor heating can be obtained at a well-tolerated IV dose. The amount of iron might be reduced with further dose–time–temperature studies or use of particles with higher SLP. Nevertheless, this high amount of iron raises issues of toxicity and clearance. With a MTD50 of 4.8 g Fe/kg, the magnetite particle used here (still investigational) is in the same range as US Food and Drug Administration-approved MNPs used as magnetic resonance imaging (MRI) contrast agents, which have been thoroughly tested for broad-spectrum toxicity, some of which have a median lethal dose (ie, lethal dose, 50% [LD50]) of 6 g/kg.Citation63 At 1.7 g Fe/kg, the amount of iron given to a human would be ~119 g Fe, 34 times the normal body iron content of 3.5 g. At this level in mice we observed no obvious clinical signs of toxicity (no weight loss or abnormal behavior) over the course of 1 year, but there was darkening of the skin that very gradually cleared over several months. The stability and slow breakdown of the particles is key to their not imposing any sudden toxic free iron load. This might be considered similar to swallowing arsenic encased in a glass bead, which would produce no adverse effects. Thus, the surprisingly large amount of iron should not be grounds for immediate dismissal of consideration for human use. Rejection should also not be based on comparison with other iron compounds, since each compound or construct has its own, often radically different, toxicity profile. In addition, if the method eradicates cancers when other methods do not, minor side effects could be tolerated. For example, cisplatin has a LD50 of 11 mg/kg IV in mice.Citation80 Scaled by body surface area, it would have a projected human equivalent LD50 of 0.89 mg/kg.Citation81 However, standard human treatment doses are 2.5 mg/kg (100 mg/m2),Citation82 2.8 times higher than the LD50 predicted from animal studies. In any case, more thorough toxicity studies are needed.
Liver uptake per gram for the MNPs is greater than for the tumor (). This is commonly the case for intravenously injected materials. It might imply that tumors near the liver should be avoided by the locally applied magnetic field, but not necessarily, since the liver regenerates and often half or more is resected surgically to remove tumors.Citation83 Liver tumors might be treated if sufficient differential tumor delivery could be achieved by targeting or hepatic artery administration.Citation67
Tumor targeting in this study was by the enhanced permeability and retention effect.Citation84,Citation85 Targeting by antibodies, peptides, porphyrins, drugs, or other tumor-binding molecules or, alternatively, targeting tumor vasculature, tumor-related, and tumor environmentCitation86 epitopes could improve tumor uptake and specificity and lower the amount needed for injection. A potential problem with previously trialed MNPs coated with dextran was their rapid removal by liver and spleen. At 1 hour post-injection, dextran MNPs had 52% of the injected dose in the liver and spleen,Citation30 compared with 16% for the MNPs used here. Another study used 20 nm antibody-targeted intravenously administered MNPs, which produced a tumor uptake of 14% injected dose per gram of tissue (id/g),Citation58 higher than our 6% id/g, but the injected amount was ~1.6 mg compared with our ~42 mg, resulting in tumor concentrations of ~0.2 mg Fe/g versus our 1.9 mg Fe/g. Their study showed delay of tumor doubling time but no complete remissions, consistent with basic studies indicating the need for higher concentrations in the tumor.Citation60,Citation61
External magnetic focusing (such as placement of external magnets or fields) to guide MNPs to an internal location is not stably possible, since external fields are strongest at their origin and MNPs move in a field gradient toward such an external source; that is, the MNPs would move toward the skin. Therefore, biotargeting appears to be the most fruitful approach to localizing MNPs to internal tumors. However, to some extent, the field can be shaped with external low-reluctance material to help avoid critical regions.Citation87
It appears that the method presented here is powerful enough to heat and ablate tumors (at least in mice), but it must be applied judiciously, as overheating can damage surrounding normal tissue due to direct heat conduction and blood-flow heat transfer. Many proteins denature at ~55°C. Controls indicated that the amount of MNPs in normal tissue did not significantly contribute to normal tissue heating, since with or without MNPs, both showed the same 36°C temperature after 2 minutes (). Optimization of a heating protocol is critical to minimizing normal surrounding tissue damage. Here, we chose to heat tumors rapidly to ablative temperatures for a short total time (~2 minutes), as opposed to heating slowly, which would allow adjacent normal tissue to equilibrate with the tumor temperature. This strategy protected the underlying leg from damage. However, other protocols might be to heat for a longer time at lower temperatures, which would lead to cellular apoptosis rather than necrosis. Theoretical thermodynamic studies have been reported that address the optimal application of magnetic hyperthermia.Citation88,Citation89 For clinical use, it may be envisioned that dose planning will be undertaken similarly to that for radiation. The iron concentrations can be mapped by MRI, computed tomography, or magnetorelaxometryCitation51,Citation90,Citation91 and, knowing the precise SLP of the particles and field strength, the heating topography can be predicted, as has been done in human magnetic nanoparticle brain tumor hyperthermia treatments.Citation76 Subjection to a tissue/blood-flow modeling program can approximate the heating profile without the need for multiple invasive thermocouples. It would be difficult to measure internal temperatures in real-time by MRI, since the induction heating equipment would have to be non-magnetic.
For clinical application, there is also concern about eddy current heating in normal tissues at high fields and frequencies.Citation10,Citation41 However, this might be countered by increasing the SLP of the particles, reducing the frequency, application to smaller diameters such as head or extremities, and lower target temperatures. Hyperthermia has long been known to be synergistic with chemotherapy and radiotherapyCitation78,Citation92–Citation94 and requires much lower temperatures (~40°C–43°C).
Conclusion
The IV delivery of biocompatible magnetic nanoparticles is now able to achieve the tumor iron concentrations needed for effective hyperthermia. With these concentrations and a high tumor to non-tumor ratio, precise tumor ablation is now possible. Because IV delivery generally loads tumors better than direct intratumoral injection, conforming to tumors’ irregular shapes, this advance in mice may be of use clinically. Combination with chemotherapy or radiotherapy should enhance their efficacy.
Acknowledgments
The authors thank Ms Natalie Muratori, Dr Yimei Zhu, Ms Lynn Lin, Thomas Zimmerman, DVM, Dr Henry M Smilowitz, Daniel N Slatkin, MD, Cat Hainfeld, and the Nanotechnology Characterization Laboratory of the National Institutes of Health for assistance.
Disclosure
J Hainfeld is a part owner of Nanoprobes. H Huang has no conflicts of interest to declare in relation to this work.
References
- HergtRDutzSRöderMEffects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermiaJ Phys Condens Matter2008203838521421693832
- RosensweigREHeating magnetic fluid with alternating magnetic fieldJ Magn Magn Mater20022521–3370374
- GilchristRKMedalRShoreyWDHanselmanRCParrottJCTaylorCBSelective inductive heating of lymph nodesAnnals of surgery1957146459660613470751
- ChanDCKirpotinDBBunnPAJrSynthesis and evaluation of colloidal magnetic iron oxides for the site-specific radiofrequency-induced hyperthermia of cancerJ Magn Magn Mater19931221–3374378
- JordanAWustPFahlingHJohnWHinzAFelixRInductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermiaInt J Hyperthermia19939151688433026
- RandRWSnowHDBrownWJThermomagnetic surgery for renal cancerProg Clin Biol Res19821006736857145996
- KumarCSMohammadFMagnetic nanomaterials for hyperthermia-based therapy and controlled drug deliveryAdv Drug Deliv Rev201163978980821447363
- LaurentSDutzSHäfeliUOMahmoudiMMagnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticlesAdv Colloid Interface Sci20111661–282321601820
- BerryCCProgress in functionalization of magnetic nanoparticles for applications in biomedicineJ Phys D Appl Phys20094222
- PankhurstQAThanhNTJonesSKDobsonJProgress in applications of magnetic nanoparticles in biomedicineJ Phys D Appl Phys20094222
- RocaAGCostoRRebolledoAFProgress in the preparation of magnetic nanoparticles for applications in biomedicineJ Phys D Appl Phys20094222
- KobayashiTCancer hyperthermia using magnetic nanoparticlesBiotechnology journal20116111342134722069094
- AmstadEReimhultENanoparticle actuated hollow drug delivery vehiclesNanomedicine (Lond)20127114516422191783
- EdelmanERBrownLKostJTaylorJLangerRModulated release from polymeric drug delivery systems using oscillating magnetic fields: in vitro and in vivo characteristicsTransactions - American Society for Artificial Internal Organs1984304454496533921
- KostJNoeckerRKunicaELangerRMagnetically controlled release systems: effect of polymer compositionJournal of biomedical materials research19851989359403880352
- KostJWolfrumJLangerRMagnetically enhanced insulin release in diabetic ratsJournal of biomedical materials research19872112136713733323204
- SuWWangHWangSPEG/RGD-modified magnetic polymeric liposomes for controlled drug release and tumor cell targetingInternational journal of pharmaceutics. 1520124261–2170181
- HosokawaTSamiMKatoYHayakawaEAlteration in the temperature-dependent content release property of thermosensitive liposomes in plasmaChemical & pharmaceutical bulletin200351111227123214600363
- MasukoYTazawaKViroonchatapanEPossibility of thermosensitive magnetoliposomes as a new agent for electromagnetic induced hyperthermiaBiol Pharm Bull19951812180218048787814
- ViroonchatapanESatoHUenoMMicrodialysis assessment of 5-fluorouracil release from thermosensitive magnetoliposomes induced by an electromagnetic field in tumor-bearing miceJ Drug Target1998553793909771619
- PeirisPMBauerLToyREnhanced delivery of chemotherapy to tumors using a multicomponent nanochain with radio-frequency-tunable drug releaseACS nano2012654157416822486623
- BabincovMAltanerovVAltanerCBergemannCBabinecPIn vitro analysis of cisplatin functionalized magnetic nanoparticles in combined cancer chemotherapy and electromagnetic hyperthermiaIEEE Trans Nanobioscience200871151918334449
- ZhangJMisraRDMagnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: core-shell nanoparticle carrier and drug release responseActa Biomater20073683885017638599
- HuSHLiaoBJChiangCSChenPJChenIWChenSYCore-shell nanocapsules stabilized by single-component polymer and nanoparticles for magneto-chemotherapy/hyperthermia with multiple drugsAdv Mater201224273627363222689346
- RenYZhangHChenBMultifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistanceInternational Journal of Nanomedicine201272261226922619560
- Le RenardPEJordanOFaesAThe in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermiaBiomaterials201031469170519878991
- ParkJAnKHwangYUltra-large-scale syntheses of monodisperse nanocrystalsNat Mater200431289189515568032
- FortinJPWilhelmCServaisJMénagerCBacriJCGazeauFSize-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermiaJ Am Chem Soc200712992628263517266310
- LeeJHJangJTChoiJSExchange-coupled magnetic nanoparticles for efficient heat inductionNat Nanotechnol20116741842221706024
- LacavaLMLacavaZGMAzevedoRBUse of magnetic resonance to study biodistribution of dextran-coated magnetic fluid intravenously administered in miceJ Magn Magn Mater200225213
- WhiteDLAicherKPTzikaAAKucharczykJEngelstadBLMoseleyMEIron-dextran as a magnetic susceptibility contrast agent: flow-related contrast effects in the T2-weighted spin-echo MRI of normal rat and cat brainMagn Reson Med199224114281313524
- KohlerNSunCFichtenholtzAGunnJFangCZhangMMethotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug deliverySmall20062678579217193123
- XieJXuCXuZLinking Hydrophilic Macromolecules to Monodisperse Magnetite (Fe(3)O(4)) Nanoparticles via Trichloro-s-triazineChem Mater200618235401540318176627
- JordanAScholzRWustPEndocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitroJ Magn Magn Mater19991941–3185196
- CarpenterEEIron nanoparticles as potential magnetic carriersJ Magn Magn Mater20012251–21720
- ParkHYSchadtMJWangLFabrication of magnetic core@ shell Fe oxide@Au nanoparticles for interfacial bioactivity and bioseparationLangmuir200723179050905617629315
- MillerKJCollettiAPapiPJMcHenryMEFe-Co-Cr nanocomposites for application in self-regulated rf heatingJ Appl Phys20101079
- ShidoYNishidaYSuzukiYKobayashiTIshiguroNTargeted hyperthermia using magnetite cationic liposomes and an alternating magnetic field in a mouse osteosarcoma modelJ Bone Joint Surg Br201092458058520357339
- PopMCosma-CachitaDBicaDTarcaATripşaMMagnetoliposomes obtained from lecithin and Fe3O4 nanoparticlesRom J Physiol1999363–423323611797938
- SkourasAMourtasSMarkoutsaEMagnetoliposomes with high USPIO entrapping efficiency, stability and magnetic propertiesNanomedicine20117557257921704597
- HilgerIHergtRKaiserWAUse of magnetic nanoparticle heating in the treatment of breast cancerIEE Proc Nanobiotechnol20051521333916441156
- ItoATanakaKHondaHAbeSYamaguchiHKobayashiTComplete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticlesJ Biosci Bioeng200396436436916233538
- MatsuokaFShinkaiMHondaHKuboTSugitaTKobayashiTHyperthermia using magnetite cationic liposomes for hamster osteosarcomaBiomagn Res Technol200421315040804
- MotoyamaJYamashitaNMorinoTTanakaMKobayashiTHondaHHyperthermic treatment of DMBA-induced rat mammary cancer using magnetic nanoparticlesBiomagn Res Technol20086218298831
- DennisCLJacksonAJBorchersJANearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermiaNanotechnology2009203939510319726837
- TanakaKItoAKobayashiTHeat immunotherapy using magnetic nanoparticles and dendritic cells for T-lymphomaJ Biosci Bioeng2005100111211516233860
- ZhaiYXieHGuHEffects of hyperthermia with dextran magnetic fluid on the growth of grafted H22 tumor in miceInt J Hyperthermia2009251657119219702
- BaselMTBalivadaSWangHCell-delivered magnetic nanoparticles caused hyperthermia-mediated increased survival in a murine pancreatic cancer modelInternational journal of nanomedicine2012729730622287840
- SuzukiMShinkaiMHondaHKobayashiTAnticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomesMelanoma research200313212913512690295
- JohannsenMGneveckowUEckeltLClinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial techniqueInt J Hyperthermia200521763764716304715
- Maier-HauffKRotheRScholzRIntracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiformeJ Neurooncol2007811536016773216
- van LandeghemFKMaier-HauffKJordanAPost-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticlesBiomaterials2009301525718848723
- HainfeldJFO’ConnorMJDilmanianFASlatkinDNAdamsDJSmilowitzHMMicro-CT enables microlocalisation and quantification of Her2-targeted gold nanoparticles within tumour regionsBr J Radiol201184100252653321081567
- HainfeldJFSmilowitzHMO’ConnorMJDilmanianFASlatkinDNGold nanoparticle imaging and radiotherapy of brain tumors in miceNanomedicine (Lond) Epub12242012
- DutzSKetteringMHilgerIMullerRZeisbergerMMagnetic particle hyperthermia - Properties of magnetic multicore nanoparticles administered to tumor tissueBiomedizinische Technik Biomedical engineering201276
- HilgerIHiergeistRHergtRWinnefeldKSchubertHKaiserWAThermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility studyInvest Radiol2002371058058612352168
- BalivadaSRachakatlaRSWangHA/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nano-particles: a mouse studyBMC Cancer20101011920350328
- DeNardoSJDeNardoGLNatarajanAThermal dosimetry predictive of efficacy of 111In-ChL6 nanoparticle AMF – induced thermoablative therapy for human breast cancer in miceJ Nucl Med200748343744417332622
- ShinkaiMLeBHondaHTargeting hyperthermia for renal cell carcinoma using human MN antigen-specific magnetoliposomesJpn J Cancer Res200192101138114511676866
- HergtRDutzSMagnetic particle hyperthermia – biophysical limitations of a visionary tumour therapyJ Magn Magn Mater20073111187192
- SamantaBYanHFischerNOShiJJerryDJRotelloVMProteinpassivated Fe(3)O(4) nanoparticles: low toxicity and rapid heating for thermal therapyJ Mater Chem200818111204120819122852
- LévyMWilhelmCSiaugueJMHornerOBacriJCGazeauFMagnetically induced hyperthermia: size-dependent heating power of γ-Fe2O3 nanoparticlesJ Phys Condens Matter2008202020413321694262
- MornetSVasseurSGrassetFDuguetEMagnetic nanoparticle design for medical diagnosis and therapyJ Mater Chem2004141421612175
- CeriottiFCeriottiGImproved direct specific determination of serum iron and total iron-binding capacityClin Chem19802623273317353288
- YangTShenCLiZHighly ordered self-assembly with large area of Fe3O4 nanoparticles and the magnetic propertiesJ Phys Chem B200510949232332323616375287
- ReardonTFAllenDGIron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performanceExp Physiol200994672073019201785
- MorozPJonesSKGrayBNTumor response to arterial embolization hyperthermia and direct injection hyperthermia in a rabbit liver tumor modelJ Surg Oncol200280314915612115798
- NomuraTShibaharaTKatakuraAMatsubaraSTakanoNEstablishment of a murine model of bone invasion by oral squamous cell carcinomaOral Oncol200743325726216920384
- GlöcklGHergtRZeisbergerMDutzSNagelSWeitschiesWThe effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermiaJ Phys Condens Matter20061838S2935
- LarsenEKNielsenTWittenbornTAccumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumorsNanoscale2012472352236122395568
- ChenBZuberiMBorgensRBChoYAffinity for, and localization of, PEG-functionalized silica nanoparticles to sites of damage in an ex vivo spinal cord injury modelJournal of biological engineering2012611822979980
- HilgerIAndräWHergtRHiergeistRSchubertHKaiserWAElectromagnetic heating of breast tumors in interventional radiology: in vitro and in vivo studies in human cadavers and miceRadiology2001218257057511161180
- SeymourLWPassive tumor targeting of soluble macromolecules and drug conjugatesCrit Rev Ther Drug Carrier Syst1992921351871386002
- MaedaHNakamuraHFangJThe EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivoAdv Drug Deliv Rev2013651717923088862
- DanquahMKZhangXAMahatoRIExtravasation of polymeric nanomedicines across tumor vasculatureAdv Drug Deliv Rev201163862363921144874
- Maier-HauffKUlrichFNestlerDEfficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiformeJ Neurooncol2011103231732420845061
- StuppRMasonWPvan den BentMJRadiotherapy plus concomitant and adjuvant temozolomide for glioblastomaThe New England journal of medicine20053521098799615758009
- HainfeldJFDilmanianFAZhongZSlatkinDNKalef-EzraJASmilowitzHMGold nanoparticles enhance the radiation therapy of a murine squamous cell carcinomaPhys Med Biol201055113045305920463371
- DutzSKetteringMHilgerIMüllerRZeisbergerMMagnetic multicore nanoparticles for hyperthermia – influence of particle immobilization in tumour tissue on magnetic propertiesNanotechnology2011222626510221576784
- LewisRSax’s Dangerous Properties of Industrial Materials1–39 edNew YorkVan Nostrand Reinhold19962722
- Reagan-ShawSNihalMAhmadNDose translation from animal to human studies revisitedFASEB J200822365966117942826
- PfizerCisplatin injection [product information]New York, NYPfizer nd [updated Apr 2012]. Available from: http://www.pfizer.com/files/products/uspi_cisplatin.pdfAccessed January 5, 2013
- AdsonMAvan HeerdenJAAdsonMHWagnerJSIlstrupDMResection of hepatic metastases from colorectal cancerArch Surg198411966476516732473
- NoguchiYWuJDuncanREarly phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissuesJpn J Cancer Res19988933073149600125
- DvorakHFNagyJADvorakJTDvorakAMIdentification and characterization of the blood vessels of solid tumors that are leaky to circulating macromoleculesAm J Pathol19881331951092459969
- YaoLDannielsJMoshnikovaApHLIP peptide targets nanogold particles to tumorsProc Natl Acad Sci USA2013110246547023267062
- IvkovRDeNardoSJDaumWApplication of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancerClin Cancer Res20051119 Pt 27093s7103s16203808
- LiangruksaMGangulyRPuriIKParametric investigation of heating due to magnetic fluid hyperthermia in a tumor with blood perfusionJ Magn Magn Mater20113236708716
- HergtRAndraWd’AmblyCGPhysical limits of hyperthermia using magnetite fine particlesIEEE Trans Magn199834537453754
- WiekhorstFSteinhoffUEberbeckDTrahmsLMagnetorelaxometry assisting biomedical applications of magnetic nanoparticlesPharm Res20122951189120222161287
- YuanYWyattCMaccariniPA heterogeneous human tissue mimicking phantom for RF heating and MRI thermal monitoring verificationPhys Med Biol20125772021203722430012
- HildebrandtBWustPAhlersOThe cellular and molecular basis of hyperthermiaCrit Rev Oncol Hematol2002431335612098606
- RaoWDengZSLiuJA review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumorsCrit Rev Biomed Eng201038110111621175406
- WustPHildebrandtBSreenivasaGHyperthermia in combined treatment of cancerLancet Oncol20023848749712147435