Abstract
Wound healing in diabetic patients is frequently hampered. Adipose-derived stem cell exosomes (ADSC-eoxs), serving as a crucial mode of intercellular communication, exhibit promising therapeutic roles in facilitating wound healing. This review aims to comprehensively outline the molecular mechanisms through which ADSC-eoxs enhance diabetic wound healing. We emphasize the biologically active molecules released by these exosomes and their involvement in signaling pathways associated with inflammation modulation, cellular proliferation, vascular neogenesis, and other pertinent processes. Additionally, the clinical application prospects of the reported ADSC-eoxs are also deliberated. A thorough understanding of these molecular mechanisms and potential applications is anticipated to furnish a theoretical groundwork for combating diabetic wound healing.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder with a global prevalence, exhibiting a rising incidence that impacts the health and lifestyles of millions.Citation1 It is predicted that by 2045, 783.2 million people worldwide will be diagnosed with DM, indicating a widespread global epidemic and a significant impact on the global population.Citation2 In addition to metabolic abnormalities, diabetes commonly gives rise to multi-system, and multi-organ complications in patients, including diabetic retinopathy, diabetic nephropathy, neurological impairments, cognitive impairment, and fatty infiltration of the pancreas.Citation3–7 One severe complication among them is non-healing wounds or lesions, which can lead to serious consequences such as limb amputation. Furthermore, the mortality rate within 5 years after amputation is reported to be as high as 80%.Citation8,Citation9 Currently, clinical management strategies for diabetic wound treatment predominantly include necrotic tissue debridement, antimicrobial interventions, limb immobilization, and wound care modalities, such as sustained negative pressure suction and the application of growth factors.Citation10 These interventions are implemented to optimize patient prognosis by enhancing healing efficacies and minimizing complications associated with diabetic wounds.Citation11,Citation12 In recent years, with the progress of stem cell research, adipose-derived stem cells (ADSCs), characterized by accessibility, ease of preparation, and robust regulatory and reparative capabilities, have been proven to exert promoting effects in the repair of various tissues and organ injuries, including hepatic and renal damage and wound healing.Citation13–16
In this context, the exosomes released by ADSCs (ADSC-exos), serving as a form of intercellular communication, have attracted considerable attention for their robust cellular functional regulatory capabilities, endowing a novel therapeutic candidate for improving wound healing in diabetic patients.Citation17,Citation18 Exosomes originated from multivesicular bodies (MVBs) formed by the invagination of cell membranes, characterized by a diameter ranging from 40 to 160 nm. Depending on the cell source, exosomes may encompass distinct components, such as RNA, lipids, and membrane proteins. Exosomes are postulated to modulate various physiological and pathological processes.Citation19,Citation20 Recent animal studies have indicated the therapeutic effects of ADSC-exos in diverse conditions, including organ injuries,Citation21,Citation22 neurodegenerative disorders,Citation23,Citation24 and tumors.Citation25,Citation26 Exploring the potential of ADSC-exos in promoting diabetic wound healing and the underlying molecular mechanisms is worthwhile.
Hence, this review systematically summarizes published literature of ADSC-exos and their effects on diabetic wound healing. The primary objective is to investigate the molecular mechanisms through which ADSC-exos promote the healing of diabetic wounds. Additionally, the review explores the application prospects of employing these exosomes. The deep understanding of this field will provide theoretical basis and applicable candidates for combating diabetic wound healing.
The Fundamental Characteristics of ADSC-Exos
In 1981, Trams et al identified vesicle structures with ATPase activity isolated from cell cultures and coined the term “exosomes”.Citation27 Subsequently, by 1983, extracellular vesicles (EVs) with an average diameter of approximately 100 nm were observed in sheep reticulocytes, marking the inception of what is now recognized as exosomes.Citation28 The composition of exosomes varies depending on the cell source. ADSC-exos exhibit diverse and distinctive biological functions. Understanding these functions and their underlying molecular mechanisms is paramount for comprehending the therapeutic effects of ADSC-exos in the context of diabetic wound healing.
Definition, Biogenesis, and Contents of Exosomes
Exosomes represent a subtype of EVs, and the International Society for Extracellular Vesicles (ISEV) defines EVs as a collective term for lipid bilayer-enclosed particles naturally released from cells, lacking a functional nucleus and the ability to self-replicate.Citation29 Among EVs, exosomes are the smallest, with a diameter ranging from approximately 40 to 160 nm, averaging around 100 nm. Other types of EVs include microvesicles and apoptotic bodies.Citation29 Virtually all cells can produce exosomes, whether in physiological or pathological states.Citation30
Exosomes originate from the continuous inward folding of the cell membrane, giving rise to Intraluminal vesicles (ILVs) via inward budding.Citation31 The membranes of ILVs continue to invaginate, accumulating within endosomes to form MVBs.Citation32 Cargoes are loaded into ILVs from the Golgi apparatus through the Endosomal sorting complex for transport (ESCRT) pathway.Citation33 However, MVB formation is not exclusively dependent on the ESCRT pathway, suggesting the existence of alternative pathways for cargo loading into ILVs.Citation34 Eventually, the membrane of MVBs fuses with the cell membrane, releasing ILVs into the extracellular environment, or undergoes lysosomal degradationCitation35 ().
Figure 1 Biogenesis, markers, and contents of exosomes. The canonical biogenesis pathway of exosomes involves the invagination of the plasma membrane, with the ESCRT facilitating cargo loading to form MVBs. Subsequent fusion of MVBs with the cellular membrane releases exosomes. Biomarkers for exosomes mainly include CD9, CD63, CD81, Alix, HSP70, and TSG101. The cargoes of exosomes are comprised of DNA, RNA (mRNA, miRNA, circRNA, and IncRNA), proteins, and lipids. ESCRT, endosomal sorting complex for transport; MVBs, multivesicular bodies; HSP70, heat shock protein 70; mRNAs, messenger RNAs; miRNAs, microRNA; circRNAs, circular RNAs; IncRNAs, long non-coding RNAs. (Figure created using Figdraw).
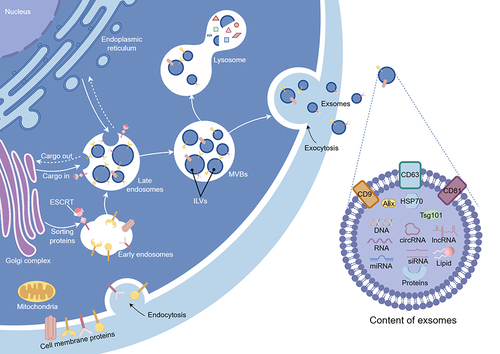
Exosomes harbor proteins, mitochondrial DNA, lipids, and RNA (comprising mRNA, miRNA, lncRNA, circRNA).Citation36,Citation37 Due to the mechanism of membrane invagination, the current understanding suggests that exosome proteins encompass membrane proteins and select cytoplasmic proteins, while lacking glycolytic enzymes and cytoskeletal proteins.Citation38,Citation39 According to the ExoCarta database (http://www.exocarta.org/), over 41,860 types of exosome proteins, 4946 types of mRNA, 2838 types of miRNA, and 1116 types of lipids have been documented.Citation40 However, this database lacks information on other ncRNAs and mitochondrial DNA. The ExoRBase database (http://www.exorbase.org/) stands as a potentially valuable supplement.Citation41
The Isolation and Identification of ADCS-Exos
Human ADSCs are typically isolated from adipose tissue obtained through surgical excision or liposuction procedures.Citation42,Citation43 Mouse ADSCs are usually isolated from adipose tissue harvested from the inguinal region.Citation44,Citation45 The enzymatic digestion method stands as the most frequently employed technique for isolating ADSCs. This process involves sequential steps, including tissue fragmentation, enzymatic digestion, filtration, centrifugation, and cultivation, for ADSC isolation.Citation42 ADSCs exhibit both self-renewal and multi-lineage differentiation capacities. ADSCs possess specific stemness markers, primarily including CD29, CD44, CD73, and CD90, accompanied with little expression of CD34 and CD45.Citation42,Citation43 Validation of successful ADSC acquisition can be confirmed through analytical methods such as Western blotting and flow cytometry.
Exosomes can be enriched from cell culture supernatants through methods such as differential centrifugation, size-exclusion chromatography, field-flow fractionation, microfiltration, or non-contact sorting immunomagnetic enrichment. Differential centrifugation has been considered as a frequently used approach for exosome isolation. Differential centrifugation is simple to perform, facilitates the extraction of large amounts of exsomes,Citation46 but the purity of exosomes in the extracts is relatively low, necessitating combination with other methods for purification.Citation47 Size-exclusion chromatography is regarded as the most effective method for enhancing exosomes purity.Citation48 The combined use of differential centrifugation and size-exclusion chromatography is expected to ensure both yield and purity, thereby enhancing the precision of subsequent analyses.Citation49,Citation50
Methods for identifying exosomes include transmission electron microscope (TEM), traditional fluorescence microscopy, super-resolution microscopy, and nanoparticle tracking. Identification can also be accomplished through immunoblotting, and mass spectrometric analysis of exosome proteins or RNA. Commonly used markers for identifying exosomes from ADSCs mainly include the a series of conserved proteins CD9, CD63, CD81, HSP70, Tsg101, and Alix.Citation20,Citation51–53
Biological Functions of ADSC-Exos
ADSCs participate in intercellular signal transduction through endocrine, autocrine, and paracrine mechanisms.Citation54 Exosomes, as crucial entities in cell signaling, play a vital role in protecting internal cargo stability, preventing the degradation of cellular signals by external conditions, and ensuring the safe delivery of cargo to target cells.Citation19 This process allows for the modulation of cellular functions. Existing literature indicates that ADSC-exos possess various functions, including immune modulation, antioxidant stress response, promotion of neovascularization, inhibition of scar formation, and anti-aging effects.
The immunomodulatory function of ADSC-exos is manifested through the regulation of macrophage polarization, promoting the transition of macrophages from a pro-inflammatory phenotype to an anti-inflammatory phenotype. This modulation contributes to the regulation of the immune microenvironment, suppressing the release of inflammatory factors, such as TNF-β and exerting protective effects on organs such as the heart, liver, lungs, and kidneys.Citation21,Citation43,Citation55,Citation56 Liu et al demonstrate that ADSC-exos can enhance the antioxidant stress capacity of myocardial cells.Citation57 Zhang et al discovered that ADSC-exos reduced ROS accumulation and inhibited apoptosis in kidney cells induced by Lipopolysaccharide (LPS), thereby exhibiting therapeutic effects on renal injury.Citation58 Li et al proved that ADSC-exos decreased high glucose (HG) induced apoptosis and senescence of vascular endothelial cells, thereby promoting the formation of local neovascularization.Citation59 ADSC-exos also regulates glucose metabolism, inhibits lipid accumulation, and modulates ceramide synthesis, and may play a therapeutic role in diabetes, obesity, and atopic dermatitis.Citation60–62
Furthermore, during the wound healing process, ADSC-exos promote wound healing by stimulating fibroblast proliferation.Citation63 ADSC-exos also inhibited scar formation by regulating extracellular matrix (ECM) remodeling and altering the ratio of type III to type I collagen, thereby facilitating scarless wound healing.Citation64 Due to their potent biological functions, ADSC-exos exhibit considerable application potential.
ADSCs offer a relatively accessible means of procurement. The diverse biological functions of ADSC-exos, coupled with their multifunctionality, position them as powerful tools in the fields of tissue engineering, stem cell therapy, and regenerative medicine.
Challenges of Diabetic Wound Healing
Diabetes is a risk factor for various disease.Citation65 Diabetes can adversely affect wound healing from multiple perspectives, often leading to chronic wounds, delayed healing, and infections. The following is a summary of the impact of diabetes on wound healing and the primary therapeutic approaches currently employed in clinical practice.
Effect of Diabetes on Wound Healing
The repair process of a wound is immediately initiated at the moment of injury. Currently the general acute wound healing process can be primarily categorized as four independent but interconnected stagesCitation66,Citation67 ().
| 1) | Hemostasis and coagulation: Blood clotting temporarily restores the continuity of tissue or skin, preventing the invasion of contaminants or bacteria into the internal tissues. Platelets and fibrin in the plasma form a temporary ECM, providing a foundation for the subsequent recruitment of inflammatory cells, fibroblasts, and others.Citation66–68 | ||||
| 2) | Inflammatory phase: Neutrophils are the first to enter the wound tissue post-injury, followed by lymphocytes and monocytes. Monocytes transform into macrophages within the tissue, which could be regulated by various cytokines, clear bacteria, and cell debris.Citation66,Citation69,Citation70 | ||||
| 3) | Proliferative phase: Around the 3rd day post-injury, after the clearance of bacteria, macrophages shift to an M2 anti-inflammatory proliferative phenotype. Macrophages signal fibroblasts and vascular endothelial cells to promote fibrin deposition and neointimal formation, thereby facilitating wound closure.Citation70,Citation71 | ||||
| 4) | Remodeling: Macrophages secrete various enzymes to promote fibrin remodeling. This step is significant in scarless wound healing.Citation70,Citation72 | ||||
These four steps do not have distinct boundaries, and interference with any of them can lead to the development of chronic wounds.Citation66 Moreover, macrophage dysfunction is associated with the formation of scar tissue.Citation73
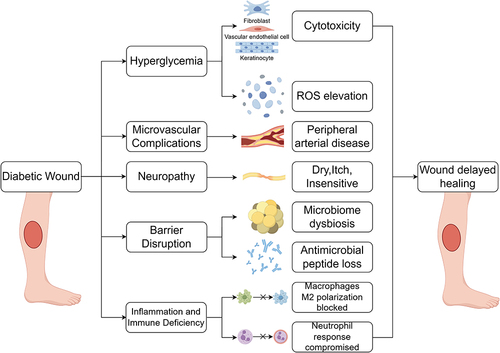
Diabetes exerts adverse effects on wound healing through various mechanisms, notably encompassing:Citation74 ()
| 1) | Hyperglycemia-induced cytotoxicity and oxidative stress: Elevated blood glucose levels lead to cellular toxicity and oxidative stress, detrimentally affecting the wound healing process. | ||||
| 2) | Microvascular pathology: Diabetes induces structural and functional changes in microvessels, impairing the vascular supply to wounds. | ||||
| 3) | Neurotoxicity: Diabetes contributes to nerve damage, resulting in impaired neural supply to wounds, sensory deficits, and neurofunctional impairment. | ||||
| 4) | Compromised antimicrobial barrier: Elevated blood glucose disrupts the immune system, rendering diabetic individuals more susceptible to wound infections. | ||||
| 5) | Wound infection and immune system dysfunction: Diabetic wounds are prone to infections, and dysfunction of the immune system further complicates the healing process. | ||||
Figure 2 Four stages of normal wound healing. The normal wound healing process consists of four distinct overlapping stages, namely hemostasis and coagulation, the inflammatory stage, the proliferative stage, and remodeling. (Figure created using Figdraw).
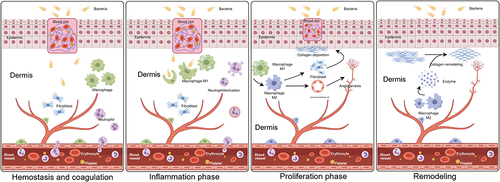
Figure 3 Various factors for diabetic wounds exhibit delayed healing. Multiple factors collectively contribute to the prolonged non-healing of diabetic wounds, mainly including the cytotoxicity of HG, microvascular complications, neuropathy, disruption of antimicrobial barriers, inflammation, and immune deficiency. HG, high glucose. (Figure created using Figdraw).
In a meta-analysis encompassing 67 articles from 33 countries with a substantial total sample size of 800,000 cases, diabetic wounds persist as a formidable challenge in the medical and health sector, particularly in low-income countries.Citation75,Citation76 Despite studies suggesting the efficacy of certain treatments, such as continuous negative pressure therapy, the complete healing rate and healing duration for chronic diabetic wounds remain unsatisfactory.Citation77,Citation78
As elucidated by advancing basic research, macrophages emerge as pivotal players in the diabetic wound healing trajectory.Citation69 The plasticity of macrophages, governed by diverse epigenetic mechanisms, plays a crucial role in orchestrating the transition from the inflammatory to the proliferative phase of wound healing.Citation79 In diabetic wounds, macrophages face challenges in smoothly transitioning from the pro-inflammatory phenotype (M1) to the anti-inflammatory proliferative phenotype (M2), resulting in sustained chronic inflammation.Citation71 Chronic inflammation affects the activities of fibroblasts, endothelial cells, and epidermal cells, hampering the formation of the ECM and impeding neovascularization, ultimately leading to delayed wound healing.Citation80–82 Consequently, fostering M2 polarization of macrophages in diabetic wounds appears to be a pivotal avenue for enhancing the healing process.Citation83
Primary Therapeutic Measures for Diabetic Wound Treatment
As per the 2019 guidelines issued by the International Working Group on the Diabetic Foot (IWGDF), the current clinical management of chronic diabetic foot wounds encompasses:Citation84,Citation85 1) Offloading to reduce local pressure, 2) Restoration of tissue perfusion, 3) Infection control, 4) Optimization of blood glucose management, 5) Wound fluid control or local continuous negative pressure therapy. Among these interventions, local continuous negative pressure therapy stands out as a globally employed measure for treating diabetic foot ulcers (DFUs) and chronic wounds.
In a small-sample clinical trial, Raghupathy et al indicated that Negative Pressure Wound Therapy (NPWT) significantly reduced the complete healing time of chronic wounds.Citation86 However, NPWT is not without limitations, including:
| 1) | Complications: Potential complications encompass bleeding (including wound oozing and vascular bleeding), local pain, malodor, wound infection, and damage to surrounding tissues. Severe complications may precipitate septic shock, intestinal fistula, and hemodynamic instability.Citation87 | ||||
| 2) | Cost Implications: The method is relatively expensive compared to optimal wound care treatments.Citation78 | ||||
| 3) | Limited Efficacy for Wounds with Arterial Insufficiency: Effectiveness may be compromised in wounds characterized by arterial blood supply issues.Citation88 | ||||
| 4) | Microcirculatory Improvement Requires Further Validation: The purported enhancement of microcirculation through this method necessitates additional validation.Citation89 | ||||
In recent years, researchers have proposed the utilization of stem cell exosomes, hydrogels, and a combination of 3D printing technologies, for diabetic wound treatment.Citation90 ADSC-exos are a type of nanomaterial that is considered to have promising clinical applications.Citation91 These therapeutic approaches are currently undergoing continuous investigation, and their progression towards clinical application requires further investigations.
ADSC-Exos Function on the Healing of Diabetic Wounds
The promotion role of ADSC-exos was observed in the healing process of normal wounds, and the development of biomaterials loaded with ADSC-exos is currently in the preclinical stage.Citation92 For diabetic wounds, ADSC-exos is a promising candidate.
ADSC-Exos in Promoting Wound Healing in Diabetic Animal Models
Experiments in animals have shown the healing effects of ADSC-exos, suggesting that there may be a similar effect in humans. The wound model of diabetic mice has been utilized to demonstrate the significant promoting effect of ADSC-exos on diabetic wound healing.Citation93–95 The healing capacity of ADSC-exos could be enhanced through low oxygen preconditioning of ADSCs.Citation96,Citation97 Other scholars have attempted different pre-treatment measures to strengthen the healing capacity of exosomes, such as overexpression circRNA or transfection hepatocyte growth factor (HGF).Citation98–100 The exploration of using biomaterials to carry ADSC-exos are emerging as a recent and promising research direction. Biomaterials loaded with exosomes can continuously release exosomes to the wound, thereby promoting diabetic wound healing.Citation101–103 These experiments have achieved a certain degree of success, and the following is a summary ().
Table 1 Summary of ADSC-Exos Promoting Diabetic Wound Healing in vivo
Model Animals and Methods
Most commonly used experimental animals include C57BL mice,Citation93,Citation96,Citation97,Citation99 Wistar rats for experiments related to biomaterials,Citation102,Citation103 and occasionally Balb/c mice for specific studies.Citation98 There are two common methods for modeling diabetic mice. The mice are induced into a diabetic state either by a single intraperitoneal injection of streptozotocin (STZ) at 60 mg/kgCitation96–99 or by intraperitoneal injection of 50 mg/kg for consecutive 5 days.Citation93 Model is successfully established when mouse blood glucose reaches 16.7 mmol/LCitation93–95,Citation100 or 250 mg/dLCitation96–99 three days after intraperitoneal injection of STZ, and the stabilizing blood glucose level for one month is a indicated time point for subsequent exploration.Citation96–99 Different reasonable STZ doses are subjected to other mouse and rat species for diabetic model construction. As for the wound models, the commonly employed models included the full-thickness injury model on the mouse back,Citation93–95,Citation100,Citation101 followed by the mouse dorsal leg.Citation96–99 Multiple-point injection of exosome solution within the wound edges is a popular method of implementation in constructing wound models. The commonly used dosage is 200 ug of exosomes dissolved in an appropriate phosphate buffer saline (PBS) solution, with injections distributed at multiple points around the wound through subcutaneous injection.Citation94,Citation96,Citation97
Results Section
Ren et al found that ADSC-exos can promote the full-thickness wound healing of diabetic mice. On the 8th day, there was a significant reduction in wound size observed compared to the untreated diabetic mice wounds. Additionally, Masson staining revealed a more extensive collagen deposition in the exosome group compared to the control group. The arrangement of collagen fibers was more orderly, resembling the wound healing level of healthy mice.Citation93 Zhang et al observed more CD34+ cells in the treated group wounds, indicating a higher production of new blood vessels after treatment with ADSC-exos. Furthermore, the levels of VCAM, IL-1, IL-6, TNF-α, and MCP-1 were reduced in the treated group, suggesting that ADSC-exos decreased the local inflammatory response in the wounds.Citation94 Wang et al also reported better collagen deposition and the formation of new microvessels in the treated group.Citation95 Through pre-treating ADSCs in a low oxygen environment for 12 hours, Shi et al observed a more pronounced healing effect of the ADSC-exos in a diabetic mouse wound. The decrease in TNF-α, IL-6, and IL-1β, along with more evident neovascularization, may be related to the enhanced ability of low oxygen exosomes to promote M2 macrophage polarization.Citation96 Hu et al also discovered that ADSC-exos with low oxygen treatment have a stronger therapeutic effect, with a more intact epithelial structure and a more orderly arrangement of collagen in wounds.Citation97 Wang et al found that overexpression of circ-Astn1 in ADSCs enhanced the healing-promoting effect of exosomes, inhibiting apoptosis in skin tissues and enriching neovascularization in wounds. Conversely, knocking down circ-Astn1 suppressed the healing-promoting effect of exosomes.Citation98 Shi et al, through overexpressing mmu_circ_0000250 in ADSCs, also obtained exosomes with stronger healing-promoting abilities, promoting the formation of new blood vessels in wounds.Citation99 Tao et al achieved similar results by transfecting HGF into ADSCs.Citation100
In vivo experiments substantiate the efficacy of ADSC-exos in promoting diabetic wound healing. Furthermore, the healing-promoting capacity of ADSC-exos is notably augmented when ADSCs undergo hypoxic preconditioning or express a specific circRNA or cytokine. The collective findings of these studies underscore the therapeutic potential of ADSC-exos in the context of diabetic wound healing, suggesting a clinical translational value. The experimental methodologies utilized in these investigations may serve as instructive benchmarks for researchers in the field.
ADSC-Exos in Improving Skin Cell Function
The efficacy of ADSC-exos in facilitating the healing of diabetic wounds has been unequivocally established. This involves ameliorating the local inflammatory milieu, promoting angiogenesis, inducing cellular matrix deposition, and orchestrating orderly arrangements, all of which demand the systematic involvement of diverse tissue cells at the wound site. The subsequent discussion provides an overview of the impact of ADSC-exos on the functional aspects of tissue cells participating in wound repair under diabetic conditions. ()
Figure 4 Promotion effect of ADSC-exos on skin cell functions. Existing cell experimental results indicate that under high-glucose conditions, ADSC-exos impacted various biological functions of skin cells, including proliferation, migration, angiogenesis, and anti-apoptosis, and could promote macrophage M2 polarization. Ultimately, ADSC-exos promote the diabetic wound healing. Exosomes from hypoxia, overexpression of specific circRNAs, or NFIC transfection pretreated ADSCs also present robust capabilities of healing-promoting effect. (Figure created using Figdraw).
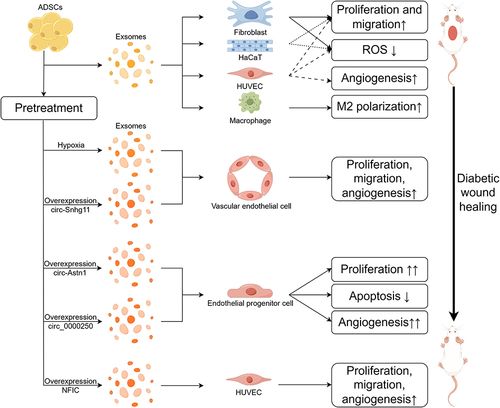
Ren et al discovered that ADSC-exos can enhance the proliferative and migratory capabilities of fibroblasts, Human umbilical vein endothelial cells (HUVECs), and HaCaT cells. ADSC-exos eliminate cell apoptosis induced by H2O2, reduce but do not completely eliminate intracellular Reactive oxygen species (ROS) generation induced by H2O2. Additionally, ADSC-exos promote the angiogenesis of HUVECs, demonstrating a protective and function-promoting effect on the aforementioned skin cells.Citation93 Zhang et al validated that the promotion of HUVEC proliferation by ADSC-exos is positively correlated with exosome concentration. Moreover, ADSC-exos enhance migration and angiogenesis functions in a high-glucose environment while reducing ROS generation induced by HG.Citation94 Wang et al also discovered the proliferative and migratory effects of angiogenesis on fibroblasts.Citation95
Diverging from this, Hu et al observed that after subjecting ADSCs to hypoxic conditions, the exosomes derived exhibited heightened efficacy in promoting endothelial cell proliferation, migration, and angiogenic capabilities.Citation97 Similarly, Wang et al, following overexpression of circ-Astn1 in ADSCs, augmented the potency of exosomes in stimulating Endothelial progenitor cell (EPC) proliferation, anti-apoptosis, and angiogenesis under hyperglycemic conditions.Citation98 Overexpression of mmu_circ_0000250 produced analogous outcomes, with Shi et al noting that the autophagy inhibitor chloroquine (CQ) could reverse this effect.Citation99 Huang et al found that ADSC-exos possessed the capability to enhance proliferation, migration, and vascularization in HUVECs. The attenuation of these capacities occurred upon knockdown of NFIC in ADSCs, whereas overexpression of NFIC bolstered ADSC-exos migratory promotion potency.Citation104 However, exploration of the impact of ADSC-exos on macrophages has been comparatively limited. Shi et al validated the capacity of ADSC-exos to promote M2 polarization of macrophages at the wound site, representing an additional cellular mechanism through which ADSC-exos contribute to the facilitation of diabetic wound healing.Citation96
ADSC-exos exhibit the ability to promote the biological functions of various cells involved in wound repair, including fibroblasts, keratinocytes, and endothelial cells. This effect is even more pronounced when ADSCs undergo hypoxic preconditioning and other modifications. However, the impact of ADSC-exos on macrophages in diabetic wound sites is currently underexplored. Additionally, the role of macrophage polarization in influencing other cell types at the wound site warrants further investigation. With ongoing research, we will soon gain a deeper understanding of the mechanisms through which ADSC-exos facilitate the diabetic wound healing.
Molecular Mechanisms of ADSC-Exos in Promoting Diabetic Wound Healing
Cells, as the fundamental units executing biological functions, are regulated by various cellular factors, and this regulatory mechanism is often intricate, involving multiple intracellular signaling pathways and transduction mechanisms. A profound understanding of the molecular regulatory mechanisms inside and outside cells during wound repair is crucial for comprehending the healing-promoting capabilities of ADSC-exos. The following is a summary from a molecular mechanism perspective. ()
Figure 5 Partial molecular mechanisms underlying the pro-healing effects of ADSC-exos in different cells. The ADSC-exo membrane protein HSP90 is bound to the fibroblast membrane protein LPR1 and promote AKT/ERK phosphorylation. ADSC-exos promote SIRT3 expression, downregulate the acetylation level of SOD2, and simultaneously upregulate ANG1, FILK1, and VEGF, while downregulate VASH1 and TSP1 in HUVECs. The ADSC-exo circ-Snhg11 is promoted by hypoxia. And circ-Snhg11 inhibit miR-144-3p, thereby promoting the protein expression of HIF-1α and NFE2L2, which in turn promote the expression of downstream proteins such as VEGF, Notch1, STAT3 and ETBR in EPCs. HIF-1α inhibits HG-induced inflammatory factors. The molecular mechanism of circRNA overexpression modified ADSC-exos in EPCs is as follows: circ-Astn1 inhibits miR-138-5p, relieves the inhibition on SIRT3, and SIRT3 deregulates FOXO1; meanwhile circ-0000250 inhibits miR-128-3p, relieves the inhibition on SIRT3, and SIRT3 promotes the expression of LC3. ADSC-exos carry NFIC into HUVECs. NFIC is directly bound to the promoter of miR-204-3p to promote miR-204-3p synthesis. And miR-204-3p inhibits the expression of HIPK2. (Figure created using Figdraw).
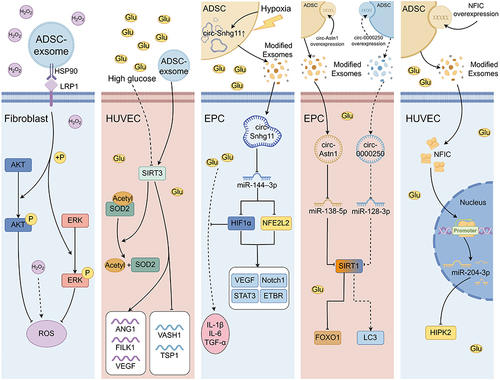
Angiogenesis: Che et al found that ADSC-exos upregulated miR-146a-5p in HUVECs, inhibited the expression of the downstream protein JAZF1, promoted the expression of VEGFA, and consequently facilitated angiogenesis in diabetic wounds.Citation105 Huang et al demonstrated that NFIC in ADSC-exos could promote miR-204-3p transcription to inhibit downstream gene HIPK2 in HUVECs, thus promoting angiogenesis.Citation104 Wang et al found higher circ-Astn1 expression in ADSCs than in EPCs. Under HG conditions, circ-Astn1 expression in EPCs decreased significantly. ADSC-overexpressing circ-Astn1 enhanced healing abilities by downregulating miR-138-5p and promoting SIRT1 to inhibit FOXO1 in EPCs, and thus regulating angiogenesis.Citation98 In the investigation involving hypoxic treatment of ADSCs, Hu et al identified the elevated levels of circ-Snhg11 in ADSC-exos, which led to miR-144-3p down-regulation in HUVECs, thereby promoting the expression of NFE2L2, HIF1α, thus up-regulating of Notch1, STAT3, and ETBR, leading to promotion of diabetic wound angiogenesis.Citation97 Liang et al showed that circ_0001052 overexpression modification of ADSC-exos downregulated miR-106a-5p, promoted FGF4 expression, activated the p38 MAPK pathway in HUVECs, thus promoting angiogenesis.Citation106 Qiu et al found that the overexpression of linc00511-modified ADSC-exos inhibited PAQR3 expression, and increased Twist1 protein levels in EPCs by inhibiting BTRC-mediated Twist1 ubiquitin degradation for promoting angiogenesis.Citation107
ROS improvement: Ren et al indicates that the surface interaction between HSP90 on ADSC-exos and LRP1 on fibroblasts activates the AKT/ERK signaling pathway, thereby enhancing the ability of fibroblasts to scavenge ROS. Disruption at any point in the HSP90, LRP1, AKT, or ERK pathways can impede this protective effect.Citation93 Zhang et al found that HG stimulation led to the downregulation of SIRT3 protein expression in HUVECs. ADSC-exos were shown to promote the expression of SIRT3 in HUVECs. Additionally, this resulted in an increase in the expression levels of SOD2, a decrease in acetylation levels, which improved the level of HG-induced oxidative stress. The mRNA levels of angiogenesis-related factors such as ANG1, FILK1 and VEGF were elevated, and mRNA levels of endogenous angiogenesis inhibitors VASH1 and TSP1 were decreased.Citation94 Shi et al confirmed similar results by overexpressing circ-0000250 in ADSCs. Circ-0000250 downregulated miR-128-3p and promoted SIRT1 expression, thus exerting a protective effect through inducting EPC autophagy.Citation99
Inflammation suppression: Shi et al observed elevated levels of circ-Snhg11 in ADSC-exos when ADSCs were treated with hypoxia. Further insights revealed that circ-Snhg11 downregulates miR-144-3p in EPCs, thereby enhancing the expression of HIF-1α, and inhibited secretion of inflammatory factors induced by HG, including IL-6, IL-1β and TNF-α. Other downstream genes of HIF-1α, including VEGF and STAT3, exhibited increased expression.Citation96
In addition to diabetic wounds, ADSC-exos have demonstrated reparative functions in other diseases, prompting exploration by researchers. Jin et al discovered that ADSC-exos promote the expression of miR-486 in foot cells, subsequently inhibiting the Smad1/mTOR signaling pathway. This inhibition elevates cellular autophagy levels, thereby ameliorating symptoms of diabetic nephropathy.Citation108 Qu et al demonstrated that ADSC-exos containing miR-181-5p can mitigate liver fibrosis induced by TGF-β1 by inhibiting the STAT3/Bcl-2/Beclin-1 pathway, thereby increasing autophagy.Citation109 Hu et al study revealed that ADSC-exosome miR-17-5p regulates abdominal aortic aneurysm progression and inflammation through the TXNIP-NLRP3 signaling pathway.Citation110 Additionally, ADSC-exos have exhibited reparative capabilities in myocardial injuryCitation55 and cerebral ischemia,Citation111 each with its unique mechanism of action.
ADSC-exos demonstrate robust reparative capabilities in skin healing, neurodegenerative disorders, ischemia-reperfusion injury, and parenchymal organ pathologies.Citation112 The intricate cellular and molecular regulatory mechanisms involved often entail the engagement of multiple signaling pathways, with substantial cross-effects between these pathways that necessitate careful consideration. Further comprehensive investigation into the interactions and molecular mechanisms of ADSC-exos with various cell types within diabetic wounds will contribute to an enhanced understanding of diabetic wound healing. Moreover, it may furnish additional evidence for the clinical application of exosome therapy and stem cell therapy.
Potential Applications of ADSC-Exos
Therapeutic effects of ADSC-exos in diabetic wounds have been largely elucidated and are important for the development of future therapeutic applications. Furthermore, modified ADSC-exos may vary in composition, including specific proteins, mRNA, miRNA, and other ncRNAs, which endows ADSC-exos with potential applications in bioengineering.Citation113
Exosome injection therapy: Direct subcutaneous injection of exosomes within the wound edge has been utilized as an intervention in diabetic wound animal models.Citation93 Research indicates that ADSC-exos promotes wound healing more effectively than stem cells.Citation114 Additionally, direct injection into the corpus cavernosum of exosomes has been reported in the intervention of erectile dysfunction in a mouse model.Citation115 Exosome injection therapy is straightforward, operationally convenient, and easy to manage but comes with drawbacks. Firstly, optimal intervention concentration for exosomes needs further exploration. Zhang et al identified 50 µg/mL as the optimal concentration for intervening with fibroblasts in vitro.Citation53 However, the optimal intervention concentration in vivo is currently unclear. Secondly, the concentration, half-life, and duration of action of exosomes at the wound site after injection, as well as the need for multiple injections before wound healing remain uncertain.
Exosome-loaded biomaterials: Loading ADSC-exos into biomaterials is another approach to intervene in diabetic wounds. Hydrogels could be particularly valued due to the excellent biocompatibility.Citation116 Hu et al loaded ADSC- exos into hydrogel materials. The exosome-loaded hydrogels showed improved wound healing ability compared to hydrogels lacking exosomes.Citation97 Furthermore, innovatively modified hydrogels offer enhanced pro-healing properties. Jiang et al loaded ADSC-exos into the matrix metalloproteinase (MMP) degradable polyethylene glycol (MMP-PEG) smart hydrogel.Citation101 Zhou et al attempted to intervene in diabetic wounds by loading ADSC-exos into a temperature-sensitive hydrogel, Pluronic F-127, demonstrating better healing promotion compared to using exosomes alone.Citation117 Wang et al developed an injectable, self-healing hydrogel (F127/OHA-EPL) containing antimicrobial peptides. F127/OHA-EPL exhibits stimulus-responsive release of ADSC-exos, synergistically promoting chronic wound healing and complete skin regeneration.Citation118 Zhang et al designed an injectable self-healing conductive hydrogel (PEG/Ag/CNT-M + E hydrogel) loaded with both ADSC-exos and metformin. PEG/Ag/CNT-M + E hydrogel stably releases metformin, silver ions, and ADSC-exos on the wound site, promoting chronic diabetic wound healing.Citation119 Hydrogels have become candidate materials for exosome delivery and wound management products with their unique properties. Novel, composition-adjustable hydrogels hold the potential to introduce efficient strategies for wound management.Citation120
Combining 3D printing technology: The main forms of innovative combinations of exosome therapies with 3D printing technology are the development of bioinks containing exosomes and the evolution of 3D printing devices. 3D-printed wound dressing scaffolds offer advantages, such as adjustable wound dressing dimensions, the ability to use various materials, and oxygen permeability.Citation121 Zhong et al combined quaternary ammonium chitosan, decellularized ECM, and gelatin to prepare GDQ bioink for 3D printing. GDQ bioink exhibits excellent mechanical properties, good antibacterial characteristics, and biocompatibility, with the ability to stably load exosomes.Citation122 Nuutila et al designed a handheld light-curing 3D printing device, using GelMA loaded with VEGF as a bio-ink for in situ 3D printing, showing significant advantages in wound healing.Citation123 Kronstadt et al reported a columnar array cell scaffold using 3D printing to achieve mesenchymal stem cell (MSC) adhesion and allow fluid permeation. This cellular scaffold could increase the yield of MSC-derived exosomes (MSC-exos) and improve the wound healing capacity of MSC-exos by continuous perfusion culture.Citation124 Further studies are needed to investigate whether this cellular scaffold has a role in increasing the yield of ADSC-exos. Khalatbary et al explored a new type of three-dimensional (3D) amniotic membrane scaffold,Citation102 providing some insights into the application of ADSC-exos. The use of bioinks containing exosomes in combination with 3D printing technology for repairing bone defects and neurological injuries has been reported.Citation125,Citation126 Unfortunately, there have been no reports of applying exosome bioinks to diabetic wounds.
Bioengineering modification of exosomes: The ability of exosomes to mediate intercellular communication is considered a potential tool for drug delivery.Citation127 Loading small molecules, such as curcumin,Citation128 doxorubicin,Citation129 paclitaxel,Citation130 into exosomes through co-incubation, is feasible strategies for enhancing the efficacy. However, the loading efficiency is not high enough, possibly due to the inclusion of various proteins and nucleic acids in exosomes.Citation131 Improvements in loading methods may enhance the loading efficiency. Currently, the frequently utilized loading method is to transfect specific target molecules into the cells, such as overexpressing circRNA in ADSCs.Citation96,Citation98 Ultrasonic loading is another method with high loading efficiency, but may disrupt the integrity of exosomes.Citation127 Other methods include electroporation, freeze-thawing, and extrusion.Citation132 Approaches to exosome modification are expected to open up new candidate treatment options for diabetic wounds.
Combination with other nanomaterials: The progress in nanotechnology and the preparation of nanomaterials have provided favorable conditions for the development of new materials for promoting diabetic wound healing. The antimicrobial, ROS regulation, and angiogenesis-promoting effects are excellent properties of nanomaterials.Citation133 Zhang et al demonstrated that polyvinyl alcohol (PVA)/alginate (Alg) nanohydrogel encapsulating human umbilical cord MSC-exos (huc-MSC-exos) could markedly promote the proliferation, migration, and angiogenesis of HUVECs, and promoted diabetic wounds.Citation134 Joint application of nanomaterials suggests new approaches to the application of ADSC-exos.
Xiong et al loaded MnO2/ε-PL nanosheets, M2 macrophage-derived exosomes, and fibroblast growth factor-2 (FGF-2) into hydrogels, for creating an injectable multifunctional hydrogel that accelerated diabetic wound healing.Citation135 Other nanomaterials include inorganic metal materials such as nano-silver, nano-gold, and nano-copper, inorganic non-metal materials such as nano-silica, nano-carbon, graphene, and organic materials like synthetic polymer nanoparticles and natural polymer nanoparticles.Citation133,Citation136 The development of novel nanomaterials and the joint application of nanomaterials with ADSC-exos may offer new approaches for diabetic wound treatment.
Conclusion and Discussion
Diabetic wounds pose challenges and complexities in the treatment, due to their unique and complex pathophysiological characteristics.Citation137 Diabetic wounds remain a severe threat to public health, impacting the quality of life for affected individuals and imposing significant socio-economic burdens.Citation138 Traditional intervention methods struggle to achieve cost-effectiveness and improve patient quality of life. Stem cells and their derivatives have shown therapeutic effects in a variety of diseases, such as tendon and bone injuries and blood vessel stenosis.Citation139,Citation140 ADSCs are a type of multipotent cells with strong proliferative and self-renewal capabilities. ADSC-exos can modulate the biological behavior of target cells, including angiogenesis, proliferation, and anti-apoptosis.Citation141
The classical model through which ADSC-exos regulate the behavior of target cells is the circRNA-miRNA-protein signaling pathway. However, there are also mechanisms whereby proteins modulate miRNAs or membrane protein interactions to activate downstream signaling pathways through. The regulatory mechanism of ADSC-exos on target cells is highly complex and requires further exploration. Additionally, the cross-talk between signaling pathways and the interaction between ADSCs and target cells via exosomes need to be verified by more rigorously designed experiments. Undoubtedly, ADSC-exos exhibit a significant pro-healing effect on diabetic wounds, holding enormous potential for diabetic wound treatment.
ADSCs are acknowledged as a reliable exosome source due to the easy accessibility and abundant tissue quantity, compared to other MSCs.Citation142 However, heterogeneity exists in exosomes. Firstly, exosomes from distinct mesenchymal sources exhibit heterogeneity, manifesting not only in variations of exosome contents, but also in functional disparities between cells. For example, Lopez-Verrilli et al discovered that exosomes from bone marrow MSCs (BMSCs) promoted axon regeneration, while exosomes from menstrual stem cells inhibited axon regeneration.Citation143 Wang et al compared the protein expression profiles of MSCs from human bone marrow, adipose tissue, and umbilical cord, revealing substantial differences in the expressed proteins among the three through bioinformatic analysis.Citation144 Secondly, heterogeneity can arise due to variations in exosome extraction methods. Dash et al characterized exosomes extracted using three different methods, which varied in quality and quantity.Citation145 Huang et al not only identified differences in characterization but also observed variations in the protein spectra and biological functions of exosomes extracted using different methods.Citation146 Thirdly, exosomes secreted by the same type of cells may have different subtypes.Citation147 This variation may be due to the existence of multiple mechanisms for the origin of exosomes within cells.Citation148 Further exploration of the heterogeneity of ADSC-exos, such as differences in exosome function and contents in different parts of adipose tissue, and the effects of different exosome isoforms on the function of target cells, will help to deepen the understanding of ADSC-exos.
Application development of ADSC-exos still encounters several challenges that need to be highlighted. Firstly, exosome extraction and purification processes present challenges, despite various methods like differential centrifugation. Generally, issues such as low exosome yield, time-intensive procedures, and high instrument costs persist. Actively developing or finding efficient, cost-effective, and balanced between purity and yield exosome purification technologies is necessary. Secondly, the safety, side effects, and adverse reactions of ADSC-exo applications remain unclear. Special attention must be paid to the potential of exosomes inducing or promoting the occurrence and progression of neoplastic disease.Citation149 Thirdly, the understanding of the underlying mechanisms remains rather poor, and more in-depth studies are needed. Furthermore, the role of exosomes in disease diagnosis remains underdeveloped; for example, peripheral blood exosomes correlate with diabetic Charcot neuroarthropathy severity.Citation150,Citation151 Well-designed large-scale clinical trials need to be conducted. Existing research data have mainly emerged from cell experiments or animal studies, and the effectiveness and safe dosage of ADSC-exos in the human body require confirmation through large-sample, multicenter, blind, randomized controlled trials. According to a search of ClinicalTrials.gov (https://clinicaltrials.gov/), there is currently only one clinical study (NCT02565264) involving exosomes in chronic wounds.
In summary, ADSC-exos represent a highly promising and versatile therapeutic candidates for diabetic wound healing. ADSC-exos can stimulate diabetic wound healing through multiple mechanisms, including the promotion of fibroblast and endothelial cell proliferation, anti-apoptotic effects, enhanced angiogenesis, and modulation of fibrous protein deposition. The molecular regulatory mechanisms underlying healing-promoting effects of ADSC-exos are complex, and further exploration of the molecular regulatory mechanisms and exosome heterogeneity would contribute to a deeper understanding of the reparative role of ADSC-exos. Continuous improvements in the extraction and purification processes of ADSC-exos, along with innovative research in biomaterials, will facilitate the discovery of effective, safe, feasible, and economical drug delivery methods and clinical applications. Well-designed randomized controlled trials (RCTs) are essential to propel the translation of exosome-based therapies from experimental research to clinical application.
Abbreviations
3D, Three-dimensional; ADSC, Adipose-derived stem cell; ADSC-exos, Exosomes released by ADSCs; Alg, Alginate; BMSC, Bone marrow mesenchymal stem cell; CQ, Chloroquine; DF, Diabetic foot; DFU, Diabetic foot ulcer; DM, Diabetes mellitus; ECM, Extracellular matrix; EPC, Endothelial progenitor cell; ESCRT, Endosomal sorting complex for transport; EV, Extracellular vesicles; FGF-2, Fibroblast growth factor-2; HG, High glucose; HGF, Hepatocyte growth factor; HUVEC, Human umbilical vein endothelial cell; IL, Intraluminal vesicle; ISEV, International Society for Extracellular Vesicles; IWGDF, International Working Group on the Diabetic Foot; LPS, Lipopolysaccharide; MMP, Matrix metalloproteinase; MMP-PEG, Matrix metalloproteinase degradable polyethylene glycol; MSC, Mesenchymal stem cell; MSC-exo, Mesenchymal stem cell-derived exosome; MVB, Multivesicular bodie; PUAO-CPO, Oxygen-releasing antioxidant polyurethane; PVA, Polyvinyl alcohol; RCT, Randomized controlled trial; ROS, Reactive oxygen species; STZ, Streptozotocin; TEM, Transmission electron microscope.
Disclosure
The authors declare that there is no conflicts of interest in this work.
Additional information
Funding
References
- Li J, Wang X, Mao H, et al. Precision therapy for three Chinese families with maturity-onset diabetes of the young (MODY12). Front Endocrinol. 2022;13:858096. doi:10.3389/fendo.2022.858096
- Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi:10.1152/physrev.00045.2011
- Mahyoub MA, Elhoumed M, Maqul AH, et al. Fatty infiltration of the pancreas: a systematic concept analysis. Front Med Lausanne. 2023;10:1227188. doi:10.3389/fmed.2023.1227188
- Zhao B, Li M, Su Y, et al. Role of transcription factor FOXM1 in diabetes and its complications (Review). Int J Mol Med. 2023;52(5). doi:10.3892/ijmm.2023.5304
- Zhou YF, Liu HW, Yang X, Li CX, Chen JS, Chen ZP. Probucol attenuates high glucose-induced Müller cell damage through enhancing the Nrf2/p62 signaling pathway. Int Ophthalmol. 2023;43(12):4595–4604. doi:10.1007/s10792-023-02859-z
- Zheng Y, Ma S, Huang Q, et al. Meta-analysis of the efficacy and safety of finerenone in diabetic kidney disease. Kidney Blood Press Res. 2022;47(4):219–228. doi:10.1159/000521908
- Chang M, Nguyen TT. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res. 2021;54(5):1080–1093. doi:10.1021/acs.accounts.0c00864
- Beyaz S, Guler UO, Bagir GS. Factors affecting lifespan following below-knee amputation in diabetic patients. Acta Orthop Traumatol Turc. 2017;51(5):393–397. doi:10.1016/j.aott.2017.07.001
- Perez-Favila A, Martinez-Fierro ML, Rodriguez-Lazalde JG, et al. Current therapeutic strategies in diabetic foot ulcers. Medicina. 2019;55(11). doi:10.3390/medicina55110714
- Lewis J, Lipp A. Pressure-relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2013;(1):Cd002302. doi:10.1002/14651858.CD002302.pub2
- Lei M, Guo X, Yao Y, et al. Trelagliptin relieved cognitive impairment of diabetes mellitus rats: involvement of PI3K/Akt/GSK-3β and inflammation pathway. Exp Gerontol. 2023;182:112307. doi:10.1016/j.exger.2023.112307
- Bacakova L, Zarubova J, Travnickova M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36(4):1111–1126. doi:10.1016/j.biotechadv.2018.03.011
- Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21(4):1.
- Hu C, Zhao L, Li L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res Ther. 2019;10(1):199. doi:10.1186/s13287-019-1310-1
- Sheashaa H, Lotfy A, Elhusseini F, et al. Protective effect of adipose-derived mesenchymal stem cells against acute kidney injury induced by ischemia-reperfusion in Sprague-Dawley rats. Exp Ther Med. 2016;11(5):1573–1580. doi:10.3892/etm.2016.3109
- Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11(1):312. doi:10.1186/s13287-020-01831-3
- Long C, Wang J, Gan W, Qin X, Yang R, Chen X. Therapeutic potential of exosomes from adipose-derived stem cells in chronic wound healing. Front Surg. 2022;9:1030288. doi:10.3389/fsurg.2022.1030288
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
- An Y, Lin S, Tan X, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi:10.1111/cpr.12993
- Zhu Z, Lian X, Su X, Wu W, Zeng Y, Chen X. Exosomes derived from adipose-derived stem cells alleviate cigarette smoke-induced lung inflammation and injury by inhibiting alveolar macrophages pyroptosis. Respir Res. 2022;23(1):5. doi:10.1186/s12931-022-01926-w
- Zhang Q, Piao C, Xu J, et al. ADSCs-exo attenuates hepatic ischemia-reperfusion injury after hepatectomy by inhibiting endoplasmic reticulum stress and inflammation. J Cell Physiol. 2023;238(3):659–669. doi:10.1002/jcp.30968
- Sheykhhasan M, Amini R, Soleimani Asl S, Saidijam M, Hashemi SM, Najafi R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomed Pharmacother. 2022;152:113224. doi:10.1016/j.biopha.2022.113224
- Hu X, Pan J, Li Y, et al. Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res Ther. 2022;13(1):21. doi:10.1186/s13287-021-02668-0
- Lou G, Chen L, Xia C, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39(1):4. doi:10.1186/s13046-019-1512-5
- Liang Z, Liu H, Zhang Y, et al. Cyr61 from adipose-derived stem cells promotes colorectal cancer metastasis and vasculogenic mimicry formation via integrin α(V) β(5). Mol Oncol. 2021;15(12):3447–3467. doi:10.1002/1878-0261.12998
- Trams EG, Lauter CJ, Heine U, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim et Bio Acta. 1981;645(1):63–70. doi:10.1016/0005-2736(81)90512-5
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi:10.1016/0092-8674(83)90040-5
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
- Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44(1):11–15. doi:10.1016/j.biocel.2011.10.005
- He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi:10.7150/thno.21945
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi:10.1038/nri855
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–452. doi:10.1038/nature07961
- Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–937. doi:10.1111/j.1600-0854.2009.00920.x
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi:10.1038/nri3622
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
- Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. doi:10.1038/s41392-020-00261-0
- Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
- Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428–445.e18. doi:10.1016/j.cell.2019.02.029
- Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428(4):688–692. doi:10.1016/j.jmb.2015.09.019
- Lai H, Li Y, Zhang H, et al. exoRBase 2.0: an atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2022:50(D1):D118–D128. doi:10.1093/nar/gkab1085
- Shen K, Wang X, Wang Y, et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 2023;62:102655. doi:10.1016/j.redox.2023.102655
- Shen K, Jia Y, Wang X, et al. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radic Biol Med. 2021;165:54–66. doi:10.1016/j.freeradbiomed.2021.01.023
- Liu M, Yang Y, Zhao B, et al. Exosomes derived from adipose-derived mesenchymal stem cells ameliorate radiation-induced brain injury by activating the SIRT1 pathway. Front Cell Dev Biol. 2021;9:693782. doi:10.3389/fcell.2021.693782
- Zhao S, Liu Y, Wang J, et al. ADSCs increase the autophagy of chondrocytes through decreasing miR-7-5p in Osteoarthritis rats by targeting ATG4A. Int Immunopharmacol. 2023;120:110390. doi:10.1016/j.intimp.2023.110390
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2. doi:10.3402/jev.v2i0.20389
- van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi:10.1124/pr.112.005983
- Baranyai T, Herczeg K, Onódi Z, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10(12):e0145686. doi:10.1371/journal.pone.0145686
- Gheinani AH, Vögeli M, Baumgartner U, et al. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci Rep. 2018;8(1):3945. doi:10.1038/s41598-018-22142-x
- Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi:10.1016/j.ymeth.2015.02.019
- Wu B, Feng J, Guo J, et al. ADSCs-derived exosomes ameliorate hepatic fibrosis by suppressing stellate cell activation and remodeling hepatocellular glutamine synthetase-mediated glutamine and ammonia homeostasis. Stem Cell Res Ther. 2022;13(1):494. doi:10.1186/s13287-022-03049-x
- Mathew A, Bell A, Johnstone RM. Hsp-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem J. 1995;308(Pt 3):823–830. doi:10.1042/bj3080823
- Zhang W, Bai X, Zhao B, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370(2):333–342. doi:10.1016/j.yexcr.2018.06.035
- Airuddin SS, Halim AS, Wan Sulaiman WA, Kadir R, Nasir NAM. Adipose-derived stem cell: ”treat or trick”. Biomedicines. 2021;9(11). doi:10.3390/biomedicines9111624
- Deng S, zhou X, Ge Z, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564. doi:10.1016/j.biocel.2019.105564
- Tian J, Cui X, Sun J, Zhang J. Exosomal microRNA-16-5p from adipose mesenchymal stem cells promotes TLR4-mediated M2 macrophage polarization in septic lung injury. Int Immunopharmacol. 2021;98:107835. doi:10.1016/j.intimp.2021.107835
- Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov. 2019;5:79. doi:10.1038/s41420-019-0159-5
- Zhang W, Zhang J, Huang H. Exosomes from adipose-derived stem cells inhibit inflammation and oxidative stress in LPS-acute kidney injury. Exp Cell Res. 2022;420(1):113332. doi:10.1016/j.yexcr.2022.113332
- Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14. doi:10.1038/s12276-018-0058-5
- Zhang X, Zhang X, Chen L, et al. Adipose mesenchymal stem cell-derived exosomes enhanced glycolysis through the SIX1/HBO1 pathway against oxygen and glucose deprivation injury in human umbilical vein endothelial cells. Curr Stem Cell Res Ther. 2023. doi:10.2174/011574888x265623230921045240
- Ji Z, Cai Z, Gu S, et al. Exosomes derived from human adipose-derived stem cells inhibit lipogenesis involving hedgehog signaling pathway. Front Bioeng Biotechnol. 2021;9:734810. doi:10.3389/fbioe.2021.734810
- Shin KO, Ha DH, Kim JO, et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells. 2020;9(3):680. doi:10.3390/cells9030680
- Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol. 2018;27(10):1170–1172. doi:10.1111/exd.13451
- Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7(1):13321. doi:10.1038/s41598-017-12919-x
- Zhu S, Li J, Zhao X. Comparative risk of new-onset hyperkalemia for antihypertensive drugs in patients with diabetic nephropathy: a Bayesian network meta-analysis. Int J Clin Pract. 2021;75(8):e13940. doi:10.1111/ijcp.13940
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res Sep-Oct. 2009;37(5):1528–1542. doi:10.1177/147323000903700531
- Gantwerker EA, Hom DB. Skin: histology and Physiology of Wound Healing. Facial Plastic Surg Clin North Am. 2011;19(3):441–453. doi:10.1016/j.fsc.2011.06.009
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi:10.1152/physrev.2003.83.3.835
- Aitcheson SM, Frentiu FD, Hurn SE, Edwards K, Murray RZ. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. 2021;26(16):4917. doi:10.3390/molecules26164917
- Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18(7):1545. doi:10.3390/ijms18071545
- Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109–116. doi:10.1016/j.trsl.2021.05.006
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: a Cellular Perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
- Zhou B, Gao Z, Liu W, Wu X, Wang W. Important role of mechanical microenvironment on macrophage dysfunction during keloid pathogenesis. Exp Dermatol. 2022;31(3):375–380. doi:10.1111/exd.14473
- Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic wound-healing science. Medicina. 2021;57:1.
- Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Anna Med. 2017;49(2):106–116. doi:10.1080/07853890.2016.1231932
- Schreml S, Berneburg M. The global burden of diabetic wounds. Br J Dermatol. 2017;176(4):845–846. doi:10.1111/bjd.15254
- Seidel D, Storck M, Lawall H, et al. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: results of the German DiaFu-RCT. BMJ Open. 2020;10(3):e026345. doi:10.1136/bmjopen-2018-026345
- Liu Z, Dumville JC, Hinchliffe RJ, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. 2018;10(10):Cd010318. doi:10.1002/14651858.CD010318.pub3
- Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol. 2017;199(1):17–24. doi:10.4049/jimmunol.1700223
- Li S, Ding X, Zhang H, Ding Y, Tan Q. IL-25 improves diabetic wound healing through stimulating M2 macrophage polarization and fibroblast activation. Int Immunopharmacol. 2022;106:108605. doi:10.1016/j.intimp.2022.108605
- Al Sadoun H. Macrophage phenotypes in normal and diabetic wound healing and therapeutic interventions. Cells. 2022;11(15):2430. doi:10.3390/cells11152430
- Di G, Du X, Qi X, et al. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6-dependent stem cell activation and macrophage switch. Invest Ophthalmol Vis Sci. 2017;58(10):4344–4354. doi:10.1167/iovs.17-21506
- Sharifiaghdam M, Shaabani E, Faridi-Majidi R, De Smedt SC, Braeckmans K, Fraire JC. Macrophages as a therapeutic target to promote diabetic wound healing. Mol Ther. 2022;30(9):2891–2908. doi:10.1016/j.ymthe.2022.07.016
- Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(1):e3266. doi:10.1002/dmrr.3266
- Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(1):e3283. doi:10.1002/dmrr.3283
- Raghupathy S, Arikrishnan A. A PROSPECTIVE RANDOMIZED TRIAL OF VACUUM-ASSISTED CLOSURE VERSUS STANDARD THERAPY OF CHRONIC NON-HEALING WOUNDS. J Evolution of Med Dent Sci. 2016;5. doi:10.14260/jemds/2016/733
- Normandin S, Safran T, Winocour S, et al. Negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164–170. doi:10.1055/s-0041-1731792
- McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum-assisted closure versus saline-moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Man. 2000;46(8):28–32, 34.
- Ubbink DT, van der Oord BM, Sobotka MR, Jacobs MJ. Effects of vacuum compression therapy on skin microcirculation in patients suffering from lower limb ischaemia. Vasa. 2000;29(1):53–57. doi:10.1024/0301-1526.29.1.53
- Bai Q, Han K, Dong K, et al. Potential applications of nanomaterials and technology for diabetic wound healing. Int J Nanomed. 2020;15:9717–9743. doi:10.2147/ijn.S276001
- Feng J, Yao Y, Wang Q, et al. Exosomes: potential key players towards novel therapeutic options in diabetic wounds. Biomed Pharmacother. 2023;166:115297. doi:10.1016/j.biopha.2023.115297
- Yuan T, Meijia L, Xinyao C, Xinyue C, Lijun H. Exosome derived from human adipose-derived stem cell improve wound healing quality: a systematic review and meta-analysis of preclinical animal studies. Int Wound J. 2023;20(6):2424–2439. doi:10.1111/iwj.14081
- Ren S, Chen J, Guo J, et al. Exosomes from adipose stem cells promote diabetic wound healing through the eHSP90/LRP1/AKT axis. Cells. 2022;11(20):3229. doi:10.3390/cells11203229
- Zhang Y, Bai X, Shen K, et al. Exosomes derived from adipose mesenchymal stem cells promote diabetic chronic wound healing through SIRT3/SOD2. Cells. 2022;11(16):1.
- Wang J, Yi Y, Zhu Y, et al. Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34(1):124–131. doi:10.7507/1002-1892.201903058
- Shi R, Jin Y, Zhao S, Yuan H, Shi J, Zhao H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed Pharmacother. 2022;153:113463. doi:10.1016/j.biopha.2022.113463
- Hu N, Cai Z, Jiang X, et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175–186. doi:10.1016/j.actbio.2022.11.057
- Wang Z, Feng C, Liu H, et al. Exosomes from circ-Astn1-modified adipose-derived mesenchymal stem cells enhance wound healing through miR-138-5p/SIRT1/FOXO1 axis regulation. World J Stem Cells. 2023;15(5):476–489. doi:10.4252/wjsc.v15.i5.476
- Shi R, Jin Y, Hu W, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318(5):C848–C856. doi:10.1152/ajpcell.00041.2020
- Cao T, Xiao D, Ji P, et al. Effects of exosomes from hepatocyte growth factor-modified human adipose mesenchymal stem cells on full-thickness skin defect in diabetic mice. Zhonghua Shao Shang Za Zhi. 2022;38(11):1004–1013. doi:10.3760/cma.j.cn501225-20220731-00330
- Jiang T, Liu S, Wu Z, et al. ADSC-exo@MMP-PEG smart hydrogel promotes diabetic wound healing by optimizing cellular functions and relieving oxidative stress. Mater Today Bio. 2022;16:100365. doi:10.1016/j.mtbio.2022.100365
- Khalatbary AR, Omraninava M, Nasiry D, et al. Exosomes derived from human adipose mesenchymal stem cells loaded bioengineered three-dimensional amniotic membrane-scaffold-accelerated diabetic wound healing. Arch Dermatol Res. 2023;315:2853–2870. doi:10.1007/s00403-023-02709-z
- Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020. doi:10.1016/j.biomaterials.2020.120020
- Huang H, Zhu W, Huang Z, Zhao D, Cao L, Gao X. Adipose-derived stem cell exosome NFIC improves diabetic foot ulcers by regulating miR-204-3p/HIPK2. J Orthop Surg Res. 2023;18(1):687. doi:10.1186/s13018-023-04165-x
- Che D, Xiang X, Xie J, Chen Z, Bao Q, Cao D. Exosomes Derived from Adipose Stem Cells Enhance Angiogenesis in Diabetic Wound Via miR-146a-5p/JAZF1 Axis. Stem Cell Rev Rep. 2024;20(4):1026–1039. doi:10.1007/s12015-024-10685-8
- Liang ZH, Pan NF, Lin SS, et al. Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther. 2022;13(1):336. doi:10.1186/s13287-022-03015-7
- Qiu J, Shu C, Li X, Ye C, Zhang WC. Exosomes from linc00511-overexpressing ADSCs accelerates angiogenesis in diabetic foot ulcers healing by suppressing PAQR3-induced Twist1 degradation. Diabet Res Clin Pract. 2021;180:109032. doi:10.1016/j.diabres.2021.109032
- Jin J, Shi Y, Gong J, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10(1):95. doi:10.1186/s13287-019-1177-1
- Qu Y, Zhang Q, Cai X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491–2502. doi:10.1111/jcmm.13170
- Hu J, Jiang Y, Wu X, et al. Exosomal miR-17-5p from adipose-derived mesenchymal stem cells inhibits abdominal aortic aneurysm by suppressing TXNIP-NLRP3 inflammasome. Stem Cell Res Ther. 2022;13(1):349. doi:10.1186/s13287-022-03037-1
- Yang H, Tu Z, Yang D, et al. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci Lett. 2022;769:136389. doi:10.1016/j.neulet.2021.136389
- Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10(1):242. doi:10.1186/s13287-019-1358-y
- Bunggulawa EJ, Wang W, Yin T, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16(1):81. doi:10.1186/s12951-018-0403-9
- Zhou Y, Zhao B, Zhang XL, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12(1):257. doi:10.1186/s13287-021-02287-9
- Chen F, Zhang H, Wang Z, et al. Adipose-derived stem cell-derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med. 2017;14(9):1084–1094. doi:10.1016/j.jsxm.2017.07.005
- Lenzini S, Bargi R, Chung G, Shin JW. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat Nanotechnol. 2020;15(3):217–223. doi:10.1038/s41565-020-0636-2
- Zhou Y, Zhang XL, Lu ST, et al. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther. 2022;13(1):407. doi:10.1186/s13287-022-02980-3
- Wang C, Wang M, Xu T, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi:10.7150/thno.29766
- Zhang Y, Li M, Wang Y, et al. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact Mater. 2023;26:323–336. doi:10.1016/j.bioactmat.2023.01.020
- Safari B, Aghazadeh M, Davaran S, Roshangar L. Exosome-loaded hydrogels: a new cell-free therapeutic approach for skin regeneration. Eur J Pharm Biopharm. 2022;171:50–59. doi:10.1016/j.ejpb.2021.11.002
- Long J, Etxeberria AE, Nand AV, Bunt CR, Ray S, Seyfoddin A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater Sci Eng C Mater Biol Appl. 2019;104:109873. doi:10.1016/j.msec.2019.109873
- Zhong Y, Ma H, Lu Y, et al. Investigation on repairing diabetic foot ulcer based on 3D bio-printing Gel/dECM/Qcs composite scaffolds. Tissue Cell. 2023;85:102213. doi:10.1016/j.tice.2023.102213
- Nuutila K, Samandari M, Endo Y, et al. In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact Mater. 2022;8:296–308. doi:10.1016/j.bioactmat.2021.06.030
- Kronstadt SM, Patel DB, Born LJ, et al. Mesenchymal stem cell culture within perfusion bioreactors incorporating 3d-printed scaffolds enables improved extracellular vesicle yield with preserved bioactivity. Adv Healthc Mater. 2023;12(20):e2300584. doi:10.1002/adhm.202300584
- Chen P, Zheng L, Wang Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi:10.7150/thno.31017
- Liu X, Zhang J, Cheng X, et al. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regen Biomater. 2023:10:rbac085. doi:10.1093/rb/rbac085
- Tenchov R, Sasso JM, Wang X, Liaw W-S, Chen C-A, Zhou QA. Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16(11):17802–17846. doi:10.1021/acsnano.2c08774
- Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
- Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi:10.1016/j.biomaterials.2013.11.083
- Yang T, Martin P, Fogarty B, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–2014. doi:10.1007/s11095-014-1593-y
- Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
- Xi XM, Xia SJ, Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76(2):61–67. doi:10.1691/ph.2021.0128
- Wang F, Zhang W, Li H, Chen X, Feng S, Mei Z. How effective are nano-based dressings in diabetic wound healing? A comprehensive review of literature. Int J Nanomed. 2022;17:2097–2119. doi:10.2147/ijn.S361282
- Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C. 2021;120:111671. doi:10.1016/j.msec.2020.111671
- Xiong Y, Chen L, Liu P, et al. All-in-one: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18(1):e2104229. doi:10.1002/smll.202104229
- Chen J, Zhao Q, Peng J, Yang X, Yu D, Zhao W. Antibacterial and mechanical properties of reduced graphene-silver nanoparticle nanocomposite modified glass ionomer cements. J Dent. 2020;96:103332. doi:10.1016/j.jdent.2020.103332
- Kong L, Zhao H, Fan J, et al. Predictors of frailty among Chinese community-dwelling older adults with type 2 diabetes: a cross-sectional survey. BMJ Open. 2021;11(3):e041578. doi:10.1136/bmjopen-2020-041578
- Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114–125. doi:10.1111/wrr.12683
- Chen Z, Jin M, He H, et al. Mesenchymal stem cells and macrophages and their interactions in tendon-bone healing. J Orthop Transl. 2023;39:63–73. doi:10.1016/j.jot.2022.12.005
- He Y, Li Q, Feng F, et al. Extracellular vesicles produced by human-induced pluripotent stem cell-derived endothelial cells can prevent arterial stenosis in mice via autophagy regulation. Front Cardiovasc Med. 2022;9:922790. doi:10.3389/fcvm.2022.922790
- Xiong M, Zhang Q, Hu W, et al. Exosomes from adipose-derived stem cells: the emerging roles and applications in tissue regeneration of plastic and cosmetic surgery. Front Cell Dev Biol. 2020;8:574223. doi:10.3389/fcell.2020.574223
- Ha DH, Kim HK, Lee J, et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020;9(5):1.
- Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi:10.1016/j.neuroscience.2016.01.061
- Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):511. doi:10.1186/s13287-020-02032-8
- Dash M, Palaniyandi K, Ramalingam S, Sahabudeen S, Raja NS. Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim Biophys Acta Biomembr. 2021;1863(2):183490. doi:10.1016/j.bbamem.2020.183490
- Huang LH, Rau CS, Wu SC, et al. Identification and characterization of hADSC-derived exosome proteins from different isolation methods. J Cell Mol Med. 2021;25(15):7436–7450. doi:10.1111/jcmm.16775
- Smith ZJ, Lee C, Rojalin T, et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533. doi:10.3402/jev.v4.28533
- Tschuschke M, Kocherova I, Bryja A, et al. Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. J Clin Med. 2020;9(2). doi:10.3390/jcm9020436
- Zhao R, Chen X, Song H, Bie Q, Zhang B. Dual role of MSC-derived exosomes in tumor development. Stem Cells Int. 2020;2020:8844730. doi:10.1155/2020/8844730
- Schara K, Štukelj R, Krek JL, et al. A study of extracellular vesicle concentration in active diabetic Charcot neuroarthropathy. Eur J Pharm Sci. 2017;98:58–63. doi:10.1016/j.ejps.2016.09.009
- Dardari D. An overview of Charcot’s neuroarthropathy. J Clin Transll Endocrinol. 2020;22:100239. doi:10.1016/j.jcte.2020.100239
