?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The goal of this study was to develop and characterize an intravaginal nanomedicine for the active targeted delivery of saquinavir (SQV) to CD4+ immune cells as a potential strategy to prevent or reduce HIV infection. The nanomedicine was formulated into a vaginal gel to provide ease in self-administration and to enhance retention within the vaginal tract. SQV-encapsulated nanoparticles (SQV-NPs) were prepared from poly(lactic-co-glycolic acid) (PLGA) and conjugated to antihuman anti-CD4 antibody. Antibody-conjugated SQV-NPs (Ab-SQV-NPs) had an encapsulation efficiency (EE%) of 74.4% + 3.7% and an antibody conjugation efficiency (ACE%) of 80.95% + 1.10%. Over 50% of total loaded SQV was released from NPs over 3 days. NPs were rapidly taken up by Sup-T1 cells, with more than a twofold increase in the intracellular levels of SQV when delivered by Ab-SQV-NPs in comparison to controls 1 hour post-treatment. No cytotoxicity was observed when vaginal epithelial cells were treated for 24 hours with drug-free Ab-NPs (1,000 μg/mL), 1% HEC placebo gel (200 mg/mL), or 1% HEC gel loaded with drug-free Ab-NPs (5 mg NPs/g gel, 200 mg/mL of gel mixture). Overall, we described an intravaginal nanomedicine that is nontoxic and can specifically deliver SQV into CD4+ immune cells. This platform may demonstrate potential utility in its application as postexposure prophylaxis for the treatment or reduction of HIV infection, but further studies are required.
Introduction
HIV/AIDS is considered to be one of the most significant global health concerns in the twenty-first century.Citation1 Current HIV incidence appears to have a disproportionate impact on women.Citation2 The latest reports indicate that women account for more than half of the HIV-infected population, and the leading cause of death among women of reproductive age is attributed to HIV infection.Citation2,Citation3 Heterosexual intercourse is identified as the main mode of HIV transmission among women.Citation2 Due to biological vulnerabilities, eg, larger surface area of the vagina and cervix in comparison to the male penis,Citation4 women are at a twofold-higher risk of infection than men when exposed to unprotected sexual intercourse.Citation2
In the absence of a vaccine, researchers have attempted to develop strategies that can prevent/reduce heterosexual transmission of HIV Current strategies include pre-/postexposure prophylacticsCitation5,Citation6 and microbicides that are applied topically vaginally or rectally prior to sexual intercourse.Citation7 Microbicides for the delivery of anti-HIV agents have been formulated in various dosage forms, including gels, films, and rings. Microbicides are intended to coat the vaginal lumen and interact with HIV extracellularly upon exposure or intracellularly. Unfortunately, these strategies do not allow the drug to attack HIV-infected immune cells specifically. To address this, our research group has developed a novel nanomedicine that can actively target the delivery of saquinavir (SQV), a protease inhibitor, to CD4+ immune cells (target cells for HIV infection).Citation8,Citation9 The nanomedicine was formulated into a vaginal gel to provide ease in self-administration and to enhance retention within the vaginal tract. SQV is the first protease inhibitor approved by the US Food and Drug Administration (FDA), and has been widely used in anti-HIV therapy due to its wide range of action. It is active against both HIV-1 and HIV-2, including strains that are resistant to reverse-transcriptase inhibitors.Citation8 SQV-encapsulated nanoparticles (SQV-NPs) were prepared from poly(lactic-co-glycolic acid) (PLGA) and conjugated to antihuman anti-CD4 antibody. NPs were characterized in terms of physicochemical properties and targeting ability. Our results showed that antibody-conjugated (Ab-SQV-NPs) had desirable particle size, encapsulation efficiency (EE%), antibody-conjugation efficiency (ACE%), and release profile, with active targeting ability of delivering SQV into CD4+ immune cells. The resulting NPs can be potentially loaded into a vaginal gel with no cytotoxicity observed on vaginal epithelial cells.
During the early stages of vaginal entry and infection, HIV substantially invades intraepithelial vaginal Langerhans cells and CD4+ T cells through trauma or epithelial transcytosis.Citation10 In an ex vivo model of the human vagina, HIV-1 virions were detected in both Langerhans cells and CD4+ T cells 2 hours after virus challenge.Citation10 In another study, simian immunodeficiency virus (SIV) infected cells were reported to be detected in the draining lymph nodes in pigtailed macaques 48 hours after intravaginal inoculation of SIV.Citation11 Therefore, after intravaginal exposure of SIV or HIV, the virus appears to be retained within local vaginal tissues for a short period of time before it reaches the lymphatic system and establishes progressive infection. This retention window provides us an opportunity to limit the virus spread and reduce HIV infection in situ. As a result, our formulation is intended to be used as a postexposure prophylactic after sexual intercourse to control and reduce HIV infection, particularly in women who are raped or those who are exposed to unprotected consensual intercourse.
Nanocarriers have been designed for the delivery of a variety of compounds for the treatment of disease.Citation12–Citation15 The extensive application of NPs in medicine is a result of their desirable attributes. The nanoscale size allows the carriers to be easily taken up by targeted cells (eg, the size allows the carrier to interact easily with functional biomolecules on the cell surface to facilitate intracellular internalization),Citation16 especially hard-to-transfect cells, such as T cells,Citation17 macrophages,Citation18 and dendritic cells,Citation19 all of which are HIV-targeted cells.Citation9 NPs also provide a large surface area that can be modified with various functional moieties to improve drug release, biodistribution, and pharmacokinetics. They can also be modified to facilitate active targeted delivery to minimize off-target toxicity. For example, functionalizing NPs with antibodies can improve cell targeting, increase intracellular uptake of drugs, reduce the required administered dose to achieve therapeutic efficacy, and minimize off-target side effects.Citation20 However, to achieve efficient intravaginal delivery and to increase patient/user adherence, NPs need to be formulated so that they can be easily administrated and retained within the vaginal lumen. Due to the physicochemical similarities between vaginal gels and cervicovaginal mucus, vaginal gels remain the preferred choice for intravaginal administration. More importantly, previous studies have established the possibility of using vaginal gels to deliver anti-HIV drug-loaded NPs as a prophylaxis.Citation21 Advantages of gel formulations include ease in self-administration and good patient compliance and acceptability.
PLGA, is an FDA-approved polymer that has been widely used in the preparation of NPs due to their biodegradability, biocompatibility, and versatility in physicochemical property modifications of NPs.Citation22,Citation23 PLGA is generally recognized as safe by the FDA, and degrades into lactic acid and glycolytic acid through the hydrolysis of ester bonds in the body.Citation23 These two monomers can either enter the tricarboxylic acid cycle for further breakdown into carbon dioxide and water or remain unchanged, and subsequently be eliminated from the body, so the degradants of PLGA are nontoxic and biocompatible.Citation24,Citation25 The hydrolytic rate of PLGA can range from days to months, depending on the physicochemical property of the PLGA, such as the molecular weight and the composition ratio of the two monomers.Citation26 Therefore, NPs fabricated from PLGA can be designed to exhibit a sustained release profile and can also be functionalized with a wide range of biomoieties, including antibodies,Citation27 aptamers,Citation28 and peptides,Citation29 as a strategy to enhance cell-targeting.
Hydroxyethylcellulose (HEC) is an FDA-approved polymer that is used in a wide range of applications, including pharmaceuticals, cosmetics, cleaning solutions, and other household products.Citation30 HEC is often used in gel formulations, particularly those for microbicide development, and has been shown to be inactive against HIV and nonirritating to vaginal tissue.Citation31 As a result, HEC is a suitable polymer for formulating NPs into a vaginal gel.
Materials and methods
Materials
Materials used for NP preparation and antibody conjugation
SQV mesylate (molecular weight 766.96 g/mol) was purchased from the US Pharmacopeial Convention (Rockville, MD, USA). Carboxylic acid terminated PLGA (lactide:glycolide ratio 50:50, molecular weight 7–17 kDa), poly(vinyl alcohol) (molecular weight 31–50 kDa), 2-(N-morpholino)ethanesulfonic acid (MES), and coumarin-6 were purchased from Sigma-Aldrich (St Louis, MO, USA). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were obtained from G- Biosciences (St Louis, MO, USA). Antihuman anti-CD4 antibody (mouse monoclonal to CD4, phycoerythrin-conjugated) and rabbit polyclonal secondary antibody to goat immunoglobulin (IgG) were purchased from Abcam (Toronto, ON, Canada). A tetramethylbenzidine (TMB) substrate kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Materials used for gel preparation
HEC (Natrosol, 250 HHX Pharm) was a kind gift from Ashland (Covington, KY, USA). Glycerol and dipotassium phosphate were purchased from Thermo Fisher Scientific.
Materials used for cell culture
Heat-inactivated fetal bovine serum was purchased from PAA (Etobicoke, ON, Canada), and Roswell Park Memorial Institute (RPMI) 1640 medium, penicillin–streptomycin, and phosphate-buffered saline (PBS) were purchased from Lonza (Allendale, NJ, USA). Keratinocyte serum-free medium and its supplements were purchased from Life Technologies (Carlsbad, CA, USA). Calcium chloride was purchased from Sigma-Aldrich.
Other materials
Ethyl acetate, methanol, and acetonitrile (high-performance liquid chromatography [HPLC] grade) were purchased from EMD Serono (Mississauga, ON, Canada). MF-Millipore Membrane (mixed cellulose esters, hydrophilic, SM 5.0 μm) was purchased from EMD Millipore (Billerica, MA, USA). (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium) (MTS) was purchased from Promega (Fitchburg, WI, USA).
High-performance liquid chromatography method for SQV quantitation
The chromatographic separation was performed on a Nova-Pak C-18 4 μm column (150 mm × 3.9 mm; Waters, Milford, MA, USA) at room temperature protected by a Nova-Pak C-18 4 μm guard column (20 × 3.9 mm; Waters) on a liquid chromatography machine (LC-2010A; Shimadzu, Kyoto, Japan). The mobile phase was composed of 45% 5 mM K2HPO4 and 55% acetonitrile (adjusted to pH 8.0 with H3PO4). The chromatographic run was performed by an isocratic elution for 10 minutes at a flow rate of 1 mL/minute, detected at a wavelength of 242 nm, and SQV was quantitated by the area under the curve (integrated with EZstart, Shimadzu, Kyoto, Japan). The standard curve of SQV was generated in the same medium and run in the same conditions as the samples.
Fabrication of SQV-encapsulated nanoparticles
SQV-NPs were prepared by a solvent-evaporation method.Citation32 In brief, SQV was dissolved in methanol, making a 10 mg/mL SQV stock solution. SQV (50 μL) was combined with 30 mg/mL of PLGA–carboxylic acid ethyl acetate solution (575 μL), forming the organic phase. The organic phase was emulsified with an aqueous phase solution containing 5% poly(vinyl alcohol) (4.38 mL) for 2 minutes on ice under continuous sonication by a microtip probe sonicator (Branson sonifier 150D; QSonica, Newtown, CT, USA). The resulting oil-in-water emulsion was then stirred for 3 hours at room temperature to evaporate the organic solvent. SQV-NPs were collected by centrifugation (20,000 g, 10 minutes, 25°C) (Sorvall RC6+; Thermo Fisher Scientific) and washed twice with autoclaved Milli-Q water to remove excess emulsifier and free drug. The resulting SQV-NPs were resuspended in 25% trehalose solution, stored at −80°C for 3 hours, and lyophilized for 24 hours (FreeZone 2.5-L freeze-dry system; Labconco, Kansas City, MO, USA). Prior to initiation of studies, NPs were rehydrated in MES buffer (pH 5.0). Coumarin 6-encapsulated NPs (C6-NPs) were prepared following the same method.
Antibody conjugation to NPs
The NP suspension in MES (2 mg/mL) was incubated with 50 μL of 1.5 mg/mL EDC and 50 μL of 1.5 mg/mL NHS solutions at 4°C for 1 hour on a rotary shaker (Roto-Shake Genie; Scientific Industries, New York, NY, USA) for carboxylic group activation. The activated NPs were incubated with 100 μL of 40 ng/mL anti-CD4 antibody or horseradish peroxidase (HRP)-labeled rabbit polyclonal secondary antibody to goat IgG at 4°C for 2 hours on a rotary shaker. The Ab-SQV-NPs were washed and collected by centrifugation to remove excess NHS, EDC, and unconjugated antibodies. The resulting NPs conjugated with antibody were resuspended in PBS and used immediately
Preparation and characterization of 1% (w/w) HEC gel
Approximately 1 g of HEC was added to 40 mL of PBS (pH 5.0) and stirred at 4°C for 2 hours until dissolved. To adjust the viscosity of the gel to be similar to gels used for microbicide evaluation, 5 g of glycerol was added and stirred until it was uniformly dispersed. The pH of the gel was adjusted to 5.0 ± 0.2 (similar to physiological vaginal pH), and the total weight was adjusted to 50 g using PBS (pH 5.0). As for loading NPs into HEC gel, Ab-SQV-NPs were resuspended in PBS (pH 5.0) to make a concentration of 10 mg/mL. The NP suspension was combined with the HEC gel and stirred at 4°C for 2 hours, resulting in a preparation containing 1% HEC gel loaded with 0.5% NPs. Homogeneity of NPs in the gel was confirmed by randomly sampling different parts of the gel containing C6-NPs and comparing the fluorescence readings of each sample.
The viscosity of the gel was determined using an AR550 Rheometer (TA Instruments, New Castle, DE, USA) with a 20 mm 2° steel cone at 37°C.
Characterization of NPs
Particle size
SQV-NPs and Ab-SQV-NPs were resuspended in autoclaved Milli-Q water. Particle size (25 μg/mL of NP suspension) was determined by dynamic light scattering using ZetaPALS (Brookhaven Instruments, Holtsville, NY, USA). Samples were tested in triplicate for three runs of 2 minutes each.
Zeta potential
SQV-NPs and Ab-SQV-NPs were resuspended in autoclaved Milli-Q water. Zeta potential (100 μg/mL of NP suspension; Smoluchowski mode) was determined by dynamic light scattering using ZetaPALS. Samples were tested in triplicate for three runs of 2 minutes each.
Surface morphology
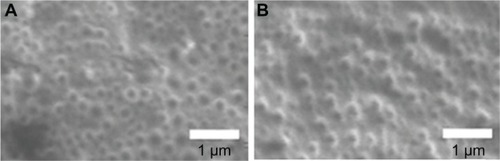
Scanning electron microscopy (SEM) was used to visualize the surface morphology and size of NPs. SEM images were taken on a Field Emission Auger Microprobe (JAMP 9500F; JEOL, Tokyo, Japan) equipped with a floating microion etching device for charge neutralization on nonconducting NP samples (5–10 mg/mL). The images were taken and analyzed using EOS 9500 image-acquisition software (Canon, Tokyo, Japan) at a voltage of 5.0 kV.
Encapsulation efficiency of NPs
To determine the EE%, the amount of unencapsulated SQV in the wash solutions was quantified by HPLC. Encapsulation efficiency was determined by the following equation:
Antibody-conjugation efficiency of anti-CD4 antibody to NPs
To determine the ACE% of anti-CD4, HRP-conjugated rabbit polyclonal secondary antibody to goat IgG was used as a surrogate for anti-CD4 antibody conjugation following the method described earlier. Various concentrations of anti-CD4 antibody were evaluated to optimize the ACE%. Unconjugated antibody recovered in the supernatant was quantified using a TMB substrate kit. TMB is a chromogenic substrate that can be oxidized by HRP. Upon reaction with HRP, the TMB yields a blue color, which further changes to yellow (with maximum absorbance at 450 nm) upon addition of a sulfuric acid stop solution (2M). The absorbance at 450 nm is proportional to the amount of HRP, which reflects the amount of antibody. Therefore, by generating a standard of HRP-conjugated antibody, the amount of HRP-conjugated antibody was quantified. ACE% was determined by the following equation:
In vitro release studies
To determine SQV release from NPs, 1 mg postlyophilized SQV-NPs were resuspended in 1 mL PBS (pH 4.6) as release medium and maintained at 37°C on a rotary shaker at a speed of 100 rpm (Incubating Orbital Shaker; VWR, Edmonton, Canada). At different time points, samples were centrifuged, and 200 μL of release medium was removed and replenished with equal amounts of fresh release medium.Citation33 Samples were filtered through a 0.2 μm GHP filter (Pall, Mississauga, Canada), and the amount of SQV released was quantified by HPLC using the method mentioned previously.
To determine SQV release from the gel, 100 μL of gel was immersed into 1 mL of PBS (pH 4.6) and incubated at 37°C on a rotary shaker. At various time points, samples were centrifuged and 200 μL of release medium was removed filtered through the 0.2 μm GHP filter and analyzed by HPLC. Release media was replenished with an equal volume of medium. To further investigate the release profile of NP-loaded gel, 200 μL of gel was placed into the donor chamber of a Franz cell with 5 mL of PBS (pH 4.6) added into the receptor chamber.Citation34 Between the donor and receptor chambers, a 5 μm semipermeable membrane (Millipore) was sandwiched to mimic the mucus mesh in the vaginal cavity. The Franz cells were incubated in a circulating water bath at 37°C. At various time points (1–24 hours), 0.5 mL of release medium was removed and replenished with an equal amount of fresh release medium. Samples were centrifuged and filtered through the 0.2 μm GHP filter, and the amount of SQV released was quantified by HPLC.
In vitro cell-targeting studies
Since intravaginal epithelial cells coexist with immune cells (CD4+ T cells, dendritic cells, and macrophages) in the vagina, a typical CD4+ cell line (Sup-T1) and a CD4− vaginal epithelial cell line (VK2/E6E7) were selected for the in vitro cell-targeting studies.Citation35 Sup-T1 cells (CD4+) were obtained from the National Institutes of Health AIDS Reagent Program and maintained at 37°C and 5% CO2 with RPMI 1640 culture media supplemented with 10% heat-inactivated fetal bovine serum and penicillin (100 U/mL)–streptomycin (100 μg/mL). Sup-T1 cells (1 × 106) were seeded in 6-well tissue culturetreated plates (Corning, New York, NY, USA) in 5 mL of culture medium on the day of the experiments.
Vaginal epithelial cells (VK2/E6E7) (CD4−) were obtained from the American Type Culture Collection and maintained at 37°C and 5% CO2, with culture media supplemented with 0.1 ng/mL human recombinant epidermal growth factor (EGF), 0.05 mg/mL bovine pituitary extract, and additional calcium chloride 44.1 mg/L and penicillin (100 U/mL)-streptomycin (100μg/mL). VK2/E6E7 cells (5 × 105) were seeded in 6-well tissue culture-treated plates (Corning) in 2.5 mL culture medium 1 day prior to the experiments.
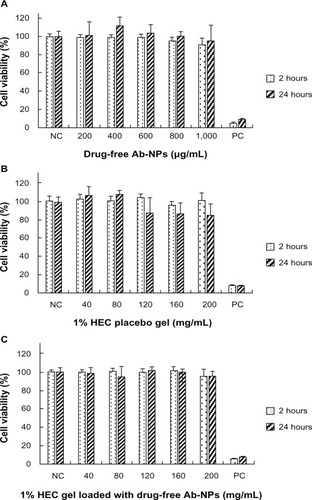
Prior to the initiation of cell-targeting studies, the impact of drug-loaded NPs (Ab-SQV-NPs) on Sup-T1 cell viability was determined. In brief, 1 × 105 cells were seeded in a 96-well plate and subjected to various concentrations of Ab-SQV-NPs (200–1,000 μg/mL) for 2 and 24 hours, respectively. Negative control was blank-cell media, and positive control was 1 M acrylamide in cell media. At the end of the treatment period 20 μL of MTS solution was added into each well and incubated for 1 hour. Interference generated by NPs was deducted by running parallel samples with NPs alone in the same media. The plate was analyzed on a microplate reader (BioTek, Winooski, VT, USA) at 490 nm.
Ab-SQV-NPs or SQV-NPs (controls) were resuspended in PBS (pH 7.4) and subjected to Sup-T1 or VK2/E6E7 cells at a final concentration of 600 μg/mL in each well. All treatment groups were maintained at 37°C, 5% CO2, and treated for 0.5, 1, 2, and 6 hours. Targeted delivery of SQV into Sup-T1 cells was evaluated by quantifying intracellular uptake of SQV In brief, at various time points, cells were collected by centrifugation at 100 g for 5 minutes, washed twice with PBS, and lysed with 200 μL of 90% dimethyl sulfoxide in PBS at room temperature for 10 minutes. The lysate was then centrifuged at 20,000 g at 4°C for 20 minutes, filtered through a GHP filter, and analyzed by HPLC. Delivery of SQV into CD4− cells (VK2/E6E7) was included as a control to determine the specificity of Ab-SQV-NP-targeted delivery. In brief, at various time points, medium containing NPs was removed and cells were washed twice with PBS, and lysed with 200 μL of 90% dimethyl sulfoxide in PBS at room temperature for 10 minutes. The lysate was collected and centrifuged at 20,000 g at 4°C for 20 minutes, filtered through a GHP filter, and analyzed by HPLC.
In vitro cellular cytotoxicity study
Cellular cytotoxicity was determined using the MTS assay. VK2/E6E7 cells were plated at 105 cells/well on 96-well tissue culture-treated plates (BD, Franklin Lakes, NJ, USA) in 100 μL culture medium. Varying concentrations of drug-free Ab-NPs (200–1,000 μg/mL), 1% HEC placebo gel (40–200 μg/mL), and 1% HEC gel loaded with drug-free Ab-NPs (5 mg NPs/g gel) (40–200 μg/mL) were premixed with culture media, added to cells, and incubated for 2 and 24 hours. Negative control was blank cell media, and positive control was 1 M acrylamide in cell media. At the end of the treatment period cells were washed replaced with fresh medium containing 20 μL of MTS solution, and incubated for 1 hour. The plate was analyzed on a microplate reader (BioTek) at 490 nm.
Statistical analysis
Student’s t-test (unpaired two-sample, unequal variance with two-tailed distribution) was performed on all results, with P < 0.05 considered significant. Data shown are expressed as means ± standard deviation.
Results
Physicochemical characterization of NPs
NP size, zeta potential, and EE% of drug-free NPs, SQV-NPs, and Ab-SQV-NPs are listed in . Average particle sizes for SQV-NPs and Ab-SQV-NPs were found to be in the range of 200–300 nm. Zeta potentials for SQV-NPs and Ab-SQV-NPs were determined to be around −18.8 ± 2.9 mV and −9.7 ± 3.1 mV respectively. EE% of SQV-NPs formulated in this study was 74.4% ± 3.7%. ACE% of SQV-NPs is shown in . ACE% of SQV-NPs was determined using antibodies of varying concentrations (10, 20 and 40 ng/mL), resulting in ACE% of 80.95% ± 1.10%, 79.91% ± 0.55%, and 74.29% ± 2.67% respectively. The ACE% decreased as the concentration of antibody increased. In terms of the amount of antibody conjugated to 1 mg of NP, there was a proportionate increase in the amount of antibody conjugated with increasing concentration of antibody added. The SEM images of SQV-NPs and Ab-SQV-NPs are shown in . SQV-NPs and Ab-SQV-NPs both appeared to be spherical in shape with a smooth surface. Furthermore, there did not appear to be any NP aggregation, and the size distribution observed from SEM images further supported the results obtained with dynamic light scattering ().
Table 1 Particle size, zeta potential and EE% of blank (drug-free) NPs, SQV-NPs, and Ab-SQV-NPs
Table 2 ACE% and antibody loading of SQV-NPs
In vitro release study of SQV from NPs and SQV from NP-loaded gel
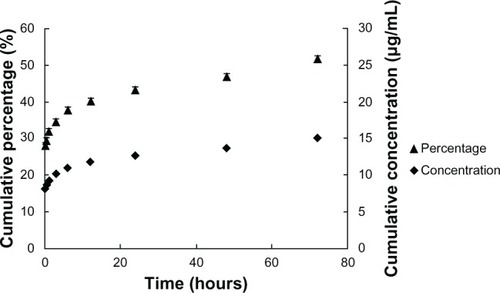
As shown in , the release of SQV from NPs was sustained throughout the entire study period. More than 50% of SQV was released after 3 days. There was a burst release of approximately 35% (10.1 μg/mL) of total loading within the first 3 hours, and 43% (12.6 μg/mL) of total loaded SQV was released after 24 hours at pH 4.6. Subsequently, the SQV-NPs demonstrated a sustained linear release profile, with approximately 2%–3% of total loaded SQV released per day.
Figure 2 In vitro cumulative release profile of saquinavir from saquinavir-encapsulated nanoparticles. The results show cumulative concentration (filled diamonds) and percentage released (filled triangles) at 37°C in phosphate-buffered saline (pH 4.6). Values represent means ± standard deviation; n = 5.
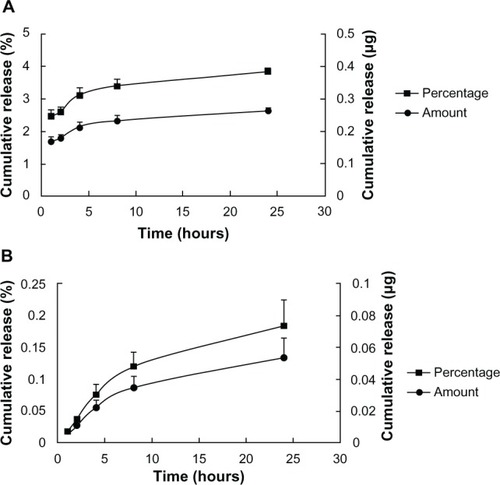
In vitro release studies performed in microcentrifuge tubes containing 1% HEC gel loaded with SQV-NPs (5 mg NPs/g gel) in PBS (pH 4.6) suggested a nearly linear release profile of SQV, with approximately 3% of total loaded SQV released within 24 hours (). shows the in vitro release profile of SQV from 1% HEC gel loaded with SQV-NPs (5 mg NPs/g gel) for the same period in PBS (pH 4.6) conducted in Franz cells. The release of SQV also exhibited a near-linear release profile after the first 24 hours, with 0.18% ± 0.04% of total loaded SQV released.
Figure 3 In vitro release study of saquinavir (SQV) from 1% hydroxyethylcellulose (HEC) gel loaded with SQV-encapsulated nanoparticles (A). Release study conducted in microcentrifuge tubes (B) release study conducted in Franz cells. The results show cumulative amounts (filled circles) and cumulative percentage released (filled squares) at 37°C in phosphate-buffered saline (pH 4.6). Values represent means ± standard deviation; n = 3.
Characterization of 1% HEC gel loaded with SQV-NPs
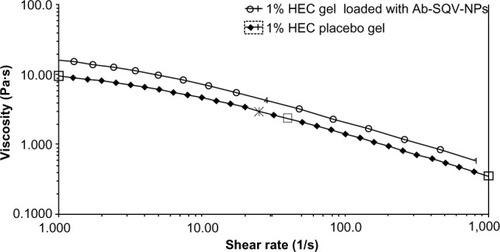
The fluorescence reading of 1% HEC gel loaded with C6-NPs coming from three different parts of the gel was 8,095, 8,321 and 7,945 respectively, indicating the NPs were homogeneously dispersed. As shown in , the flow curves for both 1% HEC placebo gel and 1% Ab-SQV-NP-loaded HEC gel (5 mg NPs/g gel) displayed non-Newtonian shear-thinning behavior. The viscosity of the 1% Ab-SQV-NP-loaded HEC gel was determined to be around 2.800 Pas at a shear rate of 60 secondsCitation1. At the same shear rate, it has been reported that over-the-counter lubricant products such as KY Jelly and Astroglide gel have viscosities of 2.765 Pas and 2.071 Pas, respectively.Citation36 Moreover, it was reported that a 1% tenofovir intravaginal gel used in a Phase II clinical trial also had a similar viscosity of 2.736 Pas.Citation37 As a result, the 1% HEC gel was an appropriate choice for our studies.
Figure 4 Steady-state flow curves of 1% hydroxyethylcellulose (HEC) placebo gel (filled diamonds), and 1% HEC gel containing antibody-conjugated saquinavir-loaded nanoparticles (Ab-SQV-NPs; 5 mg NPs/g gel, empty circles) at a measurement temperature of 37°C. Viscosity profiles were performed as a single measurement.
In vitro cell-targeting studies
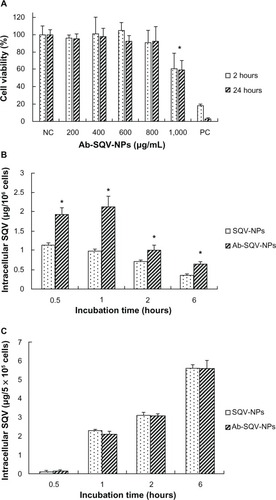
Prior to evaluating the targeting ability of Ab-NPs into CD4+ cells, Sup-T1 cells were treated with Ab-SQV-NPs to determine a drug dose that was noncytotoxic. Based on our studies, no significant effects on cell viability were observed when cells were treated with doses <800 μg/mL of Ab-SQV-NPs. As a result, a medium dose (600 μg/mL) was chosen for our cell-targeting studies. The active targeting ability of Ab-SQV-NPs was evaluated in a human CD4+ T-cell line, Sup-T1, and in a human CD4− cell line, VK2/E6E7. shows the impact of drug-loaded NPs (Ab-SQV-NPs) on Sup-T1 cell viability. No significant cytotoxicity was observed at concentrations up to 800 μg/mL, while concentrations above that (1,000 μg/mL) significantly induced cell death. compared the ability of SQV-NPs and Ab-SQV-NPs to deliver SQV intracellularly into Sup-T1 cells at various time intervals. Our data showed that SQV-NPs and Ab-SQV-NPs could achieve fast intracellular delivery of SQV into CD4+ T cells. Significant differences (P < 0.05) in the intracellular accumulation of SQV were observed in the Ab-SQV-NP group when compared to the SQV-NP group at all the time points. The intracellular concentrations of SQV delivered by Ab-SQV-NPs was 1.7-fold 2.2-fold 1.4-fold and 1.8-fold higher than unconjugated SQV-NPs at 0.5-, 1-, 2-, and 6-hour time points, respectively, suggesting that Ab-SQV-NPs could actively target the delivery of SQV into Sup-T1 cells. In contrast, no significant differences were observed in the cellular uptake of SQV by the CD4− cell line, VK2/E6E7 when delivered by SQV-NPs or Ab-SQV-NPs at any time points (). Although there was an increase in the accumulation of SQV with time, the results suggested nonspecific delivery of SQV into VK2/E6E7. More importantly, the in vitro half-maximal inhibitory concentration (IC50) of SQV against HIV-1 is reported to be 0.031 + 0.022 μM (0.02013 + 0.01476 μg/mL).Citation38 The highest nontoxic concentration of SQV-NPs (1,000 μg/mL) contained 31 μg/mL SQV which is much higher than the IC50 of SQV hence our formulation is safe and potentially effective against HIV-1.
Figure 5 (A) Cytotoxicity of antibody-conjugated saquinavir-encapsulated nanoparticles (Ab-SQV-NPs) in Sup-T1 cells. The data shown represent means ± standard deviation; n = 4. (B) Intracellular accumulation of SQV in Sup-T1 cells, and (C) intracellular accumulation of SQV in VK2/E6E7 cells; values represent means ± standard deviation; n = 5.
Note: *P < 0.05 versus SQV-NPs.
Abbreviations: NC, negative control; PC, positive control.
In vitro cytotoxicity study
According to , drug-free Ab-NPs, 1% HEC placebo gel, and 1% HEC gel loaded with drug-free Ab-NPs (5 mg Ab-NPs/g gel) appeared to have no significant impact on the cell viability of VK2/E6E7 up to concentrations of 1,000 μg/mL, 200 mg/mL, and 200 mg/mL, respectively, after exposure for 2 and 24 hours.
Discussion
NP-based therapy is a promising modality for the prevention and treatment of HIV/AIDS.Citation39 Previous studies have established effective and safe intracellular delivery of antiretroviral drugs into human peripheral blood mononuclear cells or human monocyte/macrophage by NPs.Citation40,Citation41 Further studies have also demonstrated favorable antiretroviral efficacy against HIV-1 when the drug is delivered into human monocyte-derived macrophages.Citation42 Since unprotected heterosexual vaginal intercourse has become one of the major modes of transmission, there is an increasing interest in developing intravaginal microbicides against HIV infection. For example, Zhang et al developed a pH- responsive NP formulation that can achieve increased sustained release of tenofovir in acidic environments as a potential intravaginal microbicide.Citation43 Das Neves et al also compared different types of polymeric NPs for delivering dapivirine as a microbicide, in terms of efficacy and cytotoxicity in various HIV-related cells.Citation44 Woodrow et al developed an intravaginal delivery system that can effectively silence herpes simplex virus genes with small interfering RNA.Citation33 Antibody-conjugated NPs have been long investigated for the treatment of numerous diseases, including cancer.Citation27,Citation45–Citation48 Our team is interested in extending the application of Ab-NPs for intravaginal delivery. Previous studies have developed nontargeting SQV-NPs using poly(ethylene oxide)-modified poly(epsilon-caprolactone) for delivery into a human monocyte/macrophage cell line,Citation41 and CD4-targeted lipid NPs have been shown to enhance the antiretroviral activity of indinavir.Citation49 However, our study is the first to demonstrate active targeted delivery of SQV into CD4+ cells via antibody-conjugated biodegradable NPs.
In our study, the size distribution of NPs (drug-free NPs, SQV-NPs, and Ab-SQV-NPs) were unimodal with a mean diameter of 177–280 nm, as detected by dynamic light scattering. Particle size increased slightly as drug was loaded and antibody was conjugated to NPs. The particle size of Ab-SQV-NPs could be controlled within the range of 200–500 nm (mean size 280 nm), which was previously reported to be the optimal size range for intravaginal drug delivery.Citation50 Therefore, it is rational to speculate that the Ab-SQV-NPs developed in this study have potential abilities to achieve intravaginal delivery, but further in vivo studies are required. The zeta potentials of drug-free NPs, SQV-NPs, and Ab-SQV-NPs were determined to be negative because of the presence of terminal carboxylic groups in PLGA. Encapsulation of SQV into NPs increased the zeta potential, which may be a result of SQV adsorbed onto the surface of NPs to mask the charges generated by the carboxylic groups. The zeta potential was further increased as antibody conjugated to NPs because of the direct interaction between the antibody and the carboxylic groups on the surface.Citation51 Our formulation demonstrated an SQV EE% of approximately 75%, which is higher in comparison to other SQV-encapsulated delivery systems (<60%).Citation28,Citation41,Citation52 This could be due to the fact that the salt form of SQV, SQV mesylate, has relatively high hydrophobicity (log P [o/w] = 4.1),Citation53 allowing it to enter the organic phase rather than the aqueous phase during NP preparation, hence resulting in favorable EE%. Therefore, it can be expected that the formulation established in this study can be applied to similar lipophilic drugs.
Covalent Ab-NP conjugates were achieved under the catalysis of EDC/NHS to form amide bonds through the amino groups from antibody and terminal carboxyl groups from PLGA.Citation54 ACE% results displayed a concentration-dependent trend. The highest ACE% was achieved when the lowest concentration of antibody was used, while the greatest amount of antibody conjugated to NPs was achieved when the greatest amount of antibody was added. Since NPs present a certain surface area that can accommodate limited amounts of antibody, the amount of antibody that can be conjugated to NPs will reach a saturation limit. When the amount of antibody added is below the saturation limit, theoretically all the antibody should be able to conjugate to NPs and reach an ACE% of 100. However, because some carboxyl groups on the surface remain inactivated and some antibodies are unstable in certain chemical environments, the antibodies may degrade, resulting in reduced ACE%. In our study, addition of 10 or 20 ng/mL of antibody achieved around 80% conjugation efficiency. In contrast, addition of 40 ng/mL antibody resulted in a significant reduction in ACE% (74%) suggesting that the saturation limit is somewhere between 20 and 40 ng/mL.
The normal human vaginal environment is slightly acidic (pH 3.8–4.5), due to the production of lactic acid by lactobacilli.Citation55,Citation56 As a result, our in vitro release studies were conducted in slightly acidic buffer to mimic the pH environment of the human vaginal tract. In general, the release data showed that our formulation could provide sustained release of SQV from NPs without the formation of a degradation product, as observed in HPLC chromatograms (data not shown). Previous studies have shown that the release of encapsulated therapeutic agents from a matrix is mainly attributed to the diffusion of therapeutic agents during the early stages, while the later stages of release are a result of both drug diffusion and polymer degradation.Citation57 The in vitro release of SQV from NPs indicated a controlled-release profile, with 52% of total loaded drug released after 3 days. Moreover, a 43% burst release was observed within the first 24 hours, which may be a result of SQV released from the surface of NPs. Following the burst release, a controlled-release profile was observed with a constant 2%–3% of SQV released per day.
We further investigated whether incorporation of Ab-SQV-NPs into a gel-dosage form will affect the release of SQV. Our results showed a sustained release of SQV from the Ab-SQV-NP-loaded gel system, with approximately 3% of total loading released within 24 hours. In vitro release studies were further performed in Franz cells to mimic the vaginal environment more closely. The vaginal tract is covered by mucus, a naturally produced gel-like fluid that provides protection for the underlying mucosa. Cervicovaginal mucus is a low-viscosity fluid similar to blood serum, which is continuously secreted, distributed, and discarded.Citation58 The cervicovaginal mucus is a heterogeneous and complex system of intercommunicating channels formed by mucin fibers and filled with aqueous fluid. The cross-linked mucin fibers generate thousands of 3–10 μm network-shaped meshes in mucus.Citation59 When a topical vaginal agent is applied, it first interacts with the cervicovaginal mucus followed by drug release. In our study, we wanted to mimic in vivo drug release, particularly at low volumes. As a result, in our study, a 5-μm hydrophilic membrane was chosen to be sandwiched between the donor and receptor chambers of Franz cells to mimic drug delivery within the human vaginal tract. As expected, there was a significant reduction in drug release (about 0.2% release within 24 hours) in the Franz cell model, since there was a reduction in the contact between gel and media in the recipient chamber. Release of SQV from both NPs and gel was observed in both in vitro models, demonstrating that the gel did not hinder release of SQV. Although the release of SQV from an NP-loaded gel appears low (0.18% in 24 hours), it is within the in vitro IC50 for HIV.Citation37 Future studies will include increasing the release profile of SQV from our gel system by further optimizing the viscosity of the gel or modifying the surface of the NPs.
According to the results, Sup-T1 cells treated with Ab-SQV-NPs exhibited higher amounts of SQV intracellularly than cells treated with SQV-NP, indicating specific active targeting by Ab-SQV-NPs. The targeted delivery of SQV could be achieved rapidly in Sup-T1 cells, with peak intracellular concentrations of SQV within the first hour of treatment. Interestingly, the intracellular accumulation of SQV appeared to decrease with time and was independent of the type of treatment. Previous studies have shown that SQV can be transported out of the cell by the multidrug-resistant transporter P-glycoprotein (PGP). PGP is an adenosine triphosphate-driven drug-efflux pump, which is found to be highly expressed in the mucosa of the gastrointestinal tract and brain-capillary endothelial cells,Citation60–Citation63 but weakly expressed or unexpressed in human uterus and cervix.Citation64 As a result, it has been shown to be the main barrier that limits the oral availability and tissue penetration of SQV.Citation65,Citation66 More importantly, PGP is also found to be expressed on CD4+ T lymphocytes, the major target for HIV-1 infection.Citation67,Citation68 Therefore, the decreased intracellular concentration in Sup-T1 cells may be attributed to the efflux of SQV by PGP. When developing future formulations for SQV, it may be beneficial to coencapsulate a PGP inhibitor along with SQV. To further confirm the specific targeting ability of Ab-SQV-NPs, a CD4− vaginal epithelial cell line, VK2/E6E7, was also treated with both SQV-NPs and Ab-SQV-NPs. As expected, nonspecific uptake of SQV-NPs and Ab-SQV-NPs was observed, with no significant differences detected between the two treatment groups. In addition, the intracellular accumulation of SQV increased with time in VK2/E6E7 cells, suggesting that SQV is stable in the formulation for intracellular uptake and further supporting the observation that decreased cellular accumulation of SQV in Sup-T1 cells may largely be due to the efflux of SQV.
Studies have shown that SQV can be metabolized by the cytochrome P450 (CYP450) enzyme system, primarily CYP3A4.Citation69 Currently, there appears to be a lack of studies demonstrating CYP450 expression in human T cells. As mentioned earlier, we did not detect any SQV degradants/metabolites using our HPLC method. It is possible that CYP450 plays a role in metabolizing SQV and contributes to the decreased levels of SQV in cells, but further studies are required to confirm this.
Cytotoxicity is another important aspect when evaluating an intravaginal microbicide formulation. HEC was chosen as the main polymer for our intravaginal gel formulation, because several studies have shown that it is noncytotoxic in the presence of epithelial cell lines and peripheral blood mononuclear cells.Citation36 Clinical studies using HEC-containing gels have also demonstrated a high safety profile (94.3%).Citation37 Although previous studies have investigated the individual safety profiles of PLGA NPs and HEC gel, we decided to evaluate the maximum concentration of each individual component as well as in combination (drug-free Ab-NPs, 1% HEC placebo gel, and 1% HEC gel loaded with drug-free Ab-NPs [5 mg Ab-NPs/g gel]) that can be tolerated by vaginal epithelial cells. The VK2/E6E7 cell line was able to tolerate up to 1,000 μg/mL of PLGA-NPs alone, 200 mg/mL of 1% HEC placebo gel, and 1% HEC gel loaded with drug-free Ab-NPs (5 mg Ab-NPs/g gel) before cytotoxicity was observed. As a result, our formulation appears to be safe for vaginal applications.
Conclusion
Overall, our research group has developed a nanomedicine for the active delivery of SQV to CD4+ immune cells. Our study showed that the nanomedicine can be formulated to possess favorable particle size, high EE%, and sustained drug release. More importantly, it is noncytotoxic and can significantly increase SQV uptake by CD4+ cells. The nanomedicine was further formulated into a vaginal gel to provide ease in self-administration and to enhance retention within the vaginal tract. Overall, our delivery system demonstrates potential utility as a platform for vaginal microbicide development, but further studies are required to evaluate its effectiveness in the treatment/reduction of HIV/AIDS.
Acknowledgments
This work was supported in part by grants awarded to Dr Emmanuel A Ho from the Manitoba Health Research Council and the Dr Paul HT Thorlakson Foundation Fund. Sidi Yang is grateful for the financial support received from the Faculty of Graduate Studies International Graduate Student Entrance Scholarship, and Yufei Chen is grateful for the financial support received from the Manitoba Health Research Council Graduate Studentship. The authors are grateful to Dr Xiaochen Gu for providing us access to his rheometer and Franz cells. The authors would also like to thank Deborah Chan for her help with the nanoparticle preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkerRThe global HIV/AIDS pandemic, structural inequalities, and the politics of international healthAm J Public Health200292334334611867304
- Joint United Nations Programme on HIV/AIDSUNAIDS 2011 World AIDS Day Report – 2011GenevaUNAIDS2011
- World Health OrganizationWomen and Health: Today’s Evidence – Tomorrow’s AgendaGenevaWHO2009
- HladikFHopeTJHIV infection of the genital mucosa in womenCurr HIV/AIDS Rep200961202819149993
- SmithSMPre-exposure chemoprophylaxis for HIV: it is timeRetrovirology200411615238164
- YouleMWainbergMAPre-exposure chemoprophylaxis (PREP) as an HIV prevention strategyJ Int Assoc Physicians AIDS Care (Chic)20032310210514556428
- GargABNuttallJRomanoJThe future of HIV microbicides: challenges and opportunitiesAntivir Chem Chemother200919414315019374141
- VellaSFloridiaMSaquinavir. Clinical pharmacology and efficacyClin Pharmacokinet19983431892019533981
- MillerCJShattockRJTarget cells in vaginal HIV transmissionMicrobes Infect200351596712593974
- HladikFSakchalathornPBallweberLInitial events in establishing vaginal entry and infection by human immunodeficiency virus type-1Immunity200726225727017306567
- JoagSVAdanyILiZAAnimal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDSJ Virol1997715401640239094679
- GovenderTOjewoleENaidooPMackrajIPolymeric nanoparticles for enhancing antiretroviral drug therapyDrug Deliv200815849350118720133
- MallipeddiRRohanLCProgress in antiretroviral drug delivery using nanotechnologyInt J Nanomedicine2010553354720957115
- NowacekASMcMillanJMillerRAndersonARabinowBGendelmanHENanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-Infected macrophages: implications for neuroAIDS therapeuticsJ Neuroimmune Pharmacol20105459260120237859
- PeerDInduction of therapeutic gene silencing in leukocyte-implicated diseases by targeted and stabilized nanoparticles: a mini-reviewJ Control Release20101481636820624432
- McNeilSENanotechnology for the biologistJ Leukoc Biol200578358559415923216
- GandapuUChaitanyaRKKishoreGReddyRCKondapiAKCurcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication in vitroPlos One201168e2338821887247
- DouHDestacheCJMoreheadJRDevelopment of a macrophage-based nanoparticle platform for antiretroviral drug deliveryBlood200610882827283516809617
- ReddySTRehorASchmoekelHGHubbellJASwartzMAIn vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfde) nanoparticlesJ Control Release20061121263416529839
- ArrueboMValladaresMGonzalez-FernandezAAntibody-conjugated nanoparticles for biomedical applicationsJ Nanomaterials20092009439389
- DateAAShibataAGoedeMDevelopment and evaluation of a thermosensitive vaginal gel containing raltegravir+efavirenz loaded nanoparticles for HIV prophylaxisAntiviral Res201296343043623041201
- JainRAThe manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devicesBiomaterials200021232475249011055295
- MakadiaHKSiegelSJPoly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrierPolymers (Basel)2011331377139722577513
- CrottsGParkTGProtein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issuesJ Microencapsul19981566997139818948
- KranzHUbrichNMaincentPBodmeierRPhysicomechanical properties of biodegradable poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) films in the dry and wet statesJ Pharm Sci200089121558156611042603
- CampolongoMJLuoDDrug delivery: old polymer learns new tractsNat Mater20098644744819458640
- BichoAPecaINRoqueACACardosoMMAnti-CD8 conjugated nanoparticles to target mammalian cells expressing CD8Int J Pharm20103991–2808620696228
- FarokhzadOCChengJJTeplyBATargeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivoProc Natl Acad Sci USA2006103166315632016606824
- GeldenhuysWMbimbaTBuiTHarrisonKSutariyaVBrain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancersJ Drug Target201119983784521692650
- KamelSAliNJahangirKShahSMEl-GendyAAPharmaceutical significance of cellulose: a reviewExpress Polym Lett2008211758778
- TienDSchnaareRLKangFRIn vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trialsAids Res Hum Retroviruses2005211084585316225411
- RaoJPGeckelerKEPolymer nanoparticles: preparation techniques and size-control parametersProg Polym Sci2011367887913
- WoodrowKACuYBoothCJSaucier-SawyerJKWoodMJSaltzmanWMIntravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNANat Mater20098652653319404239
- MahalingamASimmonsA PUgaonkarSRVaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1Antimicrob Agents Chemother20115541650166021245437
- HladikFMcElrathMJSetting the stage: host invasion by HIVNat Rev Immunol20088644745718469831
- RohanLCMonclaBJKunjara Na AyudhyaRPIn vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicidePlos One201052e931020174579
- Abdool KarimQAbdool KarimSSFrohlichJAEffectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in womenScience201032959961168117420643915 Erratum in:Science2011333604252421798915
- MerrillD PManionDJChouTCHirschMSAntagonism between human immunodeficiency virus type 1 protease inhibitors indinavir and saquinavir in vitroJ Infect Dis199717612652689207379
- MallipeddiRRohanLCNanoparticle-based vaginal drug delivery systems for HIV preventionExpert Opin Drug Deliv201071374820017659
- DestacheCJBelgumTChristensenKShibataASharmaADashACombination antiretroviral drugs in PLGA nanoparticle for HIV-1BMC Infect Dis2009919820003214
- ShahLKAmijiMMIntracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDSPharm Res200623112638264516969696
- NowacekASBalkundiSMcMillanJAnalyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophagesJ Control Release2011150220421121108978
- ZhangTSturgisTFYouanBBCpH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmissionEur J Pharm Biopharm201179352653621736940
- das NevesJMichielsJArienKKPolymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirinePharm Res20122961468148422072053
- YangJLeeCHParkJAntibody conjugated magnetic PLGA nanoparticles for diagnosis and treatment of breast cancerJ Mater Chem2007172626952699
- YangLLMaoHWangYASingle chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imagingSmall20095223524319089838
- PeerDKarpJMHongSFarokhzadOCMargalitRLangerRNanocarriers as an emerging platform for cancer therapyNat Nanotechnol200721275176018654426
- DinauerNBalthasarSWeberCKreuterJLangerKvon BriesenHSelective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytesBiomaterials200526295898590615949555
- EndsleyANHoRJEnhanced anti-HIV efficacy of indinavir after inclusion in CD4-targeted lipid nanoparticlesJ Acquir Immune Defc Syndr2012614417424
- das NevesJAmijiMSarmentoBMucoadhesive nanosystems for vaginal microbicide development: friend or foe?Wiley Interdiscip Rev Nanomed Nanobiotechnol20113438939921506290
- MusumeciTVenturaCAGiannoneIPLA/PLGA nanoparticles for sustained release of docetaxelInt J Pharm20063251–217217916887303
- KuoYCChenHHEntrapment and release of saquinavir using novel cationic solid lipid nanoparticlesInt J Pharm20093651–220621318848610
- SharmaPGargSPure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugsAdv Drug Deliv Rev2010624–549150219931328
- SehgalDVijayIKA method for the high-efficiency of water-soluble carbodiimide-mediated amidationAnal Biochem1994218187918053572
- LarssonPGPlatz-ChristensenJJThe vaginal pH and leucocyte/epithelial cell ratio vary during normal menstrual cyclesEur J Obstet Gynecol Reprod Biol199138139411988325
- MumperRJBellMAWorthenDRFormulating a sulfonated antiviral dendrimer in a vaginal microbicidal gel having dual mechanisms of actionDrug Dev Ind Pharm200935551552419040181
- AndersonJMShiveMSBiodegradation and biocompatibility of PLA and PLGA microspheresAdv Drug Deliv Rev199728152410837562
- OwenDHKatzDFA vaginal fluid simulantContraception1999592919510361623
- CericFSilvaDVigilPUltrastructure of the human periovulatory cervical mucusJ Electron Microsc (Tokyo)200554547948416006441
- ThiebautFTsuruoTHamadaHGottesmanMMPastanIWillinghamMCCellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissuesProc Natl Acad Sci USA19878421773577382444983
- CordoncardoCObrienJPCasalsDMultidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sitesProc Natl Acad Sci USA19898626956982563168
- ThiebautFTsuruoTHamadaHGottesmanMMPastanIWillinghamMCImmunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle proteinJ Histochem Cytochem19893721591642463300
- CordoncardoCO’BrienJPBocciaJCasalsDBertinoJRMelamedMRExpression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissuesJ Histochem Cytochem1990389127712871974900
- FlensMJZamanGJRvan der ValkPTissue distribution of the multidrug resistance proteinAm J Pathol19961484123712478644864
- HuismanMTSmitJWWiltshireHRHoetelmansRMWBeijnenJHSchinkelAHP-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavirMol Pharmacol200159480681311259625
- AlsenzJSteffenHAlexRActive apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayersPharm Res19981534234289563072
- ValentinAMorrowMPoirierRHIdentification of a potential pharmacological sanctuary for HIV type 1 in a fraction of CD4(+) primary cellsAids Res Hum Retroviruses2010261798820059395
- BiningNMillerRACytokine production by subsets of CD4 memory T cells differing in P-glycoprotein expression: effects of agingJ Gerontol A Biol Sci Med Sci1997523B137B1459158547
- EaglingVAWiltshireHWhitcombeIWABackDJCYP3A4-mediated hepatic metabolism of the HIV-1 protease inhibitor saquinavir in vitroXenobiotica200232111711824416