Abstract
Purpose
Bone defects and nonunions are major clinical skeletal problems. Growth factors are commonly used to promote bone regeneration; however, the clinical impact is limited because the factors do not last long at a given site. The introduction of tissue engineering aimed to deter the diffusion of these factors is a promising therapeutic strategy. The purpose of the present study was to evaluate the in vivo osteogenic capability of an engineered bone morphogenetic protein-4 (BMP4) fusion protein.
Methods
BMP4 was fused with a nanosized carrier, collagen-binding domain (CBD), derived from fibronectin. The stability of the CBD-BMP4 fusion protein was examined in vitro and in vivo. Osteogenic effects of CBD-BMP4 were evaluated by computer tomography after intramedullary injection without a collagen–sponge scaffold. Recombinant BMP-4, CBD, or vehicle were used as controls. Expressions of bone-related genes and growth factors were compared among the groups. Osteogenesis induced by CBD-BMP4, BMP4, and CBD was also assessed in a bone-defect model.
Results
In vitro, CBD-BMP4 was retained in a collagen gel for at least 7 days while BMP4 alone was released within 3 hours. In vivo, CBD-BMP4 remained at the given site for at least 2 weeks, both with or without a collagen–sponge scaffold, while BMP4 disappeared from the site within 3 days after injection. CBD-BMP4 induced better bone formation than BMP4 did alone, CBD alone, and vehicle after the intramedullary injection into the mouse femur. Bone-related genes and growth factors were expressed at higher levels in CBD-BMP4-treated mice than in all other groups, including BMP4-treated mice. Finally, CBD-BMP4 potentiated more bone formation than did controls, including BMP4 alone, when applied to cranial bone defects without a collagen scaffold.
Conclusion
Altogether, nanocarrier-CBD enhanced the retention of BMP4 in the bone, thereby promoting augmented osteogenic responses in the absence of a scaffold. These results suggest that CBD-BMP4 may be clinically useful in facilitating bone formation.
Introduction
Bone defects and nonunions remain considerable problems caused by tumor and trauma, and their treatment constitutes a major challenge in orthopedic reconstitution surgery.Citation1 Autologous bone graft is a standard technique for inducing bone repair; however, clinical benefits are not ensured, and collateral symptoms, including persistent site pain, nerve injury, hematoma, infection, and fracture, frequently occur.Citation2 Recent advances in the treatment methods include the use of sophisticated biocompatible scaffolds, multipotential cell populations and appropriate cellular stimulation at the affected sites. Currently, growth factor-based bone tissue engineering has attracted increasing attention.
Many growth factors induce osteogenesis.Citation3 Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGFβ) protein superfamily, and are known to play pivotal roles in the regulation of bone induction, maintenance, and repair.Citation4,Citation5 The US Food and Drug Administration has approved two BMPs – BMP2, and BMP7/OP1 – which have accompanied orthopedic surgery and are applied with an absorbable collagen sponge. The clinical benefits of BMP2 and BMP7 have been reported;Citation6,Citation7 however, several reports described the heterotopic ossification associated with the use of BMP2 and BMP7.Citation8–Citation10 To prevent heterotopic bone formation and induce successful site specific bone growth requires an approach that limits the diffusion of factors to target tissues.
BMP4 induces osteogenic differentiation of osteoblasts and osteoprogenitors and promotes bone formation,Citation11 thus playing a crucial role in the onset of bone and cartilage development and fracture repair.Citation12 In vivo BMP4 gene therapy accelerated the repair of bone fractures,Citation13 and performed as well or better when compared with BMP2.Citation14 Thus, BMP4 appears to be a viable other approach for treatment of bone defects and nonunions. BMP4 alone can be delivered by direct injection into the site of concern, but immobilized BMP4 can be localized and retained in the targeted site for longer periods and thus extend the functional half-life of this factor. Very recently, we have created a novel collagen-poly lactic-co-glycolic acid hybrid scaffold with a BMP4 fused to an additional collagen-binding domain (CBD) derived from fibronectin (CBD-BMP4).Citation15 CBD-BMP4 exhibited stronger and more stable collagen-binding activity than did wild type BMP4. This fusion protein, bound to a collagen-coated scaffold, induced osteogenic differentiation of human mesenchymal stem cells when these cells were implanted into nude mice.Citation15
In the present study, we extend our previous work and demonstrate that CBD-BMP4 is retained longer at the targeted sites compared to BMP4 alone and induced augmented bone formation even when injected without scaffold. Considering that the 100 kDa of fibronectin fragment that includes CBD (45 kDa) was reported to be about 24 nm,Citation16 CBD size can be regarded as a nanocarrier. When applied to cranial bone defects, CBD-BMP4 successfully induced new and accelerated bone formation as compared to BMP4 alone. Thus, CBD-BMP4 may be promising for the treatment of bone defects and nonunions.
Materials and methods
CBD-BMP4
CBD-BMP4 fusion protein was prepared as described.Citation15 In brief, the recombinant protein was produced by transgenic silkworms, which carried a chimeric gene encoding CBD of human fibronectin and human BMP4 (mature form). The enterokinase recognition sequence was inserted between the CBD and BMP4 sequences. The fusion protein produced by and secreted into cocoons was extracted with CaCl2 and affinity-purified by Gelatin Sepharose 4B (GE Healthcare, Waukesha, WI, USA). CBD protein (without BMP4 fusion) was prepared as described.Citation17 Control BMP4 was purchased from R&D Systems, Inc (Minneapolis, MN, USA). For some experiments, CBD-BMP4, BMP4, and CBD were labeled with HiLyte Fluor 555 (Dojindo Molecular Technologies, Inc, Kumamoto, Japan), according to the manufacturers’ instructions. In brief, NH2-reactive HiLyte Fluor 555 dissolved in 10 μL dimethyl sulfoxide was mixed with CBD-BMP4, BMP4, or CBD solution (10 μg protein in 100 μL of phosphate buffered saline [PBS]). The protein-dye solution was incubated for 10 minutes at 37°C and unreacted free dye was removed by centrifugation (8000 × g, 10 minutes) using a provided filtration tube. The labeled protein was then recovered from the filter membrane.
Characterization of CBD-BMP4
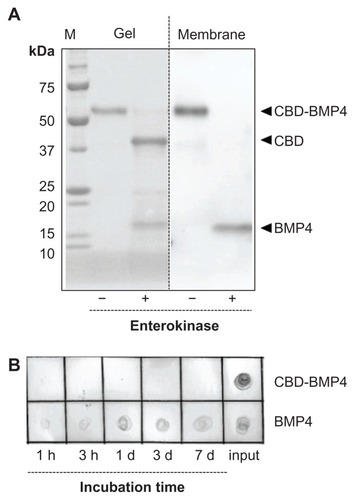
Purified protein was digested with enterokinase (EK Max, Life Technologies, Carlsbad, CA, USA), fractionated on sodium dodecyl sulfate-polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane. The gel was stained with Coomassie Brilliant Blue. The membrane was incubated with anti-BMP4 goat serum (R&D Systems, Inc), followed by incubation with horseradish peroxidase-linked antigoat immunoglobulin G antibody (Vector Laboratories, Inc, Burlingame, CA, USA), and visualized using the ECL system (GE Healthcare). To assess binding stability, CBD-BMP4 or BMP4 was mixed with 0.2% collagen solution in Eagle’s minimal essential medium, pH 7.4 (Koken Co, Tokyo, Japan), and the mixture (30 μL) was gelled at 37°C for 1 hour. PBS (150 μL) was then added and the overlaid PBS was collected after 1 hour, 3 hours, 1 day, 3 days, and 7 days. Protein released into PBS solution was dot-blotted to a nitrocellulose membrane, followed by immunostaining with goat anti-BMP4 and the secondary antibody, as above. The reaction was visualized using 4-chloronaphthol as a horseradish peroxidase substrate.
Animals
New Zealand White rabbits (female, 2.0 kg–2.5 kg) were purchased from Shimizu Laboratory Supplies (Kyoto, Japan). BALB/c mice (female, 6–8 weeks) were obtained from Charles River Laboratories (Yokohama, Japan). Animals were housed in a temperature-controlled environment with a 12-hour light/12-hour dark cycle under specific pathogen-free conditions and allowed free access to water and food. The animal care and use committee at Okayama University approved all animal experiments conducted in this study.
Collagen–sponge scaffold model
Rabbits were anesthetized with an intramuscular injection of ketamine (80 mg/kg). Both knees were shaved and draped in a sterile fashion, and a medial incision was made. A bone hole was made in the distal diaphysis of the femur with a ϕ5.0 mm drill, and a collagen sponge (3 mm cylinder, atelocollagen sponge, MIGHTY; Koken Co, Tokyo, Japan) was intramedullary implanted though the hole. The collagen sponge was presoaked with CBD-BMP4, BMP4, or CBD solution, and contained 1 μg of each protein. A sponge soaked with vehicle PBS alone was used as a control. These four groups of collagen sponges were randomly implanted into rabbit femurs (ten rabbits, 20 femurs, five femurs per each group). Rabbits were then housed in each cage without knee immobilization until the time of evaluation. Rabbits were sacrificed 4 weeks later, and the collagen sponge was retrieved from the femur, fixed in 10% formalin, decalcified in 10% ethylenediaminetetraacetic acid (EDTA), embedded in paraffin, and the sections were stained with hematoxylin–eosin. Histological sections were digitalized under a microscope, and the ossification area in the sponge was measured by image-analyzing software, WinROOF (Mitani Corp, Fukui, Japan). The peripheral area of each sponge was deselected to exclude bone ingrowth by spontaneous healing. To assess the retainment of CBD-BMP4, BMP4, or CBD in vivo, a collagen sponge containing 1 μg of each protein fluorescently labeled was implanted as described above. Sponges soaked with vehicle PBS alone were used as controls (six rabbits, 12 femurs, three femurs per each group). On days 1, 3, and 14 after the implantation, rabbits were killed, and the sponge was removed, frozen in Super CryoEmbedding Medium compound (Section-Lab Co, Hiroshima, Japan) and cut with a tungsten blade at −20°C, as described.Citation18 The sections were stained with calcein acetomethoxy (40 μg/mL; Dojindo Molecular Technologies) for bony calcium detection and evaluated under a fluorescence microscope.
Intramedullary injection model
Mice were anesthetized with ketamine (100 mg/kg). Both knees were shaved, and the skin was cleaned with 70% ethanol. A bone hole was made in the distal diaphysis of the femur with a 24 G needle, and CBD-BMP4, BMP4, or CBD (100 ng in 10 μL PBS) was injected into the medullary cavity of the right femur with a 27 G needle-tipped syringe. The left femur injected with 10 μL PBS was used as a control (five mice per each group). At 4 weeks after the injection, the mice were sacrificed, and the femurs were resected. The femurs were scanned by microcomputed tomography (micro-CT) (LaTheta LCT-200; Hitachi Aloka Medical, Ltd, Tokyo, Japan) using 48 μm slices (0.3 mm interval), and the bone mineral density (BMD) of individual trabecular bone area was calculated by accompanying image-analyzing software, LaTheta v1.20. In some mice, the bone marrow was washed with saline and the cells were stored at −80°C for messenger ribonucleic acid (mRNA) expression study. To assess the retainment of CBD-BMP4, BMP4, or CBD in vivo, each protein fluorescently labeled (1 μg in 10 μL PBS) or vehicle (10 μL PBS) was directly injected into the femur as described above (three mice per group). Mice were sacrificed on day 1, 3, and 14 after the injection and nondecalcification femurs were cut with a tungsten blade at −20°C, and the sections were stained with calcein acetomethoxy and evaluated under a fluorescence microscope.
Bone defect model
Bone defects were made in the cranial bone, as previously described.Citation19 Briefly, mice were anesthetized with ketamine (100 mg/kg), and the cranial bone was exposed. Bone defects were made in the parietal bone with a ϕ3.0 mm drill. After washing with saline, CBD-BMP4, BMP4, CBD (100 ng in 5 μL PBS), or vehicle (5 μL PBS), was applied to the defects, and the scalp was closed (five mice per each group). At 2 weeks after the surgery, the mice were killed, and the cranial bone was scanned by micro-CT in 48 μm slices. To analyze the ossification area, regions of interest were set on the bone defect area, and the accumulation of dots (counts per pixel) in the selected region of interest was measured using image-analyzing software (WinROOF; Mitani Corp). Three-dimensional images were reconstructed by the image-processing software, OsiriX (Pixmeo SARL, Bernex, Switzerland). Subsequently, the defect area was resected, fixed in 10% formalin, decalcified in 10% EDTA, embedded in paraffin, and the sections were stained with hematoxylin–eosin.
Quantitative real-time PCR
Samples were homogenized in lysis buffer (QuickGene; Fujifilm, Tokyo, Japan), and total ribonucleic acid (RNA) was isolated, according to the manufacturer’s instructions. First-strand complementary deoxyribonucleic acid (cDNA) was constructed from 2 μg of total RNA with oligo (dT) as primers,Citation12–Citation18 and the cDNAs were used as a template for polymerase chain reaction (PCR). Quantitative real-time PCR was performed with SYBR PCR master mix (Agilent Technologies, Santa Clara, CA, USA) and specific primers. To validate the SYBR Green PCR products, a dissociation step was done to verify the Tm (annealing temperature) of the SYBR Green PCR product after the PCR were run. The expression levels of each mRNA were normalized by the expression of a housekeeping gene hypoxanthine phosphoribosyltransferase. The primers used in this study are listed in .
Table 1 Primers for quantitative real-time PCR
Statistical analysis
Statistical analyses were performed using Student’s t-test for paired samples and analysis of variance for multiple samples. All data were expressed as the mean ± standard error of the mean. P < 0.05 was considered statistically significant.
Results
Characterization of CBD-BMP4
Recombinant CBD-BMP4 was purified from cocoons of transgenic silkworms. Calculated molecular sizes of CBD-BMP4, CBD, and BMP4 moieties were 52.5 kDa, 39 kDa, and 13 kDa, respectively. As confirmed by enterokinase digestion, this fusion protein consisted of CBD and BMP4. BMP4 moiety released from CBD was immunoreactive with an anti-BMP4 antibody (). In in vitro experiments, CBD-BMP4 in a collagen gel was entrapped and localized for at least 7 days while unfused BMP4 diffused out of the gel as early as 1 hour and was undetectable after 1 day (), indicating that CBD-BMP4 showed much higher collagen-binding affinity than did BMP4.
Figure 1 Characterization of CBD-BMP4. (A) Purified CBD-BMP4 was digested with enterokinase, fractionated on SDS-polyacrylamide gel, and transferred to a PVDF membrane. The gel was stained with Coomassie Brilliant Blue (left), and the membrane was immunoblotted with anti-BMP4 (right). (B) BMP4 or CBD-BMP4 was mixed with collagen solution, and the mixture was gelled at 37°C for 1 hour.
Note: Protein released into PBS solution was blotted to a nitrocellulose membrane and immunodetected with anti-BMP4.
Abbreviations: CBD, collagen-binding domain; BMP4, bone morphogenetic protein-4; SDS, sodium dodecyl sulfate; PVDF, polyvinylidene difluoride; PBS, phosphate buffered saline.
Enhanced bone formation in collagen–sponge scaffold
We next asked whether CBD-BMP4 could display enhanced collagen-binding capacity in vivo and promote bone formation more effectively than BMP4 alone. To examine this, collagen sponges containing 1 μg of CBD-BMP4, BMP4, or CBD were placed in the rabbit femur, as described in the Methods section. The immunohistological data in demonstrated that BMP4 was found localized on day 1, but was no longer visible on day 3 after the implantation. A scant calcification was seen on day 14 ().
Figure 2 Fluorescence images of a collagen sponge.
Notes: Collagen sponges containing fluorescence-labeled CBD-BMP4, BMP4, or CBD were implanted into rabbit femur. Sponges soaked with vehicle PBS were used as a control (three femurs per each group). On days 1, 3, and 14 after the implantation, the sponges were retrieved, and the sections were examined under a fluorescence microscope. HiLyte Fluor 555-labeled CBD-BMP4/BMP4/CBD is shown in red. Bone stained by calcein acetomethoxy is shown in green. Representative photographs from each group are shown.
Abbreviations: CBD, collagen-binding domain; BMP4, bone morphogenetic protein-4; PBS, phosphate buffered saline.
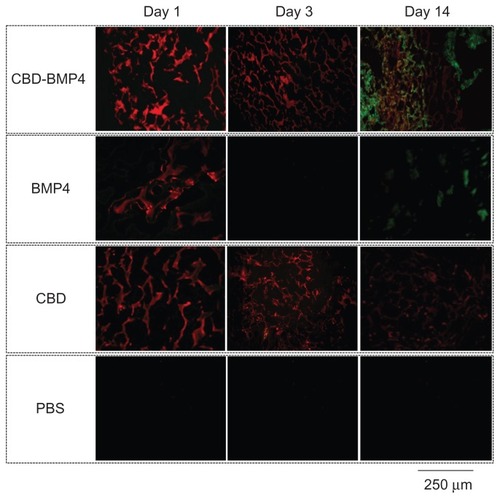
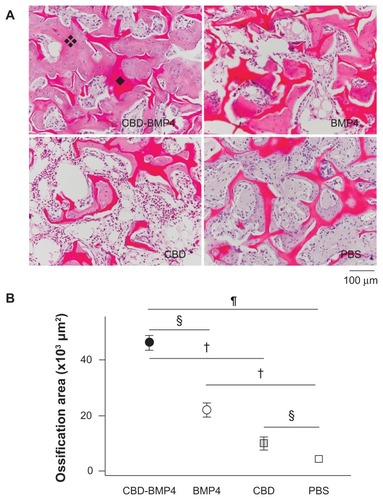
In the case of CBD-BMP4, a stronger signal was seen on day 1 relative to BMP4, and the signal intensity did not diminish by day 3. Results with CBD alone were similar to those of the fusion protein at day 3. Of note was the finding that only CBD-BMP4 appeared to induce robust calcification in the site at day 14 (). In another set of experiments, histological examination 4 weeks later revealed that CBD-BMP4 induced a thicker trabecular bone formation than BMP4 did (). Further, the ossification area formed by CBD-BMP4 injected animals was significantly larger than seen in BMP4 injected animals (). CBD induced slightly augmented bone formation as compared to the PBS control. These data clearly showed that CBD-BMP4 is retained longer at the given site in vivo, and induced bone formation more effectively than BMP4, CBD, or PBS when the fusion protein was implanted with scaffold.
Figure 3 CBD-BMP4 augments new bone formation when delivered by collagen sponges. Collagen sponges containing 1 μg of CBD-BMP4, BMP4, and CBD were implanted into rabbit femurs. Sponges soaked with vehicle PBS were used as a control (five femurs per each group). Four weeks later, the sponge was retrieved, fixed, decalcified, and the sections were stained with HE. (A) Histological sections of the collagen sponge. Representative photographs of each group are shown. ❖: New bone (note osteocyte). ◆: Collagen sponge. (B) Ossification area in the sponge was calculated (five femurs per each group).
Notes:§P < 0.05; †P < 0.01; P < 0.0001. Magnification is shown by the bar (100 μm).
Abbreviations: CBD, collagen-binding domain; BMP4, bone morphogenetic protein-4; PBS, phosphate buffered saline; HE, hematoxylin and eosin stain.
Augmented bone formation without scaffold
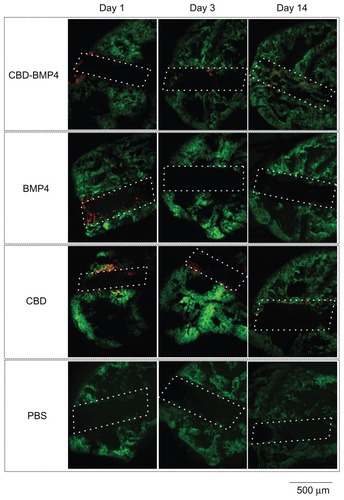
Collagen is the major constituent of the bone matrix.Citation20 The above data prompted us to investigate whether CBD-BMP4 could remain at the given site without scaffold, leading to an augmented bone formation. To address this, we first confirmed the retainment of CBD-BMP4, BMP4, and CBD after a direct injection of fluorescence-labeled proteins (1 μg in 10 μL PBS) into the medullary cavity of mouse femur. The data in demonstrated that BMP4 was not found on day 3 after the injection, whereas CBD-BMP4 was observed even after day 14. CBD remained in a similar fashion. Apparent calcification was seen only next to CBD-BMP4 on day 14 (), suggesting enhanced new bone formation by CBD-BMP4.
Figure 4 Fluorescence images of mouse femur.
Notes: Fluorescence-labeled CBD-BMP4, BMP4 or CBD (1 μg in 10 μL PBS) were injected into mouse femurs. PBS alone (10 μL) was used as a control (three mice per each group). On day 1, 3, and 14 after the injection, the femurs were resected, frozen, cut, and examined under a fluorescence microscope. HiLyte Fluor 555-labeled CBD-BMP4/BMP4/CBD is shown in red. Bone stained by calcein acetomethoxy is shown in green. Bone hole is shown in a dashed line. Shown were representative photographs from each group. Magnification is shown by the bar (500 μm).
Abbreviations: CBD, collagen-binding domain; BMP4, bone morphogenetic protein-4; PBS, phosphate buffered saline.
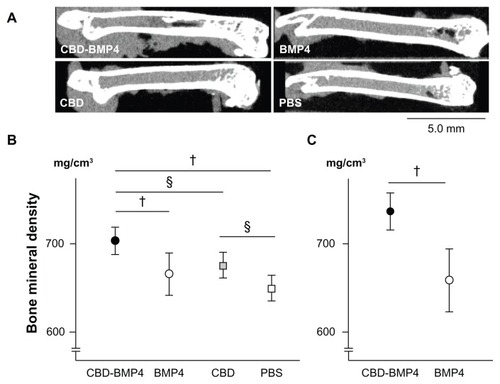
We then assessed BMD by measuring micro-CT images (). BMD in mice treated with CBD-BMP4 at 4 weeks later was increased compared to BMP4, CBD and PBS groups. There was no difference between the BMP4 and PBS groups. BMD in CBD groups was higher than was seen in PBS groups (). The BMD in CBD-BMP4 and BMP4 groups were also measured at 8 weeks after the injection and showed that BMD in CBD-BMP4 groups was higher than that observed in BMP4 groups (). BMD in the CBD-BMP4 groups at 8 weeks increased as compared to that at 4 weeks (737.1 ± 8.1 mg/cm3 versus 703.5 ± 5.9 mg/cm3; respectively, P < 0.05, five mice each). No change was found in BMP4 groups (), suggesting prolonged osteogenic activity with CBD-BMP4. These data indicate that a single injection of CBD-BMP4 augments bone formation even without scaffold.
Figure 5 CBD-BMP4 accelerates bone mineral density. CBD-BMP4, BMP4, CBD (100 ng in 10 μL PBS), or vehicle PBS (10 μL) were directly injected into mouse femurs (five mice per each group). At 4 weeks after the injection, the femurs were scanned by micro-CT. (A) Representative images from each group. Bone mineral density was calculated by micro-CT at (B) 4 weeks and (C) 8 weeks after the injection.
Notes:§P < 0.05; †P < 0.01.
Abbreviations: CBD, collagen-binding domain; BMP4, bone morphogenetic protein-4; PBS, phosphate buffered saline; CT, computed tomography.
Osteogenic gene expressions by CBD-BMP4
To better understand the molecular mechanisms underlying the augmented osteogenesis by intramedullary CBD-BMP4 treatment, bone marrow cells were harvested from the femurs at 4 weeks after therapy injection and mRNA expression of osteogenic factors were examined. Osteoblast-associated factors including alkaline phosphatase (ALP), bone sialoprotein (BSP), osteocalcin, osterix, and Runt-related transcription factor 2 (Runx2) were expressed at higher levels in the CBD-BMP4 group than in the other groups, including the BMP4 group ().
Figure 6 CBD-BMP4 increases osteogenic gene expression. Bone marrow cells were harvested at 4 weeks after the injection of CBD-BMP4, BMP4, CBD (100 ng in 10 μL PBS), or vehicle PBS (10 μL) (five mice per each group). mRNA expression for (A) osteoblast-associated factors and (B) bone growth factors were quantitated by RT-PCR. The expression levels of mRNA were normalized to HPRT.
Notes:§P < 0.05; †P < 0.01.
Abbreviations: CBD, collagen-binding domain; BMP4, bone morphogenetic protein-4; ALP, alkaline phosphatase; BSP, bone sialoprotein; PBS, phosphate buffered saline; RT-PCR, reverse transcription polymerase chain reaction; HPRT, hypoxanthine phosphoribosyltransferase.
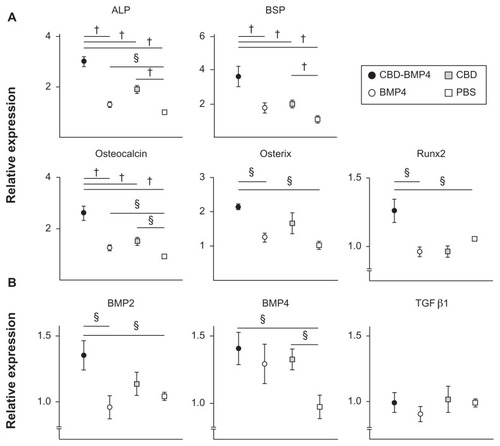
In contrast, there was no difference between the BMP4 group and the PBS control. Interestingly, the CBD group showed increased ALP, BSP, and osteocalcin expression relative to the control PBS (). Endogenous expression of growth factors was examined next, which demonstrated that the CBD-BMP4 group, but not the BMP4 group, upregulated the expression of BMP2 and BMP4, as compared to PBS control. Augmented expression of BMP4 was also found in the CBD group. No change was found in the expression level of TGFβ (). Thus, enhanced bone formation in the CBD-BMP4 group was associated with augmented expressions of osteogenic factors and growth factors. The BMP4 alone group failed to augment these factors during this time period (4 weeks after the injection), possibly due to the diffusion from the site (). CBD, which was also present at the targeted site over a longer period, might stimulate bone formation by inducing endogenous BMP4.
Accelerated bone formation in a cranial bone defect model
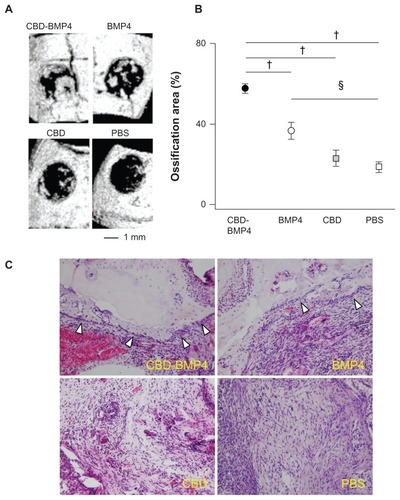
To further strengthen the in vivo osteogenesis induced by CBD-BMP4, we applied CBD-BMP4, BMP4, CBD, or vehicle in a cranial bone defect model without scaffold. BMP4 treatment demonstrated new bone formation on day 14 after the treatment (). Obviously, CBD-BMP4 showed substantial ingrowth of new bone formation. As shown in , the ossification area induced by CBD-BMP4 and BMP4 was statistically increased as compared to that by PBS control. Importantly, there was more new bone formation in the CBD-BMP4-treated group than that in the BMP4 group. Ossification area by CBD was similar to that of the PBS control (). Histological examination consistently demonstrated new bone formation lined by a layer of osteoblasts in the CBD-BMP4- and the BMP4-treated group, which was more prominent in the CBD-BMP4 group ().
Figure 7 CBD-BMP4 enhances bone formation in a cranial bone defect model. CBD-BMP4, BMP4, CBD (100 ng in 5 μL PBS), or vehicle PBS (5 μL) was applied to the defective section of cranial bone (five mice per each group). At 2 weeks after the injection, the mice were killed, and the bone defects were scanned by micro-CT. (A) Representative three-dimensional CT images from each group. (B) Ossification area was calculated by micro-CT. §P < 0.05; †P < 0.01. (C) Representative tissue sections from each group are shown (HE staining).
Note: Arrowheads indicated the layer of osteoblasts.
Abbreviations: CBD, collagen-binding domain; BMP4, bone morphogenetic protein-4; PBS, phosphate buffered saline; CT, computed tomography; HE, hematoxylin and eosin stain.
Discussion
Immobilized growth factors targeting the extracellular matrix (ECM) can be more effective than diffusible, free growth factors.Citation21 The goal of engineering a fusion protein was to deliver a functional substance that had limited diffusion over a prolonged period of time. Our novel BMP4 fusion protein with a nanosized carrier – namely, the CBD – seems to fulfill this requirement.Citation15 The stable binding of CBD to collagen led us to investigate whether this novel CBD-BMP4 by itself could enhance bone formation using bone matrix collagen as an innate scaffold.
As expected, CBD-BMP4 induced stronger bone formation than did BMP4. CBD-BMP4 remained at the injected site for at least 2 weeks, possibly through the CBD binding to bone matrix collagen, and the results demonstrated that new bone formation continues to be observed even after 8 weeks. There exist several kinds of gene-engineered binding growth factors, including BMPs.Citation21 Our CBD-BMP4 has advantages over other engineered fusion proteins. First, CBD-BMP4 induced entopic bone formation without using scaffold. As far as we know, this is the first report demonstrating accelerated bone formation by a fusion protein on its own. Previous studies reported that collagen-binding BMP2 demonstrated ectopic bone formation when applied with bone-derived matrix as a scaffold.Citation22,Citation23 Bone-derived matrix contains many factors, including native BMPs, and the complex is not fully defined, suggesting potential difficulties in clinical use. Second, CBD-BMP4 was effective even in a single low dose. In the present study, we injected 100 ng of CBD-BMP4 (≈2 pM), as preliminary experiments showed that BMP4 at this dose failed to induce appreciable bone formation at 4 weeks after the injection (not shown). Others used 8 nM CBD-BMP2 for ectopic bone formation model and 0.5 nM for the bone defect model.Citation24 Although verification of whether higher amounts of CBD-BMP4 could induce stronger bone formation in a critical-sized bone defect remains to be completed, we believe that our results predict that CBD-BMP4 will be very efficient with larger doses. In a bone-defect model, 2.5 μg to 5 μg of recombinant BMP4 was applied with collagen sponge or beta-tricalcium phosphate.Citation11 We believe our initial results also predict that, compared to other reagents, the CBD-BMP4 fusion protein will efficaciously require reduced amounts of protein to induce bone formation and thus limit possible clinical complications.
It appears that CBD-BMP4 both directly and indirectly induced bone formation. We have demonstrated that CBD-BMP4 induced osteogenic differentiation using three-dimensional cultures of human bone marrow-derived mesenchymal stem cells.Citation15 As shown in this study, CBD-BMP4 treatment upregulated the expression of all relevant osteogenic genes examined in the target site, even after 4 weeks postinjection, possibly due to the continued functional localization of the fusion protein. ALP is a major biomarker of bone formation and plays a key role in bone mineralization.Citation25 BSP is upregulated as osteoblasts mature at sites of de novo bone formation.Citation26 Osteocalcin is an ECM protein and among the most specific markers for osteoblast maturation.Citation27 Osterix is a bone-related transcription factor that functions genetically downstream of Runx2, which regulates the differentiation and/or function of osteoblasts.Citation28,Citation29 In addition, CBD-BMP4 increased the expression of endogenous BMP2 and BMP4. Thus, CBD-BMP4 stimulated de novo expressions of osteogenic genes and bone growth factors for a longer period and initiated the calcification of the ECM, leading to the prolonged bone formation.
Of note was the finding that CBD by itself somewhat induced bone formation when intramedullary injected. Similar to CBD-BMP4, CBD remained at the injected site. The CBD used in this fusion protein was from fibronectin,Citation17 which is an ECM component. ECMs and growth factors function cooperatively to stimulate osteoblast differentiation. There is evidence to suggest a role for fibronectin in the early stages of bone formation.Citation30,Citation31 Recently, it has been demonstrated that ECMs including fibronectin modified the growth patterns and induced the osteoblast differentiation of human myeloid stem cells, as evidenced by increased expressions of ALP, osteocalcin, osterix, and Runx2.Citation32 Accordingly, we showed in this study that CBD upregulated the expressions of osteogenic genes such as ALP, BSP, and osteocalcin, and growth factor BMP4. Although CBD is a segment (amino acids 260–599) of the original fibronectin,Citation15 CBD may contain fibronectin’s active site leading to osteogenesis. Thus, the osteogenic capacity of CBD-BMP4 may be partly due to the activity of CBD. A question arises as to whether the simultaneous injection of CBD and BMP4 could represent additive or synergistic osteogenic effects, which was not examined in this study. CBD-BMP4 had no osteogenic effects when applied to the defective section of cranial bone. The disparity may be due to the different location of the given site (bone marrow versus cortical bone).
There are several other concerns that were not confronted in this study. Although we believe that CBD-BMP4 binds to bone matrix collagen at the injection site, we have not shown direct evidence. It is not clear how CBD-BMP4 interacts with the BMP4 receptor at the site and how CBD-BMP4 itself affects the surrounding cells, either when acting as a complex or as individual components. CBD-BMP4 has a higher molecular weight than BMP4, which may contribute to the physics of diffusion. Further studies are necessary to explore these precise mechanisms.
Conclusion
In conclusion, our engineered CBD-BMP4 is a novel fusion protein with an exquisite ability to promote in vivo osteogenesis, even by a single injection at the targeted site both with and without scaffold. There are many ways to enhance the release of BMP4 using chemical conjugation, particle vehicle, or gene delivery, which are low cost, verified, and convenient. We believe that our fusion protein is better than others, as CBD-BMP4 can localize longer at the designated site, resulting in stronger bone formation by a single low dose of complex. This novel CBD-BMP4 may be promising clinically for the treatment of unresolved fractures, bone defects, and other bone tissue engineering and regeneration-related scenarios.
Acknowledgments
We thank Takayuki Furumatsu, Reina Tanaka, and Yuki Nakashima for their excellent technical assistance. We also thank Judith Connett (University of Michigan Medical School, Ann Arbor, MI, USA) for her critical reading of the manuscript. This work was supported in part by grants from Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (S) of KAKENHI 22220009.
Disclosure
The authors report no conflicts of interest in this work.
References
- CiernyG3rdZornKESegmental tibial defects. Comparing conventional and Ilizarov methodologiesClin Orthop Relat Res19943011181238156662
- MyeroffCArchdeaconMAutogenous bone graft: donor sites and techniquesJ Bone Joint Surg Am201193232227223622159859
- LinkhartTAMohanSBaylinkDJGrowth factors for bone growth and repair: IGF, TGF beta, and BMPBone199619Suppl 11S2S8830994
- ChenDZhaoMMundyGRBone morphogenetic proteinsGrowth Factors200422423324115621726
- MarsellREinhornTAThe role of endogenous bone morphogenetic proteins in normal skeletal repairInjury200940Suppl 3S4S720082790
- McKayWFPeckhamSMBaduraJMA comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft)Int Orthop200731672973417639384
- WhiteAPVaccaroARHallJAWhangPGFrielBCMcKeeMDClinical applications of BMP-7/OP-1 in fractures, nonunions, and spinal fusionInt Orthop200731673574117962946
- AndersonCLWhitakerMCHeterotopic ossification associated with recombinant human bone morphogenetic protein-2 (infuse) in posterolateral lumbar spine fusion: a case reportSpine (Phila Pa 1976)2012378E502E50622020605
- JosephVRampersaudYRHeterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysisSpine (Phila Pa 1976)200732252885289018246013
- KimPDLudwigSPoelstraKDugganBScaleaTGelbDEctopic bone formation in the pelvis after combined anterior and posterior fusion of the spine with osteogenic protein-1 use: a case reportJ Spinal Disord Tech201023321522020084023
- PangEKImSUKimCSEffect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect modelJ Periodontol200475101364137015562914
- LeongLMBrickellPMBone morphogenic protein-4Int J Biochem Cell Biol19962812129312969022288
- RundleCHMiyakoshiNKasukawaYIn vivo bone formation in fracture repair induced by direct retroviral-based gene therapy with bone morphogenetic protein-4Bone200332659160112810166
- Lópiz-MoralesYAbarrategiARamosVIn vivo comparison of the effects of rhBMP-2 and rhBMP-4 in osteochondral tissue regenerationEur Cell Mater20102036737821154243
- LuHKawazoeNKitajimaTSpatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivityBiomaterials201233266140614622698726
- PriceTMRudeeMLPierschbacherMRuoslahtiEStructure of fibronectin and its fragments in electron microscopyEur J Biochem198212923593637151803
- IshikawaTTeraiHKitajimaTProduction of a biologically active epidermal growth factor fusion protein with high collagen affinityJ Biochem2001129462763311275564
- KawamotoTShimizuMA method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissuesHistochem Cell Biol2000113533133910883392
- AalamiOONacamuliRPLentonKAApplications of a mouse model of calvarial healing: differences in regenerative abilities of juveniles and adultsPlast Reconstr Surg2004114371372015318051
- RobeyPGFedarkoNSHefferanTEStructure and molecular regulation of bone matrix proteinsJ Bone Miner Res19938Suppl 2S483S4878122516
- KitajimaTItoYArtificial binding growth factorsKhangGHandbook of Intelligent Scaffolds for Tissue Engineering and Regenerative MedicineSingaporePan Stanford Publishing2012337353
- ChenBLinHZhaoYActivation of demineralized bone matrix by genetically engineered human bone morphogenetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptideJ Biomed Mater Res A200780242843417013862
- ZhaoYChenBLinHThe bone-derived collagen containing mineralized matrix for the loading of collagen-binding bone morphogenetic protein-2J Biomed Mater Res A200988372573418335535
- ChenBLinHWangJHomogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2Biomaterials20072861027103517095085
- AndersonHCSipeJBHessleLImpaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient miceAm J Pathol2004164384184714982838
- BiancoPRiminucciMBonucciETermineJDRobeyPGBone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix depositionJ Histochem Cytochem19934121831918419458
- GalliMCaniggiaMOsteocalcinMinerva Med1984754224892501 Italian6334818
- NakashimaKZhouXKunkelGThe novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formationCell20021081172911792318
- NishioYDongYParisMO’KeefeRJSchwarzEMDrissiHRunx2-mediated regulation of the zinc finger Osterix/Sp7 geneGene2006372627016574347
- MoursiAMDamskyCHLullJFibronectin regulates calvarial osteoblast differentiationJ Cell Sci1996109Pt 6136913808799825
- MoursiAMGlobusRKDamskyCHInteractions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitroJ Cell Sci1997110Pt 18218721969378768
- MathewsSBhondeRGuptaPKToteySExtracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cellsDifferentiation201284218519222664173