Abstract
A new nanostructured hydroxyapatite-coated porcine acellular dermal matrix (HAp-PADM) was fabricated by a biomimetic mineralization method. Human periodontal ligament stem cells were seeded on HAp-PADM and the effects of this scaffold on cell shape, cytoskeleton organization, cell viability, and osteogenic differentiation were examined. Periodontal ligament stem cells cultured on HAp-PADM exhibited different cell shape when compared with those on pure PADM. Moreover, HAp-PADM promoted cell viability and alkaline phosphatase activity significantly. Based on quantitative real-time polymerase chain reaction, the expression of bone-related markers runt-related transcription factor 2 (Runx2), osteopontin (OPN), and osteocalcin (OCN) upregulated in the HAp-PADM scaffold. The enhancement of osteogenic differentiation of periodontal ligament stem cells on the HAp-PADM scaffold was proposed based on the research results. The results of this study highlight the micro-nano, two-level, three-dimensional HAp-PADM composite as a promising scaffold for periodontal tissue engineering.
Introduction
As one of the key elements of tissue engineering, scaffolds are used to create the three-dimensional (3D) organization for appropriate cell interactions and to serve as vehicles to deliver and retain cells and molecules. The scaffold materials studied widely for periodontal tissue engineering include chitosan,Citation1,Citation2 bioactive glass,Citation3 and silk.Citation4 Among them, porcine acellular dermal matrix (PADM) has been paid much attention because of its good biocompatibility, biodegradation properties, and easiness to prepare. PADM is mainly composed of type I and II collagen and has been successfully used in covering full-thickness burn wounds in clinical practice.Citation5 Moreover, a purified porcine skin sheet can be easily tailored to a certain size and shape for a variety of applications.Citation6 Recent studies have indicated that surface topology of biomaterials could profoundly affect cell functions,Citation7,Citation8 and participate in implanted cell lineage commitment.Citation9 A new tendency of scaffold material development is to increase the mechanical stability and to promote osteogenic commitment of cells. The assembly of bone substitute on polymer scaffold could induce seed cells to differentiate towards osteoblastsCitation9,Citation10 as well as increase mechanical strength.Citation9 As one kind of bone substitute, hydroxyapatite (HAp) has been widely used for bone regeneration and dental implant osseointegration because of its osteoconductive and osteoinductive properties.Citation11,Citation12
Currently, by a biomimetic mineralization method, the authors’ group and Guo et al successfully assembled HAp on the channel surface of PADM, and prepared a bioactive scaffold for periodontal tissue engineering (HAp-PADM).Citation6,Citation13 It was found that HAp-PADM had relatively high mechanical strength and reduced the high rate of enzymatic degradation of natural collagen, which could increase the compressive resistance and stabilize the collagen-based materials. Moreover, the HAp-PADM scaffold was biocompatible for human periodontal ligament (PDL) cells and MC3T3-E1 preosteoblasts, and the cells were able to proliferate and migrate into the scaffold.Citation6,Citation13 Therefore, this composite system combining the advantages of PADM and HAp seems to be a promising choice for periodontal tissue regeneration.
In this study, human PDL stem cells (PDLSCs) were cultured and identified, and then seeded on HAp-PADM and pure PADM scaffolds. The morphology and distribution of PDLSCs were examined with scanning electron microscope (SEM) and confocal laser scanning microscope (CLSM). In addition, methylthiazol tetrazolium (MTT) assay was used to evaluate cell viability, and alkaline phosphatase (ALP) activity was detected to determine osteogenic differentiation of PDLSCs on the scaffolds. Moreover, the expression of bone-related markers runt-related transcription factor 2 (Runx2), osteopontin (OPN), and osteocalcin (OCN) were detected by reverse transcriptase polymerase chain reaction (RT-PCR).
Material and methods
Preparation of PADM and HAp-PADM scaffolds
A PADM scaffold with natural microporous structure was decellularized through a series of agitations involving enzymes, detergents, and hyperosmotic saline treatment. The obtained PADM is used for preparation of HAp-PADM scaffolds by assembling HAp nanostructures on the channel surface of a PADM scaffold through a biomimetic chemical mineralization process in simulated body fluid. The preparation process, structural and morphological characterization, mechanical properties, and in vitro biodegradation rate of PADM and HAp-PADM scaffolds were described in detail previously.Citation6 PADM is a 3D interconnected network structure, mainly composed of collagen I, with channel diameter over 100 μm and channel wall thickness of about 10–20 μm. The HAp-PADM composite scaffold is a two-level, 3D network structure. After the biomimetic mineralization process, the composite still keeps its 3D interconnected network structure, which is the first level of the 3D network structure. A layer of HAp 3D network nanostructure with about 120 nm in channel diameter is assembled on the surface of the channel. This special structure endows the HAp-PADM composite scaffold with high strength, long-term biodegradation durability, and osteogenic differentiation promotion potential.
Collection and culture of human PDLSCs
Written approval for human PDL collection performed in this study was obtained from the Medical Ethics Committee of Medical School, Shandong University (approval number: 2010015; Jinan, People’s Republic of China) and written informed consent was obtained from each individual participant. Teeth were collected from clinically healthy premolars extracted for orthodontic reasons. PDLSCs were collected and cultured as previously described.Citation14 Briefly, PDL tissues were digested, and dissociated cell suspension was filtered and seeded at a low concentration for single cell-derived colony selection. Individual colonies were isolated with colony rings and expanded into individual vessels for further cultivation.
Immunocytochemical staining
PDL single-cell colonies were cultured on eight-well chamber slides until confluent and immunocytochemical staining for mesenchymal stem cell (MSC) marker, STRO-1, was performed as previously described.Citation14 Briefly, after fixing with 4% paraformaldehyde and sealing with 10% bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA), cells were incubated with diluted primary antibody anti-STRO-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse immunoglobulin G1 (IgG1) as a negative control, and finally with fluorescein-conjugated secondary antibody. Afterwards, the cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich). The slides were mounted and then analyzed under a fluorescence microscope (BX51, Olympus Ltd, Tokyo, Japan).
Flow cytometric analysis
PDLSCs were detached with 0.05% trypsin/ethylenediaminetetraacetic acid and resuspended in blocking buffer containing Hanks’ balanced salt solution (Sigma-Aldrich) supplemented with 5% fetal calf serum, 1% bovine serum albumin, and 5% normal human serum for 1 hour. Approximately 1 × 105 cells were incubated with specific fluorescein isothiocyanate-conjugated mouse monoclonal antibodies (10 μg/mL) for human cluster of differentiation 44 (CD44), CD73, CD90, CD105, CD166 (Becton Dickinson Biosciences, San Jose, CA, USA), CD14, CD34, CD45 (Beckman Coulter, Brea, CA, USA), or 10 μg/mL isotype-matched control 1B5 (IgG1) and 1D4.5 (IgG2a). After incubation on ice for 1 hour, cells were incubated with goat anti-mouse IgG or IgM for 45 minutes on ice. After washing, flow cytometry data were analyzed using an EPICS® XL/MCL™ flow cytometer (Beckman Coulter).
PDLSCs cultured on the scaffolds
PADM and HAp-PADM samples were cut into 8 mm × 8 mm × 0.6 mm pieces for cell culture. First, the scaffolds were sterilized using 75% ethanol for 2 hours in a 24-well plate, exposed to ultraviolet radiation for 30 minutes, washed three times with sterile phosphate buffered saline, and then transferred into 24-well culture plates and prewetted with α-minimal essential medium overnight. After 80% confluence, PDLSCs were digested with 0.25% trypsin, and 100 μL cell suspension containing 5 × 104 cells were seeded into each scaffold. After 3 hours, another 900 μL culture medium was added to each well, and the culture was maintained at 37°C in 5% carbon dioxide and 95% humidity.
SEM observation
PDLSCs morphology on HAp-PADM and PADM scaffolds was examined using an SEM (S-750; Hitachi Ltd, Tokyo, Japan). The detailed method has been described elsewhere.Citation14
CLSM observation
PDLSCs (5 × 104) were seeded into each scaffold in 24-well plastic culture plates. Forty-eight hours after initial seeding, the cell-coated samples were fixed with 3.7% formaldehyde solution for 10 minutes and then extracted with 0.1% Triton X-100 (Sigma-Aldrich) for 5 minutes and blocked with phosphate buffered saline containing 1% bovine serum albumin (Sigma-Aldrich) for 30 minutes. The samples were then stained with phalloidin conjugated to Alexa Fluor® 488 (Life Technologies, Carlsbad, CA, USA) and examined with a TCS SP2 CLSM (Leica Microsystems, Wetzlar, Germany).
Cell viability and ALP activity assay
Cell viability was determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay and ALP activity was measured by the p-nitrophenyl phosphate method as previously described.Citation14
Quantitative RT-PCR analysis of bone-related markers
Osteogenic gene expression of human PDLSCs cultured on HAp-PADM and PADM was explored by RT-PCR analysis as previously described.Citation14 Briefly, the cell-seeded HAp-PADM and PADM scaffolds were rinsed after 3, 7, and 14 days, and the cells on the scaffolds were digested and lysed with TRIzol® Reagent (Life Technologies), followed by reverse transcription using Oligo (dT) primers and SuperScript® III Reverse Transcriptase according to the manufacturer’s instructions (Life Technologies). RT-PCR was performed with RTCitation2 SYBR® Green qPCR Master Mix (SABiosciences, Frederick, MD, USA). The primer sets used (Runx2, OPN, and OCN) are shown in . Expression of β-actin was used as an internal control.
Table 1 Primer sequences for osteogenic markers
Statistical analysis
Results are presented as the mean ± standard deviation of three to nine replicates for each experiment. The statistically significant differences between groups were assessed by one-way analysis of variance. P < 0.05 was considered statistically significant.
Results
Characteristics of PDLSCs
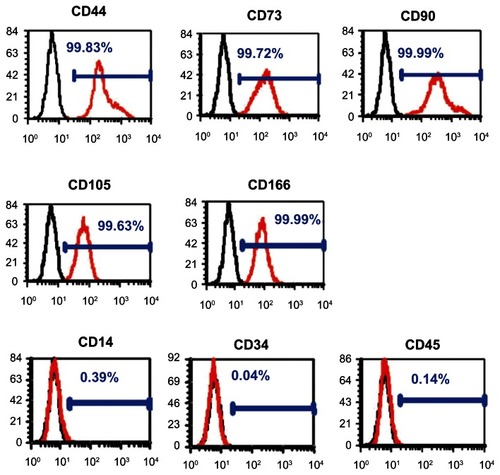
PDLSCs were identified as single-cell colonies generated from human PDL-derived cells (). This colony-forming cell population is termed as PDLSCs. Cultured PDLSCs exhibit positive staining for MSC marker STRO-1 (), which is originally identified as a marker for colony-forming osteogenic precursor cells isolated from bone marrow.Citation15 Moreover, PDLSCs were positive (defined as ≥95% positive cells) for MSC-associated surface markers CD44, CD73, CD90, CD105, and CD166, and were negative (≤5% positive cells) for hematological markers CD14, CD34, and CD45 ().
Figure 1 Characterization of human periodontal ligament stem cells. (A) Cell clusters derived from the periodontal ligament formed a single colony and were stained with 0.1% toluidine blue (scale bar: 100 μm). (B) Cultured periodontal ligament stem cells exhibited positive staining for STRO-1 (green fluorescent signals; blue signals nuclei) (scale bar: 50 μm).
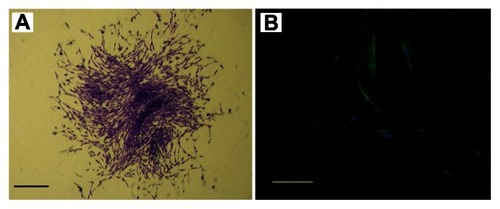
Figure 2 Flow cytometric analysis of periodontal ligament stem cells.
Notes: They were positive for mesenchymal stem cell markers CD44, CD73, CD90, CD105, and CD166 and negative for hematopoietic markers CD14, CD34, and CD45 (red line). The black line represents the isotype controls 1B5 (immunoglobulin G1) and 1D4.5 (immunoglobulin G2a).
Abbreviation: CD, cluster of differentiation.
SEM images of PDLSCs on scaffolds
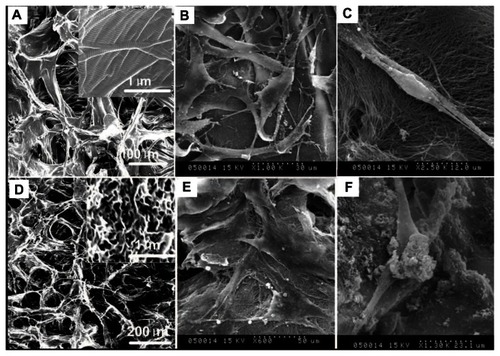
SEM images of PADM and HAp-PADM are shown in . PADM possesses a 3D porous structure with a channel diameter about 50–100 μm (). After the decellularization process, collagen in PADM still keeps its natural structure (). After the biomimetic mineralization process, HAp-PADM keeps the microstructure of PADM and forms a layer of an HAp 3D interconnected nanostructure with 120–150 nm microchannels on the surface of the microchannels of PADM (). Adhesion of human PDLSCs on the two scaffolds after 2 days cell seeding was also observed under SEM. Before being seeded on scaffolds, PDLSCs showed typical fibroblast-like morphology. However, PDLSCs adopt a polygonal shape on HAp-PADM () while those on pure PADM maintain their intrinsic spindle shapes () under SEM. PDLSCs adhered on the two scaffolds, and the morphologies of PDLSCs on the two scaffolds are different. On PADM, the vast majority of human PDLSCs appear confluent and exhibit a spindle shape, fibroblast-like morphology and possess the typical phenotype of PDLSCs (). In contrast, the shape of PDLSCs on HAp-PADM is polygonal and similar to an osteoblast phenotype (). Two cells spread completely on PADM, overlap each other, and adapt very closely to the underlying collagen fibers (), while HAp clusters can be observed on both cells and collagen fibers 2 days after cell seeding on HAp-PADM ().
Figure 3 Scanning electron microscope images of human periodontal ligament stem cells on a pure porcine acellular dermal matrix and hydroxyapatite-coated porcine acellular dermal matrix. (A) The porcine acellular dermal matrix possesses a three-dimensional porous structure with channel diameter of about 50–100 μm. (B) Periodontal ligament stem cells adhered on the porcine acellular dermal matrix uniformly and exhibited typical fibroblast-like morphology. (C) Periodontal ligament stem cells on the porcine acellular dermal matrix extended completely and intimately adhered to the underlying collagen fibrils. (D) A hydroxyapatite three-dimensional interconnected nanostructure with 120–150 nm microchannels formed on the surface of the microchannels of the porcine acellular dermal matrix. (E) Periodontal ligament stem cells exhibited osteoblast-like morphology on the hydroxyapatite-coated porcine acellular dermal matrix. (F) Hydroxyapatite clusters precipitated on the cells and collagen fibers after cells seeding on the hydroxyapatite-coated porcine acellular dermal matrix scaffold.
CLSM images of PDLSCs on scaffolds
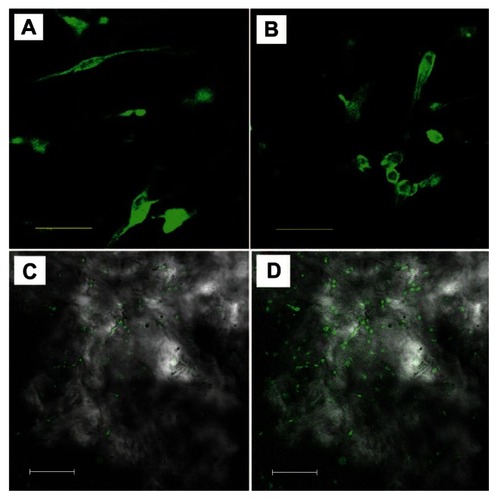
Morphology and distribution of viable human PDLSCs after 2 days culture on the scaffolds were observed under CLSM to further check the adhesion and immigration of cells on both of the scaffolds. Actin filament was stained by Alexa Fluor 488 phalloidin, which can emit green fluorescence when excited by light with a wavelength of 488 nm. show the cell morphology and distribution of viable cells on pure PDAM and HAp-PADM. Cells distribute on the framework with random arrangement due to the natural original channels of the PADM framework. In , it can be seen that some fluorescent spots are vague, which indicates that some cells are not on the focal plane. This phenomenon is the typical 3D growth of the cell, which illustrates that the cells can grow into the channels of the scaffold. The porous surface structure determines the cell position on the scaffold and causes different fluorescence intensity of cells or different parts of one cell to be in and out of focus. From low resolution CLSM images of the samples, it can be seen that cells on PADM seem less dense () than those on HAp-PADM ().
Cell viability and ALP activity
There is no statistically significant difference between the pure PADM framework and HAp-PADM scaffolds after seeding for 1 day. However, after 3, 5, and 7 days culture, the viability of cells on HAp-PADM are significantly higher than those on pure PADM (P < 0.05, P < 0.01, and P < 0.01 respectively; ). Human PDLSCs exhibited higher viability on HAp-PADM than those on pure PADM. The observation of CLSM agreed with MTT assay, and more cells adhered on the HAp-PADM scaffold.
Figure 5 Metabolic activity (methylthiazol tetrazolium assay) of periodontal ligament stem cells seeded on PADM and HAp-PADM.
Notes: Data represents mean ± standard deviation (n = 9, nine replicates per time point for each experimental condition); *P < 0.05; **P < 0.01 (PADM versus HAp-PADM).
Abbreviations: HAp-PADM, hydroxyapatite-coated porcine acellular dermal matrix; PADM, porcine acellular dermal matrix.
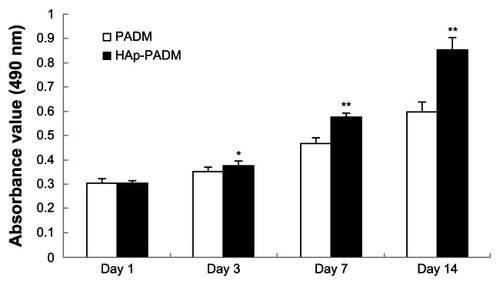
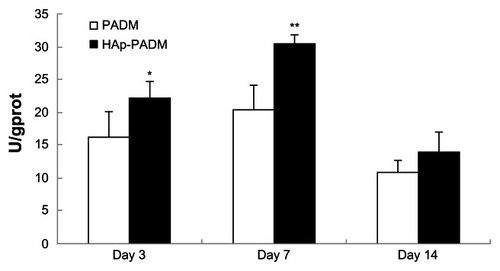
As an early marker for osteogenesis, ALP is assumed to reflect the degree of differentiation. ALP activity of cells on the two samples measured on days three, seven, and 14 shows that ALP activity peaked after 7 days of cultivation (). PDLSCs exhibited higher ALP activity on HAp-PADM than those on pure PADM on days three and seven (P < 0.05 and P < 0.01, respectively). On day 14, ALP activity decreased and there was no significant difference between the two groups.
Figure 6 Alkaline phosphatase activity of periodontal ligament stem cells seeded on PADM and HAp-PADM.
Notes: Data were normalized for total protein content to account for the effect of the different number of cells on each scaffold. Data represents mean ± standard deviation (n = 5, five replicates per time point for each experimental condition); *P < 0.05; **P < 0.01 (PADM versus HAp-PADM).
Abbreviations: HAp-PADM, hydroxyapatite-coated porcine acellular dermal matrix; PADM, porcine acellular dermal matrix.
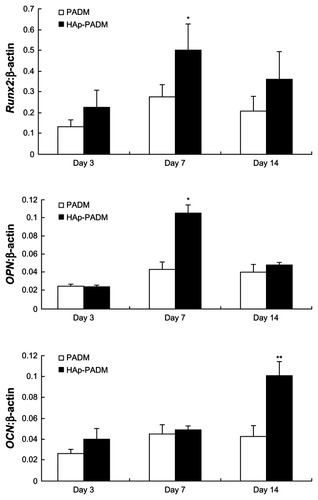
RT-PCR analysis of bone-related genes
Significantly higher expression level of Runx2 and OPN was found on HAp-PADM than on pure PADM on day seven (P < 0.05; ), followed by an expression decrease on day 14 with no significant difference at this time point. OCN was continuously upregulated on HAp-PADM and peaked on day 14. The expression level of OCN on HAp-PADM was significantly higher than that on PADM on day 14 (P < 0.01).
Figure 7 Real-time polymerase chain reaction analysis of Runx2, OPN, and OCN messenger ribonucleic acid expression in periodontal ligament stem cells on two scaffolds.
Notes: Gene expression was normalized to housekeeping gene β-actin expression. Periodontal ligament stem cells were cultivated for 3, 7, and 14 days on PADM and HAp-PADM. Data represents mean ± standard deviation (n = 3); *P < 0.05; **P < 0.01 (PADM versus HAp-PADM).
Abbreviations:OPN, osteopontin; OCN, osteocalcin; HAp-PADM, hydroxyapatite-coated porcine acellular dermal matrix; PADM, porcine acellular dermal matrix.
Discussion
Biomimetic mineralization approaches to assembling a nanostructured apatite layer throughout a 3D porous bone tissue engineering scaffold have provided an effective tool for controlling surface chemistry and geometry within a large and complex structure.Citation9 Recently, the authors’ group has successfully fabricated an HAp-PADM scaffold with a 3D two-level porous structure by assembling the HAp nanostructures on the channel surface of a PADM framework by a two-step biomimetic mineralization method. This HAp-PADM scaffold has both interconnected channels (~100 μm in diameter) formed by collagen fiber bundles and nanopores (<100 nm in diameter).Citation6
In this study, the effect of this surface biomimetic apatite nanonetwork structure of PADM on the biological characteristics and osteogenic differentiation potential of PDLSCs was examined. These characteristics were compared with PDLSCs cultured on PADM with a smooth surface.
Before being seeded on scaffolds, PDLSCs showed typical fibroblast-like morphology and expressed MSC markers CD44, CD73, CD90, CD105, and CD166. However, PDLSCs adopt a polygonal shape on HAp-PADM while those on pure PADM maintain their intrinsic spindle shapes under SEM. These results indicate that the surface and chemical composition characteristics of the scaffolds could influence the phenotype of cells. In fact, scaffold mean pore size significantly influences cell morphology and phenotypic expression.Citation16 By the assembly of HAp on PADM, the obtained HAp-PADM scaffold has a two-level porous structure with large channels (~100 μm in diameter) inherited from the purified PADM microstructure and small pores (100–120 nm in diameter) made up of self-assembled HAp on the channel surfaces.Citation6 The nano-HAp porous structure and calcium element on the channel wall may alter the phenotypic expression of PDLSCs.
The regulation of PDLSC proliferation and viability is crucial for periodontal tissue regeneration. In this study, the proliferative effect of scaffolds on PDLSCs was examined in vitro. Human PDLSCs exhibited higher viability on HAp-PADM than those on pure PADM. The observation under CLSM agreed with the MTT assay that more cells adhered on HAp-PADM scaffold. Fibroblasts bound to a wide range of pore sizes (63–150 μm) and cells increased their viability with decreasing pore size until no cells could fit into the pores.Citation17 The pore size of the pure PADM scaffold is about 100 μm, which is favorable for the attachment and ingrowth of PDLSCs. Moreover, the addition of HAp might increase the scaffold surface area and the small pores made up of the HAp nanostructure may favor the attachment and growth of cells. The promoted effect of HAp-PADM on PDLSCs viability means more pluripotent stem cells can grow on the scaffold, which will facilitate the regeneration of damaged periodontal tissue.
Most importantly, the porous self-assembled 3D continuous network-like nanostructures of HAp might dissolve in culture media in vitro and increase the concentrations of calcium and phosphorus ions. The increase of the concentrations of calcium and phosphorus ions may play an important role in the regulation of cell differentiation. For example, inorganic phosphate has been reported to be a signaling molecule of osteoblast differentiationCitation18 and regulates multiple genes during osteoblast differentiation.Citation19 As an early marker for osteogenesis, ALP is assumed to reflect the degree of differentiation. In the current study, PDLSCs cultured on HAp-PADM exhibited higher ALP activity than those on pure PADM on days three and seven.
Recent studies demonstrate that synthetically nanofabricated structure can not only affect cell shape, adhesion, migration, and proliferation, but also influence RhoA kinase-mediated cytoskeletal tension, modulation of intracellular signaling pathways that regulate transcriptional level, and gene expression.Citation20,Citation21 RT-PCR analysis for osteogenic gene expression further indicates that the surface microenvironment of the scaffolds affects osteogenic differentiation. In the current study, expression of Runx2, OPN, and OCN was upregulated on HAp-PADM. Runx2 is a key transcription factor regulating a number of other genes involved in osteoblast differentiation and bone development.Citation22OPN is a multifunctional phosphorylated glycoprotein secreted by osteoblasts at an early stage during bone development to facilitate attachment to the extracellular matrix,Citation23 while OCN is synthesized when the remodeling of the bone matrix is activated;Citation22,Citation24 this can explain why the expression of OPN reached its highest level (on day seven) earlier than OCN (on day 14) in this study. The upregulation of Runx2, OPN, and OCN further demonstrates that HAp-PADM can improve osteogenic differentiation of PDLSCs.
Altogether, the results of this study demonstrate that biomimetic HAp nanoconstructs on PADM can alter PDLSCs morphology, increase viability, and significantly enhance PDLSC osteogenic differentiation (increased ALP production and upregulation of bone-related genes). The assembly of the HAp nanostructure might increase the viability and differentiation through two approaches. First, the biomimetic mineralization procedures may change the surface topography and chemistry within the porous scaffolds (eg, scaffold surface area, pore diameter). Second, special interface interaction between surface apatite nanostructure and PDLSCs may induce osteogenic commitment. Therefore, further study will explore the biomolecular mechanisms by which nanostructured apatite influence PDLSC biobehavior, and an in vivo experiment with PDLSC-seeded HAp-PADM for repair of periodontal or bone defect may be performed.
Conclusion
Human PDLSCs were successfully isolated and cultured, and they expressed STRO-1 and were positive for MSC markers while negative for hematological markers. Moreover, when cultured on PADM with surface biomimetic HAp nanostructures, human PDLSCs exhibited a different shape from those cultured on the smooth surfaced PADM. Additionally, HAp-PADM promoted cell viability, upregulated ALP activity, and induced messenger ribonucleic acid expression of osteogenic differentiation markers of Runx2, OPN, and OCN. This study demonstrates that the surface HAp nanonetwork structure of PADM could promote the viability and differentiation of PDLSCs and is a promising scaffold for periodontal tissue engineering.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Number 81100756, NSFDYS: 5092525), Independent Innovation Foundation of Shandong University (Number 2012TS097), Science and Technology Program of Shandong Province (Number 2010GSF10248). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- SoranZAydinRSGumusdereliogluMChitosan scaffolds with BMP-6 loaded alginate microspheres for periodontal tissue engineeringJ Microencapsul201229877078022612554
- AkmanACTigliRSGumusdereliogluMNohutcuRMbFGF-loaded HA-chitosan: a promising scaffold for periodontal tissue engineeringJ Biomed Mater Res A201092395396219291690
- WuCZhouYLinCChangJXiaoYStrontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineeringActa Biomater20128103805381522750735
- DuanXTuQZhangJApplication of induced pluripotent stem (iPS) cells in periodontal tissue regenerationJ Cell Physiol2011226115015720658533
- JiongCJiakeCChunmaoHClinical application and long-term follow-up study of porcine acellular dermal matrix combined with autoskin graftingJ Burn Care Res201031228028520182383
- ZhaoHWangGHuSIn vitro biomimetic construction of hydroxyapatite-porcine acellular dermal matrix composite scaffold for MC3T3-E1 preosteoblast cultureTissue Eng Part A2011175–676577620964580
- StevensMMGeorgeJHExploring and engineering the cell surface interfaceScience200531057511135113816293749
- DalbyMJGadegaardNTareRThe control of human mesenchymal cell differentiation using nanoscale symmetry and disorderNat Mater2007612997100317891143
- WangGZhengLZhaoHIn vitro assessment of the differentiation potential of bone marrow-derived mesenchymal stem cells on genipin–chitosan conjugation scaffold with surface hydroxyapatite nanostructure for bone tissue engineeringTissue Eng Part A2011179–101341134921247339
- LiaoFChenYLiZA novel bioactive three-dimensional β-tricalcium phosphate/chitosan scaffold for periodontal tissue engineeringJ Mater Sci Mater Med201021248949619908128
- TourGWendelMMollGTcacencuIBone repair using periodontal ligament progenitor cell-seeded constructsJ Dent Res201291878979422736447
- ChengZGuoCDongWHeFMZhaoSFYangGLEffect of thin nano-hydroxyapatite coating on implant osseointegration in ovariectomized ratsOral Surg Oral Med Oral Pathol Oral Radiol20121133e48e5322669157
- GuoJWangYCaoCDziakRPrestonBGuanGHuman periodontal ligament cells reaction on a novel hydroxyapatite–collagen scaffoldDent Traumatol201329210310922681634
- GeSZhaoNWangLBone repair by periodontal ligament stem cell-seeded nanohydroxyapatite–chitosan scaffoldInt J Nanomedicine201275405541423091383
- SimmonsPJTorok-StorbBIdentification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1Blood199178155622070060
- NehrerSBreinanHARamappaAMatrix collagen type and pore size influence behavior of seeded canine chondrocytesBiomaterials199718117697769177854
- O’BrienFJHarleyBAYannasIVGibsonLJThe effect of pore size on cell adhesion in collagen–GAG scaffoldsBiomaterials200526443344115275817
- BeckGRJrInorganic phosphate as a signaling molecule in osteoblast differentiationJ Cell Biochem200390223424314505340
- BeckGRJrMoranEKnechtNInorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2Exp Cell Res2003288228830012915120
- DalbyMJBiggsMJGadegaardNKalnaGWilkinsonCDCurtisASNanotopographical stimulation of mechanotransduction and changes in interphase centromere positioningJ Cell Biochem2007100232633816888806
- YimEKDarlingEMKulangaraKGuilakFLeongKWNanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cellsBiomaterials20103161299130619879643
- ByersBAGarciaAJExogenous Runx2 expression enhances in vitro osteoblastic differentiation and mineralization in primary bone marrow stromal cellsTissue Eng20041011–121623163215684671
- SasanoYZhuJXKamakuraSKusunokiSMizoguchiIKagayamaMExpression of major bone extracellular matrix proteins during embryonic osteogenesis in rat mandiblesAnat Embryol (Berl)20002021313710926093
- ZaidiSKJavedAChoiJYA specific targeting signal directs Runx2/Cbfa1 to subnuclear domains and contributes to transactivation of the osteocalcin geneJ Cell Sci2001114Pt 173093310211590236