Abstract
Chlamydia trachomatis is a very common sexually transmissible infection in both developing and developed countries. A hallmark of C. trachomatis infection is the induction of severe inflammatory responses which play critical roles in its pathogenesis. Antibiotics are the only treatment option currently available for controlling C. trachomatis infection; however, they are efficacious only when administered early after an infection. The objectives of this study are to explore alternative strategies in the control and regulation of inflammatory responses triggered by a C. trachomatis infection. We employed silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles, which have been shown to possess anti-inflammatory properties, as our target and the in vitro mouse J774 macrophage model of C. trachomatis infection. Our hypothesis is that small sizes of Ag-PVP nanoparticles will control inflammatory mediators triggered by a C. trachomatis infection. Cytotoxicity studies using Ag-PVP nanoparticles of 10, 20, and 80 nm sizes revealed >80% macrophage viability up to a concentration of 6.25 μg/mL, with the 10 nm size being the least toxic. All sizes of Ag-PVP nanoparticles, especially the 10 nm size, reduced the levels of the prototypic cytokines, tumor necrosis factor (TNF) and interleukin (IL)-6, as elicited from C. trachomatis infected macrophages. Additionally, Ag-PVP nanoparticles (10 nm) selectively inhibited a broad spectrum of other cytokines and chemokines produced by infected macrophages. Of significance, Ag-PVP nanoparticles (10 nm) caused perturbations in a variety of upstream (toll like receptor 2 [TLR2], nucleotide-binding oligomerization-protein 2 [NOD2], cluster of differentiation [CD]40, CD80, and CD86) and downstream (IL-1 receptor-associated kinase 3 [IRAK3] and matrix metallopeptidase 9 [MMP9]) inflammatory signaling pathways by downregulating their messenger ribonucleic acid (mRNA) gene transcript expressions as induced by C. trachomatis in macrophages. Collectively, our data provides further evidence for the anti-inflammatory properties of Ag-PVP nanoparticles, and opens new possibilities for smaller sizes of Ag-PVP nanoparticles to be employed as regulators of inflammatory responses induced by C. trachomatis.
Introduction
Chlamydia trachomatis is an obligate intracellular bacterium which is implicated in a wide spectrum of diseases in humans and other mammals worldwide.Citation1–Citation3 In humans, it is the leading cause of sexually transmitted bacterial diseases in developed countries and is the main cause of blindness in developing countries.Citation3 The pathogen exists in two distinct morphological forms: the replicative, intracellular reticulate body (RB) and the infectious but metabolically inactive elementary body (EB). Infection is initiated by adherence of EBs to the host cell receptors through its ligands, including the heat shock protein 70 (Hsp70) and outer membrane protein 2 (omp2), after which they are internalized and form inclusion compartments.Citation4–Citation8 Within the inclusions, EBs differentiate into RBs for proliferation and between 48 and 72 hours post-infection, the RBs redifferentiate into EBs and are released into the extracellular space to start a new round of infection.
Mammalian cells sense the presence of invading microbial pathogens through recognition of pathogen associated molecular patterns (PAMPs). Toll like receptors (TLRs) are the major membrane bound receptors that bind PAMPs and to date, at least 11 have been identified in mammals. In addition to TLRs, mammalian cells have cytosolic surveillance for PAMPs via the nucleotide binding site/leucine-rich repeat (NBS/LRR) proteins, nucleotide binding oligomerization domain protein (NOD) 1 and 2. NOD1 is activated by a peptidoglycan motif found in gram negative bacteria, while NOD2 binds muramyl dipeptides.Citation9–Citation12 After recognition of microbial product, either by TLRs or NODs, various signaling events are triggered to allow nuclear translocation of nuclear factor kappa B (NF-κB) and subsequent upregulation of inflammatory mediators. Therefore, effective recognition of the pathogen is critical for host defense responses and initiation of the inflammatory response.
Inflammation is the primary response to infection that functions to clear the infectious agent and promote tissue repair. It is characterized by the sequential release of a series of mediators including cytokines, chemokines, and growth factors that regulate vascular permeability and recruitment of blood borne leukocytes. Increased vascular permeability also results in excessive plasma proteins that further amplify the inflammatory reaction. Therefore, the process of inflammation is intimately linked to host immune responses in an effort to resolve the infection. However, such efforts are not always successful in eliminating the infection as some pathogens persist and elicit chronic immune responses by misdirecting the immune responses to result in immunopathological diseases. Indeed, diseases caused by Chlamydia are thought to be mediated by the ensuing immunopathology.Citation13
There is much evidence to support that blindness and sterility caused by C. trachomatis are the result of inflammation based pathology.Citation13,Citation14 At the sites of C. trachomatis infection, intense inflammation is characterized by redness, edema, and mucopurulent discharge.Citation14 An intense and chronic inflammatory response and follicle necrosis arises when there is damage to the epithelium, and this is followed by local epithelial cell proliferation and scar formation.Citation14 As the infection prolongs, plasma cells and macrophages are continuously recruited and at the same time allow the release of more immune cells both from adaptive and innate immune responses.Citation15,Citation16 Many years after the infection, the physical consequences of the disease emerge which ultimately lead to visual impairment and infertility.
Antibiotics are the only available intervention strategy to treat Chlamydia infection; however, these antibiotics are effective if they are taken early during the infection. However, the silent nature of the disease does not permit patients to seek early antibiotic treatment. When Chlamydia persists, antibiotics fail to destroy the pathogen, thereby resulting in continuous damage to the host.Citation17 In a community wide antibiotic treatment trial for trachoma, both reinfection and persistent infection in humans were documented indicating loss of antibiotic efficacy.Citation18 Reactivation of persistent Chlamydia is also a problem that prevents inhibition of Chlamydia replication and weakens the effectiveness of antibiotics.Citation20,Citation21 Therefore, it is necessary to explore alternative treatment strategies to reduce the excessive production of inflammatory mediators.
The advancement of nanotechnology in medicinal applications allows opportunities to explore metal nanoparticles in the field of biology. Silver has long been known for its antimicrobial properties.Citation23–Citation27 Silver nanoparticles have been widely explored for their antimicrobial potential against both gram-negative and gram-positive bacteria,Citation26,Citation27 and as antiviral agents against human immunodeficiency virus-1,Citation28 hepatitis B virus,Citation29 respiratory syncytial virus,Citation30 herpes simplex virus type-1,Citation31 and monkeypox virus plaque formation.Citation32 Furthermore, silver nanoparticles were shown to reduce oxidative stress, and were recently described as an environmentally friendly approach to synthesize oil-based paints with antimicrobial properties.Citation33,Citation34
Silver nanoparticles also possess anti-inflammatory properties. Previously, it has been shown that silver nanoparticles decreased wound inflammation in a thermal injury animal model.Citation35,Citation36 Silver nanoparticles have also been shown to decrease the levels of inflammatory markers in animals in vivo.Citation37 Collectively, the results from these studies suggest that silver nanoparticles may be involved in altering or suppressing inflammatory events during microbial interventions, and thus play a critical role in balancing the level of inflammatory mediators secreted during infection. The ultimate goal of this study is to further enhance our knowledge of silver nanoparticles as an anti-inflammatory agent, and to provide insights into whether silver nanoparticles can be used to prevent excessive inflammatory responses, as triggered by live C. trachomatis in mouse macrophages. Herein, we used three different sizes of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles to investigate (1) the toxic effect of Ag-PVP nanoparticles to mouse J774 macrophages, (2) the ability of Ag-PVP nanoparticles to diminish the production levels of cytokines and chemokines as elicited by C. trachomatis infected macrophages, and (3) the effect of Ag-PVP nanoparticles on the messenger RNA (mRNA) gene transcript expression of macrophage receptors and other downstream inflammatory genes. The data from this study provides evidence for a role of Ag-PVP nanoparticles in regulating inflammatory mediators in C. trachomatis infected macrophages, and promotes the novel idea of Ag-PVP nanoparticles as a potential antiinflammatory molecule against C. trachomatis in vivo.
Materials and methods
Cell culture and infectivity
Mouse J774 macrophages were obtained from the American Type Culture Collection ([ATCC], Manassas, VA, USA) and cultured as already described.Citation19 C. trachomatis MoPn Nigg II was purchased from ATCC (ATCC #VR-123) and propagated as reported.Citation19 To establish infection, macrophages (106 cells/well) were seeded in 24 well plates for 24 hours after which they were infected with live C. trachomatis infectious particles (105) in 500 μL of growth media/well and incubated at 37°C with 5% CO2 for 48 hours. The optimum bacteria dose and duration of infection were determined as reported.Citation19
Preparation of Ag-PVP nanoparticles
Ag-PVP nanoparticles at 20 and 80 nm were purchased from Nanostructure and Amorphous Materials, Inc (Houston, TX, USA). Ag-PVP nanoparticles of 10 nm was previously synthesized in our laboratoryCitation30 We used Ag-PVP nanoparticles because they have been shown to be more stable and uniform in size than other coated nanoparticles, and this contributes greatly to their effectiveness in biomedical applications.Citation30
Cytotoxicity studies
The cytotoxicity of Ag-PVP nanoparticles to mouse J774 macrophages was measured using the 3-(4,5- dimeth ylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye reduction assay and the Cell-Titer 96 Cell Proliferation Assay kit (Promega, Madison, WI, USA). Mouse J774 macrophages were seeded in a 96 well plate at a density of 105 cells/well in 50 μL of media and incubated overnight at 37°C with 5% CO2. Ag-PVP nanoparticles of 10, 20, and 80 nm were added to cells in concentrations ranging from 0.39 to 200 μg/mL and after 48 hours, supernatants were removed, and cells washed two times with sterile phosphate buffered saline (PBS). MTT dye solution (15 μL) was added to each well, and cells were further incubated for 3 hours at 37°C with 5% CO2. To stop the reaction, 100 μL of solubilization solution/stop mixture was added to each well and plates were incubated for 30 minutes at room temperature. Absorbance at 570 nm was measured using a TECAN Sunrise plate reader (TECAN US Inc, Durham, NC, USA). Percent cell viability was obtained using the optical density readings of Ag-PVP nanoparticle treated cells compared to those of normal cells (control), where percent viability = [A]test/[A]control × 100, and where [A]test is the absorbance of the test sample and [A]control is the absorbance of the control sample.
Cytokine and chemokine measurement
Mouse J774 macrophages were infected with C. trachomatis for 2 days as indicated above after which the media were replaced with fresh media containing Ag-PVP nanoparticles of either 10, 20 and 80 nm sizes at concentrations of 0.39, 0.79, 1.5, 3.12, and 6.25 μg/mL. For some experiments, Ag-PVP nanoparticles (10 nm) was used at 2.5 μg/mL. Cell-free supernatants were collected after an additional 48 hour incubation following centrifugation at 450g for 10 minutes at 4°C, and stored at −80°C until used. Cytokines and chemokines were quantified using single (BD-Biosciences, San Jose, CA, USA) and multiplex (EMD Millipore Corporation, Billerica, MA, USA) ELISAs.Citation19,Citation38
Confocal microscopy
To provide physical evidence for the secretion of interleukin (IL)-6 and tumor necrosis factor (TNF), mouse J774 macrophages (2.5 × 104 cells/well) were left uninfected or infected with C. trachomatis (2.5 × 103 inclusion forming units [IFU]/well) using 6 well chamber slides. After 2 days infection, the media were replaced with fresh media supplemented with or without Ag-PVP nanoparticles and allowed to incubate for an additional 48 hours. Post Ag-PVP nanoparticle incubation, supernatants were removed cells were fixed with 2% paraformaldehyde (PFA) (USB Corporation, Cleveland OH, USA) and then permeabilized with 100% methanol. The cells were immunostained with anti-mouse TNF FITC (fluorescein isothiocyanate) and IL-6 PE (phycoerythrin) antibodies (BD Biosciences, San Jose, CA, USA) overnight at 4°C, washed three times with 1 × PBS, and stained with DAPI (4′,6-diamidino-2-phenylindole) combined with an antifade mounting solution (Life Technology, Carlsbad CA, USA). Slides were examined with a Nikon Eclipse Ti Confocal Microscope (Nikon Instrument, Melville, NY, USA) at 40× magnification.
Flow cytometry
Mouse J774 macrophages (106 cells/mL) were left uninfected or infected with C. trachomatis and after a 48 hour infection period, the media were removed and replenished with fresh media containing Ag-PVP nanoparticles and further incubated for 48 hours. The cells were blocked with Fc blocking antibody (BD Bioscience) in FACS (Fluorescence-activated cell sorting) buffer (PBS/0.1% NaN3/1.0% fetal bovine serum) for 15 minutes at 4°C, washed 2 times, and stained with fluorochrome-conjugated antibodies (50 μL in FACS buffer) against mouse CD80 (cluster of differentiation 80) (PE-Cy7) and CD86 allophycocyanin (APC) (eBiosciences, San Diego, CA, USA). The optimum concentration for all fluorochromes was predetermined in our laboratory. Cells were incubated with fluorochrome antibodies for 30 minutes at 4°C, washed 2 times, and then fixed using 2% PFA solution. Data were acquired on a BD FACS Canto II flow cytometer (BD Bioscience) with at least 105 events for each sample and analyzed using Flow-Jo software (Tree Star, Inc, Ashland, OR, USA).
RNA extraction and quantitative real time polymerase chain reaction (qRT-PCR)
Mouse J774 macrophages (3 × 106 cells/well) were infected with live C. trachomatis (3 × 105 IFU/well) in 6 well plates for 48 hours followed by replacement of fresh media containing Ag-PVP nanoparticles. RNA was extracted from the cell pellets using the Qiagen RNeasy Kit (Qiagen Inc, Valencia, CA, USA), which included a DNase-I digestion step. qRT-PCR was employed to quantify mRNA gene transcripts of various inflammatory genes using the TaqMan® RNA-to-C™ 1-step kit in combination with TaqMan® gene expression assays (Applied Biosystems-Life Technologies, Carlsbad, CA, USA) as reported.Citation38 Amplification of gene transcripts was performed according to the manufacturer’s protocol using ABI ViiA™ 7 real time PCR (Applied Biosystems) and standard amplification conditions. The relative changes in gene expression were calculated using the equation: 2−ΔΔCT where all values were normalized with respect to the “housekeeping” gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase) mRNA levels. Amplification using 100 ng RNA was performed in a volume of 20 μL. Each real time PCR assay was performed in triplicate and the results expressed as the mean ± standard deviation.
Statistical analysis
The two-tailed unpaired Student’s t-test was used to compare the data. P < 0.05 was considered significant.
Results
Cytotoxicity analysis of Ag-PVP nanoparticles
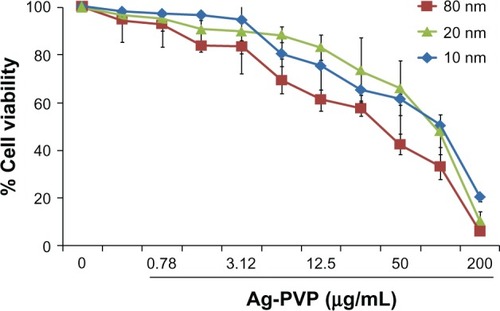
Toxicity is the most pressing problem for metal-based nanoparticles in medicinal applications. Therefore, we tested the toxicity of all sizes of Ag-PVP nanoparticles to mouse J774 macrophages prior to investigating their potential as anti-inflammatory agents against a C. trachomatis infection. The MTT results revealed that concentrations of Ag-PVP nanoparticles above 25 μg/mL were extremely toxic to cells (<40% viable cells), whereas concentrations less than 6.25 μg/mL had greater cell viability (>80% viable cells) (). We observed that Ag-PVP nanoparticles of 10 nm were less toxic to cells than the 20 and 80 nm sizes at similar concentrations (>90% viable cells) (). Our MTT assay revealed a toxicity trend and higher cell viabilities in the order of 10 nm >20 nm >80 nm for all tested concentrations. Besides giving clues on the toxicity effect of different sizes of Ag-PVP nanoparticles, the MTT results also provided the desirable concentration range (0.39–6.25 μg/mL) for all subsequent experiments.
Figure 1 Silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticle toxicity to mouse J774 macrophages is concentration dependent.
Notes: Mouse J774 macrophages were seeded in a 96 well plates at a density of 105 cells/well/50 μL in the presence or absence of Ag-PVP nanoparticles in concentrations ranging from 0.39 to 200 μg/mL. The Cell-Titer 96 cell Proliferation Assay kit was used to determine cell viability as described in the Materials and methods section. Absorbance was read at 570 nm and percent cell viability was calculated by using the optical density readings compared to normal cells. The data are representative of three separate experiments.
Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; nm, nanometer.
All sizes of Ag-PVP nanoparticles reduced IL-6 and TNF elicited from macrophages infected with live C. trachomatis
C. trachomatis infection of macrophages results in the secretion of several inflammatory mediators, amongst which are the cytokines IL-6 and TNF.Citation19 Hence, we first selected IL-6 and TNF as markers of inflammation to determine the anti-inflammatory effect of Ag-PVP nanoparticles and their secretion from C. trachomatis infected macrophages. Mouse J774 macrophages infected with live C. trachomatis showed marked infectivity that correlated with the significant secretion levels of IL-6 () and TNF () when compared to the levels from uninfected macrophages. Nevertheless, infected macrophages exposed to various concentrations of 10, 20 and 80 nm sizes of Ag-PVP nanoparticles (0.39–6.25 μg/mL) secreted less IL-6 () and TNF () in a dose dependent manner, especially at concentrations of 3.12 and 6.25 μg/mL. Additional kinetic studies revealed that Ag-PVP nanoparticles (10 nm), when used at concentrations of 2.5 and 5 μg/mL, were equally effective at reducing cytokine levels (data not shown). Evidently, Ag-PVP nanoparticles of the 10 nm size exhibited greater anti-inflammatory effect than the 20 and 80 nm sizes, and was therefore employed for further studies. The significant anti-inflammatory effect in the concentration range of 2.5 to 6.25 μg/mL ruled out the possibility that Ag-PVP nanoparticle inhibition was due to cell death since these concentrations were not toxic to cells (). These findings provide more evidence for the anti-inflammatory properties of Ag-PVP nanoparticles, and give insight to the potential of Ag-PVP nanoparticles in controlling excessive C. trachomatis inflammatory responses.
Figure 2 Silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles downregulate interleukin (IL)-6 and tumor necrosis factor (TNF) in C. trachomatis infected mouse J774 macrophages.
Notes: Mouse J774 macrophages (106 cells/mL) were seeded in 24 well plates and were either left uninfected or infected with live C trachomatis (105 inclusion forming units [IFU]/well). After 2 days post infection, Ag-PVP nanoparticles at 0.0.39 to 6.25 μg/mL were added to cell cultures and the production levels of IL-6 (A) and TNF (B) were quantified in cell-free supernatants collected 48 hours later by ELISA. *indicates significant difference (P < 0.05) between live C trachomatis and live C trachomatis + Ag-PVP nanoparticles (6.25 μg/mL) and was calculated by using the two-tailed unpaired Student’s t-test. Each bar represents the mean ± standard deviation of samples run in duplicate. The data are representative of two separate experiments.
Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; CT, live Chlamydia trachomatis; IL-6, interleukin-6; TNF, tumor necrosis factor; ELISA enzyme-linked immunosorbent assay.
![Figure 2 Silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles downregulate interleukin (IL)-6 and tumor necrosis factor (TNF) in C. trachomatis infected mouse J774 macrophages.Notes: Mouse J774 macrophages (106 cells/mL) were seeded in 24 well plates and were either left uninfected or infected with live C trachomatis (105 inclusion forming units [IFU]/well). After 2 days post infection, Ag-PVP nanoparticles at 0.0.39 to 6.25 μg/mL were added to cell cultures and the production levels of IL-6 (A) and TNF (B) were quantified in cell-free supernatants collected 48 hours later by ELISA. *indicates significant difference (P < 0.05) between live C trachomatis and live C trachomatis + Ag-PVP nanoparticles (6.25 μg/mL) and was calculated by using the two-tailed unpaired Student’s t-test. Each bar represents the mean ± standard deviation of samples run in duplicate. The data are representative of two separate experiments.Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; CT, live Chlamydia trachomatis; IL-6, interleukin-6; TNF, tumor necrosis factor; ELISA enzyme-linked immunosorbent assay.](/cms/asset/f4660c9f-c01e-414e-9e98-3cbc8ff580d4/dijn_a_44090_f0002_c.jpg)
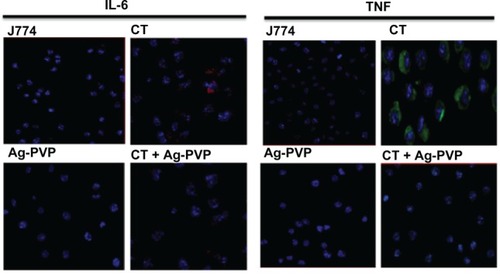
Confocal microscopy analysis confirms the effect of Ag-PVP nanoparticles on IL-6 and TNF secretion in C. trachomatis infected macrophages
Confocal microscopy was employed to provide physical evidence for the anti-inflammatory effect of Ag-PVP nanoparticles on the secretion levels of TNF and IL-6 by C. trachomatis infected macrophages, and also to support our ELISA results. In comparison to uninfected macrophages, infected macrophages had greater green and red florescence aggregating around the nucleus indicating the presence of TNF and IL-6, respectively (). Conversely, when infected macrophages were exposed to Ag-PVP nanoparticles of 10 nm, TNF and IL-6 aggregation patterns were qualitatively lower (), and thus corroborated our ELISA observations of the anti-inflammatory effect of Ag-PVP nanoparticles.
Figure 3 Microscopic analysis to confirm the effect of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles on the secretion of tumor necrosis factor (TNF) and interleukin (IL)-6 in C. trachomatis infected macrophages.
Notes: Mouse J774 macrophages (2.5 × 104 cells/well) were left uninfected or infected with C. trachomatis (2.5 × 103 IFU/well), and after 2 days infection, the media were replaced with fresh media supplemented with or without Ag-PVP nanoparticles (2.5 μg/mL). After 48 hours post Ag-PVP nanoparticle incubation, the cells were fixed with 2% paraformaldehyde and then immunostained with anti-mouse TNF (fluorescein isothiocyanate) and IL-6 (phycoerythrin) antibodies. The slides were stained with DAPI combined with an antifade mounting solution. Slides were examined using a confocal microscope (Nikon Eclipse Ti Confocal Microscope). Green (TNF) and red (IL-6) fluorescence aggregating around the nucleus (blue) indicates secretion of cytokines.
Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; CT, live Chlamydia trachomatis; IL-6, interleukin-6; TNF, tumor necrosis factor; IFU, inclusion forming units; DAPI, 4′,6-diamidino-2-phenylindole.
The effect of Ag-PVP nanoparticles on the production levels of inflammatory mediators in C. trachomatis infected J774 macrophages
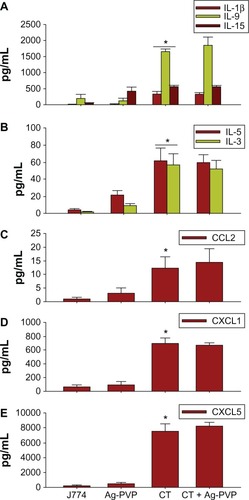
Given that C. trachomatis induces the secretion of TNF and IL-6 upon its infection of macrophages, we next investigated the effect of Ag-PVP nanoparticles on additional cytokines and chemokines using multiplex ELISA. Infected macrophages produced significant (P < 0.05) quantities of cytokines (IL-6, TNF, IL-12p70, IL-1α, IL-1β, IL-9, IL-15, GM-CSF [granulocyte-macrophage colony stimulating factor], G-CSF [granulocyte colony-stimulating factor]) (, ) and chemokines (chemokine [C-X-C motif] ligand [CXCL] 10, CXCL1, CXCL5, chemokine [C-C motif] ligand [CCL] 5, CCL2) (, ) when compared to macrophages exposed to Ag-PVP nanoparticles alone or to macrophages that remained uninfected. However, Ag-PVP nanoparticles, when added to C. trachomatis infected macrophages, significantly (P < 0.05) diminished the production levels of IL-12p70, GM-CSF, IL-1α, TNF, IL-6, G-CSF, CXCL10, and CCL5 (–) but not those of IL-1β, IL-9, IL-15, IL-5, IL-3, CCL2, CXCL1, and CXCL5 (–). Collectively, our results indicate that Ag-PVP nanoparticles have an anti-inflammatory effect on selected cytokines and chemokines triggered by C. trachomatis in macrophages.
Figure 4 Silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles downregulate the production level of various inflammatory mediators in C. trachomatis infected macrophages.
Notes: Mouse J774 macrophages (106 cells/mL) were seeded in 24 well plates and were either left uninfected or infected with live C. trachomatis (105 IFU/mL). After 2 days infection, Ag-PVP nanoparticles (2.5 μg/mL) were added to cell cultures and the production levels of various cytokines (A and B) and chemokines (C and D) were quantified in supernatants collected 2 days later by multiplex ELISA. *P < 0.05 is considered significant when compared between J774 and live C. trachomatis; **P < 0.05 is considered significant when compared between live C. trachomatis and live C. trachomatis + Ag-PVP nanoparticles. Each bar represents the mean ± standard deviation of samples run in duplicate.
Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; CCL5, chemokine (C-C motif) ligand 5; CXCL10, chemokine (C-X-C motif) ligand 10; CT, live Chlamydia trachomatis; IL, interleukin; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; TNF, tumor necrosis factor; ELISA, enzyme-linked immunosorbent assay.
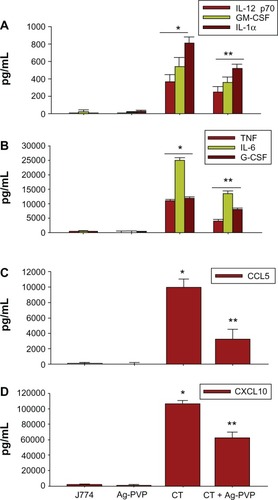
Figure 5 Failure of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles to downregulate selected inflammatory mediators in C. trachomatis infected macrophages.
Notes: Mouse J774 macrophages were stimulated and inflammatory mediators quantified as described above in . Shown are the production levels of cytokines (A and B) and chemokines (C–E). P < 0.05 is considered significant when compared between J774 and live C. trachomatis as determined by the two-tailed unpaired Student’s t-test. Each bar represents the mean ± standard deviation of samples run in duplicate. The data are representative of two separate experiments.
Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; CCL2, chemokine (C-C motif) ligand 2; CT, live Chlamydia trachomatis; CXCL, C-X-C motif chemokine ligand; IL, interleukin.
The effect of Ag-PVP nanoparticles on the mRNA gene transcript expression of TLR2 and NOD2
Mammalian cells sense the presence of invading microbial pathogens through recognition of PAMPs, which include receptors like NOD and TLR.Citation39 Since C. trachomatis induced the release of early inflammatory mediators,Citation40 we next investigated the possibility that Ag-PVP nanoparticles altered early receptor expression of NOD and TLR to reduce the production levels of cytokines triggered by C. trachomatis infected macrophages. We showed by qRT-PCR that C. trachomatis induced a 2.2 and 2.8 fold upregulation in the expression of the mRNA gene transcripts for TLR2 and NOD2 () of macrophages, respectively, in comparison to uninfected macrophages. However, in the presence of added Ag-PVP nanoparticles, the mRNA expression levels of NOD2 and TLR2 were reduced (approximately 2 fold reduction) (). Therefore, the ability of Ag-PVP nanoparticles to reduce the expression levels of these receptors strongly suggest potential mechanism(s) by which Ag-PVP nanoparticles may regulate inflammatory mediators triggered by C. trachomatis in macrophages.
Figure 6 Silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles reduce the mRNA transcription of toll like receptor 2 (TLR2), nucleotide-binding oligomerization domain-containing protein-2 (NOD2), interleukin-1 receptor associated kinases 3 (IRAK3), CD40, and matrix metallopeptidase 9 (MMP9) by C. trachomatis in mouse J774 macrophages.
Notes: Macrophages (3 × 106 cells/mL) were left uninfected or infected with live C trachomatis (3 × 105 IFU/well). After 2 days infection, Ag-PVP nanoparticles (2.5 μg/mL) were added to macrophage cultures and 2 days later total RNA was extracted. One step qRT-PCR was used to quantify the mRNA gene transcripts of TLR2 and NOD2 (A) and IRAK3, CD40, and MMP9 (B). Data shown is an average of triplicate runs. *P < 0.05 is considered significant when compared between J774 and live C trachomatis and **P < 0.05 is considered significant when compared between live C. trachomatis and live C. trachomatis + Ag-PVP nanoparticles as determined by the two-tailed unpaired Student’s t-test.
Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; CT, live Chlamydia trachomatis; IRAK3, interleukin-1 receptor associated kinases 3; MMP9, matrix metallopeptidase 9; NOD2, nucleotide-binding oligomerization domain-containing protein-2; TLR2, toll like receptor 2; CD, cluster of differentiation; qRT-PCR, quantitative real time polymerase chain reaction; mRNA, messenger ribonucleic acid.
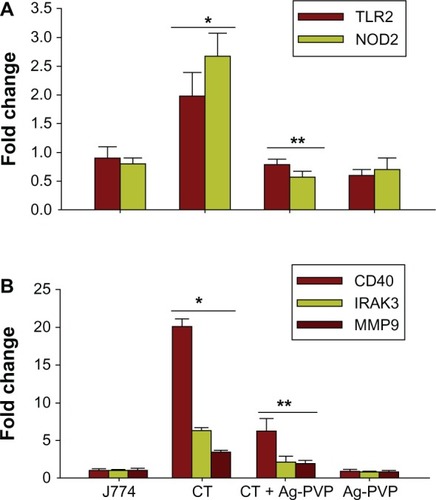
Ag-PVP downregulates the mRNA gene transcript expression of interleukin-1 receptor associated kinase (IRAK) 3, CD40, and matrix metallopeptidase (MMP) 9
Like pattern recognition receptors, perturbations in inflammatory signaling pathways may affect the secretion of cytokines and chemokines from C. trachomatis infected macrophages. Genes encoding mediators from the Toll/IL receptor immune signal transduction pathway mediate signaling from TLR to specific interleukin receptor family members, such as IRAKs, which maintains stable binding between the cytokine and its corresponding receptors. Our qRT-PCR findings clearly revealed upregulation of the mRNA expression of IRAK3 in C. trachomatis infected macrophages when compared with the expression level in uninfected macrophages (). However, when Ag-PVP nanoparticles were added to infected macrophages, the mRNA expression level of IRAK3 was markedly reduced (approximate 4 fold reduction) ().
Since the CD40 membrane protein has a role in transmitting inflammatory signaling for cytokine secretion,Citation41 we also determined the effect of Ag-PVP nanoparticles on the expression of its mRNA gene transcript levels. By qRT-PCR, we showed upregulation (20 fold increase) in the expression of CD40 at the mRNA gene transcript level in C. trachomatis infected macrophages when compared with uninfected macrophages (). Alternatively, the mRNA expression level of CD40 was reduced significantly (approximate 15 fold reduction) in the presence of Ag-PVP nanoparticles (). Taken together, the ability of Ag-PVP nanoparticles to reduce the expression levels of both IRAK3 and CD40 indicates its capacity to perturb genes encoding mediators of inflammatory signaling pathways, and to possibly further reduce inflammatory mediators in C. trachomatis infected macrophages.
The development of inflammation arising from infection with C. trachomatis is mainly due to the host immune response generated against eradicating the pathogen.Citation13–Citation16 MMPs are involved in the breakdown of extracellular matrix and thus play important roles in inflammation. We investigated whether Ag-PVP nanoparticles perturbed the expression of MMPs. Of the many MMPs involved in promoting inflammation, we focused on MMP9 because neutrophils, which are rapidly recruited during a Chlamydia infection, release MMP9 to contribute to tissue damage.Citation42 We showed by qRT-PCR a 2.5 fold increase in the level of the mRNA gene transcript expression of MMP9 in C. trachomatis infected macrophages when compared with uninfected macrophages, suggesting enhancement of MMP9 during early infection (). When Ag-PVP nanoparticles were added to infected macrophage cultures, the mRNA expression level of MMP9 reduced significantly (approximate 2 fold reduction) (), suggesting the inhibitory effect of Ag-PVP nanoparticles on MMP9 expression that additionally curtails the inflammatory process.
Ag-PVP nanoparticles reduced the expression of CD80 and CD86
Pathogens are recognized during host pathogen interactions via pattern recognition receptors, including Toll and NOD, to trigger the production of inflammatory mediators for initiating innate immune responses and the expression of costimulatory molecules for subsequent adaptive immune responses. To broaden our understanding of Ag-PVP nanoparticles on host immune responses, we tested its effect on regulating CD80 and CD86 costimulatory molecules, at both the protein and mRNA gene transcript levels, in macrophages infected with live C. trachomatis. Flow cytometric analyses revealed protein expression changes for CD86 () and CD80 () in C. trachomatis infected mouse J774 macrophages as exemplified by the peak shifts in their respective fluorescence intensities when compared to uninfected macrophages. Conversely, in the presence of added Ag-PVP nanoparticles, the peak shifted toward that of the uninfected macrophages, indicating a reduction in the expression of CD86 and CD80 by Ag-PVP nanoparticles (), and in particular for CD86.
Figure 7 Silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles downregulate the expression levels of CD80 and CD86 in C. trachomatis infected mouse J774 macrophages at both the protein and gene level.
Notes: Mouse J774 macrophages were cultured and infected as described in the Materials and methods section for flow cytometry and qRT-PCR experiments. After 2 days of infection, 2.5 μg/mL Ag-PVP nanoparticles were added to cell culture, and 2 days later, samples were analyzed by flow cytometry for protein expression and qRT-PCR for mRNA expression. Shown are the expression shifts of CD86 (A) and CD80 (B) at the protein and gene levels (C). *P < 0.05 is considered significant when compared between J774 and live C. trachomatis and **P < 0.05 is considered significant when compared between live C. trachomatis and live C. trachomatis + Ag-PVP nanoparticles as determined by the two-tailed unpaired Student’s t-test.
)Abbreviations: Ag-PVP, silver-polyvinyl pyrrolidone; CT, live Chlamydia trachomatis; CD, cluster of differentiation; qRT-PCR, quantitative real time polymerase chain reaction; mRNA, messenger ribonucleic acid.
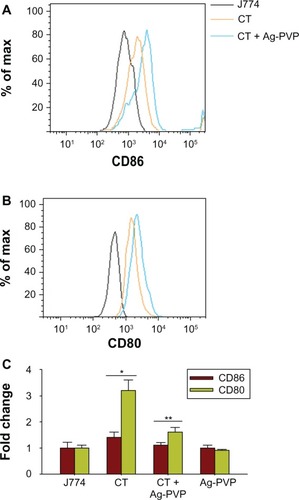
Additionally, we demonstrated by qRT-PCR a 1.5 and 3.2 fold increase in the mRNA gene transcript expression for CD86 and CD80, respectively, in C. trachomatis infected macrophages when compared with uninfected macrophages, suggesting moderate enhancement of CD80 and CD86 during infection of macrophages, especially for CD80 (). Again, the inhibitory effect of Ag-PVP nanoparticles was observed by its ability to reduce CD86, and especially CD80, mRNA expression levels in infected macrophages (). In general, our results from both the flow cytometry and qRT-PCR studies gave clues on the role of Ag-PVP nanoparticles in regulating adaptive immune responses to potentially control C. trachomatis inflammation.
Discussion
The interaction of microbes with host cells encompasses two major events causing either disease or establishment of a long-term relationship with the host.Citation13 Some pathogens manage the long-term relationship by eliciting chronic immune responses through misdirecting the normal host immune responses to cause immunopathologies as reported for C. trachomatis.Citation13,Citation14 Inflammation of an infected tissue has several beneficial effects in combating infection by recruiting cells and molecules of innate immunity to destroy the pathogen, as well as by activating lymphocytes to initiate adaptive immune responses. Continuous production of inflammatory mediators, as evidenced during a Chlamydia infection, lead to chronic inflammation and tissue damage, tubal infertility, and ectopic pregnancies, which are key characteristics of disease pathogenesis.Citation13,Citation19,Citation43,Citation44
Silver has been shown to have both antimicrobial and anti-inflammatory properties.Citation23–Citation27 Despite the many benefits of nanotechnology in biomedical applications, studies indicate that certain nanoparticles cause adverse effects because of their indiscriminate entry into various organs.Citation33 In our study, more than 80% of cells remained viable at Ag-PVP nanoparticle concentrations ranging from 0.39–3.12 μg/mL (), suggesting that these concentrations are not toxic to cells. Worthy of mention is that we used lower concentrations of silver nanoparticles in our study than what has been reported to have antimicrobial effects against viruses and bacteria.Citation29–Citation32 Also, concentrations above 25 μg/mL were highly toxic to macrophages, thus corroborating previous in vitro toxicity studies of various silver nanoparticles to mammalian cells.Citation45 We also observed that Ag-PVP nanoparticles of 10 nm were less toxic than the larger sizes (). One plausible explanation for cytotoxicity differences in the three different sizes used in our study may be attributed to the notion that small sizes of Ag-PVP nanoparticles have high mobility and do not aggregate in one location as occurs with the larger sizes.
In the burn wound model, as well as in a peritoneal adhesion model in mice, silver nanoparticles significantly lowered the levels of IL-6.Citation35,Citation37 We report here that all sizes of tested Ag-PVP nanoparticles (10, 20, and 80 nm) significantly reduced the levels of IL-6 and TNF (). The production of IL-6 and TNF is recognized as an initiator of events in the physiological alterations of inflammation to recruit and activate immune cells, such as neutrophils and macrophages, to the site of infection.Citation46 The ability of all three sizes of Ag-PVP nanoparticles to downregulate IL-6 and TNF suggests the applicability of Ag-PVP nanoparticles at broader size ranges, thus making it a potent anti-inflammatory molecule.
Ag-PVP nanoparticles not only regulated IL-6 and TNF levels, but also regulated a plethora of other cytokines and chemokines such as IL-12 p70, GM-CSF, IL-1α, G-CSF, CXCL10, and CCL5 () which can be viewed as being beneficial to the host in controlling excessive inflammatory mediators triggered by a C. trachomatis infection. Decreasing the released levels of these inflammatory mediators subsequently lowers cellular proliferation and extracellular matrix production, and thus, there is less inflammation. At the same time, other inflammatory mediators such as IL-1β, IL-9, IL-15, IL-5, IL-3, CXCL5, CXCL1, and CCL2 were not adversely affected by Ag-PVP nanoparticles (), suggesting that the anti-inflammatory effect of Ag-PVP nanoparticles is not a generalized phenomenon but rather one that is highly selective, and that may depend on the pathogen or cell type that it is exposed to. This revealing anti-inflammatory property of Ag-PVP nanoparticles permits it to be explored for a variety of disease settings as demonstrated here for our C. trachomatis in vitro model system. Even though the anti-inflammatory effect of silver nanoparticles has been confirmed in various infection models,Citation37,Citation47–Citation49 to our knowledge, this is the first study to report on its anti-inflammatory effect in a C. trachomatis infection model.
The inflammatory process is a complex phenomenon induced by different pathways and therefore many hypothetical possibilities can be explored to explain the anti-inflammatory mode of action of Ag-PVP nanoparticles. We have shown in our study that the anti-inflammatory effect of Ag-PVP nanoparticles was not attributed to apoptosis of inflammatory cells () and alternatively, that it may be due to perturbations in inflammatory signaling pathways. In this study, we observed that TLR2 and NOD2 mRNA expression levels were upregulated in C. trachomatis infected macrophages (), which is in agreement with previous findings where epithelial cells infected with C. muridarum had increased expression of NOD2 and TLR2.Citation39 Similarly, NOD2 plays a major role in recognizing microbial products of gram-negative bacteriaCitation9–Citation12 with C. trachomatis being no exception as it upregulated NOD2 in macrophages, as reported here. Both TLRs and NOD like receptors induce inflammatory signaling pathways for the secretion of inflammatory mediators. Thus, the capacity of Ag-PVP nanoparticles to reduce the expression of TLR2 and NOD2 infers less interaction between macrophages and C. trachomatis, and thereby less binding to its cognate receptors to trigger secretion of inflammatory mediators.
Additional receptors and downstream signaling events are equally vital for the secretion of inflammatory mediators. Here, we have focused on two such molecules: IRAK3 which inhibits the dissociation of IRAK1 and IRAK4 from the TLR signaling complex, and CD40, a member of the TNF receptor superfamily that is essential in mediating a variety of immune and inflammatory responses.Citation41 Both IRAK3 and CD40 were induced by C. trachomatis infected mouse macrophages, suggesting the importance of these signaling events during C. trachomatis inflammation (). That Ag-PVP nanoparticles reduced both CD40 and IRAK3 mRNA expression underscores that it may exert its anti-inflammatory effect partially by interfering with TNF and IL-1 receptors. Results from an in vitro study indicate that silver nanoparticles inhibit the interaction between glycoprotein 120 and CD4 to inhibit human immunodeficiency virus-1 infection.Citation50 Although we have not directly demonstrated the interaction of Ag-PVP nanoparticles with C. trachomatis infected macrophages, it can be speculated that inhibition of CD40 and IRAK3 by Ag-PVP nanoparticles may interfere with C. trachomatis binding to macrophages, and ultimately lower the levels of secreted inflammatory mediators. Other reported mechanisms for the anti-inflammatory actions of silver nanoparticles include regulation of NF-κB transcription in allergic airway inflammation,Citation47 and regulation of the VEGF signaling pathway in allergic airway inflammation,Citation48 or in individuals with asthma.Citation51 In our study, the capacity of Ag-PVP nanoparticles to reduce TLR2, NOD2, CD40, and IRAK3 levels suggest mechanisms subsequently leading to potential downregulation of NF-κB, and therefore lower levels of inflammatory mediators.
For any infectious disease situation, the acute inflammatory state normally represents immediate modification of the extracellular matrix which involves MMPs and other proteolytic enzymes to initiate remodeling and tissue regeneration of the affected tissue. However, as the infection persists, the presence of MMPs permit the influx of inflammatory cells with tissue damage ensuing. Therefore, persisting or excessive inflammation can indicate overexpression of MMPs, enhanced repair responses, and extracellular matrix accumulation and fibrosis. Infection in the murine model of Chlamydia urogenital infection induced excessive extracellular matrix modification with lumen occluding fibrosis, which correlated with hydrosalpinx formation and infertility.Citation42 These findings, as well as MMP expression in trachoma,Citation31 and the in vitro expression of MMPs in response to C. trachomatis infection in human fallopian tube organ culture, highlights the significance of MMPs in C. trachomatis pathogenesis.Citation13,Citation42 We observed in our study that MMP9 expression was upregulated by C. trachomatis infected macrophages as expected, but downregulated by Ag-PVP nanoparticles (). Interpretation of our results can be complex since we only tested one form of MMP. However, the ability of Ag-PVP nanoparticles to reduce MMP9 is of major significance as excessive secretion of MMP9 may contribute to the pathogenesis of tissue destruction in a wide array of diseases, including Chlamydia.Citation42 Furthermore, MMP9 contributes to tissue destruction and inflammation via degradation of matrix proteins and proteolytic activation of cytokines/chemokines during C. trachomatis infections.Citation13,Citation14,Citation42
The expression of CD80 and CD86, along with major histocompatibility class molecules, allows the presentation of microbial peptides to activate naïve CD4 T cells to produce pro-inflammatory mediators at the site of infection. CD80 and CD86 reportedly regulate innate immune responses in murine polymicrobial sepsis, which is a systemic inflammatory response to infection.Citation52 CD80 and CD86 are stimulated via CD28, while cytotoxic T lymphocyte antigen 4 serves as both a stoichiometric inhibitor of CD28-CD80/86 engagement, and directly induces immunosuppressive signals within dendritic cells.Citation53–Citation55 Indeed, the CD28-CD80/86 system is known to regulate inflammation in autoimmune diseases, where CD80/86 signals via NF-κB to induce numerous cytokines, most notable IL-6.Citation53 In vivo, deletion or blockade of CD80/86 improves survival and attenuates pro-inflammatory cytokine production in CLP.Citation56 Here, we have revealed that Ag-PVP nanoparticles reduced the expression of CD80 and CD86, both at the gene and protein levels in C. trachomatis infected macrophages (), suggesting that Ag-PVP nanoparticles have the potential to act not only on cytokines produced by innate immune cells, but also those produced by C. trachomatis activated T cells during the adaptive immune response.
Conclusion
The development of inflammation arising from infection with C. trachomatis may depend on several factors including C. trachomatis virulence, the number of bacteria in the affected tissues, and the host immune response generated against the bacteria. The advancement in nanotechnology has enabled us to utilize particles in the nanoscale range and has created new therapeutic perspectives. In the case of silver nanoparticles, the available data reveal their potential as anti-inflammatory agents in a variety of infectious disease models and we are now including C. trachomatis to the list. Collectively, our findings demonstrate that Ag-PVP nanoparticles may mediate its anti-inflammatory effect in macrophages infected with C. trachomatis by regulating various upstream surface receptors and downstream inflammatory pathway genes. Our study provides further evidence on the anti-inflammatory properties of Ag-PVP nanoparticles and opens new possibilities for small sizes of Ag-PVP nanoparticles to be employed as regulators of inflammatory responses induced by C. trachomatis infection.
Acknowledgments
The project described was supported by NSF-CREST (HRD-1241701) and NSF-HBCU-UP (HRD-1135863). We would like to thank Yvonne Williams and Lashaundria Lucas for their excellent administrative assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- StephensRSChallenge of Chlamydia researchInfect Agent Dis19931279293
- BavoilPMHsiaRCRankRGProspects for a vaccine against Chlamydia genital disease: microbiology and pathogenesisBull Inst Pasteur1996945054
- SchachterJDawsonCRThe epidemiology of trachoma predicts more blindness in the futureScand J Infect Dis1990695562
- JosephTDBoseSKA heat labile protein of Chlamydia trachomatis binds to HeLa cells and inhibits the adherence of chlamydiaeProc Nat Acad Sci USA199188405440582023955
- SwansonAFKuoCCBinding of the glycan of the major outer membrane protein of Chlamydia trachomatis to HeLa cellsInfect Immun19946224288262634
- TingLMHsiaRCHaidarisCGBavoilPMInteraction of outer envelope proteins of Chlamydia psittaci GPIC with the HeLa cell surfaceInfect Immun199563360036087642297
- SuHRaymondLRockeyDFischerEHackstadtTAldwellHDA recombinant Chlamydia trachomatis major outer membraneInfect Immun1996651113411140
- RaulstonJEPaulTRKnightSTWyrickPBLocalization of Chlamydia trachomatis heat shock proteins 60 and 70 during infection of a human endometrial epithelial cell line in vitroInfect Immun199866232323299573124
- ChamaillardMHashimotoMHorieYAn essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acidNat Immunol2002470270712796777
- GirardinSEBonecaIGCarneiroLANod1 detects a unique muropeptide from gram-negative bacterial peptidoglycanScience20033001584158712791997
- InoharaNOguraYFontalbaAHost recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn’s diseaseJ Biol Chem20032785509551212514169
- GirardinSEBonecaIGVialaJNod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detectionJ Biol Chem20032788869887212527755
- StephensRSThe cellular paradigm of chlamydial pathogensisTrends Microbiol200311445112526854
- KuoCCHost responseBarronALMicrobiology of ChlamydiaBoca RatonCRC press1988193208
- TaylorHRAn animal model of trachoma II. The importance of repeated re-infectionInvest Ophthalmol Vis Sci1982235075156749750
- PattonDLTaylorHRThe histopathology of experimental trachoma: ultrastructural changes in the conjunctival epitheliumJ Infect Dis19861538708783009637
- SkiltonRJCutcliffeLTBarlowDPenicillin induced persistence in Chlamydia trachomatis: high quality time lapse video analysis of the development cyclePLoS One20094e7723e772319893744
- BainDLChlamydial genovar distribution after community wide antibiotic treatmentJ Infect Dis20011841581158811740734
- YilmaANSinghSRFairleySJTahaMADennisVAThe anti-inflammatory cytokine, interleukin-10, inhibits inflammatory mediators in human epithelial cells and mouse macrophages to live and UV-inactivated Chlamydia trachomatisMediators Inflamm2012201252017422529524
- ByrneGIFaubionCLLymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferonInfect Immun198342115211586417024
- BeattyWLMorrisonRPByrneGIReactivation of persistent Chlamydia trachomatis infection in cell cultureInfect Immun1995631992057806358
- HoymeUBClinical significance of Crede’s prophylaxis in Germany at presentInfect Dis Obstet Gynecol19931323618476203
- KimJSKukEYuKNAntimicrobial effects of silver nanoparticlesNanomedicine200739510117379174
- LokCNHoCMChenRProteomic analysis of the mode of antibacterial action of silver nanoparticlesJ Prot Res20065916924
- MoronesJRElechiguerraJLCamachoAThe bactericidal effect of silver nanoparticlesNanotechnology2005162346235320818017
- ShahverdiARFakhimiAShahverdiHRMinaianSSynthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coliNanomedicine2007316817117468052
- SondiISalopek-SondiBSilver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteriaJ Colloid Interface Sci200427517718215158396
- SunRWRongCChungNPYHoCMLinCLSCheCMSilver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cellsChem Commun2005150595061
- LuLSunRWChenRSilver nanoparticles inhibit hepatitis B virus replicationAntivir Ther20081325326218505176
- SunLSinghAKVigKPillaiSRSinghSRSilver nanoparticles inhibit replication of respiratory syncytial virusJ Biomed Biotechnol20084149158
- Baram-PintoDShuklaSPerkasNGedankenASaridRInhibition of herpes simplex virus Type 1 infection by silver nanoparticles capped with mercaptoethane sulfonateBioconjugate Chem20092014971502
- RogersJVParkinsonCVChoiYWSpeshockJLHussainSMA preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formationNanoscale Res Lett20083129133
- AroraSJainJRajwadeJMPaknikarKMCellular responses induced by silver nanoparticles: in vitro studiesToxicol Lett20081799310018508209
- KumariAKumarPAjayanPMJohnGSilver-nanoparticle- embedded antimicrobial paints based on vegetable oilNat Mater2008723624118204453
- TainJWongKKHoCMTopical delivery of silver nanoparticles promotes wound healingChem Med Chem2007212913617075952
- SibbaldRGContreras-RuizJCouttsPFierhellerJRothmanAWooKBacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcersAdv Skin Wound Care20072054955817906429
- WongKKCheungSOHuangLFurther evidence of the anti-inflammatory effects of silver nanoparticlesChem Med Chem200941129113519405063
- GautamADixitSPhilippMTInterleukin-10 alters effect functions of multiple genes induced by Borrelia burgdorferi in macrophages to regulate Lyme disease inflammationInfect Immun2011794876489221947773
- DerbignyWAKerrMSJohnsonRMPattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell linesJ Immunol20051756065607516237102
- JohnsonRMMurine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretionInfect Immun2004723951396015213139
- GoldJAParseyMHoshinoYHoshinoSNolanACD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunityInfect Immun2003713521352812761137
- RamseyKHSigarIMSchripsemaJHShabaNCohoonKPExpression of matrix metalloproteinases subsequent to urogenital Chlamydia muridarum infection of miceInfect Immun2005736962697316177376
- GraystonJTWangSPYehLJKuoCCImportance of reinfection in the pathogenesis of trachomaRev Infect Dis198577177254070905
- MolestinaREMillerRDRamirezJASummersquillJTInfection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytesInfect Immun1999671323133010024578
- AhamedMAlSalhiMSSiddiquiMKSilver nanoparticles applications and human healthClin Chim Acta20104111841184820719239
- PaquetPPiérardGEInterleukin-6 and the skinInt Arch Allergy Immunol19961093083178634514
- ParkHSKimKHJangSAttenuation of allergic airway inflammation and hyperresponsivensess in a murine model of asthma by silver nanoparticlesInt J Nanomedicine2010550551520957173
- ParkHSKimKHJangSSilver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammationInt J Nanomedicine201071329134322457593
- SheikpranbabuSKalishwaralalKVenkataramanDEomSHParkJGurunathanSSilver nanoparticles inhibit VEGF-and IL-1-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cellsJ Nanobiotechnology20097819878566
- LaraHHAyala-NunezNVIxtepan-TurrentLRodriguez-PadillaCModel of antiviral action of silver nanoparticles against HIV-1J Nanobiotechnology20108120145735
- LeeYCLeeHKVascular endothelial growth factor in patients with acute asthmaJ Allergy Clin Immunol2001107110611398093
- NolanAKobayashiHNaveedBDifferential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsisPLoS One20094e660019672303
- OrabonaCGrohmannUBelladonnaMLFallarinoFVaccaCCD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86Nat Immunol200451134114215467723
- SharpeAHFreemanGJThe B7-CD28 superfamilyNat Rev Immunol2002211612611910893
- GrohmannUOrabonaCFallarinoFCTLA-4-Ig regulates tryptophan catabolism in vivoNat Immunol200231097110112368911
- NolanAWeidenMKellyACD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsisAm J Respir Crit Care Med200817730130817989345