Abstract
Background
The heterologous deoxyribonucleic acid (DNA) prime-adenovirus (AdV) boost vaccination approach has been widely applied as a promising strategy against human immunodeficiency virus (HIV)-1. However, the problem of inefficient delivery and lack of specificity of DNA vaccine remains a major issue. In this paper, to improve the transfection of DNA vaccine and realize dendritic cell targeting, we used mannosylated polyethyleneimine (man-PEI) as a DNA vector carrier.
Method
The DNA plasmid encoding antigen HIV gag fragment was constructed by polymerase chain reaction. Then the DNA plasmid was complexed with man-PEI. The in vitro transfection efficiency of man-PEI/DNA was analyzed on DC 2.4 cells. Mice were primed with 25 μg pVAX1-HIV gag plasmid complexed with man-PEI, 100 μg naked pVAX1-HIV gag plasmid, or empty pVAX1 vector and boosted by AdV encoding the same antigen. The antibody titer, CD4+ and CD8+ T-cell response, as well as interferon-γ and interleukin-4 levels in serum and in splenocytes culture were analyzed using flow cytometry or enzyme-linked immunosorbent assay to evaluate the immune response. To test a long-term effect of the vaccination regimen, CD8+ memory T-cell was also detected by flow cytometry.
Results
The pVAX1-HIV gag was constructed successfully. The in vitro transfection efficiency in dendritic cells was significantly higher than naked DNA plasmid. Compared with 100 μg naked DNA/AdV group, the immunoglobulin G2a antibody titer, T-cell response percentage, and cytokine production level induced by man-PEI/DNA/AdV group were significantly higher at a lower DNA dose. Also, the man-PEI/DNA could stimulate a memory CD8+ T-cell response.
Conclusion
Owing to the adjuvant effect of man-PEI, the man-PEI/pVAX1-HIV gag priming plus AdV boosting strategy proved to be a potent vaccine candidate against HIV, which could induce a stronger immune response with a lower DNA dose.
Introduction
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) has become the most catastrophic pandemic since first discovered in 1981. To date, more than 300,000 people have died of HIV/AIDS, with approximately 6500 new infections daily.Citation1 After years of efforts, though we have seen dramatic progress in treating HIV, it is still difficult to eradicate the virus.Citation2 Current options of treatment are combinations (or “cocktails”) consisting of at least three different kinds of antiretroviral agents; however, the results are far from satisfactory. To explore an effective way to slow down the spread of this pandemic is on the top of our agenda. Of all the interventions to prevent and treat diseases, vaccination seems the most promising and feasible choice.Citation2,Citation3 Recently, there has been a rapid increase of candidate vaccines for preventing the infection of HIV. Traditional vaccines are developed by inactivation or attenuation of viral pathogens. With the progress of molecular engineering, gene-based vectors including poxvirus, plasvectors, adenovirus (AdV), and adeno-associated virus, have become more competent pathogens’ carriers, exhibiting a strong impact on the magnitude of immune response.Citation4
A potent vaccine should have the ability to induce both antibody and cytotoxic T-cell responses, which are necessary for a vaccine to prevent viral infection. Ideally, an effective vaccine can abort the viral infection by stimulating high titers of antigen-specific antibodies. Meanwhile, the cytotoxic T-cell response will eliminate virus-infected cells.Citation5 Among all the gene-based vaccine candidates, a replication-defective recombinant AdV serotype 5 has been proven to be potent and widely used as a vaccine carrier for its safety and immunogenicity to elicit high titer of antigen-specific antibody and the cytotoxic T-cell response via intramuscular or subcutaneous injections. Moreover, AdV vector has the ability to stimulate the secretion of cytokines to promote the maturation of antigen-presenting cells (APCs), which will facilitate the antigen presentation process. However, the AdV vaccine still has some drawbacks. Repeated injection of AdV vaccine usually results in a high titer of serotype-specific neutralizing antibody in the immunized body, which will limit the expression of the encoded antigen and strongly impair the strength of the immune response. To circumvent this limitation of the AdV vaccination, some researchers have explored a heterologous prime–boost vaccination strategy using deoxyribonucleic acid (DNA) plasmid for priming followed by a viral vector boosting, which has shown great potential in inducing protective immunity in some experimental models and elicited a much more robust immune response than single vaccination.Citation3,Citation6–Citation8 After the efficacy of this approach was first reported by Schneider et al,Citation9 a number of investigators have demonstrated that the heterologous prime–boost strategy is capable of eliciting greater levels of immunity against a variety of tumors and pathogens than homologous prime-boost strategy or a single vaccination of the same vector.Citation7,Citation10 Moreover, the heterologous prime–boost regimen using recombinant viral vector in combination with DNA plasmid has the ability to induce Th1 (T helper cells type I) CD4+ responses.Citation7
As the priming vaccination in the prime–boost regimen (though the DNA plasmid vaccination itself has been proved to be one effective way of inducing antigen-specific Th1 and cytotoxic CD8+ T [CTL] responses by intramuscular injection and applied to generate protective immunity to various pathogens),Citation11 the strength of the immune responses induced by DNA vaccines is relatively weak compared with conventional vaccines.Citation12 It is reported that DNA immunization is incapable of generating sufficient antibody and cellular immune response to prevent the hepatitis C virus infection in a chimpanzee model,Citation13 mainly due to its low transfection efficiency and lack of specificity. Thus, the problem of inefficient delivery for the plasmid vector must be addressed before achieving a better outcome for the DNA vaccine. It is well known that APCs, such as dendritic cells (DCs), express a large amount of mannose receptor on the surface.Citation14 To enhance the specificity of drug delivery, a large number of research papers have explored different mannosylated drug delivery systems, such as mannosylated chitosan,Citation14 mannosylated liposome,Citation15 and mannosylated polyethyleneimine 25k (PEI 25k). Among them, mannosylated PEI 25k has become the center of attention for its adjuvant activity;Citation16 however, the cytotoxicity of PEI 25k strongly limits its application. To address this issue, in our previous work, we synthesized a new biodegradable mannosylated PEI polymer by linking PEI 2000 and PEI 600 using triethyleneglycol as the cross-linker, which exhibited a much lower cytotoxicity. It has been shown that this new mannosylated polyethyleneimine (man-PEI) is a promising and applicable gene-delivery carrier due to its higher gene transfection efficacy and cellular uptake compared with nonmannosylated PEI and commercial PEI 25k. Moreover, the man-PEI/DNA complex is capable of inducing an adequate upregulation of surface markers for DC maturation,Citation17 indicating that man-PEI has a certain adjuvant effect, which is crucial in DNA vaccine delivery.Citation18
To further prove this concept in vivo, we explored the adjuvanticity of man-PEI in an HIV model. We developed the DNA priming and AdV boosting strategy, using man-PEI as the DNA vaccine adjuvant. The antigen-specific antibody, interferon (IFN)-γ and interleukin (IL)-4 levels were tested. Also, the cytotoxic CD8+ and helper CD4+ T-cell responses were investigated. In addition, we analyzed the central memory T-cells (TCM) to evaluate the long-term immune response of the vaccination strategy.
Materials and methods
Plasmids and adenovirus vectors
To construct the pVAX1-HIV gag plasmid vector, an HIV-1 gag fragment was amplified from an AdV-HIV gag by polymerase chain reaction (PCR) using primers 5′-T-TTAAGCTTATGGGCGCCAGAGCCAG-3′ and 5′-TT-TTCTAGATCACTGAGAGCTGGGGTC-3′. The PCR product was then digested by restriction enzymes Xba 1 and Hind 3, and inserted into backbone vector pVAX1 (Life Technologies, Carlsbad, CA, USA), which was digested by the same enzymes. The obtained plasmid was identified by gel electrophoresis and DNA sequencing. All plasmids used for injection were amplified and purified using Qiagen endo-free Giga prep kit (Qiagen, Alameda, CA, USA) to remove endotoxins. The AdV was amplified in HEK 293T (American Type Culture Collection [ATCC], Manassas, VA, USA) cells and purified by cesium chloride gradient centrifugation. The virus particle number and plague-forming unit were determined by ultraviolet spectrophotometry and plague-forming assay, respectively.
Man-PEI/DNA complex preparation
The man-PEI/DNA complex was prepared as described in a previous paper.Citation17 Briefly, mannosylated biodegradable low molecular weight-PEI was synthesized by Sun et al.Citation17 The plasmid and man-PEI was diluted in 5% glucose solution. The DNA solution was added to man-PEI solution and vortexed for 10 seconds. Then, the mixture was incubated at room temperature for 20 minutes for the complex to form.
In vitro transfection assay
The transfection assay was conducted on DC 2.4 cells (murine DCs, ATCC), which were reported to express mannose receptor on the surface.Citation14 DC 2.4 cells were seeded in 24-well plates at 1 × 105 cells/well and incubated at 37°C, 5% CO2 overnight. The optimized nitrogen/phosphorous (N/P) ratios of the man-PEI/DNA complex and the PEI 25k/DNA complex were determined as previously described.Citation17 Naked pVAX1-HIV gag, man-PEI/pVAX1-HIV gag complex (N/P = 40), and PEI 25k/pVAX1-HIV gag complex (N/P = 15) were added to each well and incubated in serum-free Roswell Park Memorial Institute (RPMI) 1640 medium. (Hyclone, Thermo Scientific, Waltham, MA, USA) for 4 hours; both complexes were formed at the optimized N/P ratio. The DNA dose was 1 μg/well. Then, the infection solution was replaced by fresh RPMI 1640 medium containing 10% fetal bovine serum and incubated for another 40 hours. Each group was analyzed in triplicate. Then the ribonucleic acid (RNA) of each sample transfected with pVAX1-HIV gag was extracted using an RNA isolation kit (TIANGEN Biotech (Beijing) Co. Ltd., Beijing, People’s Republic of China) and reverse-transcripted into cDNA. Real-time PCR was used to detect the expression of gag fragment using primers 5′-caggtgagccagaactatcca-3′, 5′-ctcctcgttgatggtttcctt-3′. SsoFast™ EvaGreen Supermix (Bio-Rad Laboratories, Hercules, CA, USA) was used for the quantification of HIV gag transcripts. β-actin was detected as an internal reference. In addition, plasmid-expressing reporter gene lacZ was complexed with man-PEI or PEI 25k with the same N/P ratio as described above and used to transfect DC 2.4 cells. For the samples transfected with reporter gene lacZ, the results were quantified using a β-galactosidase assay. Average β-galactosidase activities were determined using the β-galactosidase enzyme assay system. The total protein content of the lysates was measured by a bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Rockford, IL, USA) using a standard of bovine serum albumin (BSA).
Mice
Six- to 8-week-old female BALB/c mice were purchased from the Laboratory Animal Center of Sichuan University and then housed under pathogen-free conditions. All animal tests were conducted according to institutional animal care and use guidelines.
Immunization
Animals were divided randomly into four groups (n = 5) and primed intramuscularly with a total volume of 100 μL divided equally between two hind quadriceps. Group 1 received 100 μg of pVAX1-HIV gag diluted in 5% glucose at week 0. Group 2 received 25 μg of pVAX1-HIV gag complexed with man-PEI. Group 3 received empty vector pVAX1 as a control. All these groups were given a boost immunization with 1 × 1010 viral particles (vp) of AdV at week 2. Group 4 received 5% glucose as a placebo. Blood was collected by retroorbital puncture before boosting vaccination. All mice were sacrificed 10 days after the last immunization for immunological assays.
Antibody ELISA
HIV gag-specific antibody responses (including total immunoglobulin [Ig]G, IgG1, and IgG2a) in serum samples were analyzed. Polystyrene 96-well plates (Corning, Incorporated, Corning, NY, USA) were coated with 100 μL of 1 μg/mL antigen HIV-1 gag P24 strain 3B (United States Biological, Swampscott, MA, USA) diluted in coating buffer and incubated at 4°C overnight. Then, plates were blocked with phosphate buffered saline (PBS) containing 1% BSA for 1 hour at 37°C. After blocking, the plates were washed three times with PBS containing 0.5% Tween-20 (PBST). Serum samples were diluted twofold serially with PBST containing 1% BSA and added to the plates for 2 hours of incubation at 37°C. After three washes, the plates were incubated with peroxidase-conjugated antimouse Ig (diluted 1:1000 in PBST-1% BSA) for 1 hour at 37°C. The plates were then washed and incubated with a 100 μL/well of tetramethylbenzidine substrate (BioTime, Inc, Beijing, People’s Republic of China) for 30 minutes at room temperature. The reaction was stopped with 2 M H2SO4. The optical density was measured at 450 nm using a Microplate Reader (VarioSkan; Thermo Fisher Scientific).
Intracellular cytokine staining
Spleens were removed and gently homogenized through a 70 μm cell strainer (BD Falcon, Franklin Lakes, NJ, USA) in 10% RPMI 1640 medium to prepare single cell suspension.Citation19 Red blood cells were lysed by ammonium–chloride–potassium lysing buffer. Splenocytes were washed by PBS and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 2-mercaptoethanol with 2 μg/mL gag peptide (the H-2 Kd-restricted immunodominant CTL epitope AMQMLKETI; GenScript USA, Inc, Piscataway, NJ, USA). Control cells were cultured with medium only. One hour later, brefeldin A was added to each sample (eBioscience, San Diego, CA, USA) and incubated for another 5 hours at 37°C. After washing, cells were incubated with 1:100 dilution of antimouse CD8a-flourescein isothiocyanate (FITC) or antimouse CD4-FITC (eBioscience) at 4°C for 30 minutes. They were washed, fixed, and permeabilized with Fixation and Permeabilization buffers (eBioscience). Then, cells were incubated in permeabilization buffer with a 1:100 dilution of antimouse IFN-γ-PE or antimouse IL-4-polyethyleneimine (PE), respectively (eBioscience). After washing, cells were diluted in PBS with 1% BSA and were analyzed for CD8+/IFN-γ+ and CD4+/IL-4+ by two-color flow cytometry (Beckman Coulter, Inc, Brea, CA, USA), respectively. For these data, the double-positive percentage in the absence of the gag peptide was subtracted from the percent of cytokines that produced by T-cells in the presence of this peptide. Data were analyzed using the Kaluza software (Beckman Coulter Inc).
Cytokine-specific ELISA
Cytokines in serum samples and in splenocytes culture supernatants were measured. IFN-γ and IL-4 were analyzed by mouse anti-IFN-γ and mouse anti-IL-4 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Optical density at 450 nm was determined. Each sample was examined in duplicate. The splenocytes were cultured in RPMI 1640 medium with supplementary 10% FBS in 96-well plates, 100 μL/well; 2 μL/ml gag peptide was added to each well and cultured for 72 hours. The supernatants were collected for ELISA.
Memory T-cell assay
Mice were primed with DNA vaccine at day 0 and boosted with AdV vaccine at day 14 (n = 5), and then the mice were sacrificed at day 40. Single splenocyte suspension was prepared as previously described. After lysis of red blood cells, splenocytes were washed with PBS and stained with CD8-FITC, CD44-PE, and CD62L-PE-Cy7 (eBioscience) at 4°C for 30 minutes without light. After washing, splenocytes were diluted in PBS and analyzed by three-color flow cytometry (Beckman Coulter Inc). Then, 6 × 105 cells were gated on CD8+, CD44+, and CD62L+. Data were analyzed using the Kaluza® software (Beckman Coulter, Inc).
Statistical analysis
All experiments were performed in triplicate, unless otherwise noted. The data were shown as the mean ± standard deviation. The differences/correlations between two groups were analyzed using Student’s t-test, and the differences between the control group and the experimental groups were analyzed using one-way analysis of variance. A P-value < 0.05 was considered to be significant.
Results
Construction and identification of pVAX1-HIV gag plasmid
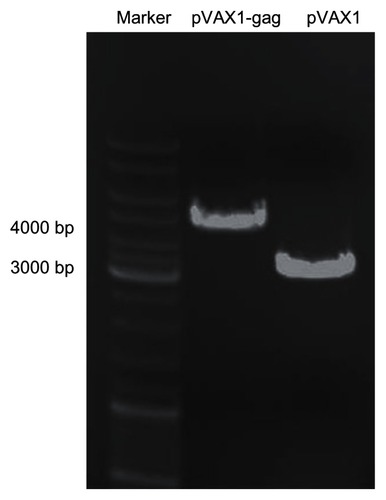
Plasmid expressing HIV-1 gag was constructed. The fragment of HIV-1 gag was amplified from AdV vector by PCR. The primers were designed with two enzyme sites: Xba 1 and Hind 3. The empty backbone plasmid was digested by the same enzymes, and then the gag fragment (1500 bp) was cloned into pVAX1 vector using T4 ligase. To confirm if the plasmid was successfully constructed, gel electrophoresis was conducted. As shown in , the pVAX1-HIV gag plasmid was constructed successfully with a length of 4500 bp, which was 1500 bp longer than the empty pVAX1 vector (3000 bp). DNA screening results proved that the sequence of pVAX1-HIV gag was correct.
Figure 1 Agarose gel electrophoresis of pVAX1-HIV gag and pVAX1 vectors.
Notes: The empty pVAX1 vector was 3000 bp. After insertion of HIV gag fragment (1500 bp), the pVAX1-HIV gag was 4500 bp.
Abbreviation: bp, base pair; HIV, human immunodeficiency virus.
Then, the constructed plasmid was transformed into Escherichia coli and incubated in Luria-Bertani (LB) culture medium. The plasmid vector was collected and purified using the Qiagen endo-free Giga prep kit (Qiagen). Ultraviolet spectrophotometry showed the concentration of the plasmid was 2.25 mg/mL. The optical density ratio of 260nm and 280nm (OD260/OD280) was 1.8, indicating the DNA sample was not contaminated by protein or RNA. The viral titration was 1 × 1013 vp/mL and 2 × 1011 plaque forming units, as determined by ultraviolet spectrophotometry and plague-forming assay, respectively.
In vitro transfection activity of man-PEI/DNA complex
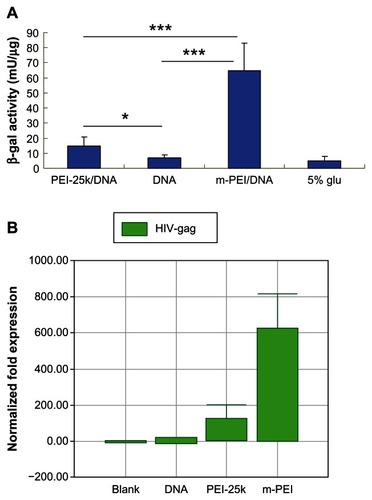
To test the transfection activity of man-PEI, transfection assay was performed on DC 2.4 cells. It was reported that mannose receptor was highly expressed on the surface of macrophages and DCs such as DC 2.4 cells. Compared with PEI 25k, the man-PEI exhibited higher transcription efficiency with lower toxicity.Citation17 Moreover, the mannosylated PEI was expected to target antigen-presenting cells via mannose and mannose receptor. For the samples transfected with reporter gene lacZ, it could be seen that the results of man-PEI/DNA exhibited the highest β-galactosidase activity (P < 0.005). The results of PEI 25k/DNA were significantly higher than naked DNA group (P < 0.05).
For the samples transfected with pVAX1-HIV gag, RNA was extracted from cells of all groups and reverse-transcripted into cDNA. Then, real-time PCR was conducted to analyze the expression of the target gene. As shown in , the man-PEI/DNA group showed the best transcription activity. The target gene expression of man-PEI/DNA group was 600 times higher than naked plasmid group and four times higher than PEI 25k/DNA group, and the gene expression of PEI 25k/DNA group was about 150 times higher than naked DNA group. It could be seen that mannosylated PEI had the ability to increase the transcription of DNA. In addition, the results proved that the plasmid we constructed was able to express the target gene.
Figure 2 In vitro transcription activity of man-PEI and PEI 25k on DC 2.4 cells. (A) Quantified by β-galactosidase assay using plasmid encoding lacZ as a reporter gene. (B) Transfected with pVAX1-HIV gag and quantified by the transcription level of HIV gag gene using real-time PCR.
Notes: The β-galactosidase activity of the m-PEI/DNA group was significantly higher than that of the naked DNA group and the PEI 25k/DNA group, ***P < 0.005. The PEI 25k/DNA group was higher than the naked DNA group, *P < 0.05.
Abbreviations: man-PEI, mannosylated polyethyleneimine; PEI 25k/DNA, polyethyleneimine 25k and DNA plasmid complex; DC, dendritic cells; DNA, deoxyribonucleic acid; m-PEI/DNA, mannosylated polyethyleneimine and DNA plasmid complex; glu, glucose; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
Detection of anti-HIV gag-specific antibody
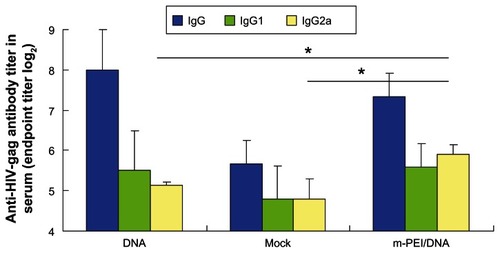
To assess whether the vaccine could induce robust specific antibody against HIV gag, the sera of immunized mice were obtained at day 14 and day 24. Anti-HIV gag-specific ELISA was performed. IgG, IgG1, and IgG2a titers were measured separately. As demonstrated in , the serum from the group primed with man-PEI/DNA and boosted with AdV 14 days later showed the highest IgG2a titer (P < 0.05). However, the differences of IgG and IgG1 titer were not significant compared with the naked plasmid and backbone plasmid groups.
Figure 3 Specific antibody titer (IgG, IgG1, IgG2a) of HIV-gag.
Notes: The titer of the 5% glucose group was used as a background level. Blood samples were diluted by twofold dilution from 1:16. The data were represented as the mean ± standard deviation of three independent experiments. The IgG2a titer of the m-PEI/DNA group was significantly higher than the naked DNA group and Mock, *P < 0.05. The IgG and IgG1 titer of the three groups were nonsignificant.
Abbreviations: Ig, immunoglobulin; HIV, human immunodeficiency virus; DNA, deoxyribonucleic acid; Mock, empty vector pVAX1; m-PEI/DNA, mannosylated polyethyleneimine and DNA plasmid complex.
For the serum obtained before boost vaccination, we compared the OD (450 nm) value of different groups since the prime vaccine alone only induced a low antibody level. Serum of the man-PEI/DNA complex group showed the highest OD value in IgG, IgG1, and IgG2a (P < 0.05) at the dilution of 1:100 (data not shown). From these results, it could be seen that the man-PEI/DNA complex could enhance the humoral immune response of immunized mice.
Induction of HIV gag-specific CD8+ T-cell response and CD4+ T-cell response
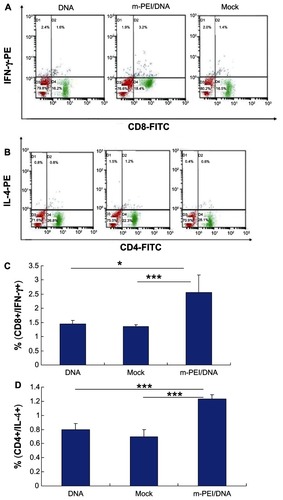
Priming with DNA vaccines and boosting with AdV vaccines could significantly augment the immune response.Citation8 Since the T-cell response played a major role in eliminating virus infection, here we analyzed HIV gag-specific CD8+ and CD4+ T-cell response. To quantify the specific T-cell response, IFN-γ expressing gag CD8+ and IL-4 expressing CD4+ T-cells were identified by intracellular cytokine staining. As shown in , the average percentage of antigen-specific CD8+ T-cells of the man-PEI/DNA/AdV group was about 2.56%, significantly higher than the other two groups. The average percentage of antigen-specific CD8+ T-cells induced by naked plasmid/AdV (1.45%) was higher than empty vector/AdV group (1.36%), but there was no significant difference between them. The results of antigen-specific CD4+ T-cells were consistent with the results of CD8+ T-cells. The man-PEI/DNA/AdV group showed the highest percentage (1.23%), which indicated that the man-PEI/DNA/AdV regimen could induce a more robust cellular immune response than naked DNA/AdV regimen. Moreover, from our in vivo preexperiment results, it was found that the higher the DNA dose complexed with man-PEI, the better the immune response it would induce, and thus the increase was almost linear.
Figure 4 Intracellular cytokine staining of splenocytes after AdV boosting immunization. (A) The CD8+/IFN-γ+ staining. (B) The CD4+/IL-4+ staining results. (C and D) The double-positive percentage of CD8+/IFN-γ+ and CD4+/IL-4+.
Notes: The data were represented as mean ± standard deviation of three independent experiments. The double-positive percentage of CD4+/IL-4+ and CD8+/IFN-γ+ of the m-PEI/DNA group was significantly higher than that of the naked DNA group and Mock, *P < 0.05; ***P < 0.005.
Abbreviations: AdV, adenovirus; IFN, interferon; PE, polyethyleneimine; DNA, deoxyribonucleic acid; m-PEI/DNA, group primed with a complex of mannosylated polyethyleneimine and DNA plasmid and boosted with AdV; Mock, group primed with empty vector pVAX1 and boosted with AdV; FITC, fluorescein isothiocyanate; IL, interleukin.
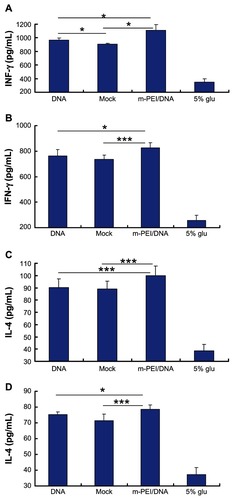
IL-4 and IFN-γ level in splenocytes culture supernatants and serum
All splenocytes were obtained and cultured as previously described in the materials and methods section. IL-4 was mainly produced by the Th2 cells, while IFN-γ was mostly secreted by Th1 cells. Cytokine levels in serum after AdV boost vaccination and in splenocytes culture supernatants were quantified using sandwich ELISA, according to the manufacturer’s instructions. As demonstrated in , the man-PEI/DNA/AdV regimen showed the highest IFN-γ level (1110 pg/mL in serum and 827 pg/mL in supernatants), which was significantly higher than that in naked DNA/AdV group (968 pg/mL in serum and 762 pg/mL in supernatants) and in the empty vector/AdV group (903 pg/mL in serum and 733 pg/mL in supernatants) (P < 0.05). Additionally, there was no significant difference in the IFN-γ production in blood serum or in the supernatant between the naked DNA/AdV group and the backbone vector/AdV group. We can see from that the IL-4 level showed the same trend. The man-PEI/DNA/AdV group showed the highest IL-4 level in both serum and supernatants (100 pg/mL in serum and 78 pg/mL in supernatants). Thus, the man-PEI/DNA/AdV regimen augmented the antigen-specific cytokine level both in serum and in the splenocytes culture supernatant of immunized mice.
Figure 5 The cytokine production of mice immunized with DNA and AdV. (A and C) The IFN-γ and IL-4 production in serum, respectively. (B and D) The IFN-γ and IL-4 production in splenocytes culture supernatant, respectively.
Notes: The data were represented as mean ± standard deviation of three independent experiments. The IFN-γ and IL-4 levels in blood serum and the splenocytes culture supernatant of the m-PEI/DNA group were significantly higher than that of the naked DNA/AdV group and Mock. The serum IFN-γ level of the DNA group was higher than that of the Mock group, *P < 0.05; ***P < 0.005.
Abbreviations: DNA, deoxyribonucleic acid; IFN, interferon; Mock, group primed with empty vector pVAX1 and boosted with AdV; m-PEI/DNA, group primed with a complex of mannosylated polyethyleneimine and DNA plasmid and boosted with AdV; glu, glucose; IL, interleukin; AdV, adenovirus.
Memory T-cell detection
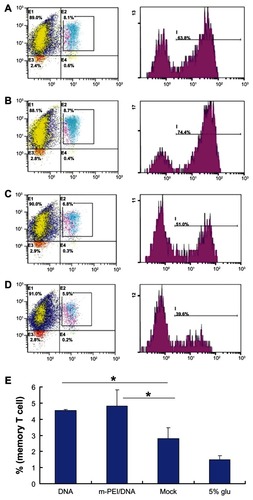
The quantity and quality of memory T-cells played a vital role in inducing a long-time immunization, which was important in eliminating viral infection. Generally, there were two types of memory T-cells: TCM and effector memory T-cells (TEM). Since TCM were predominantly found in lymphoid tissues and responded more rigorously to the secondary challenge than TEM,Citation20 here we analyzed CD8+ TCM (CD8+ CD44+ CD62L+) as an indicator of the memory T-cell response.
All groups of splenocytes were stained with CD8-FITC, CD44-PE, and CD62L-PE-Cy7. As shown in , all groups displayed a certain amount of memory T-cells. Among them, the man-PEI/DNA/AdV group showed the highest percent of TCM (4.73%) compared with both the naked DNA/AdV group (4.54%) and the empty vector/AdV group (2.8%). Though there was no significant difference between the naked DNA/AdV and man-PEI/DNA/AdV groups, these two groups showed a significantly higher percentage of TCM than the empty vector/AdV group.
Figure 6 Memory T-cell assay results. (A) The flow cytometry result of the DNA group. (B) The result of the m-PEI/DNA group. (C) The result of the Mock group. (D) The result of 5% glucose. (E) The frequency of triple-positive cells, *P < 0.05.
Note: The triple-positive percentage of the m-PEI/DNA group and the DNA group were higher than Mock, *P < 0.05.
Abbreviations: DNA, deoxyribonucleic acid; m-PEI/DNA, group primed with complex of mannosylated polyethyleneimine and DNA plasmid and boosted with AdV; Mock, group primed with empty vector pVAX1 and boosted with AdV; glu, glucose; AdV, adenovirus.
Discussion
These studies have been aimed at improving the efficacy of the heterologous DNA prime-AdV boost vaccination strategy against HIV in inducing both cellular and humoral immune responses. Previous research has shown the capability of the DNA prime-AdV boost regimen, which can elicit both antigen-specific antibody responses and cytotoxic T-cell responses.Citation12 However, some problems, such as the low transfection efficiency of the DNA vector and the lack of specificity, need to be overcome. In this paper, we used mannosylated low molecular weight PEI as a DNA vector carrier to enhance its transfection efficiency. After boosting with AdV, both the CD8+ T-cell response and the CD4+ T-cell response are enhanced significantly. Owing to the adjuvant activity of man-PEI, the man-PEI/DNA/AdV group demonstrated a more robust cellular immune response at a lower DNA plasmid dose.
PEI has been used in gene therapy for a long time since it was first introduced as a gene delivery vehicle.Citation21 Many research papers have proven that PEI was able to be used as DNA vector carriers.Citation22,Citation23 PEI, a readily available material, has the ability to bind and condense DNA. The complexes formed by PEI and DNA can protect DNA from DNase degradation and facilitate the transfection of DNA, then release DNA from endosomes to cytosol.Citation24,Citation25 The PEI/DNA complex could prolong the retention of DNA and enhance the consequent transfection of the target gene.Citation25,Citation26 Furthermore, PEI also exhibited some adjuvant activity. Garzon et alCitation27 examined the protective immune response against HIV-1 induced by the PEI/DNA complex, resulting in an improved cytokine production and antigen-specific CD8+ T-cell response. In addition, PEI has also been used in a heterologous prime–boost vaccination strategy. Huang et alCitation25 explored a heterologous approach using mucosal PEI/DNA priming and systemic recombinant TianTan vaccinia boosting. The results indicated that PEI could be used as a competent carrier for the delivery of a DNA vaccine. This idea was also supported by Wegmann et al,Citation28 who showed that PEI was a safe and efficient DNA vaccine adjuvant that induced a stronger immune response.
Thus, the adjuvanticity of PEI has been proved. In some research papers, PEI has been coupled with different ligands, such as arginine-glycine-aspartic acid (RGD) and galactose, for the purpose of targeting.Citation23 In our study, to further improve the efficacy of the DNA vaccine and to realize APC targeting, we modified PEI with a mannose. The man-PEI we used as a DNA carrier could retain the high transfection efficiency and reduce toxicity. More importantly, the mannose conjugated on man-PEI is supposed to target antigen-presenting cells, which highly express mannose receptors on their cell surface. From the data we obtained, the in vitro DNA transfection efficiency on DCs was greatly improved by man-PEI. Furthermore, after an AdV-boosting vaccination, the man-PEI/DNA complex group resulted in a better T-cell immune response and higher cytokine levels compared with the naked DNA group, whose DNA dose was four times higher than that of the man-PEI/DNA complex group. These results indicate that man-PEI has a strong adjuvant effect. Thus, we can infer from the results that man-PEI is a potent DNA vaccine carrier, which can induce a stronger immune response with a lower DNA dose.
Traditional anti-HIV-1 DNA vaccines usually generate limited antigen-specific antibody levels, mainly due to their low transfection efficiency. The boost vaccination of AdV significantly enhanced the IgG level compared with the DNA vaccination alone.Citation29 In our man-PEI/DNA complex/AdV regimen, we tested antigen-specific antibody IgG, and subtypes IgG1 and IgG2a. From the results, we can see that after AdV boosting, the IgG2a titer of the man-PEI/DNA group was higher than that of the naked DNA group (), while the IgG and IgG1 titers of the naked DNA group and the man-PEI/DNA group were nonsignificant. This may be ascribed to the dominant role the AdV played in inducing antibodies, since all groups except for the blank control, were boosted with the AdV vaccine. Generally, T helper cells mainly divide into two subtypes: Th1 and Th2 immune responses. The Th1 response, which induces the IgG2a subtype, is primarily cellular, while the Th2 immune response, which induces the IgG1 subtype, is primarily associated with antibody production.Citation10 An IgG1:IgG2a ratio ≤ 0.5 indicates a Th1-biased immune response, while a ratio ≥ 2 indicates a Th2-biased immune response. Ratios between 0.5 and 2 indicate a mixed response.Citation30 In our vaccination strategy, the IgG1:IgG2a ratio of the man-PEI/DNA priming and AdV boosting group was between 0.5 and 2 with a higher IgG2a titer, indicating that this strategy caused a mixed Th1 and Th2 response with more robust Th1-prone immunity.Citation31
Cytokines play an important role in modulating the immune response.Citation32 Different cytokines can be grouped into two types: Th1 cytokine and Th2 cytokine. IL-2, IL-12, and IFN-γ all belong to the Th1 cytokine and regulate cellular responses, while IL-4 and IL-10 are Th2 cytokines and are associated with humoral responses.Citation33 Our data showed that both INF-γ and IL-4 levels in the blood serum and splenocytes culture supernatant of the man-PEI/DNA/AdV group were higher than those of the naked DNA/AdV group. We can infer from the results that the man-PEI/DNA/AdV regimen was capable of eliciting stronger Th1 and Th2 immune responses. Moreover, intracellular cytokine staining demonstrated that cytotoxic CD8+ T-cell and helper CD4+ T-cell levels were substantially higher than in the naked DNA/AdV group. CTL cells played a vital role in eradicating virus infection.Citation34 Thus, with the help of the adjuvanticity of man-PEI, the man-PEI/DNA/AdV regimen we used in this paper was potent in eliciting both cytotoxic and helper T-cell responses.
In some research papers, the heterologous DNA/AdV vaccination regimen evoked more robust humoral and cellular immune responses with three injections of 50 μg DNA.Citation18 However, from the results we obtained, the naked DNA priming plus AdV boosting strategy induced almost the same level of immune response with empty plasmid priming and the AdV boosting group, showing no significance in terms of cytokine production and intracellular cytokine staining assay, except the serum IFN-γ levels exhibited a significant difference between the two groups ( and ). This may be due to the differences in the DNA vaccination dose and immune intervals. In our strategy, the mice were primed with one injection of 100 μg DNA plasmid, since the immune response induced by the naked DNA was too weak. It is worth noticing that the DNA dose in the man-PEI/DNA complex group is only one-quarter of that of the naked DNA group. This indicates that the mannosylated PEI has a strong adjuvant effect, and as a result, our man-PEI/DNA complex priming plus AdV boosting vaccination has the capability of inducing a more efficient immune response with a lower dose of DNA plasmid.
For an efficacious viral vaccine, the induction of neutralizing antibody and T-cell response is only temporary. When an antigen attacks the human body, it is expected to elicit an immune response – more importantly, a long-term memory. Once the same antigen attacks again, the memory T-cell will induce a quicker and stronger second expansion. CD8+ memory T-cells can differentiate between cytotoxic CD8+ T-cells, which eradicate virus-infected cells.Citation35 Therefore, the induction of potent, long-lived specific CD8+ T-cell responses is a major goal of an effective anti-HIV vaccine.Citation36 Based on the different surface markers, memory T-cells are divided into two subsets: TCM (CD62Lhigh), which are predominantly found in lymphoid tissues, and TEM (CD62Llow), which are mostly found in peripheral tissues.Citation37 Some results showed that TCM respond more vigorously than TEM.Citation20 In our work, both the group using man-PEI as the DNA vaccine carrier, but with a lower dose of plasmid, and the group with the fourfold higher plasmid dose elicit relatively high levels of CD8+ memory T-cells, indicating that the heterologous prime–boost vaccination strategy is capable of evoking a long-term immune response, which is consistent with the results from other studies,Citation38,Citation39 and more importantly, confirms that man-PEI can reduce the DNA dose substantially.
Conclusion
In summary, we used mannosylated PEI as a DNA vaccine carrier and a heterologous DNA priming plus AdV boosting vaccination strategy on immune mice. Our results indicate that the man-PEI/DNA complex/AdV regimen significantly enhances the anti-HIV immune responses with only one-quarter of the dose of DNA compared with the naked DNA/AdV group, resulting in much higher CD8+ and CD4+ T-cell responses and cytokine production levels, which make this strategy a potential anti-HIV-1 vaccine candidate. In the meantime, we have shown the adjuvanticity of mannosylated PEI, which can be used as an efficient DNA vaccine delivery carrier due to its high transfection efficacy and APC targeting effect. Future research may focus on the mechanism of vaccination, and evaluate its clinical efficacy.
Acknowledgments
We are thankful for the financial support of the National Natural Science Foundation of China (No 81173011), the Program for New Century Excellent Talents in University (NoNTEC-10-0601), and the National Science and Technology Major Project of China (No 2011ZX09401-304[4-3]). We also thank Dr Hildegund CJ Ertl from the Wistar Institute of Anatomy and Biology for her kind help in providing the replication-defective adenovirus.
Disclosure
The authors report no conflicts of interest in this work.
References
- FauciASJohnstonMIDieffenbachCWHIV vaccine research: the way forwardScience2008321588853053218653883
- DuerrAWasserheitJNCoreyLHIV vaccines: new frontiers in vaccine developmentClin Infect Dis200643450051116838241
- NabelGJHIV vaccine strategiesVaccine200220151945194711983251
- CasimiroDRChenLFuTMComparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag geneJ Virol200377116305631312743287
- ShaBEOnoratoMBartlettJASafety and immunogenicity of a polyvalent peptide C4-V3 HIV vaccine in conjunction with IL-12AIDS20041881203121615166537
- LoCYWuZMisplonJAComparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategiesVaccine200826172062207218378366
- ParkSHYangSHLeeCGYounJWChangJSungYCEfficient induction of T helper 1 CD4+ T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boostVaccine200321314555456414575768
- QiuCXuJQHIV-1/AIDS vaccine development: are we in the darknessChin Med J (Engl)20081211093994518706210
- SchneiderJGilbertSCBlanchardTJEnhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus AnkaraNat Med1998443974029546783
- KimHDJinJJMaxwellJAFukuchiKIEnhancing Th2 immune responses against amyloid protein by a DNA prime-adenovirus boost regimen for Alzheimer’s diseaseImmunol Lett20071121303817686533
- DonnellyJJUlmerJBLiuMADNA vaccinesLife Sci19976031631729000640
- MennuniCCalvarusoFFacciabeneAEfficient induction of T-cell responses to carcinoembryonic antigen by a heterologous prime-boost regimen using DNA and adenovirus vectors carrying a codon usage optimized cDNAInt J Cancer2005117344445515906358
- FornsXPayettePJMaXVaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCVHepatology200032361862510960458
- JiangHLKangMLQuanJSThe potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunizationBiomaterials200829121931193918221992
- KawakamiSSatoANishikawaMYamashitaFHashidaMMannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomesGene Ther20007429229910694809
- HowardKAAlparHOThe development of polyplex-based DNA vaccinesJ Drug Target200210214315112074541
- SunXChenSMHanJZhangZMannosylated biodegradable polyethyleneimine for targeted DNA delivery to dendritic cellsInt J Nanomedicine201272929294222745554
- WuLKongWPNabelGJEnhanced breadth of CD4 T-cell immunity by DNA prime and adenovirus boost immunization to human immunodeficiency virus Env and Gag immunogensJ Virol200579138024803115956548
- LinJZhiYMaysLWilsonJMVaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in miceJ Virol20078121118401184917715240
- WherryEJTeichgräberVBeckerTCLineage relationship and protective immunity of memory CD8 T cell subsetsNat Immunol20034322523412563257
- GodbeyWTWuKKMikosAGPoly(ethylenimine) and its role in gene deliveryJ Control Release1999602–314916010425321
- ThomasMKlibanovAMEnhancing polyethylenimine’s delivery of plasmid DNA into mammalian cellsProc Natl Acad Sci U S A20029923146401464512403826
- Kukowska-LatalloJFRaczkaEQuintanaAChenCRymaszewskiMBakerJRJrIntravascular and endobronchial DNA delivery to murine lung tissue using a novel, nonviral vectorHum Gene Ther200011101385139510910136
- BieberTMeissnerWKostinSNiemannAElsasserHPIntracellular route and transcriptional competence of polyethylenimine-DNA complexesJ Control Release2002822–344145412175756
- HuangXGXuJQQiuCMucosal priming with PEI/DNA complex and systemic boosting with recombinant TianTan vaccinia stimulate vigorous mucosal and systemic immune responsesVaccine200725142620262917280743
- GoulaDBeckerNLemkineGFRapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexesGene Ther20007649950410757023
- GarzónMRBerraondoPCrettazJInduction of gp120-specific protective immune responses by genetic vaccination with linear polyethylenimine-plasmid complexVaccine200523111384139215661387
- WegmannFGartlanKHHarandiAMPolyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigensNat Biotechnol201293088388822922673
- YangZYWyattLSKongWPMoodieZMossBNabelGJOvercoming immunity to a viral vaccine by DNA priming before vector boostingJ Virol200377179980312477888
- FeltquateDMHeaneySWebsterRGRobinsonHLDifferent T helper cell types and antibody isotypes generated by saline and gene gun DNA immunizationJ Immunol1997158522782284
- ScheerlinckJPCaseyGMcWatersPThe immune response to a DNA vaccine can be modulated by co-delivery of cytokine genes using a DNA prime-protein boost strategyVaccine20011928–294053406011427282
- KimJJSimbiriKASinJICytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIVJ Interferon Cytokine Res1999191778410048771
- TritelMStoddardAMFlynnBJPrime-boost vaccination with HIV-1 Gag protein and cytosine phosphate guanosine oligodeoxynucleotide, followed by adenovirus, induces sustained and robust humoral and cellular immune responsesJ Immunol200317152538254712928404
- PintoARFitzgeraldJCGaoGPWilsonJMErtlHCInduction of CD8+ T cells to an HIV-1 antigen upon oral immunization of mice with a simian E1-deleted adenoviral vectorVaccine2004225–669770314741162
- JacksonSSSchmitzJEKurodaMJEvaluation of CD62L expression as a marker for vaccine-elicited memory cytotoxic T lymphocytesImmunology20051164443453
- KeatingSMBejonPBerthoudTDurable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malariaJ Immunol200517595675568016237057
- RobertsADWoodlandDLCutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lungJ Immunol2004172116533653715153466
- Wille-ReeceUFlynnBJLoréKToll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primatesJ Exp Med200620351249125816636134
- MasopustDHaSJVezysVAhmedRStimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccinationJ Immunol2006177283183916818737