Abstract
Due to their ability to replicate the in vivo microenvironment through cell interaction and induce cells to stimulate cell function, three-dimensional cell culture models can overcome the limitations of two-dimensional models. Organoids are 3D models that demonstrate the ability to replicate the natural structure of an organ. In most organoid tissue cultures, matrigel made of a mouse tumor extracellular matrix protein mixture is an essential ingredient. However, its tumor-derived origin, batch-to-batch variation, high cost, and safety concerns have limited the usefulness of organoid drug development and regenerative medicine. Its clinical application has also been hindered by the fact that organoid generation is dependent on the use of poorly defined matrices. Therefore, matrix optimization is a crucial step in developing organoid culture that introduces alternatives as different materials. Recently, a variety of substitute materials has reportedly replaced matrigel. The purpose of this study is to review the significance of the latest advances in materials for cell culture applications and how they enhance build network systems by generating proper cell behavior. Excellence in cell behavior is evaluated from their cell characteristics, cell proliferation, cell differentiation, and even gene expression. As a result, graphene oxide as a matrix optimization demonstrated high potency in developing organoid models. Graphene oxide can promote good cell behavior and is well known for having good biocompatibility. Hence, advances in matrix optimization of graphene oxide provide opportunities for the future development of advanced organoid models.
Introduction
Many researchers focus on developing 3D cell culture models for organoid production since they provide advantages as valuable tools for studying disease mechanisms and testing potential treatments. The 2D cell culture does not replicate the in vivo microenvironment, as cell–cell interactions are limited and a tissue-specific architecture is lacking.Citation1 Moreover, study with references revealed that the 2D cell culture models have some disadvantages, such as the inability to control cell shape or loss of cell regulatory abilities, limitation to single-cell types, and inability to reflect the complexity of physiology in tissues, leading to bias in predicting specific tissue response.Citation2–6 Therefore, 3D culture is introduced as an alternative solution to address these disadvantages. Recent studies demonstrated that the 3D cell culture model promoted an environment that mimics an in vivo situation, stimulated cells by enhancing cell proliferation, migration, matrix creation, stem cell differentiation, and even replicated an environment of a particular tissue’s physiology and pathophysiology.Citation5–8 An organoid is a complex three-dimensional structure that can simulate various interactions, including those between cells and the matrix, the physiological function of a particular tissue that can imitate the physiology of an organ, and the composition of different cell types that can sustain cell function.Citation9–11 Organoids have demonstrated promising outcomes in recreating the native organ through metabolic activity, gene and protein expression, and tissue microfabrication.Citation12 Organoid models have been constructed for disease models that have explored pathologic agents, such as viruses, bacteria, and parasites, that cause the disease.Citation13–29 Nevertheless, there are currently certain obstacles preventing the growth of organoid needs. The drawback of organoid development is due to high cell diversity, survival in the culture system, as well as maturity and function limitations.Citation12
One of the significant elements in promoting cell differentiation and potentially reducing the populations of dying cells is the physics and chemistry of matrix optimization’s characteristics.Citation30,Citation31 The results of organoid culture are affected by matrix optimization.Citation32,Citation33 The primary function of the cell’s matrix is to sustain and promote cell development. Most organoid cultures have recently been cultivated in a matrix using matrigel.Citation34–37 Matrigel is a commercially available matrix generated from a mouse tumor that contains high levels of laminin, collagen, and heparan sulfate proteoglycans mixed with other small amount of ECM proteins and growth factor.Citation38,Citation39 Nevertheless, matrigel is limited in its ability to selectively develop organoid culture due to its varied composition. The complex composition of matrigel and thousands of identical peptides in matrigel can cause high abundances of undissolved matrigel proteins, resulting in less identification of organoid proteins; organoid proteins may be misidentified due to identical peptides from matrigel contaminants; and the abundances of organoid proteins uniquely may be estimated with bias in proteomic analysis, even the complex composition can influence cell culture in unexpected ways.Citation40,Citation41 Moreover, the complex composition of matrigel may interfere to cell behaviour, making it difficult to distinguish biological effect, and even xenogenic contaminants were detected in matrigel may restrict therapeutic potential of cell or tissue culture.Citation42 Therefore, it is crucial to develop materials as alternative materials of matrigel for organoid cultivation. However, organoid research is still in its infancy, with preliminary exploration of construction strategies, assessment, and application methods.
This review aims to investigate the significance of the latest advances in developing alternative materials for matrigel in cell culture applications and how they enhance build network systems by generating proper cell behavior. This review will focus on recent advances in using materials as an alternative for matrigel in organoid culture applications, such as tissue engineering and even disease therapy. We highlight critical studies for each application in which the materials used as matrix were directly compared to matrigel to observe cell characteristics as one of the factors to see the effects of matrix on a suitable microenvironment. Aside from that, we examine cell behavior and other factors such as cell proliferation, differentiation, and gene expression. Also, we study how they can be utilized to develop tissue systems that resemble the structure of native tissue. In the present review, we focused on graphene oxide or combined graphene oxide, as one of the materials that have succeeded in developing in the biomedical field, such as potential drug deliveryCitation43–45 and even tissue engineering.Citation46–49 Finally, we discuss the current situation of alternative materials to matrigel and give the author’s perspective on the graphene oxide potency for developing organoids with additional model applications such as investigating and studying disease, drug discovery, and even vaccine development.
Materials and Methods
Based on the purpose of this review, we have three research questions that we will explain in this review: (1) Does alternative matrigel, matrigel or combined graphene oxide in organoid culture create a more suitable microenvironment, (2) How do effects of cell proliferation, cell differentiation, and gene expressions as further parameters of behavior cell sign on organoid culture, (3) How many doses of graphene oxide have shown non-toxicity in organoid culture.
Study Design and Literature Search
A systematic literature review based on The Preferred Reporting Items for Systematic Review (PRISMA).Citation50 The searched electronic databases used SpringerLink, ScienceDirect, and Google Scholar with English language restrictions. The following keywords were used: “organoid AND cell culture AND graphene oxide OR graphene AND matrigel OR alternative matrigel AND cell behavior AND host pathogen”. We use Boolean operators OR and AND within our search.
Study Selection and Eligibility Criteria
The search was restricted to experimental studies in English published in 2012–2024, article types, area subjects, or knowledge sciences. The search results was screened by looking at article titles, abstract records, and duplicates within Mendeley software. After screening articles based on article titles and abstract records, the full text of these articles was screened based on a reference list of inclusion and exclusion criteria, as summarized in .
Table 1 Inclusion–Exclusion Criteria
Results
Search Results
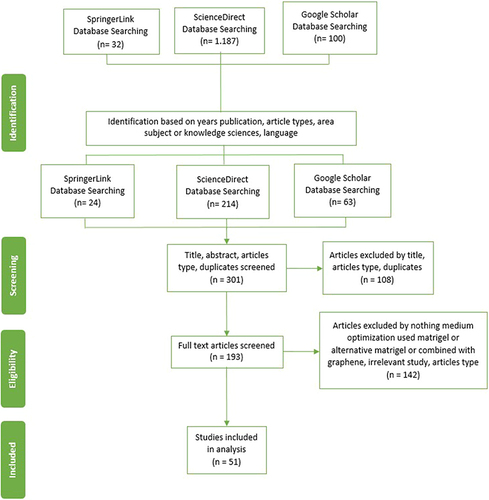
For the first search strategy, 1.319 articles were received based on years of publication in 2012–2024. Of which, 1.319 were screened based on article types, years of publication, subject area or knowledge sciences, and language. Of the remaining 301 articles, titles, abstracts, article types, and duplicates were screened, and then 193 articles were screened for full text. After reviewing the full text, 142 articles were screened based on inclusion and exclusion criteria. The remaining 51 articles were included in the analysis. The flow diagram of the results of the screening process is illustrated in .
Figure 1 Article Screening Process based on PRISMA Guidelines.Citation50
Included Studies
All 51 articles were studied in biomedical applications. Of the 32 articles related to tissue engineering or regeneration,Citation51–80 five articles refer to therapeutic agents,Citation81–85 two articles related to cancer therapy,Citation86,Citation87 1 article refers to skin therapy,Citation70 1 article refers to biosensing system,Citation88 and 10 articles refer to organoid models.Citation89–98 Matrix optimization with matrigel or alternative matrigel or combined with graphene was assessed in 51 articles. The quantity of matrigel was depicted in 13 studies,Citation53–58,Citation80,Citation88,Citation94–98 alternative matrigel in 17 studies,Citation51,Citation52,Citation63,Citation74–79,Citation81–83,Citation89–91,Citation93 and combined with graphene in 21 studies.Citation56,Citation58–62,Citation64–73,Citation84–87 Generally, all 51 articles will describe matrix optimization that affects the microenvironment in vitro, such as cell characteristics, proliferation, differentiation, and gene expression. In 18 studies, it is referred to graphene combinations, which will be presented more in detail in this review based on in vitro cell culture.Citation56,Citation58,Citation60–62,Citation64,Citation66–73,Citation84–87
Graphene Oxide as Nanomaterial for Biomedical Application
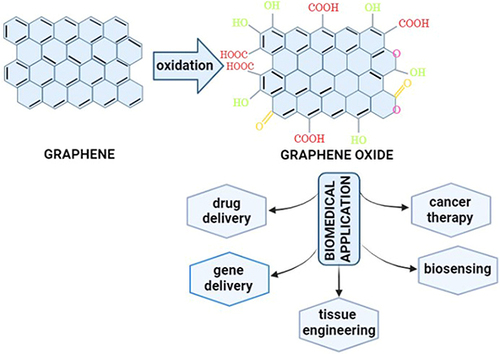
Graphene is a single layer made from sp2-hybridized carbon atoms arranged in honeycomb lattice structures.Citation99,Citation100 The sp2-hybridized carbon atoms are garnering attention in the field of biomedicine, including biosensing, drug delivery, bioimaging, tissue engineering, antimicrobial agents, and even cancer therapy.Citation101,Citation102 However, two-dimensional (2D) graphene lattice structures have some drawbacks, such as unstable chemical formations and a limited ability to interact with other molecules or materials due to graphene’s limited active component, resulting in incompatibility.Citation103 Graphene oxide (GO), a member of the graphene family, is produced via oxidation using strong oxidizing agents.Citation100 Oxidation methods for generating GO include Staudenmaier, Hofmann, Tour, and Hummers.Citation104 The Hummers method is the most often employed to produce GO by giving graphite a chemical treatment through oxidation, then dispersing and exfoliating in water or organic solubility.Citation105 Furthermore, the Hummers method is the most widely used and developed because it produces high-quality GO quickly.Citation106,Citation107
GO possesses physicochemical properties and biocompatibility due to its unique structure, which is a graphene basal plane attached with functional groups like hydroxyl (OH) and carboxylic (COOH), which leads to further functionalization and conjugation or immobilization of other nanoparticles on its surface.Citation108–112 In addition, the GO surface’s morphology, shape, size, and functional groups stimulate biological activity and may even improve cell–cell interactions.Citation113 Because GO has excellent surface functionality and is rich in oxygen-containing groups, it can be easily modified with other materials, improving solubility, selectivity, and biocompatibility.Citation100,Citation102,Citation114–116 Also, GO possesses amphiphilicity properties, which means it can be hydrophobic or hydrophilic, as well as a high affinity, which makes it helpful in interacting with biomolecules such as enzymes, peptides, DNA, and proteins in the construction of 3D models.Citation103,Citation113 Interestingly, because GO is photoluminescent and permeable to cell membranes, it can be used in drug delivery and gene delivery.Citation117 As a result, GO has been predominantly used in biomedical applications such as tissue engineering, cancer therapy, drug delivery, gene delivery, and biosensing,Citation108,Citation118–120 as illustrated in .
Graphene and graphene derivatives have been used to create a variety of biosensors due to their outstanding sensing performance (high specific surface area, extraordinary electronic properties, electron transport capabilities, and ultrahigh flexibility).Citation121 Also, due to hydrophilic oxygen-containing functional groups, GO has good water dispersibility, biocompatibility, and high affinity for certain biomolecules.Citation122 Graphene-based biosensors provide quantitative detection of cancer-related biomarkers such as DNA, miRNA, small molecules, and proteins.Citation121 Thus, GO as biosensors have been applied in surface plasmon resonance (SPR),Citation123 fluorescence resonance energy transfer (FRET)Citation124, and electrochemical-based techniques.Citation125 On the other hand, GO typically contains both hydrophobic and hydrophilic regions. In the hydrophobic region, the π–π conjugated system on the surface makes it capable of connecting multiple molecules through the non-covalent bond interactions.Citation126,Citation127 Moreover, GO has better water solubility than graphene due to its abundant hydrophilic groups, such as –O–, –COOH, –OH. These groups could form the hydrophilic region, which allows further functionalization by attaching to various molecules including protein, DNA, and RNA.Citation128,Citation129 Thus, GO met for developing future anticancer graphene drug delivery since an efficient accumulation of anticancer drugs in tumor targets/tissues, controlled cellular uptake properties, tumor-targeted drug release behavior, and selective toxicity toward the cells.
GO modified with a non-toxic cationic material and a tumor-specific monoclonal antibody (anti-EpCAM) for the delivery of survivin-siRNA (GCE/siRNA) had a strong antitumor effect in vitro, which was attributed to GCE/siRNA’s efficient antiproliferation, migration, and invasion inhibition effect.Citation85 The presence of several hybrid layers of carbon atoms (sp2) of graphene oxide has a large surface for highly efficient drug loading.Citation127 Besides that, the high degree of functionalized graphene oxide (GO) nanoparticles improves intelligent controlled release and gene silencing capability.Citation130 Moreover, GO is an effective nanocarrier that allows for the targeted delivery of small drug molecules, antibodies, nucleic acids, and peptides to the liquid or solid tumor sites.Citation130 Therefore, graphene oxide can play a significant role as a drug delivery system in gene delivery, and cancer therapy, especially as a drug carrier.Citation127,Citation130,Citation131
Tissue engineering involves the development of biotechnologically produced and functional tissues and organs for repair or replacement. Graphene oxides have excellent physical, physicochemical, and biological properties for biomedical applications due to their large surface area and ability to interact with proteins and peptides.Citation132 Therefore, GO has been successfully used to optimize scaffold architectures for a variety of organs, from skin to heart tissue.Citation113 The protein-graphene oxide structures formed either as nanocomposites or as biocomplexes. It is emphasized that the effects of carbon-containing nanostructures on protein conformation and structural stability are being investigated for applications in tissue engineering and regenerative medicine.Citation133,Citation134 The biological activity of GO nanocomposite bioconjugates also plays an important role in terms of cell viability and proliferation, as well as the ability of these constructs to sustain the formation of new and functional tissue.Citation133
Cell Behavior
For many years, in vivo animal experiments or animal models have been used in biomedical research to improve the quality of life of humans and animals through toxicity tests, diagnostic tools, and the development of new drugs or vaccines.Citation135 Furthermore, in vivo experiments were used for the characterization and analysis of molecules and biological systems, such as molecular markers for neurodegenerative diseases, models for cancer treatment, and clinical tests of drugs.Citation136–140 However, in vivo testing or animal models concern ethics, require more expenses, and involve multiple cells and agents, thereby impeding the evaluation of the intended effect,Citation141 whereas in vitro experiments do not require the technical skills needed when handling animals and are generally less expensive, quicker, and easier to perform and quantify.Citation142 Recent studies have shown that animal organoid models (eg, prostate cancer, bladder cancer) can represent tumors in vivo.Citation143,Citation144 In addition, organoid models can be used in the modeling of diseases and in transplantation or replacement studies.Citation145 Therefore, 3D culture or organoid models provide some aspects of the tissue microenvironment that could potentially be used for in vitro phenotyping to mimic in vivo situations and reduce in vivo animal experiments or animal models.
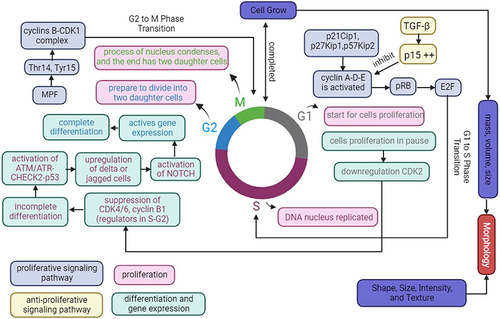
In vitro cell cultures are required for various biomedical applications, including cellular and organismic biology, drug discovery, and regenerative medicine.Citation146 3D cell culture enhances physiological formation and allows cells to recapitulate many tissue features such as shape, proliferation rate, differentiation, and even gene expression.Citation147–150 Previous studies on disease models have shown that organoids as 3D models are more physiology-relevant and pathologic. Cell number, viability, morphology, proliferation, differentiation, gene expression, and even in vivo relevance were improved by the 3D model.Citation151 Therefore, organoids as 3D models are considered capable of being developed in such a way that the unique microenvironment that occurs in vivo can be imitated the existing in vitro. is an illustration of the concepts of morphology, proliferation, differentiation, and gene expression as biological mechanisms for optimizing cell behavior in cell culture.
Cell morphology is an important aspect corresponding to the cell-cultured to understand cell behavior, such as the cell phenotype,Citation152 and it is even associated with cell functions.Citation153 The morphological characteristics of cells are assessed based on their form, size, intensity, and texture of cellular compartments.Citation154 Animal cell culture, particularly mammalian cell culture, has been classified into three fundamental types based on shape and appearance: fibroblastic, epithelial, and lymphoblastic.Citation155 Fibroblastic cells grow connected to substrates and have elongated forms. Epithelial cells have polygonal shapes with more uniform dimensions and grow in discrete patches adhering to the substrate. Lymphoblast cells are spherical form and grow in suspension, unattached to a surface. Cell shape and growth are classified according to the type of cells developed in cell culture. Cell morphology is also linked to cell proliferation since it regulates the expansion of their mass, volume, and size.Citation156 Therefore, growth speed is the time derivative of mass or volume [dV/dt], and mass or volume-specific growth rate is the growth speed divided by mass or volume [1/V dV/(dt)].Citation156
Cell growth requires proliferation. Proliferation is a process in which cells must duplicate the new cell by growing and then dividing into two equal copies by passing through the cell cycle, which includes the G0 phase, G1 phase, S phase, G2 phase, and M phase.Citation157,Citation158 Most cells in the first are stable and are in the gap (G0) phase of the cell cycle, also known as the resting cell period.Citation157,Citation158 When cell division is required, the cell will come into the G1 phase of the cell cycle.Citation157 The S (synthesis) phase occurs when DNA in the nucleus is reproduced for a limited portion of the cell cycle. The cell will then enter the second gap (G2) phase and then will prepare to divide into two daughter cells during the M (mitosis) phase. The cell will enter the M phase, in which the contents of the nucleus condense to form a visible chromosome that is pulled apart into two equal sets via an elaborately organized series of movements. In the end, the cell divides into two daughter cells. Furthermore, the M phase is divided into five stages: prophase, prometaphase, metaphase, anaphase, and telophase.Citation158
Prophase is the stage during which the paired chromosomes that were duplicated during S phase initiate condensing to form sister chromatids. Then, condensing complexes and histone phosphorylation are employed to condense chromatin, while the nuclear membrane begins to break down, and these steps are driven by the cyclin B/cyclin-dependent kinase 1 (CDK1) complex working in tandem with the Polo-like kinases (Plks). Prometaphase is the next stage in which the nuclear membrane disappears, and the spindle begins to assemble. The chromatids then align in one plane at the metaphase plate, a process known as metaphase steps. The chromatids are suitably positioned to promote the anaphase-driven separation process. As the chromosomes separate and migrate to the spindle poles, the process of cell cleavage, known as telophase, begins.
Cell proliferation is controlled by the proliferative signaling system, which propels cells into the cell cycle by activating processes early in G1, with cyclin D activation controlling the G1 phase.Citation158 The obligation to divide occurs during the G1 phase, which is regulated by cyclin-D-CDK4/6 and cyclin-E-CDK2.Citation159 Cyclin D-CDK4 and cyclin D-CDK6 have functions in the phosphorylation of retinoblastoma protein (pRB), while cyclin E-CDK2 contributes to pRB phosphorylation in late G1, which promotes cell entry into the S phase.Citation160 Furthermore, cyclin D, E, and A are regulated by the CDKI family, which includes p21Cip1, p27Kip1, and p57Kip2, whereas p21Cip1 expression is regulated by p53.Citation161 pRB regulates the G1 phase, which suppresses the activity of E2F transcription factors in mitotic cells.Citation159 As a result, the pRB-E2F pathway regulates the transition from G1 to S. The G2 to M phase transition is then mediated by the cyclin B-CDK1 complex, previously known as maturation (mitosis) promoting factor (MPF), which is dephosphorylated at Thr14 and Tyr15 by the phosphatase CDC25.Citation159,Citation160,Citation162 Furthermore, CDK1-cyclin B phosphorylates activate Wee1/Myt1.Citation163 Wee1 and Myt1 hinder mitotic progression by monitoring the G2/M phase checkpoint and providing inhibitory phosphorylation to CDK1.Citation160,Citation164 These inhibitors role significant functions in cancer cells and can even be used as cancer therapy targets.Citation164 While there are anti-proliferative signaling pathways, transforming growth factor-β (TGF-β) inhibits cell cycle entry and even encourages cells to differentiate.Citation158 TGF- β, smad signaling pathway, suppresses cell proliferation by up-regulating the CDK inhibitor p15, which inhibits cyclin E and cyclin A and even prevents the formation of transcription factor Myc, which is a key regulator of cell proliferation.Citation158
Differentiation leads to different specialized cell types, commencement through the progressive difference of developmental pathways, and completion through the consecutive programming and final elaboration of each functional cell type.Citation165 Differentiation is regulated by the cell cycle through cell cycle regulators, which directly affect the expression of differentiation genes.Citation166 The cell cycle initiates as daughter cells enter the G1 phase, which has been particularly permissive to differentiation signals and even commits to specific lineages.Citation166 While cell proliferation is paused in G1, cells remain in G1, committing to terminal differentiation into a functional cell type.Citation166,Citation167 Several cycle regulators, such as CDK2, CDK4, and CDK6 proteins, remain relatively constant at steady levels during the normal cell cycle and in quiescent, aging, and even terminally differentiated cells.Citation159 Down-regulation of CDK2 lengthens the G1 phase, resulting in differentiation, while during G1 cell cycle progression, it is mediated by the activation of CD4/6.Citation168–170 Whereas suppression of CDK4/6, cyclin B1, a regulator in the S and G2 phases, led to a rapid decrease in expression and also activated the ATM/ATR-CHECK2-p53 by incomplete differentiation to prevent abnormal cell generation.Citation171,Citation172 Additionally, DNA methylation and histone marks are inherited in S and G2 phases, indicating regulation of differentiated phenotype.Citation172 Then, cells commit to differentiation by activating notch signaling by upregulating delta or jagged cell surface ligand, which then transits to the nucleus, activating gene expression.Citation166 Therefore, cell differentiation is completed in G2 phase through cell cycle dependent.
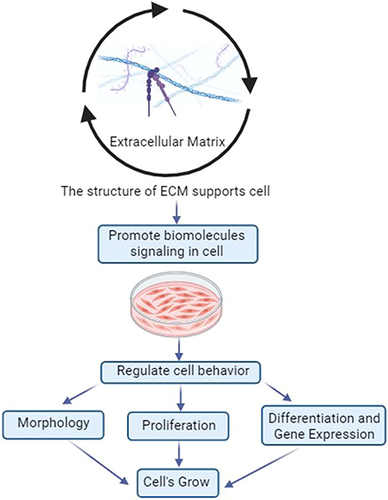
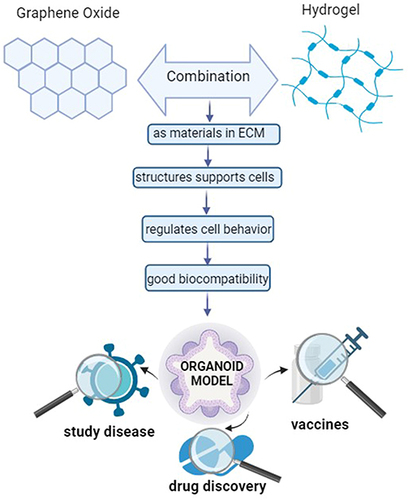
ECM roles include providing structural support cells, which have an impact on morphology, promoting cell proliferation; organizing to facilitate tissue function, which has an impact on cell differentiation, and regulating gene expression.Citation8,Citation173–175 The ECM surrounding the cells influences the topology of cell-cell, cell-matrix contact, and distribution of the signaling biomolecules.Citation176–178 The cell–matrix interaction begins cell adhesion by integrin, and then the ECM can exert physical effects to regulate cell topology by anchoring, signal transmission, and cell migration.Citation179 In addition, cell migration during environmental cues can be biochemical or physical, which can regulate differentiation.Citation180–182 Physical properties trigger mechanical cues so the ECM can regulate morphology.Citation179 Cell morphology is maintained by the regulation of gene expression via cell interactions and transcriptional regulation.Citation183,Citation184 Therefore, the structure of the ECM supports biomolecule signaling, which is integrated by the intracellular signaling pathway and provides for cell growth, proliferation, differentiation, morphology, and gene expression,Citation185 as illustrated in .
Organoid models were developed from stem cells and are grown in in vitro culture systems with extracellular matrix (ECM).Citation186 In addition, matrigel has been employed in a variety of applications including tissue engineering, assays, and organoid assembly.Citation187,Citation188 Matrigel is a gelatinous ECM protein mixture obtained from the murine Engelbreth–Holm–Swarm sarcoma.Citation189,Citation190 However, matrigel derived from the Engelbreth-Holm-Swarm mouse sarcoma exhibits significant batch-to-batch variation in grown organoids.Citation191 Furthermore, tumor matrisomes differ significantly from normal tissue, suggesting that ECM-based matrigel may not provide a tissue-specific microenvironment for organoids.Citation192 Therefore, this work has shown an alternative to matrigel, as summarized in .
Table 2 The Effects on Cell Characteristics for Cells or Organoid Culture
Study Outcomes and Discussion
Alternative Matrigel in Cell Culture or Organoid
Alternative matrigel can be used as a matrix to have effects on the microenvironment in vitro. The previous studies showed alternative materials such as gelatin, GelMa, collagen, Se-EX-Ch-COL/TA, Col-Ch, chitosan-pectin, PAMB-C-OHA, PLA-HA, PEG, GG, IGF1c-PHM, PUCL@CNT, CPSN, and RSF/Mxene,Citation51,Citation52,Citation63,Citation74–79,Citation81–83,Citation89–91,Citation93 as summarized in . The use of gelatin showed that cell aggregation is characterized by spindle-round or spheroid structures.Citation81,Citation89 These characteristics characterize this type of three-dimensional cell culture, thereby promoting an in vivo-like environment. Besides that, GelMa showed that colonoid formation is new when it is applied to organoid models.Citation90 However, the number of organoid cells in GelMa is less than that in gelatin, which is applied to organoid models. The number of organoid cells in GelMa is around 30,Citation90 while the number of organoid cells in gelatin is 39.Citation89 Similar results for collagen showed that it has characteristics of three-dimensional cell culture,Citation51,Citation82,Citation83 and even it is applied to organoid models, which shows that it can maintain cell phenotype,Citation91 and even assisted cECS-like structure formation.Citation52 Furthermore, interaction of collagen combination has an effect on tissue regeneration, which is implicated by increased cell migration.Citation52 Furthermore, cell migration is fundamental to establishing and maintaining the proper organization of multicellular organism.Citation193 In multicellular organisms, cell migration, and collective cell behaviour are essential mechanisms in morphogenesis for development. Therefore, 3D organoid culture requires collective behaviours to emerge with proper three-dimensional (3D) tissue organisation and cellular composition.Citation194
Similar results showed that chitosan combinations have effects on cells that mimic the structures of native cellsCitation63 and even cells formed spheroid as characterized by 3D models.Citation92 Additionally, it was shown that chitosan-based hydrogel formulations loaded with secretomes due to hydrophilic and hydrophobic interaction, which promotes cell migration, so it supported tubule-like blood vessel network formation for HUVECs and even elongated shaped for H9C2 cell.Citation63 Despite the fact that the chitosan combination has been successfully used for developing 3D models, the high hydrogel formulation showed that the morphology of the spheroids grown was irregular and highly heterogeneous.Citation92 Other studies showed that polycaprolactone (PCL) combination improving flexibility, so it has better physical properties and is supported for tissue manipulation and adaptation.Citation74 Additionally, silk fibroin combined with PCL improved hydrophilicity and flexibility.Citation75,Citation76 Therefore, PCL combination assistance for neuron-like structures formation,Citation74 myocardium-like structures,Citation75 and tube formation produces more honeycomb-like structures of HUVECs.Citation76 In addition to PLA-HA, PEG, GG, and also IGF1c-PHM have shown previous similar results such as forming HUVEC-like structures,Citation77 cells formed fibroblast-like,Citation78 neurospheres formation,Citation79 and even maintaining phenotype cells on organoid models.Citation93 However, the materials used as a matrix that have been developed for organoid models are still challenging, such as the fact that only a minority of cells are expressed,Citation91 histological processing is related to the difficulty of staining cells cultured on the hydrogel,Citation93 and mutation expression is insufficient to confer phenotype cells on organoid models.Citation89
Furthermore, matrigel is currently being used as a matrix for organoid models. AT2s and ileum organoids showed that formed cell epithelial characteristics successfully.Citation94,Citation97 Crypts and adults jejunum organoid showed that this model can remain viable after added bacteria.Citation95 Similar results were found for organoid models, as 3D models that improved the in vivo microenvironment showed that they formed neuroepithelium and rosette-like structures.Citation96 However, the development of organoid models showed that it is unlikely to faithfully recapitulate infection physiology when the virus acts as a pathogen is treated,Citation94 and only measurement showed viable models after being treated with bacteria after one day.Citation95 Besides that, matrix optimization with the various added biopolymers to matrigel is developing recently,Citation53–58,Citation80,Citation88,Citation98 as summarized in . The addition of biopolymers to matrigel, such as collagen-manuka honey,Citation80 PSMPs@B,Citation98 OEO-BG-PLGA/Gel,Citation53 Gel-TCP/SC,Citation54 EPO/CNP@FPH,Citation55 dL-EG,Citation56 MNSs-CNT-COOH,Citation88 AG-PCL,Citation57 and BG-GOCitation58 that is used for developing matrix for tissue engineering, regeneration, and even organoid models successfully.
Previous studies showed that there are challenges with the various added biopolymers such as the high concentration of manuka honey added to collagen-inhibited vessel formation,Citation80 the number of damaged organoids was higher after added PSMPs@B due to benzo [a] pyrene (B [a] P) enhancing toxicity,Citation98 and the high concentration of bioceramics, BG and β-TCP, did not significantly promote angiogenicity due to their toxicity, inhibitory effect on cell chemotaxis, and weak osteoinductive activity.Citation53,Citation54,Citation58 Additionally, the optimization matrix with EPO/CNP@FPH shows that it can promote the function of HUVECs, as it is signed by tubule-like blood vessel network formation and even it had effects on osteogenesis under inflammatory conditions, but it was absent in the normal condition.Citation55 A similar result showed that the optimization matrix used AG-PCL promoted the maturation of ISGCs, but further verification of their significance is needed due to complexity interaction.Citation57 Interestingly, the added graphene to matrigel had good biocompatibility, showing that GO and graphene promoted growth cells and even successfully mimicked native cells such as neuron formationCitation56 and endothelial structure branch formation.Citation58 The overall results of the studies showed that matrigel can help microenvironment in vitro related to cell characteristics similar in vivo, especially for developing organoid models.Citation94–98
Furthermore, based on this work, there are some alternative matrigel that have positive effects on cell characteristics for cell culture or organoid culture, such as gelatin, chitosan, polycaprolactone, collagen, polyethylene glycol, hyaluronic acid, ceria nanoparticle, graphene oxide, alginate, and carbon nanotubes.Citation51,Citation52,Citation55–58,Citation63,Citation74–79,Citation81–83,Citation88,Citation90,Citation93,Citation95–97 Besides that, some alternative matrigel showed negative effects on cell characteristics, such as gelatin, collagen, matrigel, optimization manuka honey, and benzo [a] pyrene (B (a) P)-loaded polystyrene microplastics, as summarized in . Citation80,Citation89,Citation91,Citation92,Citation94,Citation98 Interestingly, graphene oxide has no negative effects on cell characteristics at a certain content.Citation58 In addition, graphene oxide will be explained next section.
Materials used in graphene combinations are currently in development for tissue engineering and regeneration, therapeutic agent, and even cancer therapy.Citation59–62,Citation64–68,Citation70,Citation84,Citation86 Furthermore, combination graphene showed that cell characteristics promoted cell elongation,Citation64,Citation66,Citation84 maintained cell phenotype,Citation64,Citation68,Citation84 formed spheroidCitation61 and neurospheres,Citation59 grew like native cells,Citation60,Citation62,Citation64,Citation65,Citation67,Citation70 and even promoted breast cancer cells metastated.Citation86 Furthermore, implanted rGO did not induce DNA breakage genotoxicity at the local and systemic levels, so it can maintain cell morphology.Citation65 Various cell formations are obtained by emulating the native cells as summarized in . Additionally, the GO combination improves viscoelastic properties due to the anionic interaction of the GO and the cationic interaction of the biopolymer, so it may allow cells to mimic native cells.Citation64,Citation70 GO combination promotes migration activity of cells, reduces and even inhibits apoptotic activity, so it gives effects on cellular spheroid formation.Citation61,Citation70 Besides that, the GO combination possessing physical antibacterial function is needed for wound healing dressing application.Citation70
Table 3 The Graphene Oxide and Their Composite to Microenvironment in Cell Culture or Organoid Culture
Both the GO and rGO combinations showed that they assisted cells grow like native cells.Citation60,Citation64–67,Citation70,Citation84 The growth cell depends on contact-mediated cues which can be delivered by graphene combinations and diffusible cues in the medium.Citation67 Both GO and rGO also offer unique topographic properties that influence the cell’s biological response creating physicochemical properties in combination with additional biopolymers that allow easy tissue modification which is required in tissue engineering applications.Citation84 Additional biopolymer-to-graphene combinations also improve their mechanical properties,Citation132 such as hydrogel may support the formation of scaffolds,Citation64 and resemble MSU-1.1 cell, HeLa cell, and SH-SY5Y cell to receive the well native extracellular matrix (ECM) environment.Citation68 Therefore, graphene in combination with proper biopolymer is a potential material enabling development of organoid models.
Cell Behavior on Graphene-Based Organoid Models
Various studies investigate the effects of graphene on cell behavior as an evidence rule on improving a suitable microenvironment, as summarized in .
Table 4 Effects of Graphene Combination as Matrix Towards Proliferation, Differentiation, and Gene Expression
Cell Proliferation
All ten studies showed that graphene combinations affected cell proliferation. Graphene combinations such as graphene, GO and rGO showed that they had cell proliferation capabilities. Both GO and rGO showed that the cell proliferation ratio is 1 and even greater than.Citation60,Citation66,Citation73 Nevertheless, other studies showed that GO combinations had cell proliferation ratio less than 1.Citation58,Citation70,Citation87 Cell proliferation of BG/GO showed that the low GO content promoted cell proliferationCitation58 and similarly on GO combinations received similar result.Citation87 Other study showed that cell proliferation ratio less than 1 due to post-irradiation, indicating that radiation severely thwarted cellular growth, though growth cell for irradiated reverted to normality after extended timeframes.Citation70 However, GO showed a greater cell proliferation ratio than less rGO and graphene,Citation62,Citation68,Citation69,Citation84 in addition cell proliferation ratio with GO more than 1 is obtainedCitation69 than proliferation ratio of rGO, and graphene is more than 0.8Citation75,Citation84 and more than 0.5,Citation62 respectively. Suitable environment for proliferation cells possibly due to the increase in cell’s adhesion greater than 1000% in the present of GO because of surface functional chemical compare to rGO.Citation64,Citation69,Citation84,Citation195 Furthermore, enhanced addition properties promote cell–surface interactions, derived from an excellent ultrathin topological substrate with cell contact adhesion ability.Citation69 Besides that, the GO combination enhanced the ductility, so it displayed mechanical properties, which is beneficial for practical applications that allow cell culture.Citation70 Hydrophilic properties of graphene oxide consider responsible in cell culture medium preventing cell agglomerationCitation84 which may limit the nutrient supply and even induced oxidative stress which triggers the apoptotic pathway.Citation196 In contrast, rGO is hydrophobic which is usually promotes agglomerates in water solutions.Citation197
Additionally, graphene oxide with chitosan and a tumor-specific monoclonal antibody (anti-EpCAM) for the delivery of survivin-siRNA give tumor-targeting effectsCitation85 that cause inhibition proliferation cells. Other study reported that xanthohumol (XN) modified with PEGylated (PEG) and GO (PEG-GO@XN) can inhibit breast cancer cells.Citation86 Therefore, it reduces the production of ATP, blocks the migration and invasion of cancer cells even can suppress the metastasis of cancer cells.Citation86 Interestingly, the GO combination promoted metastatic breast cancer cells, but after adding xanthohumol (XN) to GO, it provided suppression of metastatic tumors.Citation86 Furthermore, the graphene oxide-based surfaces may influence the therapeutic agent’s applications, such as small flakes and thick graphene layers. Interestingly, small flakes of graphene oxide are 0.2–2 µm can reduce cell proliferation and induce their apoptosis.Citation84 While the most suitable surfaces for cells of graphene oxide layer thickness are 10 and 15 µg/cm2.Citation84 Similarly, an average thickness of GO of 2 nm shows biocompatibility indicated by no apparent cytotoxicity.Citation86
Cell Differentiation and Gene Expression
The biocompatibility of biomedical materials is the most important requirement. The combination of graphene has great potential for nanomedicine and biomedical applications. Recent studies have shown that graphene combinations have been widely explored for bioimaging, biosensing, and antibacterial applications, as summarized by Bellier et al.Citation198 Interestingly, there is a new study showing that inhalation of GO in humans is well tolerated and has no adverse effects.Citation199 Therefore, graphene combinations have been considered as biocompatible materials. In this review, graphene combinations have supported biomedical applications such as tissue engineering and regeneration, cancer therapy, and even therapeutic agents, as summarized in . Furthermore, graphene combinations provide a microenvironment necessary for cell differentiation and gene expression, which are important for tissue engineering. Appropriately engineered graphene materials are not only biocompatible but often greater when it comes to creating the microenvironment necessary for cell growth, differentiation, and development.Citation200–202
Cell differentiation is needed for different functions to develop multicellular cells. Therefore, cell differentiation is a cellular process for developing cell types, with appropriate functions including for developing organoids. The various cell types of differentiated cellular processes for developing mature organoids. Growth factors and chemical agents are frequently used to increase and regulate cell differentiation. Furthermore, organoids, as artificial three-dimensional cell cultures, also require growth factors and chemical agents for tissue construction. However, the use of growth factors and chemical agents has shown drawbacks such as being unstable, inefficient, and even hazardous.Citation203,Citation204 Therefore, nanomaterials have been developed currently to overcome these limitations due to their physical and chemical properties.Citation205 Nanomaterial has bioactive effects so it can improve and even control cell differentiation.Citation206 Graphene is one of the nanomaterials that has shown effects on cell differentiation. Biofunctionalization of graphene in combination with growth factor and ECM proteins may further improve biological properties.Citation84 Graphene in combination with various biomaterial effects on cell differentiation reported in ref.Citation56,Citation58,Citation60–62,Citation64,Citation66,Citation67,Citation71–73,Citation84,Citation85
Graphene combination has shown cell differentiation such as neuronal differentiation,Citation56,Citation62,Citation64,Citation66,Citation67 osteogenic and osteoblastic differentiation,Citation58,Citation60,Citation72,Citation85 angiogenic differentiation,Citation58,Citation61,Citation72,Citation73,Citation84 and cardiac differentiation.Citation62,Citation71,Citation84 Both graphene combinations, GO and rGO, have shown neuronal differentiation. In contrast, rGO has shown success in developing neuron cells, with the axon length of DRGs up to 738 μm.Citation66 Other studies of GO in combination with chitosan showed that GO can give neuritic extension from the center neurospheres about 39 μm.Citation64 Similar results showed that GO in combination with decellularized lotus petioles gives neuritic extension is more than 60 μm.Citation56 Decellularized lotus petioles in combination with GO improve carriers for neural cells.Citation56 rGO has shown longer than GO for neuronal differentiation. However, these studies showed that GO affected cell differentiation into neurons and neurospheres. However, remained acceptable for the successful development of GO in 3D cell culture. Furthermore, rGO combined with gelatin promotes osteogenic differentiation,Citation72 similarly, results GO combination showed that GO combination improves osteogenic and osteoblastic differentiationCitation58,Citation60 and even similarly only GO receives similar result.Citation85 Besides that, angiogenic differentiation has shown the capability of GO and rGO.Citation58,Citation61,Citation72,Citation73,Citation84 Furthermore, the graphene combination showed that it supported differentiating cells and the formation of functional cardiomyocytes.Citation62 Similarly, the results of the rGO combination showed that it can induce cardiac differentiation in MSCs.Citation71 GO and rGO induced hUC-MSCs differentiation into cardiomyocyte-like cells.Citation84 Moreover, GO and rGO can maintain multipotency capabilities, promote growth cells, and even maintain viability cells.Citation84 Interestingly, Nano Graphene Oxide (NGO) gave wound healing effects for tissue engineering and even regeneration, which is proved by wound area up to 20%.Citation73 Furthermore, cell differentiation could regulate the expression of proteins.Citation85 It means that the process of cellular differentiation is regulated by transcription factors. Therefore, transcription factors that affect whether or not a gene is transcribed play a role in the specialization.Citation207
In addition, studies have shown how the effects of combination graphene on cell behavior are mediated by gene expression.Citation58,Citation61,Citation62,Citation66,Citation67,Citation70–73,Citation84,Citation86 Some genes, such as SNAI1, CDH2, VIM, and TWIST1, showed that gene upregulated on PEG-GO-XN indicated the formation of endothelial-type cells into mesenchymal-type cells.Citation86 However, there is a gene downregulated, such as CDH-1 caused, by PEG-GO-XN abolishing TGF-β1. This study showed that gene upregulation and gene downregulation caused epithelial-like maintenance and even decreased the motility potential of breast cancer cells.Citation86 Therefore, XN loaded PEG-GO can suppress the metastasis of breast cancer. The other study showed genes upregulated, such as LPAR6 and YAP1, while genes downregulated, such as Lats1 and p-YAP Ser127 in HUVEC cells.Citation73 LPAR6 plays role in suggested angiogenesis, while YAP1 plays a key role in NGO-induced endothelial tip cell angiogenesis.Citation73,Citation208 In addition, LPAR6 activated the downstream Rho signaling pathway, leading to cell proliferation and migration,Citation73 whereas Lats1 and p-YAP Ser127 are genes downregulated induced the nuclear translocation of YAP as mediator of activity and cell migration.Citation73,Citation209,Citation210 Therefore, the formation, growth, and integrity of the blood vessel network are related to angiogenesis. Furthermore, other studies showed that genes upregulated related to angiogenic, such as GATA2, ENDOGLIN, VE-CADHERIN,Citation84 VEGF,Citation58 HIF1α, VEGF, and FGF2.Citation61 The expression levels of GATA2, ENDOGLIN, and VE-CADHERIN are 2.5-fold, 4.5-fold, and 3-fold, respectively.Citation84 Furthermore, this study compared with rGO and showed that the expression levels of ENDOGLIN and VE-CADHERIN are less than 5-fold and 2-fold, while the expression level of GATA2 is 4-fold.Citation84 These genes upregulated indicate that they promote the formation of long-length capillaries. Besides that, the upregulation of VEGF indicating effects to pro-angiogenic.Citation58,Citation61,Citation72 Furthermore, upregulation of VEGF and FGF2, which is considered by HIF1α as a key event that signals promoted secretion of angiogenic factors.Citation61 The expression levels of VEGF in GO combination and rGO combination are 1.5-fold and 1.75-fold, respectively.Citation61,Citation72 These results of studies showed that the expression levels of genes promoted to angiogenic of rGO are higher than GO.Citation61,Citation72,Citation84 However, other study showed that VEGF expression is upregulated, indicating that graphene combination had pro-angiogenic effects through interaction between the osteoblastic and osteoclastic progenitors, promoting angiogenesis to further favor recruitment of osteoblastic and osteoclastic.Citation58,Citation72 Therefore, these results showed a rapid wound healing area of around 50% and 80% in migration distance in combination with rGO and GO, respectively.Citation58,Citation72
Other genes upregulated such as ALP, RUNX2, and OCN indicate osteogenic differentiation due to the hydrophilic of GO significantly promoting early osteogenic differentiation.Citation58 However, these studies showed that the expression levels of ALP, RUNX2, and OCN decreased due to the high content of GO. A similar study showed that genes upregulated such as ALP, RUNX2, OCN in rGO combinations.Citation72 Furthermore, this study showed that other genes were upregulated and downregulated, such as RANKL and OPG, respectively, indicating the rGO combination has potential promotive effects on osteoclastogenesis.Citation72 Besides that, there are genes upregulated and downregulated related to cardiac gene expression that have shown the effects of graphene combination on cardiac differentiation.Citation62,Citation71,Citation84 GATA2, ACTC1, and MEF2C are genes upregulated in GO combinations with the expression level of them being 2-fold, almost 2-fold, and 1-fold, respectively.Citation84 Additionally, this study compared with rGO and showed that the level expression of GATA2, ACTC1, and MEF2C in rGO is lower than GO. These genes upregulated indicated that GO improved cardiomyogenic differentiation.Citation84 Other study showed that genes upregulated such as TrpT-2, Actn4, and Cx43 in rGO combinations, with the expression level of them is almost 5-fold, 2-fold, and 2.5-fold, respectively.Citation71 These genes indicated that Actn4 is a gene involved in the cytoskeleton, as is Cx43, a gene involved in cardiac electrical.Citation71 Besides that, genes upregulated and downregulated, such as Mef2c and oct4, respectively, in graphene combinations.Citation62 These genes upregulated related to cardiac differentiation, while genes downregulated, indicating a decrease in undifferentiated ESCs.Citation62
Besides that, other studies showed that genes regulated in neuronal differentiation.Citation66,Citation67 MBP, NGF, and Akt are genes upregulated in rGO combinations.Citation66 These genes upregulated indicated that NGF is related to neurotropic phenotype, while MBP associated with the formation of myelin sheath and Akt related to nerve regeneration.Citation66 However, this study showed that these expression levels of MBP, NGF, Akt decrease around 2.425-fold, 3.885-fold, and 2.436-fold, respectively, in rGO combinations after treatment LY294002, which is blocked activation of Akt signaling and decreased MBP and NGF.Citation66 Similar study showed that genes upregulated in rGO combinations such as Tuj1 and MAP with an increase in their levels by 1.26-fold and 1.72-fold, respectively.Citation67 Additionally, the ratio of Tuj1 in rGO combinations showed that it is higher than without rGO combinations, 35.18% and 21.33%, respectively.Citation66 Upregulation of Tuj1 and MAP indicating neuronal differentiation alongside the increase in neuritic length and even associated with the maturation of dendrites.Citation66,Citation67 Interestingly, in GO combinations with loaded XN, there are genes upregulated such as SNAIL, CDH2, VIM, and TWIST1, while genes downregulated such as CDH-1 cause the maintenance of epithelial-like features and decrease the motility potential of breast cancer.Citation86 In addition, GO combinations showed that upregulation of Nrf2 roles thwarts oxidative stress, so it effects downregulation of NLRP3 related to prevent inflammation triggering and even DNA injury, so it can reduce radiation-induced skin injury.Citation70
The overall results showed that combination graphene improved for cell behavior such as cell proliferation, cell differentiation, and gene expression.Citation56,Citation58,Citation60–62,Citation64,Citation66–73,Citation84–87 Interestingly, combination GO has the potential for developing in 3D cell culture models. The surface functional chemicals, an excellent ultrathin topological substrate with cell contact adhesion ability, hydrophilic properties, mechanical properties, and suitable size of GO, are considered for development in organoid models as 3D model cell culture.Citation64,Citation69,Citation70,Citation73,Citation84,Citation195 Additionally, the doses of GO are also considered for development in 3D cell culture models because they influence their cytotoxic effects.Citation58,Citation87 Therefore, doses of graphene oxide will be described below, along with their effects on cell viability and growth.
Doses of Graphene
Non-toxicity properties of biomaterials are considered for developing cell culture and are signed by cell viability so that they ensure good biocompatibility. Some studies have shown that doses of graphene oxide are related to cell viability and even stimulate cell growth, as summarized in .Citation60,Citation70,Citation73,Citation85–87
Table 5 Doses of Graphene Oxide Effects on Viability Cells
Viability ratio cells around 1 as shown by the doses of GO have been shown in .Citation85,Citation86 Interestingly, the various concentrations of GO (10–150 µg/mL) showed that the result of cell viability was not significantly different, namely that viability ratio cells around 1.Citation85 Similar results showed that 10 µg/mL, which is a dose of GO, received a similar ratio of cell viability as the previous study.Citation86 Therefore, these studies showed that the various concentration doses of GO showed no apparent cytotoxicity.Citation85,Citation86 Interestingly, these doses of GO tested with added inhibitor cancer cells, XN, inhibited that metastatic breast cancer was vulnerable to XN exposure due to XN inhibiting the cell viability at higher concentrations.Citation86 A similar study showed that using the various doses of GO tested to suppress cancer cells and adding a tumor-specific monoclonal antibody (anti-EpCAM) for the delivery of surviving siRNA had anti-proliferative effects.Citation85 The further study using the various doses of GO showed that the cell viability ratio around 1 and even more than 1.Citation60,Citation73 Interestingly, the doses of GO up to 400 µg/mL did not effect the viability of cells.Citation73 Additionally, this study assessed that the doses of GO are 0–50 µg/mL for wound healing and showed that doses of GO with 5 µg/mL accelerates migration and enhances endothelial cells.Citation73 However, the various doses up to 400 µg/mL showed that GO promotes the formation of capillary tubes and networks.Citation73 Other study used the doses of GO is 0.1 mg/mL showed that cell viability ratio more than 1 and even promoted cell migration.Citation60 Nevertheless, there are studies showed that cell viability ratio is less than 1.Citation70,Citation87 The dose of GO is 10 mg/mL used in GO combination tested with radiation treatment showed that it does not influence irradiated culture expansion.Citation70 Nevertheless, the cell viability ratio is less than 1 due to post-irradiation, so it influences thwarted cellular growth after 4 days, but growth for irradiated cells reverts to normality after extended timeframes.Citation70 Additionally, this study showed that GO combinations give angiogenic effects and reduced apoptotic activity, so it promotes cell migration around 50%.Citation70 Similar results showed that the cell viability ratio is less than 1 with 0–50 µg/mL, though the low doses of GO nearly by 10 µg/mL promote tumor cell proliferation.Citation87 Furthermore, the previous study showed that the doses of GO around 5–20 µg/mL of no apparent cytotoxicity in vitro assessment and even maintained cell viability at more than 80%, while the doses of GO are more than 50 and 100 µg/mL induced significant decrease in the cell viability.Citation68,Citation211
Besides that, the size of GO supported maintaining cell viability and even affected cell growth.Citation73,Citation87 The thickness of GO layers of 10 and 15 µg/cm2 increased proliferation rates.Citation84 Furthermore, several studies showed the size of GO, such as the average diameter of 100 nm and thickness of 2 nm,Citation86 diameter of 80–350 nm,Citation85 diameter of 0.5–3 µm and thickness of 0.55–1.2 nm.Citation59 In addition, GO with a size less than 100 nm showed no toxicity.Citation212 The size of GO provides for biological application because GO, especially, is more hydrophilic and has a large edge-to-area ratio, so it is enriched in carbonyl and carboxyl groups.Citation213
The overall studies showed that the doses of GO combination do not show cytotoxic cells. The previous studies have shown that the concentration of graphene oxide shown in . Besides that, some studies showed that the size, such as diameter and thickness of GO is safe for developing or growing cells. Therefore, the doses and the size of GO appropriately are considered for developing organoid models currently.
Author’s Perspective
Previous studies showed that the development of a matrix has been used for organoid models. Matrigel has been used for organoid models.Citation94,Citation95 Those studies showed that matrigel can regenerate epithelial and maintain the viability of models. Matrigel is a matrix that can support cell adhesion so suggest a cell attachment for developing the organoid model.Citation41 The composition of matrigel consists of laminin, collagen type IV, and nidogen and has been identified in 1800 peptides or proteins, so that it has various compositions.Citation40,Citation41 However, matrigel has limited manipulation so it is difficult for improving cell behavior and cannot achieve specific biological properties.Citation214 Therefore, alternative matrigel is considered for substituting matrigel. The alternative matrigel as a matrix has been used for organoid models currently. It is such as gelatin,Citation89 GelMa,Citation90 collagen,Citation91 chitosan-pectin,Citation92 and PEG.Citation93 Hydrogel as an alternative matrigel can be more flexible than matrigel for controlling cell behavior, such as cell adhesion and cell rigidity.Citation215
Gelatine, as one of the hydrogel systems, has been developed as an ECM.Citation53,Citation54,Citation81,Citation89,Citation90 Hydrogel can be used for recapitulating the ECM for several advantages, such as being highly hydrated, giving an environment to cells, and being flexible, so it is very similar to what cells experience in vivo.Citation216 The flexibility of hydrogel means that it can be combined with other biomaterials that are amenable to chemical modification and process engineering techniques that allow for control of their properties.Citation217,Citation218 The mechanical properties of hydrogel vary across the spectrum that is typical of soft tissue in the body and even much more closely match the microenvironment condition.Citation216 Additionally, the softest hydrogel influences the mechanical properties with an increase in elastic modulus, which enhances regulated gene expression, while the rate of proliferation decreases.Citation219 Besides that, some previous studies explained that gelatin for development related to cell culture in vitro can improve good cell behavior due to mimicking in vivo microenvironments.Citation72,Citation81,Citation220–222 Gelatine can provide viability for organoid models, supported growth cells, and even cell characteristics that can mimic cell structure types of organoids without changing phenotype cells.Citation89,Citation90
Interestingly, graphene oxide as an artificial extracellular matrix due to its honeycomb structures has been developing recently.Citation223 It is considered biocompatible and mechanically stable for cell growth, differentiation, and even combinations of GO in the cells to promote binding and proliferation.Citation224,Citation225 Furthermore, GO has good biocompatibility and even effects on cell growth,Citation69 cell migration,Citation64,Citation69,Citation73,Citation84,Citation86 and cell adhesion.Citation64,Citation69,Citation84 Based on this review, we found that GO has the potential to develop organoid models. Previous studies showed that GO has been used a lot in biomedical applications such as for developing tissue engineering and regeneration, as a therapeutic agent, and even for cancer therapy.Citation56,Citation58–61,Citation64,Citation69,Citation70,Citation73,Citation84–87 Furthermore, GO encourages good cell behavior, such as suitable cell characteristic,Citation58–61,Citation64,Citation70,Citation84,Citation86 cell proliferation,Citation58,Citation60,Citation69,Citation70,Citation73,Citation84,Citation87 cell differentiation,Citation58,Citation60,Citation61,Citation64,Citation73,Citation84,Citation85 and gene expression regulation.Citation58,Citation61,Citation70,Citation73,Citation84,Citation86 Cell proliferation plays an important role in growth, repair, reproduction as well as renewing damaged cells. Cell differentiation and gene expression are both related to each other for developing cell types with appropriate functions, which are regulated by transcription factors that affect whether or not gene expression relates to the function. Besides that, the structure of GO had unique properties that were preferable for cells that promote protein in culture media or cellular secretions, which improved overall cell–hydrogel interactions.Citation134,Citation226 In addition, the size of GO provides more hydrophilic properties, so it can be readily combined with other materials,Citation213 while the doses of GO suitable do not show cytotoxic cells, so it can maintain cell viability and even influence cell growth.Citation73 Therefore, GO combined with gelatin as hydrogel can be considered for developing organoids as disease models, as summarized in .
Conclusion
The development of various materials has been used a lot for biomedical applications such as tissue engineering and regeneration, as a therapeutic agent, skin therapy, biosensing system, and even cancer therapy. The development of hydrogel as an alternative to matrigel has been used a lot for developing organoid models. Gelatine is currently one of the hydrogel systems shown in the organoid model as a matrix. Their properties give them flexibility, so they can be readily combined with other materials to mimic the microenvironment in vivo. Besides that, graphene is one of the various materials that has been used a lot for tissue engineering and even drug delivery. Graphene is one of the materials that can be combined with other materials as a matrix, as has been shown in various previous studies. The overall studies related to the use of graphene, especially graphene oxide, showed that it has been successful as a material for matrix in 3D cell culture due to its good biocompatibility. Graphene oxide showed that it has good cell proliferation and differentiation, and even gene expression regulation has shown a specialization role in tissue engineering and regeneration applications. Moreover, the concentration and size of graphene oxide certainly show no cytotoxicity for developing cells. Therefore, optimizing with graphene oxide can be considered to advance the development of organoid models.
Abbreviations
ADSCs, adipose-derived mesenchymal stem cells; adECM-PDA-rGO, adipose decellularized Extracellular Matrix-polydopamine-functionalized reduced graphene oxide; AG-PCL, Alginate Gelatine-poly(ε-caprolactone); BADSCs, brown adipose-derived stem cells; BG/GO, Bioactive Glass/Graphene Oxide; cECs, Cardiac endothelial cells; CMCs-GelMa-GO-PLGA, carboxymethyl chitosan-gelatin methacryloyl-graphene oxide-Poly lactic-co-glycolic acid; CPSN, carbon nanotubes (CNTs)-containing electrospun polycaprolactone/silk fibroin nanofibers; CH-GO, Chitosan-Graphene Oxide; Col-Ch, Collagen-Chitosan; dL-EG, decellularized lotus petiole graphene oxide; EPO/CNP@FPH, erythropoietin (EPO)/ceria nanoparticle (CNP)@Fmoc-Phe3 hydrogel; EU/GL/TiO2, hybrid eumelanin/graphene-like/titanium dioxide; GCE, GO was modified with a non-toxicity cationic material (chitosan) and a tumor-specific monoclonal antibody (anti-EpCAM); GelMa, Gelatin Methacryloyl; Gel-TCP/SC, Gelatin-beta-tricalcium phosphate (β-TCP)/SrCuSi4O10 (SC); GG, Gellan gum; GO/rGO, Graphene oxide/reduced Graphene oxide; GOG, gelatin-reduced graphene oxide; HUVECs, human umbilical vein endothelial cells; H9C2, Rat cardiomyoblast cells; IGF1c-PHM, insulin-like growth factor 1 mimetic peptide supramolecular hydrogel microspheres; ISCs, Intestinal Stem Cells; iSGCs, induced sweat gland cell; MESCs, mouse embryonic stem cells; MNSs-CNT-COOH, motor neuron spheroids-carboxylated carbon nanotubes; NGO, Nano Graphene Oxide; NSCs, neural stem cells; NSPCs, neural stem/progenitor cells; OEO-BG-PLGA/Gel, oregano essential oil-Bioactive Glass-poly(L-lactide-co-glycolide)/Gelatin; PANI–GO, Polyaniline-Graphene Oxide; PAMB-C-OHA, Poly-3- amino-4-methoxybenzoic acid carboxymethyl chitosan-oxidized hyaluronic acid; PEG, polyethylene glycol; PEG-GO-XN, PEGylated-Graphene Oxide-Xanthohumol; PLA–HA, polylactic acid–hyaluronic acid; PSMPs@B, benzo [a] pyrene (B (a) P)-loaded polystyrene microplastics; PUCL@CNT, polyurethane-polycaprolactone (PUCL)@carbon nanotubes; rBMSCs, rat bone marrow stromal cells; rGO-Col, reduced Graphene Oxide-Collagen; rGOM, reduced Graphene Oxide Membrane; rGO-Mxene, reduced Graphene Oxide - Ti3C2Tx; rGO-PCL, reduced Graphene Oxide – Polycaprolactone; RGD/GO, arginine-glycine-aspartic acid peptide and Graphene Oxide; RSF/Mxene, regenerated silk fibroin/Mxene; Se-EX-Ch-COL/TA, Selenium-Exosomes-Chitosan-Collagen/Tannic Acid; SPI-SH-GA@PGO, Soy Protein Isolate-Gallic Acid-phenol and thiol cohorts@Graphene Oxide.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
This research was funded by BIMA-PFR DIKTI, grant number 3018/UN6.3.1/PT.00/2023, and also supported by an Academic Leadership Grant with contact number 1549/UN6.3.1/PT.00/2023.
References
- Urzì O, Gasparro R, Costanzo E, et al. Three-Dimensional Cell Cultures: the Bridge between In Vitro and In Vivo Models. Int J Mol Sci. 2023;24(12046):1–44. doi:10.3390/ijms241512046
- Bhadriraju K, Chen CS. Engineering Cellular Microenvironments to Improve Cell-based Drug Testing. Drug Discov Today. 2002;7(11):612–620. doi:10.1016/S1359-6446(02)02273-0
- Duval K, Grover H, Han LH, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32(4):266–277. doi:10.1152/physiol.00036.2016
- Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-based Biosensors. Assay Drug Dev Technol. 2014;12(4):207–218. doi:10.1089/adt.2014.573
- Khoruzhenko AI. 2D- and 3D-Cell Culture. Biopolym Cell. 2011;27(1):17–24. doi:10.7124/bc.00007D
- Li Z, Cui Z. Three-Dimensional Perfused Cell Culture. Biotechnol Adv. 2014;32(2):243–254. doi:10.1016/j.biotechadv.2013.10.006
- Justice BA, Badr NA, Felder RA. 3D Cell Culture Opens New Dimensions in Cell-Based Assays. Drug Discov Today. 2009;14(1–2):102–107. doi:10.1016/j.drudis.2008.11.006
- Langhans SA. Three-Dimensional In Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol. 2018;9:1–14. doi:10.3389/fphar.2018.00006
- Artegiani B, Clevers H. The Use and Application of The 3D-Organoid Technology. Hum Mol Genet. 2018;27:99–107. doi:10.1093/hmg/ddy187
- Simian M, Bissell MJ. Organoids: a Historical Perspective of Thinking in Three Dimensions. J Cell Biol. 2017;216(1):31–40. doi:10.1083/jcb.201610056
- Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Review Engineering Stem Cell Organoids. Cell Stem Cell. 2016;18(1):25–38. doi:10.1016/j.stem.2015.12.005
- Hofer M, Lutolf MP. Engineering Organoids. Nat Rev Mater. 2021;6(5):402–420. doi:10.1038/s41578-021-00279-y
- Lamers MM, Beumer J, van der VJ, et al. SARS-CoV-2 Productively Infects Human Gut Enterocytes. Science. 2020;369(6499):50–54. doi:10.1126/science.abc1669
- Hui KPY, Ching RHH, Chan SKH, et al. Tropism, Replication Competence, and Innate Immune Responses of Influenza Virus: an Analysis of Human Airway Organoids and Ex-Vivo Bronchus Cultures. Lancet Respir. 2018;6(18):846–854. doi:10.1016/s2213-2600(18)30236-4
- Li Z, Lang Y, Sakamuru S, et al. Methylene Blue Is a Potent and Broad-Spectrum Inhibitor against Zika Virus in Vitro and in Vivo. Emerg Microbes Infect. 2020;9(1):2404–2416. doi:10.1080/22221751.2020.1838954
- Zhou J, Li C, Zhao G, et al. Human Intestinal Tract Serves as an Alternative Infection Route for Middle East Respiratory Syndrome Coronavirus. SciAdv. 2017;3:1–12. doi:10.1126/sciadv.aao4966
- Sato S, Hisaie K, Kurokawa S, et al. Human Norovirus Propagation in Human Induced Pluripotent Stem Cell-Derived Intestinal Epithelial Cells. Cell Mol Gastroenterol Hepatol. 2019;7(3):686–688. doi:10.1016/j.jcmgh.2018.11.001
- Hakim MS, Chen S, Ding S, et al. Basal Interferon Signaling and Therapeutic Use of Interferons in Controlling Rotavirus Infection in Human Intestinal Cells and Organoids. Sci Rep. 2018;8(8341):1–13. doi:10.1038/s41598-018-26784-9
- Llanos-chea A, Citorik RJ, Nickerson KP, et al. Bacteriophage Therapy Testing Against Shigella flexneri in a Novel Human Intestinal Organoid-Derived Infection Model. J Pediatr Gastroenterol Nutr. 2019;68(4):509–516. doi:10.1097/mpg.0000000000002203
- Jiao Y, guo ZY, Lin Z, et al. Salmonella enteritidis Effector AvrA Suppresses Autophagy by Reducing Beclin-1 Protein. Front Immunol. 2020;11(686):1–12. doi:10.3389/fimmu.2020.00686
- Shpichka A, Bikmulina P, Peshkova M, et al. Organoids in Modelling Infectious Diseases. Drug Discov Today. 2022;27(1):223–233. doi:10.1016/j.drudis.2021.08.005
- Zhou J, Li C, Sachs N, et al. Differentiated Human Airway Organoids to Assess Infectivity of Emerging Influenza Virus. Proc Natl Acad Sci USA. 2018;115(26):6822–6827. doi:10.1073/pnas.1806308115
- Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020;181:905–913. doi:10.1016/j.cell.2020.04.004
- wen CY, Huang SX, Luisa A, et al. A Three-Dimensional Model of Human Lung Development and Disease from Pluripotent Stem Cells. Nat Cell Biol. 2017;19(5):542–549. doi:10.1038/ncb3510
- Porotto M, Ferren M, Chen YW, et al. Authentic Modeling of Human Respiratory Virus Infection In Human Pluripotent Stem Cell-Derived Lung Organoids. Am Soc Microbiol. 2019;10(3):1–13. doi:10.1128/mbio.00723-19
- Zhong NY, wen ZY, Miyakawa K, Murata S, ran ZR. Recapitulation of Hepatitis B Virus – host Interactions in Liver Organoids from Human Induced Pluripotent Stem Cells. EBioMedicine. 2018;35:114–123. doi:10.1016/j.ebiom.2018.08.014
- Baktash Y, Madhav A, Coller KE, Randall G. Single Particle Imaging of Polarized Hepatoma Organoids upon Hepatitis C Virus Infection Reveals an Ordered and Sequential Entry Process. Cell Host Microbe. 2018;23(3):382–394. doi:10.1016/j.chom.2018.02.005
- Ramani A, Müller L, Ostermann PN, et al. SARS-CoV- 2 Targets Neurons of 3 D Human Brain Organoids. EMBO J. 2020;39:1–14. doi:10.15252/embj.2020106230
- Sacramento CQ, Melo De GR, Freitas De CS, et al. The Clinically Approved Antiviral Drug Sofosbuvir Inhibits Zika Virus Replication. Sci Rep. 2017;7:1–11. doi:10.1038/srep40920
- Geuens T, Blitterswijk CA, LaPointe VLS. Overcoming Kidney Organoid Challenges for Regenerative Medicine. npj Regen Med. 2020;8:1–6. doi:10.1038/s41536-020-0093-4
- El-Badri N, Elkhenany H. Toward the Nanoengineering of Mature, Well Patterned and Vascularized Organoids. Nanomedicine. 2021;16(15):1255–1258. doi:10.2217/nnm-2021-0074
- Rauth S, Karmakar S, Batra SK, Ponnusamy MP. Recent Advances in Organoid Development and Applications in Disease Modeling. Biochim Biophys Acta - Rev Cancer. 2021;1875(2):1–37. doi:10.1016/j.bbcan.2021.188527
- Kretzschmar K, Clevers H. Organoids: modeling Development and the Stem Cell Niche in a Dish. Dev Cell. 2016;38(6):590–600. doi:10.1016/j.devcel.2016.08.014
- Mondrinos MJ, Koutzaki S, Jiwanmall E, et al. Engineering Three-Dimensional Pulmonary Tissue Constructs. Tissue Eng. 2006;12(4):717–728. doi:10.1089/ten.2006.12.717
- Xu C, Inokuma MS, Denham J, et al. Feeder-Free Growth of Undifferentiated Human Embryonic Stem Cells. Nat Biotechnol. 2001;19(10):971–974. doi:10.1038/nbt1001-971
- Qian L, Saltzman WM. Improving the Expansion and Neuronal Differentiation of Mesenchymal Stem Cells through Culture Surface Modification. Biomaterials. 2004;25(7–8):1331–1337. doi:10.1016/j.biomaterials.2003.08.013
- Lee SW, Lee HJ, Hwang HS, Ko K, Han DW, Ko K. Optimization of Matrigel-Based Culture for Expansion of Neural Stem Cells. Animal Cells Syst (Seoul). 2015;19(3):175–180. doi:10.1080/19768354.2015.1035750
- Ohashi K, Yokoyama T, Nakajima Y, Kosovsky M. Methods for Implantation of BD Matrigel TM Matrix into Mice and Tissue Fixation. BD Biosci. 2006.
- Funaki M, Janmey PA. Technologies to Engineer Cell Substrate Mechanics in Hydrogels. Elsevier Inc.; 2017; doi:10.1016/B978-0-12-802734-9.00023-8
- Wang M, Yu H, Zhang T, et al. In-Depth Comparison of Matrigel Dissolving Methods on Proteomic Profiling of Organoids. Mol Cell Proteomics. 2022;21(1):1–9. doi:10.1016/j.mcpro.2021.100181
- Kratochvil MJ, Seymour AJ, Li TL, Paşca SP, Kuo CJ, Heilshorn SC. Engineered Materials for Organoid Systems. Nat Rev Mater. 2019;4(9):606–622. doi:10.1038/s41578-019-0129-9
- Aisenbrey EA, Murphy WL. Synthetic Alternatives to Matrigel. Nat Rev Mater. 2020;5(7):539–551. doi:10.1038/s41578-020-0199-8
- Pei X, Zhou Z, Gan Z, et al. PEGylated Nano-Graphene Oxide as a Nanocarrier for Delivering Mixed Anticancer Drugs to Improve Anticancer Activity. Sci Rep Nat Res. 2020;10:1–15. doi:10.1038/s41598-020-59624-w
- Pourjavadi A, Tehrani ZM, Jokar S. Chitosan based Supramolecular Polypseudorotaxane as a pH-Responsive Polymer and Their Hybridization with Mesoporous Silica - Coated Magnetic Graphene oxide for Triggered Anticancer Drug Delivery. Polymer (Guildf). 2015;76:52–61. doi:10.1016/j.polymer.2015.08.050
- Wang LH, Sui L, Zhao PH, et al. A Composite of Graphene Oxide and Iron Oxide Nanoparticles for Targeted Drug Delivery of Temozolomide. Pharmazie. 2020;75:313–317. doi:10.1691/ph.2020.9170
- Kawamoto K, Miyaji H, Nishida E, et al. Characterization and Evaluation of Graphene Oxide Scaffold for Periodontal Wound Healing of Class II Furcation Defects in Dog. Int J Nanomed. 2018;13:2365–2376. doi:10.2147/ijn.s163206
- Jing X, yang MH, Napiwocki BN, Fang PX, Sheng TL. Mussel-Inspired Electroactive Chitosan/Graphene Oxide Composite Hydrogel with Rapid Self-Healing and Recovery. Carbon N Y. 2017;125:557–570. doi:10.1016/j.carbon.2017.09.071
- Park J, Hee J, Kim S, Jang I, Jeong S, Young J. Micropatterned Conductive Hydrogels as Multifunctional Muscle Mimicking Biomaterials: graphene-Incorporated Hydrogels Directly Patterned with Femtosecond Laser Ablation. Acta Biomater. 2019;97:141–153. doi:10.1016/j.actbio.2019.07.044
- Satapathy MK, Manga YB, Ostrikov KK, et al. Microplasma Cross-Linked Graphene Oxide-Gelatin Hydrogel for Cartilage Reconstructive Surgery. ACS Appl Mater Interfaces. 2020;12:86–95. doi:10.1021/acsami.9b14073
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Yin Y, He XT, Wang J, et al. Pore Size-Mediated Macrophage M1-to-M2 Transition Influences New Vessel Formation within the Compartment of a Scaffold. Appl Mater Today. 2020;18(100466):1–16. doi:10.1016/j.apmt.2019.100466
- Lin S, Zhu Y, Hu T, Wang K, Chen X. Novel Design of Nano-Selenium Loaded Injectable Hydrogel Combined with Mesenchymal Stem Cells-Derived Exosomes Improving Cardiac Repair and Nursing Care after Acute Myocardial Infarction. J Drug Deliv Sci Technol. 2023;87(59):1–14. doi:10.1016/j.jddst.2023.104711
- Yuan Z, Zhang L, Jiang S, et al. Anti-Inflammatory, Antibacterial, and Antioxidative Bioactive Glass-Based Nanofibrous Dressing Enables Scarless Wound Healing. Smart Mater Med. 2023;4:407–426. doi:10.1016/j.smaim.2023.01.001
- Zhang X, Wei H, Dong C, et al. 3D Printed Hydrogel/Bioceramics Core/Shell Scaffold with NIR-II Triggered Drug Release for Chemo-Photothermal Therapy of Bone Tumors and Enhanced Bone Repair. Chem Eng J. 2023;461:1–13. doi:10.1016/j.cej.2023.141855
- Li Y, Peng H, Tang W, et al. Accelerating Periodontal Regeneration through Injectable Hydrogel-Enabled Sequential Delivery of Nanoceria and Erythropoietin. Mater Des. 2023;225:1–10. doi:10.1016/j.matdes.2022.111540
- Xia N, Zhu Y, Liu R, Chen W, Zhao Y, Sun L. Decellularized Lotus Petioles Integrated Microfluidic Chips for Neural Cell Alignment Monitoring. Compos Part B Eng. 2023;255(110621):1–7. doi:10.1016/j.compositesb.2023.110621
- Yuan X, Duan X, Enhejirigala X, et al. Reciprocal Interaction between Vascular Niche and Sweat Gland Promotes Sweat Gland Regeneration. Bioact Mater. 2023;21:340–357. doi:10.1016/j.bioactmat.2022.08.021
- Qi X, Liu Y, Yin X, et al. Surface-Based Modified 3D-Printed BG/GO Scaffolds Promote Bone Defect Repair through Bone Immunomodulation. Compos Part B Eng. 2023;257(110673):1–12. doi:10.1016/j.compositesb.2023.110673
- Fraczek-Szczypta A, Jantas D, Ciepiela F, Grzonka J. Graphene Oxide-Conductive Polymer Nanocomposite Coatings Obtained by the EPD Method as Substrates for Neurite Outgrowth. Diam Relat Mater. 2020;102(107663):1–15. doi:10.1016/j.diamond.2019.107663
- Qin B, Dong H, Tang X, et al. Antisense YycF and BMP-2 Co-Delivery Gelatin Methacryloyl and Carboxymethyl Chitosan Hydrogel Composite for Infective Bone Defects Regeneration. Int J Biol Macromol. 2023;253(127233):1–14. doi:10.1016/j.ijbiomac.2023.127233
- Bayaraa O, Dashnyam K, Singh RK, et al. Nanoceria-GO-Intercalated Multicellular Spheroids Revascularize and Salvage Critical Ischemic Limbs through Anti-Apoptotic and pro-Angiogenic Functions. Biomaterials. 2023;292(121914):1–16. doi:10.1016/j.biomaterials.2022.121914
- Amantea F, Antignani G, Pota G, et al. Hybrid Eumelanin/Graphene-like/TiO2 Bio-Interface for Mouse Embryonic Stem Cell (MESC) Growth: compatibility toward Neurons and Beating Cardiomyocytes Differentiation. Appl Surf Sci. 2023;633:1–12. doi:10.1016/j.apsusc.2023.157608
- yi HS, yu LL, Cun WZ, et al. Combining HUMSC Secretome and a Conductive Hydrogel Enhances Angiogenesis and Electrical Transmission at Myocardial Infarct Sites to Support Cardiac Repair. Chem Eng J. 2023;474:1–16. doi:10.2139/ssrn.4524293
- Marapureddy SG, Hivare P, Sharma A, et al. Rheology and Direct Write Printing of Chitosan - Graphene Oxide Nanocomposite Hydrogels for Differentiation of Neuroblastoma Cells. Carbohydr Polym. 2021;269(118254):1–16. doi:10.1016/j.carbpol.2021.118254
- Zambrano-Andazol I, Vázquez N, Chacón M, et al. Reduced Graphene Oxide Membranes in Ocular Regenerative Medicine. Mater Sci Eng C. 2020;114:1–11. doi:10.1016/j.msec.2020.111075
- Yao X, Zhan L, Yan Z, et al. Non-Electric Bioelectrical Analog Strategy by a Biophysical-Driven Nano-Micro Spatial Anisotropic Scaffold for Regulating Stem Cell Niche and Tissue Regeneration in a Neuronal Therapy. Bioact Mater. 2023;20:319–338. doi:10.1016/j.bioactmat.2022.05.034
- d SDM, Barroca N, Pinto SC, et al. Decellularized Extracellular Matrix-Based 3D Nanofibrous Scaffolds Functionalized with Polydopamine-Reduced Graphene Oxide for Neural Tissue Engineering. Chem Eng J. 2023;472(144980):1–15. doi:10.1016/j.cej.2023.144980
- Wychowaniec JK, Litowczenko J, Tadyszak K, et al. Unique Cellular Network Formation Guided by Heterostructures Based on Reduced Graphene Oxide - Ti3C2Tx MXene Hydrogels. Acta Biomater. 2020;115:104–115. doi:10.1016/j.actbio.2020.08.010
- Shin MC, Kang MS, Park R, Chae SY, Han DW, Hong SW. Differential Cellular Interactions and Responses to Ultrathin Micropatterned Graphene Oxide Arrays with or Without Ordered in Turn RGD Peptide Films. Appl Surf Sci. 2021;561(150115):1–10. doi:10.1016/j.apsusc.2021.150115
- Zhou D, Liu H, Han L, et al. Paintable Graphene Oxide-Hybridized Soy Protein-Based Biogels for Skin Radioprotection. Chem Eng J. 2023;469(143914):1–12. doi:10.1016/j.cej.2023.143914
- Norahan MH, Pourmokhtari M, Saeb MR, Bakhshi B, Soufi Zomorrod M, Baheiraei N. Electroactive Cardiac Patch Containing Reduced Graphene Oxide with Potential Antibacterial Properties. Mater Sci Eng C. 2019;104(109921):1–11. doi:10.1016/j.msec.2019.109921
- Jiao D, Wang J, Yu W, et al. Biocompatible Reduced Graphene Oxide Stimulated BMSCs Induce Acceleration of Bone Remodeling and Orthodontic Tooth Movement through Promotion on Osteoclastogenesis and Angiogenesis. Bioact Mater. 2022;15:409–425. doi:10.1016/j.bioactmat.2022.01.021
- Liu W, Luo H, Wei Q, et al. Electrochemically Derived Nanographene Oxide Activates Endothelial Tip Cells and Promotes Angiogenesis by Binding Endogenous Lysophosphatidic Acid. Bioact Mater. 2022;9:92–104. doi:10.1016/j.bioactmat.2021.07.007
- Li YM, Patel KD, Han YK, et al. Electroconductive and Mechano-Competent PUCL@CNT Nanohybrid Scaffolds Guiding Neuronal Specification of Neural Stem/Progenitor Cells. Chem Eng J. 2023;466:1–18. doi:10.1016/j.cej.2023.143125
- Wei X, Wang L, Duan C, et al. Cardiac Patches Made of Brown Adipose-Derived Stem Cell Sheets and Conductive Electrospun Nanofibers Restore Infarcted Heart for Ischemic Myocardial Infarction. Bioact Mater. 2023;27:271–287. doi:10.1016/j.bioactmat.2023.03.023
- Hu ZC, Lu JQ, Zhang TW, et al. Piezoresistive MXene/Silk Fibroin Nanocomposite Hydrogel for Accelerating Bone Regeneration by Re-Establishing Electrical Microenvironment. Bioact Mater. 2023;22:1–17. doi:10.1016/j.bioactmat.2022.08.025
- Kudryavtseva V, Stankevich K, Gudima A, et al. Atmospheric Pressure Plasma Assisted Immobilization of Hyaluronic Acid on Tissue Engineering PLA-Based Scaffolds and Its Effect on Primary Human Macrophages. Mater Des. 2017;127:261–271. doi:10.1016/j.matdes.2017.04.079
- Pańczyszyn E, Jaśko M, Miłek O, et al. Gellan Gum Hydrogels Cross-Linked with Carbodiimide Stimulates Vacuolation of Human Tooth-Derived Stem Cells in Vitro. Toxicol Vitr. 2021;73(105111):1–11. doi:10.1016/j.tiv.2021.105111
- Wang Z, Jia S, Xu H, et al. IGF1c Mimetic Peptide-Based Supramolecular Hydrogel Microspheres Synergize with Neural Stem Cells to Promote Functional Recovery from Spinal Cord Injury. Nano Today. 2023;51:1–21. doi:10.1016/j.nantod.2023.101894
- Dewey MJ, Collins AJ, Tiffany A, et al. Evaluation of Bacterial Attachment on Mineralized Collagen Scaffolds and Addition of Manuka Honey to Increase Mesenchymal Stem Cell Osteogenesis. Biomaterials. 2023;294:1–14. doi:10.1016/j.biomaterials.2023.122015
- Lorant J, Saury C, Schleder C, et al. Skeletal Muscle Regenerative Potential of Human MuStem Cells following Transplantation into Injured Mice Muscle. Mol Ther. 2018;26(2):618–633. doi:10.1016/j.ymthe.2017.10.013
- Yu W, Li S, Guan X, et al. Higher Yield and Enhanced Therapeutic Effects of Exosomes Derived from MSCs in Hydrogel-Assisted 3D Culture System for Bone Regeneration. Biomater Adv. 2022;133(112646):1–12. doi:10.1016/j.msec.2022.112646
- Cougoule C, Van Goethem E, Le Cabec V, et al. Blood Leukocytes and Macrophages of Various Phenotypes Have Distinct Abilities to Form Podosomes and to Migrate in 3D Environments. Eur J Cell Biol. 2012;91:938–949. doi:10.1016/j.ejcb.2012.07.002
- Sekuła-Stryjewska M, Noga S, Dźwigońska M, et al. Graphene-Based Materials Enhance Cardiomyogenic and Angiogenic Differentiation Capacity of Human Mesenchymal Stem Cells in Vitro – focus on Cardiac Tissue Regeneration. Mater Sci Eng C. 2021;119(111614):1–18. doi:10.1016/j.msec.2020.111614
- Chen S, Zhang S, Wang Y, Yang X, Yang H, Cui C. Anti-EpCAM Functionalized Graphene Oxide Vector for Tumor Targeted SiRNA Delivery and Cancer Therapy. Asian J Pharm Sci. 2021;16(5):598–611. doi:10.1016/j.ajps.2021.04.002
- Zhang J, Yan L, Wei P, et al. PEG-GO@XN Nanocomposite Suppresses Breast Cancer Metastasis via Inhibition of Mitochondrial Oxidative Phosphorylation and Blockade of Epithelial to Mesenchymal Transition. Eur J Pharmacol. 2021;895(173866):1–9. doi:10.1016/j.ejphar.2021.173866
- Zheng Z, Halifu A, Ma J, et al. Low-Dose Graphene Oxide Promotes Tumor Cells Proliferation by Activating PI3K-AKT-MTOR Signaling via Cellular Membrane Protein Integrin ΑV. Environ Pollut. 2023;330(121817):1–11. doi:10.1016/j.envpol.2023.121817
- Ha T, Park S, Shin M, Lee JY, Choi JH, Choi JW. Biosensing System for Drug Evaluation of Amyotrophic Lateral Sclerosis Based on Muscle Bundle and Nano-Biohybrid Hydrogel Composed of Multiple Motor Neuron Spheroids and Carbon Nanotubes. Chem Eng J. 2023;463:1–9. doi:10.1016/j.cej.2023.142284
- Seino T, Kawasaki S, Shimokawa M, et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell. 2018;22(3):454–467. doi:10.1016/j.stem.2017.12.009
- Töpfer E, Pasotti A, Telopoulou A, et al. Bovine Colon Organoids: from 3D Bioprinting to Cryopreserved Multi-Well Screening Platforms. Toxicol Vitr. 2019;61(104606):1–8. doi:10.1016/j.tiv.2019.104606
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective Invasion in Breast Cancer Requires a Conserved Basal Epithelial Program. Cell. 2013;155(7):1639–1651. doi:10.1016/j.cell.2013.11.029
- Morello G, Quarta A, Gaballo A, et al. A Thermo-Sensitive Chitosan/Pectin Hydrogel for Long-Term Tumor Spheroid Culture. Carbohydr Polym. 2021;274(118633):1–13. doi:10.1016/j.carbpol.2021.118633
- Swaminathan G, Kamyabi N, Carter HE, et al. Effect of Substrate Stiffness on Human Intestinal Enteroids’ Infectivity by Enteroaggregative Escherichia coli. Acta Biomater. 2021;132:245–259. doi:10.1016/j.actbio.2021.07.024
- Huang J, Hume AJ, Abo KM, et al. SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell. 2020;27(6):962–973. doi:10.1016/j.stem.2020.09.013
- Engevik MA, Luck B, Visuthranukul C, et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut–Brain Axis. Cell Mol Gastroenterol Hepatol. 2021;11(1):221–248. doi:10.1016/j.jcmgh.2020.08.002
- Cao Y, Hu D, Cai C, et al. Modeling Early Human Cortical Development and Evaluating Neurotoxicity with a Forebrain Organoid System. Environ Pollut. 2023;337:1–14. doi:10.1016/j.envpol.2023.122624
- Chen Y, Williams AM, Gordon EB, et al. Biological Effects of Polystyrene Micro- and Nano-Plastics on Human Intestinal Organoid-Derived Epithelial Tissue Models without and with M Cells. Nanomedicine Nanotechnology, Biol Med. 2023;50:1–16. doi:10.1016/j.nano.2023.102680
- Shaoyong W, Jin H, Jiang X, et al. Benzo [a] Pyrene-Loaded Aged Polystyrene Microplastics Promote Colonic Barrier Injury via Oxidative Stress-Mediated Notch Signalling. J Hazard Mater. 2023;457(866):1–18. doi:10.1016/j.jhazmat.2023.131820
- Yang Y, Asiri AM, Tang Z, Du D, Lin Y. Graphene Based Materials for Biomedical Applications. Mater Today. 2013;16(10):365–373. doi:10.1016/j.mattod.2013.09.004
- Priyadarsini S, Mohanty S, Mukherjee S, Basu S, Mishra M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J Nanostruct Chem. 2018;8(2):123–137. doi:10.1007/s40097-018-0265-6
- Prasadh S, Suresh S, Wong R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials (Basel). 2018;11(8):1–18. doi:10.3390/ma11081430
- Dasari Shareena TP, McShan D, Dasmahapatra AK, Tchounwou PB. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018;10(53):1–34. doi:10.1007/s40820-018-0206-4
- Zare P, Aleemardani M, Seifalian A, Bagher Z, Seifalian AM. Graphene Oxide: opportunities and Challenges in Biomedicine. Nanomaterials. 2021;11:1–18. doi:10.3390/nano11051083
- Zhou X, Liang F. Application of Graphene/Graphene Oxide in Biomedicine and Biotechnology. Curr Med Chem. 2014;21(7):855–869. doi:10.2174/0929867320666131119124325
- Banerjee AN. Graphene and Its Derivatives as Biomedical Materials: future Prospects and Challenges. Interface Focus. 2018;8(3):1–22. doi:10.1098/rsfs.2017.0056
- Zaaba NI, Foo KL, Hashim U, Tan SJ, Liu WW, Voon CH. Synthesis of Graphene Oxide using Modified Hummers Method: solvent Influence. Procedia Eng. 2017;184:469–477. doi:10.1016/j.proeng.2017.04.118
- Sali S, Mackey HR, Abdala AA. Effect of Graphene Oxide Synthesis Method on Properties and Performance of Polysulfone-Graphene Oxide Mixed Matrix Membranes. Nanomaterials. 2019;9(5):1–16. doi:10.3390/nano9050769
- Sk K, Das MM, Paik P. Graphene Oxide for Biomedical Applications. J Nanomedicine Res. 2017;5(6):1–5. doi:10.15406/jnmr.2017.05.00136
- Feng P, Zhao R, Tang W, et al. Structural and Functional Adaptive Artificial Bone: materials, Fabrications, and Properties. Adv Funct Mater. 2023;33(23). doi:10.1002/adfm.202214726
- Feng P, Shen S, Yang L, Kong Y, Yang S, Shuai C. Vertical and Uniform Growth of MoS2 Nanosheets on GO Nanosheets for Efficient Mechanical Reinforcement in Polymer Scaffold. Virtual Phys Prototyp. 2023;18(1). doi:10.1080/17452759.2022.2115384
- Shuai C, Peng B, Feng P, Yu L, Lai R, Min A. In Situ Synthesis of Hydroxyapatite Nanorods on Graphene Oxide Nanosheets and Their Reinforcement in Biopolymer Scaffold. J Adv Res. 2022;35:13–24. doi:10.1016/j.jare.2021.03.009
- Shuai C, Guo W, Wu P, et al. A Graphene Oxide-Ag co-Dispersing Nanosystem: dual Synergistic Effects on Antibacterial Activities and Mechanical Properties of Polymer Scaffolds. Chem Eng J. 2018;347(2018):322–333. doi:10.1016/j.cej.2018.04.092
- Biru EI, Necolau MI, Zainea A, Iovu H. Graphene Oxide–Protein-Based Scaffolds for Tissue Engineering: recent Advances and Applications. Polymers (Basel). 2022;14(5):1–23. doi:10.3390/polym14051032
- Byun J. Emerging Frontiers of Graphene in Biomedicine. J Microb Biotechnol. 2015;25(2):145–151. doi:10.4014/jmb.1412.12045
- Sun J, Shakya S, Gong M, Liu G, Wu S. Combined Application of Graphene-Family Materials and Silk Fibroin in Biomedicine. ChemistrySelect. 2019;4(1):5745–5754. doi:10.1002/slct.201804034
- Lin J, Chen X, Huang P. Graphene-Based Nanomaterials for Bioimaging. Adv Drug Deliv Rev. 2016;105(Part B):242–254. doi:10.1016/j.addr.2016.05.013
- Trikkaliotis DG, Christoforidis AK, Mitropoulos AC, Kyzas GZ. Graphene Oxide Synthesis, Properties and Characterization Techniques: a Comprehensive Review. ChemEngineering. 2021;5(3):1–24. doi:10.3390/chemengineering5030064
- Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH. Recent Advances in Graphene-Based Biosensors. Biosens Bioelectron. 2011;26(12):4637–4648. doi:10.1016/j.bios.2011.05.039
- Kulshrestha S, Khan S, Meena R, Singh BR, Khan AU. A Graphene/Zinc Oxide Nanocomposite Film Protects Dental Implant Surfaces against Cariogenic Streptococcus Mutans. Biofouling. 2014;30(10):1281–1294. doi:10.1080/08927014.2014.983093
- Liu Z, Robinson JT, Tabakman SM, Yang K, Dai H. Carbon Materials for Drug Delivery & Cancer Therapy. Mater Today. 2011;14(7–8):316–323. doi:10.1016/S1369-7021(11)70161-4
- Bai Y, Xu T, Zhang X. Graphene-based Biosensors for Detection of Biomarkers. Micromachines. 2020;11(1). doi:10.3390/mi11010060
- Lee J, Kim J, Kim S, hee MD. Biosensors based on Graphene oxide and Its Biomedical Application. Adv Drug Deliv Rev. 2016;105(2016):275–287. doi:10.1016/j.addr.2016.06.001
- Liu X, Xu C, Fu C, et al. Graphene Oxide-Sensitized Surface Plasmon Resonance Biosensor of Porcine Reproductive and Respiratory Syndrome Virus. Molecules. 2022;27(3942):1–9. doi:10.3390/molecules27123942
- Lee J, Park IS, Kim H, Woo JS, Choi BS, Min DH. BSA as Additive: a Simple Strategy for Practical Applications of PNA in Bioanalysis. Biosens Bioelectron. 2015;69(2015):167–173. doi:10.1016/j.bios.2015.02.030
- Devi KSS, Prakash J, Tsujimura S. Graphene Oxide-based Nanomaterials for Electrochemical Bio/Immune Sensing and Its Advancements in Health Care Applications: a Review. Hybrid Adv. 2024;5(100123):1–15. doi:10.1016/j.hybadv.2023.100123
- Karlický F, Kumara Ramanatha Datta K, Otyepka M, Zbořil R. Halogenated Graphenes: rapidly Growing Family of Graphene Derivatives. ACS Nano. 2013;7(8):6434–6464. doi:10.1021/nn4024027
- Zuchowska A, Jastrzebska E, Mazurkiewicz-Pawlicka M, et al. Well-defined Graphene Oxide as a Potential Component in Lung Cancer Therapy. Curr Cancer Drug Targets. 2019;20(1):47–58. doi:10.2174/1568009619666191021113807
- Yang J, Zhai S, Qin H, Yan H, Xing D, Hu X. NIR-Controlled Morphology Transformation and Pulsatile Drug Delivery based on Multifunctional Phototheranostic Nanoparticles for Photoacoustic Imaging-guided Photothermal-Chemotherapy. Biomaterials. 2018;176:1–12. doi:10.1016/j.biomaterials.2018.05.033
- Yang L, Tseng YT, Suo G, et al. Photothermal Therapeutic Response of Cancer Cells to Aptamer-Gold Nanoparticle-Hybridized Graphene Oxide under NIR Illumination. ACS Appl Mater Interfaces. 2015;7(9):5097–5106. doi:10.1021/am508117e
- Grilli F, Gohari PH, Zou S. Characteristics of Graphene Oxide for Gene Transfection and Controlled Release in Breast Cancer Cells. Int J Mol Sci. 2022;23(12):1–43. doi:10.3390/ijms23126802
- Yao Y, Zhou Y, Liu L, et al. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front Mol Biosci. 2020;7(August):1–14. doi:10.3389/fmolb.2020.00193
- Shin SR, Li YC, Jang HL, et al. Graphene-Based Materials for Tissue Engineering. Adv Drug Deliv Rev. 2016;105:255–274. doi:10.1016/j.addr.2016.03.007
- Ricci A, Cataldi A, Zara S, Gallorini M. Graphene-Oxide-Enriched Biomaterials: a Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials (Basel). 2022;15(6):1–16. doi:10.3390/ma15062229
- Zhou X, Nowicki M, Cui H, et al. 3D Bioprinted Graphene Oxide-Incorporated Matrix for Promoting Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Carbon N Y. 2017;116:615–624. doi:10.1016/j.carbon.2017.02.049
- Andersen ML, Winter LMF. Animal models in biological and biomedical research – experimental and ethical concerns. An Acad Bras Cienc. 2019;91:1–14. doi:10.1590/0001-3765201720170238
- Yan Q, Zhang J, Liu H, et al. Anti-Inflammatory Drug Therapy Alters β-amyloid Processing and Deposition in An Animal Model of Alzheimer’s Disease. J Neurosci. 2003;23(20):7504–7509. doi:10.1523/jneurosci.23-20-07504.2003
- Van Groen T, Kadish I. Transgenic AD Model Mice, Effects of Potential Anti-AD Treatments on Inflammation and Pathology. Brain Res Rev. 2005;48(2):370–378. doi:10.1016/j.brainresrev.2004.12.026
- Howell JC, Watts KD, Parker MW, et al. Race Modifies The Relationship Between Cognition and Alzheimer’s Disease Cerebrospinal Fluid Biomarkers. Alzheimer’s Res Ther. 2017;9(1):1–10. doi:10.1186/s13195-017-0315-1
- Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC-PET Amyloid Imaging Study. Neurology. 2016;87(5):473–480. doi:10.1212/wnl.0000000000002914
- Gargiulo G. Next-Generation in vivo Modeling of Human Cancers. Front Oncol. 2018;8(429):1–18. doi:10.3389/fonc.2018.00429
- Lee SY, Lee DY, Kang JH, et al. Alternative Experimental Approaches to Reduce Animal Use in Biomedical Studies. J Drug Deliv Sci Technol. 2022;68(103131):1–21. doi:10.1016/j.jddst.2022.103131
- Moleiro AF, Conceição G, Leite-Moreira AF, Rocha-Sousa A. A Critical Analysis of the Available in Vitro and Ex Vivo Methods to Study Retinal Angiogenesis. J Ophthalmol. 2017;2017(3034953):1–19. doi:10.1155/2017/3034953
- Usui T, Sakurai M, Nishikawa S, et al. Establishment of A Dog Primary Prostate Cancer Organoid Using The Urine Cancer Stem Cells. Cancer Sci. 2017;108(12):2383–2392. doi:10.1111/cas.13418
- Elbadawy M, Sato Y, Mori T, et al. Anti-Tumor Effect of Trametinib in Bladder Cancer Organoid and The Underlying Mechanism. Cancer Biol Ther. 2021;22(5–6):357–371. doi:10.1080/15384047.2021.1919004
- Penning LC, van den Boom R. Companion Animal Organoid Technology to Advance Veterinary Regenerative Medicine. Front Vet Sci. 2023;10(14):1–12. doi:10.3389/fvets.2023.1032835
- Nicolas J, Magli S, Rabbachin L, Sampaolesi S, Nicotra F, Russo L. 3D Extracellular Matrix Mimics: fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules. 2020;21(6):1968–1994. doi:10.1021/acs.biomac.0c00045
- Koledova Z. 3D Cell Culture: method and Protocols. Methods Mol Biol. 2017;1612:325–344. doi:10.1007/978-1-4939-7021-6_1
- Ghosh S, Spagnoli GC, Martin I, et al. Three-Dimensional Culture of Melanoma Cells Profoundly Affects Gene Expression Profile: a High Density Oligonucleotide Array Study. J Cell Physiol. 2005;204(2):522–531. doi:10.1002/jcp.20320
- Li C, Kato M, Shiue L, Shively JE, Ares M, Lin RJ. Cell Type and Culture Condition-Dependent Alternative Splicing in Human Breast Cancer Cells Revealed by Splicing Sensitive Microarrays. Cancer Res. 2006;66(4):1990–1999. doi:10.1158/0008-5472.can-05-2593
- Ernst A, Hofmann S, Ahmadi R, et al. Genomic and Expression Profiling of Glioblastoma Stem Cell-like Spheroid Cultures Identifies Novel Tumor-Relevant Genes Associated with Survival. Clin Cancer Res. 2009;15(21):6541–6550. doi:10.1158/1078-0432.ccr-09-0695
- Anton D, Burckel H, Josset E, Noel G. Three-Dimensional Cell Culture: a Breakthrough in Vivo. Int J Mol Sci. 2015;16(3):5517–5527. doi:10.3390/ijms16035517
- Kuo CK, Li WJ, Tuan RS. Cartilage and Ligament Tissue Engineering: Biomaterials, Cellular Interactions, and Regenerative Strategies. Elsevier; 2013; doi:10.1016/B978-0-08-087780-8.00114-5
- Planell JA, Navarro M, Engel E. Developing Targeted Biocomposites in Tissue Engineering and Regenerative Medicine. Elsevier Ltd; 2017; doi:10.1016/b978-0-08-100752-5.00023-8
- Nassiri I, McCall MN. Systematic Exploration of Cell Morphological Phenotypes Associated with a Transcriptomic Query. Nucleic Acids Res. 2018;46(19):1–9. doi:10.1093/nar/gky626
- Cell Culture Basic. Invitrogen. Available from: https://www.vanderbilt.edu/viibre/CellCultureBasicsEU.pdf. Accessed October 14, 2023.
- Cadart C, Venkova L, Piel M, Cosentino Lagomarsino M. Volume Growth in Animal Cells Is Cell Cycle Dependent and Shows Additive Fluctuations. Elife. 2022;11:1–18. doi:10.7554/eLife.70816
- Yang N, Ray SD, Krafts K. Cell Proliferation. In: Encyclopedia of Toxicology. Elsevier; 2014:761–765. doi:10.1016/B978-0-12-386454-3.00274-8
- Berridge MJ. Cell Cycle and Proliferation. Cell Signalling Biology. 2014;6:1–45. doi:10.1042/csb0001009
- Duronio RJ, Xiong Y. Signaling Pathways That Control Cell Proliferation. Cold Spring Harb Perspect Biol. 2013;5(3):1–12. doi:10.1101/cshperspect.a008904
- Studzinski GP, Danilenko M. Differentiation and the Cell Cycle. Vitamin D. 2004;25:1635–1661. doi:10.1016/B978-012252687-9/50096-6
- Ehinger M, Bergh G, Olofsson T, Baldetorp B. Expression of the p53 Tumor Suppressor Gene Induces Differentiation and Promotes Induction of Differentiation by 1,25-Dihydroxycholecalciferol in Leukemic U-937 Cells. Blood. 1996;87(3):1064–1074. doi:10.1182/blood.V87.3.1064.bloodjournal8731064
- Sunada S, Saito H, Zhang D, Xu Z, Miki Y. CDK1 inhibitor controls G2/M phase transition and reverses DNA damage sensitivity. Biochem Biophys Res Commun. 2021;550:56–61. doi:10.1016/j.bbrc.2021.02.117
- Lemonnier T, Dupré A, Jessus C. The G2-to-M Transition from a Phosphatase Perspective: a New Vision of the Meiotic Division. Cell Div. 2020;15(1):1–17. doi:10.1186/s13008-020-00065-2
- Sargun S, Joanne DH, Jeremy F, Gordon KC. Investigating Wee1 and Myt1 Combined Inhibition as a Potential Cancer Therapeutic Strategy. Cancer Res. 2023;83. doi:10.1158/1538-7445.AM2023-5511
- Müller WA. Differentiation: a Central Topic in Developmental and Cell Biology. Naturwissenschaften. 1999;86(10):457–467. doi:10.1007/s001140050655
- Muhr J, Hagey DW. The Cell Cycle and Differentiation as Integrated Processes: cyclins and CDKs Reciprocally Regulate Sox and Notch to Balance Stem Cell Maintenance. BioEssays. 2021;43(7):1–12. doi:10.1002/bies.202000285
- Cheung TH, Rando TA. Molecular Regulation of Stem Cell Quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–340. doi:10.1038/nrm3591
- Neganova I, Zhang X, Atkinson S, Lako M. Expression and Functional Analysis of G1 to S Regulatory Components Reveals an Important Role for CDK2 in Cell Cycle Regulation in Human Embryonic Stem Cells. Oncogene. 2009;28(1):20–30. doi:10.1038/onc.2008.358
- Koledova Z, Kafkova LR, Calabkova L, Krystof V, Dolezel P, Divoky V. Cdk2 Inhibition Prolongs G1 Phase Progression in Mouse Embryonic Stem Cells. Stem Cells Dev. 2010;19(2):181–194. doi:10.1089/scd.2009.0065
- Sherr CJ, Roberts JM. Living with or without Cyclins and Cyclin-Dependent Kinases. Genes Dev. 2004;18(22):2699–2711. doi:10.1101/gad.1256504
- Gonzales KAU, Liang H, Lim YS, et al. Deterministic Restriction on Pluripotent State Dissolution by Cell-Cycle Pathways. Cell. 2015;162(3):564–579. doi:10.1016/j.cell.2015.07.001
- Vallier L. Cell Cycle Rules Pluripotency. Cell Stem Cell. 2015;17(2):131–132. doi:10.1016/j.stem.2015.07.019
- Campiglio CE, Figliuzzi M, Silvani S, et al. Influence of Culture Substrates on Morphology and Function of Pulmonary Alveolar Cells in Vitro. Biomolecules. 2021;11(5):1–13. doi:10.3390/biom11050675
- Hussey GS, Dziki JL, Badylak SF. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat Rev Mater. 2018;3(7):159–173. doi:10.1038/s41578-018-0023-x
- Walker C, Mojares E, Del Río Hernández A. Role of Extracellular Matrix in Development and Cancer Progression. Cancer. 2018;19. doi:10.3390/ijms19103028
- Wodarz A, Näthke I. Cell Polarity in Development and Cancer. Nat Cell Biol. 2007;9(9):1016–1024. doi:10.1038/ncb433
- Lee M, Vasioukhin V. Cell Polarity and Cancer - Cell and Tissue Polarity as a Non-Canonical Tumor Suppressor. J Cell Sci. 2008;121(8):1141–1150. doi:10.1242/jcs.016634
- Morrison SJ, Kimble J. Asymmetric and Symmetric Stem-Cell Divisions in Development and Cancer. Nature. 2006;441(7097):1068–1074. doi:10.1038/nature04956
- Walma DAC, Yamada KM. The Extracellular Matrix in Development. Development. 2020;147:1–74. doi:10.1242/dev.175596
- Friedl P, Gilmour D. Collective Cell Migration in Morphogenesis, Regeneration and Cancer. Nat Rev Mol Cell Biol. 2009;10(7):445–457. doi:10.1038/nrm2720
- Scarpa E, Mayor R. Collective Cell Migration in Development. J Cell Biol. 2016;212(2):143–155. doi:10.1083/jcb.201508047
- Yamada KM, Sixt M. Mechanisms of 3D Cell Migration. Nat Rev Mol Cell Biol. 2019;20(12):738–752. doi:10.1038/s41580-019-0172-9
- Bissell MJ, Barcellos-Hoff MH. The Influence of Extracellular Matrix on Gene Expression: is Structure the Message? J Cell Sci. 1987;343:327–343. doi:10.1242/jcs.1987.supplement_8.18
- Xu R, Spencer VA, Bissell MJ. Extracellular Matrix-Regulated Gene Expression Requires Cooperation of SWI/SNF and Transcription Factors. J Biol Chem. 2007;282(20):14992–14999. doi:10.1074/jbc.m610316200
- Kleinman HK, Philp D, Hoffman MP. Role of the Extracellular Matrix in Morphogenesis. Curr Opin Biotechnol. 2003;14(5):526–532. doi:10.1016/j.copbio.2003.08.002
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures In Vitro Without a Mesenchymal Niche. Nature. 2009;459(7244):262–265. doi:10.1038/nature07935
- Patel JJ, Srivastava S, Siow RCM. Tube Formation: an In Vitro Matrigel Angogenesis Assay. In: Methods in Molecular Biology. Vol. 467. Humana Press;2009:183–188. doi:10.1007/978-1-59745-241-0_10
- Lancaster MA, Renner M, Martin CA, et al. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature. 2013;501(7467):373–379. doi:10.1038/nature12517
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi:10.1016/j.semcancer.2005.05.004
- Kleinman HK, McGarvey ML, Hassell JR, et al. Basement Membrane Complexes with Biological Activity. Biochemistry. 1986;25(2):312–318. doi:10.1021/bi00350a005
- Kim S, Min S, Choi YS, et al. Tissue Extracellular Matrix Hydrogels as Alternatives to Matrigel for Culturing Gastrointestinal Organoids. Nat Commun. 2022;13(1):1–21. doi:10.1038/s41467-022-29279-4
- Henke E, Nandigama R, Ergün S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front Mol Biosci. 2020;6(160):1–24. doi:10.3389/fmolb.2019.00160
- Trepat X, Chen Z, Jacobson K. Cell Migration. Compr Physiol. 2012;2(4):2369–2392. doi:10.1002/cphy.c110012
- Yang Q, Liberali P. Collective Behaviours in Organoids. Curr Opin Cell Biol. 2021;72:81–90. doi:10.1016/j.ceb.2021.06.006
- Kim J, Choi KS, Kim Y, et al. Bioactive Effects of Graphene Oxide Cell Culture Substratum on Structure and Function of Human Adipose-Derived Stem Cells. J Biomed Mater Res. 2013;101:1–11. doi:10.1002/jbm.a.34659
- Wojtoniszak M, Chen X, Kalenczuk RJ, et al. Synthesis, Dispersion, and Cytocompatibility of Graphene Oxide and reduced Graphene Oxide. Colloids Surf B Biointerfaces. 2012;89:79–85. doi:10.1016/j.colsurfb.2011.08.026
- Gurunathan S, hoi KJ. Synthesis, Toxicity, Biocompatibility of Graphene and Graphene - related Materials. Int J Nanomed. 2016;11:1927–1945. doi:10.2147/IJN.S105264
- Bellier N, Baipaywad P, Ryu N, Lee JY, Park H. Recent Biomedical Advancements in Graphene Oxide- and reduced Graphene Oxide-based Nanocomposite Nanocarriers. Biomater Res. 2022;26(1):1–23. doi:10.1186/s40824-022-00313-2
- Andrews JPM, Joshi SS, Tzolos E, et al. First-in-Human Controlled Inhalation of Thin Graphene Oxide Nanosheets to Study Acute Cardiorespiratory Responses. Nat Nanotechnol. 2024. doi:10.1038/s41565-023-01572-3
- Lee TJ, Park S, Bhang SH, et al. Graphene Enhances The Cardiomyogenic Differentiation of Human Embryonic Stem Cells. Biochem Biophys Res Commun. 2014;452(1):174–180. doi:10.1016/j.bbrc.2014.08.062
- Kim T, Kahng YH, Lee T, Lee K, Kim DH. Graphene Films Show Stable Cell Attachment and Biocompatibility with Electrogenic Primary Cardiac Cells. Mol Cells. 2013;36(6):577–582. doi:10.1007/s10059-013-0277-5
- Wang J, Cui C, Nan H, et al. Graphene Sheet-Induced Global Maturation of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. ACS Appl Mater Interfaces. 2017;9(31):1–38. doi:10.1021/acsami.7b08777
- Malkawi AK, Alzoubi KH, Jacob M, et al. Metabolomics Based Profiling of Dexamethasone Side Effects in Rats. Front Pharmacol. 2018;9:1–14. doi:10.3389/fphar.2018.00046
- Shields LBE, Raque GH, Glassman SD, et al. Adverse Effects Associated With High-Dose Recombinant Human Bone Morphogenetic Protein-2 Use in Anterior Cervical Spine Fusion. Spine (Phila Pa 1976). 2006;31(5):542–547. doi:10.1097/01.brs.0000201424.27509.72
- Mocan T, Bartos D, Mocan L. Influence of Nanomaterials on Stem Cell Differentiation: designing an Appropriate Nanobiointerface. Int J Nanomed. 2012;7:2211–2225. doi:10.2147/ijn.s29975
- He F, Cao J, Qi J, Liu Z, Liu G, Deng W. Regulation of Stem Cell Differentiation by Inorganic Nanomaterials: recent Advances in Regenerative Medicine. Front Bioeng Biotechnol. 2021;9:1–10. doi:10.3389/fbioe.2021.721581
- Boundless. General biology. LibreTexts; 2023. Available from: https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless). Accessed October 14, 2023.
- Yasuda D, Kobayashi D, Akahoshi N, et al. Lysophosphatidic Acid – induced YAP / TAZ Activation Promotes Developmental Angiogenesis by Repressing Notch Ligand Dll4. J Clin Invest. 2019;129(10):4332–4349. doi:10.1172/jci121955
- Azad T, Ghahremani M, Yang X. The Role of YAP and TAZ in Angiogenesis and Vascular Mimicry. Cells. 2019;8:1–22. doi:10.3390/cells8050407
- xing YF, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cells. 2012;150:780–791. doi:10.1016/j.cell.2012.06.037
- Wang K, Ruan J, Song H, et al. Biocompatibility of Graphene Oxide. Nanoscale Res Lett. 2011;6(1):1–8. doi:10.1007/s11671-010-9751-6
- Lu P, Yazdi AZ, Han XX, et al. Mechanistic Insights into the Cytotoxicity of Graphene Oxide Derivatives in Mammalian Cells. Chem Res Toxicol. 2020;33:2247–2260. doi:10.1021/acs.chemrestox.9b00391
- Wei Q, Pei S, Wen G, et al. High Yield Controlled Synthesis of Nano-Graphene Oxide by Water Electrolytic Oxidation of Glassy Carbon for Metal-Free Catalysis. ACS Nano. 2019;13:1–31. doi:10.1021/acsnano.9b04447
- Hirota A, AlMusawi S, Nateri AS, Ordóñez-Morán P, Imajo M. Biomaterials for Intestinal Organoid Technology and Personalized Disease Modeling. Acta Biomater. 2021;132:1–72. doi:10.1016/j.actbio.2021.05.010
- Magno V, Meinhardt A, Werner C. Polymer Hydrogels to Guide Organotypic and Organoid Cultures. Adv Funct Mater. 2020;30(48):1–34. doi:10.1002/adfm.202000097
- Stowers RS. Advances in Extracellular Matrix-Mimetic Hydrogels to Guide Stem Cell Fate. Cells Tissues Organs. 2022;211(6):1–18. doi:10.1159/000514851
- Caliari SR, Burdick JA. A Practical Guide to Hydrogels for Cell Culture. Nat Methods. 2016;13(5):405–414. doi:10.1038/nmeth.3839
- Thiele J, Ma Y, Bruekers SMC, Ma S, Huck WTS. Anniversary Article: designer Hydrogels for Cell Cultures: a Materials Selection Guide. Adv Mater. 2013;26(1):125–148. doi:10.1002/adma.201302958
- Banerjee A, Arha M, Choudhary S, et al. The Influence of Hydrogel Modulus on the Proliferation and Differentiation of Encapsulated Neural Stem Cells. Biomaterials. 2009;30(27):4695–4699. doi:10.1016/j.biomaterials.2009.05.050
- Shahidi S, Janmaleki M, Riaz S, Sanati Nezhad A, Syed N. A Tuned Gelatin Methacryloyl (GelMA) Hydrogel Facilitates Myelination of Dorsal Root Ganglia Neurons in Vitro. Mater Sci Eng C. 2021;126:1–12. doi:10.1016/j.msec.2021.112131
- Chuang CH, Lin RZ, Tien HW, et al. Enzymatic Regulation of Functional Vascular Networks Using Gelatin Hydrogels. Acta Biomater. 2015;19:85–99. doi:10.1016/j.actbio.2015.02.024
- Tian DM, Wan HH, Chen JR, et al. In-Situ Formed Elastin-Based Hydrogels Enhance Wound Healing via Promoting Innate Immune Cells Recruitment and Angiogenesis. Mater Today Bio. 2022;15(100300):1–18. doi:10.1016/j.mtbio.2022.100300
- Ikram R, Shamsuddin SAA, Jan BM, et al. Impact of Graphene Derivatives as Artificial Extracellular Matrices on Mesenchymal Stem Cells. Molecules. 2022;27(2):1–26. doi:10.3390/molecules27020379
- Du Y, Yu M, Lu W, Kong J. Three-Dimensional (3D), Macroporous, Elastic, and Biodegradable Nanocomposite Scaffold for in Situ Bone Regeneration: toward Structural, Biophysical, and Biochemical Cues Integration. Compos Part B Eng. 2021;225:1–14. doi:10.1016/j.compositesb.2021.109270
- Chen GY, Pang DWP, Hwang SM, Tuan HY, Hu YC. A Graphene-Based Platform for Induced Pluripotent Stem Cells Culture and Differentiation. Biomaterials. 2012;33(2):418–427. doi:10.1016/j.biomaterials.2011.09.071
- Ku SH, Park CB. Myoblast Differentiation on Graphene Oxide. Biomaterials. 2013;34(8):2017–2023. doi:10.1016/j.biomaterials.2012.11.052